Abstract
Background:
Previously published cohort studies in clinical populations have suggested that obstructive sleep apnea (OSA) is a risk factor for mortality associated with cardiovascular disease. However, it is unknown whether sleep apnea is an independent risk factor for all-cause mortality in a community-based sample free from clinical referral bias.
Methods:
Residents of the Western Australian town of Busselton underwent investigation with a home sleep apnea monitoring device (MESAM IV). OSA was quantified via the respiratory disturbance index (RDI). Mortality status was determined in 397/400 participants (99.3%) after up to 14 years (mean follow-up 13.4 years) by data matching with the Australian National Death Index and the Western Australian Death Register. Univariate analyses and multivariate Cox proportional hazards modelling were used to ascertain the association between sleep apnea and mortality after adjustment for age, gender, body mass index, mean arterial pressure, total cholesterol, high-density lipoprotein cholesterol, diabetes, and medically diagnosed angina in those free from heart attack or stroke at baseline (n = 380).
Results:
Among the 380 participants, 18 had moderate-severe OSA (RDI ≥15/hr, 6 deaths) and 77 had mild OSA (RDI 5 to <15/hr, 5 deaths). Moderate-to-severe OSA was independently associated with greater risk of all-cause mortality (fully adjusted hazard ratio [HR] = 6.24, 95% CL 2.01, 19.39) than non-OSA (n = 285, 22 deaths). Mild OSA (RDI 5 to <15/hr) was not an independent risk factor for higher mortality (HR = 0.47, 95% CL 0.17, 1.29).
Conclusions:
Moderate-to-severe sleep apnea is independently associated with a large increased risk of all-cause mortality in this community-based sample.
Citation:
Marshall NS; Wong KKH; Liu PY; Cullen SRJ; Knuiman MW; Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: The Busselton Health Study. SLEEP 2008;31(8):1079-1085.
Keywords: Sleep apnea, cohort, mortality
OBSTRUCTIVE SLEEP APNEA (OSA) IS CHARACTERIZED BY REPETITIVE UPPER AIRWAY CLOSURE DURING SLEEP RESULTING IN REPEATED REVERSIBLE blood oxygen desaturation and fragmented sleep. OSA has been associated with a range of pathophysiological changes that impair cardiovascular function,1 including increased blood inflammatory markers and repeated rises in blood pressure during sleep. There is increasing evidence that OSA promotes the development of hypertension, stroke, myocardial infarction and premature death.2 However, because OSA is strongly associated with obesity and thus also with many other obesity-related diseases, it has been difficult to produce clear evidence that these associations are caused by sleep apnea and not other established causes.
Better understanding of the association between OSA and mortality risk has major public health importance.3 A number of studies have shown that approximately 25% of middle-aged men and 9% of middle-aged women stop breathing during sleep ≥ 5 times per hour.4–6 Given that the major modifiable risk factor for OSA is obesity it is likely that the prevalence of OSA is increasing.7 Thus, as a very common condition, even modest effects of sleep apnea on morbidity and mortality would be important.3
Previous reports that have independently linked OSA to mortality have all been based on patients referred to sleep clinics.8–14 Two of the most recent and frequently cited of these studies indicated that severe sleep apnea, compared to no sleep apnea, was an independent risk factor for cardiovascular mortality (odds ratio 2.87, 95% CL 1.17, 7.51)12; the other study found that sleep apnea, compared to no sleep apnea, was an independent risk factor for a composite endpoint of all-cause mortality or incident stroke (hazard ratio 1.97, 95% CL 1.12, 3.48).11 However, as these were not community recruited participants they might have been subject to a clinical referral bias that might have given a false impression of the size or significance of the true association with mortality in the community. The choice of composite or restricted mortality endpoints also failed to rule out that sleep apnea might have had some unexpected beneficial effect that reduced mortality from other causes. Data from a community-based cohort, free from clinical referral bias, would thus be useful to determine whether sleep apnea is an independent risk factor for all-cause mortality in middle-aged people.
The community of Busselton in Western Australia has been the subject of cross-sectional and follow-up health surveys since 1966.15–17 We aimed to investigate whether sleep apnea is an independent risk factor for all-cause mortality in a sample of Busselton Health Study participants recruited in 1990 to determine the community prevalence of sleep apnea.4
METHODS
In November-December 1990, 400 residents of the rural town of Busselton in the state of Western Australia who were already participants in the ongoing Busselton Health Study had their sleep disordered breathing assessed by a single night of recording in their own homes using the MESAM IV device. The Busselton Health Study is an ongoing representative and comprehensive survey of residents in the Shire of Busselton in the South-West region of Western Australia. The survey invites the participation of all individuals on the Commonwealth electoral roll. Electoral registration is compulsory for Australians ≥ 18 years of age. The MESAM IV (Madaus Medizen-Elektronik, Freiberg, Germany), is a 4-channel portable home-monitoring device used to quantify sleep disordered breathing via the measurement of snoring, heart rate, oxygen saturation, and body position. The full methods for implementation and manual scoring of this technique are described in the original prevalence paper.4 The current analyses were undertaken after approval by the University of Sydney Human Research Ethics Committee.
SAMPLE CONSTRUCTION
Men
The 2-stage sampling process has already been described elsewhere.4 Briefly, all men aged 40–65 on the Busselton Health Study Register were sent the initial sleep questionnaire (n = 758), of whom n = 486 responded. These 486 men were randomly telephoned until a sample of 311 had been recruited for an at-home overnight study (all available study appointments were filled). Five respondents who were invited to participate in the overnight study declined the test. Two hundred and ninety-four of these overnight study recordings were of adequate quality to score, and 293 men had matched longitudinal data.
Women
All women aged 40–65 on the register (n = 810) were sent the sleep questionnaire, of whom n = 537 responded. At-home overnight studies were undertaken in equal numbers from the 3 categories of snoring (never, sometimes, and almost always/always, each n = 38) so as to ensure some cases of OSA. Women were randomly telephoned until a sample of 114 had been recruited (all available study appointments were filled). Six women who were telephoned did not participate. One hundred and six of these recordings were of adequate quality to score, and 104 women had matched longitudinal data. Less women than men were sampled for financial and logistic reasons.5
Outcome: Mortality and Vitality Ascertainment
Mortality status and time to death was ascertained via the last available match (2004) between the cohort and the Western Australian Health Research Linked Database that contains linked death and morbidity data for all people who resided in Western Australia since 1980.18 The system includes interstate deaths via the National Death Index.19 Survival was confirmed via listings in the telephone directory, direct contact with relatives, or listing on the electoral role (which is compulsory in Australia). Cause of death as listed on the death certificates was also matched.
Exposure Variables
Exposure to sleep apnea was quantified by the respiratory disturbance index (RDI), which is calculated by summing the total number of respiratory disturbances and dividing by the participant estimated hours of sleep to give an event rate per hour. Respiratory disturbances were defined as oxygen desaturations of ≥ 3% from the preceding baseline level that were accompanied by either a) an increased heart rate ≥ 10 beats/min and/or b) a burst of snoring associated with commencement and termination of the desaturation event (i.e., an audible apnea). Hours of sleep were estimated using lights off and lights on time—a method that would have overestimated sleep time and therefore caused a systematically lower estimation of RDI than would have been estimated via traditional polysomnography (PSG).
Validation in 10 patients with a wide OSA severity range who had been referred for PSG investigation of sleep disordered breathing at the Royal Prince Alfred Hospital, Sydney, Australia, closely correlated simultaneous MESAM IV recordings (ICC = 0.98). Thus scoring of MESAM IV data for RDI by this method gave a very close approximation to simultaneously measured but independently scored standard PSG measured RDI (including pressure transducer measured airflow). The MESAM IV has also been validated against PSG by other investigators.20,21
Anthropometric variables were measured by study investigators on the evening before the sleep study and included height, weight, abdominal height while supine (sagittal diameter), as well as neck, waist, and hip circumference. Blood pressure was then measured twice via an electronic sphygmomanometer (Spacelabs, Redmond, WA) with measurements at ≥ 5 min apart in the supine position and after the participant had been lying quietly for ≥ 10 min. The mean of these measurements was then calculated.
History of stroke, heart attack, diabetes, and medically diagnosed angina were measured via self-report questionnaire. Where these medical history questions had not been answered a participant was assumed to have not been diagnosed with the condition. Smoking status was ascertained via questionnaire by asking whether the patient was a current, former, or never smoker. Former and current smokers were queried about smoking duration and use in order to calculate pack years. Alcohol consumption patterns were queried in order to calculate the approximate grams of alcohol consumption per week. Fasting blood samples were taken the morning after the overnight study for blood glucose and total and HDL cholesterol and analyzed using standard assay methods at the Western Australian State Health laboratories.
Data Handling and Statistical Analyses
Analyses were undertaken (NSM, KKHW supervised by PYL and MWK) using SAS (v 9.1 SAS institute NC, USA). We categorized sleep apnea as a continuous variable and also by using standard clinical cut-points for severity.22 No or subclinical sleep apnea served as the reference category (i.e., 0 to 4 respiratory disturbances per hour), and the 2 exposed categories were mild (RDI 5 to < 15) and moderate-to-severe OSA (RDI ≥ 15). We were unable to examine the association of severe OSA with mortality because only 3 participants had an RDI ≥ 30 at baseline.
Previously established risk factors for mortality were analyzed with the variables transformed or parameterized to maximize model fit (using AIC criteria) and to reduce the chance that any observed association between sleep apnea and mortality was due to poor controlling of known risk factors.
Mean arterial pressure (2/3*Systolic + 1/3*Diastolic), gave the best blood pressure fit to observed mortality. The best fit for body habitus was BMI classified into normal/overweight/obese (< 25, 25 to < 30, or ≥ 30 kg/m2). Smoking defined categorically (never, ex, current) had the best fit, whereas pack years was not a significant mortality risk factor.
Univariate associations between continuous risk factors were investigated with Cox proportional hazards models using chi-square tests for significance (PROC PHREG). Categorical risk factors for mortality were undertaken (in PROC LIFETEST) with visually inspected Kaplan-Meier curves and log-rank tests for determining statistical significance and also with Cox models. All risk factors were also investigated for their association with sleep apnea severity categories using chi-square, Fisher exact, ANOVA or Kruskal-Wallis tests, where appropriate.
Multivariate mortality models were built using Cox regression and confirmed by best-subset variable selection. Regardless of univariate association the following risk factors were forced into the fully adjusted model because of known associations with OSA or mortality: age, gender, obesity, smoking status, blood pressure, total cholesterol, high density lipoprotein (HDL) cholesterol, angina and diabetes. Other risk factors were examined for independent association with mortality when they exhibited some evidence of a univariate association with either mortality or with sleep apnea (P < 0.1). Fasting blood glucose was associated with sleep apnea (see Table 1). However, it was not associated with mortality (P > 0.5) when added to the fully adjusted model and did not change the size and significance of the association between sleep apnea and mortality. It was not included in the final model because of a number of missing values.
Table 1.
Association of Sleep Apnea with Mortality Risk Factors
| Baseline Confounder Mean (SD) | No OSA (RDI <5) n = 285 | Mild OSA RDI (5 to <15) n = 77 | Moderate-Severe OSA (RDI ≥15) n = 18 | P Value For Difference |
|---|---|---|---|---|
| Females % (n, cases) | 27.4 (78) | 24.7 (19) | 27.8 (5) | 0.89† |
| Age (years) | 52.6 (7.5) | 54.3 (7.2) | 55.1 (8.2) | 0.09 |
| Body mass index (kg/m2) | 26.2 (3.7) | 27.9 (4.1) | 34.3 (7.3) | < 0.001 |
| Current smokers % (n, cases) | 15.4 (44) | 19.5 (15) | 5.6 (1) | 0.38 |
| Pack years | 12.9 (19.7) | 16.8 (22.3) | 9.4 (16.1) | 0.21‡ |
| Waist-hip ratio | 0.90 (0.08) | 0.92 (0.09) | 0.94 (0.06) | 0.02 |
| Alcohol consumption (g/week) | 86.2 (104.3) | 101.2 (118.9) | 112.3 (113.3) | 0.37‡ |
| Mean arterial pressure | 112.4 (11.8) | 117.1 (12.7) | 118.1 (14.2) | 0.003 |
| High density lipoprotein Cholesterol | 1.26 (0.33) | 1.17 (0.34) | 1.14 (0.29) | 0.053 |
| Triglycerides | 1.51 (1.09) | 1.77 (1.32) | 1.75 (1.07) | 0.19 |
| Total cholesterol | 5.9 (1.0) | 6.0 (1.1) | 5.9 (0.8) | 0.68 |
| Fasting glucose | 5.6 (0.8) | 5.8 (0.6) | 6.7 (2.6) | < 0.001‡ |
| Diabetes % (n, cases) | 2.5 (7) | 2.6 (2) | 11.1 (2) | 0.11† |
| Doctor diagnosed angina % (n, cases) | 7.4 (21) | 2.6 (2) | 16.7 (3) | 0.08† |
Figures represent means and SD unless otherwise stated. No participants with unascertained mortality (n = 3) and none with either a heart attack or stroke at baseline (n = 17) were analyzed. Categorical variables tested with chi square or Fisher exact (marked with † where moderate-severe OSA compared to mild and no OSA) and continuous variables with ANOVA or with a Kruskal-Wallis test as appropriate (marked with‡).
A partially adjusted model where blood pressure was not controlled for was also presented for comparison with the fully adjusted model as OSA is a known cause of hypertension and thus such models are properly presented in investigations of sleep apnea cohorts.11,12,23,24 We also prepared a model where only variables that were significant predictors of mortality in the main model were entered. All three of unadjusted, partially adjusted and fully adjusted models yielded similar conclusions (see results).
Schoenfeld residuals were used to confirm the proportional hazards assumption and Martingale residuals to ensure that continuous variables were approximately linear in their association with mortality risk.25,26
Additionally we performed a number of secondary analyses. Firstly, all deaths occurring within the first 2 years of follow-up were removed from the analysis to exclude the remote possibility that sleep apnea was itself caused by another covert and ultimately fatal unknown disease. Secondly, we explored the relationship between OSA severity and mortality further by arbitrarily removing the 2% most extreme RDI values, to ensure that any relationship between RDI and hazard ratio was not driven entirely by a few outliers.
RESULTS
Those participants who indicated at baseline that they had already had a stroke (n = 4) or a heart attack (n = 13) were excluded from mortality analyses which is consistent with a previous investigation.11 Inclusion of these 17 individuals in models which statistically controlled for previous heart attack and stroke did not change the size or significance of the association between sleep apnea and mortality. This left a total of 380 participants (n = 102 females), of whom 33 had died. These 380 participants with a mean 13.4 years of observation yielded a total of 5073.1 person-years of observation. However, in the primary model a further 6 participants did not have complete data and were thus excluded (none of these participants had died).
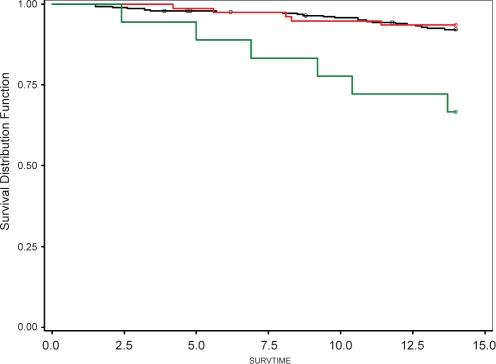
Eighteen participants had moderate-to-severe sleep apnea, 6 of whom died (33.3% mortality, 215.6 person years = 2.78 deaths per 100 person years of observation). Seventy-seven participants had mild OSA, 5 of whom died (6.5% mortality, 1045.6 person years = 0.48 deaths per 100 person years). Two-hundred and eighty-five participants did not have OSA, 22 of whom died (7.7% mortality, 3811.9 person years = 0.58 deaths per 100 person years). A Kaplan-Meier plot (Figure 1) and log-rank test indicated that this univariate association was significant (P < 0.001). The observed association suggested that RDI be investigated as a categorical variable.22 We have however examined RDI as a linear variable in some sub-analyses. Determining whether this relationship is truly linear or curvilinear will require larger data sets.
Figure 1.
Green line indicates the survival distribution for participants with moderate-severe sleep apnea (RDI ≥15), the Red line those with mild sleep apnea (RDI 5–14) and the Black line those with no sleep apnea (RDI <5). Circles indicate censored observations. The visually apparent difference between the Green line and the Black line is statistically significant (P < 0.001, see also Table 2 and Table 3 for multivariate confirmation).
Table 1 lists the association of important mortality risk factors with sleep apnea severity. Table 2 lists the univariate associations between these risk factors and mortality. Table 3 shows the association between categories of sleep apnea severity and mortality before and after adjustment for all other identified risk factors and confounders (the association between these risk factors and mortality in the adjusted models can be found in Supplemental Table 1). All models yielded a significant relationship between sleep apnea and mortality. There were no significant interaction effects between sleep apnea and gender, age, smoking status, or mean arterial pressure (P > 0.4). A potential interaction between HDLC and gender was also nonsignificant when tested (P = 0.7). However, given the relatively small sample size, there is limited statistical power to detect potential effect modifications.
Table 2.
Univariate Risk Factors for Mortality
| Baseline Risk Factor | Unit of Measure | Hazard Ratio (95% CL) | P value |
|---|---|---|---|
| Sleep apnea | Per 10 units of Respiratory Disturbance Index | 1.55 (1.17, 2.06) | < 0.01 |
| Sleep apnea categories | <5 events/hr | 1.00 (REF) | |
| 5 to <15 events/hr | 0.83 (0.31, 2.19) | 0.70 | |
| ≥ 15 events/hr | 4.96 (2.01, 12.23) | < 0.001 | |
| Gender | Female | 1.00 (REF) | |
| Male | 1.68 (0.69, 4.07) | 0.25 | |
| Diabetes | No | 1.00 (REF) | |
| Yes | 4.01 (1.22, 13.14) | 0.02 | |
| Angina | No | 1.00 (REF) | |
| Yes | 1.91 (0.67, 5.44) | 0.25 | |
| Age | Per decade | 3.58 (1.97, 6.52) | < 0.001 |
| Mean arterial pressure | Per 10 mm Hg | 1.67 (1.29, 2.16) | < 0.001 |
| High density lipoprotein cholesterol | mmol/L | 0.33 (0.10, 1.05) | 0.06 |
| Total cholesterol | mmol/L | 0.92 (0.66, 1.28) | 0.62 |
| Triglycerides | mmol/L | 1.07 (0.82, 1.41) | 0.62 |
| Smoking | Per 10 pack years | 1.16 (1.02, 1.32) | 0.02 |
| Smoker categories | Never | 1.00 (REF) | |
| Past | 1.31 (0.56, 3.08) | 0.54 | |
| Current | 3.81 (1.65, 8.82) | < 0.01 | |
| Fasting glucose | mmol/L | 1.05 (0.76, 1.45) | 0.76 |
| Body mass index | Per BMI unit | 1.01 (0.94, 1.09) | 0.74 |
| Body mass categories | Normal <25 | 1.00 (REF) | |
| Overweight 25-<30 | 0.91 (0.42, 2.00) | 0.81 | |
| Obese ≥30 | 1.10 (0.43, 2.80) | 0.84 |
Figures are derived from univariate Cox models. REF = reference category; BMI = body mass index; CL = confidence limits
Table 3.
Independent Association of Sleep Apnea with Mortality after Adjustment for Other Mortality Risk Factors
| Sleep Apnea Severity | Unadjusted Hazard Ratio (95% CL) | Partially Adjusted Hazard Ratio (95% CL)† | Fully Adjusted Hazard Ratio (95% CL)‡ |
|---|---|---|---|
| Moderate-to-severe sleep apnea | 4.96 (2.01, 12.23)* | 4.40 (1.48, 13.07)** | 6.24 (2.01, 19.39)*** |
| Mild sleep apnea | 0.83 (0.31, 2.19) | 0.62 (0.23, 1.69) | 0.47 (0.17, 1.29) |
| No sleep apnea | 1.00 (REF) | 1.00 (REF) | 1.00 (REF) |
†Adjusted for age, gender, body mass index (normal, overweight, obese), smoking status (never, ex, current), total cholesterol, high density lipoprotein cholesterol, diabetes (yes/no), doctor diagnosed angina. ‡Adjusted for all of the above plus mean arterial pressure. *P < 0.001, **P = 0.008, ***P = 0.002. REF = reference category. CL = confidence limits
Supplemental Table 1.
Independent Risk Factors for Mortality after Adjustment for Sleep Apnea
| Risk Factor | Partially Adjusted Hazard Ratio (95% CL)† | Partially adjusted P value | Fully Adjusted Hazard Ratio (95% CL)‡ | Fully Adjusted P value |
|---|---|---|---|---|
| Gender (Male vs Female) | 1.16 (0.43, 3.09) | 0.77 | 0.92 (0.33, 2.56) | 0.87 |
| Age (per decade) | 3.23 (1.79, 5.84) | < 0.001 | 2.85 (1.60, 5.09) | < 0.001 |
| Mean arterial pressure (per 10 mm Hg) | N/A | N/A | 1.87 (1.36, 2.57) | 0.001 |
| High density lipoprotein cholesterol (per mmol/L) | 0.31 (0.08, 1.18) | 0.09 | 0.22 (0.06, 0.87) | 0.03 |
| Total cholesterol (per mmol/L) | 0.88 (0.62, 1.25) | 0.48 | 0.80 (0.55, 1.16) | 0.25 |
| Diabetes | 2.54 (0.67, 9.68) | 0.17 | 1.03 (0.22, 4.88) | 0.97 |
| Angina | 1.32 (0.45, 3.91) | 0.62 | 1.65 (0.57, 4.82) | 0.36 |
| Smoking categories: | ||||
| Never | 1.00 (REF) | |||
| Past | 0.88 (0.35, 2.20) | 0.78 | 0.79 (0.31, 2.03) | 0.79 |
| Current | 3.48 (1.38, 8.75) | 0.008 | 4.17 (1.62, 10.77) | 0.003 |
| Body mass categories: | ||||
| Normal <25 | 1.00 (REF) | |||
| Overweight 25 to <30 | 0.84 (0.36, 1.97) | 0.69 | 0.74 (0.33, 1.70) | 0.48 |
| Obese ≥30 | 0.71 (0.23, 2.22) | 0.55 | 0.33 (0.10, 1.11) | 0.07 |
Adjusted for sleep apnea and all other variables in the table except blood pressure.
Adjusted for sleep apnea and all other variables in the table. N/A Not Applicable. CL = confidence limits
An additional model constructed by entering only the significant risk factors from the main model (age, sleep apnea, blood pressure, HDL cholesterol, and smoking) also showed that the association between sleep apnea and mortality remained significant (adj. HR 3.9, 95% CL 1.5, 10.0).
Examination of the Kaplan-Meier curve (see Figure 1) indicates that the groups with no and mild OSA exhibited statistically comparable survival. For this reason and as a confirmatory analysis, no and mild sleep apnea were combined into a single reference category for the multivariate modelling. In the resultant model moderate-severe OSA remained independently associated with a higher rate of mortality (adj. HR = 7.35, 95% CL 2.40, 22.49).
When RDI was entered into the full model as a continuous linear variable instead of a categorical variable it was a significant independent predictor of mortality (HR per ten units of RDI = 1.72, 95% CL 1.13, 2.62). While this is consistent with a linear dose-response relationship between severity of sleep apnea (RDI) and mortality, superior model fit was achieved by using categorized RDI suggesting, but not proving, that the dose-response relationship may be curvilinear.
There is a remote possibility that sleep apnea could be caused by another covert and ultimately fatal unknown disease. As such we removed all deaths occurring within the first 2 years of follow-up from the analysis (n = 2). This did not markedly change the HR associated with moderate-to-severe OSA (adj. HR 6.93, 95% CL 2.20, 21.86).
To exclude the effects of outliers on the analysis, we secondarily recalculated fully-adjusted mortality hazard ratios after removing from the highest severity category all participants with RDI ≥ 30 (n = 3, adj. HR = 5.5, 95% CL 1.6, 18.3) and then 2% of all participants with the most extreme sleep apnea (n = 8 with RDI ≥ 23, adj. HR = 11.1, 95% CL 2.3, 54.7): as shown, the association between moderate-severe OSA and mortality remained significant.
Cause of death was also matched via death certificates. These data did not indicate a predominant cause of death that could be linked to OSA. However, cause of death data on death certificates can be prone to misclassification.19
DISCUSSION
Moderate-to-severe sleep apnea was associated with 33% mortality over 14 years compared to 6.5% and 7.7% mortality in people with mild or no sleep apnea, respectively. This association remained significant after adjustment for age, gender, mean arterial pressure, total cholesterol, high density lipoprotein cholesterol, body mass, diabetes, angina, and smoking status. The observed adjusted hazard ratio was large (6.24, 95% CL 2.01, 19.39) and was roughly equivalent here in size to that of an increase of 17.5 years of age or 29 mm Hg in mean arterial pressure. This is the first report of an independent association between all-cause mortality and obstructive sleep apnea (OSA) in a population-based cohort and confirms observations from clinic-based studies where the potential effects of clinical referral bias could not be ruled out.8–14
In the two most cited of these recent clinical cohorts,11,12 OSA was a significant independent risk factor for CVD-related mortality12 and a composite endpoint of all-cause mortality and incident stroke.11 The analyses from the Busselton Health Study confirm and extend these previous findings. Firstly, the hypothesized effect of OSA now extends to all-cause mortality. This may be explained in part by longer mean follow-up (13.4 years) compared to the previous studies,11,12 allowing manifestation of effects that accumulate slowly over time. The Busselton study included both men and women, unlike one of the previous cohorts.12 Most importantly, this study is community-based and thus free of the potential effects of clinical referral bias where patients have been selected for their high likelihood for having sleep apnea and/or are selected because they have evidence of conditions known or suspected to be associated with or caused by sleep apnea. In particular, the potential confounding effects of sleep apnea treatment are less likely to be an issue in this non-clinic population. Indeed the strength of the mortality association with moderate-to-severe OSA (HR = 6.24) is markedly stronger here (although with wider confidence limits) than in the 2 clinical cohorts where mortality rates approximately 2–3 times as high were observed in those with severe OSA. This could indicate that any clinical referral bias in those cohorts might be acting to cause underestimation of the true difference in mortality between individuals with and without OSA. Despite these differences in design and in the size of the observed effect, these 3 cohort studies from 3 different countries have all found that severe sleep apnea in middle-aged people is a risk factor for mortality after controlling for a wide range of potential confounders.
Mild OSA might have greater public health significance than moderate-severe OSA because it is far more common.6 Previous cohorts have usually reported that morbidity/mortality increases in a roughly linear fashion with the severity of OSA.12,23,24 However, in the present study, mild OSA was not associated with significantly greater mortality than no OSA (hazard ratio = 0.47, 95% CL 0.17, 1.29). Determination of whether mild OSA is on balance harmful, helpful, or of no significance requires further investigation in larger cohorts. However, the upper limit of our confidence interval does not support the hypothesis of a strong association of mild OSA with mortality.
Sleep apnea was measured using the MESAM IV device rather than polysomnography. This device had very close agreement with standard PSG (ICC = 0.98) in the original prevalence study as well as being validated by other research groups.4,20,21 Moreover, the prevalence estimate using this method in Busselton was in very close agreement (26% versus 24%) with the PSG measured prevalence estimate from the widely accepted prevalence estimate from the Wisconsin Sleep Cohort.5 Finally, the ability of the MESAM IV device to predict mortality, demonstrated in this study, provides strong evidence that this is a valid OSA measurement technique in community-based epidemiological investigations.
We did not have information about, nor did we attempt to control for, any effects of sleep apnea treatment on mortality. Treatment effects are unlikely to explain either the strength or significance of the observed association as any beneficial sleep apnea treatment should have reduced the differences in mortality between the sleep apnea and no sleep apnea groups. There is empirical support for this assumption as treatment of severe sleep apnea has been shown to be associated with a reduced risk of CVD mortality compared to non-treatment in a number of studies (for review see2,12). Moreover, access to diagnostic and treatment services for sleep apnea in Australia, while better than most countries, still only exposes around 2% of the population to diagnosis and treatment, and has been less prevalent in Western Australia.27 Furthermore, access to treatment in rural towns, such as Busselton, is likely to be substantially lower than nationwide estimates.
It is possible that these findings might have been sensitive to the misclassification of mortality in the group with moderate-to-severe OSA, as this group had 6 deaths from 18 cases. However, death certificate data in Australia rarely misclassifies alive people as having died.19 Non-recording of deaths that had occurred in the non-OSA group would have had little effect on the hazard estimate, and more deaths in the moderate-severe OSA group would have further strengthened the reported association. Nor are these findings due to the effects of extremely severe sleep apnea cases inflating the hazard associated with moderate-severe OSA. Excluding severe cases of OSA (RDI ≥ 30) or excluding the 2% of the most severe cases (RDI ≥ 23, i.e., 8 out of 18 cases) did not change the significance of the independent association in the fully adjusted model. Although these data indicate both a large and statistically significant effect, replication, particularly of the magnitude of the effect, by other population-based sleep apnea cohorts5,28 will be important as it is in all observational studies. The small number of exposed cases in this study (n = 18) may be regarded as a weakness, but this figure reflects the prevalence of moderate-severe OSA in the community.
Assuming that moderate-to-severe OSA is independently confirmed as a risk factor for all-cause mortality it might therefore be added to the list of standard mortality risk factors. Obesity, hypertension, and male gender are associated with both higher mortality risk and sleep apnea.29 Thus it is possible that some of the risk traditionally associated with these 3 factors might actually be attributable to sleep apnea, and sleep apnea might therefore become recognized as an important additional mortality risk factor. Our finding that moderate-severe OSA is an independent risk factor for all-cause mortality also highlights the need for high-quality randomized controlled trials of treatment/s for sleep apnea that are powered to detect primary disease prevention or reduced mortality risk.
ACKNOWLEDGMENTS
The authors would like to thank Graham Maier for programming the data match that made these analyses possible and Michael Hunter for assistance with initiating the project and both Dr. Hunter and Prof Judy Simpson for useful comments on the analyses. The Busselton Population Medical Research Foundation gave us access to the population and data and we would also like to thank the community of Busselton for their long-standing support of the study.
Dedication: This manuscript is dedicated to the memory of Dr. Helen Bearpark who collected the baseline exposure data presented here with the expressed intention of longitudinal study. She died in a tragic road accident in December 1996.
Financial Support: PYL supported by Australian NHMRC Career Development Award 511929. Research supported by Australian NHMRC grants to RRG 264598, 202916.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Phillips B. Sleep-disordered breathing and cardiovascular disease. Sleep Med Rev. 2005;9:131–40. doi: 10.1016/j.smrv.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Marin J, Carrizo S. Mortality in obstructive sleep apnea. Sleep Med Clin. 2007;2:593–601. [Google Scholar]
- 3.Phillipson EA. Sleep apnea—a major public health problem. N Engl J Med. 1993;328:1271–3. doi: 10.1056/NEJM199304293281712. [DOI] [PubMed] [Google Scholar]
- 4.Bearpark H, Elliot L, Grunstein R, et al. Snoring and sleep apnea: A population study in Australian men. Am J Respir Crit Care Med. 1995;151:1459–65. doi: 10.1164/ajrccm.151.5.7735600. [DOI] [PubMed] [Google Scholar]
- 5.Young T, Palta M, Dempsey J, et al. The occurrence of sleep disordered breathing among middle aged adults. New Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 6.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: A population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 7.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99:1592–9. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 8.Campos-Rodriguez F, Pena-Grinan N, Reyes-Nunez N, et al. Mortality in obstructive sleep apnea-hypopnea patients treated with positive airway pressure. Chest. 2005;128:624–33. doi: 10.1378/chest.128.2.624. [DOI] [PubMed] [Google Scholar]
- 9.He J, Kryger M, Zorick F, et al. Mortality and apnea index in obstructive sleep apnea: experience in 385 male patients. Chest. 1988;94:9–14. [PubMed] [Google Scholar]
- 10.Lavie P, Herer P, Peled R, et al. Mortality in sleep apnea patients- a multivariate analysis of risk factors. Sleep. 1995;18:149–57. doi: 10.1093/sleep/18.3.149. [DOI] [PubMed] [Google Scholar]
- 11.Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 12.Marin J, Carrizo S, Vincente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 13.Marti S, Sampol G, Munoz X, et al. Mortality in severe sleep apnoea/hypopnoea syndrome patients: Impact of treatment. Eur Respir J. 2002;20:1511–8. doi: 10.1183/09031936.02.00306502. [DOI] [PubMed] [Google Scholar]
- 14.Lavie P, Herer P, Lavie L. Mortality risk factors in sleep apnoea: A matched case-control study. J Sleep Res. 2007;16:128–34. doi: 10.1111/j.1365-2869.2007.00578.x. [DOI] [PubMed] [Google Scholar]
- 15.Cullen K, Stenhouse N, Welborn T, et al. Chronic respiratory disease in a rural community. Lancet. 1968;2:657–60. doi: 10.1016/s0140-6736(68)92507-5. [DOI] [PubMed] [Google Scholar]
- 16.Knuiman M, James A, Divitini M, et al. Lung function, respiratory symptoms and mortality: Results from the Busselton health study. Ann Epidemiol. 1999;9:297–306. doi: 10.1016/s1047-2797(98)00066-0. [DOI] [PubMed] [Google Scholar]
- 17.Knuiman M, James A, Divitini M, et al. Longitudinal study of risk factors for habitual snoring in a general adult population: The Busselton health study. Chest. 2006;130:1779–83. doi: 10.1378/chest.130.6.1779. [DOI] [PubMed] [Google Scholar]
- 18.Holman C, Bass A, Rouse I, et al. Population-based linkage of health records in Western Australia: Development of a health services research linked database. Aust NZ J Public Health. 1999;23:453–9. doi: 10.1111/j.1467-842x.1999.tb01297.x. [DOI] [PubMed] [Google Scholar]
- 19.Magliano D, Liew D, Pater H, et al. Accuracy of the Australian national death index: Comparison with adjudicated fatal outcomes among Australian participants in the long-term intervention with pravastatin in ischaemic disease (lipid) study. Aust NZ J Public Health. 2003;27:649–53. doi: 10.1111/j.1467-842x.2003.tb00615.x. [DOI] [PubMed] [Google Scholar]
- 20.Esnaola S, Duran J, Infante-Rivard C, et al. Diagnostic accuracy of a portable recording device (MESAM IV) in suspected obstructive sleep apnoea. Eur Respir J. 1996;9:2597–605. doi: 10.1183/09031936.96.09122597. [DOI] [PubMed] [Google Scholar]
- 21.Stoohs R, Guilleminault C. Mesam 4: An ambulatory device for the detection of patients at risk for obstructive sleep apnea syndrome (OSAS) Chest. 1992;101:1221–7. doi: 10.1378/chest.101.5.1221. [DOI] [PubMed] [Google Scholar]
- 22.American Academy of Sleep Medicine Taskforce. Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 23.Peppard P, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. New Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 24.Shahar E, Whitney C, Redline S, et al. Sleep disordered breathing and cardiovascular diseases: Cross sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 25.Liu PY, Swerdloff RS, Christenson PD, et al. Rate, extent, and modifiers of spermatogenic recovery after hormonal male contraception: An integrated analysis. Lancet. 2006;367:1412–20. doi: 10.1016/S0140-6736(06)68614-5. [DOI] [PubMed] [Google Scholar]
- 26.Therneau T, Grambsch P. New York: Springer-Verlag; 2000. Modelling survival data: Extending the Cox model. [Google Scholar]
- 27.Marshall NS, Wilsmore BR, McEvoy RD, et al. Polysomnography in Australia- trends in provision. J Clin Sleep Med. 2007;3:281–4. [PMC free article] [PubMed] [Google Scholar]
- 28.Quan S, Howard B, Iber C, et al. The Sleep Heart Health Study: Design, methods and rationale. Sleep. 1997;20:1077–85. [PubMed] [Google Scholar]
- 29.Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291:2013–6. doi: 10.1001/jama.291.16.2013. [DOI] [PubMed] [Google Scholar]