Abstract
C-type lectins such as DC-SIGN and L-SIGN, which bind mannose-enriched carbohydrate modifications of host and pathogen proteins, have been shown to bind glycoproteins of several viruses and facilitate either cis or trans infection. DC-SIGN and L-SIGN are expressed in several early targets of arbovirus infection, including dendritic cells (DCs) and cells of the reticuloendothelial system. In the present study, we show that DC-SIGN and L-SIGN can function as attachment receptors for Sindbis (SB) virus, an arbovirus of the Alphavirus genus. Human monocytic THP-1 cells stably transfected with DC-SIGN or L-SIGN were permissive for SB virus replication, while untransfected controls were essentially nonpermissive. The majority of control THP-1 cells were permissive when attachment and entry steps were eliminated through electroporation of virus transcripts. Infectivity for the DC-SIGN/L-SIGN-expressing cells was largely blocked by yeast mannan, EDTA, or a DC-SIGN/L-SIGN-specific monoclonal antibody. Infection of primary human DCs by SB virus was also dependent upon SIGN expression by similar criteria. Furthermore, production of virus particles in either C6/36 mosquito cells or CHO mammalian cells under conditions that limited complex carbohydrate content greatly increased SB virus binding to and infection of THP-1 cells expressing these lectins. C6/36-derived virus also was much more infectious for primary human DCs than CHO-derived virus. These results suggest that (i) lectin molecules such as DC-SIGN and L-SIGN may represent common attachment receptor molecules for arthropod-borne viruses, (ii) arbovirus particles produced in and delivered by arthropod vectors may preferentially target vertebrate host cells bearing these or similar lectin molecules, and (iii) a cell line has been identified that can productively replicate alphaviruses but is deficient in attachment receptors.
In a natural infection, arthropod-borne viruses (arboviruses) are introduced into the skin of a vertebrate host by the bite of an infected mosquito. Viruses of the Alphavirus genus, Togaviridae family, have adapted to exploit cells of the macrophage-dendritic cell (DC) lineage for dissemination to regional lymph nodes draining the site of inoculation in the skin (13, 28, 36, 37). However, it is unclear by what mechanism alphaviruses target DCs. Analysis of alphavirus receptor usage is complicated by the broad cell tropisms of these viruses in vitro and the rapid acquisition of envelope glycoprotein mutations in laboratory strains that confer high-efficiency attachment to cell-surface heparan sulfate (HS) structures (2, 3, 24). Such mutations promote efficient binding to cell surfaces and high levels of infectivity per virus particle in vitro, but are attenuating in vivo (2, 22-24). In contrast, the virulent consensus wild-type strains do not bind efficiently to HS or cell surfaces and exhibit much lower infectivity for cultured cells than HS-binding laboratory strains (2, 22, 24). While the high-affinity laminin receptor protein has been shown to enhance infectivity of cells by cell culture-adapted, presumably HS-binding, alphavirus strains (27, 46), a receptor capable of mediating infection by wild-type alphaviruses has yet to be identified. Similar issues have been encountered for arboviruses in the Flaviviridae family, until recently, when dengue virus was shown to use C-type lectins as attachment receptors leading to productive infection of DCs (30, 44).
The C-type lectin family of Ca2+-dependent carbohydrate binding proteins have a wide range of biological functions. High-level expression of the C-type lectin DC-SIGN (for DC-specific ICAM-3 grabbing non-integrin) has been demonstrated on immature DC and macrophage subpopulations abundant in the dermis of the skin, at mucosal surfaces, and in lymph nodes and peripheral tissues (40, 42). In addition to their role in DC migration and T-lymphocyte activation via interaction with ICAM-3 (14), DC-SIGN molecules function as “scavenger” receptors. Microorganisms invading peripheral tissues are sensed and captured via conserved pathogen-associated molecular patterns and then internalized for processing and antigen presentation (8). A DC-SIGN homologue termed L-SIGN (for liver/lymph node-specific ICAM-3 grabbing non-integrin) or DC-SIGNR (DC-SIGN related) is expressed on endothelial cells in liver sinusoids, lymph node, gastrointestinal tract, and placenta (1, 41). Endothelial cells have been speculated to play a similar role to macrophages and DCs in antigen capture and clearance from the blood (10). DC-SIGN and L-SIGN bind preferentially to high-mannose oligosaccharides through a carbohydrate recognition domain in their C termini (5, 9, 29).
A number of viruses with heavily glycosylated envelope proteins, including Ebola virus (39), hepatitis C virus (HCV) (33), human cytomegalovirus (17), and human immunodeficiency virus (HIV) (15, 31, 32), have been demonstrated to bind cells via DC-SIGN/L-SIGN molecules with the interaction facilitating trans infection of neighboring permissive cells and/or enhancing cis infection of the lectin-bearing cell. The presence of immature, mannose-containing carbohydrate structures, not fully processed during transport through the Golgi apparatus, appears to be required for efficient binding to SIGN lectins, perhaps explaining why some glycosylated viruses do not bind DC-SIGN (39). Ebola virus GP, HIV gp120, and HCV E1 and E2 envelope proteins are highly glycosylated, containing hybrid and high-mannose sugars (26, 33). Modulation of N-glycans on HIV Env or Ebola virus GP dramatically affected DC-SIGN infectivity enhancement, and murine leukemia virus, which typically does not interact efficiently with DC-SIGN/L-SIGN, could do so when produced in the presence of a mannosidase I inhibitor (26). Notably, high-mannose and Man3GlnNAc2-containing carbohydrates, both of which are DC- and L-SIGN ligands (9), represent the most extensively processed carbohydrate modifications of Sindbis (SB) virus glycoproteins derived from mosquito cells (18, 19).
In the light of these previous findings, we reasoned that use of C-type lectins for DC entry might be a general property of arboviruses and that the virus-receptor interaction might be enhanced for virus derived from invertebrates, which represent the natural source of initially infecting virus particles. In the present study, we have identified DC-SIGN and L-SIGN as cell-surface proteins able to mediate the binding to and infection of cells by the wild-type consensus sequence SB virus strain TR339. Furthermore, TR339 virions produced in mosquito cells or under conditions that limit the processing of viral carbohydrate modifications exhibit greatly increased binding to and infectivity for DC-SIGN/L-SIGN-expressing cells as compared with mammalian cell-produced virus. Thus, in the case of arboviruses, pattern recognition receptor (PRR) proteins of the C-type lectin family can distinguish self from nonself via the carbohydrate modifications of arthropod-derived glycoproteins, and arboviruses derived from cells of the arthropod vector can use these molecules as infection receptors. We also identify human THP-1 cells, a premyelocyte cell line derived from a leukemia patient (45), as a cell type that, while highly permissive for alphavirus replication, lacks cell-surface receptors capable of promoting efficient entry of wild-type or cell-adapted SB virus.
MATERIALS AND METHODS
Cell lines.
Baby hamster kidney (BHK-21; ATCC CCL-10) cell lines were maintained in alpha minimal essential medium (αMEM) supplemented with 10% donor calf serum (DCS), 2.9-mg/ml tryptose phosphate, 0.29-mg/ml l-glutamine, and 100-U/ml penicillin-0.05-mg/ml streptomycin (pen/strep) (37°C, 5% CO2). Chinese hamster ovary K1 (CHOK1) cells were maintained in Ham's F12 supplemented with 10% fetal bovine serum (FBS), 0.29-mg/ml l-glutamine, and pen/strep (37°C, 5% CO2). The Lec1 CHO (ATCC CRL1735) and wild-type parental Pro-5 CHO cell lines (ATCC CRL-1781) were maintained in αMEM, supplemented with 10% FBS, 0.29-mg/ml l-glutamine, and pen/strep (37°C, 5% CO2). The Aedes albopictus-derived cell line C6/36 was maintained in αMEM, supplemented with 10% FBS and 0.29-mg/ml l-glutamine (30°C, 5% CO2). DF-1 chicken fibroblasts (ATCC CCL-12203) and human Hep-2 HeLa cell derivatives (ATCC CCL-23) were maintained in Dulbecco's minimal essential medium (DMEM) with 10% FBS, l-glutamine, and pen/strep as described above and incubated at 39 and 37°C, respectively.
Parental control THP-1 cells and THP-1 cells stably transfected with human DC-SIGN (THP DC-SIGN) or DC-SIGN with a cytoplasmic truncation (THP DC-SIGNΔ35) were generously provided by Dan Littman (New York University School of Medicine, New York) (25), and THP-1 cells expressing L-SIGN/DC-SIGNR (THP L-SIGN) were provided by Vineet KewalRamani (National Cancer Institute, Frederick, Md.) (1). Cell surface expression of DC-SIGN, DC-SIGNΔ35, and L-SIGN was confirmed by immunofluorescent staining and flow cytometric analyses with the DC-SIGN/L-SIGN cross-reactive anti-DC-SIGN monoclonal antibody (MAb) 612 (47) or isotype control antibody (R&D Systems).
MDDCs.
Human peripheral blood mononuclear cells (PBMCs) were isolated from normal donors over a Ficoll-Hypaque gradient according to standard procedures (44). Monocytes were purified from PBMCs by a 1-h adherence step at 37°C in cRPMI. Purified cells were then cultured in 800-U/ml human granulocyte-macrophage colony-stimulating factor (hu-GM-CSF) (Peprotech) and 1,000-U/ml human interleukin-4 (hu-IL-4) (Peprotech) for 6 days. Loosely adherent cells (DCs) were harvested by pipetting and concentrated by centrifugation. DC-SIGN expression on human monocyte-derived DCs (MDDCs) was quantitated by antibody staining and flow cytometry as described for THP-1 cells. Infection and infectivity-blocking conditions were as described for THP-1 cells; however, infection rates were quantitated by counting of green fluorescent protein (GFP)-positive (fluorescence setting) versus total cells (bright-field setting) in 24-well plates (Corning) by using a Nikon TE-300 fluorescence microscope with an Endow GFP filter.
Viruses.
The construction of the SB AR339 consensus sequence pTR339 plasmid and mutant pS1K70 plasmid (22, 24) and the E2S1GFP double subgenomic promoter virus expressing GFP (pE2S1GFP) (K. D. Ryman, W. B. Klimstra, and R. E. Johnston, submitted for publication) has been described previously. Virus derived from the pE2S1GFP plasmid begins with the prefix “39GFP.” The E2S1 sequence differs from the AR339 consensus sequence only at E1 position 72, and virus derived from this clone has cell attachment and mouse virulence phenotypes identical to those of the AR339 consensus sequence virus, TR339 (36). The S1K70GFP double subgenomic promoter virus expressing GFP was constructed by a BssHII-to-XhoI restriction fragment exchange from E2S1GFP into pS1K70. Infectious viral RNA was generated by in vitro transcription with mMessage mMachine (Ambion) from XhoI-linearized virus plasmid DNA templates and transfected into cells by electroporation. Progeny virus particles released into supernatant were clarified by centrifugation and stored at −80°C in single-use aliquots. To prepare various virus stocks, TR339, S1K70, E2S1GFP, or S1K70GFP transcripts were electroporated into either CHO K1 or the CHOK1 normal clone Pro5 (viruses derived from either cell line were designated 39GFP-C or 39-C) or C6/36 (39GFP-M or 39-M viruses) cells. Mammalian cell-derived stocks with altered glycosylation were generated by electroporation of Lec1 CHO cells (39GFP-L or 39-L viruses) with TR339 or E2S1GFP virus RNA or by similar electroporation of CHO Pro-5 control cells, which were cultured in 1 mM 1-deoxymannojirimycin (1-DMJ; Sigma) after electroporation (39GFP-DMJ or 39-DMJ viruses). Progeny virions were harvested 24 h postelectroporation, and their titer was determined by standard BHK cell plaque assay.
Virus growth curves.
For one-step growth curves, triplicate wells containing ∼106 THP-1 cells in 50 μl of phosphate-buffered saline (PBS)-1% DCS with Ca2+-Mg2+ (virus buffer [VB]) suspension were infected at a multiplicity of infection (MOI) of 1 PFU of TR339 per cell derived from C6/36 or normal CHO cell lines (39-M or 39-C viruses). After 1 h of infection at 4 or 37°C, cells were washed three times in VB, and the medium was replaced. At each time point, progeny virions in 10-μl aliquots of supernatant from triplicate wells were independently quantitated by standard BHK plaque assay, and titers were expressed as BHK PFU per milliliter.
Flow cytometric analysis of infected cells.
Triplicate wells containing ∼5 × 105 THP-1 cells were infected with equal BHK cell titer PFU of 39GFP or S1K70GFP double-promoter viruses expressing GFP in a total of 50 μl for 1 h at 37°C (MOI of ∼0.5 PFU per cell). Twelve to 14 h after infection, virus-infected and mock-infected THP-1 cells were washed three times in VB by pelleting in V-bottom 96-well plates (Sarstedt), fixed for 10 min at 4°C with 4% paraformaldehyde (in PBS), and resuspended in fluorescence-activated cell sorter (FACS) buffer (PBS-Ca-Mg-2% DCS) at ∼2 × 106 cells per ml. The percentage of GFP-positive cells was quantitated by flow cytometric analysis on a FACSCalibur flow cytometer (Flow Cytometry Facility, Louisiana State University Health Sciences Center—Shreveport). Expression of GFP was monitored by fluorescence microscopy and recorded photographically at constant camera settings to facilitate comparison of GFP fluorescence intensity between samples. Fluorescence micrographs were taken on a Nikon TE-300 fluorescence microscope with an Endow GFP filter.
Blocking of infectivity.
In dose-response experiments, triplicate wells of THP-1, CHOK1, or Hep2 cell lines were treated with 0.2 to 200 μg of mannan per ml (Sigma), 0.2 to 20 μg of anti-DC-SIGN MAb 612 per ml (47), or isotype control antibody (R&D Systems). In other experiments, the cell lines or MDDCs were treated with 200-μg/ml mannan (Sigma), 20-μg/ml anti-DC-SIGN MAb 612 (47) or isotype control antibody (R&D Systems), or 5 mM EDTA for 30 min at room temperature prior to infection with GFP-expressing viruses.
Virus attachment assays.
Production of [35S]methionine-labeled virus and the assay for cell attachment were completed as previously described (22, 24), with the following exceptions. Radiolabeled TR339 or S1K70 viruses were produced by infection (MOI of >5) of cultures of C6/36 (39-M virus), CHO Pro5 (39-C virus), or Lec1 (39-L virus) cells or cells treated after infection with 1 mM 1-DMJ (39-DMJ virus). Viruses were purified from clarified cell supernatants by using a discontinuous sucrose gradient (20%/60% [wt/wt] in TNE buffer [50 mM Tris-HCl, 100 mM NaCl, 1 mM EDTA]), followed by pelleting through 20% sucrose in TNE buffer. Radiolabeled virus particles were resuspended in HEPES-buffered RPMI 1640 medium supplemented with 1% fetal bovine serum (FBS) and stored at −80°C.
THP-1 control and L-SIGN-, DC-SIGN-, and DC-SIGNΔ35-transfected THP-1 cell attachment assays were conducted in 96-well V-bottom plates. Approximately 105 cpm of each radiolabeled virus diluted in VB was mixed with 106 THP-1 cells in a total volume of 50 μl, and this mixture was incubated at 4°C for 1 h with gentle agitation. Cells were washed three times with VB and resuspended in 200 μl of VB, and 35S radioactivity of l00 μl of the resuspended cells was quantitated with a liquid scintillation counter (Beckman).
RESULTS
Cell-surface expression of DC-SIGN and L-SIGN on THP-1 cells is correlated with SB virus infection.
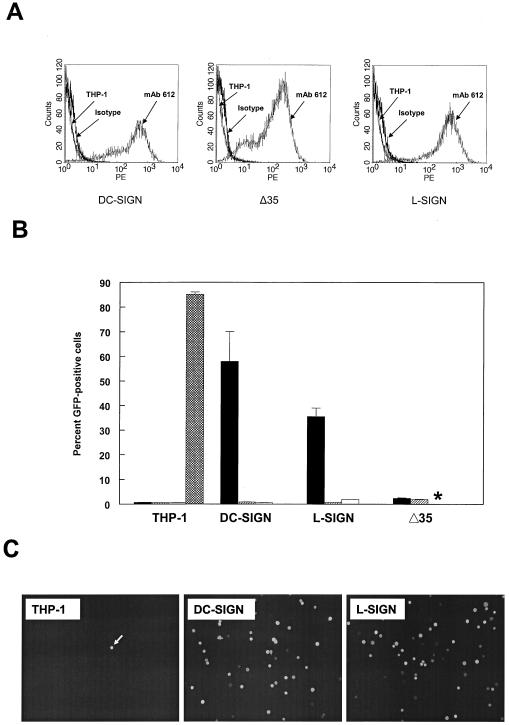
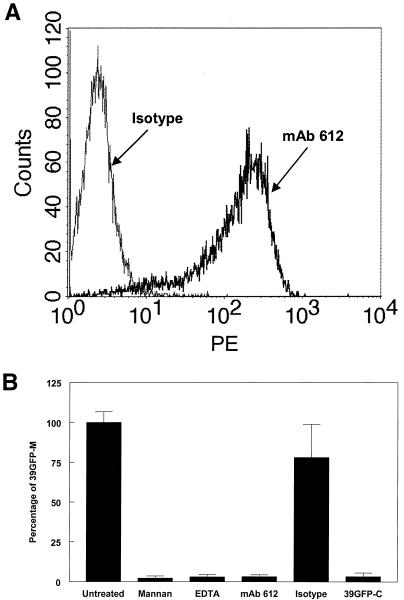
We had previously observed that yeast mannan, a competitive inhibitor of carbohydrate binding to C-type lectins, could inhibit the infection of the murine macrophage/monocyte cell line RAW 264.7 by the consensus sequence, wild-type SB virus strain TR339 (data not shown). Therefore, we reasoned that C-type lectins might serve as alphavirus receptors. To study the role of the C-type lectins in SB virus entry, we used DC-SIGN- and L-SIGN-transfected THP-1 (THP-1 DC-SIGN and THP-1 L-SIGN, respectively) cell lines and the corresponding nontransfected THP-1 cells as controls (1, 25). We also used the Δ35 THP-1 cell line, which expresses DC-SIGN lacking 35 amino acids of the cytosplasmic tail (25). This mutation has been shown to prevent the endocytosis of DC-SIGN and therefore would show dependence of infection upon endocytosis of the receptor. We observed similar levels of staining of the cross-reactive MAb 612 (R&D Systems) (Fig. 1A) on DC-SIGN- and L-SIGN-transfected THP-1 cells. Although the Δ35 THP-1 cells exhibited similar percentage of positive cells to the DC-SIGN and L-SIGN cells, the mean fluorescence intensity was approximately threefold lower (data not shown), suggesting either an effect of the mutation on antibody binding or the level of expression of mutant protein was lower.
FIG. 1.
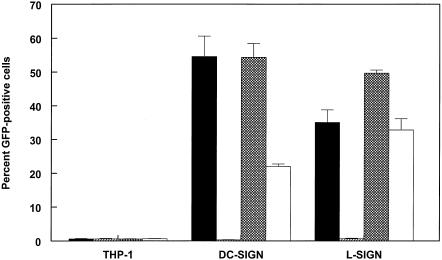
DC-SIGN/L-SIGN expression and SB virus infection of THP-1 cells. (A) Histogram of flow cytometric analysis of cell surface DC-SIGN/L-SIGN expression on THP-1 control and transfected cells. Control THP-1 cells were stained with MAb 612, and transfected cells were stained with MAb 612 or the isotype control MAb. (B) Infectivity of 39GFP-M (solid bars), 39GFP-C (hatched bars), or CHOK1-derived HS binding mutant (S1K70GFP) SB virus (open bars) for control or transfected THP-1 cells and electroporation efficiency of TR339GFP transcripts (crosshatched bars) for control THP-1 cells. *, S1K70 infectivity not measured. (C) Fluorescence micrographs (magnification, ×100) of THP-1 cells infected with 39GFP-M virus. Equal numbers of each THP-1 cell type were infected with 39GFP-M as described in Materials and Methods and then diluted 10-fold into 96-well plates, incubated for 12 h, and photographed on the fluorescence microscope. Bright-field observations confirmed that each view shown contained similar numbers of cells. The arrow indicates a rare GFP-positive cell in control THP-1 cells.
Initially, we infected the THP-1 cells with the 39GFP-C (E2S1GFP derived from CHO cells designated 39GFP-C; similarly derived normal TR339 designated 39-C) virus derived by electroporation of mammalian CHOK1 cells. While numbers of green cells were slightly increased by 12 h postinfection (hpi) in the presence of DC- or L-SIGN molecules (as high as ∼5.0% in one experiment versus <1.0% for THP-1 cells), infection rates were low in repeated experiments (Fig. 1B). In contrast, infection of the DC- or L-SIGN-expressing THP-1 cells with virus derived from C6/36 mosquito cells (GFP-expressing virus designated 39GFP-M; normal virus designated 39-M) resulted in high levels of infectivity for the cells reaching as high as 60 or 70% GFP positive for L-SIGN- or DC-SIGN-expressing cells, respectively (Fig. 1B and see Fig. 3). Mean fluorescence intensities of DC-SIGN- and L-SIGN-expressing THP-1 cells infected with 39GFP-M were similar (data not shown). Infection rates for the DC-SIGN Δ35 cells were low but reproducibly higher than those for the THP-1 control cells. The dependence of infection and GFP expression on the presence of a suitable receptor and the high permissivity of the control THP-1 cells for SB replication were demonstrated by electroporation of cells with in vitro-transcribed E2S1GFP RNA (Fig. 1B). More than 80% of the cells expressed GFP 12 h after electroporation. In all of the virus infection experiments, THP-1 cells were infected with equivalent BHK cell PFU of 39GFP-C or 39GFP-M virus. It is of note that the BHK cell titers of virus stocks produced on either cell type did not differ in the BHK cell plaque assay titer by more than twofold. Control THP-1 cells obtained from either laboratory (D. Littman or V. KewalRamani) were essentially nonpermissive regardless of virus production conditions (<1.0% positive by flow cytometry); however, GFP-positive cells were occasionally observed (Fig. 1C). In addition, control THP-1 cells transfected with an empty pcDNA3.1 vector (Invitrogen) and selected for G418 resistance exhibited similar rates of infection (data not shown). Assessment of the temperature dependence of infection with 39GFP-M virus on the THP-1 cells indicated that interactions with DC-SIGN were equally efficient at 37 or 4°C; however, infectivity for L-SIGN-expressing cells was ∼4-fold lower at 4°C (Fig. 2).
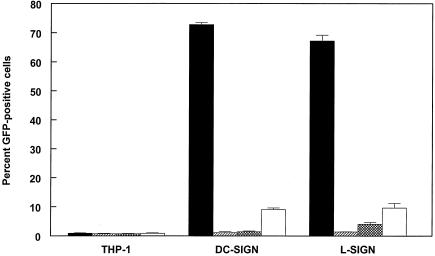
FIG. 3.
Infectivity of 39GFP-M (solid bars), 39-GFP-C (hatched bars), or 39GFP derived from THP-1 (crosshatched bars) or DF-1 (open bars) cells for control THP-1 or DC-SIGN/L-SIGN-expressing cells.
FIG. 2.
Effect of infection temperature on infectivity of 39GFP-M for THP-1 cells. Solid bars, 37°C; hatched bars, 4°C.
To determine if SIGN-dependent infection of THP-1 cells was independent of the HS-binding phenotype of SB strains, we infected the cells with a CHOK1 cell-derived GFP-expressing S1K70 mutant of TR339 that attaches efficiently to cell surface HS and exhibits much greater infectivity than TR339 for cells in such lines as BHK and CHOK1 (22). Efficient binding to and high infectivity for CHOK1 cells by this cell culture-adapted virus are due to a glutamic acid-to-lysine mutation at position 70 of the E2 glycoprotein protein that confers efficient interaction with HS (22). In contrast to the high infectivity of this virus for CHOK1 (22) and several other cell types, including cells of human origin (data not shown), the S1K70 virus exhibited very low rates of infection of control THP-1 cells that were similar to those of 39GFP-C by flow cytometry analysis (Fig. 1B).
We further tested the infectivity of virus particles derived from human THP-1 monocyte and avian DF-1 fibroblast cells, since vertebrate species-specific variation in viral carbohydrate modifications has been documented for alphaviruses (19). In addition to mosquitoes, rodents, humans, and birds can be infected with alphaviruses, and natural circulation is found between mosquitoes and rodent or bird reservoir hosts (16). The 39GFP virus derived from THP-1 cells exhibited low infectivity for DC-SIGN-expressing cells, similar to CHOK1 cell-derived virus, and slightly increased infectivity for L-SIGN-expressing cells (Fig. 3). SB virus derived from DF-1 chicken fibroblasts exhibited approximately fivefold and twofold higher infectivity for DC- or L-SIGN-expressing THP-1 cells, respectively, than the mammalian cell-derived viruses.
SIGN-expressing THP-1 cells are productively infected.
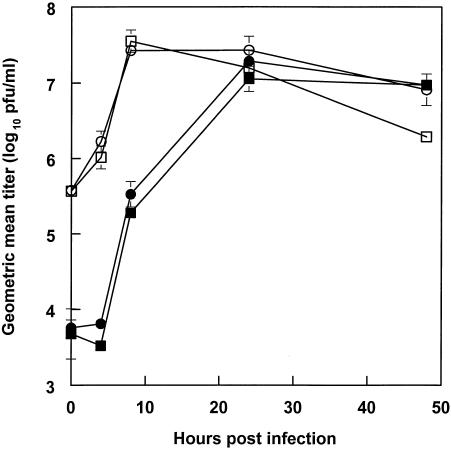
To confirm that GFP expression correlated with a complete virus replication cycle, we completed growth curves infecting DC- or L-SIGN-expressing cells with 39-M or 39-C. Supporting the GFP expression results, the 39-M virus exhibited rapid production and release of infectious progeny in DC- or L-SIGN-expressing cells (Fig. 4). The 39-C virus was clearly able to infect the SIGN-expressing cells; however, a longer time was required for virus titers to reach levels similar to 39-M. Despite extensive washing of infected cells, titers of supernatants taken immediately after infection (time zero) were always much higher for the 39-M virus, presumably reflecting the much greater binding of this virus by the DC-SIGN/L-SIGN-expressing cells. By 24 to 48 hpi, titers of 39-C from these cells were similar to those of 39-M, indicating that the CHOK1-derived virus could infect, replicate, and spread. Infection of the cells with the 39GFP-C virus also showed spread of the GFP signal by 48 hpi (data not shown).
FIG. 4.
Growth of TR339 in cultures of transfected THP-1 cells. DC-SIGN-transfected cells (squares) or L-SIGN-transfected cells (circles) were infected with 39-M (open symbols) or 39-C (solid symbols) virus.
As described above, infection of DC-SIGN/L-SIGN-expressing THP-1 cells with the 39GFP-C virus was inefficient. It is possible that initiation of subsequent rounds of infection of THP-1 cells was enhanced in growth curves by altered carbohydrate modifications of virus produced in initially infected THP-1 cells, resulting in more efficient interactions with SIGN molecules and enhanced growth. However, as indicated above, THP-1-derived virus was similar to CHOK1-derived virus in infectivity for DC-SIGN/L-SIGN-expressing cells. This suggests that the initial infection rates measured with CHOK1-derived virus were sufficient to promote multiple rounds of virus growth.
Notably, modest increases in titer were observed in control THP-1 cells infected with either C6/36-derived or CHOK1-derived viruses, particularly after 24 hpi, suggesting that the viruses could infect the cells (data not shown). Therefore, the THP-1 cells or at least some cells within the population (<1% by flow cytometric analysis), were permissive for SB virus infection. We observed that substantial increases in titer were correlated with the appearance of small-plaque variants of TR339 in plaque assays. Therefore, it is likely that TR339 can adapt to more efficient infection of THP-1 cells. Considering the poor infectivity of TR339 with the BHK-/CHO cell-adaptive E2 K70 mutation (Fig. 1B), it is likely that THP-1 cell-adaptive mutations differ from those selected on BHK or CHOK1 cells.
Limited processing of carbohydrate in SB virus glycoproteins correlates with efficient infection of SIGN-expressing THP-1 cells.
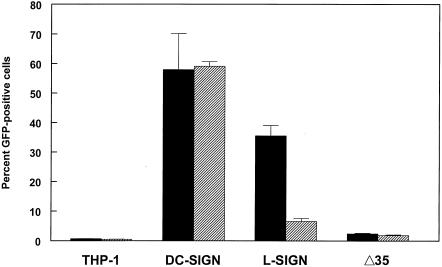
Production of virus in C6/36 arthropod cells was correlated with efficient infection of SIGN-expressing THP-1 cells. To determine whether this effect was due to differences in carbohydrate processing between mosquito and vertebrate cells, rather than another property of C6/36-derived virus stocks, we analyzed GFP-expressing TR339 produced in G1cNAc-T1 glycosyltransferase-deficient Lec1 CHO cells (GFP-expressing virus designated 39GFP-L; normal virus designated 39-L) in which N-linked glycosylation is blocked at the Man5GlcNAc2 intermediate (43), or in Lec1 parental control Pro5 CHO cells treated with 1-DMJ (GFP-expressing virus designated 39GFP-DMJ; normal virus designated 39-DMJ) an inhibitor of the enzyme α-mannosidase, which removes high-mannose carbohydrate chains (11). Both of these treatments decrease the extent of processing of glycoprotein carbohydrate modifications, resulting in enhanced mannose content and the absence of complex carbohydrates containing sialic acid and galactose. In this way, carbohydrate modifications resemble those produced during virus replication in mosquito cells (18). Titers of TR339 GFP virus produced under these conditions were similar (less than fourfold difference) in BHK cell plaque assays to control CHO-derived virus (data not shown). In contrast, a large increase in infectivity was observed for both treatments with SIGN-expressing THP-1 cells (Fig. 5). The 39GFP-L virus exhibited infectivity equal to or greater than that of C6/36-derived virus on both cell types, while the 39GFP-DMJ virus was ∼40 and ∼94% as infectious as C6/36-derived virus on DC-SIGN- and L-SIGN-transfected cells, respectively. Lower infectivity of 39GFP-DMJ for DC-SIGN-bearing versus L-SIGN-bearing cells may reflect differences in recognition of modified carbohydrates by the DC-SIGN/L-SIGN molecules.
FIG. 5.
Infectivity of 39GFP-M (solid bars), 39GFP-C (hatched bars), 39GFP-L (crosshatched bars), or 39GFP-DMJ (open bars) virus for control THP-1 or DC-SIGN/L-SIGN-expressing cells.
Infectivity of TR339 for SIGN-expressing cells can be blocked with inhibitors of DC-SIGN/L-SIGN binding.
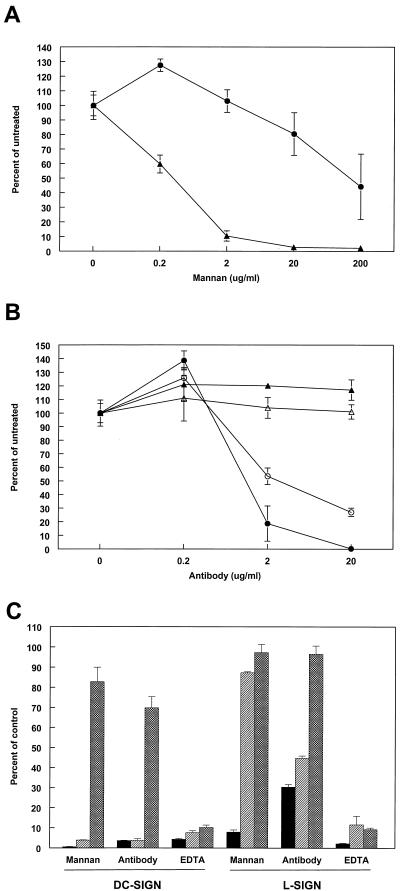
Efficient infection of THP-1 cells was dependent upon the expression of DC-SIGN or L-SIGN. To further confirm the specificity of virus interaction with these molecules, we performed infectivity experiments with 39GFP-M in the presence of 50 mM EDTA, increasing concentrations of yeast mannan, or increasing concentrations of the cross-reactive anti-DC-SIGN MAB 612 or an isotype-matched control (Fig. 6). These three treatments disrupt carbohydrate interactions with DC- or L-SIGN molecules (15, 47). Incubation with mannan or the anti-DC-SIGN antibody during infection resulted in a dose-dependent reduction in numbers of GFP-positive cells with both L- and DC-SIGN-expressing cells, whereas the isotype control antibody had no inhibitory effect (Fig. 6A and B). In dose-response experiments, mannan and anti-DC-SIGN antibody were less effective with L-SIGN-expressing cells, suggesting a different interaction with SB virus carbohydrates. In repeated experiments, inhibition of SB virus infection of L-SIGN-expressing cells by the highest concentration of mannan (200 μg/ml) was variable, ranging from ∼60% to >90% (Fig. 6A and C). EDTA treatment during infection was consisted for both cell types and resulted in a >90% reduction in GFP-positive cells (Fig. 6C). The 39GFP-L virus produced similar results with DC-SIGN-expressing cells but was not competed by mannan and exhibited partial competition with the cross-reactive 612 MAb on L-SIGN-expressing cells. The 39GFP-DMJ virus did not exhibit significant competition with mannan or antibody treatment on either cell type (Fig. 6C); however, the virus was blocked ∼90% by EDTA on both cell types. The results of these experiments suggest that the carbohydrate structures of virus prepared in C6/36, Lec1, or CHO cells treated with 1-DMJ interact with DC-SIGN/L-SIGN molecules in very different ways; however, the infectivity of each virus for THP-1 cells is dependent upon DC-SIGN/L-SIGN molecule expression.
FIG. 6.
Infectivity of 39GFP for cells in the presence of DC-SIGN/L-SIGN competitors or EDTA. (A) Infectivity of 39GFP-M virus for THP-1 cells expressing DC-SIGN (solid triangles) or L-SIGN (solid circles) in the presence of increasing concentrations of mannan. (B) Infectivity of 39GFP-M virus for THP-1 cells expressing DC-SIGN (solid symbols) or L-SIGN (open symbols) in the presence of increasing concentrations of MAb 612 (circles) or an isotype-matched control MAb (triangles). (C) Infectivity of 39GFP-M (solid bars), 39GFP-L (hatched bars), or 39GFP-DMJ (crosshatched bars) virus for THP-1 cells in the presence of 200 μg of mannan per ml, 20 μg of MAb 612 per ml, or 5 mM EDTA. The control for mannan- or EDTA-treated cells was untreated cells. The control for MAb 612 was 20 μg of isotype-matched MAb per ml.
We also completed experiments to test the similarity of virus interaction with receptors present on human Hep-2 epithelial cells or CHOK1 hamster ovary fibroblasts, both cell types that are permissive to mammalian and mosquito cell-derived SB virus. In repeated experiments under infection conditions identical to those for THP-1 cells, 39GFP-M or 39GFP-C infected only approximately 5 or 10% of the Hep-2 or CHOK1 cells, respectively. Limited infection most likely reflects the characteristic weak binding of TR339-derived viruses to these cell types (22, 24). In contrast to the effects of these treatments on SIGN-expressing THP-1 cells, mannan had little inhibitory effect upon infectivity of mammalian- or mosquito-derived virus for the cells (data not shown). We have previously tested the capacity of a range of carbohydrate molecules to block infectivity of mammalian cell-derived TR339 for CHOK1 cells and found no significant effect (data not shown). Treatment with EDTA had no effect on infectivity of either virus for Hep-2 cells but could depress infectivity of 39GFP-C and 39GFP-M virus for CHOK1 cells by 50 or 70%, respectively, suggesting a partially calcium- or magnesium-dependent entry process.
Virus binding to cells is increased in the presence of DC-SIGN or L-SIGN molecules.
To determine if the increase in infectivity of SB for THP-1 cells expressing DC-SIGN or L-SIGN was due to greater cell binding, in vitro attachment assays were performed with [35S]methionine-radiolabeled virus. Results of assays with 39-M showed clearly that increased infectivity correlated directly with increased binding to the cells (Fig. 7). The 39-M virus bound 30- to 70-fold more efficiently to SIGN-expressing cells than THP-1 controls. However, the level of mosquito cell-derived virus binding to L-SIGN-expressing cells was generally two- to threefold less than that to DC-SIGN-expressing cells. Since virus binding assays were performed at 4°C, this result correlates with the reduced infectivity observed at 4°C with L-SIGN-expressing cells (Fig. 2). In contrast with 39-M, the 39-C virus bound poorly (25- to 50-fold less with DC- or L-SIGN-expressing cells) to THP-1 cells regardless of SIGN expression. In the absence of SIGN expression, TR339 bound poorly to all cell types assayed, independent of virus production conditions. This phenotype is consistent with previous results (22, 24) and is typical even for cell types such as CHOK1, which are efficiently infected by TR339. Virus produced in Lec1 cells and in normal Pro5 CHO cells treated with 1-DMJ exhibited greatly increased binding to the SIGN-expressing cells only, exhibiting similar levels of attachment to C6/36-derived virus. This suggests that the improvement in infectivity and binding of virus derived from C6/36 cells is due to the altered processing of glycoprotein carbohydrate modifications. Together, these data indicate that the infectivity increases observed with SB virus with increased mannose carbohydrate content on SIGN-expressing THP-1 cells are due to enhanced attachment of virus particles to cells resulting in productive infection.
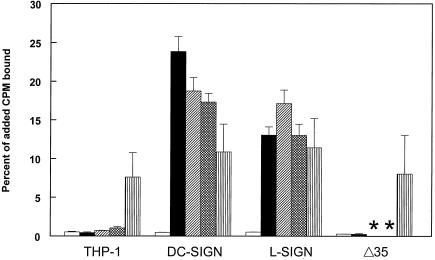
FIG. 7.
Binding of radiolabeled TR339 or S1K70 virus to THP-1 control and DC-SIGN/L-SIGN-transfected cells. The viruses used were 39-C (open bars), 39-M (solid bars), 39-L (hatched bars), or 39-DMJ (crosshatched bars) and S1K70 (striped bars), which was derived from CHO cells. *, binding of TR339 derived from Lec1- and 1-DMJ-treated Pro5 CHO cells not tested on Δ35 THP-1 cells.
Surprisingly, the truncated form of DC-SIGN did not increase binding of 39-M to the Δ35 cells. However, this result is consistent with the poor infectivity observed with 39GFP-M (Fig. 1B). As shown above (Fig. 1A), the level of expression of the Δ35 form of DC-SIGN was approximately threefold lower than that with full-length DC- or L-SIGN. Thus, low infectivity for these cells could arise from low receptor expression or an effect of the truncation mutation on the carbohydrate-binding capacity of the mutant DC-SIGN molecule. For these reasons, we were unable to directly test whether the endocytosis of DC-SIGN is required for infectivity.
To determine if HS was present on THP-1 cells and capable of binding laboratory-adapted SB, we completed binding assays using radiolabeled S1K70 virus derived from CHO cells. This virus would not be expected to interact efficiently with SIGN molecules. S1K70 bound to the control THP-1 cells >25-fold more efficiently than mammalian or mosquito cell-produced TR339 and, with the L-SIGN-expressing cells, exhibited binding efficiency virtually identical to that of the highly infectious C6/36-derived TR339. Similar results were obtained with other HS-binding SB virus mutants (data not shown). This result suggests that HS structures capable of binding laboratory-adapted SB virus are present on THP-1 cells and can bind the virus independent of DC-/L-SIGN expression. However, as indicated above, the infectivity of mammalian cell-produced S1K70 for the THP-1 cells was similar to that of mammalian cell-produced TR339, which exhibited very little binding. Therefore, it is likely that a factor required for efficient infection of HS-binding SB is expressed at low levels or not at all on THP-1 cells.
SB virus infection of primary human DCs is DC-SIGN dependent.
To determine if DC-SIGN molecules could serve as SB virus entry receptors in the context of natural receptor expression, we assessed infectivity of 39GFP-C or 39GFP-M for primary MDDCs cultured with GM-CSF and IL-4 to generate high-level cell-surface expression of DC-SIGN (34). More than 90% of the cultured MDDCs were positive for SIGN expression by MAb 612 staining (Fig. 8A). In control experiments, approximately 30% of the MDDCs infected with 39GFP-M exhibited GFP expression at 6 hpi. The 39GFP-M virus was >30-fold more infectious for the MDDCs than the 39-GFP-C virus (Fig. 8B). In addition, infectivity of the 39GFP-M virus was competed efficiently with mannan (200 μg/ml), 50 mM EDTA, or MAb 612 (20 μg/ml). Competition with these reagents was more effective than that observed with transfected THP-1 cells, perhaps reflecting higher levels of SIGN expression on the THP-1 cells (data not shown).
FIG. 8.
Flow cytometric analysis of cell surface DC-SIGN expression and infectivity of 39GFP viruses for primary human DCs. (A) Histogram showing MAb and isotype control antibody reactivity with primary human DCs. (B) Relative infectivity of 39GFP-M and 39GFP-C for human DCs and relative blocking of DC infection by 39GFP-M by 200 μg of mannan per ml, 5 mM EDTA, 20 μg of MAb 612 or isotype control antibody per ml. Approximately 30% of the cells infected with 39GFP-M were positive for GFP at 6 hpi.
DISCUSSION
We have demonstrated that SB virus can utilize DC-SIGN or L-SIGN as attachment receptors, resulting in productive infections of THP-1 cells and primary human DCs bearing these molecules. These in vitro results suggest that attachment factors such as DC-SIGN and L-SIGN might be responsible for the characteristic pattern of cell types and organs that are predominantly infected during early stages of alphavirus infection by efficiently capturing virus and promoting cell entry. DC-SIGN is expressed on dermal DCs and macrophages, which often encounter invading arboviruses in the skin. L-SIGN is expressed on sinusoidal endothelial cells, which would capture any virus inoculated into the small capillaries of the skin. Interaction with these receptors is presumably mediated by binding of N-linked carbohydrate modifications of the E2 or E1 proteins to carbohydrate recognition domains of the lectin molecules. Cells stably transfected with the cDNAs for DC-SIGN or L-SIGN were nearly 2 orders of magnitude more permissive than control THP-1 cells for SB virus derived from mosquito cells or produced in mammalian cells under conditions in which processing to complex carbohydrates was inhibited. The increased infectivity was specifically correlated with an increase in attachment to the cells as measured in radiolabeled virus binding assays. Specificity of the lectin-carbohydrate interaction was demonstrated by blocking of infectivity for the SIGN-expressing cells with yeast mannan, EDTA, or a MAb that cross-reacted with DC-SIGN and L-SIGN. Infectivity of virus for and binding of virus to cells bearing L-SIGN were lower than those for DC-SIGN, although flow cytometric analysis with the cross-reactive MAb 612 indicated similar levels of cell surface expression of the two molecules. Furthermore, while DC-SIGN-mediated infection was unaffected, infectivity for the SIGN-expressing cells was reduced fourfold when infection was conducted at 4°C. It is likely that the interaction of mosquito cell-derived SB virus carbohydrates with DC-SIGN is of higher affinity than that of L-SIGN.
Virus particles produced in mosquito cells, Lec1 CHO cells, or Pro5 CHO cells treated with 1 mM 1-DMJ were 10- to 70-fold more infectious for and bound more efficiently to the SIGN-expressing cells than virus produced in normal CHO cells. These results are consistent with the carbohydrate binding specificities of DC- and L-SIGN, which bind most efficiently to high-mannose carbohydrates and also bind Man3GlcNAc2, but do not bind complex carbohydrate structures terminating in galactose and sialic acid (9). The synthesis and processing of asparagine-linked oligosaccharides have been compared on SB virus grown in Aedes albopictus C6/36 mosquito cells versus vertebrate cells (18, 19). SB virus produced in BHK or CHO cells possesses predominantly complex N-linked carbohydrate at E2 Asn 196, high-mannose carbohydrate at E2 Asn 318, complex carbohydrate at E1 Asn 139, and either complex carbohydrate (BHK) or a mixture of complex and high-mannose carbohydrates (CHO) at E1 Asn 245 (19). Man3GlcNAc2 structures, which arise from high-mannose oligosaccharides, constitute the most extensively processed oligosaccharides in C6/36 and other invertebrate cells (18). When SB virus is grown in C6/36 cells, the DC-/L-SIGN ligand Man3GlcNAc2 is preferentially located at sites that have complex-type oligosaccharides on virus grown in vertebrate cells (18). Similar to mosquito cells, complex carbohydrate processing is absent in CHO cells deficient in N-acetylglucosaminyltransferase-I (e.g., Lec1) and high-mannose Man5GlcNAc2 is the predominant carbohydrate on SB virions (19). The specific composition of carbohydrates present on SB virus derived from cells treated with 1-DMJ has not been determined; however, such modifications should also be predominantly of the high-mannose type (21). Our infectivity blocking data reveal clear differences in the interactions of C6/36-, Lec1-, and DMJ-treated cell-derived viruses with DCs and, particularly, L-SIGN-expressing cells, which likely reflect the presence (C6/36) or absence (Lec1, 1-DMJ) of Man3GlcNAc2 and, potentially, the trimmed (Lec1) versus untrimmed (1-DMJ) structure of high-mannose carbohydrates. However, the consistent feature of these viruses is the absence of complex carbohydrates. Together, these data suggest that virus produced in and delivered by arthropod vectors would, due to enhanced high-mannose and Man3GlcNAc2 content, bind more efficiently to DC- and L-SIGN and therefore preferentially target cells bearing these molecules. C-type lectin molecules provide pathogen-associated molecular pattern recognition activity to immune system sentinel cells as well as host cell adhesion and signaling functions. However, it has been unclear how these molecules distinguish “self” from “nonself” carbohydrates (10). Our results suggest that with arboviruses, this distinction may be made by virtue of the carbohydrate-processing phenotype of the arthropod vector. In the THP-1 model system and in primary human DCs, arboviruses are able to usurp this recognition system to initiate infection of the cell.
Interestingly, SB virus derived from chicken embryo fibroblast cells differs from that from mammalian cells, possessing predominantly high-mannose carbohydrates at Asn 245 of E1 (19). Our data indicate that SB virus derived from DF-1 chicken fibroblasts is modestly more infectious than mammalian virus for SIGN-bearing THP-1 cells. Avian species are the natural reservoir host of SB group viruses (4), and, therefore, their carbohydrate-processing phenotypes could conceivably function during the transmission of virus to the arthropod vector. Invertebrates possess proteins with C-type lectin carbohydrate recognition domains similar to those of mammals (6); however, it is unknown whether invertebrate lectins can serve as arbovirus receptors.
In addition to the divergence in carbohydrate processing between evolutionarily distant organisms noted above, viral glycoprotein carbohydrate modifications may also differ between more closely related species and even within individual tissues of the same species. Humans, Old World monkeys and apes lack α-galactosyl carbohydrate modifications due to an enzyme deficiency, while these modifications are found on proteins produced in New World monkeys and non-primates (12). Repik and coworkers (35) determined the carbohydrate composition of eastern equine encephalitis alphavirus produced on African green monkey Vero cells versus mouse 3T3 cells and found that the murine cell-derived virus expressed α-galactosyl epitopes that bound a natural human anti-α-galactosyl antibody that actually neutralized eastern equine encephalitis alphavirus infectivity. Furthermore, HIV gp120 and Ebola virus glycoprotein carbohydrate modifications can exhibit different types of carbohydrate processing, depending upon the human host cell type in which they were produced, and this property has been correlated with altered C-type lectin interactions (26). Thus, lectin-dependent virus targeting could alter during different phases of replication within a single host or between closely related hosts. It will be of interest to determine if carbohydrate processing differs between mammalian cells commonly used to culture alphaviruses or if any differences in processing exist between mosquito species that could be correlated with their competence in transmitting virus to mammalian hosts.
An additional factor is whether or not alphavirus types may differ in carbohydrate processing or carbohydrate exposure to potential receptors. MacDonald and Johnston (28) observed differences in the numbers, morphologies, and locations of Venezuelan equine encephalitis virus-infected cells in the draining lymph nodes of mice, depending upon whether virus particles expressed wild-type or mutant E1 or E2 glycoproteins, and proposed that this might reflect use of different attachment and entry pathways. It is possible that mutations in the E1 or E2 proteins could alter interactions of carbohydrate chains with cell surface lectin molecules or possibly affect the processing of viral carbohydrates during virus maturation. Likewise, there may be differences in carbohydrate processing between individual alphavirus types encoded by the primary sequence of the glycoprotein, such that the tropism for cell types expressing specific lectins may differ. In studies with SB virus and other carbohydrate addition substrates, the extent of carbohydrate processing in a given cell type was linked to the accessibility of the high-mannose chain within the folded glycoprotein structure (20). In our studies, SB virus produced in mammalian CHOK1 or BHK (data not shown) cells bound poorly to and was only marginally infectious for DC- and L-SIGN-expressing THP-1 cells. In other experiments, we have found that nonpropagative Venezuelan equine encephalitis virus replicon particles produced in mammalian BHK cells exhibit low infectivity for DC-SIGN-expressing THP-1 cells but are nearly as infectious for L-SIGN-expressing cells as TR339 virus produced in mosquito cells (unpublished observations). We are currently assessing the carbohydrate structures and lectin interactions of other alphaviruses and the relationship of these phenomena to infection of cells in vivo and the pathogenesis of alphavirus disease.
Electroporation, infection, and growth curve data clearly indicate that normal THP-1 cells are highly permissive for wild-type SB virus replication but lack cell surface receptors capable of promoting efficient binding and entry and therefore are predominantly nonpermissive. These cells are also poorly infected by HS-binding SB virus mutants, yet binding data suggest that the THP-1 cells possess structures on their surfaces that we presume represent HS structures capable of binding the S1K70 virus in the absence of SIGN molecules. These results suggest that either a coreceptor or specific HS species may be required for HS-mediated productive infection and that this structure is not expressed sufficiently on THP-1 cells. However, we did observe increases in titer in control THP-1 cells in growth curve experiments that were correlated with the appearance of small-plaque variants. Therefore, at least a subset the THP-1 cells are permissive to SB virus infection. Nonetheless, the identification of an attachment/entry-defective cell line that can be rendered highly permissive by transfection of a receptor is a significant advancement in the study of alphavirus receptor biology. Previously, due to the very-broad-cultured cell tropism of alphaviruses, receptor expression experiments had been done in already highly permissive cells (46), making unequivocal determination of activity difficult. Use of THP-1 cells will now permit rapid screening of host cell protein expression libraries and, perhaps, rapid identification of other alphavirus receptor molecules.
We propose that the results of these studies and the studies of Tassaneetrithep et al. and Navarro-Sanchez et al. with dengue virus (30, 44) may reveal an important pathway used by arboviruses to initiate infection of a vertebrate host, particularly if virus particles are delivered by an arthropod vector. Additionally, demonstration of the use of lectins as attachment receptors may begin to unravel the conundrum of the arbovirus receptor in that evolutionarily conserved structures such as carbohydrates can act to mediate infection of cells, possibly representing a mechanism for bridging infection of invertebrates and vertebrates. In addition to DC-SIGN, there are other C-type lectin molecules present on DCs (7, 10), and C-type and other lectins are found on macrophage, endothelial, and other cell types (10, 38). However, it is important to note that our data do not distinguish between attachment and entry steps of the infection process. While we have shown that DC-SIGN/L-SIGN expression promotes virus binding to cells that leads to infection, it remains possible that other proteins facilitate entry of bound virus.
The replication cycle of alphaviruses within a single vertebrate host requires that virus produced by initially infected cells is capable of attaching to and infecting cells of a number of tissues, including periosteum, skeletal muscle, and neurons, and virus transferred from the vertebrate bloodstream to a feeding arthropod vector must initiate replication in the vector gut epithelium (16). Our data evaluating alphavirus binding to and infectivity for several types of rodent, mosquito, and human cells (22, 24; data not shown) suggest that there are C-type lectin-independent pathways to infection available to native alphaviruses. The characteristics of these pathways are (i) very inefficient binding to cell surfaces, which may reflect weak receptor interaction or limited receptor abundance; (ii) relatively low infectivity per virion, an underlying consequence of poor binding; (iii) resistance to competition with mannan and other carbohydrates; and (iv) substantial resistance to disruption with EDTA. Whether or not these pathways involve carbohydrate attachment to additional lectin molecules or another type of receptor remains to be determined.
Finally, the results described herein have relevance to the design and production of virus or virus-like particles used as alphavirus vaccine and gene therapy vectors. It is very likely that targeting of such particles to C-type lectin-expressing cells such as DCs can be increased by production under conditions that limit host cell processing of viral carbohydrate modifications. Incorporation of additional N-linked carbohydrate attachment sites into the virus structural glycoproteins could also increase the number of potential ligands for lectin interaction.
Acknowledgments
These studies were supported by NIH grant R01 AI22186-16.
We thank D. Littman and V. KewalRamani for the DC- and L-SIGN-transfected THP-1 cell lines. We thank Deborah Chervenak and Lijia Yin at the LSUHSC Flow Cytometry Core Facility for assistance with flow cytometry figures. Janice Anderson and Paul Schuetze provided excellent technical assistance.
REFERENCES
- 1.Bashirova, A. A., T. B. Geijtenbeek, G. C. van Duijnhoven, S. J. Van Vliet, J. B. Eilering, M. P. Martin, L. Wu, T. D. Martin, N. Viebig, P. A. Knolle, V. N. KewalRamani, Y. van Kooyk, and M. Carrington. 2001. A dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN)-related protein is highly expressed on human liver sinusoidal endothelial cells and promotes HIV-1 infection. J. Exp. Med. 193:671-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernard, K. A., W. B. Klimstra, and R. E. Johnston. 2000. Mutations in the E2 glycoprotein of Venezuelan equine encephalitis virus confer heparan sulfate interaction, low morbidity, and rapid clearance from blood of mice. Virology 276:93-103. [DOI] [PubMed] [Google Scholar]
- 3.Byrnes, A. P., and D. E. Griffin. 1998. Binding of Sindbis virus to cell surface heparan sulfate. J. Virol. 72:7349-7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, J.-P., J. H. Strauss, E. G. Strauss, and T. K. Frey. 1996. Characterization of the rubella virus nonstructural protease domain and its cleavage site. J. Virol. 70:4707-4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curtis, B. M., S. Scharnowske, and A. J. Watson. 1992. Sequence and expression of a membrane-associated C-type lectin that exhibits CD4-independent binding of human immunodeficiency virus envelope glycoprotein gp120. Proc. Natl. Acad. Sci. USA 89:8356-8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dodd, R. B., and K. Drickamer. 2001. Lectin-like proteins in model organisms: implications for evolution of carbohydrate-binding activity. Glycobiology 11:71R-79R. [DOI] [PubMed] [Google Scholar]
- 7.Dzionek, A., Y. Sohma, J. Nagafune, M. Cella, M. Colonna, F. Facchetti, G. Gunther, I. Johnston, A. Lanzavecchia, T. Nagasaka, T. Okada, W. Vermi, G. Winkels, T. Yamamoto, M. Zysk, Y. Yamaguchi, and J. Schmitz. 2001. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J. Exp. Med. 194:1823-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engering, A., T. B. Geijtenbeek, S. J. Van Vliet, M. Wijers, E. van Liempt, N. Demaurex, A. Lanzavecchia, J. Fransen, C. G. Figdor, V. Piguet, and Y. van Kooyk. 2002. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J. Immunol. 168:2118-2126. [DOI] [PubMed] [Google Scholar]
- 9.Feinberg, H., D. A. Mitchell, K. Drickamer, and W. I. Weis. 2001. Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science 294:2163-2166. [DOI] [PubMed] [Google Scholar]
- 10.Figdor, C. G., Y. van Kooyk, and G. J. Adema. 2002. C-type lectin receptors on dendritic cells and Langerhans cells. Nat. Rev. Immunol. 2:77-84. [DOI] [PubMed] [Google Scholar]
- 11.Fuhrmann, U., E. Bause, G. Legler, and H. Ploegh. 1984. Novel mannosidase inhibitor blocking conversion of high mannose to complex oligosaccharides. Nature 307:755-758. [DOI] [PubMed] [Google Scholar]
- 12.Galili, U., S. B. Shohet, E. Kobrin, C. L. Stults, and B. A. Macher. 1988. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J. Biol. Chem. 263:17755-17762. [PubMed] [Google Scholar]
- 13.Gardner, J. P., I. Frolov, S. Perri, Y. Ji, M. L. MacKichan, M. J. zur Megede, M. Chen, B. A. Belli, D. A. Driver, S. Sherrill, C. E. Greer, G. R. Otten, S. W. Barnett, M. A. Liu, T. W. Dubensky, and J. M. Polo. 2000. Infection of human dendritic cells by a Sindbis virus replicon vector is determined by a single amino acid substitution in the E2 glycoprotein. J. Virol. 74:11849-11857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geijtenbeek, T. B., A. Engering, and Y. van Kooyk. 2002. DC-SIGN, a C-type lectin on dendritic cells that unveils many aspects of dendritic cell biology. J. Leukoc. Biol. 71:921-931. [PubMed] [Google Scholar]
- 15.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. Van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587-597. [DOI] [PubMed] [Google Scholar]
- 16.Griffin, D. E. 2001. Alphaviruses, p. 917-962. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott-Williams and Wilkins, Philadelphia, Pa.
- 17.Halary, F., A. Amara, H. Lortat-Jacob, M. Messerle, T. Delaunay, C. Houles, F. Fieschi, F. Arenzana-Seisdedos, J. F. Moreau, and J. Dechanet-Merville. 2002. Human cytomegalovirus binding to DC-SIGN is required for dendritic cell infection and target cell trans-infection. Immunity 17:653-664. [DOI] [PubMed] [Google Scholar]
- 18.Hsieh, P., and P. W. Robbins. 1984. Regulation of asparagine-linked oligosaccharide processing. Oligosaccharide processing in Aedes albopictus mosquito cells. J. Biol. Chem. 259:2375-2382. [PubMed] [Google Scholar]
- 19.Hsieh, P., M. R. Rosner, and P. W. Robbins. 1983. Host-dependent variation of asparagine-linked oligosaccharides at individual glycosylation sites of Sindbis virus glycoproteins. J. Biol. Chem. 258:2548-2554. [PubMed] [Google Scholar]
- 20.Hsieh, P., M. R. Rosner, and P. W. Robbins. 1983. Selective cleavage by endo-beta-N-acetylglucosaminidase H at individual glycosylation sites of Sindbis virion envelope glycoproteins. J. Biol. Chem. 258:2555-2561. [PubMed] [Google Scholar]
- 21.Kaushal, G. P., and A. D. Elbein. 1994. Glycosidase inhibitors in study of glycoconjugates. Methods Enzymol. 230:316-329. [DOI] [PubMed] [Google Scholar]
- 22.Klimstra, W. B., H. W. Heidner, and R. E. Johnston. 1999. The furin protease cleavage recognition sequence of Sindbis virus PE2 can mediate virion attachment to cell surface heparan sulfate. J. Virol. 73:6299-6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klimstra, W. B., K. D. Ryman, K. A. Bernard, K. B. Nguyen, C. A. Biron, and R. E. Johnston. 1999. Infection of neonatal mice with Sindbis virus results in a systemic inflammatory response syndrome. J. Virol. 73:10387-10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klimstra, W. B., K. D. Ryman, and R. E. Johnston. 1998. Adaptation of Sindbis virus to BHK cells selects for use of heparan sulfate as an attachment receptor. J. Virol. 72:7357-7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwon, D. S., G. Gregorio, N. Bitton, W. A. Hendrickson, and D. R. Littman. 2002. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity 16:135-144. [DOI] [PubMed] [Google Scholar]
- 26.Lin, G., G. Simmons, S. Pöhlmann, F. Baribaud, H. Ni, G. J. Leslie, B. S. Haggarty, P. Bates, D. Weissman, J. A. Hoxie, and R. W. Doms. 2003. Differential N-linked glycosylation of human immunodeficiency virus and Ebola virus envelope glycoproteins modulates interactions with DC-SIGN and DC-SIGNR. J. Virol. 77:1337-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ludwig, G. V., J. P. Kondig, and J. F. Smith. 1996. A putative receptor for Venezuelan equine encephalitis virus from mosquito cells. J. Virol. 70:5592-5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacDonald, G. H., and R. E. Johnston. 2000. Role of dendritic cell targeting in Venezuelan equine encephalitis virus pathogenesis. J. Virol. 74:914-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell, D. A., A. J. Fadden, and K. Drickamer. 2001. A novel mechanism of carbohydrate recognition by the C-type lectins DC-SIGN and DC-SIGNR. Subunit organization and binding to multivalent ligands. J. Biol. Chem. 276:28939-28945. [DOI] [PubMed] [Google Scholar]
- 30.Navarro-Sanchez, E., R. Altmeyer, A. Amara, O. Schwartz, F. Fieschi, J. L. Virelizier, F. Arenzana-Seisdedos, and P. Despres. 2003. Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses. EMBO Rep. 4:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pöhlmann, S., F. Baribaud, B. Lee, G. J. Leslie, M. D. Sanchez, K. Hiebenthal-Millow, J. Munch, F. Kirchhoff, and R. W. Doms. 2001. DC-SIGN interactions with human immunodeficiency virus type 1 and 2 and simian immunodeficiency virus. J. Virol. 75:4664-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pohlmann, S., E. J. Soilleux, F. Baribaud, G. J. Leslie, L. S. Morris, J. Trowsdale, B. Lee, N. Coleman, and R. W. Doms. 2001. DC-SIGNR, a DC-SIGN homologue expressed in endothelial cells, binds to human and simian immunodeficiency viruses and activates infection in trans. Proc. Natl. Acad. Sci. USA 98:2670-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pöhlmann, S., J. Zhang, F. Baribaud, Z. Chen, G. J. Leslie, G. Lin, A. Granelli-Piperno, R. W. Doms, C. M. Rice, and J. A. McKeating. 2003. Hepatitis C virus glycoproteins interact with DC-SIGN and DC-SIGNR. J. Virol. 77:4070-4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Relloso, M., A. Puig-Kroger, O. M. Pello, J. L. Rodriguez-Fernandez, G. de la Rosa, N. Longo, J. Navarro, M. A. Munoz-Fernandez, P. Sanchez-Mateos, and A. L. Corbi. 2002. DC-SIGN (CD209) expression is IL-4 dependent and is negatively regulated by IFN, TGF-beta, and anti-inflammatory agents. J. Immunol. 168:2634-2643. [DOI] [PubMed] [Google Scholar]
- 35.Repik, P. M., J. M. Strizki, and U. Galili. 1994. Differential host-dependent expression of alpha-galactosyl epitopes on viral glycoproteins: a study of eastern equine encephalitis virus as a model. J. Gen. Virol. 75:1177-1181. [DOI] [PubMed] [Google Scholar]
- 36.Ryman, K. D., W. B. Klimstra, K. B. Nguyen, C. A. Biron, and R. E. Johnston. 2000. Alpha/beta interferon protects adult mice from fatal Sindbis virus infection and is an important determinant of cell and tissue tropism. J. Virol. 74:3366-3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryman, K. D., L. J. White, R. E. Johnston, and W. B. Klimstra. 2002. Effects of PKR/RNase L-dependent and alternative antiviral pathways on alphavirus replication and pathogenesis. Viral Immunol. 15:53-76. [DOI] [PubMed] [Google Scholar]
- 38.Sheikh, H., H. Yarwood, A. Ashworth, and C. M. Isacke. 2000. Endo180, an endocytic recycling glycoprotein related to the macrophage mannose receptor is expressed on fibroblasts, endothelial cells and macrophages and functions as a lectin receptor. J. Cell Sci. 113:1021-1032. [DOI] [PubMed] [Google Scholar]
- 39.Simmons, G., J. D. Reeves, C. C. Grogan, L. H. Vandenberghe, F. Baribaud, J. C. Whitbeck, E. Burke, M. J. Buchmeier, E. J. Soilleux, J. L. Riley, R. W. Doms, P. Bates, and S. Pohlmann. 2003. DC-SIGN and DC-SIGNR bind Ebola glycoproteins and enhance infection of macrophages and endothelial cells. Virology 305:115-123. [DOI] [PubMed] [Google Scholar]
- 40.Soilleux, E. J., R. Barten, and J. Trowsdale. 2000. DC-SIGN; a related gene, DC-SIGNR; and CD23 form a cluster on 19p13. J. Immunol. 165:2937-2942. [DOI] [PubMed] [Google Scholar]
- 41.Soilleux, E. J., L. S. Morris, B. Lee, S. Pohlmann, J. Trowsdale, R. W. Doms, and N. Coleman. 2001. Placental expression of DC-SIGN may mediate intrauterine vertical transmission of HIV. J. Pathol. 195:586-592. [DOI] [PubMed] [Google Scholar]
- 42.Soilleux, E. J., L. S. Morris, G. Leslie, J. Chehimi, Q. Luo, E. Levroney, J. Trowsdale, L. J. Montaner, R. W. Doms, D. Weissman, N. Coleman, and B. Lee. 2002. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. J. Leukoc. Biol. 71:445-457. [PubMed] [Google Scholar]
- 43.Stanley, P., and L. Siminovitch. 1977. Complementation between mutants of CHO cells resistant to a variety of plant lectins. Somatic Cell Genet. 3:391-405. [DOI] [PubMed] [Google Scholar]
- 44.Tassaneetrithep, B., T. H. Burgess, A. Granelli-Piperno, C. Trumpfheller, J. Finke, W. Sun, M. A. Eller, K. Pattanapanyasat, S. Sarasombath, D. L. Birx, R. M. Steinman, S. Schlesinger, and M. A. Marovich. 2003. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J. Exp. Med. 197:823-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsuchiya, S., M. Yamabe, Y. Yamaguchi, Y. Kobayashi, T. Konno, and K. Tada. 1980. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 26:171-176. [DOI] [PubMed] [Google Scholar]
- 46.Wang, K.-S., R. J. Kuhn, E. G. Strauss, S. Ou, and J. H. Strauss. 1992. High-affinity laminin receptor is a receptor for Sindbis virus in mammalian cells. J. Virol. 66:4992-5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu, L., T. D. Martin, R. Vazeux, D. Unutmaz, and V. N. KewalRamani. 2002. Functional evaluation of DC-SIGN monoclonal antibodies reveals DC-SIGN interactions with ICAM-3 do not promote human immunodeficiency virus type 1 transmission. J. Virol. 76:5905-5914. [DOI] [PMC free article] [PubMed] [Google Scholar]