Abstract
4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) is a potent lung carcinogen present in both unburned tobacco and cigarette smoke. The sum of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and its glucuronides, referred to as total NNAL, is an established urinary biomarker of human NNK uptake. Metabolic activation of NNK to DNA adducts proceeds via α-hydroxylation pathways, and 4-oxo-4-(3-pyridyl)butanoic acid (keto acid) and 4-hydroxy-4-(3-pyridyl)butanoic acid (hydroxy acid) are the principal end products of these pathways in rodents and primates. The purpose of this study was to determine NNK metabolic activation in smokers, as measured by the sum of keto acid and hydroxy acid, relative to total NNAL. To specifically identify NNK-derived keto acid and hydroxy acid, which are also formed extensively from nicotine, we added [pyridine-D4]NNK to cigarettes that were originally low in NNK, and measured the deuterium-labeled metabolites in the urine of people who smoked these cigarettes. The total amount of [pyridine-D4]keto acid plus [pyridine-D4]hydroxy acid averaged 4.00±2.49 nmol/24 h, while the average amount of total [pyridine-D4]NNAL was 0.511±0.368 nmol/24 h. The results of this study demonstrate for the first time that NNK metabolic activation is a quantitatively significant pathway in smokers, accounting for about 86% of total urinary excretion of NNK metabolites. The large interindividual variation in the excreted [pyridine-D4]keto acid and [pyridine-D4]hydroxy acid among 20 smokers strongly support our hypothesis that some smokers activate NNK more extensively than others, and that the ratio between biomarkers of metabolic activation and detoxification at a given dose of NNK could be a potential indicator of cancer risk.
Keywords: NNK, biomarkers, metabolic activation, cancer
INTRODUCTION
Numerous investigations indicate that tobacco-specific nitrosamines play an important role in cancer induction by tobacco products (reviewed in 1 and 2). 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) is one of the most prevalent of these compounds present in both unburned tobacco and cigarette smoke, and is a remarkably effective lung carcinogen in laboratory animals, inducing lung tumors in rodents independent of the route of administration (1). NNK and polycyclic aromatic hydrocarbons are believed to be major causative agents for lung cancer in smokers (2-4). 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), a metabolite of NNK, is also a pulmonary carcinogen. Both compounds require metabolic activation to exert their carcinogenic effects.
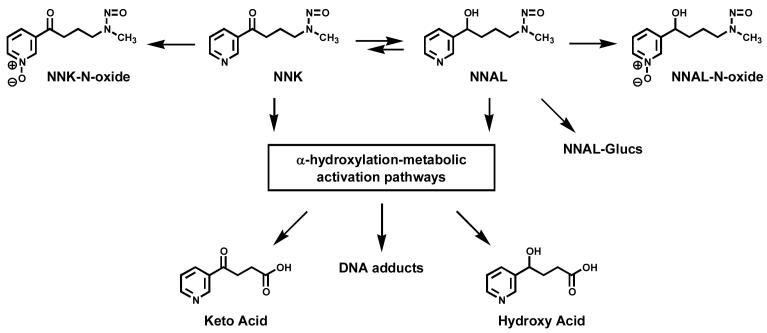
NNK and NNAL metabolism, adduct formation, and detoxification have been investigated in a number of studies involving laboratory animals and humans, and are now quite well-understood (1). Figure 1 presents an overview of NNK and NNAL metabolism and DNA adduct formation. Although there are quantitative differences, many of the same reactions are observed in both laboratory animals and humans. Studies in rodents and primates show that NNK is rapidly metabolized and distributed to all tissues (5-9). Overall, three major routes of NNK metabolism are consistently observed in vivo: carbonyl reduction, pyridine oxidation, and α-hydroxylation (1). Reduction of the NNK carbonyl group produces NNAL, which can be partially converted back to NNK, the NNK-NNAL equilibrium favoring NNAL in rodents, primates, and humans (8-13). Major metabolic pathways and carcinogenicity of NNAL are similar to those of NNK, and therefore it is not a detoxified metabolite of NNK (1,14-16). A unique feature of NNAL metabolism is the formation of NNAL-glucuronides (NNAL-Glucs), which is an important detoxification pathway for NNK and NNAL (1,9,11). Glucuronidation of NNAL at the pyridine nitrogen forms NNAL-N-Gluc, while that at the carbinol oxygen gives NNAL-O-Gluc (17). Levels of NNAL-Glucs are generally higher than those of NNAL in human urine. Pyridine-N-oxidation results in formation of NNK-N-oxide and NNAL-N-oxide in rodents and primates (9,11). In humans, urinary levels of NNAL-N-oxide were less than 20% of those of NNAL-Glucs, while NNK-N-oxide and unchanged NNK have not been detected (13,18). Metabolic activation of NNK and NNAL to DNA adducts proceeds via α-hydroxylation pathways (Figure 1). The DNA adducts, if unrepaired, cause miscoding leading to gene mutations and cancer. 4-Oxo-4-(3-pyridyl)butanoic acid (keto acid) and 4-hydroxy-4-(3-pyridyl)butanoic acid (hydroxy acid) are the principle end products of the NNK α-hydroxylation pathways in rodents and primates (1,9,11,19-21). Both of these metabolites can be measured in human urine; however they cannot be used to evaluate the extent of NNK α-hydroxylation in smokers because they are also formed extensively from nicotine, and levels of nicotine in cigarettes are much higher than those of NNK (22,23).
Figure 1.
Overview of NNK metabolism and DNA adduct formation as determined by studies in laboratory animals and humans (for more details see ref. 1).
In this study, we address the important question of the extent of NNK metabolic activation in humans, which, for the reasons discussed above, has never been previously reported. Our hypothesis is that individuals who activate NNK more extensively will be at higher risk for lung cancer. The key factor in the evaluation of NNK metabolic activation would be distinguishing urinary keto acid and hydroxy acid as formed from NNK, versus nicotine. Since hydroxy acid is chiral, it was suggested that one enantiomer would be formed preferentially from NNK, while the formation of the other would result from nicotine, and studies in rats demonstrated that this was plausible (22). However, in contrast to the studies in rats, hydroxy acid was found to be a more abundant nicotine metabolite in humans, and even the minor enantiomer, as formed from nicotine, was much higher in concentration than that which would be produced from NNK (23). Therefore, we took advantage of the commercial availability of a low-nicotine cigarette called Quest, which also has relatively low NNK levels (24). We added [pyridine-D4]NNK (Figure 2) to the tobacco of Quest cigarettes, such that the total NNK concentration (unlabeled plus deuterated) in the tobacco of these cigarettes was comparable to that of a standard cigarette. We recruited smokers of standard brands to smoke the cigarettes containing [pyridine-D4]NNK, and then quantified [pyridine-D4]NNAL, [pyridine-D4]NNAL-Glucs, and the sum of [pyridine-D4]hydroxy acid and [pyridine-D4]keto acid in the urine of these individuals. Data from this study thus provide the first direct information on the extent of NNK metabolic activation in smokers.
Figure 2.
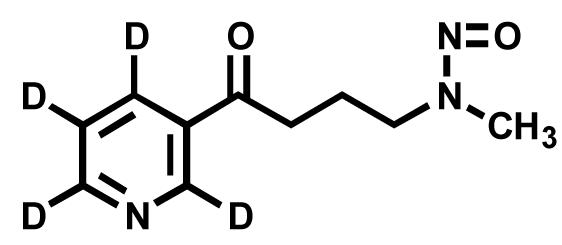
Structure of [pyridine-D4]NNK.
METHODS AND MATERIALS
Caution
NNK, [pyridine-D4]NNK, NNAL, and [pyridine-D4]NNAL are carcinogenic and mutagenic and should be handled with extreme care, using appropriate protective clothing and ventilation at all times.
Chemicals and Enzymes
NNAL and 5-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (C5-NNAL) were purchased from Toronto Research Chemicals Inc. (Toronto, Ontario, Canada). NNK, 5-(methylnitrosamino)-1-(3-pyridyl)-1-pentanone (C5-NNK), [13C6]NNAL, [pyridine-D4]NNAL, and hydroxy acid were synthesized as previously described (19, 22, 25-29). β-Glucuronidase (type IX-A from Escherichia coli) was purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO). [Pyridine-D4]Ethyl nicotinate was purchased from CDN Iostopes (Quebec, Canada). Methyl-5-methyl nicotinate was obtained from Lancaster (Alfa Aesar, Ward Hill Road, MA). NaBH4 and all other chemicals were purchased from Sigma-Aldrich Chemical Co. (Milwaukee, WI).
[Pyridine-D4]NNK
The synthesis of [pyridine-D4]NNK was carried out essentially as previously described (28), but starting with [pyridine-D4]ethyl nicotinate. Properties of [pyridine-D4]NNK: mp 66-68 °C, literature (ref. 30), 63-65 °C; NMR δ (CDCl3) 4.3 (t, 2H, CH2-N-N=O, E-isomer), 3.8 (s, CH3-N-N=O, Z isomer), 3.7, (t, 2H, CH2-N-N=O, Z-isomer), 3.07 (s, CH3-N-N=O, E-isomer), 3.05 (t, O=C-CH2, E-isomer), 2.9 (t, O=C-CH2,Z-isomer), 2.2 (m, O=C-CH2-CH2, E-isomer), 1.9 (m, O=C-CH2-CH2, Z-isomer). Positive ion ESI-MS m/z 212 [M + H]+, MS/MS of m/z 212, m/z 182 [M - N=O]+, 126 [M - C3H5N]+.
A concentrated stock of 1.0 μg/μl [pyridine-D4]NNK in ethanol was prepared. Spiking solution (15.0 ng/μl [pyridine-D4]NNK in H2O) was prepared by adding 15 μl of stock to 985 μl HPLC-grade H2O.
4-Hydroxy-4-(5-methyl-3-pyridyl)butanoic acid (5-methylhydroxy acid)
This was prepared from 5-methyl methyl nicotinate essentially as described (29). Spectral properties: NMR δ ((CD3)2SO) 8.27 (d, 2H, Pyr-2H, Pyr-6H), 7.5 (s, 1H, Pyr-4H), 4.5 (m, 1H, CH-OH), 2.8 (s, 3H, Pyr-CH3), 2.2 (t, 2H, CH2-COOH), 1.8 (t, 2H, CH2-CH2-COOH). Positive ion ESI-MS m/z (relative intensity) 196 [M + H]+ (63), 178 [M + H - H2O]+ (100), 132 [M - CH3O3]+ (30).
[Pyridine-D4]hydroxy acid
[Pyridine-D4]hydroxy acid was synthesized as described for 5-methylhydroxy acid, except that [pyridine-D4]ethyl nicotinate was used as the starting material. HPLC purification was carried out on a 250 mm × 10 mm, C-18 Vydac 201TP 10μm column (Separations Group, Hesperia, CA) eluted at 3 mL/min, using isocratic elution with 0.15% trifluoroacetic acid in H2O for 20 min, giving [pyridine-D4]hydroxy acid, retention time 7 min. NMR δ ((CD3)2SO) 4.8 (m, 1H, CH-OH), 2.3 (t, 2H, CH2-COOH), 1.9 (m, 2H, CH2-CH2-COOH). Positive ion ESI-MS m/z (relative intensity) 186 [M + H]+ (40), 168 [M + H - H2O]+ (100), 122 [M - CH3O3]+ (63).
Study cigarettes
Deuterium is non-radioactive and non-hazardous, and its substitution in the pyridine ring of NNK is not expected to have an effect on NNK metabolic activation or carcinogenicity (15,28). The use of these cigarettes was approved by the U.S. Food and Drug Administration.
Quest 1 cigarettes were ordered in four batches through www.smokeplaza.com and www.smokes-spirits.com. The addition of [pyridine-D4]NNK to the cigarettes was carried out with a specially designed microsyringe applicator system which uniformly distributed 21 μl of 15.0 ng/μl [pyridine-D4]NNK spiking solution along the tobacco rod of the cigarette. The spiked cigarettes were conditioned in a humidity controlled chamber at 25 °C and 60% relative humidity for 2 days. Then they were packed in their original packs, 20 cigarettes per pack. The packs were put in original Quest 1 cartons (10 packs per carton) and stored at 4 °C.
Subjects and urine collection
The study was approved by the University of Minnesota Research Subjects’ Protection Programs Institutional Review Board: Human Subjects Committee. Subjects were daily “light”, non-menthol cigarette smokers recruited through newspaper advertisements. They were initially screened over the telephone, and potential subjects were scheduled for a screening visit at the University of Minnesota Tobacco Use Research Center. At the screening visit, they signed a consent form and completed several questionnaires on demographics, smoking and health history. Subjects were excluded if they had poor or unstable physical or mental health which would affect study participation. Eligible subjects were asked to collect a 24 h urine sample the day prior to their next scheduled visit. At that visit, they were given the study cigarettes and instructed to smoke only those cigarettes over the following 7-day period. Beginning on the 5th day of smoking the study cigarettes, subjects were asked to collect their 24 h urine on each of the next 3 days and to keep each 24 h urine collection in a separate container. After completing each day’s collection, subjects were required to bring the sample to the Tobacco Use Reseach Center.
Analyses
Analysis of the study cigarettes
To prepare tobacco samples, three cigarettes were cut open, the tobacco was mixed, and two 500 mg samples were analyzed.
Total NNK
Prior to spiking with [pyridine-D4]NNK, the tobacco of Quest 1 cigarettes was analyzed for NNK using a model 5890 gas chromatograph (GC)(Hewlett Packard, Palo Alto, CA) interfaced with a model 610 Thermal Energy Analyzer (TEA) (Orion Research, Beverly, MA) as described elsewhere (24). The same method was used to analyze total NNK (NNK plus [pyridine-D4]NNK) content in the study cigarettes after their spiking with [pyridine-D4]NNK.
[Pyridine-D4]NNK
To distinguish between non-deuterated NNK and [pyridine-D4]NNK, we used gas chromatography-tandem mass spectrometry (GC-MS/MS). Tobacco samples were prepared in the same manner as for analysis of non-deuterated NNK (24), and 2 μl of the prepared sample was injected into the GC-MS/MS system. The analysis was carried out with a Finnigan TSQ-7000 instrument operated in the positive ion electron ionization mode. Daughter ion scans were performed to monitor the transitions m/z 177 → m/z 146 and m/z 177 → m/z 118 for NNK; m/z 181 → m/z 150 and m/z 181 → m/z 122 for [pyridine-D4]NNK; and m/z 191 → m/z 134 and m/z 191 → m/z 162 for C5-NNK, operating Q3 in the selected ion monitoring mode at a scan rate 0.5 scan/sec. The gas chromatograph (HP Model 5890) was equipped with a 15 m × 0.25 mm i.d. DB 1301 column (0.25 μm film thickness) from J & W Scientific connected to a 2 m × 0.32 mm i.d. deactivated precolumn. The injection mode was splitless, the constant flow rate was 2.0 ml/min He, and the injection port temperature was 225 °C. The temperature program was as follows: 80 °C for 2 min; then 20 °C per min to 155 °C; then 2 °C per min to 190 °C; and then 20 °C per min to 250 °C. The final temperature was held for 5 min.
Analysis of NNAL and [pyridine-D4]NNAL in the urine of study participants
Total NNAL and total [pyridine-D4]NNAL
Urine (3 ml) was placed in a 10 ml glass centrifuge tube, the pH was adjusted to 6-7 if necessary, and 20 pg [13C6]NNAL was added as internal standard. β-Glucuronidase (10,000 units in 500 μl HPLC-grade H2O) was added to each sample, and the mixture was incubated at 37 °C overnight. The next day, the mixture was applied to a 5-ml ChemElut cartridge (Varian, Harbor City, CA), eluted with 2 × 8 ml CH2Cl2 into a 15-ml glass centrifuge tube, and the combined eluants were concentrated to dryness (Speedvac concentrator). The dry residue was redissolved in 1 ml H2O, adjusted to pH 2-3 by adding 100 μl 1N HCl, and the mixture was applied to a 60 mg Oasis MCX cartridge (Waters Corp., Milford, MA) activated with 5 ml CH3OH and equilibrated with 10 ml H2O. The cartridges were washed with 5 ml 1N HCl, 5 ml CH3OH, and 5 ml H2O:CH3OH:NH4OH (90:5:5) and these washings were discarded. Then, the analytes were eluted from the Oasis MCX cartridges with 5 ml H2O:CH3OH:NH4OH (30:65:5) and the eluant was concentrated to dryness. The dry residues were transferred to autosampler vials with CH3OH, dried, and stored at -20 °C until analysis by LC-ESI-MS/MS. Prior to analysis, samples were re-dissolved in 10 μl of 2% CH3OH in H2O, and 5 μl were injected.
LC-ESI-MS/MS was carried out with a Finnigan TSQ Quantum Discovery Max instrument (Thermo Electron Corp., Waltham, MA) interfaced with an Agilent Model 1100 capillary high-performance liquid chromatography system and a Model 1100 micro autosampler (Agilent, Palo Alto, CA). The high-performance liquid chromatograph was fitted with a 150 × 0.5 mm ZORBAX SB C18 RR 3.5 μm column (Agilent) eluted isocratically with 35% MeOH in H2O for 20 min at a flow rate of 10 μl / min. The column was maintained at 25 °C. MS/MS variables were as follows: positive ion electrospray mode with selected reaction monitoring for m/z 210 → m/z 180 ([M+H]+ → [M+H - NO]+) for NNAL, m/z 214 → m/z 184 for [pyridine-D4]NNAL, and m/z 216 → m/z 186 for [13C6]NNAL at 0.5 amu scan width. The collision gas was Ar at a pressure of 1 mTorr, with a collision energy of 10 eV. The quadrupoles were operated at a resolution of 0.7 amu.
Free NNAL and free [pyridine-D4]NNAL
The method was practically identical to that described for total NNAL and total [pyridine-D4]NNAL, except that the samples were not treated with β-glucuronidase prior to their extraction on ChemElut cartridges.
NNAL-Glucs and [pyridine-D4]NNAL-Glucs
These were determined by subtracting amounts of free NNAL and free [pyridine-D4]NNAL from total NNAL and total [pyridine-D4]NNAL, respectively.
Analysis of the sum of [pyridine-D4]keto acid and [pyridine-D4] hydroxy acid in urine
This was performed by a modification of the previously described method (23), as follows.
Reduction of [pyridine-D4]keto acid to [pyridine-D4]hydroxy acid
Urine (3 ml) was treated with NaBH4 overnight at room temperature, as previously described (23). The next day, the excess NaBH4 was destroyed by dropwise addition of 1N HCl, and the samples were then analyzed as described for [pyridine-D4]hydroxy acid.
[Pyridine-D4]hydroxy acid
Fifty ng 5-methylhydroxy acid internal standard was added to the neutralized urine that had been treated with NaBH4. Each sample was applied to an activated 200 mg Strata X cartridge (Phenomenex Inc., Torrance, CA), the [pyridine-D4]hydroxy acid was eluted with 6 ml of 20% CH3OH, and the eluant was concentrated to dryness. One ml CH3OH was added to the dry residue, and the sample was concentrated to dryness. This was repeated twice to ensure complete removal of H2O. The dry residue was dissolved in 1 ml of freshly prepared 3% concentrated H2SO4 in CH3OH, and the mixture was allowed to stand overnight at room temperature. This step converts very polar [pyridine-D4]hydroxy acid to its methyl ester, which further can be extracted into a nonpolar organic solvent and subjected to normal phase extraction, thus leading to less chemical noise and lower ion suppression upon sample analysis by LC-ESI-MS/MS. The conversion is nearly quantitative (22). The next day, the mixture was neutralized by addition of 2.0 ml 5% (w/v) NaHCO3 and loaded on a 5 ml ChemElut cartridge. The methyl ester was eluted with 2 × 8 ml CH2Cl2, and the eluant was concentrated to dryness. The dry residue was immediately redissolved in 500 μl CH2Cl2 and loaded on a 500 mg Sep Pak Silica cartridge (Waters) pre-equilibrated with 4 ml CH2Cl2. The cartridge was washed with 4 ml CH2Cl2 and 4 ml CH2Cl2/EtOAc (50:50) - these fractions were discarded. The methyl ester of [pyridine-D4]hydroxy acid was eluted with 8 ml of EtOAc:CH3OH (90:10), and the eluant was concentrated to dryness. The dry residue was immediately redissolved in 1 ml H2O and 100 μl 1N HCl, and the mixture was incubated at 40 °C for 2 h. This step hydrolyzed the methyl ester back to [pyridine-D4]hydroxy acid, which was further purified on a 60 mg Oasis MCX cartridge activated with 5 ml CH3OH and pre-equilibrated with 10 ml H2O. The cartridge was washed with 3 ml 1N HCl and 3 ml of 80:20 CH3OH/1N HCl, which were discarded, and [pyridine-D4]hydroxy acid was eluted with 6 ml of CH3OH:H2O:NH4OH (20:75:5). The eluant was concentrated to dryness, and the residue was transferred in two 100-μl aliquots of acetonitrile to a 45-μm nylon Spin-X LC filtration vial. The filtrate was transferred to a glass micro-insert autosampler vial, concentrated to dryness, and stored at -20 °C until analysis by LC-ESI-MS/MS.
LC-ESI-MS/MS was carried out with a Finnigan TSQ Quantum Discovery Max instrument (Thermo Electron Corp., Waltham, MA) interfaced with an Agilent Model 1100 capillary high-performance liquid chromatography system and a Model 1100 micro autosampler (Agilent, Palo Alto, CA). The high performance liquid chromatograph was fitted with a 100 × 0.5 mm ZORBAX SB C18 1.8 μm column (Agilent) maintained at 35 °C and eluted over the course of 35 min at a flow rate of 9 μl/min. The following gradient program was used: 10 mM ammonium acetate for 2 min, then a linear gradient over the course of 5 min to 95% 10 mM ammonium acetate/5% acetonitrile which was held for 5 min; then a linear gradient over the course of 10 min to 65% 10 mM ammonium acetate/35% acetonitrile; then return to 100% 10 mM ammonium acetate in 5 min and hold for 8 min to equilibrate the column for the next injection. Based on the MS of the analytes, the following MS/MS variables were used: positive ion electrospray mode with selected reaction monitoring for m/z 182 → m/z 164 plus m/z 182 → m/z 118 for hydroxy acid, m/z 186 → m/z 168 plus m/z 186 → m/z 122 for [pyridine-D4]hydroxy acid, and m/z 196 → m/z 178 plus m/z 196 → m/z 132 for 5-methylhydroxy acid, at 0.2 amu scan width. The collision gas was Ar at a pressure of 1 mTorr, with a collision energy of 10 eV. The quadrupoles were operated at a resolution of 0.7 amu.
Statistical analysis
The Pearson correlation was used to measure the association between the ratios of [pyridine-D4]NNAL-Glucs/free [pyridine-D4]NNAL and NNAL-Glucs/free NNAL, both on the log scale.
RESULTS
Study cigarettes
Quest cigarettes are available in three varieties: Quest 1, low nicotine; Quest 2, extra low nicotine; and Quest 3, nicotine free. Our previous studies demonstrated that levels of NNK are similar in the tobacco of Quest 1 and 2, and lower in the nicotine-free Quest 3 (24). Since the nicotine content in Quest 1 is the highest of the three Quest varieties, we chose it for the trial because we expected that our smokers would not try to compensate for the lack of nicotine by smoking their usual cigarettes or by increasing the number of study cigarettes smoked per day (thus increasing their uptake of NNK).
The mean (±SD) NNK content in four batches of Quest 1 cigarettes was 0.225 (± 0.07) μg/g tobacco, and the average tobacco weight per cigarette was 0.623 g (Table 1). Based on the reported average NNK levels in popular commercial cigarette brands (0.750 μg/g tobacco, ref. 24), we added 0.315 μg [pyridine-D4]NNK to each study cigarette, so that the amount of total (deuterated plus unlabeled) NNK in these cigarettes would be ∼ 0.750 μg/g tobacco. The results of the analyses of the study cigarettes before and after spiking are summarized in Table 1. The spiked cigarettes from batch 2 were also analyzed for [pyridine-D4]NNK. They contained 0.527 μg/g tobacco, or 0.323 μg/cigarette.
Table 1. NNK content in Quest cigarettes before and after spiking with [pyridine-D4]NNK.
| Batch | Average tobacco weight per cigarette, g | NNK | |||
|---|---|---|---|---|---|
| Before spiking | After spiking | ||||
| μg/g tobacco | μg/cigarette | μg/g tobacco | μg/cigarette | ||
| 1 | 0.614 | 0.301 | 0.182 | 0.810a | 0.497 |
| 2 | 0.626 | 0.142 | 0.089 | 0.692b | 0.424 |
| 3 | 0.624 | 0.262 | 0.164 | 0.752a | 0.461 |
| 4 | 0.629 | 0.193 | 0.121 | 0.764a | 0.468 |
| Mean for all batches | 0.623 | 0.225 | 0.139 | 0.755 | 0.463 |
Total NNK (the sum of [pyridine-D4]NNK and unlabeled NNK) measured by GC-TEA
Sum of [pyridine-D4]NNK and unlabeled NNK measured separately by GC-MS/MS
Subjects
Twenty subjects were recruited for the study. Eleven (55%) were male and 15 (75%) were white. Their average age was 34 years (range 20-56). The average (±SD) number of study cigarettes smoked per day during the trial, as reported by the participants, was 28 (±9). Six subjects reported smoking a total of 1-19 cigarettes of their own brand during the trial period, in addition to the reported number of study cigarettes.
Urine analysis
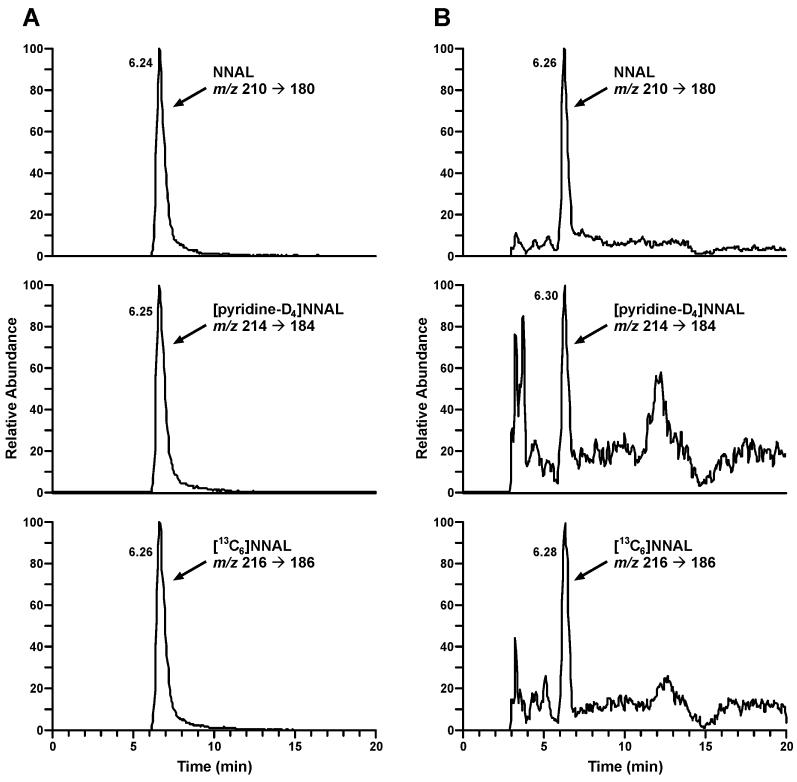
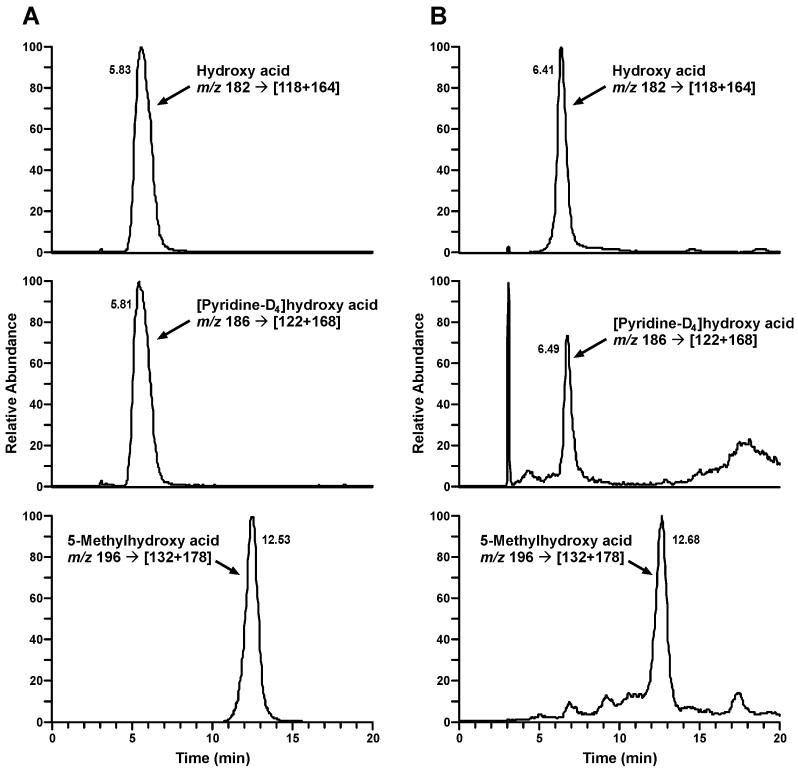
Deuterated biomarkers were not detected in the baseline urine samples. The mean level of unlabeled total NNAL in the baseline urine was 2.99 ± 1.75 nmol/24 h. In the urine collected during the days 5-7 of smoking the spiked Quest 1 cigarettes these levels slightly decreased, averaging 2.23 ± 1.23 nmol/24h. Typical LC-MS/MS traces from the analysis of NNAL, [pyridine-D4]NNAL, hydroxy acid, and [pyridine-D4]hydroxy acid in the urine of a study participant after 5 days of smoking the spiked Quest 1 cigarettes are illustrated in Figures 3 and 4. The results of the analyses of the deuterated biomarkers are summarized in Table 2. Despite the quite variable amounts of deuterated metabolites among the trial participants, the sum of the products of [pyridine-D4]NNK α-hydroxylation pathways was the prevalent biomarker in all subjects and all urine samples analyzed. Thus, the total amount of [pyridine-D4]keto acid plus [pyridine-D4]hydroxy acid averaged 4.00 ± 2.49 nmol/24h (geometric mean, 2.87 nmol/24h), while the average amount of total [pyridine-D4]NNAL was 0.511 ± 0.368 nmol/24h (geometric mean, 0.407 nmol/24h). There was also a significant day-to-day variability of the deuterated biomarkers of α-hydroxylation pathways within subjects (average CV 44.5%), while the levels of total [pyridine-D4]NNAL showed lower intraindividual variation (average CV 23.1%).
Figure 3.
Chromatograms obtained upon LC-ESI-MS/MS analysis of total NNAL and total [pyridine-D4]NNAL in the urine of a study participant after 5 days of smoking cigarettes spiked with [pyridine-D4]NNK: A, standard mix and B, a smoker’s urine sample.
Figure 4.
Chromatograms obtained upon LC-ESI-MS/MS analysis of hydroxy acid and [pyridine-D4]hydroxy acid in the urine of a study participant after 5 days of smoking cigarettes spiked with [pyridine-D4]NNK: A, standard mix and B, a smoker’s urine sample.
Table 2. Summary statistics for deuterated biomarkers analyzed in 20 study participants.
| Biomarker | nmol/24h | |||
|---|---|---|---|---|
| Average (SD) | Median | Min/Max | Geometric Mean (95% CI) | |
| Total [pyridine-D4]NNALa | 0.511 (0.368) | 0.398 | 0.121/1.70 | 0.407 (0.301, 0.550) |
| Free [pyridine-D4]NNAL | 0.197 (0.183) | 0.149 | 0.051/0.874 | 0.149 (0.109, 0.204) |
| [Pyridine-D4]NNAL-Gluc | 0.314 (0.207) | 0.241 | 0.070/0.827 | 0.246 (0.178, 0.340) |
| [Pyridine-D4]keto acid plus [pyridine-D4]hydroxy acidb | 4.00 (2.49) | 4.18 | 0.780/8.02 | 2.87 (1.99, 4.13) |
Free [pyridine-D4]NNAL plus [pyridine-D4]NNAL-Glucs
Urine samples were treated with NaBH4 and analyzed for [pyridine-D4]hydroxy acid
To further investigate the α-hydroxylation pathways of [pyridine-D4]NNK, we performed separate analysis of [pyridine-D4]keto acid and [pyridine-D4]hydroxy acid in 3 randomly selected subjects. The analysis for [pyridine-D4]hydroxy acid was accomplished by excluding the NaBH4 reduction step from the sample preparation procedure. The amount of [pyridine-D4]keto acid was determined by subtracting [pyridine-D4]hydroxy acid from the total amount of [pyridine-D4]keto acid plus [pyridine-D4]hydroxy acid detected with NaBH4 reduction. Non-deuterated keto acid and hydroxy acid were also analyzed in the same subjects. The results are summarized in Table 3. [Pyridine-D4]keto acid accounted for an average of 28.2 % of the total amount of the deuterated products of α-hydroxylation pathways, which was quite similar to the 22.5 % average contribution of non-deuterated keto acid to the total amount of unlabeled keto acid plus hydroxy acid found in the same urine samples.
Table 3. Separate analysis of deuterated and unlabeled keto acid and hydroxy acid in 3 study participants.
| Subject # | Day on study cigarettes | [Pyridine-D4]-labeled metabolites, pmol/mL urine | [Pyridine-D4] keto acid as % of total | Unlabeled metabolites, pmol/mL urine | Keto acid as % of total | ||||
|---|---|---|---|---|---|---|---|---|---|
| Keto acid plus hydroxy acida | Hydroxy acidb | Keto acidc | Keto acid plus hydroxy acida | Hydroxy acidb | Keto acidc | ||||
| 1 | 3 | 3.00 | 2.27 | 0.733 | 24.4 | 2720 | 2380 | 343 | 12.6 |
| 4 | 3.97 | 3.13 | 0.833 | 21.0 | 2820 | 2400 | 429 | 15.2 | |
| 5 | 2.50 | 2.13 | 0.367 | 14.7 | 1390 | 1250 | 139 | 10.0 | |
| Mean for 3 days | 20.0 | 12.6 | |||||||
| 9 | 3 | 2.13 | 1.80 | 0.333 | 15.6 | 2370 | 1840 | 536 | 22.6 |
| 4 | 7.33 | 5.70 | 1.63 | 22.3 | 4460 | 3780 | 672 | 15.1 | |
| 5 | 4.10 | 2.60 | 1.50 | 36.6 | 2950 | 2040 | 911 | 30.9 | |
| Mean for 3 days | 24.8 | 22.9 | |||||||
| 13 | 3 | 4.47 | 2.47 | 2.00 | 44.8 | 7460 | 4670 | 2790 | 37.4 |
| 4 | 7.63 | 4.57 | 3.07 | 40.2 | 7350 | 5090 | 2270 | 30.8 | |
| 5 | 1.83 | 1.20 | 0.63 | 34.5 | 4350 | 3160 | 1190 | 27.4 | |
| Mean for 3 days | 39.8 | 31.9 | |||||||
| Mean for 3 subjects | 28.2 | 22.5 | |||||||
Urine samples were treated with NaBH4 and analyzed for hydroxy acid
Urine samples were analyzed for hydroxy acid without NaBH4 reduction step.
The amount of keto acid was calculated by subtracting hydroxy acid from the sum of keto acid and hydroxy acid.
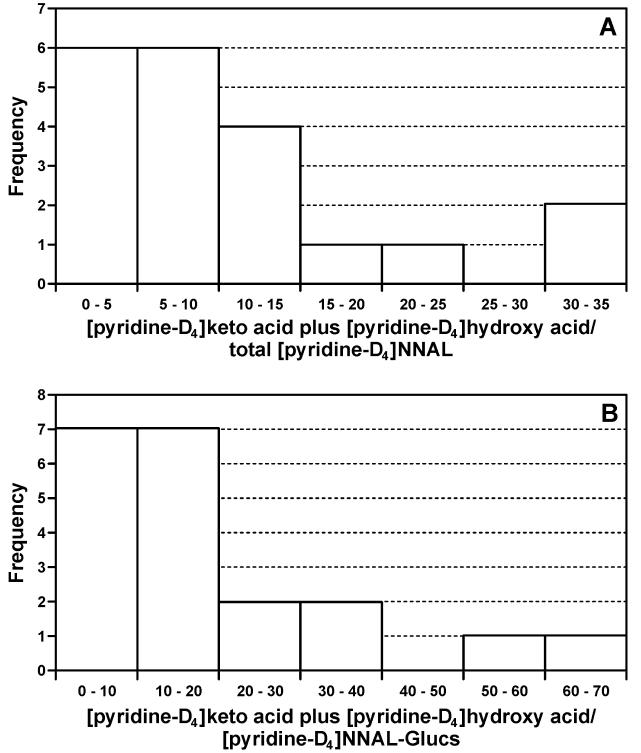
Important ratios between the measured biomarkers are summarized in Table 4. The average ratios of [pyridine-D4]NNAL-Glucs to free [pyridine-D4]NNAL (1.90 ± 0.95) and non-deuterated NNAL-Glucs to free NNAL (2.01 ± 0.91) were similar and were highly correlated in the trial participants (r = 0.72, P<0.001). The ratio of the sum of [pyridine-D4]keto acid plus [pyridine-D4]hydroxy acid to total [pyridine-D4]NNAL ranged from 0.99 to 33.4, averaging 10.8 ± 9.16 (geometric mean, 7.04). The ratio of the sum of deuterated α-hydroxylation biomarkers to the product of [pyridine-D4]NNK detoxification, [pyridine-D4]NNAL-Gluc, varied from 1.35 to 62.8, averaging 18.8 ± 16.8 (geometric mean, 11.7). Frequency plots of these ratios are illustrated in Figure 5.
Table 4. Summary statistics for biomarker ratios.
| Ratio | Average (SD) | Median | Min/Max | Geometric Mean (95% CI) |
|---|---|---|---|---|
| [Pyridine-D4]NNAL-Gluc/free [pyridine-D4]NNAL | 1.90 (0.950) | 1.49 | 0.960/3.89 | 1.65 (1.32, 2.06) |
| NNAL-Gluc/free NNAL | 2.01 (0.910) | 1.59 | 0.860/3.61 | 1.78 (1.44, 2.20) |
| Sum of [pyridine-D4]NNK α-hydroxylation productsa/ total [pyridine-D4]NNAL | 10.8 (9.16) | 7.97 | 0.990/33.4 | 7.04 (4.64, 10.7) |
| Sum of [pyridine-D4]NNK α-hydroxylation products/ [pyridine-D4]NNAL-Glucs | 18.8 (16.8) | 13.0 | 1.35/62.8 | 11.7 (7.47, 18.2) |
[Pyridine-D4]keto acid plus [pyridine-D4]hydroxy acid
Figure 5.
Frequency distributions of the ratios between measured urinary biomarkers in 20 smokers: A, [pyridine-D4]keto acid plus [pyridine-D4]hydroxy acid to total [pyridine-D4]NNAL; B, [pyridine-D4]keto acid plus [pyridine-D4]hydroxy acid to [pyridine-D4]NNAL-Glucs.
DISCUSSION
The purpose of this study was to determine the extent of NNK metabolic activation in smokers, as measured by the sum of the major end products of this pathway, keto acid and hydroxy acid, relative to total NNAL. To specifically identify NNK-derived keto acid and hydroxy acid, which are also metabolites of nicotine, we added [pyridine-D4]NNK to cigarettes that were originally low in NNK, and measured the deuterium-labeled metabolites in the urine of people who smoked these cigarettes. The results demonstrate for the first time that the tobacco-specific lung carcinogen NNK is extensively metabolically activated in smokers.
Metabolic activation of NNK occurs largely via cytochrome P450-catalyzed α-hydroxylation (i.e., hydroxylation of the carbons adjacent to the N-nitroso group), which does not involve modifications in the pyridine ring (1). Therefore, deuterium substitution in the pyridine ring of NNK is not expected to have an effect on NNK metabolic activation or carcinogenicity. Similarly, this substitution is not expected to affect the formation of [pyridine-D4]NNAL via carbonyl reduction of [pyridine-D4]NNK.
The levels of unlabeled total NNAL in the baseline urine were similar to the earlier reported mean total NNAL in 27 smokers (3.14 nmol/24 h, ref.31). After switching to the spiked Quest 1, which is relatively low in unlabeled NNK, urinary excretion of unlabeled total NNAL slightly decreased, however remained higher than that of total [pyridine-D4]NNAL in the same urine samples. Thus, the average (±SD) ratio of unlabeled to labeled total NNAL in the urine collected during days 5-7 of smoking [pyridine-D4]NNK-spiked cigarettes was 5.92 (±3.88) whereas it should have been about 0.4 based on the amounts of NNK and [pyridine-D4]NNK in the spiked cigarettes (Table 1). There could be a number of factors that contributed to the relatively high levels of unlabeled NNAL. First, it is possible that during the trial period, our smokers were compensating for the lack of nicotine in Quest cigarettes by smoking their regular brands in addition to the study cigarettes. Another possibility is that NNAL formed prior to the beginning of the study was still being excreted in the urine of our smokers, and contributed to the total unlabeled NNAL measured during the trial period (31). Exposure to secondhand smoke (SHS) could also contribute to the higher levels of unlabeled NNAL compared to deuterated NNAL. All studies reported to date show significantly higher amounts of total NNAL in the urine of SHS exposed humans than unexposed controls (reviewed in 32), and one recent publication has presented results of a study which demonstrated that additional amounts of NNK can be formed in the SHS (33). Despite the unexpected relative amounts of deuterated and unlabeled NNAL, the ratio of [pyridine-D4]NNAL-Glucs to free [pyridine-D4]NNAL was very close to that of NNAL-Glucs to free NNAL in each urine sample (Table 3), supporting the concept that deuterated and non-deuterated NNK are metabolized in a similar way in humans.
The most important result of this study is the quantitation of the sum of urinary metabolites that resulted from [pyridine-D4]NNK α-hydroxylation pathways - [pyridine-D4]hydroxy acid and [pyridine-D4]keto acid. The average contribution of [pyridine-D4]keto acid to the sum of these metabolites was 28.2% (Table 3), which is consistent with the previously reported extensive conversion of keto acid to hydroxy acid in humans, and 15% keto acid contribution to the total keto acid and hydroxy acid in the urine of smokers (23). The relative amounts of the sum of α-hydroxylation biomarkers and total [pyridine-D4]NNAL observed here (Table 2) indicate that α-hydroxylation is the major pathway of NNK metabolism in smokers. This provides further insights into the overall NNK metabolic profile in human urine and its differences from those observed in studies with laboratory animals. Relative amounts of the urinary biomarkers of NNK major metabolic pathways in mice, rats, primates, and humans are compared in Table 5. Because of differences in routes of administration and doses applied in different studies, these data are not exactly comparable; however, they still provide some useful information. A notable similarity in the urinary NNK metabolic profiles in humans and animals is the prevalence of the products of α-hydroxylation pathways: the sum of keto acid and hydroxy acid accounts for 58, 54, 67.5, and 86% of the urinary excretion of NNK metabolites in mice, rats, primates, and humans, respectively (Table 5). However, the relative contributions of keto acid and hydroxy acid to the total amount of these biomarkers are different. Thus, keto acid accounts for 42, 74, 37, and 28% of total keto acid plus hydroxy acid in mice, rats, primates, and humans, respectively, suggesting that carbonyl reductase activity for keto acid is greater in humans than in the studied animals. This idea is supported by a comparative study of several carbonyl-containing drugs, which demonstrated more efficient ketone reduction in human than in mouse and rat liver (34). The extent of carbonyl reduction, glucuronidation, and pyridine-N-oxidation also varies in rodents, primates, and humans. For instance, neither NNAL nor NNAL-Glucs was detected in the urine of mice or rats treated with low NNK doses (11), indicating that pyridine-N-oxidation predominates over glucuronidation or urinary excretion of NNAL in rodents. Conversely, the results obtained for primates and humans suggest that carbonyl reduction and glucuronidation are more important metabolic pathways than pyridine-N-oxidation, glucuronidation being an important detoxification pathway for NNAL (Table 5).
Table 5. Urinary excretion of NNK metabolites in mice, rats, primates, and humans.
| Metabolic pathway | Metabolite | % of urinary excretion | |||
|---|---|---|---|---|---|
| micea | ratsa | primatesb | humansc | ||
| Carbonyl reduction + glucuronidation | Free NNAL NNAL-O-Gluc NNAL-N-Gluc Sum of NNAL-Glucs Total NNAL |
NDd NAe NA ND ND |
ND NA NA ND ND |
1 NA NA 19.4 20.4 |
2.8 5.1 4.4 9.5 12.3 |
| Pyridine-N-oxidation | NNK-N-oxide NNAL-N-oxide Total N-oxides |
7 6 13 |
14 12 26 |
6.7 4.7 11.4 |
ND 1.7 1.7 |
| α-Hydroxylation | Keto acid Hydroxy acid Total keto acid plus hydroxy acid |
23 35 58 |
40 14 54 |
25.1 42.4 67.5 |
24 62 86 |
Data for urinary metabolites excreted at low NNK doses (11)
From ref. 9
Combined data from ref. 13, 17, and the current study (based on geometric means for deuterated metabolites), and assuming that there are no other NNK metabolic pathways contributing to urinary metabolites.
ND, not detected
NA, not analyzed
Our finding that total NNAL is present in human urine in relatively low amounts as compared to NNK-derived keto acid and hydroxy acid does not diminish the importance of total NNAL as a biomarker of human NNK uptake. Most investigations to date demonstrate a significant correlation between total NNAL and cotinine in human urine (1,18,35-37). Cotinine, a major nicotine metabolite in humans, is widely used as a biomarker of human nicotine uptake, even though its urinary levels account for only 10-15% of the nicotine dose (38). We also have demonstrated a significant positive correlation between cigarettes per day and cotinine, and between cigarettes per day and NNAL in the urine of smokers (39). Therefore, total NNAL can serve as an indicator of NNK uptake, just as cotinine serves as an indicator of nicotine uptake from tobacco exposure.
Considering the 23% transfer rate of NNK from tobacco to cigarette smoke reported elsewhere (40) and an average of 28 spiked cigarettes smoked per day, our smokers were exposed to about 9.6 nmol [pyridine-D4]NNK per day. Thus, the average amount of total [pyridine-D4]NNAL excreted by the study participants over the 24-h period accounts for approximately 5%, while the average amount of [pyridine-D4]hydroxy acid accounts for about 42%, of the estimated average [pyridine-D4]NNK exposure. Calculations based on the geometric means for the corresponding biomarkers (Table 2) change these numbers to 4 and 30%, respectively. These calculations, even though quite approximate and potentially greatly affected by the misreported number of spiked cigarettes smoked by the study participants, as well as by the individual patterns of smoking, show a reasonably consistent relationship between the estimated dose of [pyridine-D4]NNK and the amounts of deuterated metabolites detected in the urine of our subjects.
The more than 10-fold variation in the levels of the sum of [pyridine-D4] keto acid plus [pyridine-D4]hydroxy acid, more than 30-fold variation in the [pyridine-D4]keto acid plus [pyridine-D4]hydroxy acid/total [pyridine-D4]NNAL ratio, and more than 45-fold variation in the [pyridine-D4]keto acid plus [pyridine-D4]hydroxy acid/[pyridine-D4]NNAL-Glucs ratio among the 20 smokers strongly support our hypothesis that some smokers activate NNK more extensively than others, and that the ratio between biomarkers of metabolic activation and detoxification at a given dose of NNK could be a potential indicator of cancer risk. We are planning studies in which these smokers could be identified by smoking cigarettes to which [pyridine-D4]NNK has been added.
In summary, the results of this study demonstrate for the first time that NNK metabolic activation is a quantitatively significant pathway in smokers, accounting for about 86% of total urinary excretion of NNK metabolites. In the future, [pyridine-D4]NNK could be used in a biomarker strategy to identify smokers who activate NNK more efficiently, and, therefore, might be at an increased risk of developing lung cancer.
Acknowledgements
We thank David Wen and Bradley Lieberman for technical assistance, Dr. Peter W. Villalta for help with mass spectrometry techniques, Siyi Zhang for help with NMR analysis, Brad Hochalter for valuable advice in the method development, and Bruce Lindgren for statistical analyses. We also thank Bob Carlson for editorial assistance.
Grant support: This study was supported by grant no. CA-81301 from the National Cancer Institute.
References
- 1.Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol. 1998;11(6):560–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- 2.Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1999;91:1194–210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 3.Pfeifer GP, Denissenko MF, Oliver M, et al. Tobacco smoke carcinogens, DNA damage, and p53 mutations in smoking-related cancers. Oncogene. 2002;21:7435–51. doi: 10.1038/sj.onc.1205803. [DOI] [PubMed] [Google Scholar]
- 4.Hecht SS. Tobacco carcinogens, their biomarkers, and tobacco-induced cancer. Nat Rev Cancer. 2003;3:733–44. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 5.Tjalve H, Castonguay A. The in vivo tissue disposition and in vitro target-tissue metabolism of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in Syrian golden hamsters. Carcinogenesis. 1983;4:1259–65. doi: 10.1093/carcin/4.10.1259. [DOI] [PubMed] [Google Scholar]
- 6.Castonguay A, Tjalve H, Trushin N, Hecht SS. Perinatal metabolism of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in C57B1 mice. J Natl Cancer Inst. 1984;72:1117–26. [PubMed] [Google Scholar]
- 7.Staretz ME, Hecht SS. Effects of phenethyl isothiocyanate on the tissue distribution of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and metabolites in F344 rats. Cancer Res. 1995;55:5580–8. [PubMed] [Google Scholar]
- 8.Castonguay A, Tjalve H, Trushin N, et al. Metabolism and tissue distribution of tobacco-specific N-nitrosamines in the marmoset monkey (Callithrix jacchus) Carcinogenesis. 1985;6:1543–50. doi: 10.1093/carcin/6.11.1543. [DOI] [PubMed] [Google Scholar]
- 9.Hecht SS, Trushin N, Reid-Quinn CA, et al. Metabolism of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in the Patas monkey: pharmacokinetics and characterization of glucuronide metabolites. Carcinogenesis. 1993;14:229–36. doi: 10.1093/carcin/14.2.229. [DOI] [PubMed] [Google Scholar]
- 10.Adams JD, LaVoie EJ, Hoffmann D. On the pharmacokinetics of the tobacco-specific N-nitrosamines in Fisher rats. Carcinogenesis. 1985;6:509–511. doi: 10.1093/carcin/6.4.509. [DOI] [PubMed] [Google Scholar]
- 11.Morse MA, Eklind KI, Toussaint M, et al. Characterization of a glucuronide metabolite of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and its dose-dependent excretion in the urine of mice and rats. Carcinogenesis. 1990;11:1819–23. doi: 10.1093/carcin/11.10.1819. [DOI] [PubMed] [Google Scholar]
- 12.Adams JD, LaVoie EJ, O’Mara-Adams KJ, et al. Pharmacokinetics of N’-nitrosonornicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in laboratory animals. Cancer Lett. 1985;28:195–201. doi: 10.1016/0304-3835(85)90075-8. [DOI] [PubMed] [Google Scholar]
- 13.Carmella SG, Borukhova A, Akerkar SA, Hecht SS. Analysis of human urine for pyridine-N-oxide metabolites of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, a tobacco-specific lung carcinogen. Cancer Epidemiol Biomarkers Prev. 1997;6:113–20. [PubMed] [Google Scholar]
- 14.Castonguay A, Lin D, Stoner GD, et al. Comparative carcinogenicity in A/J mice and metabolism by cultured mouse peripheral lung of N’-nitrosonornicotine, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and their analogues. Cancer Res. 1983;43:1223–9. [PubMed] [Google Scholar]
- 15.Hecht SS, Jordan KG, Choi C-I, Trushin N. Effects of deuterium substitution on the tumorigenicity of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in A/J mice. Carcinogenesis. 1990;11:1017–20. doi: 10.1093/carcin/11.6.1017. [DOI] [PubMed] [Google Scholar]
- 16.Rivenson A, Hoffmann D, Procopszyk B, et al. Induction of lung and exocrine pancreas tumors in F344 rats by tobacco-specific and Areca-derived N-nitrosamines. Cancer Res. 1988;48:6912–7. [PubMed] [Google Scholar]
- 17.Carmella SG, Le KA, Upadhyaya P, Hecht SS. Analysis of N- and O- glucuronides of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in human urine. Chem Res Toxicol. 2002;15:545–50. doi: 10.1021/tx015584c. [DOI] [PubMed] [Google Scholar]
- 18.Carmella SG, Akerkar S, Richie JP, Jr, Hecht SS. Intraindividual and interindividual differences in the metabolites of the tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in smokers’ urine. Cancer Epidemiol Biomarkers Prev. 1995;4:635–42. [PubMed] [Google Scholar]
- 19.Hecht SS, Young R, Chen CB. Metabolism in the F344 rat of 4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone, a tobacco specific carcinogen. Cancer Res. 1980;40:4144–50. [PubMed] [Google Scholar]
- 20.Desai D, Kagan SS, Amin S, et al. Identification of 4-(methylnitrosamino)-1-[3-(6-hydroxypyridyl)]-1-butanone as a urinary metabolite of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in the rat. Chem Res Toxicol. 1993;6:794–9. doi: 10.1021/tx00036a007. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann D, Casonguay A, Rivenson A, Hecht SS. Comparative carcinogenicity and metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and N’-nitrosonornicotine in Syrian golden hamsters. Cancer Res. 1981;41:2386–93. [PubMed] [Google Scholar]
- 22.Trushin N, Hecht SS. Stereoselective metabolism of nicotine and tobacco-specific N-nitrosamines to 4-hydroxy-4-(3-pyridyl)butanoic acid in rats. Chem Res Toxicol. 1999;12:164–171. doi: 10.1021/tx980213q. [DOI] [PubMed] [Google Scholar]
- 23.Hecht SS, Hatsukami DK, Bonilla LE, Hochalter JB. Quantitation of 4-oxo-4-(3-pyridyl)butanoic acid and enantiomers of 4-hydroxy-4-(3-pyridyl)butanoic acid in human urine: a substantial pathway of nicotine metabolism. Chem Res Toxicol. 1999;12:172–9. doi: 10.1021/tx980214i. [DOI] [PubMed] [Google Scholar]
- 24.Stepanov I, Jensen J, Hatsukami D, Hecht SS. Tobacco-specific nitrosamines in new tobacco products. Nicotine Tobacco Res. 2006;8(2):309–13. doi: 10.1080/14622200500490151. [DOI] [PubMed] [Google Scholar]
- 25.Amin S, Desai D, Hecht SS, Hoffmann D. Synthesis of tobacco-specific N-nitrosamines and their metabolites and results of related bioassays. Crit. Rev Toxicol. 1996;26:139–47. doi: 10.3109/10408449609017927. [DOI] [PubMed] [Google Scholar]
- 26.Stepanov I, Carmella SG, Hecht SS, Duca G. Analysis of tobacco-specific nitrosamines in Moldovan cigarette tobacco. J Agric Food Chem. 2002;59:2793–7. doi: 10.1021/jf011552j. [DOI] [PubMed] [Google Scholar]
- 27.Stepanov I, Feuer R, Jensen J, et al. Mass spectrometric quantitation of nicotine, cotinine, and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in human toenails. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2378–83. doi: 10.1158/1055-9965.EPI-06-0265. [DOI] [PubMed] [Google Scholar]
- 28.Hecht SS, Lin D, Castonguay A. Effects of α-deuterium substitution on the mutagenicity of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) Carcinogenesis. 1983;4:305–10. doi: 10.1093/carcin/4.3.305. [DOI] [PubMed] [Google Scholar]
- 29.McKennis H, Schwartz SL, Turnbull LB, et al. The metabolic formation of γ-(3-pyridyl)-γ-hydroxybutiric acid and its possible intermediary role in the mammalian metabolism of nicotine. J Biol Chem. 1964;239:3981–9. [PubMed] [Google Scholar]
- 30.Hecht SS, Chen CB, Dong M, et al. Studies on non-volatile nitrosamines in tobacco. Beiträge zur Tabakforschung. 1997;9:1–6. [Google Scholar]
- 31.Hecht SS, Carmella SG, Chen M, et al. Quantitation of urinary metabolites of a tobacco-specific lung carcinogen after smoking cessation. Cancer Res. 1999;59:590–6. [PubMed] [Google Scholar]
- 32.Hecht SS. Carcinogen derived biomarkers: applications in studies of human exposure to secondhand tobacco smoke. Tobacco Control. 2003;13:i48–i56. doi: 10.1136/tc.2002.002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schick SF, Glantz S. Concentrations of the carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in sidestream cigarette smoke increase after release into indoor air: results from unpublished tobacco industry research. Cancer Epidemiol Biomarkers Prev. 2007;16(8):1547–53. doi: 10.1158/1055-9965.EPI-07-0210. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed NK, Felsted RL, Bachur NR. Comparison and characterization of mammalian xenobiotic ketone reductases. J Pharmacol Exp Ther. 1979;209:12–9. [PubMed] [Google Scholar]
- 35.Hecht SS. Human urinary carcinogen metabolites: biomarkers for investigating tobacco and cancer. Carcinogenesis. 2002;23(6):907–22. doi: 10.1093/carcin/23.6.907. [DOI] [PubMed] [Google Scholar]
- 36.Hecht SS, Carmella SG, Murphy SE, et al. A tobacco-specific lung carcinogen in the urine of men exposed to cigarette smoke. New Engl J Med. 1993;329:1543–6. doi: 10.1056/NEJM199311183292105. [DOI] [PubMed] [Google Scholar]
- 37.Murphy SE, Carmella SG, Idris AM, Hoffmann D. Uptake and metabolism of carcinogenic levels of tobacco-specific nitrosamines by Sudanese snuff dippers. Cancer Epidemiol Biomarkers Prev. 1994;3:423–8. [PubMed] [Google Scholar]
- 38.Benowitz NL, Jacob P., 3rd. Metabolism of nicotine to cotinine studied by a dual stable isotope method. Clin Pharmacol Ther. 1994;56:483–93. doi: 10.1038/clpt.1994.169. [DOI] [PubMed] [Google Scholar]
- 39.Joseph AM, Hecht SS, Murphy SE, et al. Relationship between cigarette consumption and biomarkers of tobacco toxin exposure. Cancer Epidemiol Biomarkers Prev. 2005;14(12):2963–8. doi: 10.1158/1055-9965.EPI-04-0768. [DOI] [PubMed] [Google Scholar]
- 40.Fischer S, Spiegelhalder B, Eisenbarth J, Preussmann R. Investigations on the origin of tobacco-specific nitrosamines in mainstream smoke of cigarettes. Carcinogenesis. 1990;11(5):723–30. doi: 10.1093/carcin/11.5.723. [DOI] [PubMed] [Google Scholar]