Abstract
Objective
A subset of fibromyalgia (FM) patients have a dysfunctional hypothalamic–pituitary–insulin-like growth factor 1 (IGF-1) axis, as evidenced by low serum levels of IGF-1 and a reduced growth hormone (GH) response to physiologic stimuli. There is evidence that pyridostigmine (PYD) improves the acute response of GH to exercise in FM patients. The purpose of this study was to evaluate the clinical effectiveness of 6 months of PYD and group exercise on FM symptoms.
Methods
FM patients were randomized to 1 of the following 4 groups: PYD plus exercise, PYD plus diet recall but no exercise, placebo plus exercise, and placebo plus diet recall but no exercise. The primary outcome measures were the visual analog scale (VAS) score for pain, tender point count, and total myalgic score. Secondary outcome measures were the total score on the Fibromyalgia Impact Questionnaire (FIQ) and FIQ VAS scores for individual symptoms (fatigue, poor sleep, stiffness, and anxiety), as well as quality of life (QOL) and physical fitness (lower body strength/endurance, upper and lower body flexibility, balance, and time on the treadmill).
Results
A total of 165 FM patients completed baseline measurements; 154 (93.3%) completed the study. The combination of PYD and exercise did not improve pain scores. PYD groups showed a significant improvement in sleep and anxiety in those who completed the study and in QOL in those who complied with the therapeutic regimen as compared with the placebo groups. Compared with the nonexercise groups, the 2 exercise groups demonstrated improvement in fatigue and fitness. PYD was generally well tolerated.
Conclusion
Neither the combination of PYD plus supervised exercise nor either treatment alone yielded improvement in most FM symptoms. However, PYD did improve anxiety and sleep, and exercise improved fatigue and fitness. We speculate that PYD may have improved vagal tone, thus benefiting sleep and anxiety; this notion warrants further study.
Fibromyalgia (FM) is a disorder characterized by persistent widespread pain and tender points (1). However, most FM patients have multiple symptoms (2). While the concept of central sensitization is increasingly accepted as an important pathophysiologic aberration in FM patients, the factors that contribute to its persistence are not well-characterized (3). FM patients have low levels of physical fitness and often experience post-exertional pain, fatigue, and delayed onset of muscle soreness (4,5). Muscle tissue may be an important source of postexertional symptom flares through the mechanism of muscle microtrauma (6,7). It has been hypothesized that reduced insulin-like growth factor 1 (IGF-1) levels may predispose some FM patients to the development of exercise-induced muscle microtrauma (8). Approximately one-third of FM patients have low levels of IGF-1 (9,10), the major effector molecule that mediates the anabolic effect of growth hormone (GH) on muscle (11).
Treatment of FM patients with GH has been reported to be of some clinical benefit (12), but GH is seldom prescribed because of its high costs and concerns regarding its long-term usage (13). GH secretion in FM patients can be acutely increased by the use of pyridostigmine (PYD; an acetylcholine esterase inhibittor) in combination with exercise (14). Regular low-grade exercise has an established role in the management of FM patients (4) and is also a stimulus for GH secretion (15,16). The aim of this study was to explore whether the long-term use of PYD and regular exercise could improve symptoms in FM patients by stimulating the hypothalamic–pituitary–IGF-1 axis.
Patients and Methods
Study design
The study was a single-center, 6-month, randomized, blinded, controlled clinical trial with a 2 × 2 × 2 design (training exercise × PYD × time). Patients were randomly assigned to 1 of 4 groups (PYD plus exercise, PYD plus diet recall but no exercise, placebo plus exercise, and placebo plus diet recall but no exercise) by the study team's statistician (NAP), who had no contact with the subjects. Age, sex, and body mass index (BMI) were block factors in the random assignment.
The laboratory portion of the study was conducted at the Clinical Translational Research Institute (CTRI) of Oregon Health & Science University. The training exercise intervention was conducted in a specially designed suspended wood-floor aerobics studio at the School of Nursing. The diet recall interviews for attention control were conducted in a private office at the School of Nursing. The Institutional Review Board and CTRI at Oregon Health & Science University approved the protocol, and all study subjects provided their written informed consent.
Study inclusion criteria
The target population consisted of adults of both sexes who were ages 18–65 years, had been diagnosed as having primary FM according to the American College of Rheumatology (ACR) criteria (1), and were medically able to engage in an exercise program. The diagnosis of FM was confirmed at the initial laboratory visit.
Study exclusion criteria
Patients with any of the following conditions were excluded from the study: 1) current or past cardiovascular, pulmonary, neurologic, endocrine, or renal disease that would preclude involvement in an exercise program (specifically, hypertension, chronic obstructive pulmonary disease, uncontrolled asthma, hypothyroidism, severe depression, pituitary disease, or surgery); 2) current use of the study drug, high-dose β-blockers (which could significantly affect the normal physiologic response to exercise), or systemic steroids (which might affect GH secretion); 3) currently exercising >30 minutes/week; 4) score of ≥29 on the revised Beck Depression Inventory (BDI-R) as modified for use in FM patients; 5) BMI >45 kg/m2; 6) women who were pregnant or nursing; 7) planned elective surgery during the study period; or 8) ongoing, unresolved litigation.
Population sampling and setting
Participants were randomly selected from a large database of FM patients who have been seen upon referral to our university tertiary care center. The probability of adequate representation of low income and minority participants was increased by planned oversampling from the database in zip code areas known to have a higher percentage of minority populations.
Power analysis
Data from previous studies (13,16) were used to calculate the study power and sample size. In the acute exercise plus PYD study (14), serum GH was the primary outcome variable and was found to be a mean ± SD of 0.57 ± 0.82 ng/ml at rest and 4.70 ± 3.80 ng/ml after PYD plus exercise in a sample of 20 women with FM.
Assuming the same effect size for the placebo plus no exercise group as compared with the PYD plus no exercise group in our proposed study, a total of 30 participants in each group would provide statistical power >0.95 for an alpha level of 0.05. A sample size of 30 in each group would also provide adequate power for testing changes in pain, FM symptoms, and quality of life (QOL). Based on these data, we planned to enroll 144 patients (36 per group at baseline), which would allow for 18% attrition over the study period.
Study assessments
Demographic data, such as age, weight, height, BMI, estrogen status (menopause status and/or use of estrogen), current medications, and duration of FM were obtained at baseline.
Pain was the primary outcome variable. A combination of 3 pain measures was evaluated before and after the study intervention: the visual analog scale (VAS) for pain, which is a subscale of the Fibromyalgia Impact Questionnaire (FIQ), the number of tender points, and the total myalgic score. The number of tender points and the total myalgic score were measured at 18 sites as described in the ACR criteria for FM (1). The degree of tenderness at each site was based on the patient's response (scored 0–3, where 0 = no pain and 3 = withdrawal from the examiner) to digital application of pressure at a rate of 0–4 kg over a period of 4 seconds at the 18 specific tender points. This test was performed by a single examiner. The 10-cm VAS for pain (a subscale of the FIQ) rated the patient's perception of pain intensity over the previous 7 days, with anchors at 0 (no pain) and 10 (very severe pain).
Other FM symptoms were measured with the FIQ. The FIQ is a 10-item instrument that uses a 0–10-cm VAS to measure physical functioning and symptoms of pain, fatigue (0 = no tiredness and 10 = extreme tiredness), sleep (0 = awakening unrefreshed and 10 = awakening well-rested), stiffness (0 = no stiffness and 10 = very stiff), anxiety (0 = no anxiety and 10 = very anxious), and depression (0 = no depression and 10 = very depressed), along with levels of disability and overall well-being during the previous week. Total FIQ scores range from 0–100, with higher values indicating a more negative impact of FM. The FIQ has been extensively validated and has shown good sensitivity to change (17). In this study, the reliability (Cronbach's alpha) of the total myalgic score was 0.87, and the reliability (Cronbach's alpha) of the total FIQ was 0.88.
The level of depression was evaluated with the BDI-R (18). The BDI-R is a 21-item scale that measures mood and behaviors characteristic of depression. Potential participants who scored ≥29 were excluded from the study and referred for more appropriate therapy (19). In this study, the reliability (Cronbach's alpha) of the BDI-R was 0.87.
The Quality of Life Scale is a 16-item Likert-type scale that measures well-being and satisfaction in multiple domains of life. Scores for each item range from 1 (terrible) to 7 (delighted). Possible scores range from 6 to 112, with higher scores indicating better well-being and quality of life. The Quality of Life Scale has been validated in a sample of FM patients (internal consistency reliability α = 0.82–0.88; test-retest reliability r = 0.84) and is sensitive to change in FM patients (20,21). The reliability (Cronbach's alpha) of the Quality of Life Scale in this study was 0.90.
Flexibility, strength/endurance, and balance were assessed with field measures. Upper body flexibility was measured by asking participants to reach behind their head with 1 arm, extending their reach as far below the neck as possible. While that arm was extended behind them, the participants were asked to reach behind their back with the other arm, extending their reach up toward the scapula as far as possible. While both arms were behind the subject's back, the space between the extended fingertips was measured (in cm). Higher numbers indicated greater impairment of flexibility. The best of 3 attempts was recorded.
Lower body flexibility was measured by asking seated participants to extend 1 arm toward their toes on the same side of the body. The space between the fingertips and toes was measured (in cm). Higher numbers indicated greater impairment of flexibility. Negative numbers were recorded if the subject was able to reach beyond the toes. The best of 3 attempts was recorded.
Lower body strength/endurance was measured by asking participants who were seated in a wooden chair to cross their arms in front of their chest and to rise from a seated position to a standing position as many times as possible in 30 seconds. The first attempt was recorded.
Balance was measured while participants were standing with their arms at their sides and with their eyes open. They were asked to lift 1 foot no more than 3 inches from the floor and to maintain their balance in this position as long as possible. The duration was measured (in seconds). Poorer balance resulted in lower scores. The best of 3 attempts was recorded.
Study procedures
The study patients completed the treadmill testing to V̇o2 max and blood draws as previously described (22). Lower body strength/endurance, upper and lower body flexibility, and balance were then measured, and questionnaires were administered.
Study subjects were randomized via stratified block (age in 5-year blocks, BMI in 3-point blocks, and sex) into 4 treatment groups: PYD plus exercise, PYD plus diet recall but no exercise (exercise control group), placebo plus exercise (drug control group), and placebo plus diet recall but no exercise (drug and exercise control group). The dosage of PYD bromide (Mestinon; kindly provided by Valeant Pharmaceuticals, Costa Mesa, CA) or identical placebo tablets (also provided by Valeant Pharmaceuticals) was titrated to the full dosage of 180 mg/day over the following 11 days. The titration protocol was one-half pill on day 1, increasing by one-half pill each day until the patient was taking 1 pill (60 mg) 3 times a day. The total dose was based on our previous single-dose trial (14) and clinical experience (23). Patients were instructed to take the study drug in the morning, 1 hour prior to exercise class (based on our pilot data), and at bedtime to maximize GH release during stages 3 and 4 sleep, when 70–80% of GH is produced (24). Patients completed a monthly log, recording exercise periods, study drug intake, food intake, and any side effects they might be experiencing. Each month, information from the logs was used to confirm the dose of training exercise, to monitor for compliance with the medication regimen, to monitor for adverse events, and to document side effects of the drug.
Training exercise treatment protocol
The training exercise was supervised by an instructor certified by the American Council on Exercise as a Clinical Exercise Specialist; this instructor was not responsible for collecting outcome data. Exercises were group-based (10–20 per group), 60-minutes in duration, and were conducted 3 times each week for 6 months. The format included low-impact, nonrepetitive cardioaerobics training for 30 minutes, strength training for 10 minutes, flexibility training for 5 minutes, balance training for 5 minutes, and relaxation for 10 minutes. The intensity goal was 40–50% of the patient's maximum heart rate or a perceived exertion of 10–12 of a total of 20, as measured by the Borg Scale.
Progressive strength training was accomplished with 3 variations of elastic bands (kindly provided by Thera-Band, Akron, OH) and free weights. All major muscle groups were exercised while minimizing work over the head, keeping movements near the midline of the body, and reducing the speed of concentric contractions compared with the speed of eccentric contractions. Weights were relaxed down or bands were slackened between contractions, allowing for delayed return to resting baseline state (25), as detailed elsewhere (26). Flexibility training to the point of gentle tension included static and nonballistic stretches. Balance training was both static and dynamic, progressing from balancing with both feet on a flat surface, balancing with 1 foot on a flat surface, and then balancing on boards/discs (kindly provided by Thera-Band) to challenge plantar proprioception.
Progressive relaxation was achieved without muscle tensing and included guided imagery with breathing awareness. The intensity of exercises were purposefully lower than the intensity described in the guidelines of the American Academy of Sports Medicine, based on our recognition of baseline physical deconditioning in FM patients that predisposes them to enhanced delayed muscle pain, symptom flare, and aggravation of FM tender point areas.
Protocol for attention control
All patients who were not randomized to an exercise group received weekly telephone calls and a 2-hour monthly visit from a registered nurse. The study subjects provided dietary data by completing objective diet recall surveys (27) and provided a narrative diet history.
All study subjects returned at the end of the 6-month study for a second treadmill test. The procedures were the same as at baseline, with 1 key difference: one half of the study subjects were given 1 60-mg tablet of PYD and the other half were given placebo, depending on their group assignment, 1 hour before stepping on the treadmill. In the event that a patient requested to withdraw from the study, the final laboratory visit was conducted within 2 weeks of withdrawal, if the patient agreed to this.
Statistical analysis
The purpose of this study was to test whether daily PYD plus supervised exercise would improve clinical symptoms of FM as a result of increased levels of IGF-1. The primary outcome measures were the FIQ pain VAS score, the tender point count, and the total myalgic score. Secondary outcome measures were scores on the total FIQ and individual VAS items of the FIQ, as well as QOL, lower body strength/endurance, balance, flexibility, and time on the treadmill. The analyses were conducted on data from patients who completed the study and not on the intent-to-treat population. Preliminary analyses included one-way analyses of variance and chi-square tests to examine statistical differences between the 4 groups at baseline. The main hypotheses of the study were tested using 2 × 2 (PYD versus placebo) × 2 (exercise versus no exercise) analysis of covariance (ANCOVA), controlling for the baseline value of the variable of interest. The adjusted means that were used for the ANCOVAs and are reported in the text are the part of the posttest scores that could not be predicted by the pretest scores or the residualized posttest scores.
Results
Characteristics of the study patients
One hundred sixty-five patients with FM completed the baseline assessments. These patients were randomized into 1 of 4 groups: 43 were randomized to PYD plus exercise, 42 to PYD plus diet recall but no exercise, 39 to placebo plus exercise, and 41 to placebo plus diet recall but no exercise. Eleven of these 164 enrolled patients failed to attend the 6-month followup visit (Figure 1).
Figure 1.
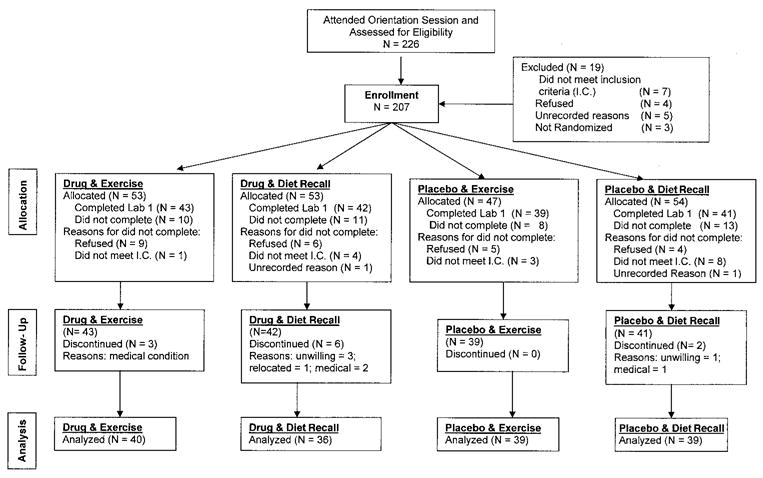
Distribution of the study patients from initial contact to completion of the study. A total of 165 patients with fibromyalgia completed the baseline measurements, and 154 patients completed the study. Patients were assigned to 1 of 4 groups: pyridostigmine plus exercise, pyridostigmine plus diet recall but no exercise, placebo plus exercise, and placebo plus diet recall but no exercise (see Patients and Methods for details). Adapted, with permission, from ref. 22.
The demographic features of the 165 patients who completed the baseline assessments are shown in Table 1. There were no statistically significant differences at baseline between the 4 groups. Estrogen status did not correlate with any symptom, fitness variable, or physiologic variable. For descriptive purposes, the raw means and SDs for all outcomes are presented in Tables 2 and 3, by drug intervention groups. The adjusted means from the ANCOVAs, which were the basis of the statistical tests, are presented in the text.
Table 1.
Baseline demographic, clinical, and laboratory data in the 165 fibromyalgia patients, by intervention group*
| Placebo | Pyridostigmine | ||||
|---|---|---|---|---|---|
| Total
(n = 165) |
No group exercise
(n = 41) |
Group exercise
(n = 39) |
No group exercise
(n = 42) |
Group exercise
(n = 43) |
|
| % female/male | 97/3 | 100/0 | 95/5 | 93/7 | 100/0 |
| % Caucasian/non-Caucasian | 93/7 | 88/12 | 90/10 | 93/7 | 100/0 |
| % premenopausal/postmenopausal | 64/36 | 61/39 | 62/38 | 75/25 | 60/40 |
| Age, years | 49.45 ± 8.05 | 49.78 ± 7.87 | 49.62 ± 7.65 | 49.31 ± 7.89 | 49.12 ± 8.95 |
| Duration of fibromyalgia, years | 15.39 ± 10.65 | 14.91 ± 10.62 | 16.91 ± 11.94 | 14.81 ± 9.74 | 14.97 ± 10.48 |
| Beck Depression Inventory score | 9.32 ± 6.40 | 8.97 ± 6.51 | 10.58 ± 6.81 | 9.99 ± 5.90 | 7.85 ± 6.27 |
| No. of tender points | 17.2 ± 1.31 | 17.39 ± 0.97 | 17.21 ± 1.13 | 16.88 ± 1.70 | 17.00 ± 1.29 |
| Total myalgic score | 36.92 ± 8.53 | 38.85 ± 7.76 | 38.00 ± 8.88 | 35.17 ± 9.41 | 35.83 ± 7.75 |
| Total FIQ score | 58.10 ± 15.92 | 58.58 ± 17.80 | 59.64 ± 16.02 | 56.59 ± 14.34 | 57.64 ± 15.74 |
| Pain VAS score | 6.48 ± 2.20 | 6.46 ± 2.25 | 6.95 ± 2.13 | 5.88 ± 2.24 | 6.63 ± 2.14 |
| Fatigue VAS score | 7.93 ± 1.87 | 7.78 ± 2.24 | 8.16 ± 1.60 | 7.90 ± 1.91 | 7.91 ± 1.72 |
| Sleep VAS score | 8.01 ± 2.08 | 7.78 ± 2.15 | 7.82 ± 1.93 | 8.43 ± 2.00 | 8.02 ± 2.21 |
| Stiffness VAS score | 7.22 ± 2.21 | 7.54 ± 2.29 | 7.26 ± 2.23 | 6.95 ± 2.22 | 7.12 ± 2.14 |
| Anxiety VAS score | 4.88 ± 2.98 | 4.80 ± 2.89 | 5.00 ± 3.11 | 5.10 ± 2.87 | 4.63 ± 3.12 |
| Depression VAS score | 3.57 ± 2.73 | 3.66 ± 3.13 | 3.71 ± 2.67 | 4.05 ± 2.51 | 2.93 ± 2.55 |
| BMI, kg/m2 | 29.79 ± 6.25 | 30.22 ± 6.54 | 30.08 ± 6.30 | 28.99 ± 6.01 | 29.01 ± 6.12 |
| % body fat at 7 sites, by skinfold test | 34.59 ± 8.02 | 35.54 ± 6.89 | 35.32 ± 8.60 | 33.36 ± 8.54 | 34.25 ± 8.03 |
| V̇o2 max, ml/kg | 21.73 ± 4.88 | 20.4 ± 4.52 | 21.39 ± 4.91 | 22.95 ± 4.74 | 22.11 ± 5.13 |
| Total time on treadmill, seconds | 618.24 ± 282.42 | 533.02 ± 259.77 | 581.49 ± 284.23 | 715.0 ± 292.0 | 638.8 ± 270.0 |
| Upper body flexibility, cm | 8.81 ± 10.77 | 8.76 ± 11.50 | 9.71 ± 11.73 | 7.31 ± 10.41 | 9.55 ± 9.67 |
| Lower body flexibility, cm | 0.64 ± 12.42 | −0.61 ± 12.43 | −0.44 ± 14.07 | 3.07 ± 12.10 | 0.43 ± 11.12 |
| Balance, seconds | 29.51 ± 41.45 | 23.78 ± 27.62 | 32.85 ± 54.98 | 31.48 ± 43.29 | 30.02 ± 36.90 |
| Lower body strength/endurance† | 11.12 ± 3.70 | 10.59 ± 3.15 | 10.46 ± 4.23 | 12.07 ± 4.18 | 11.29 ± 3.00 |
| GH at rest, ng/ml | 0.67 ± 1.65 | 0.47 ± 0.78 | 0.72 ± 2.01 | 0.53 ± 1.14 | 0.94 ± 2.22 |
| GH after treadmill exercise (at V̇o2 max), ng/ml | 1.76 ± 3.53 | 1.41 ± 2.94 | 1.01 ± 1.26 | 2.95 ± 5.31 | 1.61 ± 3.09 |
| GH at rest 1 hour after treadmill exercise, ng/ml | 0.42 ± 0.80 | 0.63 ± 1.28 | 0.27 ± 0.44 | 0.40 ± 0.58 | 0.37 ± 0.62 |
| IGF-1, ng/dl | 142.10 ± 57.98 | 140.46 ± 52.07 | 126.73 ± 63.32 | 148.44 ± 56.26 | 151.5 ± 58.93 |
| IGFBP-3, ng/dl | 4.75 ± 1.18 | 4.97 ± 1.3 | 4.61 ± 1.21 | 4.5 ± 1.11 | 4.91 ± 1.06 |
None of the comparisons between the placebo and pyrodistigmine groups showed a significant difference. No tests of significance were conducted for sex or race because the numbers in 1 or more cells were <5. Except where indicated otherwise, values are the mean ± SD. FIQ = Fibromyalgia Impact Questionnaire; VAS = visual analog scale (0–10 cm); BMI = body mass index; V̇o2 max = maximum oxygen consumption (i.e., highest rate at which oxygen can be taken up and utilized during exercise); GH = growth hormone; IGF-1 = insulin-like growth factor 1; IGFBP-3 = insulin-like growth factor binding protein 3.
Represents the number of times subjects could rise from a seated position in 30 seconds.
Table 2.
Raw means and SDs for quality of life and fibromyalgia symptoms in the fibromyalgia patients at baseline and followup, by drug intervention group*
| Followup | ||||||
|---|---|---|---|---|---|---|
| Baseline | Placebo | Pyridostigmine | ||||
| Placebo, mean ± SD | Pyridostigmine, mean ± SD | No. of patients | Mean ± SD | No. of patients | Mean ± SD | |
| Quality of life | ||||||
| No training exercise | 70.80 ± 13.87 | 74.62 ± 13.95 | 38 | 72.10 ± 14.14 | 36 | 77.06 ± 16.24 |
| Training exercise | 68.78 ± 15.47 | 75.30 ± 15.54 | 38 | 71.44 ± 15.93 | 39 | 82.00 ± 11.47 |
| Total myalgic score | ||||||
| No training exercise | 38.44 ± 7.87 | 34.36 ± 9.25 | 38 | 34.07 ± 10.75 | 36 | 28.92 ± 12.62 |
| Training exercise | 37.87 ± 8.96 | 36.38 ± 7.82 | 38 | 33.47 ± 10.36 | 39 | 31.23 ± 10.04 |
| No. of tender points | ||||||
| No training exercise | 17.34 ± 0.99 | 16.81 ± 1.77 | 38 | 16.08 ± 2.76 | 36 | 14.83 ± 4.27 |
| Training exercise | 17.24 ± 1.13 | 17.00 ± 1.34 | 38 | 16.29 ± 2.48 | 39 | 15.92 ± 3.25 |
| Total FIQ score | ||||||
| No training exercise | 58.79 ± 18.48 | 56.02 ± 14.77 | 38 | 53.16 ± 19.93 | 35 | 47.80 ± 21.66 |
| Training exercise | 59.96 ± 16.10 | 58.25 ± 15.42 | 38 | 53.66 ± 21.29 | 39 | 44.01 ± 20.73 |
| Pain VAS score | ||||||
| No training exercise | 6.42 ± 2.33 | 5.97 ± 2.14 | 38 | 5.84 ± 2.53 | 34 | 5.29 ± 2.73 |
| Training exercise | 6.97 ± 2.15 | 6.72 ± 2.05 | 37 | 5.59 ± 2.92 | 39 | 5.00 ± 2.31 |
| Fatigue VAS score | ||||||
| No training exercise | 7.74 ± 2.32 | 7.69 ± 1.92 | 38 | 6.95 ± 2.49 | 35 | 7.23 ± 2.91 |
| Training exercise | 8.19 ± 1.61 | 7.87 ± 1.75 | 37 | 6.62 ± 2.69† | 39 | 5.97 ± 2.32† |
| Sleep VAS score | ||||||
| No training exercise | 7.76 ± 2.20 | 8.26 ± 2.08 | 38 | 7.34 ± 2.56 | 35 | 6.66 ± 3.00† |
| Training exercise | 7.89 ± 1.90 | 7.97 ± 2.32 | 37 | 6.92 ± 2.89 | 38 | 6.00 ± 2.86† |
| Stiffness VAS score | ||||||
| No training exercise | 7.47 ± 2.35 | 7.09 ± 2.19 | 38 | 6.84 ± 2.80 | 35 | 6.06 ± 2.85 |
| Training exercise | 7.35 ± 2.19 | 7.33 ± 1.77 | 37 | 6.11 ± 2.94 | 39 | 5.69 ± 2.60 |
| Anxiety VAS score | ||||||
| No training exercise | 4.82 ± 2.99 | 4.86 ± 2.70 | 38 | 4.29 ± 2.98 | 35 | 3.14 ± 3.14† |
| Training exercise | 5.14 ± 3.04 | 4.67 ± 3.17 | 37 | 4.57 ± 3.04 | 39 | 3.26 ± 2.80† |
| Depression VAS score | ||||||
| No training exercise | 3.76 ± 3.21 | 3.91 ± 2.65 | 38 | 2.95 ± 2.66 | 35 | 2.83 ± 2.54 |
| Training exercise | 3.76 ± 2.53 | 2.90 ± 2.63 | 37 | 4.32 ± 3.26 | 39 | 2.85 ± 2.86 |
Of the 4 intervention groups, the 2 groups receiving pyridostigmine were analyzed separately from the 2 groups receiving placebo. FIQ = Fibromyalgia Impact Questionnaire; VAS = visual analog scale (0–10 cm).
P < 0.006-0.001.
Table 3.
Raw means and SDs for fitness outcomes in the fibromyalgia patients at baseline and followup, by drug intervention group*
| Followup | ||||||
|---|---|---|---|---|---|---|
| Baseline | Placebo | Pyridostigmine | ||||
| Placebo, mean ± SD | Pyridostigmine, mean ± SD | No. of patients | Mean ± SD | No. of patients | Mean ± SD | |
| BMI, kg/m2 | ||||||
| No training exercise | 30.16 ± 6.68 | 29.17 ± 5.77 | 38 | 30.48 ± 7.21 | 35 | 28.85 ± 5.38 |
| Training exercise | 31.08 ± 6.30 | 29.01 ± 6.13 | 39 | 30.62 ± 6.15 | 40 | 28.87 ± 6.25 |
| % body fat at 7 sites, by skinfold test | ||||||
| No training exercise | 35.53 ± 7.05 | 33.66 ± 8.28 | 38 | 36.10 ± 7.55 | 35 | 33.72 ± 8.14 |
| Training exercise | 35.32 ± 8.60 | 33.94 ± 7.91 | 39 | 35.78 ± 8.28 | 39 | 33.89 ± 8.47 |
| V̇o2 max, ml/kg | ||||||
| No training exercise | 20.68 ± 4.54 | 23.13 ± 4.84 | 37 | 19.07 ± 3.65 | 34 | 21.38 ± 4.42 |
| Training exercise | 21.39 ± 4.91 | 21.74 ± 4.65 | 38 | 20.83 ± 5.16 | 40 | 20.83 ± 4.22 |
| Total time on treadmill, seconds | ||||||
| No training exercise | 548.95 ± 256.15 | 721.49 ± 282.23 | 38 | 514.89 ± 270.31 | 35 | 729.43 ± 326.11 |
| Training exercise | 581.49 ± 284.23 | 632.95 ± 265.92 | 39 | 630.13 ± 318.88 | 40 | 673.63 ± 239.40 |
| Upper body flexibility, cm | ||||||
| No training exercise | −8.74 ± 11.71 | −8.20 ± 10.37 | 38 | −10.13 ± 10.54 | 35 | −6.94 ± 9.91 |
| Training exercise | −9.71 ± 11.73 | −9.23 ± 9.82 | 38 | −7.66 ± 11.48 | 39 | −6.77 ± 9.21 |
| Lower body flexibility, cm | ||||||
| No training exercise | −0.29 ± 12.82 | 3.37 ± 11.17 | 38 | 0.97 ± 13.18 | 35 | 2.66 ± 13.60 |
| Training exercise | −0.44 ± 14.07 | 0.38 ± 10.97 | 39 | 4.00 ± 12.31† | 39 | 5.46 ± 12.80† |
| Lower body strength/endurance‡ | ||||||
| No training exercise | 10.62 ± 3.26 | 12.20 ± 4.47 | 37 | 10.95 ± 3.81 | 35 | 13.26 ± 5.31 |
| Training exercise | 10.42 ± 4.28 | 11.11 ± 3.01 | 38 | 12.42 ± 4.07 | 38 | 13.00 ± 3.85 |
| Balance, seconds | ||||||
| No training exercise | 23.58 ± 28.27 | 35.20 ± 46.33 | 38 | 34.11 ± 40.84 | 35 | 48.49 ± 69.06 |
| Training exercise | 32.85 ± 54.98 | 25.97 ± 28.69 | 39 | 73.82 ± 78.54† | 38 | 62.21 ± 47.96† |
Of the 4 intervention groups, the 2 groups receiving pyridostigmine were analyzed separately from the 2 groups receiving placebo. BMI = body mass index; V̇o2 max = maximum oxygen consumption (i.e., highest rate at which oxygen can be taken up and utilized during exercise).
P < 0.006−0.001.
Represents the number of times subjects could rise from a seated position in 30 seconds.
Results of the primary outcome measures
The primary aim of this study, that daily PYD plus supervised exercise would improve pain scores (as determined with the FIQ VAS scores, the number of tender points, or the total myalgic score) was not substantiated by the results. For the pain VAS score, the interaction of PYD and training exercise (F[1,143] = 0.04, P = 0.849), the main effect of PYD (F[1,143] = 0.97, P = 0.325), and the main effect of exercise (F[1,143] = 2.39, P = 0.124) failed to reach significance. For the total myalgic score, the interaction of PYD and training exercise (F[1,146] = 0.10, P = 0.751), the main effect of PYD (F[1,146] = 1.13, P = 0.289), and the main effect of exercise (F[1,146] = 0.05, P = 0.831) failed to reach significance. Additionally, for the number of tender points, the interaction of PYD and training exercise (F[1,146] = 0.29, P = 0.592), the main effect of PYD (F[1,146] = 0.41, P = 0.525), and the main effect of exercise (F[1,146] = 1.78, P = 0.0184) failed to reach significance.
Results of the secondary outcome measures
Patients who completed the study demonstrated no significant improvements in QOL, total FIQ score, FIQ stiffness VAS score, or FIQ depression VAS score. Thus, no further details on these analyses will be presented. However, there were improvements in several other outcome measures: fatigue, sleep, and anxiety levels, lower body flexibility, and balance.
Fatigue VAS scores
For fatigue, neither the interaction of PYD and training exercise nor the main effect of PYD was significant. However, the main effect of exercise training on fatigue was significant (F[1,144] = 7.66, P = 0.006). Collapsing the 4 groups into 2 according to exercise and no exercise, the groups who participated in the training exercise reported less fatigue (adjusted mean ± SEM VAS scores 6.19 ± 0.26) than did the no training exercise groups (adjusted mean ± SEM VAS scores 7.20 ± 0.26) at followup, controlling for baseline levels of fatigue.
Sleep VAS scores
For sleep, neither the interaction of PYD and training exercise nor the main effect of training exercise was significant. However, the main effect of PYD on sleep was significant (F[1,143] = 8.38, P = 0.004) (Figure 2). Collapsing the 4 groups into 2 according to PYD and placebo treatment, the PYD-treated groups reported less nonrefreshing sleep (adjusted mean ± SEM VAS scores 6.20 ± 0.26) than did the placebo-treated groups (adjusted mean ± SEM VAS scores 7.25 ± 0.25) at followup, controlling for baseline sleep values.
Figure 2.
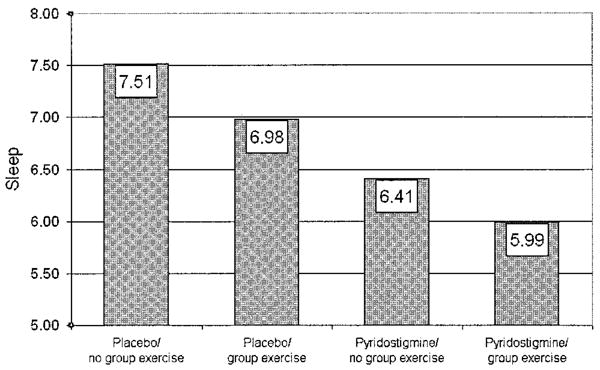
Improvement in sleep quality with pyridostigmine therapy in patients with fibromyalgia. Patients were assigned to 1 of 4 groups: placebo plus diet recall but no exercise (placebo/no group exercise), placebo plus exercise (placebo/group exercise), pyridostigmine plus diet recall but no exercise (pyridostigmine/no group exercise), and pyridostigmine plus exercise (pyridostigmine/group exercise). Sleep was assessed using a 0–10-cm visual analog scale, where lower scores indicate better sleep. Values are the adjusted mean.
Anxiety VAS scores
For anxiety, neither the interaction of PYD and training exercise nor the main effect of training exercise was significant. However, the main effect of PYD on anxiety was significant (F[1,144] = 7.02, P = 0.009) (Figure 3). Collapsing the 4 groups into 2 according to PYD and placebo treatment, the PYD-treated groups reported less anxiety (adjusted mean ± SEM VAS scores 3.25 ± 0.30) than did the placebo-treated groups (adjusted mean ± SEM VAS scores 4.37 ± 0.30) at followup, controlling for baseline levels of anxiety.
Figure 3.
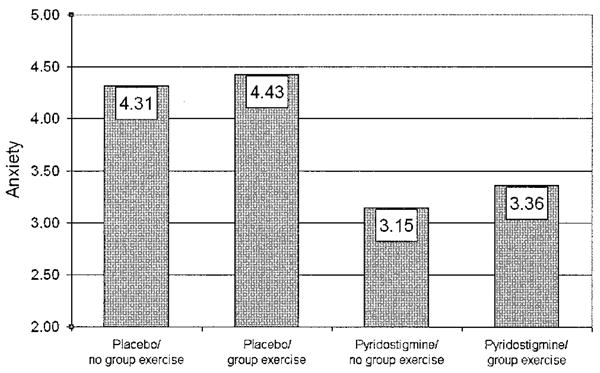
Improvement in anxiety levels with pyridostigmine therapy in patients with fibromyalgia. Patients were assigned to 1 of 4 groups: placebo plus diet recall but no exercise (placebo/no group exercise), placebo plus exercise (placebo/group exercise), pyridostigmine plus diet recall but no exercise (pyridostigmine/no group exercise), and pyridostigmine plus exercise (pyridostigmine/group exercise). Anxiety symptoms were assessed using a 0–10-cm visual analog scale, where lower scores indicate less anxiety. Values are the adjusted mean.
Lower body flexibility
For lower body flexibility, neither the interaction of PYD with training exercise nor the main effect of PYD was significant. However, the main effect of exercise training on lower body flexibility was significant (F[1,146] = 7.81, P = 0.006). Collapsing the 4 groups into 2 according to exercise and no exercise, the groups who participated in the training exercise had better lower body flexibility (adjusted mean ± SEM cm 5.28 ± 1.02) than did the no training exercise groups (adjusted mean ± SEM cm 1.17 ± 1.05) at followup, controlling for baseline lower body flexibility values.
Balance
For balance, neither the interaction of PYD with training exercise nor the main effect of PYD was significant. However, the main effect of exercise training on balance was significant (F[1,145] = 10.64, P = 0.001). Collapsing the 4 groups into 2 according to exercise and no exercise, the groups who participated in the training exercise had better balance (adjusted mean ± SEM seconds 67.93 ± 5.71) than did the no training exercise groups (adjusted mean ± SEM seconds 41.23 ± 5.87) at followup, controlling for baseline balance values.
Findings in patients who were compliant with the PYD regimen
The subjects who took ≥75% of their PYD pills (n = 133) were analyzed separately. This group was found to have a significant improvement in QOL (F[1,126] = 7.97, P = 0.006). Collapsing the 4 groups into 2 according to PYD and placebo treatment, the PYD-treated groups reported higher QOL scores (adjusted mean ± SEM 78.39 ± 1.37) than did the placebo-treated groups (adjusted mean ± SEM 73.26 ± 1.16) at followup, controlling for baseline QOL values.
Findings in patients who were compliant with the exercise regimen
We also examined the effect of the intervention for patients who complied with the training exercise regimen by excluding participants who attended <50% of the exercise classes (n = 123). No significant effects for the interaction or the main effect of PYD emerged in these exercise compliance analyses. However, the main effects of training exercise emerged as significant: V̇o2 max (F[1,114] = 7.91, P = 0.006), time on the treadmill (F[1,117] = 7.68, P = 0.006), upper body flexibility (F[1,115] = 12.31, P = 0.001), and lower body strength/endurance (F[1,115] = 6.81, P = 0.010). The training exercise groups had higher V̇o2 max values (adjusted mean ± SEM 21.75 ± 0.44 ml/kg) than did the no training exercise groups (adjusted mean ± SEM 20.14 ± 0.36 ml/kg) at followup, controlling for baseline V̇o2 max values. The training exercise groups had a longer time on the treadmill (adjusted mean ± SEM 707.01 ± 26.53 seconds) than the no training exercise groups (adjusted mean ± SEM 611.93 ± 21.71 seconds) at followup, controlling for baseline time on the treadmill. The training exercise groups had better upper body flexibility (adjusted mean ± SEM cm −4.80 ± 0.86) than did the no training exercise groups (adjusted mean ± SEM cm −8.69 ± 0.69) at followup, controlling for baseline upper body flexibility levels. The training exercise groups had better lower body strength/endurance (adjusted mean ± SEM number of rises from seated position 13.33 ± 0.45) than did the no training exercise groups (adjusted mean ± SEM number of rises from seated position 11.79 ± 0.37) at followup, controlling for baseline lower body strength/endurance.
To explore relationships between serum markers at baseline and symptoms of FM, we examined their correlations. We found no significant correlation between the total IGF-1 level or the age-adjusted IGF-1 level and any symptoms of FM. However, we did find a significant correlation between GH levels at V̇o2 max and the pain (r = −0.21, P = 0.006) and stiffness (r = −0.27, P = 0.001) scores. Correlations between serum markers (GH, IGF-1, and IGFBP-3), fitness levels, and anthropomorphic measures have been described elsewhere (22).
Safety
In a previous study based on data obtained from these patients, we reported in detail on the side effects of PYD in the study participants (22). A significantly larger proportion of the PYD-treated group reported abdominal pain, diarrhea/loose stools, and muscle cramping or twitching. These effects were common, but were cited as the reason for study discontinuation in only 2 of the 11 patients who did not complete the study, perhaps indicating that the severity of the side effects was generally tolerable (22).
Discussion
The rationale for this study was that the daily use of pyridostigmine and regular exercise would stimulate the hypothalamic–pituitary–IGF-1 axis in FM patients and the resulting increase in IGF-1 levels would result in improvement in FM symptoms. As we have previously reported (22), the combination of PYD and regular exercise failed to increase IGF-1 levels, which were low for their age in 58% of the study population. As might have been predicted from this negative response, we found that the primary outcome measures of FIQ pain VAS score, tender point count, and total myalgic score did not improve in the present study. Furthermore, there was no improvement in the following secondary outcome measures: total FIQ score, FIQ fatigue VAS score, FIQ stiffness VAS score, FIQ depression VAS score, QOL, BMI, or percentage of body fat. However, there was a significant improvement in both FIQ restorative sleep VAS score and FIQ anxiety VAS score in the 2 PYD-treated groups, whereas the 2 exercise groups experienced improvements in fatigue VAS score, flexibility, balance, lower body strength/endurance, V̇o2 max, and time on the treadmill.
The improvements in anxiety levels and sleep quality (“awoke well rested”) in subjects taking PYD are intriguing. This is unlikely to be a spurious statistical finding resulting from multiple comparisons, since the predetermined significant P value was set at 0.01 rather than 0.05. We speculate that these improvements may be related to an augmentation of parasympathetic tone as a consequence of the daily use of PYD (28) and regular exercise (29). There is now persuasive evidence that FM patients have dysautonomia, as evidenced by results of heart rate variability studies, tilt-table testing (30), and sympathetic skin responses (31–34). Two common clinical manifestations of dysautonomia are neurally mediated hypotension and postural orthostatic tachycardia syndrome (35,36). Both of these dysautonomia syndromes have shown improvement with regular use of PYD (36). The observed improvement in restorative sleep was unexpected and may be related to recent observations that vagal stimulation can improve alertness and reduce daytime sleepiness (37,38). On the other hand, GH is known to stimulate slow-wave sleep (24), and a transient surge in GH levels related to the nighttime dose of PYD is another possibility. The observed improvement in anxiety is consistent with many reports of reduced heart rate variability in anxiety disorders (39,40) and the ability of PYD to improve heart rate variability (28,41). There is some preliminary evidence that increasing heart rate variability through biofeedback benefits some clinical features of FM (42).
This study is the first to attempt to combine training exercise and a medication in an effort to manipulate the GH–IGF-1 axis in patients with FM and to measure changes in symptoms. It is only the second study to combine a drug and exercise in a randomized controlled trial in FM patients. In an abstract published in 1992, Isomeri et al (43) reported improvement in the pain VAS score with amitriptyline and exercise in FM patients. One other exercise study reported on IGF-1 levels in a randomized controlled strength training intervention conducted for 21 weeks (44). Despite improvements in muscle mass, lower body strength/endurance, and neural recruitment, there was no improvement in IGF-1 levels.
In a previous article using data from this study group (22), we reported that strenuous exercise plus PYD promoted an acute surge in GH levels during testing, but did not increase IGF-1 levels. However, the exercise level achieved during the tri-weekly classes did have beneficial effects in terms of fatigue, flexibility, balance, lower body strength/endurance, V̇o2 max, and time on the treadmill. Presumably, these benefits of exercise, which have been described in many previous studies, are not a result of changes in the hypothalamic–GH–IGF-1 axis. In our previously published review of 46 other published trials of exercise in FM patients (4), fitness and physical function were most often improved as a result of aerobic or mixed-type training programs. Pain, however, was not consistently improved in these trials. At higher exercise intensity, frequency, and duration (exercise dose), there was often a worsening of pain, whereas a lower exercise dose often resulted in clinical improvements. Some of these trials had attrition rates between 30% and 87%, limiting the interpretation of the results and highlighting the difficulty in maintaining compliance in exercise trials in FM. The exercise portion of this study confirms that group-based, mixed-type exercise training in FM is possible and that the attrition rates are low (9%).
The study had several limitations. First, we may not have selected the most sensitive measurement of pain. At the time this study was funded, pain was commonly measured with a single VAS score, a total myalgic score, and a count of tender points. Newer recommendations suggest that the brief pain index and the patient's global assessment of change be used in FM intervention studies (45). Furthermore, there is increasing evidence that the number of tender points is not particularly sensitive to change in the average FM patient. Another possible limitation of the study is that the dose of PYD may not have been adequate or the duration of the trial sufficiently long. To our knowledge, there has never been a PYD dosage-escalation study or placebo comparison in FM patients. We chose the dosage of PYD based on our previous experience with a single-dose protocol and the clinical experience of other FM patient healthcare providers (23).
A future trial with PYD could use an unblinded investigator who adjusts the dosage until the IGF-1 level is normalized, along with a similar adjustment in the dosing of the placebo medication. In the previous study of injectable recombinant GH, there was normalization of the serum IGF-1 level within 4 weeks, but symptom improvements began at 4 months and were still continuing at 9 months (12). This lag time for improvement in symptoms is consistent with the known kinetics of muscle anabolism in patients taking GH (46). In general, 6 months of PYD at a dosage of 60 mg 3 times a day plus FM-tailored group exercise for 60 minutes 3 times a week was safe and well-tolerated. The side effects of the drug, including loosening of stool and increased tearing and salivation, were common, but were well-tolerated by the study subjects. The side effect of abdominal pain was somewhat problematic and was cited by 2 patients as the reason for discontinuing the study.
Overall, our study provides additional objective evidence of abnormalities in the GH–IGF-1 axis in patients with FM, but the combination of PYD and exercise at the dosage and duration tested was not able to increase IGF-1 levels or to improve most FM symptoms, except for anxiety and sleep quality. Future trials aimed toward manipulating the GH–IGF-1 axis in FM patients may include exercise and somatostatin-blocking agents, such as PYD, in combination with GH-releasing hormone secretagogues. Based on the results of this study, it would be reasonable to prospectively study the effects of long-term PYD therapy on heart rate variability and possible interactions with sleep and anxiety levels.
Acknowledgments
We thank Cheryl Hryciw, MS, FNP, Laura Cirotski, MS, RN, Edit Serfozo, MPH, project director, and Janice Hoffman, BS, clinical exercise specialist, our study participants, and their families.
Supported by the NIH (National Institute of Nursing Research grant 5R01-NR-8150-4 and General Clinical Research Center grant M01-RR-000334). Pyridostigmine and placebo were provided by Valeant Pharmaceuticals. Exercise equipment was provided by Thera-Band.
Footnotes
Author Contributions: Dr. Jones had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study design. Jones, Burckhardt, Deodhar, Perrin, Bennett.
Acquisition of data. Jones.
Analysis and interpretation of data. Jones, Burckhardt, Deodhar, Bennett.
Manuscript preparation. Jones, Burckhardt, Deodhar, Perrin, Hanson, Bennett.
Statistical analysis. Jones, Perrin, Hanson.
References
- 1.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia: report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–72. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 2.Bennett RM, Jones J, Turk DC, Russell IJ, Matallana L. An internet survey of 2,596 people with fibromyalgia. BMC Musculoskelet Disord. 2007;8:27. doi: 10.1186/1471-2474-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price DD, Staud R. Neurobiology of fibromyalgia syndrome. J Rheumatol Suppl. 2005;75:22–8. [PubMed] [Google Scholar]
- 4.Jones KD, Adams D, Winters-Stone K, Burckhardt CS. A comprehensive review of 46 exercise treatment studies in fibromyalgia (1988–2005) Health Qual Life Outcomes. 2006;4:67. doi: 10.1186/1477-7525-4-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Staud R, Robinson ME, Price DD. Isometric exercise has opposite effects on central pain mechanisms in fibromyalgia patients compared with normal controls. Pain. 2005;118:176–84. doi: 10.1016/j.pain.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Bennett RM, Jacobsen S. Muscle function and origin of pain in fibromyalgia. Baillieres Clin Rheumatol. 1994;8:721–46. doi: 10.1016/s0950-3579(05)80045-7. [DOI] [PubMed] [Google Scholar]
- 7.Gibson W, Arendt-Nielsen L, Graven-Nielsen T. Delayed onset muscle soreness at tendon-bone junction and muscle tissue is associated with facilitated referred pain. Exp Brain Res. 2006;174:351–60. doi: 10.1007/s00221-006-0466-y. [DOI] [PubMed] [Google Scholar]
- 8.Bennett RM. Adult growth hormone deficiency in patients with fibromyalgia. Curr Rheumatol Rep. 2002;4:306–12. doi: 10.1007/s11926-002-0039-4. [DOI] [PubMed] [Google Scholar]
- 9.Bennett RM, Cook DM, Clark SR, Burckhardt CS, Campbell SM. Hypothalamic-pituitary-insulin-like growth factor-I axis dysfunction in patients with fibromyalgia. J Rheumatol. 1997;24:1384–9. [PubMed] [Google Scholar]
- 10.Jones KD, Deodhar P, Lorentzen A, Bennett RM, Deodhar AA. Growth hormone perturbations in fibromyalgia: a review. Semin Arthritis Rheum. 2007;36:357–79. doi: 10.1016/j.semarthrit.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Frost RA, Lang CH. Regulation of insulin-like growth factor-I in skeletal muscle and muscle cells. Minerva Endocrinol. 2003;28:53–73. [PubMed] [Google Scholar]
- 12.Bennett RM, Clark SC, Walczyk J. A randomized, double-blind, placebo-controlled study of growth hormone in the treatment of fibromyalgia. Am J Med. 1998;104:227–31. doi: 10.1016/s0002-9343(97)00351-3. [DOI] [PubMed] [Google Scholar]
- 13.Ur E, Serri O, Legg K, Murphy LJ, Ezzat S. Canadian guidelines for the management of adult growth hormone deficiency. Clin Invest Med. 2006;29:83–90. [PubMed] [Google Scholar]
- 14.Paiva ES, Deodhar A, Jones KD, Bennett R. Impaired growth hormone secretion in fibromyalgia patients: evidence for augmented hypothalamic somatostatin tone. Arthritis Rheum. 2002;46:1344–50. doi: 10.1002/art.10209. [DOI] [PubMed] [Google Scholar]
- 15.De Vries WR, Abdesselam SA, Schers TJ, Maas HC, Osman-Dualeh M, Maitimu I, et al. Complete inhibition of hypothalamic somatostatin activity is only partially responsible for the growth hormone response to strenuous exercise. Metabolism. 2002;51:1093–6. doi: 10.1053/meta.2002.34697. [DOI] [PubMed] [Google Scholar]
- 16.De Vries WR, Schers TJ, Ait AS, Osman-Dualeh M, Maitimu I, Koppeschaar HP. Involvement of endogenous growth hormone-releasing hormone (GHRH) in the exercise-related response of growth hormone. Int J Sports Med. 2003;24:208–11. doi: 10.1055/s-2003-39093. [DOI] [PubMed] [Google Scholar]
- 17.Bennett R. The Fibromyalgia Impact Questionnaire (FIQ): a review of its development, current version, operating characteristics and uses. Clin Exp Rheumatol. 2005;23 Suppl 39:S154–62. [PubMed] [Google Scholar]
- 18.Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 19.Burckhardt CS, O'Reilly CA, Wiens AN, Clark SR, Campbell SM, Bennett RM. Assessing depression in fibromyalgia patients. Arthritis Care Res. 1994;7:35–9. doi: 10.1002/art.1790070108. [DOI] [PubMed] [Google Scholar]
- 20.Burckhardt CS, Clark SR, Bennett RM. Fibromyalgia and quality of life: a comparative analysis. J Rheumatol. 1993;20:475–9. [PubMed] [Google Scholar]
- 21.Flanagan JC. A research approach to improving our quality of life. Am Psychol. 1978;33:138–47. [Google Scholar]
- 22.Jones KD, Deodhar AA, Burckhardt CS, Perrin NA, Hanson GC, Bennett RM. A combination of 6 months of treatment with pyridostigmine and triweekly exercise fails to improve insulin-like growth factor-I levels in fibromyalgia, despite improvement in the acute growth hormone response to exercise. J Rheumatol. 2007;34:1103–11. [PubMed] [Google Scholar]
- 23.Wallace DJ. Fibromyalgia: unusual historical aspects and new pathogenic insights. Mt Sinai J Med. 1984;51:124–31. [PubMed] [Google Scholar]
- 24.Van Cauter E, Plat L, Copinschi G. Interrelations between sleep and the somatotropic axis. Sleep. 1998;21:553–66. [PubMed] [Google Scholar]
- 25.Elert J, Kendall SA, Larsson B, Mansson B, Gerdle B. Chronic pain and difficulty in relaxing postural muscles in patients with fibromyalgia and chronic whiplash associated disorders. J Rheumatol. 2001;28:1361–8. [PubMed] [Google Scholar]
- 26.Jones KD, Clark SR. Individualizing the exercise prescription for persons with fibromyalgia. Rheum Dis Clin North Am. 2002;28:419–36. doi: 10.1016/s0889-857x(01)00010-2. [DOI] [PubMed] [Google Scholar]
- 27.Connor SL, Gustafson JR, Sexton G, Becker N, Artaud-Wild S, Connor WE. The Diet Habit Survey: a new method of dietary assessment that relates to plasma cholesterol changes. J Am Diet Assoc. 1992;92:41–7. [PubMed] [Google Scholar]
- 28.Dewland TA, Androne AS, Lee FA, Lampert RJ, Katz SD. Effect of acetylcholinesterase inhibition with pyridostigmine on cardiac parasympathetic function in sedentary adults and trained athletes. Am J Physiol Heart Circ Physiol. 2007;293:H86–92. doi: 10.1152/ajpheart.01339.2006. [DOI] [PubMed] [Google Scholar]
- 29.Jurca R, Church TS, Morss GM, Jordan AN, Earnest CP. Eight weeks of moderate-intensity exercise training increases heart rate variability in sedentary postmenopausal women. Am Heart J. 2004;147:e21. doi: 10.1016/j.ahj.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 30.Raj SR, Brouillard D, Simpson CS, Hopman WM, Abdollah H. Dysautonomia among patients with fibromyalgia: a noninvasive assessment. J Rheumatol. 2000;27:2660–5. [PubMed] [Google Scholar]
- 31.Cohen H, Neumann L, Alhosshle A, Kotler M, Abu-Shakra M, Buskila D. Abnormal sympathovagal balance in men with fibromyalgia. J Rheumatol. 2001;28:581–9. [PubMed] [Google Scholar]
- 32.Martinez-Lavin M, Hermosillo AG, Rosas M, Soto ME. Circadian studies of autonomic nervous balance in patients with fibromyalgia: a heart rate variability analysis. Arthritis Rheum. 1998;41:1966–71. doi: 10.1002/1529-0131(199811)41:11<1966::AID-ART11>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 33.McLean SA, Williams DA, Stein PK, Harris RE, Lyden AK, Whalen G, et al. Cerebrospinal fluid corticotropin-releasing factor concentration is associated with pain but not fatigue symptoms in patients with fibromyalgia. Neuropsychopharmacology. 2006;31:2776–82. doi: 10.1038/sj.npp.1301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ulas UH, Unlu E, Hamamcioglu K, Odabasi Z, Cakci A, Vural O. Dysautonomia in fibromyalgia syndrome: sympathetic skin responses and RR interval analysis. Rheumatol Int. 2006;26:383–7. doi: 10.1007/s00296-005-0007-1. [DOI] [PubMed] [Google Scholar]
- 35.Jacob G, Biaggioni I. Idiopathic orthostatic intolerance and postural tachycardia syndromes. Am J Med Sci. 1999;317:88–101. doi: 10.1097/00000441-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Kaufmann H, Bhattacharya K. Diagnosis and treatment of neurally mediated syncope. Neurologist. 2002;8:175–85. doi: 10.1097/00127893-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Malow BA, Edwards J, Marzec M, Sagher O, Ross D, Fromes G. Vagus nerve stimulation reduces daytime sleepiness in epilepsy patients. Neurology. 2001;57:879–84. doi: 10.1212/wnl.57.5.879. [DOI] [PubMed] [Google Scholar]
- 38.Rizzo P, Beelke M, De Carli F, Canovaro P, Nobili L, Robert A, et al. Chronic vagus nerve stimulation improves alertness and reduces rapid eye movement sleep in patients affected by refractory epilepsy. Sleep. 2003;26:607–11. doi: 10.1093/sleep/26.5.607. [DOI] [PubMed] [Google Scholar]
- 39.Gorman JM, Sloan RP. Heart rate variability in depressive and anxiety disorders. Am Heart J. 2000;140 Suppl 4:77–83. doi: 10.1067/mhj.2000.109981. [DOI] [PubMed] [Google Scholar]
- 40.Piccirillo G, Elvira S, Bucca C, Viola E, Cacciafesta M, Marigliano V. Abnormal passive head-up tilt test in subjects with symptoms of anxiety power spectral analysis study of heart rate and blood pressure. Int J Cardiol. 1997;60:121–31. doi: 10.1016/s0167-5273(97)00088-0. [DOI] [PubMed] [Google Scholar]
- 41.Raj SR, Black BK, Biaggioni I, Harris PA, Robertson D. Acetylcholinesterase inhibition improves tachycardia in postural tachycardia syndrome. Circulation. 2005;111:2734–40. doi: 10.1161/CIRCULATIONAHA.104.497594. [DOI] [PubMed] [Google Scholar]
- 42.Hassett AL, Radvanski DC, Vaschillo EG, Vaschillo B, Sigal LH, Karavidas MK, et al. A pilot study of the efficacy of heart rate variability (HRV) biofeedback in patients with fibromyalgia. Appl Psychophysiol Biofeedback. 2007;32:1–10. doi: 10.1007/s10484-006-9028-0. [DOI] [PubMed] [Google Scholar]
- 43.Isomeri R, Mikkelsson M, Latikka P. Effects of amitriptyline and cardiovascular fitness training on the pain of fibromyalgia patients [abstract] Scand J Rheumatol Suppl. 1992;94:47. [Google Scholar]
- 44.Valkeinen H, Hakkinen K, Pakarinen A, Hannonen P, Hakkinen A, Airaksinen O, et al. Muscle hypertrophy, strength development, and serum hormones during strength training in elderly women with fibromyalgia. Scand J Rheumatol. 2005;34:309–14. doi: 10.1080/03009740510018697. [DOI] [PubMed] [Google Scholar]
- 45.Mease PJ, Clauw DJ, Arnold LM, Goldenberg DL, Witter J, Williams DA, et al. Fibromyalgia syndrome. J Rheumatol. 2005;32:2270–7. [PubMed] [Google Scholar]
- 46.Wallymahmed ME, Foy P, Shaw D, Hutcheon R, Edwards RH, MacFarlane IA. Quality of life, body composition and muscle strength in adult growth hormone deficiency: the influence of growth hormone replacement therapy for up to 3 years. Clin Endocrinol (Oxf) 1997;47:439–46. doi: 10.1046/j.1365-2265.1997.2801076.x. [DOI] [PubMed] [Google Scholar]


