Abstract
The human papillomavirus type 16 (HPV-16) E7 oncoprotein rapidly induces centrosome duplication errors in primary human cells, thereby increasing the propensity for multipolar mitoses, which can lead to chromosome missegregation and aneuploidy. We analyzed a series of HPV-16 E7 mutants and demonstrate that this biological activity of the E7 oncoprotein is mediated by sequences encompassing the core pRB binding site but is independent of its ability to inactivate the retinoblastoma tumor suppressor protein pRB and the related pocket proteins p107 and p130. In addition, interaction of E7 with the S4 subunit of the 26S proteasome and dysregulation of cdc25A transcription are also dispensable for the induction of centrosome duplication errors. Consistent with these results, expression of HPV-16 E7 induces abnormal centrosome duplication in a cell line that lacks functional pRB and in mouse embryo fibroblasts that are deficient for pRB, p107, and p130. These results demonstrate that the molecular mechanism whereby HPV-16 E7 induces centrosome duplication errors is independent of its ability to inactivate pRB, p107, and p130 or to interact with the S4 proteasome subunit.
Infection with “high-risk” human papillomaviruses (HPVs) is associated with anogenital neoplasia—in particular, cervical cancer (reviewed in reference 53). During malignant progression, the HPV genome is frequently integrated into host cell chromosomes, and as a consequence only the viral oncoproteins E6 and E7 are consistently retained and expressed in cervical cancers (reviewed in reference 32). HPV E6 and E7 exert their transforming activities by subverting critical antiproliferative control mechanisms. By binding and destabilizing the retinoblastoma tumor suppressor protein (pRB) and the related pocket proteins p107 and p130 (14, 18), the HPV type 16 (HPV-16) oncoprotein E7 thwarts E2F-mediated transcriptional repressor functions that normally restrain G1/S cell cycle progression (3, 15). In addition, HPV-16 E7 can interfere with p21Cip1- and p27Kip1-mediated inhibition of cdk2 activity (17, 24, 40, 52). HPV-16 E7 also dysregulates cdc25A expression (35), and the Drosophila cdc25 homologue string is necessary for completion of daughter centriole assembly (48). Aberrant expression of cyclin E and cyclin A and abnormal patterns of cdk2 activity have been detected in HPV-16 E7-expressing cells (29, 40, 43, 51). The cooperating HPV-16 oncoprotein E6 induces accelerated proteasomal degradation of the p53 tumor suppressor (42), thereby bypassing p53-induced antiproliferative cellular defense responses in reaction to unscheduled proliferative signals triggered by expression of the HPV E7 oncoprotein. Since HPVs replicate their genomes in terminally differentiated keratinocytes, the transforming activities of E6 and E7 likely reflect their functions during the viral life cycle to induce and/or maintain a replication-competent cellular milieu in these normally growth-arrested host epithelial cells. Coexpression of HPV E6 or E7 extends the life span of primary human keratinocytes and facilitates cellular immortalization (20, 33). High-risk HPV-immortalized keratinocytes are nontumorigenic at low passage numbers but can undergo malignant conversion upon long-term passaging or exposure to additional carcinogens (11, 23). Similarly, progression of high-risk HPV induced premalignant lesions to invasive cervical cancers in vivo occurs relatively infrequently and is associated with additional alterations of the host cell genome (reviewed in reference 27). In vitro, high-risk HPV E6- and E7-expressing cell populations are genomically unstable and prone to develop distinct chromosomal alterations when selected for resistance to N-(phosphonoacetyl)-l-aspartate (PALA). HPV-16 E6-expressing cells acquire PALA resistance by amplifying the chromosomal region harboring the resistance locus. High-risk HPV E7-expressing cells become resistant by gaining additional copies of the entire chromosome that encodes the PALA resistance element and develop aneuploidy (49). This result is consistent with an earlier report demonstrating that expression of the HPV E7 oncoprotein leads to chromosome segregation defects (19). We have previously shown that mitotic infidelity in HPV E7-expressing host cells is increased by formation of multipolar mitotic spindles (13). Multipolar mitoses in cervical lesions have long been recognized as histomorphological hallmarks of high-risk HPV infection (7). Expression of high-risk HPV E7 induces centrosome duplication errors by uncoupling centrosome duplication from the cell division cycle (13). In contrast, centrosome abnormalities in high-risk HPV E6-expressing cells accumulate in parallel with other morphological signs of genomic instability and defective cytokinesis (12). Centrosomes are the major microtubule-organizing centers in animal and human cells and contribute importantly to mitotic spindle formation and function (reviewed in reference 47). In order to ensure bipolarity of cell division, the single centrosome of a G1 cell duplicates precisely once prior to mitosis in intimate synchrony with the cell division cycle (22), followed by an intrinsic block of rereplication (50). Centrosome abnormalities have been detected in a variety of human tumors (39), including HPV-associated premalignant cervical lesions and cancers (13, 45), and are believed to contribute to mitotic defects and aneuploidy, the most common form of chromosomal instability in human tumors (41).
Inactivation of pRB is not required for HPV-16 E7-induced abnormal centrosome duplication.
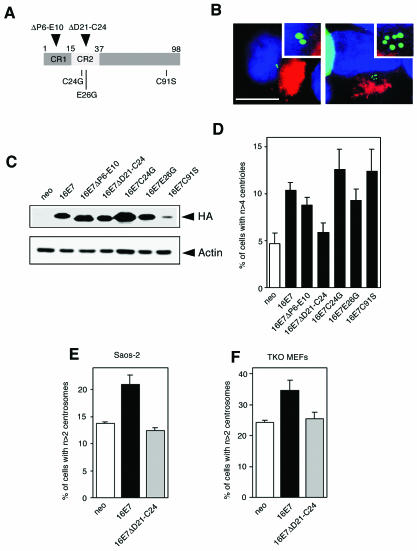
It was previously shown that the HPV-16 E7 ΔD21-C24 mutant, which contains a deletion of the core pRB binding site within the conserved region 2 homology domain and is defective for pocket protein binding (34, 37) and degradation (18, 25), is unable to induce aberrant centrosome duplication (13). To analyze in more detail whether HPV-16 E7-induced abnormal centrosome duplication and interaction with pRB may be linked, we tested several additional HPV-16 E7 mutants (Fig. 1A). The transformation-deficient HPV-16 E7 ΔP6-E10 mutant lacks a segment within the conserved region 1 homology domain and is pocket protein binding competent (34, 37) but defective for inducing pocket protein degradation (18, 25). The HPV-16 E7 C24G and E26G mutants are defective for pRB binding and degradation but retain the ability to bind and destabilize p107 (9, 10, 18). Since centrosome duplication is dependent on proteasome-mediated protein degradation (16), we also tested the HPV-16 E7 C91S mutant, which retains the ability to bind and degrade pocket proteins (18) but is defective for binding to the S4 subunit of the 26S proteasome (2) (Fig. 1A).
FIG. 1.
(A) Schematic representation of HPV-16 E7 mutants used in this study. CR, conserved region. (B) Visualization of centrioles in U-2 OS cells stably expressing centrin-GFP and transiently transfected with empty vector (left) or HPV-16 E7 (right). Mitochondrial DsRED was used as transfection marker. Nuclei were stained with DAPI. Bar, 10 μm. (C) Immunoblot detection of hemagglutinin-tagged full-length and mutant HPV-16 E7 oncoproteins. The actin immunoblot is shown as a loading control. (D) Quantitation of the proportion of U-2 OS/centrin-GFP cells exhibiting abnormal centriole numbers at 48 h after transfection with either empty vector (neo), wild-type HPV-16 E7 or E7 mutants. Each bar shows the average plus the standard error for at least three independent experiments. (E) Quantitation of the proportion of Saos-2 cells exhibiting abnormal centrosome numbers at 48 h after transfection with either empty vector (neo), wild-type HPV-16 E7, or the HPV-16 E7 ΔD21-C24 mutant. Each bar shows the average plus the standard error for at least three independent experiments. (F) Quantitation of the proportion of TKO MEFs exhibiting abnormal centrosome numbers at 48 h after transfection with either empty vector (neo), wild-type HPV-16 E7, or the HPV-16 E7 ΔD21-C24 mutant. Each bar shows the average plus the standard error for at least three independent experiments.
Effects on centrosome duplication were assessed by using a population of the p53- and pRB-positive human osteosarcoma cell line U-2 OS which was engineered to stably express centrin-green fluorescent protein (GFP) (kindly provided by M. Bornens, Institut Curie, Paris, France) (36). In these U-2 OS/centrin-GFP cells, individual centrioles are readily detectable, allowing accurate determination of the centriole and centrosome duplication status (12) (Fig. 1B). Cells grown in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum, 50 U of penicillin per ml, and 50 μg of streptomycin per ml were transiently transfected by calcium phosphate coprecipitation (4) with 10 μg of pCMVBamH1-Neo-based plasmids (1) carrying wild-type or mutant HPV-16 E7 genes tagged with a hemagglutinin epitope at their carboxyl termini (18). One microgram of a mitochondrial DsRED fluorescent protein-encoding vector (Clontech) was cotransfected as a transfection marker. Cells were harvested at 48 h posttransfection, and expression of wild-type and mutant HPV-16 E7 proteins was assessed by immunoblot analysis as previously described (24) (Fig. 1C). Cells grown on coverslips were analyzed for centriole numbers. Briefly, cells were fixed in 4% paraformaldehyde for 5 min followed by permeabilization in phosphate-buffered saline (PBS) containing 1% Triton X-100 for 5 min at room temperature. After a final rinse with PBS, nuclei were counterstained with 4′,6′-diamidino-2-phenylindole (DAPI). Transfected cells were visually selected by mitochondrial DsRED expression, and the number of GFP-labeled centrioles was analyzed by epifluorescence microscopy. At least 100 cells each from three independent experiments were assessed, and cells containing more than four centrioles were counted as abnormal. Similar to results in a previous report (12), the background level of U-2 OS/centrin-GFP with abnormal centriole numbers was 4.7%. Also consistent with earlier results (12), expression of the wild-type HPV-16 E7 oncoprotein caused a 2.2-fold increase in the proportion of cells with abnormal centriole numbers (10.4%, versus 4.7% in empty-vector controls; P ≤ 0.005) (Fig. 1B and D). As expected (13), expression of the pocket protein binding site mutant HPV-16 E7 ΔD21-C24 did not induce centriole duplication errors (5.9 versus 4.7% in controls; P > 0.05). In contrast, expression of the pocket protein binding-competent, degradation-defective HPV-16 E7 ΔP6-E10 mutant caused a 1.9-fold increase in cells with supernumerary centrioles (8.8%, versus 4.7% in controls; P ≤ 0.05). Expression of the pRB binding- and degradation-deficient, p107 binding- and degradation-competent HPV-16 E7 C24G and E26G mutants caused significant 2.7- and 2-fold increases, respectively, in cells with centriole duplication errors (12.6 and 9.3%, respectively, versus 4.7% in controls; P ≤ 0.05). Since the HPV-16 E7 C24G mutant is defective for dysregulating cdc25A transcription (35), this activity of E7 is not connected to induction of centrosome abnormalities. Even though it is expressed at reduced levels (Fig. 1C), the S4 proteasome subunit binding-deficient HPV-16 E7 C91S mutant was also able to induce a 2.6-fold increase in centrosome duplication errors (12.4%, versus 4.7% in controls; P ≤ 0.05). These results demonstrate that binding and/or degradation of pRB, interaction with the S4 subunit of the 26S proteasome, and dysregulation of cdc25A expression by E7 are not necessary for the induction of centrosome duplication errors.
HPV-16 E7 induces centrosome duplication errors in cells that lack pRB function.
To confirm that HPV-16 E7-induced centrosome duplication errors are independent of pRB inactivation, we transiently expressed wild-type HPV-16 E7 and the pocket protein binding- and degradation-deficient HPV-16 E7 ΔD21-C24 mutant in the human osteosarcoma cell line Saos-2, which lacks functional pRB and p53 (5, 44) but expresses p107 and p130 at levels similar to those in pRB-expressing cells (6) (Fig. 1E). Cells were cultured and transiently transfected as described above and processed for immunofluorescence microscopy for the pericentriolar marker γ-tubulin (46) at 48 h after transfection. Briefly, cells grown on coverslips were fixed in 4% paraformaldehyde for 15 min and permeabilized with 1% Triton X-100 in PBS for 15 min at room temperature. Cells were blocked by using 10% normal donkey serum and incubated with a 1:2,000 dilution of a monoclonal anti-γ-tubulin antibody (GTU-88; Sigma) overnight at 4°C. Cells were then incubated with a rhodamine red-labeled donkey anti-mouse immunoglobulin G secondary antibody (Jackson Immunoresearch) for 2 h at 37°C, and nuclei were counterstained with DAPI. Cells containing more than two centrosomes were counted in three independent experiments. Although the background level of centrosome abnormalities in Saos-2 cells was higher than in U-2 OS cells (compare Fig. 1D and E), expression of HPV-16 E7 in Saos-2 cells still resulted in a 1.5-fold increase of the proportion of cells showing abnormal centrosome numbers (20.9%, versus 13.7% in vector-transfected controls; P ≤ 0.01) (Fig. 1E). In contrast, expression of the HPV-16 E7 ΔD21-C24 mutant did not cause an increase of numerical centrosome abnormalities. Even though the relative increase of centrosome abnormalities in response to E7 expression was lower in the pRB-deficient Saos-2 cells (1.5-fold) than in the pRB-positive U-2OS cells (2.2-fold), in combination with the mutational analysis (Fig. 1D), these results demonstrate that the ability of HPV-16 E7 to induce abnormal centrosome numbers does not strictly depend on pRB inactivation.
HPV-16 E7 induces centrosome duplication errors in TKO MEFs.
Since our mutational analyses indicated that the HPV-16 E7 C24G and E26G mutants that retain the ability to bind and degrade p107 also remain competent for induction of numerical centrosome abnormalities (Fig. 1D), we next determined whether the capacity of E7 to inactivate p107 and p130 might be linked to induction of centrosome duplication errors. Hence, we analyzed the ability of HPV-16 E7 to induce numerical centrosome abnormalities in cells that are deficient for all three pRB family members. Mouse embryo fibroblasts (MEFs) in which pRB, p107, and p130 had been genetically deleted (i.e., triple-knockout [TKO] MEFs; kindly provided by H. te Riele, The Netherlands Cancer Institute, Amsterdam, The Netherlands) (8) were grown in Dulbecco's modified Eagle medium containing 10% fetal calf serum, 50 U of penicillin per ml, and 50 μg of streptomycin per ml. Early-passage cells were transiently transfected with 2 μg of E7 expression plasmid DNA by using Lipofectamine (Invitrogen). Cells were cotransfected with 0.2 μg of a DsRED-encoding plasmid as a transfection marker. Immunofluorescence staining for γ-tubulin was performed at 48 h posttransfection as described above except that a fluorescein isothiocyanate-conjugated donkey anti-mouse immunoglobulin secondary antibody (Jackson Immunoresearch) was used at a 1:100 dilution. Despite a high level of centrosome abnormalities in vector-transfected control TKO MEFs, transient transfection of wild-type HPV-16 E7 resulted in a 1.4-fold increase of the proportion of cells with abnormal centrosome numbers (34.6%, versus 24.4% in controls; P ≤ 0.05). Interestingly, however, expression of the HPV-16 E7 ΔD21-C24 mutant did not induce an increase in centrosome abnormalities (Fig. 1F). Hence, expression of the HPV-16 E7 oncoprotein can induce centrosome duplication errors in cells that do not contain any pocket proteins. We hasten to add, however, that the ability of HPV-16 E7 to induce abnormal centrosome numbers was somewhat less pronounced in the pRB-negative Saos-2 cells and TKO MEFs than in pRB-positive U-2 OS cells (1.5-fold in Saos-2 cells and 1.4-fold in TKO MEFs versus 2.2-fold in U-2 OS/centrin-GFP cells) and that Saos-2 cells and TKO MEFs already contain a high background level of centrosome abnormalities (Fig. 1E and F). Hence, our findings may indicate that even though HPV-16 E7-induced abnormal centrosome duplication is independent of pocket protein inactivation per se, degradation of these negative growth regulators by E7 may contribute to the ability to induce centrosome duplication errors. Nevertheless, these results are consistent with an earlier study demonstrating that inactivation of pRB by constitutive hyperphosphorylation is not sufficient to yield numerical centrosome abnormalities (38). Importantly, however, our results demonstrate that the HPV-16 E7 ΔD21-C24 mutant is unable to induce centrosome duplication errors in pocket protein-deficient cells and hence that this E7 domain mediates activities other than pocket protein binding. Centrosome duplication is intimately linked to the cell division cycle, at least in part through cyclin/cdk2 activity (22), and dysregulation of cyclin/cdk2 activity has been demonstrated to cause aberrant rounds of centrosome duplication in various model systems (21, 26, 30, 31). In addition to increasing cyclin A and E expression through pocket protein inactivation, HPV-16 E7 can interact with and inactivate Cdk inhibitors (17, 24, 40, 52), including p21Cip1. In our hands, the ability of E7 to inactivate p21Cip1 was dependent on the integrity of amino acids D21 to C24 (24). Since ectopic expression of p21Cip1 (as well as p27Kip1) can block centrosome duplication (26) and loss of p21Cip1 results in abnormal centrosome numbers (28), our results are consistent with the model that inactivation of p21Cip1 and/or p27Kip1 by HPV-16 E7 may importantly contribute to the ability of HPV-16 E7 to induce centrosome duplication errors.
Acknowledgments
We are grateful to M. Bornens and H. te Riele for sharing reagents.
This work was supported by PHS grant CA66980 to K.M. S.D. was supported by a research fellowship from the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Baker, S. J., S. Markowitz, E. R. Fearon, J. K. Willson, and B. Vogelstein. 1990. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science 249:912-915. [DOI] [PubMed] [Google Scholar]
- 2.Berezutskaya, E., and S. Bagchi. 1997. The human papillomavirus E7 oncoprotein functionally interacts with the S4 subunit of the 26 S proteasome. J. Biol. Chem. 272:30135-30140. [DOI] [PubMed] [Google Scholar]
- 3.Boyer, S. N., D. E. Wazer, and V. Band. 1996. E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway. Cancer Res. 56:4620-4624. [PubMed] [Google Scholar]
- 4.Chen, C., and H. Okayama. 1987. High-efficiency transformation of mammalian cells by plasmid DNA. Mol. Cell. Biol. 7:2745-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, P. L., Y. M. Chen, R. Bookstein, and W. H. Lee. 1990. Genetic mechanisms of tumor suppression by the human p53 gene. Science 250:1576-1580. [DOI] [PubMed] [Google Scholar]
- 6.Cheng, L., F. Rossi, W. Fang, T. Mori, and D. Cobrinik. 2000. Cdk2-dependent phosphorylation and functional inactivation of the pRB-related p130 protein in pRB(−), p16INK4A(+) tumor cells. J. Biol. Chem. 275:30317-30325. [DOI] [PubMed] [Google Scholar]
- 7.Crum, C. P., H. Ikenberg, R. M. Richart, and L. Gissman. 1984. Human papillomavirus type 16 and early cervical neoplasia. N. Engl. J. Med. 310:880-883. [DOI] [PubMed] [Google Scholar]
- 8.Dannenberg, J.-H., A. van Rossum, L. Schuijff, and H. te Riele. 2000. Ablation of the retinoblastoma gene family deregulates G1 control causing immortalization and increased cell turnover under growth-restricting conditions. Genes Dev. 14:3051-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies, R., R. Hicks, T. Crook, J. Morris, and K. Vousden. 1993. Human papillomavirus type 16 E7 associates with a histone H1 kinase and with p107 through sequences necessary for transformation. J. Virol. 67:2521-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demers, G. W., E. Espling, J. B. Harry, B. G. Etscheid, and D. A. Galloway. 1996. Abrogation of growth arrest signals by human papillomavirus type 16 E7 is mediated by sequences required for transformation. J. Virol. 70:6862-6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiPaolo, J. A., C. D. Woodworth, N. C. Popescu, V. Notario, and J. Doninger. 1989. Induction of human cervical squamous cell carcinoma by sequential transfection with human papillomavirus 16 DNA and viral Harvey ras. Oncogene 4:395-399. [PubMed] [Google Scholar]
- 12.Duensing, S., A. Duensing, C. P. Crum, and K. Munger. 2001. Human papillomavirus type 16 E7 oncoprotein-induced abnormal centrosome synthesis is an early event in the evolving malignant phenotype. Cancer Res. 61:2356-2360. [PubMed] [Google Scholar]
- 13.Duensing, S., L. Y. Lee, A. Duensing, J. Basile, S. Piboonniyom, S. Gonzalez, C. P. Crum, and K. Munger. 2000. The human papillomavirus type 16 E6 and E7 oncoproteins cooperate to induce mitotic defects and genomic instability by uncoupling centrosome duplication from the cell division cycle. Proc. Natl. Acad. Sci. USA 97:10002-10007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dyson, N., P. Guida, K. Münger, and E. Harlow. 1992. Homologous sequences in adenovirus E1A and human papillomavirus E7 proteins mediate interaction with the same set of cellular proteins. J. Virol. 66:6893-6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dyson, N., P. M. Howley, K. Munger, and E. Harlow. 1989. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 243:934-937. [DOI] [PubMed] [Google Scholar]
- 16.Freed, E., K. R. Lacey, P. Huie, S. A. Lyapina, R. J. Deshaies, T. Stearns, and P. K. Jackson. 1999. Components of an SCF ubiquitin ligase localize to the centrosome and regulate the centrosome duplication cycle. Genes Dev. 13:2242-2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Funk, J. O., S. Waga, J. B. Harry, E. Espling, B. Stillman, and D. A. Galloway. 1997. Inhibition of CDK activity and PCNA-dependent DNA replication by p21 is blocked by interaction with the HPV-16 E7 oncoprotein. Genes Dev. 11:2090-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez, S. L., M. Stremlau, X. He, J. R. Basile, and K. Münger. 2001. Degradation of the retinoblastoma tumor suppressor by the human papillomavirus type 16 E7 oncoprotein is important for functional inactivation and is separable from proteasomal degradation of E7. J. Virol. 75:7583-7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashida, T., and S. Yasumoto. 1991. Induction of chromosome abnormalities in mouse and human epidermal keratinocytes by the human papillomavirus type 16 E7 oncogene. J. Gen. Virol. 72:1569-1577. [DOI] [PubMed] [Google Scholar]
- 20.Hawley-Nelson, P., K. H. Vousden, N. L. Hubbert, D. R. Lowy, and J. T. Schiller. 1989. HPV16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. EMBO J. 8:3905-3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hinchcliffe, E. H., C. Li, E. A. Thompson, J. L. Maller, and G. Sluder. 1999. Requirement of Cdk2-cyclin E activity for repeated centrosome reproduction in Xenopus egg extracts. Science 283:851-854. [DOI] [PubMed] [Google Scholar]
- 22.Hinchcliffe, E. H., and G. Sluder. 2001. “It takes two to tango”: understanding how centrosome duplication is regulated throughout the cell cycle. Genes Dev. 15:1167-1181. [DOI] [PubMed] [Google Scholar]
- 23.Hurlin, P. J., P. Kaur, P. P. Smith, N. Perez-Reyes, R. A. Blanton, and J. K. McDougall. 1991. Progression of human papillomavirus type 18-immortalized human keratinocytes to a malignant phenotype. Proc. Natl. Acad. Sci. USA 88:570-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones, D. L., R. M. Alani, and K. Munger. 1997. The human papillomavirus E7 oncoprotein can uncouple cellular differentiation and proliferation in human keratinocytes by abrogating p21Cip1-mediated inhibition of cdk2. Genes Dev. 11:2101-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones, D. L., D. A. Thompson, and K. Münger. 1997. Destabilization of the RB tumor suppressor and stabilization of p53 contribute to HPV type 16 E7-induced apoptosis. Virology 239:97-107. [DOI] [PubMed] [Google Scholar]
- 26.Lacey, K. R., P. K. Jackson, and T. Stearns. 1999. Cyclin-dependent kinase control of centrosome duplication. Proc. Natl. Acad. Sci. USA 96:2817-2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowy, D. R., and P. M. Howley. 2001. Papillomaviruses, p. 2231-2264. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 28.Mantel, C., S. E. Braun, S. Reid, O. Henegariu, L. Liu, G. Hangoc, and H. E. Broxmeyer. 1999. p21cip1/waf-1 deficiency causes deformed nuclear architecture, centriole overduplication, polyploidy, and relaxed microtubule damage checkpoints in human hematopoietic cells. Blood 93:1390-1398. [PubMed] [Google Scholar]
- 29.Martin, L. G., G. W. Demers, and D. A. Galloway. 1998. Disruption of the G1/S transition in human papillomavirus type 16 E7-expressing human cells is associated with altered regulation of cyclin E. J. Virol. 72:975-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsumoto, Y., K. Hayashi, and E. Nishida. 1999. Cyclin-dependent kinase 2 (Cdk2) is required for centrosome duplication in mammalian cells. Curr. Biol. 9:429-432. [DOI] [PubMed] [Google Scholar]
- 31.Meraldi, P., J. Lukas, A. M. Fry, J. Bartek, and E. A. Nigg. 1999. Centrosome duplication in mammalian somatic cells requires E2F and Cdk2-cyclin A. Nat. Cell Biol. 1:88-93. [DOI] [PubMed] [Google Scholar]
- 32.Munger, K., and P. M. Howley. 2002. Human papillomavirus immortalization and transformation functions. Virus Res. 89:213-228. [DOI] [PubMed] [Google Scholar]
- 33.Munger, K., W. C. Phelps, V. Bubb, P. M. Howley, and R. Schlegel. 1989. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J. Virol. 63:4417-4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Münger, K., B. A. Werness, N. Dyson, W. C. Phelps, E. Harlow, and P. M. Howley. 1989. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 8:4099-4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen, D. X., T. F. Westbrook, and D. J. McCance. 2002. Human papillomavirus type 16 E7 maintains elevated levels of the cdc25A tyrosine phosphatase during deregulation of cell cycle arrest. J. Virol. 76:619-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paoletti, A., M. Moudjou, M. Paintrand, J. L. Salisbury, and M. Bornens. 1996. Most of centrin in animal cells is not centrosome-associated and centrosomal centrin is confined to the distal lumen of centrioles. J. Cell Sci. 109:3089-3102. [DOI] [PubMed] [Google Scholar]
- 37.Phelps, W. C., K. Munger, C. L. Yee, J. A. Barnes, and P. M. Howley. 1992. Structure-function analysis of the human papillomavirus type 16 E7 oncoprotein. J. Virol. 66:2418-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piboonniyom, S., S. Duensing, N. W. Swilling, P. W. Hinds, and K. Münger. 2003. Abrogation of the retinoblastoma tumor suppressor checkpoint during keratinocyte immortalization is not sufficient for induction of centrosome-mediated genomic instability. Cancer Res. 63:476-483. [PubMed] [Google Scholar]
- 39.Pihan, G. A., A. Purohit, J. Wallace, H. Knecht, B. Woda, P. Quesenberry, and S. J. Doxsey. 1998. Centrosome defects and genetic instability in malignant tumors. Cancer Res. 58:3974-3985. [PubMed] [Google Scholar]
- 40.Ruesch, M. N., and L. A. Laimins. 1998. Human papillomavirus oncoproteins alter differentiation-dependent cell cycle exit on suspension in semisolid medium. Virology 250:19-29. [DOI] [PubMed] [Google Scholar]
- 41.Salisbury, J. L., C. M. Whitehead, W. L. Lingle, and S. L. Barrett. 1999. Centrosomes and cancer. Biol. Cell 91:451-460. [PubMed] [Google Scholar]
- 42.Scheffner, M., B. A. Werness, J. M. Huibregtse, A. J. Levine, and P. M. Howley. 1990. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 63:1129-1136. [DOI] [PubMed] [Google Scholar]
- 43.Schulze, A., B. Mannhardt, K. Zerfass-Thome, W. Zwerschke, and P. Jansen-Durr. 1998. Anchorage-independent transcription of the cyclin A gene induced by the E7 oncoprotein of human papillomavirus type 16. J. Virol. 72:2323-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shew, J. Y., B. T. Lin, P. L. Chen, B. Y. Tseng, T. L. Yang-Feng, and W. H. Lee. 1990. C-terminal truncation of the retinoblastoma gene product leads to functional inactivation. Proc. Natl. Acad. Sci. USA 87:6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skyldberg, B., K. Fujioka, A. C. Hellstrom, L. Sylven, B. Moberger, and G. Auer. 2001. Human papillomavirus infection, centrosome aberration, and genetic stability in cervical lesions. Mod. Pathol. 14:279-284. [DOI] [PubMed] [Google Scholar]
- 46.Stearns, T., L. Evans, and M. Kirschner. 1991. Gamma-tubulin is a highly conserved component of the centrosome. Cell 65:825-836. [DOI] [PubMed] [Google Scholar]
- 47.Urbani, L., and T. Stearns. 1999. The centrosome. Curr. Biol. 9:R315-R317. [DOI] [PubMed] [Google Scholar]
- 48.Vidwans, S. J., M. L. Wong, and P. H. O'Farrell. 1999. Mitotic regulators govern progress through steps in the centrosome duplication cycle. J. Cell Biol. 147:1371-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.White, A. E., E. M. Livanos, and T. D. Tlsty. 1994. Differential disruption of genomic integrity and cell cycle regulation in normal human fibroblasts by the HPV oncoproteins. Genes Dev. 8:666-677. [DOI] [PubMed] [Google Scholar]
- 50.Wong, C., and T. Stearns. 2003. Centrosome number is controlled by a centrosome-intrinsic block to reduplication. Nat. Cell Biol. 5:539-544. [DOI] [PubMed] [Google Scholar]
- 51.Zerfass, K., A. Schulze, D. Spitkovsky, V. Friedman, B. Henglein, and P. Jansen-Durr. 1995. Sequential activation of cyclin E and cyclin A gene expression by human papillomavirus type 16 E7 through sequences necessary for transformation. J. Virol. 69:6389-6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zerfass-Thome, K., W. Zwerschke, B. Mannhardt, R. Tindle, J. W. Botz, and P. Jansen-Durr. 1996. Inactivation of the cdk inhibitor p27KIP1 by the human papillomavirus type 16 E7 oncoprotein. Oncogene 13:2323-2330. [PubMed] [Google Scholar]
- 53.zur Hausen, H. 1996. Papillomavirus infections—a major cause of human cancers. Biochim. Biophys. Acta 1288:F55-F78. [DOI] [PubMed] [Google Scholar]