Abstract
Neurite extension and growth cone navigation are guided by extracellular cues that control cytoskeletal rearrangements. However, understanding the complex signaling mechanisms that mediate neuritogenesis has been limited by the inability to biochemically separate the neurite and soma for spatial proteomic and bioinformatic analyses. Here, we apply global proteome profiling in combination with a neurite purification methodology for comparative analysis of the soma and neurite proteomes of neuroblastoma cells. The spatial relationship of 4,855 proteins were mapped, revealing networks of signaling proteins that control integrins, the actin cytoskeleton, and axonal guidance in the extending neurite. Bioinformatics and functional analyses revealed a spatially compartmentalized Rac/Cdc42 signaling network that operates in conjunction with multiple guanine-nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs) to control neurite formation. Interestingly, RNA interference experiments revealed that the different GEFs and GAPs regulate specialized functions during neurite formation, including neurite growth and retraction kinetics, cytoskeletal organization, and cell polarity. Our findings provide insight into the spatial organization of signaling networks that enable neuritogenesis and provide a comprehensive system-wide profile of proteins that mediate this process, including those that control Rac and Cdc42 signaling.
Keywords: GAPs, GEFs, neuritogenesis, proteomics, Rho GTPase
Neuritogenesis is a dynamic process involving the extension of long, thin protrusions called neurites that will subsequently differentiate into long axons or an elaborate dendritic arbor. This highly polarized process occurs through the segmentation from the soma periphery of a microtubule-rich shaft capped with a growth cone, which is itself characterized by an actin-rich lamellipodium with numerous filopodial extensions and integrin-mediated adhesive contacts (1). Understanding this process is crucial, because it is necessary for proper wiring of the brain and nerve regeneration, and has been linked to numerous neurodegenerative diseases (2).
Although cultured neurons randomly form neurites in vitro, in vivo this process is orchestrated by gradients of chemoattractants and/or extracellular matrix proteins that precisely guide neurite initiation and advancement. This occurs in a polarized and highly controlled manner and relies on spatially regulated mechanisms for gradient sensing, membrane trafficking, integrin-mediated adhesion, and organization of the actin-microtubule cytoskeletons (3). Although progress has been made in identifying such spatially regulated signals, this work has been limited primarily to single cell analyses, using imaging-based techniques, precluding a large-scale view of these signaling events during neuritogenesis.
Recently, we described a method for the purification of pseudopodia from migrating cells, using a microporous filter system (4). This model system, combined with contemporary large-scale protein mass spectrometry (LC-MS/MS), provided global insight into the spatial organization of the signaling networks that control this process. Here, we extend this technique to neuroblastoma cells enabling the specific isolation of the neurite and soma compartments for biochemical analyses. Comparative analysis of the neurite and soma proteomes revealed the spatial relationship of thousands of proteins and specific signaling networks that operate in these distinct cellular compartments. In addition, using bioinformatics and cell-based RNA interference approaches, we address a fundamental question pertinent to Rho family small GTPases, which couple extracellular cues to the cytoskeleton during neuritogenesis (3). Although it is clear that Rac and Cdc42 play a fundamental role in regulating neurite extension, it is not understood why regulation of their activity relies on redundant upstream regulators such as guanine-nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs) (5, 6). These proteins are widely expressed and outnumber their GTPase targets by a factor of three (5, 6). This prompted us to investigate the spatial complexity of the Rac/Cdc42 signaling networks in the soma and neurite and determine the functional relevance of multiple neurite-enriched GEFs and GAPs. Surprisingly, we find that the different Cdc42/Rac GEFs and GAPs control unique cellular functions, which cooperate to fine tune neurite formation, rather than solely regulating neurite extension as proposed previously (3).
Results
Biochemical Purification of Neurites and Somata.
To specifically isolate neurite and soma proteins, we used NIE-115 neuroblastoma cells as a model system for neuritogenesis. These cells can be grown in large numbers for large-scale biochemical analysis and have been well characterized for their neuron-like properties. Upon serum starvation in neuron differentiation medium, these cells express the neurofilament protein and readily extend neurite-like processes that are morphologically similar to that of primary neurons (7). Indeed, these neurites form actin-rich growth cones connected to a tubulin-rich shaft that can respond to directional cues including immobilized extracellular matrix proteins (ECM) and soluble extension and collapsing factors (8). When plated on the top of 3.0-μm porous filters coated on the lower surface with the ECM laminin, neurite extension occurs through the small pores exclusively to the lower surface of the filter [Fig. 1A and supporting information (SI) Appendix, Fig. 4A]. This response occurs within 24 h (Fig. 1 B and C), exclusively on the laminin matrix (SI Appendix, Fig. 4B), is mediated by β1-integrins (SI Appendix, Fig. 4C), and is robust with >80% of somata extending neurites (SI Appendix, Fig. 4D) with a mean of 1.8 ± 0.8 neurites per cell (five fields of view, n = 85 cells, data not shown). Polarized neurite extension also works on fibronectin (SI Appendix, Fig. 4E) and with pheochromocytoma PC-12 cells on laminin and fibronectin (SI Appendix, Fig. 4 F and G). The polarized neurites and their somata can then be stained and visualized by microscopy or manually separated from either side of the filter into the appropriate lysis buffer for protein concentration determination and biochemical analyses as described in Material and Methods. Confocal imaging through the upper and lower filter surface illustrates the high level of morphological polarity (Fig. 1D) achieved by these cells after stimulation with ECM proteins and reveals a typical neurite morphology consisting of an F-actin rich growth cone with numerous filopodia attached to a dense tubulin-rich shaft (Fig. 1C). Biochemical and signaling polarity are illustrated by the selective localization of nuclear histones in the soma and elevated phosphotyrosine (Fig. 1E), Cdc42, Rac (Fig. 1F), and ERK activation (Fig. 1G) in the neurite compartment. It is important to note that, because the neurite fraction contains much less protein than the soma fraction (typically 40 μg of neurite versus 800 μg of soma lysate for 1.2 × 106 cells) and because multiple neurites often emanate from one neuron, direct comparison of neurite-soma equivalents on a per-filter basis is not possible. However, as described for the pseudopodia purification system (4), this issue can be easily resolved by assuming equal protein density in the neurite and soma fractions, which allows for normalization based on protein concentration. As expected, when equal amounts of neurite and soma lysate were analyzed from cells expressing green fluorescent protein (GFP), which acts as a soluble exogenous protein marker, GFP was equally abundant in the neurite and soma fractions (SI Appendix, Fig. 5A). This is also true for the cytosolic protein Erk2, which serves as a loading control in these biochemical experiments (SI Appendix, Fig. 5A). However, the possibility remained that our protein profile reflected an enrichment of plasma membrane versus cytosolic proteins, rather than real changes in protein abundance in both subdomains. This was excluded by the finding that an exogenously transfected GFP-fusion with the C-terminal CAAX domain of K-Ras4b, which localizes exclusively to the plasma membrane, was equally abundant in the neurite and soma fractions (SI Appendix, Fig. 5A). Together, these findings demonstrate that microporous filters coated with ECM proteins provide a simple system to selectively purify large numbers of neurites from their somata for biochemical analysis.
Fig. 1.
Neurite purification assay, biochemical, and proteomic analyses. (A) Schematic of microporous filter system. (B) Neurite lysate protein amount on the filter bottom was determined for the indicated times from filters coated with laminin on the top, the bottom, or both sides. Standard deviations from three independent experiments are shown. (C) Fluorescence micrographs of α-tubulin (red) and F-actin (green, phalloidin) immunostained neurites extending to the lower filter surface for the indicated times. (Scale bar, 20 μm.) (D) 3D reconstruction and volume rendering of a confocal series of α-tubulin stained neurons on filter. (E) Equal amounts of neurite and soma lysates were separated by SDS/PAGE and either silver stained or Western blotted for phosphotyrosine (pY). (F) Rac and Cdc42 activity (GTP-Cdc42, GTP-Rac) was determined from equal amounts of neurite and soma protein fractions, using a GST-PBD pulldown assay and Western blot analysis. ERK served as a protein loading control. (G) Erk activity in neurite and soma fractions was determined by Western blot analysis with phosphospecific antibodies to the phosphorylated activated form of ERK. (H) Gene ontology analysis of the most significant canonical pathways present in the neurite (blue, 10 of 39 shown), soma (yellow), or equally distributed proteins (red). Green dotted line represents significance threshold as measured by Fishers's test (P < 0.05).
Characterization of the Neurite and Soma Proteomes.
To quantify relative changes in protein abundance of specific proteins in the neurite and soma proteomes, we used two dimensional liquid chromatography-coupled tandem MS (LC-MS/MS) to analyze equal amounts of soma and neurite lysate. By calculating the ratio of peptide spectrum counts (neurite/soma) assigned to all of the peptides for each protein in each fraction, it is possible to quantify the relative abundance for a given protein in the two samples. A detailed description of this system is provided in Material and Methods and in our previous work on the pseudopod proteome (4). In total, 4855 proteins were identified from at least two peptides. A protein was considered to be increased/decreased in a particular fraction if its neurite/soma ratio changed by 2-fold or greater or if it was detected in only one fraction (unique). All other proteins were considered to be equally abundant in the two fractions. Using these criteria, 1,229 proteins were enriched in the neurite, 1,676 proteins were enriched in the soma, and 1,950 proteins were equally distributed (SI Appendix, Fig. 5B). A complete list of these proteins is found in SI Table 1. To validate our proteomics data, we used quantitative Western blotting and densitometry to confirm the relative changes in protein abundance measured by LC-MS/MS (SI Appendix, Fig. 5C). There was good correlation of protein abundance ratios detected by the two different methods for several protein classes including cytoskeletal proteins (actin), cytoplasmic signaling proteins (p130Cas), and internal membrane proteins (β-COP) (SI Appendix, Fig. 5C). However, in a few cases (Cdc42, RhoA, and Dock4) a correlation was not apparent. This likely reflects differences in the protein solubility properties of the mass spectrometry compatible urea lysis buffer compared with the highly efficient 1% SDS buffer used for Western blot analysis. Also, using the gene ontology resource Babelomics (9), we performed a proteome-wide analysis of protein subcellular localization (SI Appendix, Fig. 5D). Results from these studies revealed the expected subcellular distribution of neurite and soma proteins, which provides additional validation of our biochemical approach. Finally, several reported neurite marker proteins MAP1b (2.5×), MAP2 (5×), Tau (7.7×), kinesin-1 (4.7×), and kinesin-3 (3.5×) (1) were enriched in the neurite fraction. Together, these findings confirm our previous observations that LC-MS/MS can be used to determine quantitative differences in protein abundance between two subproteomes obtained from fractionated cells.
Spatial Organization of Signaling Networks in Neurites and Somata.
To find the functional interrelationship of the proteins enriched in the neurite and soma proteomes and determine whether they compartmentalize into distinct signaling networks, we used the Ingenuity Pathways Analysis (IPA) program and the Ingenuity Pathways Knowledge Base (IPKB), which is a system wide database of biological pathways created from multiple relationships of proteins, genes, and diseases (10). The IPA program can analyze large genomic or proteomic datasets to find the most statistically significant canonical pathways and protein networks relevant to the dataset based on the calculated probability score by searching the IPKB. The neurite proteome contained 39 canonical pathways and the soma proteome contained 7 pathways, whereas the equally distributed protein pool contained 5 pathways (Fig. 1H and SI Appendix, Fig. 5E). Interestingly, functional annotation of the neurite and soma proteomes revealed a high degree of spatial separation of signaling pathways between the two different cellular compartments. For example, pathways that regulate axon guidance, the actin cytoskeleton, integrin, and ephrin signaling are highly represented in the neurite, whereas pathways that regulate estrogen receptor signaling, cell cycle entry, and ubiquitination are dominant in the soma. This asymmetrical representation of signaling networks likely reflects the highly polarized state of the neuron and its dedication to the neuritogenesis process. Consistent with high Erk activity in the neurite (Fig. 1G), an ERK/MAP signaling pathway was found in the neurite. An unexpected finding was that apoptosis, JNK, and PDGF signaling pathways are highly represented in the neurite relative to the soma (Fig. 1H and SI Appendix, Fig. 5E). These pathways have not been previously linked to neurite formation. It is notable that such an analysis does not imply that all of the components of a defined signaling network are solely restricted to one subcellular domain. For example, there are 6 proteins with the “axonal guidance signaling” descriptor enriched in the soma versus 64 in the neurite. Conversely, there are 15 proteins with the “estrogen receptor signaling” descriptor enriched in the soma versus 8 in the neurite. Further description of the signaling networks present in the neurite and in the soma are provided in SI Tables 2 and 3 and SI Appendix, Figs. 6 and 7 and SI Text.
Analysis of Rac and Cdc42 Signaling in Neuritogenesis.
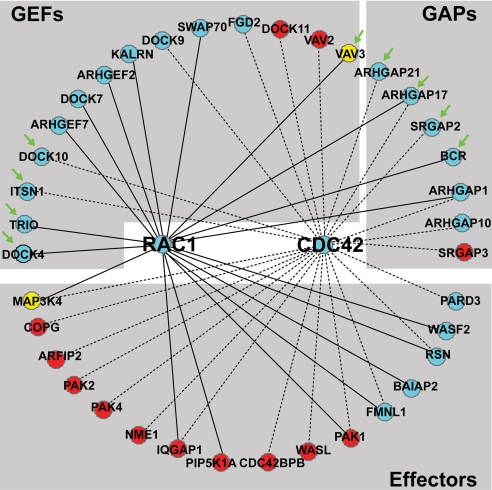
Previous work has shown that Rac and Cdc42 regulate neuritogenesis by coupling extracellular guidance cues to organization of the actin cytoskeleton. Although the activation of these two GTPases has been correlated with neurite extension (3), a critical question remains as to how they interact with multiple upstream regulators and downstream effectors to achieve spatial regulation of the actin cytoskeleton. Therefore, we mined the proteomics datasets to identify all possible upstream regulators (GEFs and GAPs) and downstream effectors that could interact with Rac and Cdc42, using IPKB and the gene ontology resource Babelomics (9). This revealed a complex putative Rac/Cdc42 signaling network with 13 GEFs, 7 GAPs, and 16 downstream effectors (Fig. 2; detailed information about each protein is found in SI Appendix, Table 4). Interestingly, the majority of the GEFs (11 of 14) and GAPs (6 of 7) were enriched in the neurite, which is consistent with the highly elevated Rac and Cdc42 activity in this domain (Fig. 1F). Our findings strongly suggest that multiple signaling mechanisms regulate GTPase activation in the neurite compartment. However, of the 16 downstream effectors, only 5 were neurite-enriched, whereas 10 were equally distributed, and 1 was enriched in the soma. Thus, the neurite enrichment of Rac/Cdc42 effectors does not necessarily correlate with the spatially increased GTPase activity. However, most effectors are regulated through intramolecular interactions independently of changes in protein levels (11).
Fig. 2.
Potential Rac and Cdc42 interactomes. Interactome was generated as described in Material and Methods. Proteins are named by their official gene symbol according to Entrez Gene. Proteins with decreasing enrichment values are ordered from left to right. Subcellular localization is color-coded: blue, neurite enriched proteins; red, equally distributed proteins; yellow, soma enriched proteins. Solid lines represent interactions with Rac, whereas dotted lines represent interactions with Cdc42. Green arrows point to proteins functionally analyzed by siRNA in this study. A detailed description of each protein can be found in SI Appendix, Table 4.
Functional Characterization of Rac and Cdc42 GEFs and GAPs.
The biological role of individual Rac and Cdc42 GEFs and GAPs are beginning to be elucidated, but our understanding of how these molecules are spatially organized within the dynamic cell at the systems level has not been addressed. Our proteomic analysis suggests that multiple GEF and GAP pathways operate in the neurite to regulate Rac and Cdc42 activity. This prompted us to determine which of the Rac and Cdc42 GEFs and GAPs are functionally required for neuritogenesis, using RNA interference and cell-based assays of neurite formation. For these studies, we picked the four most neurite-enriched GEFs (Dock4, Trio, Itsn1, and Dock10), the four most neurite-enriched GAPs (ArhGAP21, ArhGAP30, SrGAP2, and BCR), and (as a control) the only soma-enriched GEF (Vav3) (Fig. 2, green arrows). We also analyzed ArhGAP30, a neurite-enriched, previously uncharacterized GAP of which the specificity is unknown. Of these, the GEFs Trio, Itsn-l, and Vav3 (12) and the GAPs SrGAP2, BCR, and ArhGAP17 have been linked to neuritogenesis or other neuronal functions (12–17). The GEFs Dock4 and Dock10 and the GAPs ArhGAP21 and ArhGAP30 have not yet been linked to neuronal functions.
Potent knockdown was observed as measured by Western blot analysis or quantitative PCR (SI Appendix, Fig. 8), and in all cases, siRNA transfected cells were viable and readily attached to the ECM proteins. Surprisingly, silencing of none of the different GEFs and GAPs prevented neurite formation. Rather, in 7 of 10 knockdowns (all but Trio, ArhGAP21, and Vav3), a potent increase in neurite length was observed (SI Appendix, Fig. 9). Other parameters, such as branching and number of neurites, were not affected (data not shown). These findings suggest that there is a high level of redundancy built into the Rac/Cdc42 regulatory network that mediates neurite formation and that the various GEFs and GAPs play a more subtle role in fine tuning neuritogenesis. However, the possibility remains that the small residual level of protein present after knockdown is sufficient to drive neurite extension. To investigate this possibility, we examined the details of neurite formation of the various GEF and GAP knockdown cells with time-lapse video microscopy and immunofluorescence staining of the actin-tubulin cytoskeleton. These studies revealed distinct phenotypes that affected neuritogenesis in different ways and are summarized in SI Appendix, Table 5.
Time-lapse analyses showed that neurons depleted of the GAP ArhGAP30 or of the GEFs Dock4 and Dock10 displayed defects in neurite protrusion/retraction events compared with control siRNA transfected or wild-type cells (Fig. 3A and SI Movies 1–3). Specifically, these neurites displayed a more persistent and straight path leading to an overall increase in neurite length (Fig. 3B). A similar phenotype was observed when the GEF Itsn-l or the GAP ArhGAP17 were silenced although the alteration in the extension/retraction events was somewhat milder (SI Movies 4 and 5) with some residual retraction events. Although the extension/retraction kinetics were altered in these cells, we did not detect changes in the actin-tubulin cytoskeleton by immunofluorescence (data not shown). Finally, silencing of the neurite-enriched GAP ArhGAP21 did not affect neuritogenesis (data not shown) nor did knockdown of the soma-enriched GEF Vav3 (SI Movie 6).
Fig. 3.
Morphodynamics and cytosketetal changes of cells transfected with the indicated GEF and GAP siRNAs. (A) Time-lapse series of control and ArhGAP30 siRNA transfected neurons. Neurite tip trajectory is shown in red. (Scale bar, 50 μm.) (B) Representative neurite tip tracks of control, ArhGAP30, Dock4, and Dock10 siRNA transfected cells. (Scale bar, 100 μm.) (C) Control, SrGAP2, and Trio siRNA transfected cells were allowed to spread for 8 h on laminin-coated coverslips and immunostained for actin (green), tubulin (red), and nucleus (blue). (D) Fluorescence micrographs of growth cone morphologies associated with BCR and SrGAP2 knockdowns. Cells were immunostained for actin (green) and tubulin (red). White arrows indicate high-density arrays of filopodia. The bar graph shows the occurrence of growth cones with “low” or “high” filopodia density from neurites longer than one soma length scored on four different regions of the coverslip from a 24-h time point. P values between control and specific knockdowns were computed by using a t test. (E) Time-lapse analysis of neurite formation of SrGAP2 and Trio knockdown cells. Red arrows indicate phase refractile globular structures indicative of retracting neurites. (Scale bar, 20 μm.)
In contrast to the regulators described above, silencing of the GAPs SrGAP2 and BCR or the GEF Trio strongly impacted the actin cytoskeleton as observed by immunofluorescence staining. For both SrGAP2 and BCR, a potent defect of protrusion/retraction events leading to more persistent neurite extension was again observed (SI Movie 7 and data not shown). Knockdown of the Cdc42-specific GAP SrGAP2 increased initial cell spreading on the ECM with the formation of prominent lamellipodia characterized by a dense array of peripheral filopodia (Fig. 3C). Time-lapse analysis showed that this occurred ≈3 h after plating (SI Movie 7) and resulted in a 2.3-fold increase in cell surface area when compared with control cells (n = 25 cells each condition, P < 0.01, measured 3 h after plating). This increase in spreading then rapidly resolved. Also associated with this phenotype was an increase in the formation of large fan-shaped growth cones with prominent arrays of dense filopodia (Fig. 3 D and E and SI Movie 7). BCR knockdown led to a similar but less potent phenotype. However, this response was not associated with changes in cell spreading (data not shown). Silencing of the GEF Trio led to a defect in cell spreading (20% decrease in cell surface area compared with control cells at the 24-h time point, n = 140 cells for each condition, P < 0.01) with the formation of long and poorly organized filopodia emanating from the neurites (Fig. 3C). Time-lapse analysis revealed that these neurites were highly unstable and frequently collapsed, but immediately extended again (Fig. 3E and SI Movie 8). However, these neurites were eventually able to compensate for the defect and by 24 h displayed neurites as long as those from control cells (SI Appendix, Fig. 9). Together, these findings indicate that these GEFs and GAPs play an important role in regulating various Rac and Cdc42 dependent cellular functions that cooperate to fine tune neurite formation.
The fact that knockdown of Rac and Cdc42-specific GEFs and GAPs only led to impairment of subtle cellular functions during neuritogenesis, prompted us to investigate whether Rac and Cdc42 were necessary for neurite formation. It is important to note that inhibition of Rac and Cdc42 activity has been commonly achieved by using overexpression of dominant negative (DN) GTPase constructs. These constructs act by sequestering multiple GEFs, which can affect nonspecifically multiple GTPase pathways (18). Thus, we silenced endogenous Rac1 and Cdc42, using RNA interference, and then evaluated neurite extension (summarized in SI Appendix, Table 5). In undifferentiated cells (i.e., in the presence of serum), knockdown of Rac or Cdc42 proteins (SI Appendix, Fig. 10A) reduced cell spreading (SI Appendix, Fig. 10 B and C) without any impairment in cell viability (data not shown). Surprisingly, unlike with expression of DN Rac or Cdc42, which inhibits neurite extension (7), siRNA knockdown of Rac or Cdc42 protein did not significantly inhibit neurite outgrowth. In fact, Cdc42 knockdown actually led to a significant increase in neurite length (SI Appendix, Fig. 10 D and E). Also, no obvious changes in the actin cytoskeleton were observed in cells with reduced Rac or Cdc42 protein levels. Indeed, these cells exhibited normal growth cones and filopodial structures when compared with control cells (SI Appendix, Fig. 10 F and G). The increase in neurite length in Cdc42 knockdown cells was due to a loss of neurite protrusion and retraction cycles (SI Movie 9) as described above for knockdown of the Cdc42 specific regulators Itsn-1, Dock10, and ArhGAP17. Rac knockdown did not significantly alter neurite dynamics (SI Movie 10). These results indicate that reducing Rac and Cdc42 protein levels does not significantly impair neuritogenesis under these conditions. This may be due to functional compensation by the structurally related GTPases, Rac3, Tc10, and TcL (19, 20).
Discussion
The present study introduces a simple method to selectively isolate neurites from the somata of neuron-like neuroblastoma cells in large scale for biochemical analyses. This opens up unique possibilities to not only globally monitor the spatial organization of proteins in these two different cellular compartments but also directly examine protein regulatory activities involving posttranslational modifications (e.g., phosphorylation, ubiquitination) and protein–protein interactions that dictate neuritogenesis. This will be important in the future, because neurite localization of a protein does not necessarily mean that it should be functionally important for neuritogenesis (as observed for ArhGAP21). Furthermore, many proteins could be activated locally by posttranslational modifications but still have homogeneous subcellular distribution. This approach complements traditional imaging methodologies used to examine protein compartmentalization and should readily transfer to other cell types, guidance and collapsing agents.
Our findings provide a spatial view of the neurite and soma proteomes of neuroblastoma cells extending neurite-like processes in response to a directional cue. This revealed a prominent asymmetry in the signaling pathways present in both subcellular domains with pathways involved in sensing extracellular cues, regulation of actin, and adhesion dynamics being enriched in the neurite. Our findings that the neurite contains a highly compartmentalized and complex set of potential Cdc42 and Rac GEFs and GAPs highlight an important question in this field. Why are there multiple GEFs and GAPs present in the cell to regulate the same GTPase? Our functional analyses show that these regulators control different cellular functions during neuritogenesis that cooperate to fine tune neurite extension. Thus, rather than Rac and Cdc42 regulating neurite extension per se, our results suggest a more complex scenario in which different GEFs and GAPs control the activity of multiple GTPase pools with distinct functions in time and space. The finding of upstream regulators that impact directly on different aspects of the cytoskeleton or that act independently of it suggests that such spatiotemporal GTPase pools couple with distinct downstream effectors to regulate the cytoskeleton, membrane trafficking, or generation of polarity at precise subcellular localizations. It is notable that most studies of neuritogenesis have relied on overexpression of DN GTPase mutants to link a specific GTPase to a particular cellular function (3). However, this approach can titrate out GEFs indiscriminately, which could globally inhibit various neurite functions. This may explain the discrepancy with the more subtle phenotypes observed in our study. This is consistent with gene targeting studies, which show that Rac1 knockout does not impair cell migration (21) and that Cdc42 knockout does not impair filopodium formation and cell migration (18). These findings, together with our results, suggest the possible involvement of compensatory mechanisms involving structurally related GTPases with redundant functions such as Rac3, which is known to be expressed in the developing brain (20), and the Cdc42-related proteins TC10 and TCL, which control filopodium formation during N1E-115 neuronal differentiation (19). These GTPases might also respond to the different GEFs and GAPs reported here. Alternatively, GEFs and GAPs might also regulate signaling mechanisms that operate independently of Rho family GTPases during neuritogenesis. In any case, the lack of neurite protrusion/retraction events associated with Cdc42 knockdown is consistent with the phenotypes obtained from the knockdown of the Cdc42-specific GEFs Dock10 and Itsn-1 and the GAP ArhGAP17.
Although our findings provide initial insight into the signaling complexity of this system, additional work will be necessary to fully appreciate the various levels of regulation of the Rac/Cdc42 signaling module containing various GEFs, GAPs, and related GTPases. In this regard, it will not be possible to rescue our siRNA defects by using overexpression of mutant active forms of Rho family GTPases, because this would lead to global GTPase activation throughout the entire cell. Rather, this will require methods that reveal signaling in space and time, including biochemical approaches, like the neurite purification assay or the use of fluorescent probes that measure spatiotemporal dynamics of GTPase activation. Finally, the finding that the soma-enriched GEF Vav3 is not involved in neurite extension in our model but is essential for nerve growth factor-induced neuritogenesis (12) suggests that different GTPase signaling programs can be activated depending on the extracellular cue that triggers neuritogenesis. Our neurite purification system provides a model to explore the different signaling mechanisms that drive neuritogenesis in response to various biological cues. Although neuroblastoma cells and the filter purification system recapitulate many aspects of neuritogenesis, it will be important to confirm these findings in primary neurons. Further optimization of the sensitivity of the large-scale proteomic methods described here should make it possible to achieve similar results with smaller amounts of cell lysate obtained from isolated primary neurons.
In summary, the ability to purify the neurite and its integration with contemporary proteomics allowed us to spatially map the neurite and soma proteomes, creating a comprehensive database for investigation of neuritogenesis and cell polarization. Proteomic and functional analyses revealed a complex Rac and Cdc42 regulatory network consisting of multiple GEFs and GAPs that regulate distinct cellular processes during neuritogenesis. Future work to integrate our findings with emerging genomic and proteomic datasets representing human disease states will provide valuable insight into neuropathology and provide important resources to develop disease-related diagnostics.
Materials and Methods
Neurite Sample Preparation and Biochemical Analysis.
N1E-115 neuroblastoma cells (American Tissue Culture Collection) were cultured in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) supplemented with 10% FBS, 1% Gln, and 1% gentamycin. For differentiation, N1E-115 cells were starved for 24 h in serum-free media optimized for neuroblastoma neuronal differentiation (Millipore–Chemicon). Cells were then detached, and 1.2 × 106 cells were replated on a 2.4-cm wide 3-μm microporous filter that had previously been coated on the bottom surface with 10 μg/ml laminin (Millipore–Chemicon) for 2 h at 37°C. Cells were then allowed to extend neurites through the pores for 24 h in serum-free media. The microporous filters were then fixed by a 20-min incubation in ice-cold methanol, and the neurites on the filter bottom or the somata on the filter top or where scraped away, using a cotton swab. The remaining structures were then solubilized in the appropriate lysis buffer. For routine Western blot analysis, a 1% SDS buffer containing protease inhibitors and 2 mM Vanadate was used. This typically yielded 30–40 μg of neurite versus 800 μg of soma sample, necessitating the pooling of multiple filters for any biochemical experiment. For proteomics studies, a buffer containing 40 mM Tris (pH 8.4), 7 M urea, 2 M thiourea, 0.5% Nonidet P-40, 2 mM Vanadate, 50 mM Calyculin A, and protease inhibitors were used. Five milligrams of neurite and soma each were generated for the proteomics analysis. Multiple soma and neurite preparations were pooled to generate the 5 mg of each fraction needed here. The samples were then digested with trypsin, and the resulting peptides were desalted and quantified. For active Rac and Cdc42 pulldowns, which necessitates native conditions, the fixation step was omitted and the microporous filters were allowed to cool to 4°C. After the scraping procedure, solubilization and pulldowns were then performed as described by the manufacturer (Millipore–Upstate). The pulldown experiments were performed on 200 μg of lysate. Note that the native conditions used here were not able to solubilize all Rac1 and Cdc42 as with the 1% SDS buffer. Thus, a GTPase pool is not soluble under these conditions and is not accessible for immunoprecipitation. See SI Appendix, SI Text for additional information.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Monica Holcomb for early contributions to this research project; Konstantin Stoletov for help with Imaris software; and Dr. H. Katoh, M. Umeda, and W. Balch for providing antibodies. This work was supported by Susan G. Komen Foundation Grant PDF0503999 (to Y.W.), National Institutes of Health Grants GM068487 (to R.L.K.) and CA097022 (to R.L.K.), and Cell Migration Consortium Grants GM064346 (to R.L.K.) and RR018522 (to R.D.S). The Environmental Molecular Sciences Laboratory is a U.S. Department of Energy national scientific user facility located at Pacific Northwest National Laboratory, a multiprogram national laboratory operated by Battelle for the U.S. Department of Energy under Contract DE-AC05-76RL01830.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0706545105/DC1.
References
- 1.Dehmelt L, Halpain S. J Neurobiol. 2004;58:18–33. doi: 10.1002/neu.10284. [DOI] [PubMed] [Google Scholar]
- 2.Jones LL, Oudega M, Bunge MB, Tuszynski MH. J Physiol. 2001;533:83–89. doi: 10.1111/j.1469-7793.2001.0083b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.da Silva JS, Dotti CG. Nat Rev Neurosci. 2002;3:694–704. doi: 10.1038/nrn918. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Ding SJ, Wang W, Jacobs JM, Qian WJ, Moore RJ, Yang F, Camp DG, 2nd, Smith RD, Klemke RL. Proc Natl Acad Sci USA. 2007;104:8328–8333. doi: 10.1073/pnas.0701103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Mata R, Burridge K. Trends Cell Biol. 2007;17:36–43. doi: 10.1016/j.tcb.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Peck J, Douglas GT, Wu CH, Burbelo PD. FEBS Lett. 2002;528:27–34. doi: 10.1016/s0014-5793(02)03331-8. [DOI] [PubMed] [Google Scholar]
- 7.Sarner S, Kozma R, Ahmed S, Lim L. Mol Cell Biol. 2000;20:158–172. doi: 10.1128/mcb.20.1.158-172.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kozma R, Sarner S, Ahmed S, Lim L. Mol Cell Biol. 1997;17:1201–1211. doi: 10.1128/mcb.17.3.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Shahrour F, Minguez P, Tarraga J, Montaner D, Alloza E, Vaquerizas JM, Conde L, Blaschke C, Vera J, Dopazo J. Nucleic Acids Res. 2006;34:W472–W476. doi: 10.1093/nar/gkl172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calvano SE, Xiao W, Richards DR, Felciano RM, Baker HV, Cho RJ, Chen RO, Brownstein BH, Cobb JP, Tschoeke SK, et al. Nature. 2005;437:1032–1037. doi: 10.1038/nature03985. [DOI] [PubMed] [Google Scholar]
- 11.Bishop AL, Hall A. Biochem J. 2000;348(2):241–255. [PMC free article] [PubMed] [Google Scholar]
- 12.Aoki K, Nakamura T, Fujikawa K, Matsuda M. Mol Biol Cell. 2005;16:2207–2217. doi: 10.1091/mbc.E04-10-0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Estrach S, Schmidt S, Diriong S, Penna A, Blangy A, Fort P, Debant A. Curr Biol. 2002;12:307–312. doi: 10.1016/s0960-9822(02)00658-9. [DOI] [PubMed] [Google Scholar]
- 14.Furuta B, Harada A, Kobayashi Y, Takeuchi K, Kobayashi T, Umeda M. J Neurochem. 2002;82:1018–1028. doi: 10.1046/j.1471-4159.2002.01021.x. [DOI] [PubMed] [Google Scholar]
- 15.Hussain NK, Jenna S, Glogauer M, Quinn CC, Wasiak S, Guipponi M, Antonarakis SE, Kay BK, Stossel TP, Lamarche-Vane N, McPherson PS. Nat Cell Biol. 2001;3:927–932. doi: 10.1038/ncb1001-927. [DOI] [PubMed] [Google Scholar]
- 16.Laurent CE, Smithgall TE. Exp Cell Res. 2004;299:188–198. doi: 10.1016/j.yexcr.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Wong K, Ren XR, Huang YZ, Xie Y, Liu G, Saito H, Tang H, Wen L, Brady-Kalnay SM, Mei L, et al. Cell. 2001;107:209–221. doi: 10.1016/s0092-8674(01)00530-x. [DOI] [PubMed] [Google Scholar]
- 18.Czuchra A, Wu X, Meyer H, van Hengel J, Schroeder T, Geffers R, Rottner K, Brakebusch C. Mol Biol Cell. 2005;16:4473–4484. doi: 10.1091/mbc.E05-01-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abe T, Kato M, Miki H, Takenawa T, Endo T. J Cell Sci. 2003;116:155–168. doi: 10.1242/jcs.00208. [DOI] [PubMed] [Google Scholar]
- 20.Corbetta S, Gualdoni S, Albertinazzi C, Paris S, Croci L, Consalez GG, de Curtis I. Mol Cell Biol. 2005;25:5763–5776. doi: 10.1128/MCB.25.13.5763-5776.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wells CM, Walmsley M, Ooi S, Tybulewicz V, Ridley AJ. J Cell Sci. 2004;117:1259–1268. doi: 10.1242/jcs.00997. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.