Abstract
We describe a heteromolecular single crystal that exhibits three reversible and concerted reorganizations upon heating and cooling. The products of the reorganizations are conformational polymorphs. The reorganizations are postulated to proceed through three motions: (i) alkyl translations, (ii) olefin rotations, and (iii) rotational tilts. The motions are akin to rack-and-pinion gears at the molecular level. The rack-like movement is based on expansions and compressions of alkyl chains that are coupled with pinion-like 180° rotations of olefins. To accommodate the movements, phenol and thiophene components undergo rotational tilts about intermolecular hydrogen bonds. The movements are collective, being propagated in close-packed repeating units. This discovery marks a step to understanding how organic solids can support the development of crystalline molecular machines and devices through correlated and collective movements.
Keywords: cocrystal, hydrogen bond, molecular machine, solid-state chemistry, supramolecular chemistry
Molecular and supramolecular systems that exhibit movements under influences of external stimuli are of growing interest to chemists, physicists, and material scientists (1, 2). Such systems are expected to form a structural and dynamic basis of molecule-based machines and devices. In this context, it has been noted that the precise control of molecular and supramolecular organization that is required to bring together molecules that function as a working unit (3–7) can be fully realized in the solid state (8, 9). Such movements have been described by Garcia-Garibay to occur in solids via three possible pathways: free-volume processes, volume-conserving processes, or correlated motions (8–10). Correlated motions are considered to be of the greatest challenge to realize and develop because such motions require concerted displacements of multiple components (11–13) within a tightly condensed medium (14, 15).
Motions among molecules can be manifested either autonomously or collectively. In the former, the moving molecules are completely isolated from the surrounding environment (13), whereas, in the latter, the movements occur within repeating units (14, 15). An ideal medium to study motions of molecules is a single crystal, where the atomic-level structural information provided by x-ray diffraction can provide insight into the nature of movements (8). Movements in crystals that occur without disruption of the crystal lattice proceed via a single-crystal-to-single-crystal (SCSC) transformation. Despite being of enormous structural value, SCSC transformations are rare because the buildup of strain that accompanies the movements of atoms in such solids often result in a loss of single crystallinity and lattice structure (15). SCSC transformations that do not involve the formation or breakage of covalent bonds and result in a new crystal phase exhibit polymorphism. Although molecular and supramolecular systems that undergo autonomous movements have been the focus of a number of recent studies (e.g., ratchets, brakes) (1, 2), systems that exhibit collective behavior have been markedly less studied, which is, perhaps, not surprising considering the subtleties of movements in solids and the burgeoning nature of the field of crystal engineering, particularly as it relates to dynamics (15).
With this in mind, we report here a heteromolecular single crystal that exhibits concerted and collective motions upon application of an external stimulus in a cooling and heating cycle. The motions of the components, which interact via hydrogen bonds, are reversible and consist of three distinct movements: (i) alkyl translations, (ii) olefin rotations, and (iii) aromatic tilting. We show that the movements in the single crystal, which occur despite a lack of free volume, are akin to collective rack-and-pinion gears at the molecular level. We have characterized the movements as three SCSC transformations, with the solids being related as conformational polymorphs (16–18). The development and study of the concerted movements and single-crystal transformations may be considered a step to understanding how movements in molecular solids can support the development of correlated and collective crystalline molecular machines and devices (15).
Results and Discussion
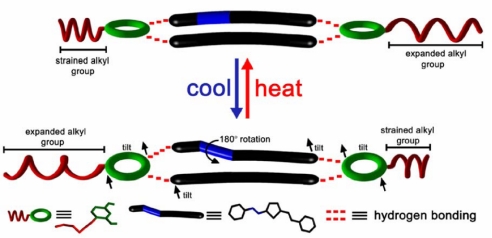
The single crystal that is the focus of this study is 2(DPVT)·2(4HR) [DPVT, all-trans-2,5-bis(4-ethenylpyridyl)thiophene; 4HR, 4-hexylresorcinol]. The two molecules self-assemble to form a four-component molecular aggregate held together by four O H⋯N hydrogen bonds. The DPVT molecules are stacked in a face-to-face geometry by the 4HR molecules. The single crystal, in general, is composed of alkyl chains, olefinic bonds, and aromatic rings (Fig. 1). On cooling and subsequent heating, the alkyl chains of 4HR undergo reversible expansions and compressions (19–23), a carbon–carbon double bond (C
H⋯N hydrogen bonds. The DPVT molecules are stacked in a face-to-face geometry by the 4HR molecules. The single crystal, in general, is composed of alkyl chains, olefinic bonds, and aromatic rings (Fig. 1). On cooling and subsequent heating, the alkyl chains of 4HR undergo reversible expansions and compressions (19–23), a carbon–carbon double bond (C C) of DPVT undergoes a reversible rotation of 180°, and the aromatic rings of 4HR and DPVT undergo reversible tilting about the hydrogen bonds. Although the translations, rotations, and tilts of the kind described here are known to proceed in the solid state (18, 24–28), the three processes have, to our knowledge, not been observed in the same solid. Our x-ray structural data also suggests that the tilting of the aromatics precedes the translations and rotations of the alkyl groups and C
C) of DPVT undergoes a reversible rotation of 180°, and the aromatic rings of 4HR and DPVT undergo reversible tilting about the hydrogen bonds. Although the translations, rotations, and tilts of the kind described here are known to proceed in the solid state (18, 24–28), the three processes have, to our knowledge, not been observed in the same solid. Our x-ray structural data also suggests that the tilting of the aromatics precedes the translations and rotations of the alkyl groups and C C bond.
C bond.
Fig. 1.
Schematic of the alkyl translational motion, olefin rotation, and aromatic group tilts within the tightly packed heteromolecular crystal.
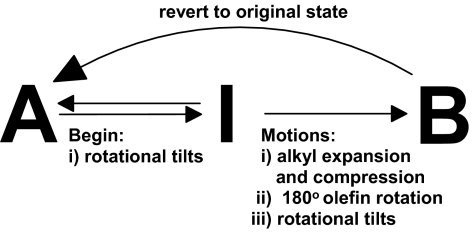
The single crystal 2(DPVT)·2(4HR) exhibits three phases: A, B, and I (Table 1). The three phases are conformational polymorphs. An x-ray structure determination at room temperature afforded a structure in the space group P21/c, which we term phase A. When the crystal was cooled and held at 220 K for 15 min, the crystal converted to a new stable phase in the space group P2/c, which we term phase B. The conversion was evidenced by the complete disappearance of the original diffraction intensity of phase A and the appearance of phase B. Warming the crystal to 240 K, or above, resulted in the restoration of phase A within minutes. Remarkably, the cycle could be repeated multiple times without loss of the single crystallinity. When cooled to 190 K, the formation of phase B did not occur for 8 h. Moreover, upon immediate cooling of phase A to 180 K, phase B did not form after 24 h. Instead, a third, intermediate phase in the space group P21/c, which we term phase I, formed after 24 h (Scheme 1). Phase I also converted to phase B upon being held at 180 K for a total of 40 h. These observations are consistent with phase I being captured as a metastable phase. Phase B was stable indefinitely below 230 K.
Table 1.
Crystallographic parameters for phases A, B, and I
| Parameter | Phase A | Phase B | Phase C |
|---|---|---|---|
| Crystal system | Monoclinic | Monoclinic | Monoclinic |
| Space group | P21/c | P2/c | P21/c |
| a, Å | 8.647(1) | 16.640(2) | 17.254(2) |
| b, Å | 21.097(2) | 10.606(1) | 21.111(2) |
| c, Å | 28.838(3) | 29.457(3) | 28.905(3) |
| β, ° | 92.622(5) | 93.605(5) | 92.604(5) |
| Volume, Å3 | 5,255.4(2) | 5,188.1(9) | 10,517(2) |
| Z | 8 | 8 | 16 |
| ρcalc, g/cm3 | 1.23 | 1.24 | 1.22 |
| R1 | 0.050 | 0.048 | 0.118 |
| No. of reflections I > 2σ(I) | 5,091 | 5,991 | 3,577 |
Scheme 1.
Relationships of phases A, B, and I.
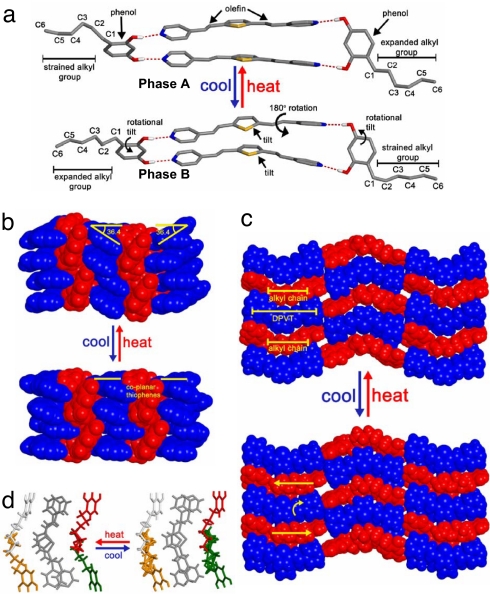
The asymmetric unit of phase A consists of two molecules of DPVT and two molecules of 4HR. The DPVT and 4HR molecules form a four-component assembly held together by four O H⋯N hydrogen bonds (O
H⋯N hydrogen bonds (O H⋯N distances: 2.76–2.78 Å) (Fig. 2a). The assembly exhibits pseudo-C2 symmetry and, therefore, is chiral. The chirality arises from the C-shape of the DPVT molecules, which lie stacked and parallel, and an antiparallel arrangement of the 4HR molecules within the aggregate. The chiral aggregates are present as a racemate. The C
H⋯N distances: 2.76–2.78 Å) (Fig. 2a). The assembly exhibits pseudo-C2 symmetry and, therefore, is chiral. The chirality arises from the C-shape of the DPVT molecules, which lie stacked and parallel, and an antiparallel arrangement of the 4HR molecules within the aggregate. The chiral aggregates are present as a racemate. The C C bonds of each assembly lie parallel with separations of 3.60–3.90 Å (Fig. 2a). The aromatic rings of 4HR are inclined by 75.6° and 56.5° with respect to the DPVT molecules. Both DPVT molecules are surrounded on each side by two hexyl groups (Fig. 2c). One hexyl group of one 4HR molecule exists in a strained conformation that is based on three gauche (C2–C3, C3–C4, and C4–C5) interactions. The atoms of one gauche conformation (C4–C5) exhibit disorder (occupancy C4–C5: 46% antiparallel, 54% gauche), which results in an average bond strain energy of 2.1 kJ/mol (29). The disorder may be static or dynamic; nevertheless, the total strain energy of the alkyl chain, as based on the sum of all steric interactions within the chain, is 9.7 kJ/mol. The hexyl group of the second 4HR is ordered and contains only a single gauche interaction (C3–C4), which corresponds to 3.8 kJ/mol. The aggregates pack in a herringbone motif, as defined by the 36.4° tilt of the thiophene rings (Fig. 2b). This packing leads to an environment that is deficient in free volume and, thus, is tightly packed. Indeed, the packing coefficient of phase A is 0.67, which is in line with organic solids composed of aromatic and hydrocarbon functionalities (30).
C bonds of each assembly lie parallel with separations of 3.60–3.90 Å (Fig. 2a). The aromatic rings of 4HR are inclined by 75.6° and 56.5° with respect to the DPVT molecules. Both DPVT molecules are surrounded on each side by two hexyl groups (Fig. 2c). One hexyl group of one 4HR molecule exists in a strained conformation that is based on three gauche (C2–C3, C3–C4, and C4–C5) interactions. The atoms of one gauche conformation (C4–C5) exhibit disorder (occupancy C4–C5: 46% antiparallel, 54% gauche), which results in an average bond strain energy of 2.1 kJ/mol (29). The disorder may be static or dynamic; nevertheless, the total strain energy of the alkyl chain, as based on the sum of all steric interactions within the chain, is 9.7 kJ/mol. The hexyl group of the second 4HR is ordered and contains only a single gauche interaction (C3–C4), which corresponds to 3.8 kJ/mol. The aggregates pack in a herringbone motif, as defined by the 36.4° tilt of the thiophene rings (Fig. 2b). This packing leads to an environment that is deficient in free volume and, thus, is tightly packed. Indeed, the packing coefficient of phase A is 0.67, which is in line with organic solids composed of aromatic and hydrocarbon functionalities (30).
Fig. 2.
X-ray crystal structures. (a) A single 2(DPVT)·2(4HR) assembly of phase A and B that highlights the two different alkyl group configurations. (b) Space-filling view that highlights the herringbone packing of phase A and the coplanar packing of phase B. (c) Arrangement of the DPVT (blue) and 4HR (red) molecules of phase A and B. (d) Wire-frame view that illustrates the ordered and disordered alkyl groups (white, red, green, orange) in phases A and B.
Cooling and holding the single crystal at 190 K for 8 h afforded phase B, which is in the space group P2/c. The asymmetric unit consists of two complete molecules of DPVT and two complete molecules of 4HR. As in phase A, the two DPVT and 4HR molecules make up chiral, four-component aggregates held together by four O H⋯N hydrogen bonds (O
H⋯N hydrogen bonds (O H⋯N distances: 2.76–2.80 Å) (Fig. 2a), which means that the hydrogen bonds were maintained during the transition from phase A to phase B. As a consequence of the cooling, the molecules of the single crystal undergo a number of concerted reorganizations in the solid.
H⋯N distances: 2.76–2.80 Å) (Fig. 2a), which means that the hydrogen bonds were maintained during the transition from phase A to phase B. As a consequence of the cooling, the molecules of the single crystal undergo a number of concerted reorganizations in the solid.
The most strained hexyl group of phase A undergoes two different and crystallographically separate expansions to yield two different conformations in phase B. The first expansion yields an ordered, relaxed chain with a single gauche conformation (C3–C4), which corresponds to 3.8 kJ/mol of strain energy (Fig. 2d, red). The second expansion yields a disordered chain with one antiparallel and three partial gauche conformations (occupancy C1–C2: 100% gauche, occupancy C2–C3: 48% gauche, occupancy C3–C4: 100% antiparallel, and occupancy C4–C5: 24% gauche) for a total of 6.5 kJ/mol of strain energy (Fig. 2d, green). Thus, the first and second expansions involve the release of 5.9 kJ/mol and 3.2 kJ/mol of energy, respectively. The second and more relaxed hexyl chain of phase A also experiences two different and crystallographically separate processes. In one case, the chain does not exhibit movement and, thus, remains ordered, containing a single gauche interaction (C3–C4) (Fig. 2d, white). In the second case, the chain undergoes a compression to give one antiparallel and three partial gauche conformations (occupancy C1–C2: 100% gauche, occupancy C2–C3: 48% gauche, occupancy C3–C4: 100% antiparallel, and occupancy C4–C5: 24% gauche). This finding corresponds to a buildup of 2.7 kJ/mol of energy for a total strain energy of 6.5 kJ/mol (Fig. 2d, orange). The expansions and compression mean that the C and H atoms of the alkyl groups experience translational movements. It is these four events involving two crystallographically different chains that account for the reduction in crystal symmetry from phase A to phase B.
In addition to translational movements of alkyl groups, a C C bond of one olefin undergoes a rotation of 180° (Fig. 3) (24–27). This motion places the stacked C
C bond of one olefin undergoes a rotation of 180° (Fig. 3) (24–27). This motion places the stacked C C bonds in a criss-cross conformation with separations of 3.5–4.0 Å. The C
C bonds in a criss-cross conformation with separations of 3.5–4.0 Å. The C C bond that rotates is the only C
C bond that rotates is the only C C bond surrounded on both sides by alkyl chains that experience translational motion. The remaining three C
C bond surrounded on both sides by alkyl chains that experience translational motion. The remaining three C C bonds are stationary, being surrounded on at least one side by a group that does not move upon cooling. The olefin that rotates is completely surrounded by well ordered alkyl groups in phase A, with the C
C bonds are stationary, being surrounded on at least one side by a group that does not move upon cooling. The olefin that rotates is completely surrounded by well ordered alkyl groups in phase A, with the C H(olefin) H atoms exhibiting a tongue-in-groove fit with the C
H(olefin) H atoms exhibiting a tongue-in-groove fit with the C H(alkyl) moieties (Fig. 3c). We postulate that the rotation of the C
H(alkyl) moieties (Fig. 3c). We postulate that the rotation of the C C bond is accompanied by van der Waals repulsions involving the alkyl groups that experience the translations. Such concerted motions between the alkyl and olefinic components are not expected to generate free volume (22). The packing coefficient slightly increases to 0.68 on going from phase A to B. The concerted motions, therefore, are expected to be correlated.
C bond is accompanied by van der Waals repulsions involving the alkyl groups that experience the translations. Such concerted motions between the alkyl and olefinic components are not expected to generate free volume (22). The packing coefficient slightly increases to 0.68 on going from phase A to B. The concerted motions, therefore, are expected to be correlated.
Fig. 3.
X-ray crystal structures. (a) Phase A. (b) Phase B, highlighting the correlated motions of the alkyl and olefin groups (blue). (c) van der Waals surfaces (red) of the alkyl and olefinic components for phase A.
Although the hexyl chains experience translations and a C C bond experiences a rotation, the aromatic rings of the 4HR and DPVT molecules undergo tilts. In particular, the 4HR molecules tilt by 4.6° and 12.8° to become inclined by 88.4° and 61.1°, respectively, with respect to the DPVT molecules. The thiophene rings of the DPVT molecules also tilt by 36.4° to lie coplanar along the b axis (Fig. 2b). This tilting disrupts the herringbone packing of phase A. Indeed, that the hydrogen bonds remain intact and accommodate the tilting of the rings during the transition of phase A to phase B attests to the strength and flexibility of the noncovalent force.
C bond experiences a rotation, the aromatic rings of the 4HR and DPVT molecules undergo tilts. In particular, the 4HR molecules tilt by 4.6° and 12.8° to become inclined by 88.4° and 61.1°, respectively, with respect to the DPVT molecules. The thiophene rings of the DPVT molecules also tilt by 36.4° to lie coplanar along the b axis (Fig. 2b). This tilting disrupts the herringbone packing of phase A. Indeed, that the hydrogen bonds remain intact and accommodate the tilting of the rings during the transition of phase A to phase B attests to the strength and flexibility of the noncovalent force.
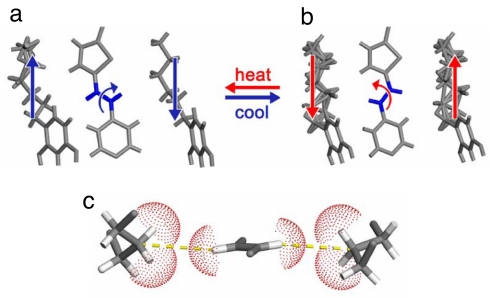
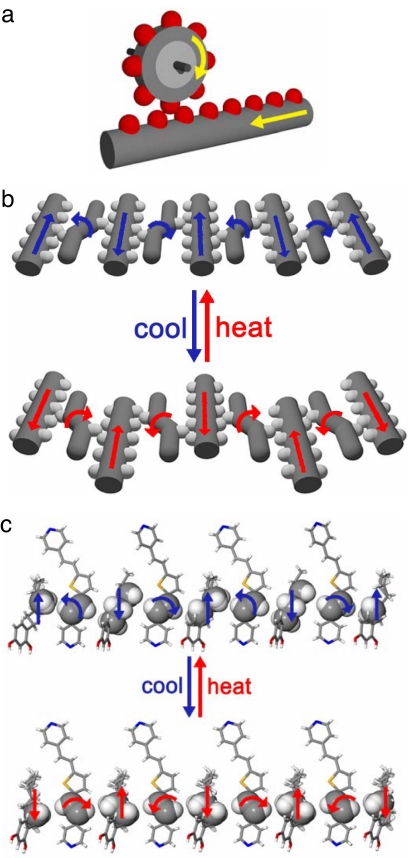
Phases A and B are conformational polymorphs. Although expansions and compressions of alkyl groups and rotations of C C bonds have been reported in the solid state, in this case both movements have been observed in the same solid. Moreover, the concerted reorganizations of the components of the single crystal can be considered analogous to collective rack-and-pinion gears at the molecular level (Fig. 4) (31). In this setting, the alkyl groups play the role of a rack gear that experiences translational movement. Owing to the van der Waals contacts between the alkyl groups and C
C bonds have been reported in the solid state, in this case both movements have been observed in the same solid. Moreover, the concerted reorganizations of the components of the single crystal can be considered analogous to collective rack-and-pinion gears at the molecular level (Fig. 4) (31). In this setting, the alkyl groups play the role of a rack gear that experiences translational movement. Owing to the van der Waals contacts between the alkyl groups and C C bond, the movement of the rack gear supports a correlated rotation of a olefinic pinion gear, which results in a conversion of linear motion to rotary motion at the molecular scale. The reorganizations are propagated infinitely throughout the single crystal, which makes the movement collective. The tilting experienced by the aromatics between the two phases can be considered to support the transfer of the concerted translations and rotations, which is afforded by the strength and flexibility of the hydrogen bonds.
C bond, the movement of the rack gear supports a correlated rotation of a olefinic pinion gear, which results in a conversion of linear motion to rotary motion at the molecular scale. The reorganizations are propagated infinitely throughout the single crystal, which makes the movement collective. The tilting experienced by the aromatics between the two phases can be considered to support the transfer of the concerted translations and rotations, which is afforded by the strength and flexibility of the hydrogen bonds.
Fig. 4.
Illustrations of rack-and-pinion gear (a), schematic (b), and x-ray crystal structure (c) of the components of 2(DPVT)·2(4HR) behaving as rack-and-pinion gears.
On immediate cooling phase A to 180 K, a new set of diffraction intensities was observed after a period of 24 h. The intensities for phase B were not observed during this period. This new phase, which is in the space group P21/c, is a third conformational polymorph that we term phase I. The asymmetric unit contains four independent DPVT and four independent 4HR molecules, which effectively doubles the size of the unit cell. The change in the unit cell is the result of slight tilts (<3°) of the 4HR and DPVT molecules. The tilts disrupt the symmetry of the hydrogen-bonded aggregates. On going from phase A to phase I, the most strained alkyl group of phase A becomes more disordered, whereas the second chain remains ordered. The overall energetic strain in the alkyl groups, however, remains constant. The packing coefficient of phase I is 0.66, which is less than that of both phase A (0.67) and phase B (0.68). Phase I converts to phase B on being held at 180 K for 40 h. This means that phase I was trapped as a metastable phase between phases A and B. The slight tilts in phase I, thus, precede the translational and rotational movements, as well as the larger tilts, of phase B. Similar to phase B, warming of phase I to 240 K results in the restoration of phase A within minutes.
In this article, we have described a heteromolecular single crystal that exhibits three conformational polymorphs related by reversible SCSC transformations. The transitions occur through translations, rotations, and tilts that are akin to collective rack-and-pinion gears at the molecular level. The molecular movements are concerted, being among alkyl, olefinic, and aromatic functionalities. That the movements occur in the absence of free volume is consistent with the motion being correlated. These observations attest to the degree of motion that can be experienced by a single crystal and suggests that solids that exhibit more complex motion expected for the construction of molecular machines and devices can be pursued and developed (32, 33).
Materials and Methods
All chemicals were purchased from Aldrich, unless otherwise noted. 4-Vinylpyridine was distilled before use. A round-bottom flask was charged with 2,5-diiodothiophene (1.65 g, 5 mmol), 4-vinylpyridine (1.55 g, 15 mmol), PdCl2(PPh3)2 (175 mg, 5 mol %), and 0.3 ml of tributylamine in 75 ml of 57 g/liter K2CO3 solution. The mixture was kept under reflux conditions for a period of 8 h. The solid product was collected by filtration and washed with H2O. The product was taken into 300 ml of CHCl3, washed two times with H2O, and dried (MgSO4), and the solvent was removed on a rotary evaporator to yield ≈900 mg of an orange solid. The product was purified by column chromatography using neutral alumina and CH2Cl2 as the eluent. DPVT then was recrystallized from CH3CN and passed through neutral alumina by using 96:4 diethyl ether:methanol. Subsequent recrystallization from CH3CN yielded a crystalline compound (200 mg, 14.2% yield).
Single crystals of 2(DPVT)·2(4HR) were synthesized via slow evaporation of a 1:1 solution of DPVT and 4HR in acetonitrile over the course of 1 day. The solid was characterized via 1H NMR spectroscopy and single-crystal x-ray diffraction. All solid-state transformations were monitored via single-crystal x-ray diffraction. All crystal data were measured on a Nonius Kappa CCD single-crystal x-ray diffractometer. Structure solution, refinement, and packing coefficients were accomplished with the SHELX-97 software package (34). Crystallographic data and refinement details have been deposited with the Cambridge Structural Database, Cambridge Crystallographic Data Centre [CSD reference nos. 641325 (Phase A), 641326 (Phase B), and 641327 (Phase I)]. Crystal data for Phase A: monoclinic, P21/c, a = 8.647(1) Å, b = 21.097(2) Å, c = 28.838(3) Å, β = 92.622(5)°, V = 5,255.4(2) Å3, Z = 8, ρcalc = 1.23 g/cm3, R1 = 0.050 for 5,091 reflections with I > 2σ(I). Crystal data for Phase B: monoclinic, P2/c, a = 16.640(2) Å, b = 10.606(1) Å, c = 29.457(3) Å, β = 93.605 (5)°, V = 5,188.1(9) Å3, Z = 8, ρcalc = 1.24 g/cm3, R1 = 0.048 for 5,991 reflections with I > 2σ(I). Crystal data for Phase I: monoclinic, P21/c,a = 17.254(2) Å, b = 21.111(2) Å, c = 28.905(3) Å, β = 92.604(5)°, V = 10,517(2) Å3, Z = 16, ρcalc = 1.22 g/cm3, R1 = 0.118 for 3,577 reflections with I > 2σ(I).
ACKNOWLEDGMENTS.
We are grateful to the University of Iowa Mathematical and Physical Sciences Funding program for support of this work.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates have been deposited in the Cambridge Structural Database, Cambridge Crystallographic Data Centre, Cambridge CB2 1EZ, United Kingdom [CSD reference nos. 641325 (Phase A), 641326 (Phase B), and 641327 (Phase I)].
References
- 1.Kay ER, Leigh DA, Zerbetto F. Angew Chem Int Ed. 2007;46:72–191. doi: 10.1002/anie.200504313. [DOI] [PubMed] [Google Scholar]
- 2.Kottas GS, Clarke LI, Horinek D, Michl J. Chem Rev. 2005;105:1281–1376. doi: 10.1021/cr0300993. [DOI] [PubMed] [Google Scholar]
- 3.Feringa BL, van Delden RA, Koumura N, Geertsema EM. Chem Rev. 2000;100:1789–1816. doi: 10.1021/cr9900228. [DOI] [PubMed] [Google Scholar]
- 4.Iwamura I, Mislow K. Acc Chem Res. 1988;21:175–182. [Google Scholar]
- 5.Kelly TR, Bowyer MC, Bhaskar KV, Bebbington D, Garcia A, Lang F, Kim MH, Jette MP. J Am Chem Soc. 1994;116:3657–3658. [Google Scholar]
- 6.Mislow K. Chemtracts Org Chem. 1988;2:151–174. [Google Scholar]
- 7.Balzani V, Credi A, Raymo FM, Stoddart JF. Angew Chem Int Ed. 2000;39:3348–3391. doi: 10.1002/1521-3773(20001002)39:19<3348::aid-anie3348>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 8.Khuong T-AV, Nunez JE, Godinez CE, Garcia-Garibay MA. Acc Chem Res. 2006;39:413–422. doi: 10.1021/ar0680217. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Garibay MA. Proc Natl Acad Sci USA. 2005;102:10771–10776. doi: 10.1073/pnas.0502816102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Natarajan A, Mague JT, Venkatesan K, Arai T, Ramamurthy V. J Org Chem. 2006;71:1055–1059. doi: 10.1021/jo0520644. [DOI] [PubMed] [Google Scholar]
- 11.Karlen SD, Garcia-Garibay MA. Top Curr Chem. 2005;262:179–227. [Google Scholar]
- 12.Jarowski PD, Houk KN, Garcia-Garibay MA. J Am Chem Soc. 2007;129:3110–3117. doi: 10.1021/ja0637907. [DOI] [PubMed] [Google Scholar]
- 13.Shirai Y, Osgood AJ, Zhao Y, Kelly KF, Tour JM. Nano Lett. 2005;5:2330–2334. doi: 10.1021/nl051915k. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Flood AH, Bonvallet PA, Vignon SA, Northrop BH, Tseng H-R, Jeppesen JO, Huang TJ, Brough B, Baller M, et al. J Am Chem Soc. 2005;127:9745–9759. doi: 10.1021/ja051088p. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Garibay MA. Angew Chem Int Ed. 2007;46:8945–8947. doi: 10.1002/anie.200702443. [DOI] [PubMed] [Google Scholar]
- 16.Bernstein J. Polymorphism in Molecular Crystals. Oxford: Clarendon; 2002. [Google Scholar]
- 17.Dunitz JD. Acta Crystallogr B. 1995;51:619–631. [Google Scholar]
- 18.Hollingsworth MD, Peterson ML, Pate KL, Dinkelmeyer BD, Brown ME. J Am Chem Soc. 2002;124:2094–2095. doi: 10.1021/ja010327f. [DOI] [PubMed] [Google Scholar]
- 19.Hannak RB, Farber G, Konrat R, Krautler B. J Am Chem Soc. 1997;119:2313–2314. [Google Scholar]
- 20.Hollingsworth MD, Werner-Zwanziger U, Brown ME, Chaney JD, Huffman JC, Harris KDM, Smart SP. J Am Chem Soc. 1999;121:9732–9733. [Google Scholar]
- 21.Ajami D, Rebek J., Jr J Am Chem Soc. 2006;128:15038–15039. doi: 10.1021/ja064233n. [DOI] [PubMed] [Google Scholar]
- 22.Rebek J., Jr Chem Commun. 2007;2007:2777–2789. doi: 10.1039/b617548a. [DOI] [PubMed] [Google Scholar]
- 23.Guillaume F, El Baghdadi A, Dianoux AJ. Phys Scr T. 1993;49:691–698. [Google Scholar]
- 24.Harada J, Ogawa K. J Am Chem Soc. 2001;123:10884–10888. doi: 10.1021/ja011197d. [DOI] [PubMed] [Google Scholar]
- 25.Harada J, Ogawa K. J Am Chem Soc. 2004;126:3539–3544. doi: 10.1021/ja038203l. [DOI] [PubMed] [Google Scholar]
- 26.Harada J, Harakawa M, Ogawa K. Acta Crystallogr B. 2004;60:589–597. doi: 10.1107/S0108768104016623. [DOI] [PubMed] [Google Scholar]
- 27.Ohba S, Hosomi H, Ito Y. J Am Chem Soc. 2001;123:6349–6352. doi: 10.1021/ja0034287. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt A, Kababya S, Appel M, Khatib S, Botoshansky M, Eichen Y. J Am Chem Soc. 1999;121:11291–11299. [Google Scholar]
- 29.Solomons TWG, Fryhle CB. Organic Chemistry. 7th Ed. New York: Wiley; 1998. p. 152. [Google Scholar]
- 30.Eckhardt CJ, Gavezzotti A. J Phys Chem B. 2007;111:3430–3437. doi: 10.1021/jp0669299. [DOI] [PubMed] [Google Scholar]
- 31.Chiaravalloti F, Gross LC, Rieder K-H, Stojkovic SM, Gourdon A, Joachim C, Moresco F. Nat Mater. 2007;6:30–33. doi: 10.1038/nmat1802. [DOI] [PubMed] [Google Scholar]
- 32.Kobatake S, Takami S, Muto H, Ishikawa T, Irie M. Nature. 2007;446:778–781. doi: 10.1038/nature05669. [DOI] [PubMed] [Google Scholar]
- 33.Irie M, Kobatake S, Horichi M. Science. 2001;291:1769–1772. doi: 10.1126/science.291.5509.1769. [DOI] [PubMed] [Google Scholar]
- 34.Sheldrick GM. SHELXL-97: A Program for Crystal Structure Refinement. Göttingen, Germany: Univ of Göttingen; 1997. [Google Scholar]