Abstract
Armadillos (Dasypus novemcinctus) manifest the full histopathological spectrum of leprosy, and are hosts of choice for in vivo propagation of Mycobacterium leprae. Though potentially useful as a model of leprosy pathogenesis, few armadillo specific reagents exist. We have identified a region of high homology to the interferon gamma (IFN-γ) of other mammals within the recently published armadillo whole genomic sequence. cDNA was made from ConA-stimulated armadillo peripheral blood mononuclear cells (PBMC), amplified, and cloned into a pET expression vector for transformation and over-expression in E. coli. The recombinant protein (rDnIFN-γ) was characterized by western blot and its biological function confirmed with biosassays including intracellular killing of Toxoplasma gondii and induction of indoleamine 2, 3-dioxygenase activity. In using rIFN-γ to activate macrophages from mice, humans or armadillos, similar to humans, rIFN-γ-activated armadillo MΦ did not produce nitrite and or inhibit the viability of M. leprae in vitro. Conversely, murine rIFN-γ-activated mouse MΦ produced high levels of nitrite and killed intracellular M. leprae in vitro. These data indicate that the response of armadillo MΦto rDnIFN-γ is similar to that which occurs in humans, and demonstrates a potentially important value of the armadillo as a model in leprosy research.
Keywords: Dasypus novemcinctus, interferon, leprosy, Mycobacterium leprae, armadillo
1. INTRODUCTION
Leprosy continues to be an important public health problem for the developing world and an estimated 2-3 million people currently live with deformity brought by their disease [1]. Though significant progress has been made in reducing new case presentations [2], improved diagnostic tests and therapeutic regimen are still needed. Two major obstacles impeding the progress of research in these areas have been our inability to cultivate Mycobacterium leprae (the etiological agent for leprosy) on artificial media in the laboratory, and the lack of a robust animal model for studying this infection [3].
Nine-banded armadillos (Dasypus novemcinctus) are the only immunologically intact animal species that exhibits high susceptibility to M. leprae. Like man, armadillos manifest leprosy over a broad clinical and histopathological spectrum that is classifiable from lepromatous to tuberculoid [4], and they have been developed as the hosts of choice for in vivo propagation of leprosy bacilli [5] [6]. Because of their unique natural susceptibility to infection with M. leprae, armadillos could be valuable models of leprosy pathogenesis and help advance development of new diagnostic tests, immunotherapies or vaccines [6]. Unfortunately, because of their exotic nature and scant commercial value, relatively few armadillo-specific immunological reagents have been generated, and consequently few translational benefits actually have been realized with this model to date.
Resistance to M. leprae is mediated through cellular immune processes and involves a complex interplay of cytokines and chemokines. Prominent among these is interferon gamma (IFN-γ), which stimulates macrophages (MΦ) to up-regulate antimicrobial, anti-tumour, and antigen processing and presentation pathways [7]. In rodent immune systems, activation of MΦ by IFN-γ results in effective growth restriction and clearance of mycobacteria with production of reactive nitrogen intermediates (RNI) as effector molecules [8;9]. However this potent antimicrobial mechanism varies from species to species. Human IFN-γ-activated peripheral blood MΦ demonstrate little or no production of nitric oxide (NO)[10;11] and are unable to kill several different mycobacterial species.
The IFN-γ genes of many other mammals have been cloned and over-expressed in E. coli [12;13]. Commercially available recombinant IFN-γ proteins and antibodies also are available for a variety of species, but they rarely exhibit functional cross reactivity between species and it has not been possible to monitor the production of IFN-γ among armadillos over the course of infection by M. leprae. However, because of the armadillo's evolutionary and medical significance, a low (2 X) coverage of the D. novemcinctus genome sequence (http://www.ncbi.nlm.nih.gov/BLAST) was recently published, and more extensive 6 X sequence coverage also is underway. Genomic sequence data is an invaluable resource for the identification and generation of specific immunological reagents [14] and exploitation of the armadillo sequence data can significantly benefit efforts to advance these animals as models for leprosy. We probed the available sequence data for an armadillo homolog to human IFN-γ, and report here the sequence, cloning, expression, biological activity and development of associated specific reagents of recombinant D. novemcinctus IFN-γ (rDnIFN-γ). In addition, we also used these reagents to examine the functional character of armadillo IFN-γ-activated armadillo MΦ to live M. leprae and compared their function to both human and mouse activated macrophages.
2. MATERIALS AND METHODS
2.1. Identification of DnIFN-γ
Bioinformatic tools were used to identify the putative coding sequence of DnIFN-γ. The amino acid sequence of Homo sapiens IFN-γ (GI: 56786138) and tBLASTn (http://www.ncbi.nlm.nih.gov/BLAST/) were used to search for homologous translated sequences in the D. novemcinctus whole genome sequence (WGS)[15]. The putative coding region of DnIFN-γ (GI: DQ094083) was found in two D. novemcinctus genomic contigs (GI: 64640499 and GI: 64640497). The partial genomic sequence was used to derive a putative cDNA and a corresponding translation for the putative amino acid sequence was identified using ExPASy Transalate tool (http://us.expasy.org/tools/dna.html). The cDNA and the amino acid sequence were submitted to BLAST to compare homology to other IFN-γ molecules [15].
2.2. Generation of D. novemcinctus cDNA
Armadillo peripheral blood mononuclear cells (PBMC) were purified from 8 mL peripheral blood collected in BD Vacutainer® CPT Mononuclear Cell Preparation Tubes (BD Biosciences, San Jose, CA) and mononuclear cells were isolated after centrifugation (1600 × g for 45 mins, 25°C). The mononuclear cell layer was removed, washed 3 X with cold PBS, resuspended in culture medium (RPMI 1640 medium containing 2mM glutamine and HEPES) supplemented with 20% fetal bovine serum (FBS), and plated at 2 × 106 cells/mL in a T75 tissue culture flask. The cells were stimulated with ConA (Sigma-Aldrich, St. Louis, MO) at a final concentration of 5 μg/mL for 4 h at 37°C. Aliquots of the ConA-stimulated cells were washed 3 X in cold PBS, resuspended in 500 μL cold PBS, snap frozen in liquid nitrogen, and stored at −70°C for RNA purification. Total RNA was purified from these cells using the FASTRNA™ kit and the FastPrep® FP120 Instrument and manufacturer's recommendations (Q-Biogene, Carlsbad, CA). The cDNA was generated from 1 μg total RNA using the Advantage RT-for-PCR kit with random hexamers (BD Biosciences Clontech, Palo Alto, CA) in a final volume of 50 μL according to the manufacturer's recommendations.
2.3. DnIFN-γ Amplification and Recombinant Plasmid Construction
Primers (DnIFNγ-F 5′-AGAAAAGATCAGCCAAGTCC-3′ and DnIFNγ-R 5′ TTCAAATATTACAGGGAGGATG 3′) (BIOMEDD, Baton Rouge, LA) and armadillo cDNA from Con A-stimulated PBMCs were used with high fidelity polymerase, (Pfu, Strategene, La Jolla, CA), and PCR to generate a fragment encoding the entire DnIFN-γ cDNA. This product was purified using QIAquick columns (QIAgen, Valencia, CA) and verified by automated DNA sequencing using an ABI prism 3310 DNA sequencer (Applied Biosystems, Foster City, CA) (BIOMMED). DNA encoding the mature peptide (the protein without the signal peptide) was amplified from the cDNA using primers containing the “topo” sequence (CACC) on the 5′ terminus (DnIFN-γTOPOF: 5′CACCTGCTACTGCCAGGCCAC3′ and DnIFNγTOPO-R: 5′CAAATATTACAGGGAGGATGACCA3′) and ligated into pET 200/DTOPO® vector (Invitrogen, Carlsbad, CA) using standard procedures. The recombinant plasmid was transformed into E. coli BL21star competent cells (Invitrogen) according to manufacturer's recommendations. Clones were identified by antibiotic selection and further characterized using DnIFNγ PCR/direct sequencing.
2.4. DnIFNγ Protein Expression and Characterization
rDnIFNγ protein was produced from a positive clone using the following protocol. Recombinant bacteria were grown to mid-log phase (OD600= 0.6) and induced with a final concentration of 1 mM isopropyl β-D-thiogalactopyranoside (IPTG) (Sigma-Aldrich). The expressed protein was purified using a Ni-NTA Purification System (Invitrogen) and dialyzed using the recommended, denaturing regimen (Invitrogen). The rDnIFN-γ was refolded as described by Jeevan et al. [16] and concentrated using a Vivaspin 15 mL concentrator with a 10 kDa molecular weight cut-off (Vivascience, Hanover, Germany). The recombinant protein was separated by SDS-PAGE using a 4% to 20% gradient Novex TBE-urea polyacrylamide gel (Invitrogen), stained with Coomassie® Brilliant Blue (Bio-Rad, Hercules, CA), and compared to Kaleidoscope pre-stained polypeptide standards (Bio-Rad). Western blot analysis using rabbit polyclonal antibody prepared with synthetic DnIFN-γ peptide epitopes (below) was used to verify the size and presence of the recombinant product.
2.5. Generation of anti-rDnIFN-γ Antibodies
Polyclonal antibodies (Abgent, Inc, San Diego, CA) were prepared in rabbits against selected synthetic DnIFN-γ peptide epitopes without Freund's complete adjuvant. Based on the deduced amino acid sequence, two epitopes Anti-DnIFN-γ #1 (LKNWKEESDKKIIQS) and Anti-DnIFN-γ #2 (PKSNLRKRKRSQSTF) were selected using in silico predictions of antigenicity. The complexity, hydropathy (Hopp-Woods method) [17], ß-turns (Chou-Fasman method) [18], flexibility, and accessibility of epitopes in the deduced DnIFN-γ amino acid sequence were assessed to generate the regions of the protein most likely to produce antibodies.
Monoclonal antibodies (Mabs) to DnIFN-γ were produced by immunizing Balb/c mice (Harlan, Indianapolis, IN) intra-peritoneally (IP) with 50 μg of the purified recombinant protein in 1:1 TiterMax Gold (Titermax, Inc., Norcross, Ga.) twice in 3-week intervals followed by a final IP injection of 20 μg protein in PBS. Hybridomas were made by fusing primed mouse spleen cells and the myeloma B-cell line SP2/0 and cultured in hypoxanthine, aminopterin and thymidine (HAT) selection medium using a protocol previously described [19]. Hybridoma culture supernatants were screened in an ELISA with either rDnIFN-γ or synthetic peptides. HRP-Rabbit anti-mouse IgG conjugate or HRP-Goat anti-Rabbit IgG (Zymed laboratories, San Francisco, CA) was used to detect the positive clones. Specific antibody reactive sites were determined by ELISA using synthetic 15mer peptides overlapping by 5 amino acids each (15 × 5) and extending over the entire length of the DnIFN-γ (Mimotopes: PharmAus, Ltd., Nedlands, Aus.).
2.6. DnIFN-γ RT-PCR assay
Semi-quantitative RT-PCR assays were developed for detection of DnIFN-γ and DnG3PDH gene transcripts. The following PCR primers were designed from cDNA sequences of DnIFN-γ (Assession # DQ094083) and the contig containing DnG3PDH (Assession # 64811560) using Primer Express software: DnIFNγ-F 5′GAATTACACGGGCTATCTCTTAGCTT3′, DnIFNγ-R 5′ AAGGTCGGCCTGGCAGTAG3′, DnG3PDH-F 5′ AATGGGCATCCCATCACTAT CT3′. Both gene fragments were amplified in PCR from the cDNA of PBMC both stimulated and unstimulated with ConA for 8 hr and specific parameters specified by the Primer Express software. The resultant amplicons were separated on a Novex® 10% TBE mini-gel (Invitrogen) and visualized after ethidium bromide staining using GelDoc2000 Instrument (Bio-Rad). The relative quantity of DnIFN-γ transcripts was estimated by comparing the intensity of the amplicon bands of ConA-stimulated to that of its non-stimulated control for both assays. Amplicons were purified and the DNA sequence of each amplicon was obtained to verify the gene fragments.
2.7. Preparation of MΦ monolayers
Armadillo PBMC were isolated with BD Vacutainer® CPT Mononuclear Cell Preparation Tubes (BD Biosciences) as described above and the mononuclear cells were resuspended in culture medium containing 20% FBS. Cell viability was determined using trypan blue exclusion. Human PBMC were separated over Ficoll-Hypaque (Pharmacia) and resuspended in culture medium supplemented with 10% autologous serum. Cell suspensions were seeded individually into 24 well culture plates (0.5 mL/ well) containing 13 mm round LUX cover slips at a concentration of 4 × 106 cells/mL. Resident murine (Balb/c) peritoneal cells were obtained by lavage, and adherent peritoneal MΦ were cultured on coverslips in culture medium supplemented with 10% FBS. All cultures were incubated at 37°C with 5% CO2 for 7 days to allow differentiation of monocytes to MΦ. Media was changed at least once for all cultures before performing the bioassays.
2.8. MΦ activation
MΦ were activated by replacing the medium in each well with culture medium and appropriate serum supplement along with either rDnIFN-γ (125.0 ng), rHuman IFN-γ (R&D Systems, Minneapolis, MN) (500 U/ml) or rMurine IFN-γ (Genzyme, Cambridge, MA) (500 U/ml). LPS (Sigma-Aldrich) at 5 ng/mL was used as the second signal and plates were incubated overnight at 37°C. Controls with LPS alone showed no activation.
2.9. Toxoplasma killing assay
Bioassays for the IFN-γ-mediated killing of intracellular Toxoplasma gondii were performed as described before [20;21]. Briefly, T. gondii strain RH was maintained by serial 2-day i.p. passage in Swiss-Webster mice (Harlan), harvested from the ascites fluid, and resuspended at 1-1.5 × 106 T. gondii/mL in culture medium containing the appropriate serum. Normal and activated MΦ monolayers were washed with PBS and 0.5 mL of the T. gondii suspension was added to each well. The plates were incubated for 1 h at 37°C and the coverslips were washed and transferred to a new plate with fresh medium. Some cover slips from both controls and activated cells were fixed and stained (Dif-Quick; Dade Behring, Inc., Newark, DE). The rest of the plates were incubated for 20 h at 37°C to accommodate the growth of the T. gondii. At this time the remaining cover slips were fixed, stained, and the individual tachyzoites in rosettes were enumerated.
2.10. Determination of M. leprae viability
Viable M. leprae were harvested from the footpads of athymic nu/nu mice and determined to be free of other microbial contaminants as described previously [22]. The viability of M. leprae was assessed by the oxidation of [14C] palmitate in radiorespirometry using a procedure described before [23].
2.11. Determination of nitrite production
The MΦ culture supernatants were collected in 2 ml centrifuge tubes and stored at −70°C until the nitrite assay was performed. Nitrite production was determined using the Griess Reagent System (Promega, Madison, WI) according to the manufacturer's recommendations. Briefly, 50 μl of the culture supernatants were added in duplicate to a 96-well plate, 50μl of 1% sulfanilamide solution in 5% phosphoric acid was added and the plate was incubated for 10 min 25°C, protected from light. After incubation, 50μl of 0.1% N-1-napthylethylenediamine dihydrochloride solution was added, the plate was incubated for additional 10 min, and absorbance was measured with a plate reader (Bio-Rad) at 520-550 nm.
2.12. Indoleamine 2, 3-dioxygenase activity
rDnIFN-γ bioactivity also was assessed by measuring indoleamine 2, 3-dioxygenase (IDO) activity. Armadillo MΦ monolayers were stimulated with serial dilutions of rDnIFN-γ at 33°C for 72 h. For blocking experiments, monolayers were stimulated with 7.0 μg/mL rDnIFN-γ in the presence of serial dilutions of the polyclonal antibody, anti-DnIFN-γ #1 (500 μL/well) and L-tryptophan (500 μM/well). Supernatants were harvested, combined with 250 μL of 30% trichloroacetic acid, vortexed and heated to 50° C for 30 min to hydrolyze N-formylkynurenine to L-kynurenine. Samples were centrifuged at 10,000 rpm for 5 min and a 125 μl aliquot of the supernatant was added to 125μl of Ehrlich reagent (100 mg of p-dimethylbenzaldehyde + 5 ml of glacial acetic acid), loaded in a 96-well plate and absorbance read at 470 nm.
2.13. Protein Quantification
Protein concentrations were determined spectrophotometrically using BCA Protein Assay Kit (Peirce, Rockford, IL) according to the manufacturer's recommendation.
2.14. Statistical Analyses
The means and standard deviations of groups and replicate samples were compared in ANOVA and Tukey-Kramer t-tests using InStat statistical software (GraphPad Software, Inc, San Diego, CA).
3. RESULTS
3.1. DnIFN-γ Sequence analyses
The 510 bp cDNA sequence encoding DnIFN-γ was identified in the D. novemcinctus WGS, verified by direct PCR/DNA sequencing of armadillo cDNA and translated in silico into a 166 aa protein (Fig. 1). ClustalW multiple alignment analysis of the putative 166 aa DnIFN-γ and other mammalian IFN-γs demonstrated that the DnIFN-γ protein had the greatest homology to Equus caballus (horse) (E-value: 1e-70, 89% match), and a lesser homology to Camelus bactrianus (bactrian camel) (E-value: 9e-69, 87% match), Lama glama (llama) (E-value: 3e-67, 86% match), and Canis familiaris (dog) (E-value: 6e-67, 86% match) (Fig.2). Commercially available IFN-γs from porcine (E-value: 4e-55, 66% match), human (E-value: 2e-49, 62% match) and mouse (E-value: 1e-28, 43% match) were examined for potential homology to DnIFN-γ. Porcine (Sus scrofa) IFN-γ was seen to be the most similar (Fig. 2) and subsequent studies showed porcine IFN-γ also to be effective for activating armadillo MΦ (data not shown).
Figure 1.
Putative DnIFN-γ cDNA (GI: DQ094083) and predicted protein. Italicized characters indicate the putative signal peptide.
Figure 2.
Clustal W alignment of DnIFN-γ-IFN-γ Dasypus (Dasypus novemcinctus) with Equus (Equus caballus), Camelus ( Camelus bactrianus), Lama (Lama glama), Canis (Canis familiaris), Sus (Sus scrofa), Homo (Homo sapiens), and Mus (Mus musculus) IFN-γs. Epitopes used to generate polyclonal antibodies =Anti-DnIFN-γ #1 and Anti-DnIFN-γ #2 are indicated by bold text. The signal peptide is indicated in italics. “*” indicates positions which have a single, fully conserved residue. “:” indicates that one of the following “strong” groups is fully conserved: STA NEQK NHQK NDEQ QHRK MILV MILF HY FYW. “.” indicates that one of the following “weaker” groups is fully conserved: CSA ATV SAG STNK STPA SGND SNDEQK NDEQHK NEQHRK FVLIH FYM.
3.2. Protein Purification
When expressed as recombinant protein, the rDnIFN-γ was found in the insoluble fraction of the E. coli lysate (data not shown). The size of the purified rDnIFN-γ protein (21.2 kDa) was determined in silico and by analyzing the size of the recombinant protein in Coomassie Blue-stained SDS PAGE gels (Fig. 3A) and in Western blot analysis (Fig. 3C) using rabbit polyclonal antibodies (antibodies Anti-DnIFN-γ #1 (Fig. 3B) and Anti-DnIFN-γ #2 (Fig. 3D). The rDnIFN-γ protein appeared larger than the other calculated, mature, native IFN-γ's produced without glycosylation: Sus scrofa 17.1 kDa [24], Homo sapiens 17.5 kDa [25], and Mus musculus 15.8 kDa [26]. This increase in molecular weight likely was due to the inclusion of the 6 X His-tag and enterokinase recognition site (M R G S H H H H H H G M A S M T G G Q Q M G R D L Y D D D D K D H P F T) added to the mature polypeptide sequence and starting at residue C-21 in the recombinant product (Fig. 1).
Figure 3.
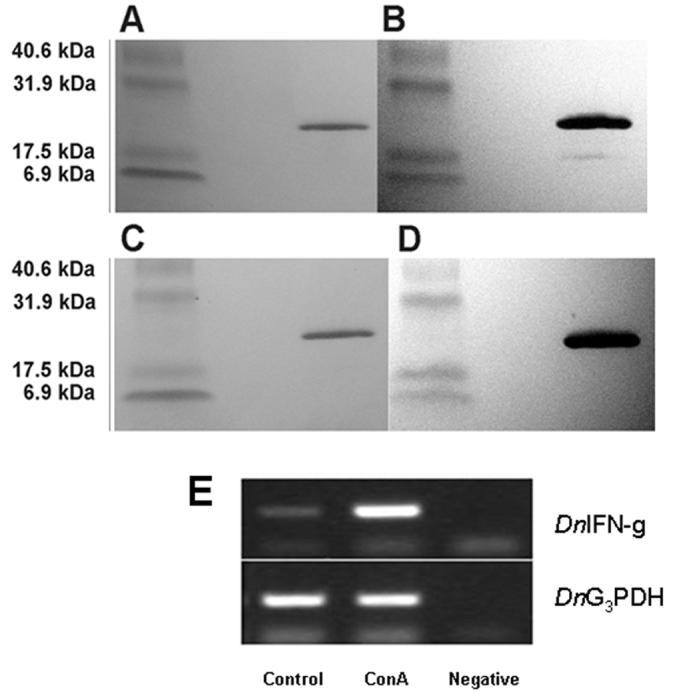
(A and C). Purified rDnIFN-γ (21.22 kDa) separated on a 4% to 20% gradient polyacrylamide gel and stained with Coomassie Blue Brilliant; (B and D) Western blots with polyclonal antibodies Anti-DnIFN-g #1 and Anti-DnIFN-g #2, respectively. E. Semi-quantitative RT-PCR analysis of DnIFN-γ gene expression in armadillo PBMC: Control, non-stimulated armadillo PBMC cDNA; ConA, ConA-stimulated armadillo PBMC cDNA; Negative, buffer control; DnIFN-γ, DnIFN-γ PCR products; DnG3PDH, DnG3PDH housekeeping gene PCR products.
3.3. rDnIFN-γ Antibodies
Although both rabbit polyclonal DnIFN-γ antibodies reacted specifically with their synthetic peptides and were useful in identifying the recombinant product by western blot analysis (Fig. 3 B, D), unfortunately, they failed to show sufficient reactivity when used in combination in capture ELISA for quantifying protein production. The hybridoma production resulted in only one monoclonal antibody that was reactive with armadillo rDnIFN-γ. While it reacted specifically with its synthetic 15mer peptide LKVQRKAVNELFKVM, it also failed to work in combination with the polyclonal antibodies in capture ELISA (data not shown).
3.4. DnIFN-γ gene transcript analysis
Stimulation of armadillo PBMC with ConA resulted in the induction of increased levels of DnIFN-γ gene transcription as indicated by the increase in the DnIFN-γ amplicons over that of the non-stimulated control using the DnIFN-γ RT-PCR assay (Fig 4).
Figure 4.
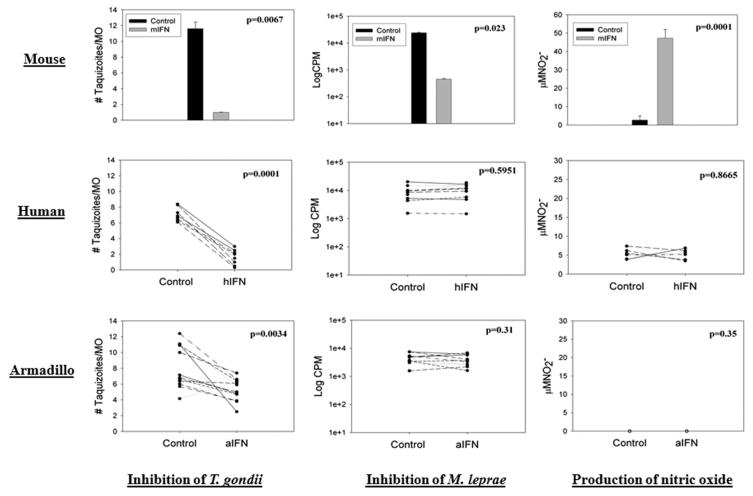
Inhibition of intracellular growth of T. gondii, viability of M. leprae, and Nitrite production in mouse, human and armadillo ṂΦ. MΦ were unstimulated (Control) or activated for 24 h with the appropriate rIFNγ + 5 ng/ml LPS (ACT) prior to challenge with T. gondii or M. leprae. Growth of T. gondii indexed by enumerated intracellular rosettes, and viability of M. leprae indexed with radiorespirometry of 14C-palmitate. Nitrites were assessed on supernatants using the Griess reagent system. Results are representative of three independent experiments in mouse, 8 different human donors, and 4 different armadillos.
3.5. Effect of rDnIFN-γ on IDO activity
In order to determine if DnIFN-γ was biologically active we investigated its ability to induce IDO activity in armadillo MΦ. Results demonstrated that armadillo MΦ stimulated with rDnIFN-γ induced IDO activity whereas MΦ cultured in medium alone produced no L-kynurenine (Table 1). The peak production (104.95 μg/mL L-kynurenine) was induced in armadillo MΦ with 7.0 μg/mL rDnIFN-γ. Polyclonal antibody, anti-DnIFN-γ #1, at 385 μg/mL, successfully blocked IDO activity after stimulation with these maximal levels of rDnIFN-γ, with the resulting 11.2 μg/mL L-kynurenine indicating effective inhibition of the rDnIFN-γ biological activity. Titered results showed 50% and 25% inhibition with 200ug and 50ug pAb concentrations respectively.
Table 1.
Indoleamine 2,3-dioxygenase (IDO) production by armadillo MΦ
| Concentration of rDnIFN-γ (μg/mL) |
L-Kynurenine production (± SD) in armadillo MΦ (μg/mL)a |
|---|---|
| 7.0 | 103.6 ± 43.7 |
| 1.6 | 31.3 ± 28.8 |
| 0.36 | 4.7 ± 1.7 |
| 0.078 | 3.7 ± 1.2 |
| 0.0156 | 3.6 ± 1.1 |
| 0.0 | 3.7 ± 0.9 |
| 7.0 + anti-IFN-γ | 11.2 ± 2.6 |
IDO activity was measured by L-Kynurenine production.
IDO production by armadillo MΦ showed a dosal response to stimulation with rDnIFN-g, and was significantly inhibited with anti-IFN-γ antibody.
3.6. Effect of DnIFN-γ on intracellular growth of T. gondii.
A T. gondii killing assay was used to determine the ability of rDnIFN-γ to activate armadillo MΦ antimicrobial activity. Results demonstrated that mouse MΦ supported growth of intracellular T. gondii while IFN-γ activated MΦ significantly limited parasite growth (P = 0.0067) (Fig. 5). Human MN-derived MΦ from eight different donors supported growth of intracellular T. gondii while IFNγ-activated MΦ significantly limited parasite growth (P = 0.001) (Fig. 5). Armadillo MΦ from four animals were permissive for the intracellular growth of T. gondii; in contrast, armadillo MΦ activated with rDnIFN-γ markedly limited the growth of the intracellular protozoan over a 20 h period (P = 0.0034). Controls with LPS alone did not activate armadillo MΦ to kill T. gondii (data not shown)
3.7. Effect of DnIFN-γ on M. leprae viability
To further evaluate the ability of DnIFN-γ to activate armadillo MΦ, we also examined their antimycobacterial properties, comparing these to activated mouse and human MΦ. The metabolism of M. leprae was significantly reduced (p=0.023) in mouse MΦ activated with rIFN-γ 24 hours prior to M. leprae challenge compared to normal mouse MΦ (Fig. 5). In contrast, no inhibition of M. leprae metabolic activity (P = 0.31) was seen in bacilli recovered from activated human MΦ (Fig. 5) or activated armadillo MΦ (P = 0.59) (Fig. 5).
3.8. Effect of rDnIFN-γ on nitrite production
Mouse MΦ activated with murine rIFN-γ and LPS generated copious amounts of nitrites compared to normal MΦ (P = 0.0001) (Fig.5). In contrast, no significant increase in nitrite production (P = 0.866) was seen in activated human MΦ (Fig. 5) or armadillo MΦ after activation with rDnIFN-γ and LPS (Fig. 5).
4. DISCUSSION
IFN-γ is a pleiotropic molecule produced primarily by T lymphocytes that plays an important role in resistance to intracellular pathogens via its ability to activate MΦ for antimicrobial activity. Therefore, IFN-γ is one of the key cytokines necessary for the study of immunologic processes in the armadillo leprosy model. The data presented here demonstrate that the mature DnIFN-γ has been successfully produced, purified and that it possesses immunological activity.
The DnIFN-γ shows high homology to other mammalian IFN-γ as evidenced by their low Expect (E) values. However, glycosylation prediction analysis using NetNGlyc (http://www.cbs.dtu.dk/services/NetNGlyc/) demonstrated a lower N-glycosylation level in DnIFN-γ compared to that of other IFN-γ such as H. sapiens and Mus musculus (data not shown). It has been shown that glycosylation of the other mammalian IFN-γ may increase bioactivity [24] [25], but it apparently was not necessary for DnIFN-γ function as evidenced by the appropriate biological properties we observed.
One such biological property studied was the induction of IDO activity. Human MΦ activated with IFN-γ produce IDO which rapidly depletes intracellular tryptophan and has been associated with an inhibitory effect on the growth of intracellular pathogens [27;28]. In the IDO pathway, tryptophan is decyclized to L-Kynurenine which can be measured spectrophotometrically and used as an indicator of IFN-γ activity [29]. In the present study DnIFN-γ appropriately up-regulated IDO activity in armadillo MΦ, and polyclonal antibodies (anti-DnIFN-γ #1), made against a synthetic peptide epitope of rDnIFN-γ, effectively blocked IDO activity.
We also examined the production of RNI by MΦ following IFN-γ activation. MΦ activated with IFN-γ and a microbe-derived trigger, such as LPS, generate inducible nitric oxide synthase (iNOS) which catalyzes the formation of nitric oxide radical (NO) from L-arginine [30]. NO induces cytotoxic effects via its ability to inhibit iron and iron/sulfur containing enzymes involved in cellular respiration and DNA synthesis [31]. The end products of iNOS activity are nitrites, nitrates, and citrulline that can be measured in culture supernatants. Activated mouse peritoneal MΦ produce large quantities of nitric oxide [30;32], and we and others have previously shown that such MΦ inhibit both T. gondii [20] and M. leprae in an RNI dependent manner [8;33]. In contrast, peripheral blood derived human MΦ produce little nitric oxide after activation with IFN [10;11]. In this study we showed that armadillo MΦ, like their human counterparts, did not generate nitrites after activation with DnIFN-γ. Interestingly, RNI, as measured by nitrotyrosine staining, have been demonstrated in human tissues at sites of infection, including leprosy lesions [34;35] and M. tuberculosis infected lungs [36]. Whether or not armadillos express RNI in leprosy lesions is currently under investigation.
One of the most important functions of the activated MΦ is the killing of intracellular pathogens. This complex process is a culmination of a series of events that ultimately affords protection to the host. Previous reports have demonstrated a role for the enhanced microbicidal activity of the IFN-γ-activated mouse MΦ against a variety of mycobacterial pathogens, including M. tuberculosis [37] and M. leprae[38]. However, unlike the murine system, IFN-γ does not appear to activate human MΦ to kill or inhibit mycobacteria such as: M. tuberculosis [37], M. avium[39] and M. phlei [40]. In the well-controlled studies from Crowle's group [41], MdMΦactivated by IFN-γ efficiently killed the intracellular protozoan Leishmania but not M. tuberculosis. In the present study we explored killing of another intracellular protozoan, T. gondii in parallel with M. leprae by IFN-γ-activated MΦ from human, mice and armadillos. Our results confirm the finding that IFN-γ-activated mouse and human MΦcan kill intracellular protozoa [42;43] and we also showed that activated armadillo MΦ are microbicidal for T. gondii. In contrast however, while activated mouse MΦreadily cope with M. leprae, neither IFN-γ-activated human nor DnIFN-γ-activated armadillo MΦ killed or inhibited the leprosy bacillus.
Armadillos, like humans, manifest leprosy over a broad histopathological spectrum, and are considered potentially valuable models for studying leprosy pathogenesis, investigating susceptibility and resistance to M. leprae, monitoring the evolution of the immune response to this pathogen, and developing new diagnostic assays and vaccines. The in vitro response of MΦ to IFN-γ activation shown in this study highlights interesting similarities between humans and armadillos in their cell mediated immune response and affirms an important value of this animal as a model for the study of human leprosy.
Besides man, nine-banded armadillos are the only immunologically intact animal hosts that exhibit natural susceptibility to high levels of infection with M. leprae. Like man, armadillos also manifest their leprosy over a broad spectrum of clinical and histopathological responses. The factors which underlie their unique shared susceptibility to M. leprae, or that trigger the immune response of individual animals to manifest the disease over such a diverse clinical spectrum remain mysteries. Access to the armadillo whole genomic sequence will greatly benefit our ability to develop additional armadillo immunological reagents. Availability of rDnIFN-γand other cytokines, probes and cell markers that are now in development will help advance these animals as important models in leprosy research.
Acknowledgements
The authors thank Kyle Andrews, Tana Pittman, Heidi Zhang, Roena Stevenson and Tamara Chouljenko for their technical assistance. We express our appreciation to Dr. Jean Chang at the Broad Institute for her assistance and advice regarding the Dasypus novemcinctus sequence and to Hyosun Cho of the D. N. McMurray lab at Texas A & M with information on refolding guinea pig IFN-γ. This study was supported in part by the National Hansen's Disease Programs and funds from the National Institute for Allergy and Infectious Disease, contract number Y1-AI-2646-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lockwood DN, Suneetha S. Leprosy: too complex a disease for a simple elimination paradigm. Bull of World Health Organ. 2005;83(3):230–5. [PMC free article] [PubMed] [Google Scholar]
- 2.Global leprosy situation, 2007. Wkly Epidemiol Rec. 2007;25(82):225–32. [PubMed] [Google Scholar]
- 3.Scollard DM, Adams LB, Gillis TP, Krahenbuhl JL, Truman RW, Williams DL. The continuing challenges of leprosy. Clin Microbiol Rev. 2006;9(2):338–81. doi: 10.1128/CMR.19.2.338-381.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Job CK, Sanchez RM, Hastings RC. Manifestations of experimental leprosy in the armadillo. Am J Trop Med Hyg. 1985;34:151–61. doi: 10.4269/ajtmh.1985.34.151. [DOI] [PubMed] [Google Scholar]
- 5.Kirchheimer WF, Storrs EE, Binford CH. Attempts to establish the Armadillo (Dasypus novemcinctus linn.) as a model for the study of leprosy. II. Histopathologic and bacteriologic post-mortem findings in lepromatoid leprosy in the Armadillo. Int J Lepr Other Mycobact Dis. 1972;40:229–42. [PubMed] [Google Scholar]
- 6.Truman RW, Sanchez R. “Armadillos: models for leprosy”. Lab Animal. 1993;22(1):28–32. [Google Scholar]
- 7.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–89. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 8.Adams LB, Franzblau SG, Vavrin Z, Hibbs JB, Jr, Krahenbuhl JL. L-arginine-dependent macrophage effector functions inhibit metabolic activity of Mycobacterium leprae. J. Immunol. 1991;147:1642–6. [PubMed] [Google Scholar]
- 9.Chang J, Xing Y, Magliozzo RS, Bloom BR. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992;175(4):1111–22. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bermudez L, Covaro EG, Remington J. Infection of murine macrophages with Toxoplasma gondii is associated with release of transforming growth factor beta and downregulation of expression of tumor necrosis factor receptors. Infect Immun. 1993;61:4126–30. doi: 10.1128/iai.61.10.4126-4130.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneemann M, Schoedon G, Hofer S, Blau N, Guerrero L, Schaffner A. Nitric oxide synthase is not a constituent of the antimicrobial armature of human mononuclear phagocytes. J Infect Dis. 1993;167:1358–63. doi: 10.1093/infdis/167.6.1358. [DOI] [PubMed] [Google Scholar]
- 12.Slobbe L, Lockhart E, Kelly J, Buchan G. The production and biological assessment of cervine interferon gamma. Cytokine. 2000;12:1211–7. doi: 10.1006/cyto.2000.0690. [DOI] [PubMed] [Google Scholar]
- 13.Zucker K, Lu P, Asthana D, et al. Production and characterization of recombinant canine interferon-gamma from Escherichia coli. J Interferon Res. 1993;13:91–7. doi: 10.1089/jir.1993.13.91. [DOI] [PubMed] [Google Scholar]
- 14.Adams JE, Pena MT, Gillis TP, Williams DL, Adams LB, Truman RW. Expression of nine-banded armadillo (Dasypus novemcinctus) interleukin-2 in E.coli. Cytokine. 2005;32:219–25. doi: 10.1016/j.cyto.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeevan A, McFarland CT, Yoshimura T, et al. Production and characterization of guinea pig recombinant gamma interferon and its effect on macrophage activation. Infect Immun. 2006;74:213–24. doi: 10.1128/IAI.74.1.213-224.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hopp TP, Woods KR. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981;78:3824–8. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chou PY, Fasman GD. Prediction of protein conformation. Biochemistry. 1974;13:222–45. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- 19.Liddell JE, Cryer A. In: A Practical guide to monoclonal antibodies. Liddell JE, Cryer A, editors. John Wiley & Sons Ltd; West Sussex, England: 1993. p. 188. [Google Scholar]
- 20.Adams LB, Hibbs JB, Jr, taintor R, Krahenbuhl JL. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role of synthesis of inorganic nitrogen oxides from L-arginine. J. Immunol. 1990;144:2725–9. [PubMed] [Google Scholar]
- 21.Sibley LD, Adams LB, Fukutomi Y, Krahenbuhl JL. Tumor necrosis factor-alpha triggers antitoxoplasmal activity of IFN-gamma primed macrophages. J Immunol. 1991;147:2340–5. [PubMed] [Google Scholar]
- 22.Truman RW, Krahenbuhl JL, Viable M. leprae as a research reagent. Int J Lepr Other Mycobact Dis. 2001;69:1–12. [PubMed] [Google Scholar]
- 23.Hagge DA, Ray NA, Krahenbuhl JL, Adams LB. An in vitro model for the lepromatous leprosy granuloma: fate of Mycobacterium leprae from target macrophages after interaction with normal and activated effector macrophages. J Immunol. 2004;172:7771–9. doi: 10.4049/jimmunol.172.12.7771. [DOI] [PubMed] [Google Scholar]
- 24.Dijkmans R, Vandenbroeck K, Beuken E, Billiau A. Sequence of the porcine interferon-gamma (IFN-gamma) gene. Nucleic Acids Res. 1990;18:4259. doi: 10.1093/nar/18.14.4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gray PW, Leung DW, Pennica D, et al. Expression of human immune interferon cDNA in E. coli and monkey cells. Nature. 1982;295:503–8. doi: 10.1038/295503a0. [DOI] [PubMed] [Google Scholar]
- 26.Gray PW, Goeddel DV. Cloning and expression of murine immune interferon cDNA. Proc Natl Acad Sci U S A. 1983;80:5842–6. doi: 10.1073/pnas.80.19.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlin JM, Borden EC, Sondel PM, Byrne GI. Interferon-induced indoleamine 2,3-dioxygenase activity in human mononuclear phagocytes. J Leukoc Biol. 1989;45:29–34. doi: 10.1002/jlb.45.1.29. [DOI] [PubMed] [Google Scholar]
- 28.Hucke C, MacKenzie CR, Adjogble KD, Takikawa O, Daubener W. Nitric oxide-mediated regulation of gamma interferon-induced bacteriostasis: inhibition and degradation of human indoleamine 2,3-dioxygenase. Infect Immun. 2004;72:2723–30. doi: 10.1128/IAI.72.5.2723-2730.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Werner-Felmayer G, Werner ER, Fuchs D, Hausen A, Reibnegger G, Wachter H. Characteristics of interferon induced tryptophan metabolism in human cells in vitro. Biochim Biophys Acta. 1989;1012:140–7. doi: 10.1016/0167-4889(89)90087-6. [DOI] [PubMed] [Google Scholar]
- 30.Hibbs JB, Jr, Vavrin Z, Taintor RR. L-arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J Immunol. 1987;138:550–65. [PubMed] [Google Scholar]
- 31.Thomas SR, Mohr D, Stocker R. Nitric oxide inhibits indoleamine 2,3-dioxygenase activity in interferon-gamma primed mononuclear phagocytes. J Biol Chem. 1994;269:14457–64. [PubMed] [Google Scholar]
- 32.Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407–12. [PubMed] [Google Scholar]
- 33.Adams LB, C. K. Job, Krahenbuhl JL. Role of inducible nitric oxide synthase in resistance to Mycobcterium leprae in mice. Infect Immun. 2000;68:5462–5. doi: 10.1128/iai.68.9.5462-5465.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schon T, Hernandez-Pando R, Baquera-Heredia J, et al. Nitrotyrosine localization to dermal nerves in borderline leprosy. Br J Dermatol. 2004;150:570–4. doi: 10.1046/j.1365-2133.2004.05764.x. [DOI] [PubMed] [Google Scholar]
- 35.Schon T, Hernandez-Pando RH, Negesse Y, Leekassa R, Sundqvist T, Britton S. Expression of inducible nitric oxide synthase and nitrotyrosine in borderline leprosy lesions. Br J Dermatol. 2001;145:809–15. doi: 10.1046/j.1365-2133.2001.04491.x. [DOI] [PubMed] [Google Scholar]
- 36.MacMicking JD, North RJ, LaCourse R, Mudgett JS, Shah SK, Nathan CF. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci U S A. 1997;94:5243–8. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rook GA, Steel WJ, Ainsworth M, Champion BR. Activation of macrophages to inhibit prolifearation of Mycobacterium tuberculosis: comparison of the effects of recombinant gamma-interferon on human monocytes and murine peritoneal macrophages. Immunol. 1986;19:333–38. [PMC free article] [PubMed] [Google Scholar]
- 38.Sibley LD, Franzblau SG, Krahenbuhl JL. Intracellular fate of Mycobacterium leprae in normal and activated mouse macrophages. Infect Immun. 1987;55:680–5. doi: 10.1128/iai.55.3.680-685.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toba H, Crawford JT, Ellner JJ. Pathogenicity of Mycobacterium avium for human monocytes: absence of macrophage-activating factor activity of gamma interferon. Infect Immun. 1989;57:239–44. doi: 10.1128/iai.57.1.239-244.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robertson AK, Andrew PW. Interferon gamma fails to activate human monocyte-derived macrophages to kill or inhibit the replication of a non-pathogenic mycobacterial species. Microb Pathog. 1991;11:283–8. doi: 10.1016/0882-4010(91)90032-6. [DOI] [PubMed] [Google Scholar]
- 41.Douvas GS, Looker DL, Vatter AE, Crowle AJ. Gamma interferon activates human macrophages to become tumoricidal and leishmanicidal but enhances replication of macrophage-associated mycobacteria. Infect Immun. 1985;50:1–8. doi: 10.1128/iai.50.1.1-8.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sibley LD, Adams LB, Krahenbuhl JL. Macrophage interactions in toxoplasmosis. Res Immunol. 1993;144:38–40. doi: 10.1016/s0923-2494(05)80095-1. [DOI] [PubMed] [Google Scholar]
- 43.Nathan CF, Murray HW, Wiebe ME, Rubin BY. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983;158:670–89. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]