Abstract
This manuscript describes the fabrication and manipulation of millimeter-scale spheres fabricated from ionotropic hydrogels that are crosslinked with paramagnetic metal ions (e.g., Ho3+). These ionotropic hydrogels experience a force in a magnetic field gradient that correlates with the concentration of the paramagnetic cations crosslinking the polymer. In an externally applied magnetic field, the paramagnetic hydrogel spheres assemble into ordered arrays or confined geometrical structures in the regions of highest magnetic field. These spheres can be separated from heterogeneous mixtures of diamagnetic materials using a simple bar magnet. Two applications using these recoverable hydrogel spheres were demonstrated: i) When prepared with embedded indicator dyes bound to paper, the spheres were used as colorimetric sensors for pH. ii) When prepared with embedded activated carbon powder, they were used to remove organic materials from aqueous solutions.
Keywords: Alginic Acid, Holmium, Self-Assembly, Ion Chromatography, Magnetism
Introduction
This manuscript describes the fabrication and manipulation of millimeter-scale spheres made from ionotropic hydrogels that are crosslinked with paramagnetic metal ions (e.g., Ho3+). These ionotropic hydrogels experience a force in a magnetic field gradient that reflects the paramagnetism of the ions crosslinking the polymer. (Most magnetically responsive hydrogels are made with embedded superparamagnetic particles in the polymer matrix.) Although the forces on paramagnetic materials are weaker than those on superparamagnetic materials, we can separate spheres that are crosslinked with paramagnetic ions from spheres crosslinked with diamagnetic ions (e.g., La3+ or Al3+). The magnetic response allows for collection of the hydrogel spheres from aqueous or heterogeneous mixtures for further use or analysis. In an externally applied magnetic field, the paramagnetic hydrogel spheres assemble into ordered arrays or confined geometrical structures in the regions of highest magnetic field. We demonstrated two applications for these hydrogel spheres: i) as colorimetric sensors for pH, when prepared with embedded paper fibers containing bound indicator dyes; and ii) as sorbents for the removal of organic molecules from aqueous solutions, when prepared with embedded activated carbon powder. In both applications, the spheres can be recovered with a bar magnet and reused.
Ionotropic hydrogels
Ionotropic hydrogels are polymers that form hydrated gel matrices in the presence of metal cations. Most multivalent cations will crosslink aqueous solutions of alginic acid (AA)—a linear copolymer of α-L-guluronic acid (G) and β-D-mannuronic acid (M) residues—to form hydrogels.1,2 A wide variety of ions form complexes with AA, including alkaline earth metals (e.g., Ca2+, Sr2+, Ba2+), transition metals (e.g., Pb2+, Cu2+, Cd2+, Zn2+, Ni2+, Al3+, Fe3+, Sn4+), and lanthanide metals (e.g., La3+, Nd3+, Eu3+).3,4
Polymers other than AA also form ionotropic hydrogels.5 Pectin6 and carboxymethyl cellulose (CMC)7 present carboxylate groups and gel in the presence of metal cations. The polysaccharide chitosan,8 which bears an amino group on each residue, and κ- and ι-carrageenan,6,9,10 both of which bear sulfate groups, also form ionotropic hydrogels. Aside from these polysaccharides, poly(bis(4-carboxyphenoxy)phosphazene)11 is a widely used polycarboxylate that gels in the presence of metal cations. These ionotropic hydrogels have been used as cell scaffolds (e.g., for islet cells),12–14 delivery systems for pharmaceuticals,7,15 sorbents for the sequestration of metals in contaminated aqueous solutions,16 substrates for microfluidics,17 and components in the production of foods and beverages.18
Structure of AA Hydrogels
Cozzi and coworkers developed three techniques to measure the affinity of AA for metal cations:4 i) The first monitored the change in pH upon the addition of a metal cation to a solution of alginic acid.19 ii) The second determined the amount of metal cation required to form a precipitate from a solution of alginic acid.19 iii) The third measured the relative rate of migration of the metal ion to the aqueous solvent front during thin- layer chromatography through a film of AA.20,21 These methods yield similar trends for the strengths of complexation of ions with alginic acid: Pb2+ > Ba2+ > Fe3+, Al3+ > Cu2+, Cd2+ > Ca2+ > Zn2+, Co2+, Ni2+ > Mn2+, Mg2+ > K+ > Na+ > Li+.4,19–21 Monovalent alkali earth metals (e.g., Na+ and K+) and some divalent cations—Mn2+ and Mg2+—do not crosslink AA to form hydrogels.4
There is a growing body of research aimed at defining the structure of and the mechanism of crosslinking in alginate hydrogels.1 The mechanism of crosslinking is the coordination of carboxylic acids and hydroxyl groups on the polymer to the metal ions. While the conformations of MM and MG blocks in AA are roughly linear, the GG blocks are “puckered”.15,22 Rees et al. observed that these puckered sequences create cavities that can be occupied by cations.22 This configuration is called the “egg-box model,” as the crosslinking cations (the “eggs”) sit in the cavities found in GG blocks of the polymer. It is the most widely accepted model for the structure of alginate hydrogels.
Hydrogels and Magnetism
Magnetically-responsive hydrogels can be manipulated by an applied inhomogeneous magnetic field. A common approach for fabricating magnetically-responsive hydrogels is to create a two-phase system in which ferromagnetic or superparamagnetic particles are either suspended in the polymer matrix before gelation23–25 or synthesized in preformed hydrogels.26,27 The magnetic particles decrease the transparency of the hydrogels and alter the porosity of the material.
Only a few examples exist in which ionotropic hydrogels incorporate paramagnetic cations as the crosslinking agents. One application of hydrogels crosslinked with paramagnetic ions (e.g., Cu2+ or Mn2+) is in the determination of structures of the hydrogels by NMR spectroscopy.28,29 Another application is the use of these magnetic hydrogels as coatings on implantable devices to enhance the contrast for magnetic imaging.30,31 All reported uses of magnetic ionotropic hydrogels are passive—none take advantage of the positive magnetic response of the hydrogels for actuation or manipulation.
Experimental Design
We selected Ho3+ as the paramagnetic ion to crosslink the aqueous polymer solutions into hydrogels because it offers two distinct advantages over main-group paramagnetic cations. First, Ho3+ has one of the largest magnetic susceptibilities (χ ~ +0.44 cm3/mol) of any ionic species.32 Since the magnetic susceptibilities of the organic polymer and water are negative and small, the positive magnetic response of these hydrogel spheres arises solely from the presence of Ho3+ cations. The strength of the magnetic force is linearly proportional to the concentration of the Ho3+ bound within the gel. Second, the affinity of AA for cations typically increases with charge. The Ho3+-AA complex is strong enough for the hydrogel spheres to remain intact in aqueous solutions (pH ~7) for long periods of time (over one year) and retain their positive magnetic response.
Results and Discussion
Crosslinking and formation of paramagnetic ionotropic hydrogels
We produced millimeter-sized hydrogel spheres by crosslinking anionic polymers in aqueous solutions with Ho3+ cations based on a standard procedure.13 We injected droplets of the solution of polymer (typically 2% w/v) from a 27-gauge needle into an aqueous solution of Ho(NO3)3 (concentrations 10–500 mM), buffered with 10 mM Tris, pH 7.5. The viscosity of the solution and the gauge of the needle determined the size of the droplet, and correspondingly, of the hydrogel sphere. The spheres we fabricated were 2–5 mm in diameter.
We investigated the effect of pH on the production of the hydrogel beads. Using either buffered solutions of polymers in water (pH 7.5 or greater) or unbuffered solutions of polymer (pH ~6, for the sodium salt of AA), stable hydrogels could form so long as the polymer did not precipitate with the change in pH. At low values of pH (~1), some of the polymers were insoluble. The pH of the aqueous solution of Ho3+ (over the range 4–10) did not affect the formation of stable hydrogels.
We surveyed a number of ionomers—polymers that present bound ions and mobile counterions—for their ability to form stable hydrogels with Ho3+ (Table 1). Of the ionotropic polymers we investigated, AA, CMC, ι-carrageenan, and polygalacturonic acid (PG) formed stable hydrogel spheres in the presence of Ho3+. Poly(bis(4-carboxyphenoxy)phosphazene) produced hydrogel particles, but instead of spheres, either shells or toroids formed depending on the height from which the droplets fell into the aqueous solution containing holmium cations. Unlike the other hydrogels, poly(bis(4-carboxyphenoxy)phosphazene) formed fragile gels that could not be manipulated without destruction of the material.
Table 1.
Polymers that form hydrogels in the presence of Ho3+ cations and those that fail.1
| Form Gels with Ho3+ | Do Not Form Gels with Ho3+ |
|---|---|
| alginic acid | hyaluronic acid |
| carboxymethyl cellulose | chitosan |
| ι-carrageenan | κ-carrageenan |
| polygalacturonic acid | dextran sulfate |
| poly(bis(4-carboxyphenoxy phosphazene)) | polystyrene sulfonate |
| poly(vinyl phosphonic acid) | |
| poly(acrylic acid) | |
| chondroitin sulfate |
See Supplemental Figure 3 for structures of the polymers.
Not every polymer reported in the literature to form hydrogels in the presence of metal cations formed a stable hydrogel in the presence of Ho3+ (Table 1). Vercruysse, et al. reported that hyaluronic acid formed a “reverse transition” hydrogel in the presence of several lanthanides, with a critical temperature of gelation dependent on the molecular weight of the polymer and on the metal cation.33 We did not observe hyaluronic acid to produce this type of gel with Ho3+. Also, κ-carrageenan—a polymer that gels in high concentrations of potassium—did not gel in the presence of Ho3+, and neither did chitosan, a polymer that is soluble only when its pendant amine groups are protonated (i.e., pH < 6.3).34
The anionic polymers dextran sulfate, polystyrene sulfonate, poly(vinyl phosphonic acid) (2–40% w/v), poly(acrylic acid) (2–35% w/v), and chondroitin sulfate (2–10% w/v) did not form stable hydrogels with Ho3+. Dextran sulfate, polystyrene sulfonate, and poly(vinyl phosphonic acid) precipitated out of solution in the presence of 100 mM Ho3+, but we do not consider these precipitates to be gels as they do not maintain their structural integrity. Poly(acrylic acid) and chondroitin sulfate remained soluble in the presence of holmium, even for [Ho3+] > 1 M.
Characterization of the paramagnetic hydrogels
In order to compare the affinity of AA for Ho3+ relative to other ions, we modified the procedure for thin-layer chromatography (TLC) described by Cozzi, et al.20 by performing liquid chromatography (LC) on a column of acidified AA. Our use of LC instead of TLC offered the advantages of more accurate quantitative data, improved reproducibility by reusing the same column instead of homemade single-use plates, and easier analysis by substituting UV-vis detection for chemical developing reagents.
To compare our data to those of Cozzi, we calculated Rf values based on the ratio of eluant (0.1 M HCl) required to elute nitrate, a marker for the solvent front, to the volume required to elute a metal analyte. Chromatographic analysis of Ni2+, Cu2+, and Fe3+ gave Rf values of 0.79, 0.59, and 0.19, respectively (for an example chromatogram, see Supplemental Figure 1). These data are similar to the results of Cozzi, et al. Analysis of Ho3+ gave an Rf value of 0.62. This result suggests that the affinity of AA for Ho3+ is markedly lower than for trivalent cations of transition metals such as Fe3+ (0.19, which we measured) and Cr3+ (0.39, as reported in the literature).20,21
Using UV-vis spectroscopy, we measured the concentration of holmium inside of the Ho3+-AA spheres and calculated the magnetic susceptibility per sphere. First, we fabricated a set of AA spheres in three different concentrations of Ho3+—10 mM, 100 mM, and 500 mM. The AA spheres were subsequently washed for several hours to extract any unbound Ho3+. A 5-mL aqueous solution of EDTA (pH 7.4) dissolved 100 AA spheres from each of the three sets. Using the absorption peak for Ho3+ at 451 nm, we measured the absorbance of each solution and determined an average value for the [Ho3+] per sphere. The concentration of Ho3+ per sphere—roughly 100 mM—was similar for the spheres made from the 100-mM and 500-mM solutions. The 10-mM solution produced spheres with a lower concentration of Ho3+—roughly 85 mM. These concentrations correspond to magnetic susceptibilities of +0.042 and +0.037, respectively.
Separation and manipulation of hydrogel spheres
Rare-earth permanent magnets (e.g., NdFeB) attract paramagnetic hydrogel spheres suspended in diamagnetic media and allow the spheres to be manipulated. The magnetic force acting on a paramagnetic sphere in a diamagnetic medium is linearly proportional to the concentration of paramagnetic ions within the sphere (eq. 1.), where χS and χl are the magnetic susceptibilities of the sphere and the liquid,
| (1) |
respectively (we assume χl for a diamagnetic liquid to be negligible), V is the volume of the sphere, μ0 is the magnetic permeability of free space, and (B⃗⋅∇⃗)B⃗ is the product of the magnetic field and its gradient.
To test the effect of Ho3+ incorporation on the magnetic response of the hydrogels, we crosslinked CMC in solutions containing both paramagnetic cations (Ho3+) and diamagnetic cations (La3+). These cations were selected because they are trivalent lanthanides, so we expect both to interact with CMC with similar binding affinities. We crosslinked CMC in six different solutions of Ho3+ and La3+ ions; each solution contained a different mole fraction of [Ho3+] but an identical total concentration of cations ([La3+] + [Ho3+]). During fabrication, we left the spheres to soak for 12 hours and assumed that the relative concentration of the crosslinking cations in the hydrogel was similar to that of the bulk solution. After washing the spheres in deionized water for 2 hours, for each batch of AA spheres, we measured the furthest distance from which a hydrogel sphere was attracted to the surface of the NdFeB magnet (Supplemental Figure 2) when the orientation of the magnet was parallel to the benchtop (i.e., normal to the gravitational force). As a control, we crosslinked AA spheres with diamagnetic La3+ cations only; these spheres were not attracted to the surface of the magnet.
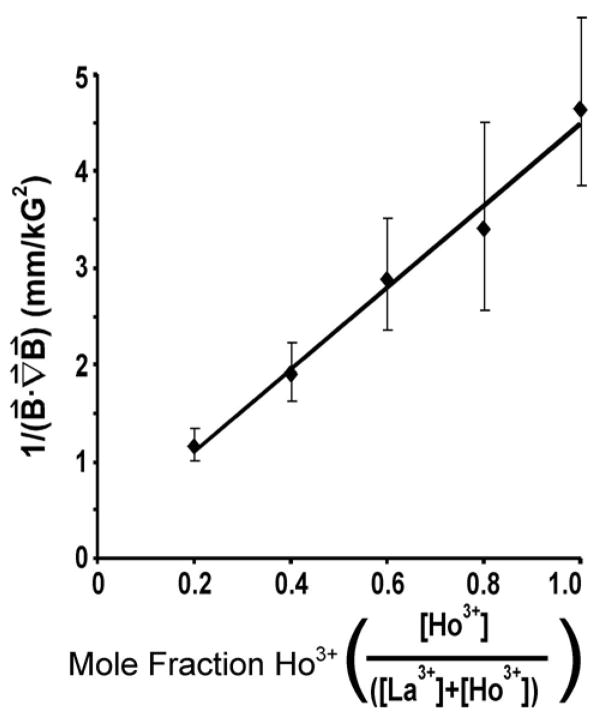
The distance from which a paramagnetic sphere is attracted to a magnet is a function of the orientation and strength of the magnet. We, therefore, assumed that the minimum force required to attract a paramagnetic sphere would scale linearly with the extent of Ho3+ incorporation in the sphere when the distribution of the magnetic field was held constant. The sphere will only move when the magnetic force is greater than the viscous drag force on the sphere, since the gravitational force can be neglected, as it is orthogonal to the magnetic force. We measured the magnetic field strength normal to the surface of the magnet with a hand-held magnetometer and calculated the magnetic field gradient from these measurements.35 The graph of the mole fraction of Ho3+ in the crosslinking solution versus the inverse of the product of the magnetic field and its gradient shows a linear relationship (from eq. 1), which confirms that the minimum force required to attract a paramagnetic CMC sphere is constant (Figure 1). The time that is required for a paramagnetic sphere to attach to the surface of a bar magnet will depend on the distance of the sphere from the magnet, the strength of the applied field, the gradient of the field, the magnetic susceptibility of the paramagnetic hydrogel, viscous forces (which depend on the effective radius of the particle and the viscosity of the solution), and the force of gravity.
Figure 1.
The magnetic susceptibility is controlled by the extent of incorporation of a paramagnetic ion into a hydrogel. A) A set of CMC spheres was crosslinked with different relative concentrations of paramagnetic Ho3+ and diamagnetic La3+. The graph plots the inverse of the dot product of the magnetic field and its gradient against the mole fraction of Ho3+ cations. The inverse proportionality is linear, as predicted by equation 1. Error bars represent one standard deviation based on the data from seven measurements.
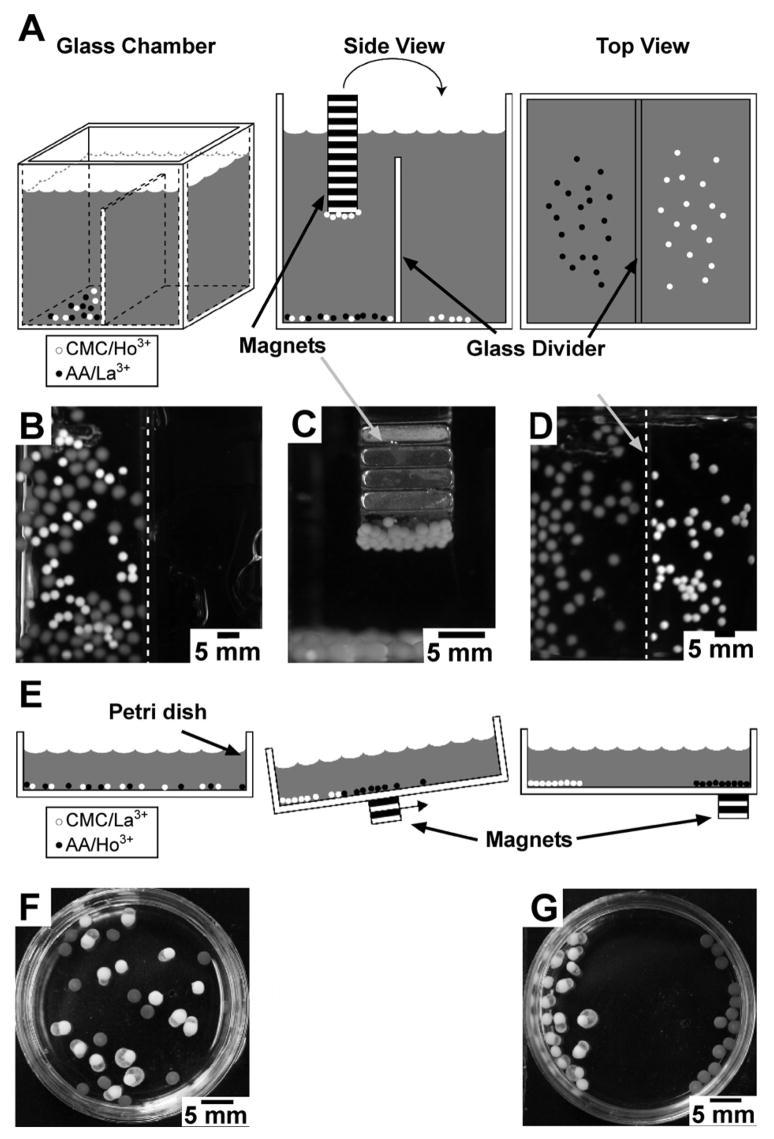
Figure 2 shows two different types of separations (using a NdFeB bar magnet) of hydrogel spheres crosslinked with paramagnetic ions from hydrogel spheres crosslinked with diamagnetic ions. In the first type of separation, a magnet was immersed in a mixed suspension of i) paramagnetic CMC spheres (2–3 mm in diameter) crosslinked with Ho3+ and ii) diamagnetic AA spheres (3–4 mm in diameter) crosslinked with Al3+ (Figure 2A). When we brought the permanent magnet into contact with the mixture of spheres, only the paramagnetic Ho3+-CMC spheres were attracted to the magnet and could be removed from the container (together with adhering water) (Figure 2C). In order for the spheres to break the surface tension of the water without falling from the magnet, the magnets had to be withdrawn slowly and at an angle. The Al3+-AA spheres were not attracted to the magnet. The paramagnetic hydrogel spheres formed no more than a monolayer on the surface of the magnet; its strength was too weak to enable spheres farther from the surface of the magnet to be lifted from the bottom of the chamber. We could release the magnetic spheres by either shaking the magnet in solution or by directing a stream of water from a wash bottle at the beads (Figure 2D). To transfer all of the paramagnetic CMC spheres, we repeated this process of attracting paramagnetic spheres to a magnet, transporting them once bound, and releasing them in a second chamber. Diamagnetic AA spheres did not transfer.
Figure 2.
Separations of paramagnetic hydrogel spheres from diamagnetic hydrogel spheres using rare-earth magnets. A) A set of schematics—three-dimensional, side-view, and top-view—illustrating the method of separation used in (B)–(D) in which the magnets contact the spheres in solution. B) An optical image of the initial mixture of paramagnetic CMC spheres (white, Ho3+) and diamagnetic AA spheres (gray, Al3+) placed in a glass chamber (A). B–D) Upon immersion of a magnet in the mixture, the paramagnetic spheres were strongly attracted to the surface of the magnets, from which they were transferred selectively to a second chamber. The dotted white line highlights the glass divider separating the two chambers. E) A schematic illustration of the second type of separation, in which a magnet was placed under the dish where it does not contact the spheres. F–G) Optical images of the mixture of paramagnetic AA spheres (gray, Ho3+) and diamagnetic CMC spheres (white, La3+) in a Petri dish (E) before and after separation by an external rare-earth magnet.
In the second type of separation, the magnet did not come in contact with either the solution or the hydrogel spheres. In this case, we used the same polymers as before but switched the crosslinking cations. We placed a suspension of a mixture of paramagnetic AA spheres (3–4 mm in diameter) crosslinked with Ho3+, and diamagnetic CMC spheres (3–5 mm in diameter) crosslinked with La3+, into a Petri dish (Figure 2E). After tilting the dish to allow for all of the spheres to settle near one edge (Figure 2F), we passed a NdFeB magnet along the underside of the Petri dish. Only the paramagnetic spheres followed the magnet, yielding an in-plane, spatially-resolved separation of the two types of spheres (Figure 2G).
For the separation methods described above, the percent recovery was 100%, as we isolated all of the paramagnetic spheres from the mixture without contamination by any of the diamagnetic spheres. The number of attract-and-detach cycles required to transfer the paramagnetic spheres quantitatively depended on the surface area of the magnet and the strength of the magnetic field. The experiment depicted in Figures 2A–2D required 3–4 cycles to transfer all of the paramagnetic spheres (~50) from one chamber to the other.
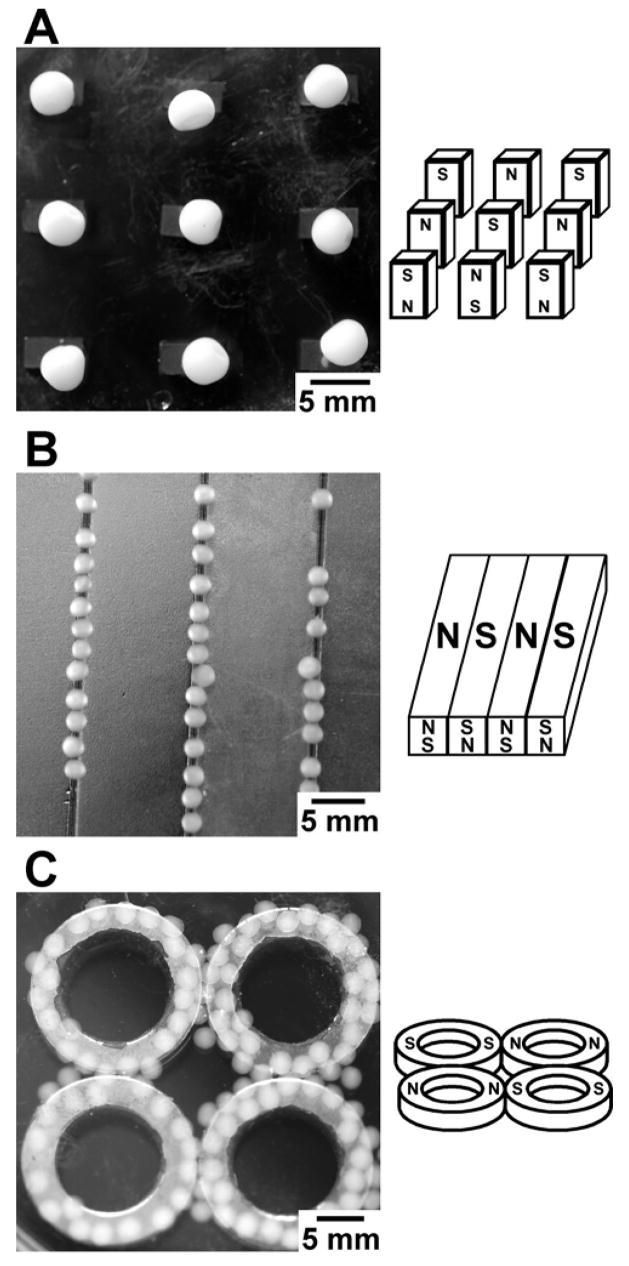
We could also manipulate the paramagnetic hydrogels such that they assembled in the presence of an inhomogeneous magnetic field in the region of highest field strength. We placed several spheres into a Petri dish containing deionized water over a set of rare-earth magnets and gently agitated the dish by hand. Figure 3 depicts three different polymer hydrogel spheres (CMC, ι-carrageenan, and AA), each having assembled into a geometrical pattern under the influence of the external magnetic field. The size and spacing of the magnets can alter the regions of highest magnetic field, and thus, can be used to control the final position of the spheres. The highest field from an array of small magnets spaced several diameters apart is directly over each magnet (Figure 3A), whereas the highest field for similarly poled, large magnets adjacent to each other is over the gap between the magnets (Figure 3B). The geometrical constraints on the pattern of assembly appear to be limited only by the profile of the magnetic field that can be generated on a length scale comparable to the diameter of the hydrogel spheres.
Figure 3.
The self-assembly of the paramagnetic hydrogel spheres using different geometries and configurations of NdFeB magnets. Schematic drawings on the right illustrate the polarity of the magnets. The spheres collect over the region of highest magnetic field. A) Carboxymethyl cellulose spheres assembled into an open lattice structure of small magnetic posts. B) Spheres of ι-carrageenan assembled into lines over the intersection between two adjacent magnets with opposite polarity. The magnets were immersed in the solution containing the spheres. C) AA spheres aligned with a set of ring magnets only in the region directly above each magnet. In this geometry, there is essentially zero magnetic field in the center of each ring and in the center of the cluster of rings.
Colorimetic pH Detection using Hydrogel Spheres
We produced paramagnetic hydrogel spheres with embedded colorimetric pH indicators that can register the pH of a medium and be recovered magnetically. A pulp of pH paper in which the indicators were covalently bound was prepared by pureeing a suspension of the paper in water. (In experiments where the indicator was not covalently attached to the paper (or the hydrogel), the indicator diffused into solution.) We dissolved AA (2% w/v) in the aqueous solution containing the pulp, and then fabricated AA spheres using a 100-mM solution of Ho3+. We made three sets of these spheres, each with a different pH indicator. These spheres change color when placed in aqueous solutions of various pH. We soaked the spheres in solutions having a pH of 0 (1 M HCl), 6.2 (1 M Bis-Tris), 9.9 (1 M CAPS), and 14 (1 M NaOH) (Figure 4). The spheres changed color within seconds and retained their color when soaked in the solutions of pH 0, 6.2, and 9.9 for >100 hours. In the pH 14 solution, the indicator dye degraded after ~6 hours.
Figure 4.

An optical image of the same set of three types of hydrogel spheres with embedded fibers of pH paper containing different covalently-bound indicators in solutions titrated to four different values of pH: 0, 6.2, 9.9, and 14 (from left to right). Each tube contains three spheres (one of each set).
The presence of Ho3+ cations does not interfere with the color changes of the pH paper, and the presence of the embedded fibers neither disrupts the crosslinking nor the paramagnetic response of the hydrogel. These spheres, therefore, can be removed or separated by a rare earth bar magnet as discussed earlier (Figure 2). This experiment demonstrates that magnetic hydrogels can be used to introduce sensors into an aqueous system, and that the spheres can be retrieved and reused without contaminating the sample.
Hydrogel Spheres as Retrievable Sorbents
Paramagnetic AA spheres can host other materials with useful properties. Activated carbon powder is used as a decolorizing agent and a common sorbent for removing undesirable organic molecules from aqueous solutions. In such applications, the carbon must be removed from the system (usually) by filtration; however, in heterogenous mixtures or large bodies of water, filtration is not possible. The paramagnetic hydrogels prepared with embedded carbon powder have three useful features: i) The aqueous solution easily diffuses through the hydrogel matrix and exposes the carbon powder to the solution and its contaminants. ii) The carbon powder, like the pH paper, does not interfere with the paramagnetism of the hydrogel. iii) The hydrogel, along with the carbon powder and the adsorbed contaminants, can be removed by external magnets.
As a demonstration of this application of paramagnetic hydrogels, we placed three sets of fifteen 3–5 mm spheres into a 10-mL aqueous solution of crystal violet dye (0.1 mg/mL). The first set was a control containing AA spheres crosslinked by Ho3+ with no embedded carbon. The second set contained Ho3+-AA spheres with 5% (w/v) carbon powder, and the third set contained Ho3+-AA spheres with 10% (w/v) carbon powder. After shaking the three vials for two hours, we measured the UV absorption of each solution (Supplemental Figure 4) and compared their relative absorption at 590 nm. For the carbon-free control set, the relative ratio of the absorbance of the final solution to the initial solution was 0.945. The decrease in observed concentration of dye is most likely due to dilution resulting from adding spheres, which contained adhered water, to the solution of dye. The ratio for the solution containing the 5%-carbon-powder hydrogel spheres to the initial solution was 0.087, and the ratio for the solution containing the 10%-carbon-powder hydrogel spheres to the initial solution was 0.036. The embedded carbon powder efficiently removed the dye contaminant from the aqueous solution. This experiment demonstrates that paramagnetic hydrogels can be employed as a matrix for sorbents that remove organic contaminants from aqueous solutions.
Conclusion
We have demonstrated the ability to fabricate magnetically-responsive hydrogels that are crosslinked with paramagnetic ions. Paramagnetic hydrogel spheres assembled in magnetic field gradients and can be used as a matrix to introduce recoverable sensors or reagents to aqueous mixtures.
In comparison to other magnetic hydrogels, these materials have several advantages, including: i) they are translucent, and thus, simple to assay (unlike Fe3O4, which is black); ii) they gel rapidly (in <1 sec of contact with a solution of paramagnetic cations), which allows for simple, fast fabrication; and iii) they are able to incorporate solid materials and be used as sensors or sorbents. These paramagnetic ionotropic hydrogels also have their disadvantages, including that i) the magnetic response of the spheres is at least an order of magnitude weaker than that of hydrogel spheres incorporating superparamagnetic particles, and ii) some buffers (e.g., phosphate anions) precipitate the holmium from the spheres and cause them to dissolve slowly.
Owing to the simplicity of their fabrication, ease of use, and low toxicity, paramagnetic hydrogels crosslinked with Ho3+ represent a new class of functional materials available for use as environmentally-friendly detection systems.
Experimental Section
Crosslinking and formation of paramagnetic ionotropic hydrogels
The polymers (alginic acid (AA) (Aldrich); carboxymethyl cellulose (CMC) (Aldrich); polygalacturonic acid (PG) (Aldrich); ι-carrageenan (Aldrich)) that successfully formed hydrogels were initially dissolved in water (2% w/v). The hydrogel spheres were formed by dropping the dissolved polymer solution (5–10 mL) through a 27-gauge needle (Becton Dickinson & Co.) into an aqueous solution of Ho(NO3)3 (10–500 mM, Aldrich) and left to soak for 30 minutes. The spheres were subsequently soaked in 40 mL of deionized water for at least 2 hours and rinsed to remove any free Ho3+. This washing process was repeated at least three times.
Preparation of Acidified Alginic Acid
This procedure was based on the work by Cozzi et al.20,21 A solid portion of the sodium salt of alginic acid (23 g, Sigma-Aldrich) was suspended in 150 mL of 3 M HCl and stirred at room temperature for 8 h. The liquid phase was decanted and replaced by 100 mL of 3 M HCl and the mixture was stirred for an additional 3 h. The solid was isolated by vacuum filtration through a large frit of sintered glass and washed with deionized water. The acidified alginic acid product was stored as an aqueous slurry.
Ion Exchange Chromatography of Metals on Alginic Acid
All chromatographic experiments were conducted on a Varian Star Model HPLC Instrument equipped with a variable- pressure solvent delivery pump (Rainin Dynamax SD-300) and a UV-vis dual wavelength detector (Rainin Dynamax UV-D II). Chromatography was performed isocratically using 0.1 M HCl as the eluant. A glass column (length = 20 cm, i.d. = 1 cm) suitable for liquid chromatography at low pressure (<30 psi) was packed with a slurry of acidified alginic acid. The alginic acid was compressed by setting the rate of flow at 0.8 mL/min. at a pressure of 20 psi. Additional portions of alginic acid were added until the final height of the packed stationary phase was 19 cm. The column was washed with 0.1 M HCl at a flow rate of 0.4 mL/min. for 12 hours prior to its initial use.
The procedure for loading the analyte onto the column involved opening the column, removing the excess mobile phase, and allowing the remaining eluant to drain until level with the top of the stationary phase. At this point, a 100-μL aliquot of a 50-mM solution of the metal ion in 0.1 M HCl spiked with 2 mM KNO3 was injected onto the top of the column and allowed to drain until level with the top of the stationary phase. An additional 100 μL of eluant was added and allowed to drain, then the void space of the column was filled completely with eluant and the HPLC instrument was programmed to deliver eluant through the column at 0.4 mL/min. with a pressure of 15 psi. The elution of nitrate (NO3− ) and the metal ions was monitored by UV-vis detection at 220 nm and 393 nm. Each metal ion was chromatographed in triplicate, and the resulting Rf values were associated with 90% confidence intervals of ±0.016 or less.
Determination of Magnetic Susceptibility of AA Spheres
Sets of AA spheres were crosslinked with aqueous solutions of 10 mM, 100 mM, or 500 mM Ho3+. One hundred spheres of each set were dissolved in 5 mL of a 0.5-M solution of EDTA (Aldrich). The absorption spectrum of each solution was taken with a UV-vis spectrophotometer (HP 8453), and the baseline of each spectrum was corrected to zero absorption—we believe the dissolved alginate salt in the samples resulted in Rayleigh scattering that shifted the baseline up.
Separation and manipulation of hydrogel spheres
A set of NdFeB magnets (Master Magnetics Inc.) was placed in an aqueous solution such that the polarization of the magnetic field was parallel to the benchtop. A single hydrogel sphere was placed at a distance of 2 cm from the surface of the magnet and manually brought closer to the magnet until the magnetic force attracted the sphere to the surface of the magnet. The furthest distance at which the magnetic force could attract the sphere was recorded. The measurement was repeated at least seven times for each type of hydrogel sphere. The strength of the magnetic field was measured with a hand-held magnetometer (Sypris 6010).
Colorimetric Detection of pH using Hydrogel Spheres
ColorpHast pH paper (EMD Chemicals Inc.) was removed from 100 plastic strips and soaked in 25 mL of hot water for at least 2 hours. Neither the paper nor the indicator dyes dissolved or leached into solution. The mixture containing the paper was shredded with an electric food processor (Cuisinart MM–2M) for 30 seconds to create pulp. AA was dissolved into the wet pulp (2% w/v) and crosslinked in a 100-mM Ho3+ solution. A 16-gauge needle (Becton Dickinson & Co.) was required to form the drops of the polymer/pulp solution in order to avoid clogging. After rinsing, the spheres were placed in each of four solutions having different pH.
Hydrogel Spheres as Retrievable Sorbents
Carbon powder decolorizing agent (Eastman Kodak)—5% w/v or 10% w/v—was dispersed in a 2% w/v aqueous solution of AA. The solution of AA-containing carbon was crosslinked in a 100-mM solution of Ho3+ (Ho(NO3)3). Three sets of fifteen spheres, each set containing a different carbon loading (0%, 5%, or 10%), were added to separate vials containing 10 mL of a 0.1 mg/mL aqueous solution of crystal violet dye (Sigma). The solutions containing the spheres were shaken in scintillation vials on a vortex mixer (VWR) for 2 hours. UV-vis spectra of the supernatants were recorded on a UV-vis spectrophotometer (HP 8453) and compared to the absorption of the initial 0.1 mg/mL solution of crystal violet.
Supplementary Material
A sample chromatogram from the LC analysis of metal ions, data pertaining to the positive magnetic response of the spheres, chemical structures of the monomer units for the polymers used in this study, and UV-vis spectra demonstrating that the Ho3+-AA spheres with embedded carbon powder function as sorbents are provided as Supporting Information. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
The authors thank Dr. Raquel Perez-Castillejos for useful discussions and assistance with the figures and Dr. Lara Estroff for useful conversations. P.J.B. gratefully acknowledges support in the form of predoctoral fellowships from the National Science Foundation (NSF) and the Harvard Origins-of-Life Initiative. This work was supported by the NIH (award #GW065364), DARPA, and the Office of Naval Research.
References
- 1.Usov AI. Usp Khim. 1999;68:1051–1061. [Google Scholar]
- 2.Martinsen A, Skjakbraek G, Smidsrod O. Biotechnol Bioeng. 1989;33:79–89. doi: 10.1002/bit.260330111. [DOI] [PubMed] [Google Scholar]
- 3.DeRamos CM, Irwin AE, Nauss JL, Stout BE. Inorg Chim Acta. 1997;256:69–75. [Google Scholar]
- 4.Muzzarelli RAA. Natural Chelating Polymers. Pergamon Press; Oxford: 1973. [Google Scholar]
- 5.Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Adv Mater. 2006;18:1345–1360. [Google Scholar]
- 6.Navratil M, Domeny Z, Hronsky V, Sturdik E, Smogrovicova D, Gemeiner P. Anal Biochem. 2000;284:394–400. doi: 10.1006/abio.2000.4700. [DOI] [PubMed] [Google Scholar]
- 7.Sungur S. Art Cells, Blood Subs, Immob Biotech. 1999;27:279–290. doi: 10.3109/10731199909117700. [DOI] [PubMed] [Google Scholar]
- 8.Berger J, Reist M, Mayer JM, Felt O, Peppas NA, Gurny R. Eur J Pharm Biopharm. 2004;57:19–34. doi: 10.1016/s0939-6411(03)00161-9. [DOI] [PubMed] [Google Scholar]
- 9.Michel AS, Mestdagh MM, Axelos MAV. Int J Biol Macromol. 1997;21:195–200. doi: 10.1016/s0141-8130(97)00061-5. [DOI] [PubMed] [Google Scholar]
- 10.Morris VJ, Belton PS. Prog Food Nutr Sci. 1982;6:55–66. [Google Scholar]
- 11.Andrianov AK, Cohen S, Visscher KB, Payne LG, Allcock HR, Langer R. J Controlled Release. 1993;27:69–77. [Google Scholar]
- 12.Wang T, Lacik I, Brissova M, Anilkumar AV, Prokop A, Hunkeler D, Green R, Shahrokhi K, Powers AC. Nat Biotechnol. 1997;15:358–362. doi: 10.1038/nbt0497-358. [DOI] [PubMed] [Google Scholar]
- 13.Lim F, Sun AM. Science. 1980;210:908–910. doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- 14.Rowley JA, Madlambayan G, Mooney DJ. Biomaterials. 1999;20:45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 15.Tonnesen HH, Karlsen J. Drug Dev Ind Pharm. 2002;28:621–630. doi: 10.1081/ddc-120003853. [DOI] [PubMed] [Google Scholar]
- 16.Davis TA, Volesky B, Mucci A. Water Res. 2003;37:4311–4330. doi: 10.1016/S0043-1354(03)00293-8. [DOI] [PubMed] [Google Scholar]
- 17.Cabodi M, Choi NW, Gleghorn JP, Lee CSD, Bonassar LJ, Stroock AD. J Am Chem Soc. 2005;127:13788–13789. doi: 10.1021/ja054820t. [DOI] [PubMed] [Google Scholar]
- 18.Navratil M, Gemeiner P, Klein J, Sturdik E, Malovikova A, Nahalka J, Vikartovska A, Domeny Z, Smogrovicova D. Art Cells, Blood Subs, Immob Biotech. 2002;30:199–218. doi: 10.1081/bio-120004340. [DOI] [PubMed] [Google Scholar]
- 19.Cozzi D, Desideri PG, Lepri L. J Chromatogr. 1969;40:130–137. doi: 10.1016/s0021-9673(01)96629-4. [DOI] [PubMed] [Google Scholar]
- 20.Cozzi D, Desideri PG, Lepri L, Ciantell G. J Chromatogr. 1968;35:396–404. doi: 10.1016/s0021-9673(01)82401-8. [DOI] [PubMed] [Google Scholar]
- 21.Cozzi D, Desideri PG, Lepri L, Ciantell G. J Chromatogr. 1968;35:405–415. doi: 10.1016/s0021-9673(01)82402-x. [DOI] [PubMed] [Google Scholar]
- 22.Grant GT, Morris ER, Rees DA, Smith PJC, Thom D. FEBS Lett. 1973;32:195–198. [Google Scholar]
- 23.Chatterjee J, Haik Y, Chen CJ. Colloid Polym Sci. 2003;281:892–896. [Google Scholar]
- 24.Mayer CR, Cabuil V, Lalot T, Thouvenot R. Adv Mater. 2000;12:417–420. [Google Scholar]
- 25.Paneva D, Stoilova O, Manolova N, Rashkov I. E-Polymers. 2004:060. [Google Scholar]
- 26.Zhang JG, Xu SQ, Kumacheva E. J Am Chem Soc. 2004;126:7908–7914. doi: 10.1021/ja031523k. [DOI] [PubMed] [Google Scholar]
- 27.Kroll E, Winnik FM, Ziolo RF. Chem Mater. 1996;8:1594–1596. [Google Scholar]
- 28.Emmerichs N, Wingender J, Flemming HC, Mayer C. Int J Biol Macromol. 2004;34:73–79. doi: 10.1016/j.ijbiomac.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Wang ZY, Zhang QZ, Konno M, Saito S. Biopolymers. 1993;33:703–711. [Google Scholar]
- 30.Barry SE, Steiert M, Goodwin AA, Lam A, Hsu AW. World Patent WO 2005/065724 A1. 2005. [Google Scholar]
- 31.Zhong S-P, Sahatjian RA, Ma E. US Patent Application 2003/0100830 A1. 2003. [Google Scholar]
- 32.Berger LI, editor. CRC Handbook of Chemistry and Physics. 85. CRC Press LLC; Boca Raton, FL: 2000. [Google Scholar]
- 33.Vercruysse KP, Li H, Luo Y, Prestwich GD. Biomacromolecules. 2002;3:639–643. doi: 10.1021/bm020026d. [DOI] [PubMed] [Google Scholar]
- 34.Kumar G, Smith PJ, Payne GF. Biotechnol Bioeng. 1999;63:154–165. doi: 10.1002/(sici)1097-0290(19990420)63:2<154::aid-bit4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 35.We assumed that the components of the magnetic field parallel to the surface were negligible and do not contribute significantly to either the field or the gradient.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A sample chromatogram from the LC analysis of metal ions, data pertaining to the positive magnetic response of the spheres, chemical structures of the monomer units for the polymers used in this study, and UV-vis spectra demonstrating that the Ho3+-AA spheres with embedded carbon powder function as sorbents are provided as Supporting Information. This material is available free of charge via the Internet at http://pubs.acs.org.