Abstract
The route of transmission of Helicobacter pylori, which is usually acquired in childhood and is one of the most common bacterial infections in humans, remains undetermined. Mapping the distribution of H. pylori genotypes within families could help to determine the routes of transmission and risk factors. Here we describe a noninvasive method for obtaining H. pylori DNA isolates from the feces of children. Children presenting with gastrointestinal symptoms at the Royal Hospital for Sick Children were tested for gastric H. pylori colonization by using the 13C-urea breath test (UBT) and were asked to provide fecal samples, which were tested for H. pylori by using the HpSA fecal antigen test. DNA was purified from fecal samples by using a novel method of gene capture with subsequent H. pylori PCR analysis. Fifteen UBT-positive and 15 UBT-negative children participated in the study. The positive and negative predictive values for the assay were 80 and 100%, respectively. Fecal DNA purification followed by H. pylori PCR analysis is an effective tool for harvesting H. pylori DNA isolates from the feces of children. This technique may be developed to allow the diagnosis and noninvasive genotyping of H. pylori in children and their families.
Helicobacter pylori is one of the most common bacterial pathogens of humans (8). Primarily acquired in early life, it can be associated with both malnutrition (17) and growth retardation in children (14). Colonization is usually lifelong and may lead to chronic gastritis, duodenal ulceration (12), and gastric cancer in later life (4).
Despite many years of research into H. pylori, its mode(s) of transmission is not fully understood. Epidemiological studies have demonstrated the importance of close person-to-person contact and intrafamilial spread (3), but it remains uncertain whether transmission occurs primarily through the fecal-oral or gastric-oral route (13).
Publication of the complete genome sequence of H. pylori (21) and advances in molecular genotyping methods make possible the genotypic analysis of H. pylori DNA isolates within individuals, families, and communities (2). Studies suggest that transmission within families occurs most readily between siblings of similar ages (6) and is uncommon between couples (18). However, many questions remain unanswered, such as the relative importance of vertical or horizontal transmission and the risk of transmission from outside the family group. Large-scale genotyping studies could answer some of the epidemiological questions, but these are difficult to perform, mainly due to a lack of easily accessible H. pylori DNA.
H. pylori DNA is currently obtained during endoscopy (or a string test) from the stomach either directly or following bacterial culturing. These methods are invasive and inappropriate for studies involving children, which would be of particular epidemiological interest.
Fecal material could provide an easily accessible source of H. pylori DNA. H. pylori has been cultured from the feces of experimentally colonized individuals (13) and from children in The Gambia (20), but such culturing does not appear to be easy for the majority of patients. Several studies have detected H. pylori antigens in fecal material (15, 22, 23), but this technique cannot provide information beyond the presence or absence of antigens.
Although H. pylori colonizes only the stomach, it must pass through the intestine in a viable form, as it has been reported to colonize Meckel's diverticulum and other sites of acid-secreting epithelium (5). Its fate during further passage in the gastrointestinal tract remains unclear (25). Reported success rates for the detection of H. pylori DNA in feces vary from 25 to 100% (9, 16, 24). This variability is probably due to H. pylori degradation in the gastrointestinal tract and/or the presence of inhibitors.
Human feces contain inhibitory compounds of bacterial and/or plant origin, such as complex polysaccharides, that readily inhibit the further processing of purified DNA (10). Diets free of fruit and vegetables can reverse fecal inhibition (11), but such regimens are inappropriate for large-scale genotyping studies. A number of DNA purification methods have been developed to remove inhibitors; these involve organic extraction and ethanol precipitation (7), the use of macroporous polypropylene filters (1), and modified commercial kits. All of them have met with varying success, and a standardized method for the optimal recovery of H. pylori DNA isolates from feces has yet to be developed.
Recently, Shuber et al. (16) published a protocol that specifically enriches H. pylori DNA from complex fecal DNA mixtures. So far, this technique has demonstrated (for a small number of subjects) 100% sensitivity and specificity (16). Although this new technique showed promise, it included a proprietary initial purification step that may have hindered other groups from repeating the work. Here we describe the development of a nonproprietary, noninvasive H. pylori DNA purification protocol that is suited to studies involving children and that should open the way to the genotyping of H. pylori within families.
MATERIALS AND METHODS
Subjects.
Based on the results of the 13C-urea breath test (UBT), 30 children (15 positive and 15 negative for H. pylori) were studied. They were selected from children attending the Royal Hospital for Sick Children (Glasgow, Scotland) for a UBT as part of an investigation of gastrointestinal problems.
UBT.
Following an overnight fast, each subject ingested an oral dose of 100 mg of 13C-urea (99 atom% excess; CK Gas, Berks, United Kingdom), administered in 20 ml of 15% Polycose (Abbott Laboratories, Dublin, Ireland). Baseline breath samples were collected by asking the subject to blow with a straw into an Exetainer (Labco, High Wycombe, United Kingdom) before and at 30 and 45 min after ingestion of the labeled urea. The abundance of 13C in breath CO2 was measured by continuous-flow isotope ratio mass spectrometry (20/20; PDZ Europa, Crewe, United Kingdom) against a reference gas (3% CO2 in N2; Air Products Special Gases, Crewe, United Kingdom), which was calibrated against a secondary reference traceable to the international standard (Vienna Pee Dee Belemnite). The enrichment of the postdose sample was calculated by subtracting the abundance of the baseline sample from that of the postdose sample. An excess in 13C enrichment of more than 40.0 ppm (delta over baseline, 3.5‰ 13C) was regarded as diagnostic of H. pylori colonization (19).
Fecal analysis.
Each child provided a fecal sample from home by using a collection device, which fitted over the toilet, to transfer the sample directly into a sterile plastic bag. Immediately after collection, fecal samples were stored at −80°C until analysis.
An initial analysis for the presence of H. pylori in feces was performed by using an enzyme immunoassay as directed by the manufacturer (Premier Platinum HpSA; Meridian Diagnostics Inc., Cincinnati, Ohio). Briefly, diluted fecal samples and a peroxidase-conjugated polyclonal antibody were added to microwells coated with anti-H. pylori antibodies and incubated for 1 h at 24°C. The wells were cleansed with wash buffer (supplied in the kit) to remove any unbound material, and a substrate solution was added before a further 10 min of incubation. The reaction was stopped by the addition of a stop solution (sulfuric acid), and the absorbance was measured spectrophotometrically at 450 nm (Multiskan Acent; Thermo Life Sciences, Basingstoke, United Kingdom) (15).
For DNA analysis, fecal samples were thawed and processed immediately by homogenization in sterile phosphate-buffered saline (0.01 M phosphate buffer, 0.0027 M potassium chloride, 0.317 M sodium chloride [pH 7.4]) in a Stomacher 400 (Seward Medical, London, United Kingdom) for 10 min at room temperature to produce 20% (wt/vol) fecal slurries.
Fecal slurries were centrifuged at 20,000 × g for 30 min (Heraeus Biofuge Stratos centrifuge; Kendro Laboratory Products, Sollentum, Germany), and the supernatant was removed carefully so that the pellet was left undisturbed. Previous studies had shown that H. pylori was concentrated in a creamy white layer at the top of the pellet (12), which was removed by using a plastic Pasteur pipette. The suspension was transferred to a sterile 2-ml microcentrifuge tube (Hybaid, Middlesex, United Kingdom) and modified by the addition of an equal volume of PrepMan Ultra (Applied Biosystems, Cheshire, United Kingdom), which has been formulated for the removal of PCR-quality DNA from foodstuffs. Samples were boiled for 10 min, allowed to cool for 2 min, and then centrifuged at 20,000 × g for 10 min (Heraeus Biofuge Stratos) to pellet the particulates.
Total nucleic acid was separated from the protein by the phenol-chloroform-isoamyl alcohol (PCI) method with modifications as follows. Fecal concentrates were modified by the addition of an equal volume of PCI (25:24:1; Sigma-Aldrich, Dorset, United Kingdom) and mixed by inversion before centrifugation at 20,000 × g for 3 min (EBA 12 Centrifuge; Hettich Zentrifugen, Tuttlingen, Germany). The upper (aqueous) phase was transferred to a fresh 2-ml microcentrifuge tube, and the process was repeated once more. Trace PCI was removed from the aqueous phase by treatment as described above but with the addition of chloroform instead of PCI. Total nucleic acid was harvested by precipitation with 0.1 volume of ice-cold 3 M sodium acetate and 2 volumes of ice-cold 100% ethanol (Sigma-Aldrich). Precipitation was performed on ice for 30 min and followed by centrifugation at 16,000 × g for 15 min (EBA 12 Centrifuge), air drying, and resuspension of samples in 266 μl of ultrapure water (Sigma-Aldrich). RNA was removed by the addition of 40 U of RNase ONE (Promega, Southampton, United Kingdom) and incubation for 1 h at 37°C. The enzyme was removed by precipitation with sodium acetate and ethanol as described above.
Gene capture was performed by using an H. pylori-specific biotinylated capture probe (HpS1 [5-GGG GAG TAC GGT CGC AAG ATT AAA ACT CAA AGG AAT A-3]), which targeted the 16S rRNA gene of H. pylori (16) and which was supplied by Cruachem (Glasgow, United Kingdom). Total fecal DNA suspensions were mixed with 300 μl of 6 M guanidine thiocyanate (Sigma-Aldrich) and 20 nmol of the capture probe and incubated overnight at 25°C. H. pylori DNA sequences were harvested by using 10 μl of paramagnetic polystyrene beads coated with Streptavidin (Dynabeads M-280- Streptavidin; Dynal ASA, Oslo, Norway) and washed three times in wash buffer (0.1 M Tris-HCl, 0.01 M EDTA, 1 M sodium chloride, 0.1% [vol/vol] Tween 20; Sigma-Aldrich). Final harvesting was performed at 85°C (for 6 min), and H. pylori DNA was transferred to a clean tube.
PCR reagents were supplied by AbGene (Surrey, United Kingdom). Each PCR consisted of reaction buffer, 1.5 mM MgCl2, 200 μM deoxynucleoside triphosphates, forward and reverse primers at 0.5 μM each, 1.25 U of Hotstart DNA polymerase, and 10 μl of target DNA (final reaction volume, 50 μl). The following primers were synthesized by Cruachem: HpF (5-GCG ACC TGC TGG AAC ATT AC-3) and HpR (5-CGT TAG CTG CAT TAC TGG AGA-3) (7). All experiments included both a positive control (purified H. pylori DNA) and a negative control (the template DNA was substituted with sterile water).
Thermal cycling was performed with a PTC-200 Peltier thermal cycler (MJ Research, Waltham, Mass.). The thermal cycling parameters were as follows: an initial denaturation at 94°C for 15 min (prolonged heating at this temperature was required to activate the Hotstart DNA polymerase enzyme); 40 cycles of denaturation at 94°C for 1 min, annealing at 60°C for 1 min, and extension at 72°C for 1 min; and a final extension at 72°C for 5 min. All PCR products were analyzed by gel electrophoresis in a 3% (wt/vol) agarose gel (containing 0.6 μg of ethidium bromide ml−1) in 0.5× Tris-acetate- EDTA buffer. Electrophoresis was carried out for 1 h at 50 V (RunOne electrophoresis cell; Embitec, San Diego, Calif.), and DNA bands were examined under UV illumination.
One H. pylori strain, five nonpyloric Helicobacter strains, and one Campylobacter jejuni strain were cultivated on Columbia blood agar base (Oxoid, Hants, United Kingdom) supplemented with 7% defibrinated horse blood (TCS Biosciences, Buckingham, United Kingdom) for 7 days under microaerobic conditions (37°C) in gas jars (Becton Dickinson, Oxford, United Kingdom). The total biomass was harvested, resuspended in phosphate-buffered saline, and enumerated by microscopy (at a magnification of ×1,000 [phase contrast]) under oil immersion with an Axioscope microscope (Leica Microsystems, Milton Keynes, United Kingdom) and a cell counting chamber (Sigma-Aldrich) according to the manufacturer's instructions.
The specificity of the PCR alone was determined with 1-μl volumes of cultivated bacterial cells. The specificity of the assay (DNA purification followed by PCR) was determined by seeding 20% fecal slurries made from samples from an H. pylori-negative control subject (as determined by the UBT and HpSA test) with bacterial cells. The resultant fecal slurries were purified, and the DNA was subjected to PCR.
The sensitivity of the assay was determined by seeding fecal slurries made from samples from an H. pylori-negative control subject (determined by the UBT and HpSA test) with serial dilutions of H. pylori cells. The resultant slurries were purified, and the DNA was assayed by PCR. The sensitivity was defined as the lowest concentration of H. pylori cells (per gram of feces) that resulted in PCR amplification.
RESULTS
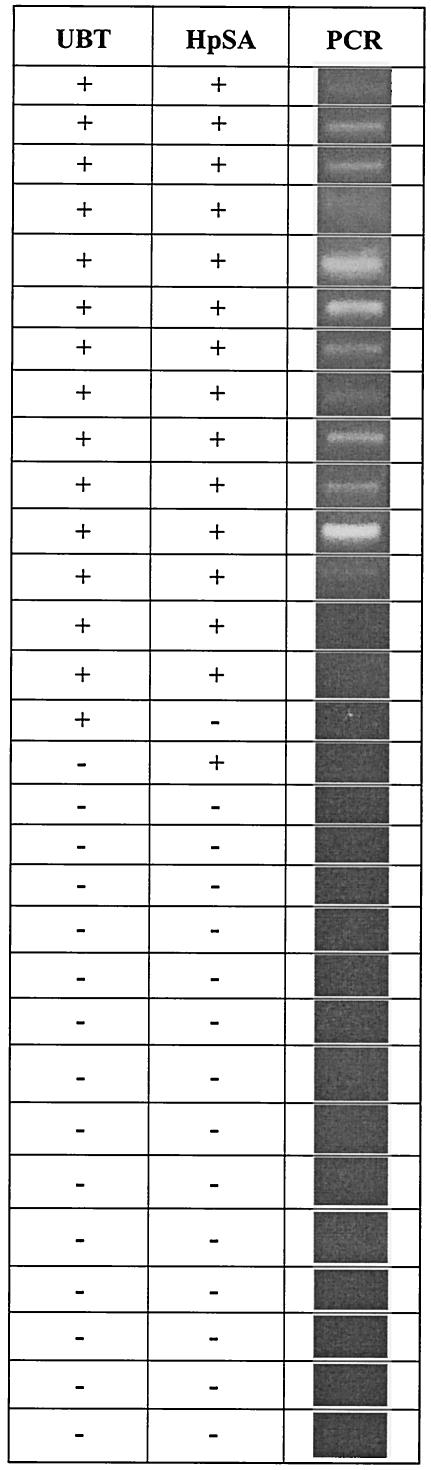
Based on the results of the UBT, 30 children (15 positive and 15 negative for H. pylori) were selected for the study. They differed in no significant way in sex, age, or socioeconomic status. Fourteen children were positive and 14 were negative in both the UBT and the HpSA test. One child was UBT negative but HpSA test positive, and one child was UBT positive but HpSA test negative. There were no equivocal HpSA test results. The positive and negative predictive values were 93% (Fig. 1).
FIG. 1.
Data set showing results for H. pylori PCR compared to the UBT and the HpSA test. The overall positive and negative predictive values for the HpSA and PCR compared to the UBT were 93 and 93%, respectively, and 80 and 100%, respectively.
Twelve children were positive and 15 were negative in both the UBT and PCR. One child was UBT positive but HpSA test and PCR negative, and one child was UBT and PCR negative but HpSA test positive. Two children were positive in the UBT and HpSA test but negative in PCR. The positive predictive value was 80%, and the negative predictive value was 100% (Fig. 1).
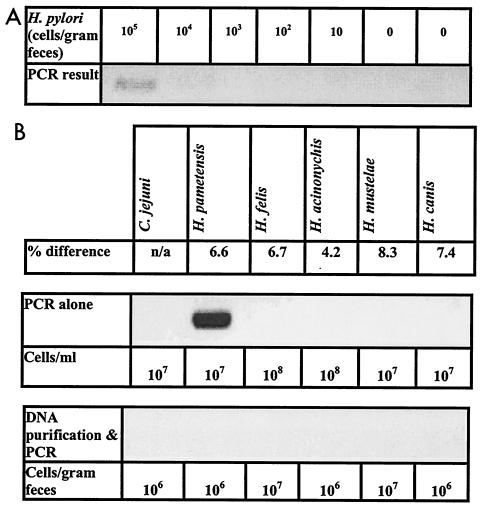
The PCR assay amplified DNA directly from one (H. pametensis) out of the five nonpyloric Helicobacter strains and one C. jejuni strain tested. Fecal slurries made from samples from an H. pylori-negative subject and seeded with these species did not yield positive PCR results following DNA purification. The limit of sensitivity of the assay was 105 H. pylori cells/g of feces (Fig. 2).
FIG. 2.
Determination of PCR assay specificity and sensitivity. Specificity was tested by using five nonpyloric Helicobacter strains and one C. jejuni strain. Percent differences between the Helicobacter spp. were determined by comparison of 16S rRNA genes as reported by Solnick and Vandamme (16a). The sensitivity of the assay was determined by purification and amplification of serial dilutions of H. pylori cells seeded into fecal slurries from samples from an H. pylori-negative subject. (A) Sensitivity of the assay; (B) specificity of the PCR alone and the assay (DNA purification followed by PCR).
DISCUSSION
The development of noninvasive tests for H. pylori has traditionally concentrated on diagnostic tests because of a reluctance to perform invasive procedures on young individuals unless absolutely necessary. Noninvasive tests report the presence or absence of H. pylori but provide no further information, such as which genotypes are present.
The ability to genotype H. pylori from children without the need for invasive procedures would be a major advance and would make possible both the surveillance of H. pylori in families and the detection of antibiotic resistance in patients whose treatment has failed, without the need for a second endoscopy. There is also the possibility of screening for the presence of virulence genes in asymptomatic carriers so that the risk of later complications can be determined. For all of the above reasons, there is a need to develop noninvasive robust methods for harvesting H. pylori DNA from colonized subjects.
We utilized a PCR protocol developed by Gramley et al. (7) to determine the efficacy of a novel fecal DNA purification method that was reported by Shuber et al. (16) and that utilized several steps (including gene capture) to yield pure H. pylori DNA for PCR analysis. The original protocol developed by Shuber et al. (16) included an initial fecal cleanup step, which was the intellectual property of the Exact Science Corporation (Maynard, Mass.). We replaced this step with a novel system for initial fecal cleanup that involved concentrating bacteria from fecal slurries by centrifugation, a procedure which previous work had shown could be used to culture bacteria from feces (20).
The PCR used in this study (7) amplified DNA directly from one nonpyloric Helicobacter strain (Fig. 2). However, fecal slurries seeded with bacteria closely related to H. pylori and subjected to DNA purification did not yield any positive PCR results. The gene capture step used during DNA purification was selective for H. pylori. The assay developed during this study was also found to be a sensitive tool, with a limit of sensitivity of 105 H. pylori cells/g of feces (Fig. 2).
Using feces from children with a known H. pylori status, we investigated the efficacy of this new protocol for the recovery of PCR-quality H. pylori DNA. The H. pylori status of the children was determined by using the UBT, which is the standard noninvasive diagnostic test (19). The presence or absence of H. pylori in feces was verified by using the HpSA fecal antigen test (15). A comparison of the three assays demonstrated a high degree of agreement. However, both the HpSA test and PCR failed to detect the presence of H. pylori in the feces of one and three UBT-positive children, respectively (Fig. 1). The lack of agreement between the HpSA test and PCR suggests that the problem was not the intermittent shedding of H. pylori. The finding of children who were UBT positive but PCR negative may indicate that in 20% of our cases, the purification protocol failed to provide DNA of adequate quality and/or quantity for analysis. In contrast, Shuber et al. (16) reported a positive predictive value of 100% for adult feces.
Children's feces are more difficult to work with because of a greater variety in volume and consistency, and our failure to obtain a positive predictive value of 100% may have been due to the quality of the fecal samples studied. Further work is under way with a larger number of children to investigate the effect of sample quality and to improve the positive predictive value of this novel extraction method.
For investigation of the feces of UBT-negative children, the PCR assay had a negative predictive value of 100%, while that for the HpSA test was 93%; these results suggest that the fecal PCR assay may be a more reliable tool than the HpSA test for verifying the successful eradication of H. pylori following antibiotic therapy (Fig. 1).
We conclude that fecal DNA purification involving gene capture coupled with PCR is an effective tool for obtaining H. pylori DNA from the feces of children.
This method has the potential to be a useful tool for investigating the genotypes of H. pylori harvested from feces. It should allow the study of genotypes of H. pylori in families to determine the route of transmission of this important human pathogen.
Acknowledgments
W. G. MacKay was supported by Chief Scientist Office grant CZB/4/22. R. N. Ndip was supported by Commonwealth Scholarship and Fellowship grant CMCF-2000-23.
We are indebted to Christine Slater (University of Glasgow) for the UBT analysis.
REFERENCES
- 1.Cavallini, A., P. Notarnicola, P. Berloco, A. Lippolis, and A. Di Leo. 2000. Use of macroporous polypropylene filter to allow identification of bacteria by PCR in human fecal samples. J. Microbiol. Methods 39:265-270. [DOI] [PubMed] [Google Scholar]
- 2.Colding, H., S. H. Hartzen, H. Roshanisefat, L. P. Andersen, and K. A. Krogfelt. 1999. Molecular methods for typing of Helicobacter pylori and their applications. FEMS Immunol. Med. Microbiol. 24:193-199. [DOI] [PubMed] [Google Scholar]
- 3.Drumm, B., G. I. Perez-Perez, M. J. Blaser, and P. M. Sherman. 1990. Intrafamilial clustering of Helicobacter pylori infection. N. Engl. J. Med. 322:359-363. [DOI] [PubMed] [Google Scholar]
- 4.Eurogast Study Group. 1993. An international association between Helicobacter pylori infection and gastric cancer. Lancet 341:1359-1362. [PubMed] [Google Scholar]
- 5.Finn, L. S., and D. L. J. Christie. 2001. Helicobacter pylori and Meckel's diverticula. J. Pediatr. Gastroenterol. Nutr. 32:150-155. [DOI] [PubMed] [Google Scholar]
- 6.Goodman, K. J., and P. Correa. 2000. Transmission of Helicobacter pylori among siblings. Lancet 355:358-362. [DOI] [PubMed] [Google Scholar]
- 7.Gramley, W. A., A. Asghar, H. F. Frierson, and S. M. Powell. 1999. Detection of Helicobacter pylori DNA in fecal samples from infected individuals. J. Clin. Microbiol. 37:2236-2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holcombe, C., B. A. Omotata, J. Eldridge, and D. M. Jones. 1992. Helicobacter pylori, the most common chronic bacterial infection in Africa: a random serological study. Am. J. Gastroenterol. 87:28-30. [PubMed] [Google Scholar]
- 9.Li, C. F., T. Z. Ha, D. A. Ferguson, D. S. Chi, R. G. Zhao, N. R. Patel, G. Krishnaswamy, and E. Thomas. 1996. A newly developed PCR assay of H. pylori in gastric biopsy, saliva, and feces—evidence of high prevalence of H. pylori in saliva supports oral transmission. Dig. Dis. Sci. 41:2142-2149. [DOI] [PubMed] [Google Scholar]
- 10.Monteiro, L., D. Bonnemaison, A. Vekris, K. G. Petry, J. Bonnet, R. Vidal, J. Cabrita, and F. Megraud. 1997. Complex polysaccharides as PCR inhibitors in feces: Helicobacter pylori model. J. Clin. Microbiol. 35:995-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monteiro, L., N. Gras, R. Vidal, J. Cabriti, and F. Megraud. 2001. Detection of Helicobacter pylori DNA in human feces by PCR: DNA stability and removal of inhibitors. J. Microbiol. Methods 45:89-94. [DOI] [PubMed] [Google Scholar]
- 12.National Institutes of Health. 1994. Helicobacter pylori and peptic ulcer disease, p. 1-22. NIH Consensus Statement. National Institutes of Health, Bethesda, Md.
- 13.Parsonnet, J., H. Shmuely, and T. Haggerty. 1999. Fecal and oral shedding of Helicobacter pylori from healthy infected adults. JAMA 23:2240-2245. [DOI] [PubMed] [Google Scholar]
- 14.Patel, P., M. A. Mendall, S. Khulusi, T. C. Northfield, and D. P. Strachan. 1994. Helicobacter pylori infection in childhood: risk factors and effect on growth. Br. Med. J. 309:1119-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shepherd, A. J., C. L. Williams, C. P. Doherty, M. Hossack, T. Preston, K. E. L. McColl, and L. T. Weaver. 2000. Comparison of an enzyme immunoassay for the detection of Helicobacter pylori antigens in the feces with the urea breath test. Arch. Dis. Child. 83:268-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shuber, A. P., J. J. Ascano, K. A. Boynton, A. Mitchell, H. F. Frierson, W. El-Rifai, and S. M. Powell. 2002. Accurate, noninvasive detection of Helicobacter pylori DNA from stool samples: potential usefulness for monitoring treatment. J. Clin. Microbiol. 40:262-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16a.Solnick, J. V., and P. Vandamme. 2001. Taxonomy of the Helicobacter genus, p. 39-51. In H. L. T. Mobley, G. L. Mendz, and S. L. Hazell (ed.), Helicobacter pylori: physiology and genetics. ASM Press, Washington, D.C. [PubMed]
- 17.Sullivan, P. B., J. E. Thomas, D. G. W. Wight, G. Neale, E. J. Eatham, T. Corrah, N. Lloyd-Evans, and B. M. Greenwood. 1990. Helicobacter pylori infection in Gambian children with chronic diarrhoea and malnutrition. Arch. Dis. Child. 65:189-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki, J., H. Muraoka, I. Kobayasi, T. Fujita, and T. Mine. 1999. Rare incidence of interspousal transmission of Helicobacter pylori in asymptomatic individuals in Japan. J. Clin. Microbiol. 37:4174-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas, J. E., A. Dale, M. Harding, W. A. Coward, T. J. Cole, P. B. Sullivan, D. I. Campbell, B. F. Warren, and L. T. Weaver. 1999. Interpreting the 13C-urea breath test among a large population of young children from a developing country. Pediatr. Res. 46:147-151. [DOI] [PubMed] [Google Scholar]
- 20.Thomas, J. E., G. Gibson, M. Darboe, A. Dale, and L. T. Weaver. 1992. Isolation of Helicobacter pylori from human feces. Lancet 340:1094-1095. [DOI] [PubMed] [Google Scholar]
- 21.Tomb, J. F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. X. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, E. K. Hickey, D. E. Berg, J. D. Gocayne, T. R. Utterback, J. D. Peterson, J. M. Kelley, M. D. Cotton, J. M. Weldman, C. Fujii, C. Bowman, L. Watthey, E. Wallin, W. S. Hayes, J. M. Weidman, C. Fujii, M. Borodovsky, P. D. Karp, H. O. Smith, C. M. Fraser, and J. C. Venter. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 22.Trevisani, L., S. Sartori, F. Galvani, A. Dentale, M. Caselli, M. Ruina, and B. Bigoni. 1998. Evaluation of a new enzyme immunoassay for detecting Helicobacter pylori in feces. Gut 43(Suppl. 2):A47-A48. [DOI] [PubMed] [Google Scholar]
- 23.Trevisani, L., S. Sartori, M. Ruina, M. Caselli, M. R. Rossi, F. Costa, M. Bellini, G. Iaquinto, N. Gardullo, and A. Todisco. 1999. Helicobacter pylori stool antigen test—clinical evaluation and cost analysis of a new enzyme immunoassay. Dig. Dis. Sci. 44:2303-2306. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe, T., S. Tomita, M. Kudo, M. Kurokawa, A. Orino, A. Todo, and T. Chiba. 1998. Detection of Helicobacter pylori gene by means of immunomagnetic separation-based polymerase chain reaction in feces. Scand. J. Gastroenterol. 33:1140-1143. [DOI] [PubMed] [Google Scholar]
- 25.Weaver, L. T., A. J. Shepherd, C. P. Doherty, K. E. L. McColl, and C. L. Williams. 1999. Helicobacter pylori in the feces? Q. J. Med. 92:361-364. [DOI] [PubMed] [Google Scholar]