Abstract
Maturation of neuronal synapses is thought to involve mitochondria. Bcl-xL protein inhibits mitochondria-mediated apoptosis but may have other functions in healthy adult neurons in which Bcl-xL is abundant. Here, we report that overexpression of Bcl-xL postsynaptically increases frequency and amplitude of spontaneous miniature synaptic currents in rat hippocampal neurons in culture. Bcl-xL, overexpressed either pre or postsynaptically, increases synapse number, the number and size of synaptic vesicle clusters, and mitochondrial localization to vesicle clusters and synapses, likely accounting for the changes in miniature synaptic currents. Conversely, knockdown of Bcl-xL or inhibiting it with ABT-737 decreases these morphological parameters. The mitochondrial fission protein, dynamin-related protein 1 (Drp1), is a GTPase known to localize to synapses and affect synaptic function and structure. The effects of Bcl-xL appear mediated through Drp1 because overexpression of Drp1 increases synaptic markers, and overexpression of the dominant-negative dnDrp1-K38A decreases them. Furthermore, Bcl-xL coimmunoprecipitates with Drp1 in tissue lysates, and in a recombinant system, Bcl-xL protein stimulates GTPase activity of Drp1. These findings suggest that Bcl-xL positively regulates Drp1 to alter mitochondrial function in a manner that stimulates synapse formation.
Keywords: Bcl-2, synaptic transmission, mitochondria, cell death, ABT-737
During the initial stages of synaptic development, vesicles containing neurotransmitter aggregate into clusters within the presynaptic axons (1). Although neurotransmitter can be released before contact with the postsynaptic cell (2), enlargement and stabilization of the presynaptic vesicle cluster after postsynaptic contact underlie the development of high-fidelity release of neurotransmitter (3–5). Despite this understanding, many of the molecular players and mechanisms in synapse development are unknown. Here, we detail effects on synapse formation of the Bcl-2 family member, Bcl-xL, and we suggest that dynamin-related protein 1 (Drp1) is a downstream effector of Bcl-xL.
Anti- and proapoptotic Bcl-2 family proteins regulate the permeability of outer mitochondrial membranes during apoptosis, a key control point for amplification of the death pathway (6–8). The antiapoptotic members (such as Bcl-xL, Bcl-2, and Mcl-1) are widely thought to protect against cell death by directly or indirectly counteracting the proapoptotic family members, such as Bax and Bak (9). In addition, a number of studies suggest that Bcl-2 family proteins regulate activities of cells that are not undergoing apoptosis and in particular, may control the strength of synaptic transmission in neurons (10). The amount of Bcl-xL protein in the developing brain increases at approximately the same time that the size of presynaptic vesicle clusters increases (11–13). Synaptic boutons often contain mitochondria to which endogenous Bcl-xL is localized (14). Injection of recombinant Bcl-xL protein into presynaptic terminals of the squid giant synapse potentiates synaptic transmission within minutes and speeds recovery of neurotransmission from tetanus-induced depression (15). Although the mechanisms are unknown, the effects of Bcl-xL on synaptic potentiation at the squid synapse were suggested to result from increased release of ATP from mitochondria, consistent with the observation that injection of ATP into presynaptic terminals mimics the effects of recombinant Bcl-xL protein (15, 16). Increased ATP could facilitate recycling of synaptic vesicles (17) and enhance development of a large “reserve pool” of vesicles to maintain transmission during prolonged or high-frequency firing.
One important aspect of synaptic development is localization of mitochondria near synapses (17, 18). This process occurs in part through activity of the mitochondrial fission protein Drp1, which facilitates mitochondrial division to generate new mitochondria that are inserted into developing synaptic sites (17, 19). In the dendrites of mammalian neurons, inhibition of Drp1 by transfection of a dominant-negative mutant, dnDrp1-K38A, inhibits mitochondrial localization to dendritic spines (19). Drp1 is a large GTPase that is required for normal division of mitochondria in growth and development (20, 21), but it also has a role in promoting programmed cell death in mammals, flies, worms, and yeast (22–25). Drp1 colocalizes with Bax on mitochondria and was reported to interact with Bcl-2 family proteins.
Using electrophysiological measurements and light and electron microscopy, we demonstrate here that Bcl-xL overexpression in cultured hippocampal neurons increases the numbers of mitochondria, synaptic vesicle clusters, and synapses. Furthermore, Bcl-xL increases colocalization of mitochondria and vesicle clusters at synapses. Conversely, depletion/inhibition of endogenous Bcl-xL, using shRNA or treatment with a small molecular inhibitor of Bcl-xL, ABT-737, reduces the number of axonal vesicle clusters and mitochondria. Bcl-xL may act through the mitochondrial fission protein Drp1 to increase synapse formation. Biochemical studies suggest that formation of a Bcl-xL-Drp1 complex increases Drp1 GTPase activity, which is likely to play a role in mitochondrial localization to sites of maturing synapses.
Results
Overexpression of Bcl-xL Enhances Synaptic Activity of Rat Hippocampal Neurons in Culture.
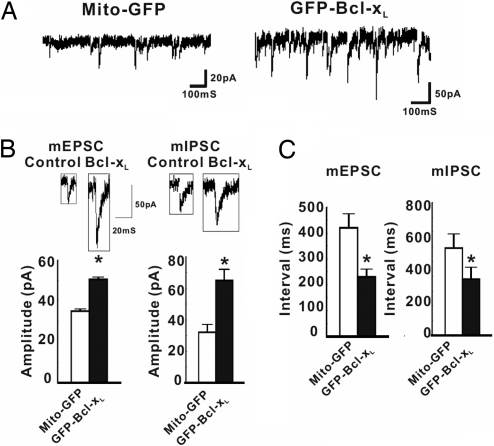
To extend our earlier findings in squid, we determined whether Bcl-xL influences synaptic activity in mammalian cells. Low-density cultures of rat hippocampal neurons were transfected at 5 days in vitro (DIV5) with plasmids expressing Bcl-xL tagged at the N terminus with green fluorescent protein (GFP-Bcl-xL) or the Mito-GFP control, in which the mitochondrial targeting sequence of COX4 is fused to GFP. At 12 days posttransfection (DIV17), neurons with green somata were voltage-clamped at their resting potential in the presence of 1 μM tetrodotoxin to block action potentials (26), and miniature postsynaptic currents were recorded (Fig. 1A). Transfection efficacy was low (1–5%), so it is likely that most axons synapsing on the recorded (transfected) cells came from nontransfected cells and that actions on the presynaptic axons were retrograde from the transfected cells. In cells expressing GFP-Bcl-xL, the amplitude of both excitatory and inhibitory spontaneous events was increased compared with Mito-GFP-transfected controls, suggesting that Bcl-xL increases the number of postsynaptic receptors per synapse or possibly the amount of transmitter in presynaptic vesicles (Fig. 1B). Postsynaptic Bcl-xL also decreased the interval between both miniature excitatory and inhibitory postsynaptic currents (mEPSCs and mIPSCs, respectively; Fig. 1C), indicating that Bcl-xL produces an increase in the frequency of vesicle fusion events, ascribable to an increase in synapse number (see below; Fig. 2 H and I).
Fig. 1.
Bcl-xL alters the spontaneous release of neurotransmitter. (A) Representative recordings at −70 mV of spontaneous mEPSCs and mIPSCs of rat hippocampal neurons transfected (DIV5) with Mito-GFP (control) or GFP-Bcl-xL. (B) Recordings and quantified amplitudes of mEPSCs and mIPSCs for all experiments, as in A (DIV17) (n = 6 cells for GFP-Bcl-xL; n = 8 for Mito-GFP). (C) Quantified intervals between events for all experiments. *, P < 0.05, determined by Student's t test.
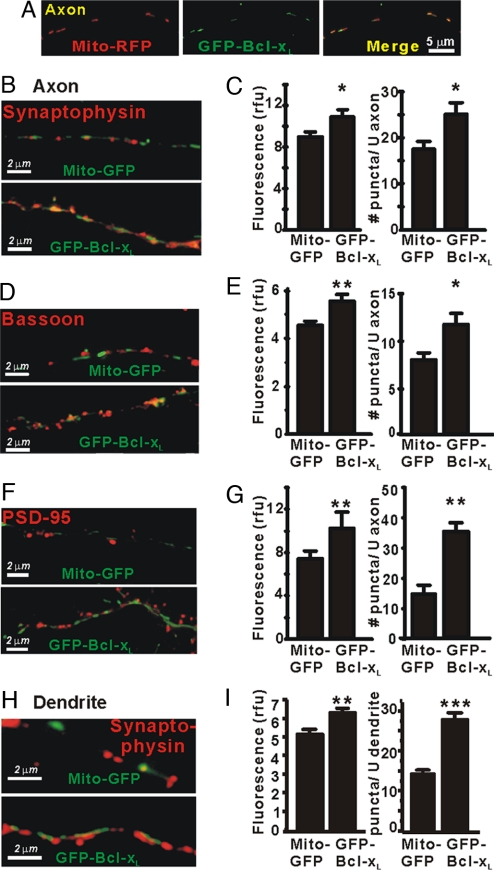
Fig. 2.
Bcl-xL induces synapse formation in cultured hippocampal neurons. (A) Fluorescence microscopy of an axon (DIV13) of a cultured hippocampal neuron cotransfected (DIV5) with Mito-RFP (red) and GFP-Bcl-xL (green) and the merge of the two images. (B and C) Immunofluorescence microscopy for synaptophysin in axons of transfected neurons. Fluorescence intensity of puncta in relative fluorescence units (rfu) and their number per unit length (≈35 μm) of axon are presented as mean ± SEM for n = 15 neurons per group in three independent experiments. *, P < 0.05; **, P < 0.01, Student's t test. (D and E) Immunofluorescence microscopy for bassoon as in B and C. (F and G) Immunofluorescence microscopy for PSD-95 as in B and C. (H and I) Immunofluorescence microscopy for synaptophysin puncta apposed to dendrites of transfected neurons as in B and C. Synapse formation is highly likely in H, but dendrites may or may not be present in B, D, and F.
Bcl-xL Overexpression Induces Synapse Formation in Cultured Neurons.
To pursue the role of Bcl-xL in regulating synaptic activity, we quantified several synaptic parameters. At 7–8 days posttransfection, the fluorescence patterns of cotransfected GFP-Bcl-xL and Mito-RFP were punctate and nearly completely overlapping in axons (Fig. 2A) and in dendrites [supporting information (SI) Fig. 7], confirming mitochondrial localization of Bcl-xL. During embryonic development and during maturation in culture, presynaptic vesicle clusters can first be mobile precursors or “orphans” that are not associated with postsynaptic densities (PSDs) in an opposing cell before formation of mature excitatory synapses (4, 27). To examine the effects of presynaptic Bcl-xL on formation of either precursor vesicle clusters or synapses, parallel cultures of hippocampal neurons were transfected at DIV5 with GFP-Bcl-xL or Mito-GFP and immunostained (DIV13) for presynaptic proteins. In three independent experiments, axons expressing GFP-Bcl-xL had significantly increased immunofluorescence staining for synaptophysin, a component of synaptic vesicle membranes (12) (Fig. 2 B and C) and for the scaffolding protein, bassoon, also found at both precursor vesicle clusters and presynaptic active zones (4) (Fig. 2 D and E). Untransfected axons synapsing on untransfected dendrites did not show the increases in synaptophysin and bassoon seen in nearby axons expressing GFP-Bcl-xL (not shown). Fluorescence was quantified as the number of puncta per unit length of axon and as mean fluorescence intensity (see Materials and Methods and ref. 27). The effects of Bcl-xL on synaptophysin staining were already apparent by 2 days posttransfection (DIV7) but were more obvious at 14 and 21 days in culture after synapses had matured (SI Fig. 8). By this parameter, the controls appeared to reach a lower steady-state level and did not catch up with Bcl-xL-overexpressing cells during the life span of the cultures. These data indicate that presynaptic Bcl-xL overexpression increases number and size of vesicle clusters and of bassoon immunoreactive puncta.
To determine whether presynaptic Bcl-xL overexpression in the axon induces postsynaptic maturation in the dendrites of adjacent untransfected (GFP-negative) cells, cultures were stained for PSD-95, a major protein of the postsynaptic density at excitatory synapses (4). There was a pronounced increase in the number and fluorescence intensity of PSD-95-stained puncta along the axon of GFP-Bcl-xL-transfected cells (Fig. 2 F and G), indicating the presence of mature PSDs in the dendrites of adjacent untransfected cells. In contrast, appositions of untransfected axons to untransfected dendrites showed fewer synaptophysin and PSD-95 puncta than appositions of Bcl-xL-expressing axons to untransfected dendrites (not shown). Expression of GFP-Bcl-xL in dendrites also increased synapse number and size at contacts with untransfected axons (based on synaptophysin staining) (Fig. 2 H and I). This difference could account for the ≈2-fold greater frequency of mPSPs illustrated in Fig. 1. We conclude that overexpression of Bcl-xL in either axon or dendrite significantly increases synapse formation in cultured hippocampal neurons.
Endogenous Bcl-xL Increases the Number and Size of Presynaptic Vesicle Clusters.
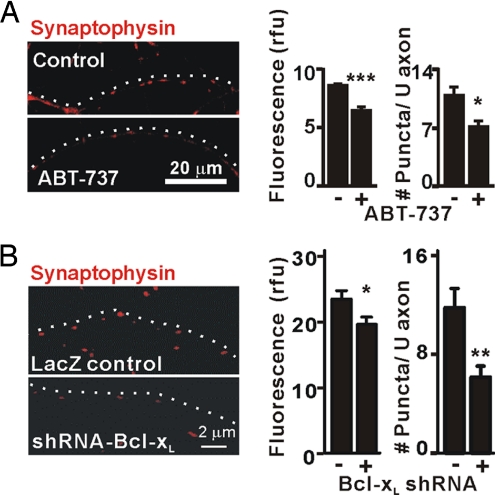
To verify that endogenous Bcl-xL promotes synapse formation, as does overexpressed GFP-Bcl-xL, hippocampal cultures (DIV5) were treated with 1 μM ABT-737, a small-molecule inhibitor designed to inhibit antiapoptotic activity by occupying the BH3-binding groove of Bcl-xL (28, 29). At DIV14, these cultures showed reduced synaptophysin staining intensity and number of puncta in the axons, indicating that endogenous Bcl-xL is required for normal development of vesicle clusters (Fig. 3A). Furthermore, ABT-737 did not enhance cell death measured by propidium iodide staining, even at 5 μM, which greatly exceeds the Ki of ≤1 nM (SI Fig. 9) (28).
Fig. 3.
Bcl-xL is required for synapse formation. (A) Immunofluorescence microscopy for synaptophysin in axons of untransfected hippocampal neurons (DIV14) with or without 1 μM ABT-737 added at DIV5. Data were quantified as in Fig. 2C for three independent experiments (n = 6 cells per group). *, P < 0.05; ***, P < 0.001, Student's t test. White dots indicate the course of the axon. (B) Immunofluorescence microscopy for synaptophysin in axons of hippocampal neurons (DIV14) that were transfected (DIV5) with bcl-x hairpin RNA (pCAG-lacZ-shRNA-Bcl-xL) or control vector (pCAG-lacZ). Synaptophysin intensity (rfu) and number of puncta per 20 μm of axon in three independent experiments were quantified (mean ± SEM; n = 12 neurons per group). *, P < 0.05; **, P < 0.01; ***, P < 0.001, Student's t test.
Although Bcl-xL is reportedly the most abundant Bcl-2 family member expressed in mature neurons and in postnatal brain (11), ABT-737 also binds to the antiapoptotic proteins Bcl-2 and Bcl-w (but not the antiapoptotic protein Mcl-1) (29). To verify the specific role of endogenous Bcl-xL in synapse formation, Bcl-xL protein was depleted by using a short hairpin RNA (shRNA) that effectively depressed endogenous Bcl-xL in mouse IMCD cells (SI Fig. 10) (30). The axons of hippocampal neurons transfected with a bcl-x shRNA plasmid expressing LacZ to mark transfected cells (1 μg), but not the control LacZ plasmid, had significantly decreased staining intensity and number of synaptophysin puncta in 14-day cultures (Fig. 3B). Dendrites in which Bcl-xL had been knocked down also showed a marked decrease in synaptophysin puncta, ascribable to a decrease in synapse formation on those dendrites (SI Fig. 11). Using Hoechst stain to mark nuclei and propidium iodide to mark dead cells we confirmed that Bcl-xL knockdown did not compromise cell viability (90.9% for shRNA-Bcl-xL and 92.2% for control, n = 110 cells per group). The ABT-737 and RNAi results strongly suggest that endogenous Bcl-xL participates in synapse formation during maturation of hippocampal neurons in culture.
Bcl-xL Induces Localization of Mitochondria to Presynaptic Sites.
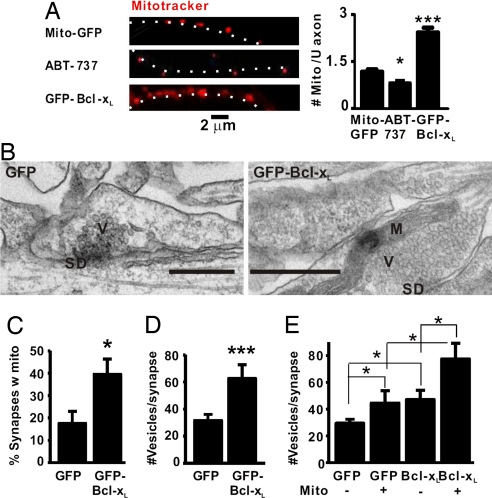
Morphological studies have demonstrated that approximately half of all presynaptic terminals contain mitochondria (31, 32). However, mitochondria are mobile and can move along dendrites and into dendritic spines in an activity-dependent manner (19). The mechanisms of mitochondrial recruitment to synapses are currently not understood. Given its mitochondrial localization, Bcl-xL might have a role in localization of mitochondria to synapses pre- and/or postsynaptically. Treatment with the Bcl-xL inhibitor ABT-737 significantly decreased the number of mitochondria per unit length of axon (Fig. 4A, Middle). Conversely, GFP-Bcl-xL overexpression significantly increased the number of mitochondria (Bottom). These data showing that endogenous Bcl-xL and Bcl-xL overexpressed in axons increase the number of mitochondria in axons (Fig. 4A), together with the evidence above showing that presynaptic Bcl-xL induces synapse formation (Fig. 2 F and G), suggest that Bcl-xL induces the targeting of axonal mitochondria to synapses.
Fig. 4.
Bcl-xL increases the number of mitochondria per unit length of axon and their colocalization to presynaptic sites. (A) Fluorescence microscopy of transfected axons in hippocampal cultures stained with MitoTracker Red. Neurons were either transfected as indicated or treated with ABT-737 (DIV5 for both). White dots beside the axon images show the axon trajectories. The number of MitoTracker-stained mitochondria per 6 μm of axon (mean ± SEM) was quantified for both control and ABT-737 (n = 6 neurons per group) and for GFP-Bcl-xL (n = 5 in each of three independent experiments for a total of 15 neurons). *, P < 0.05; ***, P < 0.001. (B) Representative electron micrographs of synaptic profiles identified by a synaptic density between neurons (DIV13) expressing GFP only or GFP-Bcl-xL. Data are quantified in C–E. V, vesicles; SD, synaptic density; M, mitochondrion. (Scale bars: 500 nm.) (C) Percentage of synaptic profiles containing a mitochondrion. (D) Number of vesicles per synaptic profile. (E) The number of vesicles per synaptic profile was greater if a mitochondrion was present and if the neuron expressed GFP-BclxL. *, P < 0.05; ***, P < 0.001.
To test whether Bcl-xL increases localization of mitochondria to presynaptic sites, lentiviruses encoding GFP-Bcl-xL or GFP alone were used to transduce the neurons (DIV5) with ≈100% efficacy, and these cultures fixed at DIV13 were examined by electron microscopy. The percentage of sections through synapses that contained a presynaptic mitochondrion increased from <20% to ≈40% with GFP-Bcl-xL (Fig. 4 B and C). In addition, there was a 2-fold increase in the number of vesicles present in the synaptic profiles of GFP-Bcl-xL-containing axons (Fig. 4D), and both GFP-Bcl-xL and control synapses had a greater number of vesicles if a mitochondrion was present at the synapse (Fig. 4E). We conclude that Bcl-xL stimulates mitochondrial localization to synaptic boutons and increases vesicle number and that the presence of mitochondria increases the size of the vesicle clusters, consistent with the increase in synapse formation and the electrophysiological data (Fig. 1).
Bcl-xL-Induced Changes at Synapses Are Facilitated by Drp1.
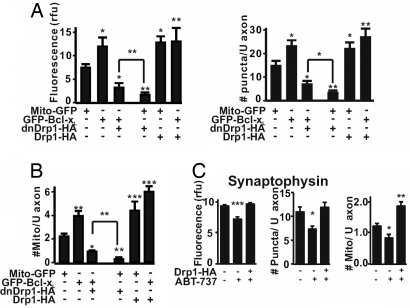
Drp1 is a large GTPase that is required for mitochondrial division (20) and for targeting of mitochondria to newly developing synaptic mitochondrial sites (17, 19, 20). Therefore, we explored the possibility that the effects of Bcl-xL on axonal mitochondria and synapse formation are mediated through Drp1. GFP-Bcl-xL was cotransfected with the dominant-negative inhibitor of Drp1, dnDrp1-K38A (HA-tagged) (33) into hippocampal neurons (DIV5), and axons were evaluated (DIV13) for synaptophysin staining and number of mitochondria (Fig. 5 A and B and SI Fig. 12). dnDrp1-K38A markedly reduced the action of GFP-Bcl-xL overexpression to increase intensity and number of synaptophysin-positive puncta and number of mitochondria (Fig. 5 A and B, first three bars, and SI Fig. 12). dnDrp1-K38A also reduced the action of endogenous Bcl-xL based on a comparison with the Mito-GFP control (first and fourth bars and SI Fig. 12). These results suggest that Drp1 acts downstream of endogenous Bcl-xL in regulation of synaptic vesicle and mitochondrial number. However, in the presence of dnDrp1-K38A, overexpression of GFP-Bcl-xL did increase these parameters relative to endogenous Bcl-xL in the Mito-GFP-transfected cells (bars 3, 4). Consistent with a role for Drp1 in mitochondrial fission, the mitochondria were longer in dnDrp1-K38A transfected cells (SI Fig. 12).
Fig. 5.
Drp1-dependent function of Bcl-xL in synapse formation and mitochondrial localization. (A) Quantified immunofluorescence intensity (rfu, Left) and number of synaptophysin puncta per 20 μm of axon (mean ± SEM, Right) for three independent experiments as in Fig. 2 (n = total of 12 neurons per group). *, P < 0.05; **, P < 0.01 compared with Mito-GFP control, Student's t test. Significance of difference between Mito-GFP and GFP-Bcl-xL each with dnDrp1-K38A is also indicated. (B) Quantified number of mitochondria per 6 μm of axon in three independent experiments (mean ± SEM; n = 10 neurons per group). *, P < 0.05; **, P < 0.01; ***, P < 0.001, Student's t test and comparisons as in A. (C) Quantified immunofluorescence for synaptophysin intensity (rfu) and number of puncta per 20 μm of axon and number of mitochondria per 6 μm of axon of hippocampal neurons (DIV13) transfected with Drp1-HA (DIV5) and/or treated with ABT-737 (DIV5) (means ± SEM, n = 6 cells per group in each of three independent experiments). *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared with control.
One explanation of these data is that Bcl-xL is a positive regulator of Drp1. However, there may be some Drp1 activity in the absence of Bcl-xL (see Fig. 6C). If so, the synaptic effects caused by ABT-737 inhibition of Bcl-xL may be reversed by overexpression of Drp1 (Figs. 4A and 5C). Overexpression of Drp1 did reverse the ABT-737-induced reduction in synaptophysin labeling and increased the number of mitochondria above control, consistent with a downstream mode of action of Drp1 (Fig. 5C).
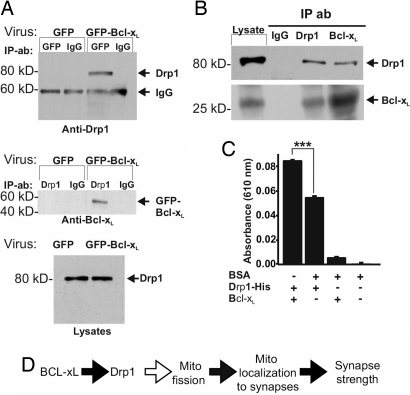
Fig. 6.
Bcl-xL and Drp1 interact. (A) (Top and Middle) Immunoblots of reciprocal coimmunoprecipitation experiments on hippocampal neurons (DIV13) transduced with viral vectors (DIV5) as indicated (n = 2). Antibodies used for precipitation (IP) and for immunoblots are indicated. (Top) Drp1 was coprecipitated with GFP-Bcl-xL, brought down by a GFP antibody. (Bottom) Blot of endogenous Drp1 in the starting lysates for immunoprecipitations above. (B) Immunoblots for endogenous Bcl-xL and endogenous Drp1 from adult rat brain after immunoprecipitation with the antibodies indicated. Each panel shows the starting lysate, control IgG precipitate, and the indicated precipitates probed with Drp1 and Bcl-xL antibodies. (C) Relative amounts of phosphate produced by GTPase activity in 1 h with purified recombinant proteins. Shown are results of three independent experiments (mean ± SEM). BSA had no GTPase activity above background, and this signal was subtracted from the other values. ***, P < 0.001. (D) Model of Bcl-xL-induced synaptic function. Black arrows indicate steps addressed in this work.
Bcl-xL Binds Drp1 and Modulates Its GTPase Activity.
To determine whether Bcl-xL can be a direct regulator of Drp1, we performed coimmunoprecipitations on lysates of hippocampal neurons transduced with lentiviruses encoding GFP-Bcl-xL or GFP alone. Immunoprecipitation of GFP-Bcl-xL with anti-GFP antibody coprecipitated endogenous Drp1 (Fig. 6A, Top). Drp1 was not coprecipitated from GFP-only samples. In the reciprocal experiment, immunoprecipitation of endogenous Drp1 coprecipitated GFP-Bcl-xL but not GFP alone (Fig. 6A, Middle, and data not shown), despite similar levels of Drp1 in both lysates (Fig. 6A, Bottom). This interaction was verified for endogenous Bcl-xL in adult rat whole brain. Anti-Drp1 antibody coprecipitated endogenous Bcl-xL protein from brain lysate, and conversely, anti-Bcl-xL antibody coprecipitated endogenous brain Drp1 (Fig. 6B).
Drp1 associates with the outer mitochondrial membrane to induce GTP-dependent mitochondrial fission. To investigate the possibility that Bcl-xL could alter the GTPase activity of Drp1, an in vitro GTPase assay (see Materials and Methods) was performed with purified recombinant full-length Bcl-xL and full-length human Drp1 (amino acids 1–699 plus a His tag for purification). Bcl-xL, which had little GTPase activity itself, increased Drp1 GTPase activity by ≈60%, relative to a control protein BSA (Fig. 6C, first two bars). BSA itself had no effect on Drp1 (data not shown). These findings demonstrate that Bcl-xL causes a significant increase in GTPase activity of Drp1 but that Drp1 has substantial basal activity in the absence of Bcl-xL. Bcl-xL may be a novel type of GTPase-activating protein for Drp1.
Discussion
We have shown that endogenous and overexpressed Bcl-xL induce synapse formation and increase the number of axonal mitochondria, the size and number of synaptic vesicle clusters, and recruitment of mitochondria to synapses in axons of hippocampal neurons matured in culture. In addition, dnDrp1-K38A, a dominant-negative inhibitor of Drp1, greatly reduces the effects of Bcl-xL on synaptic vesicle clusters and mitochondrial number, suggesting that Bcl-xL-induced synapse formation is mediated at least in part by Drp1. Endogenous complexes containing Bcl-xL and Drp1 were identified, and Bcl-xL increases the GTPase activity of Drp1 in vitro. Therefore, Bcl-xL may increase the number of mitochondria in axons and increase their localization to presynaptic domains by activating Drp1. These activities may be rate-limiting in synapse maturation.
Bcl-xL overexpression in presynaptic axons also affects the postsynaptic neuron as indicated by PSD-95 induction in adjacent dendrites. Bcl-xL overexpression in dendrites also increases the size and number of synaptophysin puncta contacting the dendrite and increases the amplitude of mEPSCs and mIPSCs, consistent with the possibility that Bcl-xL increases responses to transmitter release through effects on postsynaptic receptor insertion. The frequency of mPSCs is also increased consistent with the increase in number of synapses. The augmentation of synaptic structure and function that we observe with Bcl-xL overexpression could be caused by enhanced synaptic development or by mechanisms involved in other forms of synaptic plasticity.
Prodeath Factors Have Neuronal Functions Distinct from Cell Death.
Despite the contribution of Drp1 to cell death (22), we found that transfection with Drp1 promotes formation of synaptic vesicle clusters and presumably synapses without reducing cell viability. Although a decrease in mitochondrial fission produced by down-regulation of Drp1 can protect cells from death (34), up-regulation of Drp1 can also inhibit apoptosis (33), and in dendrites it induces increased number of dendritic spines (19). Down-regulation of Drp1 also alters mitochondrial targeting to the synapse and compromises synaptic function during high rates of transmission (17). Our data suggest that Drp1 may contribute to formation of vesicle pools in the developing synapse. Several studies have also suggested that both pro- and antideath Bcl-2 family proteins have physiological functions other than regulation of programmed cell death (15, 35–38).
Mitochondrial Targeting May Regulate Synapse Formation and Stabilization.
Mitochondria have recently been shown to regulate short-term potentiation of neurotransmitter release after a period of frequent synaptic activity (10) and to influence vesicle replenishment after intense activity (17). Mitochondria arrive at sites distant from the cell body by transport along microtubules (39) and respond to local axonal activation of growth factor receptors that stimulate mitochondrial movement to the site of growth factor stimulation (40, 41). In a growing neurite, mitochondrial localization may occur during the process of synapse formation, where mitochondria would then be in a position to provide energy for future and ongoing synaptic activity. Alterations in mitochondrial biogenesis, fission, and subsequent distribution may play critical roles in metabolic support for synaptic functions (42, 43), and a Bcl-xL–Drp1 complex may distribute new mitochondria to developing synaptic sites.
Bcl-xL May Regulate Availability of ATP for Synaptic Transmission.
Synaptic activity increases the rate of both glycolysis and respiration (44), presumably to provide energy for multiple ATP-dependent steps in transmitter synthesis, vesicle preparation, and recycling (45–47). Bcl-xL may increase the availability of ATP by increasing the permeability of the mitochondrial outer membrane or other mechanism (15, 16). We presume that mitochondria preferentially localize to sites of increased activity, in part through action of a Bcl-xL–Drp1 complex, and that ATP production is required for formation of new synapses and maintenance of activity. Furthermore, mitochondrial length and number per unit length along a neurite may be only an approximate measure of mitochondrial function, and long-lasting changes in synaptic transmission and synapse formation may depend on changes in mitochondrial bioenergetics and protein interactions mediated by Bcl-xL.
Materials and Methods
Details beyond the descriptions here and in Results are given in SI Materials and Methods.
Low-density cultures of rat hippocampal neurons were prepared as described in refs. 27 and 48 and contained few glial cells.
Spontaneous postsynaptic currents at the resting membrane potential (−70 mV) were recorded by whole-cell patch-clamp at 22–25°C. Recording electrodes contained 120 mM potassium gluconate, 8 mM NaCl, 0.5 mM EGTA, 10 mM Hepes, 2 mM MgATP (pH 7.3) with KOH. The external solution [1 μM tetrodotoxin, 150 mM NaCl, 5 mM KCl, 2.5 mM CaCl2, 5.5 mM Hepes-acid, 4.5 mM Hepes-Na, and 10 mM glucose (pH 7.3) with NaOH, 285–300 mosmol−1] was perfused continuously over the cells. With these solutions, both mEPSCs and mIPSPs were inward at the resting potential. They were distinguished by the slower decay time of mIPSPs and were sorted on a computer-based template by using pClamp 9.0 (Molecular Devices) according to ref. 26.
Axons were identified by using phase contrast (SI Fig. 13). Axons were longer than dendrites and unbranching and smaller in diameter. Image analysis was performed by using mIPLab software (Scanalytics) as described in ref. 27. Background fluorescence of each image was subtracted. Fluorescent puncta with an area up to 0.29 μm2 (10 pixels) were outlined and measured. For every punctum, the software determined a region of interest comprising a maximum of 10 pixels (0.29 μm2) of the highest fluorescent intensity. The summed fluorescence correlates with area (up to 0.29 μm2) and depth of the labeled structure (27). Puncta with <5 pixels were excluded. MitoTracker Red (25 nM; Molecular Probes) or transfection with plasmids encoding a mitochondrially targeted fluorescent peptide (Mito-RFP) were used to image mitochondria. Analysis of synaptophysin immunofluorescence in axons apposed to transfected dendrites was performed in Adobe Photoshop. For immunofluorescence intensity, in Fig. 2 H and I, a small box of predetermined pixel number was drawn over each punctum, and the average fluorescence within the box was measured.
See SI Materials and Methods for antibodies used in immunolabeling and Western blotting. Western blotting and immunoprecipitation were carried out as described in ref. 49.
For transmission electron microscopy, virally transduced cells in culture were fixed in 2.5% glutaraldehyde, stained with 2% uranyl acetate (SPI Supplies), dehydrated in ethanol, and embedded in Embed 812 epoxy resin (EMS). Ultrathin (60-nm) sections were stained with 2% uranyl acetate and 1% lead citrate and examined in a Tecnai 12 Biotwin electron microscope (FEI Company).
Drp1 GTPase activity was assayed in vitro with recombinant Bcl-xL and Drp1 by Cytophos phosphate assay (Cytoskeleton).
Supplementary Material
ACKNOWLEDGMENTS.
We thank R. Fitzsimonds, S. Krueger, A. Kolar, and L. K. Kaczmarek for discussions and manuscript preparation; and S. Krueger and A. Kolar, P. Casara and T. Le Diguarher for technical assistance. This work was supported by an American Heart Established Investigator award (to E.A.J.), an American Federation for Aging Research grant (to E.A.J.), and National Institutes of Health Grants NS037402 (to M.V.L.B.), NS045876 (to E.A.J.), NCRR P41 RR001395 (to P.J.S.S.), and NS037402 (to J.M.H.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0711647105/DC1.
References
- 1.Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annu Rev Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- 2.Young SH, Poo MM. Spontaneous release of transmitter from growth cones of embryonic neurones. Nature. 1983;305:634–637. doi: 10.1038/305634a0. [DOI] [PubMed] [Google Scholar]
- 3.Chow I, Poo MM. Release of acetylcholine from embryonic neurons upon contact with muscle cell. J Neurosci. 1985;5:1076–1082. doi: 10.1523/JNEUROSCI.05-04-01076.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang W, Benson DL. Development and molecular organization of dendritic spines and their synapses. Hippocampus. 2000;10:512–526. doi: 10.1002/1098-1063(2000)10:5<512::AID-HIPO2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 5.Ziv NE, Garner CC. Cellular and molecular mechanisms of presynaptic assembly. Nat Rev Neurosci. 2004;5:385–399. doi: 10.1038/nrn1370. [DOI] [PubMed] [Google Scholar]
- 6.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 8.Dejean LM, et al. Oligomeric Bax is a component of the putative cytochrome c release channel MAC, mitochondrial apoptosis-induced channel. Mol Biol Cell. 2005;16:2424–2432. doi: 10.1091/mbc.E04-12-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galonek HL, Hardwick JM. Upgrading the Bcl-2 network. Nat Cell Biol. 2006;8:1317–1319. doi: 10.1038/ncb1206-1317. [DOI] [PubMed] [Google Scholar]
- 10.Jonas E. Bcl-xL regulates synaptic plasticity. Mol Interv. 2006;6:208–222. doi: 10.1124/mi.6.4.7. [DOI] [PubMed] [Google Scholar]
- 11.Krajewska M, et al. Dynamics of expression of apoptosis-regulatory proteins Bid, Bcl-2, Bcl-X, Bax, and Bak during development of murine nervous system. Cell Death Differ. 2002;9:145–157. doi: 10.1038/sj.cdd.4400934. [DOI] [PubMed] [Google Scholar]
- 12.Fletcher TL, Cameron P, De Camilli P, Banker G. The distribution of synapsin I, synaptophysin in hippocampal neurons developing in culture. J Neurosci. 1991;11:1617–1626. doi: 10.1523/JNEUROSCI.11-06-01617.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verderio C, et al. Synaptogenesis in hippocampal cultures. Cell Mol Life Sci. 1999;55:1448–1462. doi: 10.1007/s000180050384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufmann T, et al. Characterization of the signal that directs Bcl-x(L), but not Bcl-2, to the mitochondrial outer membrane. J Cell Biol. 2003;160:53–64. doi: 10.1083/jcb.200210084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jonas EA, et al. Modulation of synaptic transmission by the Bcl-2 family protein Bcl-xL. J Neurosci. 2003;23:8423–8431. doi: 10.1523/JNEUROSCI.23-23-08423.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vander Heiden MG, et al. Bcl-xL promotes the open configuration of the voltage-dependent anion channel and metabolite passage through the outer mitochondrial membrane. J Biol Chem. 2001;276:19414–19419. doi: 10.1074/jbc.M101590200. [DOI] [PubMed] [Google Scholar]
- 17.Verstreken P, et al. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–378. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 18.Guo X, et al. The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron. 2005;47:379–393. doi: 10.1016/j.neuron.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 19.Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Shaw JM, Nunnari J. Mitochondrial dynamics and division in budding yeast. Trends Cell Biol. 2002;12:178–184. doi: 10.1016/s0962-8924(01)02246-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen H, Chan DC. Emerging functions of mammalian mitochondrial fusion and fission. Hum Mol Genet. 2005;14:R283–R289. doi: 10.1093/hmg/ddi270. [DOI] [PubMed] [Google Scholar]
- 22.Frank S, et al. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1:515–525. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- 23.Bossy-Wetzel E, et al. Mitochondrial fission in apoptosis, neurodegeneration, and aging. Curr Opin Cell Biol. 2003;15:706–716. doi: 10.1016/j.ceb.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 24.Jagasia R, Grote P, Westermann B, Conradt B. DRP-1-mediated mitochondrial fragmentation during EGL-1-induced cell death in C. elegans. Nature. 2005;433:754–760. doi: 10.1038/nature03316. [DOI] [PubMed] [Google Scholar]
- 25.Goyal G, et al. Role of mitochondrial remodeling in programmed cell death in Drosophila melanogaster. Dev Cell. 2007;12:807–816. doi: 10.1016/j.devcel.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fitzsimonds RM, Song HJ, Poo MM. Propagation of activity-dependent synaptic depression in simple neural networks. Nature. 1997;388:427–428. doi: 10.1038/41267. [DOI] [PubMed] [Google Scholar]
- 27.Krueger SR, Kolar A, Fitzsimonds RM. The presynaptic release apparatus is functional in the absence of dendritic contact and highly mobile within isolated axons. Neuron. 2003;40:945–957. doi: 10.1016/s0896-6273(03)00729-3. [DOI] [PubMed] [Google Scholar]
- 28.Oltersdorf T, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 29.Letai A. Restoring cancer's death sentence. Cancer Cell. 2006;10:343–345. doi: 10.1016/j.ccr.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 30.Hon H, Rucker EB, III, Hennighausen L, Jacob J. Bcl-xL is critical for dendritic cell survival in vivo. J Immunol. 2004;173:4425–4432. doi: 10.4049/jimmunol.173.7.4425. [DOI] [PubMed] [Google Scholar]
- 31.Shepherd GM, Harris KM. Three-dimensional structure and composition of CA3→CA1 axons in rat hippocampal slices: Implications for presynaptic connectivity and compartmentalization. J Neurosci. 1998;18:8300–8310. doi: 10.1523/JNEUROSCI.18-20-08300.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waters J, Smith SJ. Mitochondria and release at hippocampal synapses. Pflügers Arch. 2003;447:363–370. doi: 10.1007/s00424-003-1182-0. [DOI] [PubMed] [Google Scholar]
- 33.Szabadkai G, et al. Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis. Mol Cell. 2004;16:59–68. doi: 10.1016/j.molcel.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 34.Lee YJ, et al. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell. 2004;15:5001–5011. doi: 10.1091/mbc.E04-04-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jonas EA, Hardwick JM, Kaczmarek LK. Actions of BAX on mitochondrial channel activity and on synaptic transmission. Antioxid Redox Signal. 2005;7:1092–1100. doi: 10.1089/ars.2005.7.1092. [DOI] [PubMed] [Google Scholar]
- 36.Jonas EA, et al. Proapoptotic N-truncated Bcl-xL protein activates endogenous mitochondrial channels in living synaptic terminals. Proc Natl Acad Sci USA. 2004;101:13590–13595. doi: 10.1073/pnas.0401372101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jonas EA, Hickman JA, Hardwick JM, Kaczmarek LK. Exposure to hypoxia rapidly induces mitochondrial channel activity within a living synapse. J Biol Chem. 2005;280:4491–4497. doi: 10.1074/jbc.M410661200. [DOI] [PubMed] [Google Scholar]
- 38.Fannjiang Y, et al. BAK alters neuronal excitability and can switch from anti- to prodeath function during postnatal development. Dev Cell. 2003;4:575–585. doi: 10.1016/s1534-5807(03)00091-1. [DOI] [PubMed] [Google Scholar]
- 39.Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J Cell Sci. 2005;118:5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chada SR, Hollenbeck PJ. Mitochondrial movement and positioning in axons: The role of growth factor signaling. J Exp Biol. 2003;206:1985–1992. doi: 10.1242/jeb.00263. [DOI] [PubMed] [Google Scholar]
- 41.Chada SR, Hollenbeck PJ. Nerve growth factor signaling regulates motility and docking of axonal mitochondria. Curr Biol. 2004;14:1272–1276. doi: 10.1016/j.cub.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 42.Karbowski M, Youle RJ. Dynamics of mitochondrial morphology in healthy cells and during apoptosis. Cell Death Differ. 2003;10:870–880. doi: 10.1038/sj.cdd.4401260. [DOI] [PubMed] [Google Scholar]
- 43.Perfettini JL, Roumier T, Kroemer G. Mitochondrial fusion and fission in the control of apoptosis. Trends Cell Biol. 2005;15:179–183. doi: 10.1016/j.tcb.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 44.De Belleroche JS, Bradford HF. Metabolism of beds of mammalian cortical synaptosomes: Response to depolarizing influences. J Neurochem. 1972;19:585–602. doi: 10.1111/j.1471-4159.1972.tb01376.x. [DOI] [PubMed] [Google Scholar]
- 45.Heidelberger R. ATP is required at an early step in compensatory endocytosis in synaptic terminals. J Neurosci. 2001;21:6467–6474. doi: 10.1523/JNEUROSCI.21-17-06467.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakaba T, Neher E. Involvement of actin polymerization in vesicle recruitment at the calyx of Held synapse. J Neurosci. 2003;23:837–846. doi: 10.1523/JNEUROSCI.23-03-00837.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tobaben S, et al. A trimeric protein complex functions as a synaptic chaperone machine. Neuron. 2001;31:987–999. doi: 10.1016/s0896-6273(01)00427-5. [DOI] [PubMed] [Google Scholar]
- 48.Brewer GJ. Isolation and culture of adult rat hippocampal neurons. J Neurosci Meth. 1997;71:143–155. doi: 10.1016/s0165-0270(96)00136-7. [DOI] [PubMed] [Google Scholar]
- 49.Coligan JE. Current Protocols in Protein Science. New York: Wiley; 1995. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.