Noninvasive detection of tumors at an early stage by magnetic resonance imaging (MRI) needs highly sensitive contrast agents. Synthesized from solution phase and coated with various hydrophilic molecules, iron oxide nanoparticles (IONPs) can be well stabilized in biological systems and used for T2 contrast enhancement in MRI.1–3 However, commonly used IONP contrast agents require nonspecific uptake by mononuclear phagocytes to improve the local contrast, and at the hydrodynamic size of over 50 nm, these particles have very limited extravasation ability and are subject to easy uptake by the reticuloendothelial system (RES),4,5 which undermines severely their targeting specificity. To overcome the problems of nonspecific uptake and to enhance the extra-vascular ability, even smaller hydrodynamic sizes are desired.5,6 Despite recent synthetic progresses, making small (<10 nm hydrodynamic size) IONPs with required biocompatibility and targeting capability is still extremely challenging.
Here, we present a direct synthesis of ultrasmall Fe3O4 NPs (< 10 nm in hydrodynamic diameter) and demonstrate their in vivo tumor-specific targeting capability. The Fe3O4 NPs with 4.5 nm core size were synthesized by thermal decomposition of iron pentacarbonyl, Fe(CO)5, followed by air oxidation. Different from any of the previous synthesis methods,7 the current preparation used a new ligand 4-methylcatechol (4-MC) as the surfactant. More importantly, the 4-MC coated IONP surface was directly conjugated with a peptide, c(RGDyK), via the Mannich reaction, rendering the particles stable in physiological environment. The synthesis is illustrated in Scheme 1. The c(RGDyK)-MC-Fe3O4 NPs have an overall diameter of ∼8.4 nm and are stable in physiological conditions. They can target specifically to integrin αvβ3-rich tumor cells. When administrated intravenously, these c(RGDyK)-MC-Fe3O4 NPs accumulate preferentially in tumor cells, which are readily tracked by MRI.
Scheme 1.
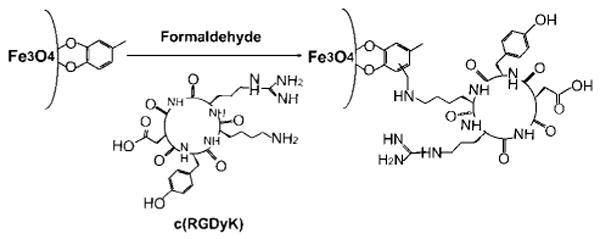
Schematic Illustration of Coupling c(RGDyK) Peptide to Fe3O4 NPs
Fe3O4 NPs were synthesized by thermal decomposition of Fe(CO)5 in benzyl ether in the presence of 4-MC followed by air oxidation.8 Because of the formation of a strong chelate bond between iron and a catechol unit,9 4-MC forms a tight thin coating layer around the NP surface. The sizes of the NPs (from 2.5–5 nm as shown in Figure S1) were tunable by the MC/Fe ratio. Figure 1A shows the high resolution transmission electron microscopy (HRTEM) image of the 4.5 nm Fe3O4 NPs. The inverse spinel structure of the Fe3O4 NPs was characterized by X-ray diffraction (XRD) analysis (Figure S2). The 4-MC coating was confirmed by FTIR (Figure 1B), from which three aromatic C=C stretch peaks at 1602, 1521, and 1415 cm−1 are attributed to the coated 4-MC and are right-shifted compared to those from the free 4-MC (1623, 1527, and 1448 cm−1). Peaks at 624, 594, and 445 cm−1 are typical Fe—O absorption bands.10 The MC-Fe3O4 NPs were well dispersed in chloroform (CHCl3) or dimethylformamide (DMF), ready for further functionalization.
Figure 1.
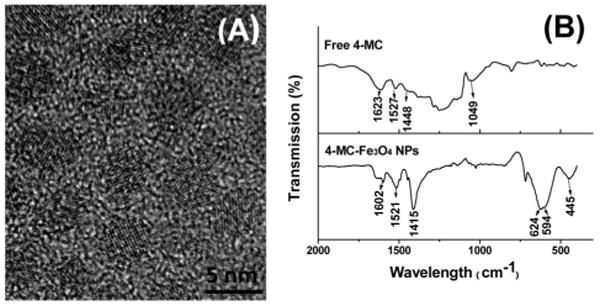
(A) HRTEM image of the as-synthesized, 4.5 nm MC-Fe3O4 NPs; (B) FTIR spectra of the free 4-MC and MC-Fe3O4 NPs.
The aromatic ring of 4-MC around the Fe3O4 NPs can be directly coupled with amine (NH2) group via a Mannich reaction, offering a direct method for Fe3O4 functionalization. Here we choose to conjugate a cyclic RGD peptide, c(RGDyK), to the NP surface. This head-to-tail cyclized RGD peptide has been proven to target specifically to the activated endothelial cells and various tumor cells expressing cell adhesion molecule integrin αvβ3.11 The coupling via a Mannich reaction was performed by mixing the MC-Fe3O4 NPs with c(RGDyK) and formaldehyde (HCHO) in DMF (Scheme 1).8
The RGD coated Fe3O4 NPs are stable for months without precipitation in aqueous dispersion (Figure S3). The overall size of the particles, measured by dynamic light scattering (DLS) in water, is 8.4 ±1.0 nm (Supporting Information, Figure S3), close to the simple addition of the ∼4.5 nm core (Figure 1A) and ∼2 nm coating of 4-MC + c(RGDyK). The matrix-assisted laser desorption ionization-mass spectrometry (MALDI-MS) shows various M/Z peaks (Figure S4) that confirm the successful conjugation of c(RGDyK) to 4-MC. The number of RGD peptide per particle was estimated to be 100–200.8
To study integrin targeting specificity of the c(RGDyK)-MC-Fe3O4 NPs, we incubated these NPs with both U87MG human glioblastoma (high αvβ3) and MCF-7 human breast cancer (low αvβ3) cell lines at 37 °C for 30 min. The iron concentration within the cells were analyzed by inductively coupled plasma atomic emission spectroscopy (ICP-AES).8 Figure 2A shows the Fe uptake for U87MG cells, which is about 5-fold higher that of MCF-7 cells. The accumulation of NPs in U87MG cells is effectively inhibited in the presence of the blocking dose (2 μM) of c(RGDyK), further demonstrating integrin specificity of c(RGDyK)-MC-Fe3O4 NPs. The c(RGDyK)-MC-Fe3O4 NPs also show good binding ability to integrin. This is confirmed by the displacement competitive binding assay using αvβ3 positive U87MG cells, as shown in Figure 2B. The c(RGDyK)-MC-Fe3O4 NPs inhibit 125I-echistatin binding to integrin in a concentration dependent manner. The IC50 value, used to measure the affinity of the binding agents, is calculated to be 23 ± 5 nM for NPs, which is significantly higher than monomeric RGD peptide (250 ± 60 nM). The enhanced binding affinity is from the multivalent effect as discussed previously.3,12
Figure 2.
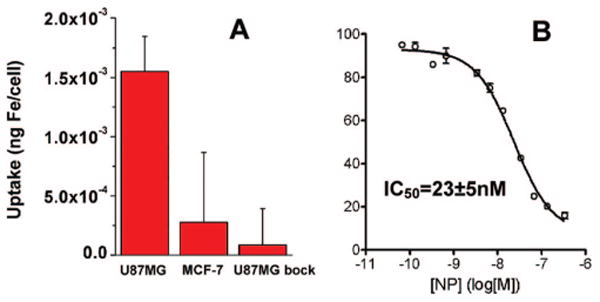
(A) Cell uptake of c(RGDyK)-MC-Fe3O4 NPs by U87MG, MCF-7, and U87MG + c(RGDyK) block, and (B) the c(RGDyK)-MC-Fe3O4 NP concentration dependent replacement of 125I-echistatin on U87MG cells.
The targeting ability of the c(RGDyK)-MC-Fe3O4 NPs to integrin αvβ3 in vivo was evaluated by T2-weighted fast spin–echo MR imaging with mice bearing U87MG tumors. The r2 relaxavity of the c(RGDyK)-MC-Fe3O4 NPs was measured to be 165 mM−1s−1 (Figure S5), which is larger than that of the commercial Feridex NPs (104 mM−1 s−1) with similar core size.13 This larger r2 value is likely caused by the stronger field perturbation around the NPs due to the thin c(RGDyK) coating. The tumor MR signal intensity decreased significantly (42 ± 5%) after injection of c(RGDyK)-MC-Fe3O4 NPs (Figure 3A,B). Such signal reduction was inhibited (15 ± 3%) in the presence of a blocking dose of c(RGDyK) peptide (10 mg/kg) (Figure 3C), proving the specificity of the particle targeting in vivo. To further investigate the particle distribution in various organs, the animals were sacrificed immediately after the MR scan at 4 h time point, and Prussian blue staining was employed to visualize the disposition of the c(RGDyK)-MC-Fe3O4 NPs without and with the blocking dose of c(RGDyK) peptide (Figure 3D,E). Prominent blue spots on the U87MG tumor slices were observed (Figure 3D) and the blue spots were reduced in the presence of free c(RGDyK) (Figure 3E), further confirming that the accumulation of c(RGDyK)-MC-Fe3O4 NPs was mediated by integrin αvβ3 binding. Additionally, the c(RGDyK)-MC-Fe3O4 NPs were rarely seen in kidneys and muscle, indicating that the NPs sustained enough circulation time for targeting, even though some deposition in both liver and spleen were seen (Figure S6). The NPs in the tumor were mostly localized on the integrin expressing tumor vasculature and tumor cells with little to no macrophage uptake (Figure S7).
Figure 3.
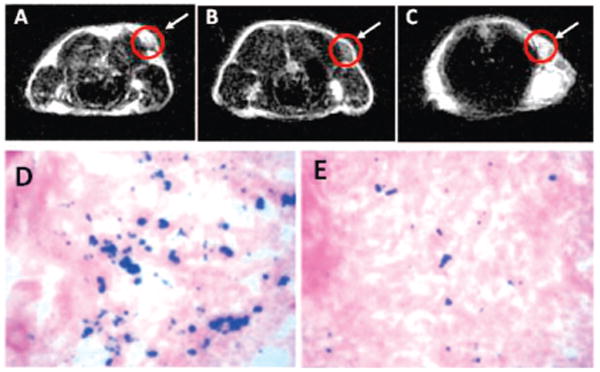
MRI of the cross section of the U87MG tumors implanted in mice: (A) without NPs, (B) with the injection of 300 μg of c(RGDyK)-MC-Fe3O4 NPs, and (C) with the injection of c(RGDyK)-MC-Fe3O4 NPs and blocking dose of c(RGDyK); and Prussian blue staining of U87MG tumors in the presence of (D) c(RGDyK)-MC-Fe3O4 NPs and (E) c(RGDyK)-MC-Fe3O4 NPs plus blocking dose of c(RGDyK).
This report introduces a novel way of synthesizing and functionalizing ultrasmall Fe3O4 NPs as contrast agents for potential in vivo tumor detection using MRI. The Fe3O4 NPs are synthesized by thermal decomposition of Fe(CO)5 in the presence of 4-MC followed by air oxidation. Mannich reaction is applied to couple NH2-terminated peptide c(RGDyK), and the c(RGDyK)-MC-Fe3O4 NPs show the desired biocompatibility and specificity to U87MG tumor cells. Compared to the free RGD peptide, the c(RGDyk)-MC-Fe3O4 NPs with desired size could dramatically increase the cellular uptake due to the multivalent binding. This new NP functionalization strategy can be readily extended to couple other bioactive molecules with amine functional group to Fe3O4 NPs. Further work on extravasation ability of the biofunctionalized IONPs for target-specific delivery is underway.
Supplemental Material
Acknowledgments
The work was supported by NIH/NCI 1R21CA12859-01 (S.S.), U54CA119367/R21CA121842 (X.C.) and in part by DOE/EPSCoR DE-FG02-07ER36374 (S.S.).
Footnotes
Supporting Information Available: Fe3O4 NP synthesis, surface functionalization and characterization. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Pankhurst QA, Connolly J, Jones SK, Dobson J. J Phys D: Appl Phys. 2003;36:R167–R181. [Google Scholar]; (b) Lee JH, Huh YM, Jun Y, Seo J, Jang J, Song HT, Kim S, Cho EJ, Yoon HG, Suh JS, Cheon J. Nat Med. 2007;13:95–99. doi: 10.1038/nm1467. [DOI] [PubMed] [Google Scholar]
- 2.Jun YW, Huh YM, Choi JS, Lee JH, Song HT, Kim S, Yoon S, Kim KS, Shin JS, Suh JS, Cheon J. J Am Chem Soc. 2005;127:5732–5733. doi: 10.1021/ja0422155. [DOI] [PubMed] [Google Scholar]
- 3.Montet X, Funovics M, Montet-Abou K, Weissleder R, Josephson L. J Med Chem. 2006;49:6087–6093. doi: 10.1021/jm060515m. [DOI] [PubMed] [Google Scholar]
- 4.Moghimi SM, Hunter AC, Murray JC. Pharm Rev. 2001;53:283–318. [PubMed] [Google Scholar]
- 5.Neuberger T, Schopf B, Hofmann H, Hofmann M, von Rechenberg B. J Magn Mag Mater. 2005;293:483–496. [Google Scholar]
- 6.Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Ipe BI, Bawendi MG, Frangioni JV. Nat Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Park J, An KJ, Hwang YS, Park JG, Noh HJ, Kim JY, Park JH, Hwang NM, Hyeon T. Nat Mater. 2004;3:891–895. doi: 10.1038/nmat1251. [DOI] [PubMed] [Google Scholar]; (b) Sun SH, Zeng H, Robinson DB, Raoux S, Rice PM, Wang SX, Li GX. J Am Chem Soc. 2004;126:273–279. doi: 10.1021/ja0380852. [DOI] [PubMed] [Google Scholar]
- 8.See Supporting Information.
- 9.Xu CJ, Xu KM, Gu HW, Zheng RK, Liu H, Zhang XX, Guo ZH, Xu B. J Am Chem Soc. 2004;126:9938–9939. doi: 10.1021/ja0464802. [DOI] [PubMed] [Google Scholar]
- 10.Ma M, Zhang Y, Yu W, Shen HY, Zhang HQ, Gu N. Colloids Surf A: Physicochem Eng Asp. 2003;212:219–226. [Google Scholar]
- 11.(a) Li ZB, Cai WB, Cao QZ, Chen K, Wu ZH, He LN, Chen XY. J Nucl Med. 2007;48:1162–1171. doi: 10.2967/jnumed.107.039859. [DOI] [PubMed] [Google Scholar]; (b) Cai WB, Shin DW, Chen K, Gheysens O, Cao QZ, Wang SX, Gambhir SS, Chen XY. Nano Lett. 2006;6:669–676. doi: 10.1021/nl052405t. [DOI] [PubMed] [Google Scholar]
- 12.Jiang W, Kim BYS, Rutka JT, Chan W. Nat Nanotechnol. 2008;3:145–150. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- 13.Seo WS, Lee JH, Sun XM, Suzuki Y, Mann D, Liu Z, Terashima M, Yang PC, McConnell MV, Nishimura DG, Dai HJ. Nat Mater. 2006;5:971–976. doi: 10.1038/nmat1775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


