Abstract
Study Objectives:
Clinical rating scales, self-reports, and diagnostic instruments measuring depression often inquire about daytime fatigue and tiredness. Excessive daytime sleepiness refers specifically to the tendency to feel drowsy or fall asleep during waking hours and is considered conceptually and operationally independent from the fatigue, tiredness, and sleeping difficulties that characterize depression. The objective of this study was to examine whether daytime sleepiness assessed using the Epworth Sleepiness Scale and depressive symptoms assessed using the Geriatric Depression Scale are genetically related.
Design/Setting:
Cross-sectional data were collected via questionnaire in 1998–2000.
Participants:
Population-based sample of more than 5,000 male elderly twins aged 69–82 years old at the time of survey.
Interventions:
N/A
Measurements and Results:
There was evidence for moderate heritability for daytime sleepiness (36.9%) and depressive symptoms (30.7%). There was evidence for a significant genetic correlation (0.40) between the 2 measures, suggesting that both daytime sleepiness and depressive symptoms have some genes in common. The genetic correlation was reduced to 0.21 after adjustment for several covariates.
Conclusions:
The results showed that the often reported phenotypic correlation between daytime sleepiness and depressive symptoms is due, in part, to modest overlap in genetic factors, at least in elderly men. However, the majority of individual variation in daytime sleepiness and depressive symptoms, in particular after covariate adjustment, was attributable to individual-specific environmental factors.
Citation:
Lessov-Schlaggar CN; Bliwise DL; Krasnow RE; Swan GE; Reed TR. Genetic Association of Daytime Sleepiness and Depressive Symptoms in Elderly Men. SLEEP 2008;31(8):1111-1117.
Keywords: aged, depression, genetics, sleep disorders, twins
CLINICAL RATING SCALES AND SELF-REPORTS MEASURING DEPRESSION OFTEN INQUIRE ABOUT FATIGUE AND TIREDNESS. INSTRUMENTS ASKING ABOUT such phenomena include: the Schedule for Affective Disorders and Schizophrenia (tiredness),1 Beck Depression Inventory (tiredness),2 Montgomery-Åsberg Rating Scale for Depression (lassitude),3 Hamilton Rating Scale for Depression (component of somatic symptoms),4 the Depression scale of the Symptom Check-List 90 (low energy),5 Centers for Epidemiologic Studies Depression Scale (CES-D) (“could not get going”),6 Zung Depression Scale (fatigue),7 the Geriatric Depression Scale (GDS) (lack of energy),8 and diagnostic major depressive disorder (fatigue and sleep problems).9 Although somewhat varied in the nature of the behaviors that they assess, none of these instruments specifically inquire about the tendency to feel sleepy during normal waking hours of the day or excessive daytime sleepiness (EDS). EDS is considered to be conceptually and operationally independent from fatigue and tiredness that characterize depression. For example, some studies measuring EDS physiologically using the multiple sleep latency test or other daytime nap testing have failed to find that depressed or psychiatric hypersomnia patients are physiologically sleepier than comparison groups.10–12
Epidemiologic studies of individuals aged as young as 15 y to as old as 100 y show significant association between increased daytime sleepiness and diagnostic depression13–15 or depressive symptomatology.16,17 Studies in older individuals (55+ years of age) also support a relationship between daytime sleepiness and depression.18–23 However, other cross-sectional studies have reported no relationship in older individuals.24,25 Longitudinal investigations show evidence that sleep disturbances (most commonly insomnia and hypersomnia) predict later depression/depressive symptomatology.26–29
Both daytime sleepiness and depressive symptomatology are moderately heritable, with heritability coefficients on the order of 38% to 48% for daytime sleepiness30–34 and 16% to 55% for depressive symptoms.35–42
Although not all studies demonstrate a relationship between sleepiness and depressed mood, the majority of the evidence suggests at least a modest association between the 2 phenomena, and significant heritability for both phenotypes. This study sought to examine whether the relationship between self-reported daytime sleepiness and depressives symptomatology may be due to common genetic factors, using cross-sectional data in a large epidemiologic sample of elderly male twins. Twin studies allow determination of the extent to which correlated behaviors, such as daytime sleepiness and depressed mood, are influenced by the same or different genetic and environmental factors.
METHODS
Sample
Twins in this study were part of the National Academy of Sciences National Research Council (NAS-NRC) World War II Twin Registry.43 Multiple births of white males occurring between 1917 and 1927 in the US were identified through birth registries. Based on national statistics of multiple births, 93% of all multiple births were identified. Birth certificate records were matched with the Department of Veterans Affairs Index File to identify twin brothers who both served in the military during World War II. This selection yielded 15,924 eligible twin pairs. Prior to entry into military service, pairs where one or both brothers had history of chronic childhood illness were excluded. Zygosity assignment was initially made based on questionnaire responses about physical similarity, which was shown to be 95% accurate based on serologic assays in a subsample.43
As part of collaboration with the Swedish Twin Registry, a health behavior questionnaire survey was administered to the NAS-NRC twin panel in 1969–1972 when the twins were 45–55 years old. This survey included assessment of demographic characteristics, cigarette smoking and alcohol use, diet, and history of medical conditions. Subsequent expanded questionnaire surveys were conducted in 1983–85 when the twins were 58–68 years old and in 1998–2000 when they were 69–82 years old. At that time, the survey was sent to complete twin pairs or to surviving single twins who had completed both earlier epidemiologic questionnaires for a total of 6109 questionnaires (69% response rate). The 1998–2000 survey included assessment of daytime sleepiness and depressive symptoms, the main variables of interest in the present investigation. Daytime sleepiness data were available from both twins of 817 MZ and 743 DZ twin pairs; depressive symptoms data were available from both twins of 755 MZ and 683 DZ twin pairs. Both daytime sleepiness and depressive symptoms were available from both twins of 637 MZ and 581 DZ twin pairs.
The study protocol was reviewed and approved by the Indiana University-Purdue University at Indianapolis institutional review board. The survey instrument was edited and approved by the NAS-NRC Twins Committee. At the time, the study was judged exempt since there were no names on the questionnaire and the data were entered directly from the questionnaire with the entry person not having any knowledge of who the respondent was. Thus no informed consent was judged necessary.
Measures
Epworth Sleepiness Scale
Daytime sleepiness was assessed using the ESS,44 a subjective, short, self-administered survey about the likelihood of dozing in any of 8 situations: sitting and reading; watching TV; sitting, inactive in a public place (e.g., a theater or a meeting); as a passenger in a car for an hour without a break; lying down to rest in the afternoon when circumstances permit; sitting and talking to someone; sitting quietly after a lunch without alcohol; in a car, while stopped for a few minutes in the traffic. Response categories include 0 (would never doze), 1 (slight chance of dozing), 2 (moderate chance of dozing), and 3 (high chance of dozing). Subjects are instructed to respond specifically in relation to dozing off, as opposed to just feeling tired. ESS summary scores range 0–24; its clinical threshold is ≥11, though a threshold of ≥16 has been shown to be more clinically useful.45 The ESS is one-dimensional, has high test-retest reliability and internal consistency, and moderate construct validity shown by its correlation with the objective multiple sleep latency test (MSLT) reported in some studies.44–48
Although other questionnaires, such as the Karolinska Sleepiness Scale49 and the Stanford Sleepiness Scale50 have been devised, these scales are used largely for measurements of sleepiness on a state, rather than trait, basis. For these reasons, we elected to use the ESS in this study. The Psychomotor Vigilance Task51,52 is another approach that has proven useful for the measurement of sleepiness; however, it was impractical for us to administer this to the very large number of study participants.
Geriatric Depression Scale
Depressive symptoms were assessed using the GDS,8 which was originally a 30-item scale developed to fill a need for screening of depressive symptomatology in elderly individuals. In the NAS-NRC twin sample, a shortened 15-item version of the GDS was used (GDS-15).53 GDS-15 items ask about life satisfaction, mood, energy levels, helplessness, and hopelessness with Yes/No response categories and scale score range 0–15, with a clinical threshold >5. The GDS-15 has acceptable reliability and validity54 and strong sensitivity and specificity in relation to diagnostic major depression.55
The distributions of both ESS and GDS-15 scales were skewed and were log transformed prior to analysis.
Covariates
Previous studies16,19,22,23 and preliminary analysis identified correlates of ESS total score that were subsequently controlled for in the genetic analysis. These correlates included: (1) body mass index (BMI)—higher BMI was associated with more daytime sleepiness (r = 0.12, P < 0.01); (2) activities of daily living (ADL) which assessed the subject's self-report on degree of limitation (yes, limited a lot; yes, limited a little; no, not limited at all) on any of 10 possible daily activities including vigorous activities (running, lifting heavy objects); moderate activities (moving a table, pushing a vacuum cleaner); lifting or carrying groceries; climbing several flights of stairs; climbing one flight of stairs; bending, kneeling, or stooping; walking more than a mile; walking several blocks; walking one block; and bathing or dressing oneself – greater summary score of limitations in ADL was associated with more daytime sleepiness (r = 0.20, P < 0.01); (3) current smoking—mean ESS scores were lower in current smokers (6.4 ± 3.8 SD) compared to nonsmokers (7.1 ± 3.9 SD, P < 0.01); (4) alcohol use, defined based on measures of frequency and amount of alcohol consumed including beer, wine, and liquor, categorized participants as never drinkers, former drinkers (last drink more than one year ago), light drinkers (< 1 drink/day if drinking daily, 1–2 drinks per day if drinking < daily), moderate drinkers (1–2 drinks per day if daily, up to 6 drinks/day if < daily), and heavy drinkers (3 or more drinks per day if daily, more than 6 drinks/day if < daily)—greater drinking severity was associated with lower daytime sleepiness (r = −0.08, P < 0.01); (5) snoring frequency with 5 response categories ranging from never to almost always—more frequent snoring was associated with more daytime sleepiness (r = 0.19, P < 0.01); and (6) lifetime history (ever/never) of heart attack, stroke, diabetes, and sleep apnea—for each condition, mean ESS scores were higher in those with positive history (7.7 ± 4.1 SD for heart attack; 8.0 ± 4.4 SD for stroke; 7.6 ± 4.2 SD for diabetes; and 8.9 ± 4.4 SD for sleep apnea) compared to those with negative history (all means = 7.0 ± 3.9 SD, all P < 0.01). Age was not associated with ESS score (r = 0.01), but was included as a covariate because of evidence of the change of prevalence of daytime sleepiness with age in other studies.13,16,17 The aim of these preliminary analyses was to make sure that potentially important variables were included as covariates in the genetic analysis. The significance of the covariates was tested in the genetic analysis as well.
Statistical Analysis
Twin Methodology
In standard twin methodology using methods of maximum likelihood, phenotypic variance can be attributed to additive genetic factors (A), which are perfectly correlated in monozygotic (MZ) twins and are correlated on average about 0.5 in dizygotic (DZ) twins; non-additive genetic factors (D) such as dominance (interaction of gene alleles at the same locus) or epistasis (interaction of alleles at different loci), which are perfectly correlated in MZ twins and correlated 0.25 in DZ twins; shared environmental factors (C) common to members of a twin pair such as family, school, friends, or neighborhood; and non-shared environmental factors including measurement error (E), that are unique to each twin. In twin models, MZ and DZ twins are assumed to be perfectly correlated vis-à-vis the shared environmental effects they experience. A, D, and C contribute to twin pair similarity, while E contributes to twin pair dissimilarity. Because non-additive and shared environmental effects are confounded in twin studies, they cannot be simultaneously modeled.
Bivariate Twin Model
Prior to fitting a bivariate model to the data, the phenotypic correlation between the ESS and GDS-15 scores was estimated. Presence of a relationship between 2 measures is necessary to support the validity of conducting bivariate genetic analysis. A bivariate correlated factor model was used that quantifies the relative contribution of genetic (A) and environmental (C and E) influences on variance of each phenotype and on covariance across phenotypes (i.e., genetic and environmental correlations). A perfect genetic (ra) or shared environmental (rc) correlation between phenotypes suggests complete overlap in genetic or environmental influences; a zero correlation suggests independent influences for each phenotype; a correlation between 0 and 1 suggests some degree of common genetic or environmental influences. The correlated factors model was fitted to observed raw data allowing for incorporation of the effect of covariates on ESS and GDS-15 means into the models. Model fit was evaluated using the −2 Log Likelihood statistic, and principles of model parsimony were applied where models with fewer parameters were preferred if they showed no significant worsening of fit compared to the full model. The difference in model fit statistics between alternative models is distributed as a chi-square; the significance of this difference was evaluated using the likelihood-ratio chi-square difference test. Thus, the contribution of genetic or shared environmental components to phenotypic variance was evaluated by setting either parameter to zero. Significant worsening of the fit of the reduced model containing the constrained parameters relative to the full model was indicated by a significant (P < 0.05) likelihood-ratio chi-square difference, at the degrees of freedom difference between the same 2 models. The significance contribution of genetic and environmental correlations to phenotypic covariance was similarly evaluated, except that correlations were set either to 0 or to 1; if either of these constraints resulted in worsening of model fit, correlations were freely estimated.
Analysis was conducted twice—first a best fitting model was identified for the relationship between ESS and GDS-15; next, covariates were incorporated into the best fitting model. The significance of the association of each covariate with each of the ESS and GDS-15 dependent variables was tested by setting the appropriate regression coefficient to zero. Genetic analysis was conducted using the structural equation modeling program Mx.56
RESULTS
Mean and prevalence estimates of sample characteristics are shown in Table 1. Mean ESS and GDS-15 scores are consistent with rates reported in other studies of older Caucasian men.23,53,57 The phenotypic correlation between ESS and GDS-15 scores was modest but statistically significant (rph = 0.17, P < 0.01). The ESS-GDS rph was computed based on data from all the twins surveyed in 1998–2000, which included pairs of twins as well as single twins whose cotwin did not participate at this survey. The genetic analyses, on the other hand, included only data from complete twin pairs, that is both twins of a pair had to have been surveyed in 1998–2000, hence the smaller sample size available for genetic analysis.
Table 1.
Sample characteristics at time of survey (1998–2000).
| Characteristics | n | Mean (SD) | Prevalence (%) | Range |
|---|---|---|---|---|
| Outcomes | ||||
| ESS | 5381 | 7.05 (3.9) | 0–24 | |
| GDS | 5168 | 2.16 (2.4) | 0–15 | |
| Covariates | ||||
| Age | 5777 | 74.3 (2.8) | 69–82 | |
| BMI | 5868 | 26.5 (3.5) | 14.6–47.9 | |
| ADL | 5553 | 15.3 (5.1) | 10–30 | |
| Alcohol Use | 5641 | |||
| Never | 14.1 | |||
| Former | 20.0 | |||
| Light | 44.7 | |||
| Moderate | 15.4 | |||
| Heavy | 5.8 | |||
| Current smoking | 6025 | 7.6 | ||
| Snoring frequency | 4603 | 2.68 (1.1) | 1–5 | |
| Heart attack (lifetime) | 5575 | 17.7 | ||
| Stroke (lifetime) | 5578 | 10.0 | ||
| Diabetes (lifetime) | 5592 | 15.1 | ||
| Sleep apnea (lifetime) | 5947 | 3.9 | ||
ESS: Epworth Sleepiness Scale; GDS: Geriatric Depression Scale; BMI: body mass index; ADL: activities of daily living; SD: standard deviation
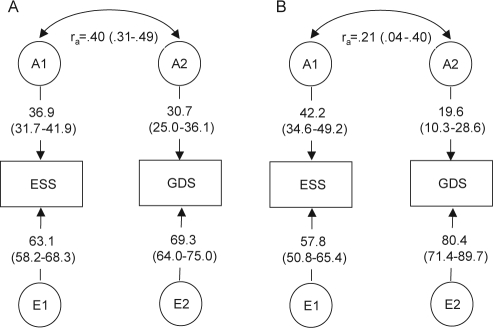
Standardized parameter estimates for the best fitting correlated factors model before incorporation of covariates are shown in Figure 1, Panel A. Phenotypic variance of both the ESS and GDS-15 was significantly heritable (A1 = 36.9% and A2 = 30.7%, respectively). There was no evidence for a significant contribution of shared environmental influences on either measure. The possible contribution of non-additive genetic effects (D) was also tested and was not significant. The ESS and GDS-15 were significantly genetically correlated (ra = 0.40), suggesting overlap in genetic factors. A correlation between the non-shared environmental effects was not modeled (i.e, it was constrained to 0).
Figure 1.
Path diagrams for the best fitting bivariate genetic models for ESS and GDS in the NAS-NRC twin sample before (Panel A) and after (Panel B) adjustment for covariates. Numbers in parentheses are the 95% confidence limits of the estimates.
Genetic and environmental parameter estimates after inclusion of covariates is shown in Figure 1, Panel B. After testing the significance of the association of each of the 10 covariates with ESS and GDS-15, we found that just 3 of 10 measures (ADL, snoring frequency, and lifetime history of diabetes) were significantly associated with either the ESS or GDS-15. Greater limitation in ADLs was associated with more daytime sleepiness (regression coefficient β = 0.019 [95% CI: 0.014, 0.025]) and more depressive symptoms (β = 0.062 [95% CI: 0.055, 0.069]); greater frequency of snoring was associated with more daytime sleepiness (β = 0.099 [95% CI: 0.077, 0.121]); and lifetime history of diabetes was associated with more depressive symptoms (β = 0.123 [95% CI: 0.03, 0.22]).
Including covariates into the model reduced the genetic correlation between ESS and GDS-15 to ra = 0.21 and resulted in somewhat larger ESS heritability (42.2%) and lower GDS-15 heritability (19.6%) (Figure 1, Panel B). The decreased magnitude of overlap in genetic influences after incorporation of covariates suggests that ADL, snoring frequency, and lifetime history of diabetes may account for part of the genetic relationship between ESS and GDS-15.
DISCUSSION
This cross-sectional epidemiologic study of elderly male twins supports the existence of a modest correlation between subjectively assessed daytime sleepiness and depressive symptoms similar to results reported in other studies of elderly individuals.18,19,21–23 Whitney and colleagues23 examined the relationship of ESS to a number of factors, including the CES-D, in a sample of adults aged 65 and older, and showed results separately by sex, thus most closely approximating the measures and sample demographics in our study. In multivariate models, Whitney and colleagues23 showed that in men, each unit increase in CES-D score was associated with 0.13 units increase in the ESS score, a modest relationship; in addition, their multivariate model accounted for only 10% of the total variance in ESS in men. In other elderly samples, depression was associated with about 2-fold or lower increase in risk for daytime sleepiness (risk range 1.1–2.3).18–22,24 The strength of the association may also depend on how daytime sleepiness or depression are defined in the different studies.58 Taken together, results from this study and others support a modest relationship between daytime sleepiness and depressive symptoms in elderly individuals.
The present investigation in a genetically informative sample extends previous findings by showing that daytime sleepiness and depressive symptoms are modestly genetically correlated, suggesting the presence of genes that affect both measures. The magnitude of the genetic correlation was reduced after adjusting for significant covariates (ADL, snoring frequency, and lifetime history of diabetes), suggesting that these covariate measures account for part of the genetic relationship between ESS and GDS-15. In addition, it is possible that the decrease in GDS-15 heritability magnitude after covariate adjustment was due to removal of GDS-15 genetic variation that was shared with its significant covariates (ADL and lifetime history of diabetes), which are also significantly heritable in this sample (data not shown).
Computation of the phenotypic correlation is based on the total variance for each measure, variance that is both genetic and environmental. The genetic correlation is based only on the portion of the total phenotypic variance of each of the ESS and GDS-15 that is influenced by genes. The two types of correlations are related in that the genetic correlation provides evidence for a mechanism that may help explain the causes of the phenotypic relationship.
The present investigation also showed modest but significant heritability for the ESS and GDS-15, and no evidence for a significant influence of shared environmental factors, consistent with previous reports on the magnitude of heritable influences on daytime sleepiness in this sample of twins30,31 and on depressive symptoms in other studies of older twins.37,38,40 In a subsample of the NAS-NRC twins, heritability of depressive symptoms measured using the CES-D was around 50%.35,39
Heritability represents the total effect of several or many genes that account for observed individual differences in behavior. There are a few published gene association studies of daytime sleepiness, conducted in largely Caucasian samples, parallel to the ethnic/racial distribution of our sample, and none were conducted in elderly, general population samples. Association was found between daytime sleepiness in narcoleptic patients and variation in the catechol-O-methyltransferase (COMT) gene involved in dopamine degradation59 and between excessive daytime sleepiness and variation in the orexin/hypocretin system gene OX2R involved in sleep regulation.60 No association was found between the apolipoprotein E (APOE) ϵ4 allele and ESS scores.61
In relation to gene association studies of depressive symptoms, a handful of published studies using Caucasian elderly samples show a role of genes in the serotonergic system, a major target for antidepressant medication. Associations have been reported between depressive symptoms and variation in the serotonin receptor 2a gene (5-HTR2A), in the tryptophan hydroxylase gene (TPH) responsible for serotonin synthesis, in the vesicular monoamine transporter 2 gene (VMAT2) responsible for serotonin transport, and in the monoamine oxidase A gene (MAO-A) responsible for serotonin degradation.62–64 No association between variation in the serotonin transporter gene (5-HTT) and depressive symptoms has been found.62–64
The fact that history of diabetes and reported snoring account for at least some of the genetic correlation between ESS and GDS-15 implies that sleep apnea may well be the condition underlying the relationships that we have observed here. For example, we have already demonstrated a genetic component of physiologically measured sleep apnea in a smaller subset of this twin cohort (heritability coefficients of 36% to 37%).65 Diabetes and insulin resistance are well-recognized correlates of sleep apnea.66–68 Cross-sectional studies showing relationships between depression and sleep apnea symptoms69 or diagnoses,70 as well as at least one longitudinal study71 showing incident depression associated with physiologically monitored, untreated sleep apnea are consistent with sleep apnea as the condition underlying the relationships noted in our study. On the other hand, in our previous analyses of the genetic versus environmental factors shared by EDS, obesity and reported snoring, we found that EDS shared only 30% of genetic variance with snoring (as a sleep apnea proxy) and even less (6%) with obesity.31 Thus, the genetic overlap between EDS and depression is unlikely to be subsumed entirely by their relationship to sleep apnea.
Several limitations to this study need to be considered. First, a statistical genetic correlation does not necessarily imply a biological correlation.72 From a genetic perspective, evidence for a biological relationship would be seen if variation in a given gene affects both excessive daytime sleepiness and depressive symptoms in the same individual. The interpretation of the genetic correlation assumes that alleles common to two phenotypes have the same effect size, identical frequencies, and identical direction of effects (i.e., increased or decreased number of alleles) for both phenotypes.72
Second, different results may be expected with an objective measure of sleepiness, as subjective and objective sleepiness may relate to different physiological processes. Though total ESS score is significantly modestly related to sleep latency as measured by the MSLT in patient populations,44–48 no relationship has also been reported in some patient groups46,73,74 and in a non-patient sample.75 Therefore, the observed genetic relationship may or may not apply to physiological or objective sleepiness.
Third, a cross-sectional association as shown in this study does not imply a causal influence of one measure on another. The present study suggests instead, that other factors, such as genetic influences, may account, at least in part, for the relationship between daytime sleepiness and depressive symptoms. It would be interesting to examine the genetic relationship between daytime sleepiness and depression in clinically depressed individuals and using objective measures of sleepiness. To the degree that depression is heritable and that objective sleepiness measures more closely reflect underlying biology, it could be hypothesized that a stronger genetic relationship would be observed.
Fourth, findings may not be generalizable to women, to other ethnic/racial groups, or to clinical samples. However, analyses stratified by sex23,24 and a study in elderly Asian men18 support the existence of a relationship between excessive daytime sleepiness and depressive symptoms in women and in racial/ethnic groups other than Caucasian. Depressed patients also report excessive levels of daytime sleepiness.13–15,76
Twin studies and gene association studies support the conclusion that there are significant genetic influences on daytime sleepiness and depressive symptoms. The results in this paper suggest the existence of pleiotropic genes associated with daytime sleepiness and depressive symptoms (as well as perhaps significant covariates). The majority of individual variation in daytime sleepiness and depressive symptoms in particular after covariate adjustment, is attributable to individual-specific environmental factors, however, which comprises a broad category of possibilities, from lifestyle choices and habits (e.g., diet, exercise, drug use, work shifts) to sporadic events such as travel or acute illness.
ACKNOWLEDGMENTS
Study design and data collection were conducted at and supported by internal funds from Indiana University School of Medicine. Data analysis and manuscript preparation was conducted at and supported by internal funds from SRI International. The authors are grateful to the twins for their long term commitment to this study. The authors are indebted to Dorit Carmelli, PhD, for her leadership.
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. Bliwise has received research support from Takeda; has consulted for Cephalon, Gerson Lehrman Group, Neurocrine, Pfizer, Takeda, and Sepracor; and has participated in speaking engagements for Boehringer Ingelheim, Takeda, School of Sleep Medicine, and Sleep Medicine Education Initiative. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Endicott J, Spitzer RL. A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch Gen Psychiatry. 1978;35:837–44. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- 2.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 3.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Derogatis LR, Lipman RS, Covi L. SCL-90: an outpatient psychiatric rating scale--preliminary report. Psychopharmacol Bull. 1973;9:13–28. [PubMed] [Google Scholar]
- 6.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 7.Zung WW. A Self-Rating Depression Scale. Arch Gen Psychiatry. 1965;12:63–70. doi: 10.1001/archpsyc.1965.01720310065008. [DOI] [PubMed] [Google Scholar]
- 8.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 9.APA. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 10.Nofzinger EA, Thase ME, Reynolds CF, 3rd, et al. Hypersomnia in bipolar depression: a comparison with narcolepsy using the multiple sleep latency test. Am J Psychiatry. 1991;148:1177–81. doi: 10.1176/ajp.148.9.1177. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds CF, 3rd, Coble PA, Kupfer DJ, Holzer BC. Application of the multiple sleep latency test in disorders of excessive sleepiness. Electroencephalogr Clin Neurophysiol. 1982;53:443–52. doi: 10.1016/0013-4694(82)90009-8. [DOI] [PubMed] [Google Scholar]
- 12.Vgontzas AN, Bixler EO, Kales A, Criley C, Vela-Bueno A. Differences in nocturnal and daytime sleep between primary and psychiatric hypersomnia: diagnostic and treatment implications. Psychosom Med. 2000;62:220–6. doi: 10.1097/00006842-200003000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Bixler EO, Vgontzas AN, Lin HM, Calhoun SL, Vela-Bueno A, Kales A. Excessive daytime sleepiness in a general population sample: the role of sleep apnea, age, obesity, diabetes, and depression. J Clin Endocrinol Metab. 2005;90:4510–5. doi: 10.1210/jc.2005-0035. [DOI] [PubMed] [Google Scholar]
- 14.Breslau N, Roth T, Rosenthal L, Andreski P. Daytime sleepiness: an epidemiological study of young adults. Am J Public Health. 1997;87:1649–53. doi: 10.2105/ajph.87.10.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohayon MM, Caulet M, Philip P, Guilleminault C, Priest RG. How sleep and mental disorders are related to complaints of daytime sleepiness. Arch Intern Med. 1997;157:2645–52. [PubMed] [Google Scholar]
- 16.Hublin C, Kaprio J, Partinen M, Heikkila K, Koskenvuo M. Daytime sleepiness in an adult, Finnish population. J Intern Med. 1996;239:417–23. doi: 10.1046/j.1365-2796.1996.475826000.x. [DOI] [PubMed] [Google Scholar]
- 17.Theorell-Haglow J, Lindberg E, Janson C. What are the important risk factors for daytime sleepiness and fatigue in women? Sleep. 2006;29:751–7. doi: 10.1093/sleep/29.6.751. [DOI] [PubMed] [Google Scholar]
- 18.Barbar SI, Enright PL, Boyle P, et al. Sleep disturbances and their correlates in elderly Japanese American men residing in Hawaii. J Gerontol A Biol Sci Med Sci. 2000;55:M406–11. doi: 10.1093/gerona/55.7.m406. [DOI] [PubMed] [Google Scholar]
- 19.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18:425–32. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein IB, Ancoli-Israel S, Shapiro D. Relationship between daytime sleepiness and blood pressure in healthy older adults. Am J Hypertens. 2004;17:787–92. doi: 10.1016/j.amjhyper.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Hays JC, Blazer DG, Foley DJ. Risk of napping: excessive daytime sleepiness and mortality in an older community population. J Am Geriatr Soc. 1996;44:693–8. doi: 10.1111/j.1532-5415.1996.tb01834.x. [DOI] [PubMed] [Google Scholar]
- 22.Quan SF, Katz R, Olson J, et al. Factors associated with incidence and persistence of symptoms of disturbed sleep in an elderly cohort: the Cardiovascular Health Study. Am J Med Sci. 2005;329:163–72. doi: 10.1097/00000441-200504000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Whitney CW, Enright PL, Newman AB, Bonekat W, Foley D, Quan SF. Correlates of daytime sleepiness in 4578 elderly persons: the Cardiovascular Health Study. Sleep. 1998;21:27–36. doi: 10.1093/sleep/21.1.27. [DOI] [PubMed] [Google Scholar]
- 24.Newman AB, Enright PL, Manolio TA, Haponik EF, Wahl PW. Sleep disturbance, psychosocial correlates, and cardiovascular disease in 5201 older adults: the Cardiovascular Health Study. J Am Geriatr Soc. 1997;45:1–7. doi: 10.1111/j.1532-5415.1997.tb00970.x. [DOI] [PubMed] [Google Scholar]
- 25.Ohayon MM, Vecchierini MF. Normative sleep data, cognitive function and daily living activities in older adults in the community. Sleep. 2005;28:981–9. [PubMed] [Google Scholar]
- 26.Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411–8. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- 27.Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psychiatry. 2003;160:1147–56. doi: 10.1176/appi.ajp.160.6.1147. [DOI] [PubMed] [Google Scholar]
- 28.Livingston G, Blizard B, Mann A. Does sleep disturbance predict depression in elderly people? A study in inner London. Br J Gen Pract. 1993;43:445–8. [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts RE, Shema SJ, Kaplan GA, Strawbridge WJ. Sleep complaints and depression in an aging cohort: A prospective perspective. Am J Psychiatry. 2000;157:81–8. doi: 10.1176/ajp.157.1.81. [DOI] [PubMed] [Google Scholar]
- 30.Carmelli D, Bliwise DL, Swan GE, Reed T. A genetic analysis of the Epworth Sleepiness Scale in 1560 World War II male veteran twins in the NAS-NRC Twin Registry. J Sleep Res. 2001;10:53–8. doi: 10.1046/j.1365-2869.2001.00241.x. [DOI] [PubMed] [Google Scholar]
- 31.Carmelli D, Bliwise DL, Swan GE, Reed T. Genetic factors in self-reported snoring and excessive daytime sleepiness: a twin study. Am J Respir Crit Care Med. 2001;164:949–52. doi: 10.1164/ajrccm.164.6.2012001. [DOI] [PubMed] [Google Scholar]
- 32.Desai AV, Cherkas LF, Spector TD, Williams AJ. Genetic influences in self-reported symptoms of obstructive sleep apnoea and restless legs: a twin study. Twin Res. 2004;7:589–95. doi: 10.1375/1369052042663841. [DOI] [PubMed] [Google Scholar]
- 33.Watson NF, Goldberg J, Arguelles L, Buchwald D. Genetic and environmental influences on insomnia, daytime sleepiness, and obesity in twins. Sleep. 2006;29:645–9. doi: 10.1093/sleep/29.5.645. [DOI] [PubMed] [Google Scholar]
- 34.Heath AC, Kendler KS, Eaves LJ, Martin NG. Evidence for genetic influences on sleep disturbance and sleep pattern in twins. Sleep. 1990;13:318–35. doi: 10.1093/sleep/13.4.318. [DOI] [PubMed] [Google Scholar]
- 35.Carmelli D, Swan GE, Kelly-Hayes M, Wolf PA, Reed T, Miller B. Longitudinal changes in the contribution of genetic and environmental influences to symptoms of depression in older male twins. Psychol Aging. 2000;15:505–10. doi: 10.1037//0882-7974.15.3.505. [DOI] [PubMed] [Google Scholar]
- 36.Gatz M, Pedersen NL, Plomin R, Nesselroade JR, McClearn GE. Importance of shared genes and shared environments for symptoms of depression in older adults. J Abnorm Psychol. 1992;101:701–8. doi: 10.1037//0021-843x.101.4.701. [DOI] [PubMed] [Google Scholar]
- 37.Jansson M, Gatz M, Berg S, et al. Gender differences in heritability of depressive symptoms in the elderly. Psychol Med. 2004;34:471–9. doi: 10.1017/s0033291703001375. [DOI] [PubMed] [Google Scholar]
- 38.Johnson W, McGue M, Gaist D, Vaupel JW, Christensen K. Frequency and heritability of depression symptomatology in the second half of life: evidence from Danish twins over 45. Psychol Med. 2002;32:1175–85. doi: 10.1017/s0033291702006207. [DOI] [PubMed] [Google Scholar]
- 39.McCaffery JM, Niaura R, Swan GE, Carmelli D. A study of depressive symptoms and smoking behavior in adult male twins from the NHLBI twin study. Nicotine Tob Res. 2003;5:77–83. doi: 10.1080/14622200307259. [DOI] [PubMed] [Google Scholar]
- 40.McGue M, Christensen K. Genetic and environmental contributions to depression symptomatology: evidence from Danish twins 75 years of age and older. J Abnorm Psychol. 1997;106:439–48. doi: 10.1037//0021-843x.106.3.439. [DOI] [PubMed] [Google Scholar]
- 41.Kendler KS, Walters EE, Truett KR, et al. Sources of individual differences in depressive symptoms: analysis of two samples of twins and their families. Am J Psychiatry. 1994;151:1605–14. doi: 10.1176/ajp.151.11.1605. [DOI] [PubMed] [Google Scholar]
- 42.Silberg JL, Heath AC, Kessler R, et al. Genetic and environmental effects on self-reported depressive symptoms in a general population twin sample. J Psychiatr Res. 1990;24:197–212. doi: 10.1016/0022-3956(90)90010-n. [DOI] [PubMed] [Google Scholar]
- 43.Jablon S, Neel JV, Gershowitz H, Atkinson GF. The NAS-NRC twin panel: methods of construction of the panel, zygosity diagnosis, and proposed use. Am J Hum Genet. 1967;19:133–61. [PMC free article] [PubMed] [Google Scholar]
- 44.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 45.Chervin RD, Aldrich MS, Pickett R, Guilleminault C. Comparison of the results of the Epworth Sleepiness Scale and the Multiple Sleep Latency Test. J Psychosom Res. 1997;42:145–55. doi: 10.1016/s0022-3999(96)00239-5. [DOI] [PubMed] [Google Scholar]
- 46.Fong SY, Ho CK, Wing YK. Comparing MSLT and ESS in the measurement of excessive daytime sleepiness in obstructive sleep apnoea syndrome. J Psychosom Res. 2005;58:55–60. doi: 10.1016/j.jpsychores.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 47.Johns MW. Sleepiness in different situations measured by the Epworth Sleepiness Scale. Sleep. 1994;17:703–10. doi: 10.1093/sleep/17.8.703. [DOI] [PubMed] [Google Scholar]
- 48.Mediano O, Barcelo A, de la Pena M, Gozal D, Agusti A, Barbe F. Daytime sleepiness and polysomnographic variables in sleep apnoea patients. Eur Respir J. 2007;30:110–13. doi: 10.1183/09031936.00009506. [DOI] [PubMed] [Google Scholar]
- 49.Akerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 50.Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10:431–6. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 51.Kribbs NB, Pack AI, Kline LR, et al. Effects of one night without nasal CPAP treatment on sleep and sleepiness in patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:1162–8. doi: 10.1164/ajrccm/147.5.1162. [DOI] [PubMed] [Google Scholar]
- 52.Van Dongen HP, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 2004;27:423–33. [PubMed] [Google Scholar]
- 53.Sheikh JI, Yesavage JA. Geriatric depression scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–73. [Google Scholar]
- 54.Jang Y, Small BJ, Haley WE. Cross-cultural comparability of the Geriatric Depression Scale: comparison between older Koreans and older Americans. Aging Ment Health. 2001;5:31–7. doi: 10.1080/13607860020020618. [DOI] [PubMed] [Google Scholar]
- 55.Almeida OP, Almeida SA. Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int J Geriatr Psychiatry. 1999;14:858–65. doi: 10.1002/(sici)1099-1166(199910)14:10<858::aid-gps35>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 56.Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 6th ed. Richmond, VA: Virginia Commonwealth University; 2002. [Google Scholar]
- 57.Sanford SD, Lichstein KL, Durrence HH, Riedel BW, Taylor DJ, Bush AJ. The influence of age, gender, ethnicity, and insomnia on Epworth sleepiness scores: a normative US population. Sleep Med. 2006;7:319–26. doi: 10.1016/j.sleep.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 58.Young TB. Epidemiology of daytime sleepiness: definitions, symptomatology, and prevalence. J Clin Psychiatry. 2004;65(Suppl 16):12–6. [PubMed] [Google Scholar]
- 59.Dauvilliers Y, Neidhart E, Lecendreux M, Billiard M, Tafti M. MAO-A and COMT polymorphisms and gene effects in narcolepsy. Mol Psychiatry. 2001;6:367–72. doi: 10.1038/sj.mp.4000911. [DOI] [PubMed] [Google Scholar]
- 60.Thompson MD, Comings DE, Abu-Ghazalah R, et al. Variants of the orexin2/hcrt2 receptor gene identified in patients with excessive daytime sleepiness and patients with Tourette's syndrome comorbidity. Am J Med Genet B Neuropsychiatr Genet. 2004;129:69–75. doi: 10.1002/ajmg.b.30047. [DOI] [PubMed] [Google Scholar]
- 61.Caselli RJ, Reiman EM, Hentz JG, Osborne D, Alexander GE, Boeve BF. A distinctive interaction between memory and chronic daytime somnolence in asymptomatic APOE e4 homozygotes. Sleep. 2002;25:447–53. [PubMed] [Google Scholar]
- 62.Christiansen L, Tan Q, Iachina M, et al. Candidate gene polymorphisms in the serotonergic pathway: influence on depression symptomatology in an elderly population. Biol Psychiatry. 2007;61:223–30. doi: 10.1016/j.biopsych.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 63.Grunblatt E, Loffler C, Zehetmayer S, et al. Association study of the 5-HTTLPR polymorphism and depression in 75-year-old nondemented subjects from the Vienna Transdanube Aging (VITA) study. J Clin Psychiatry. 2006;67:1373–8. doi: 10.4088/jcp.v67n0907. [DOI] [PubMed] [Google Scholar]
- 64.Jansson M, Gatz M, Berg S, et al. Association between depressed mood in the elderly and a 5-HTR2A gene variant. Am J Med Genet B Neuropsychiatr Genet. 2003;120:79–84. doi: 10.1002/ajmg.b.20016. [DOI] [PubMed] [Google Scholar]
- 65.Carmelli D, Colrain IM, Swan GE, Bliwise DL. Genetic and environmental influences in sleep-disordered breathing in older male twins. Sleep. 2004;27:917–22. doi: 10.1093/sleep/27.5.917. [DOI] [PubMed] [Google Scholar]
- 66.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85:1151–8. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 67.Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165:670–6. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 68.Resnick HE, Redline S, Shahar E, et al. Diabetes and sleep disturbances: findings from the Sleep Heart Health Study. Diabetes Care. 2003;26:702–9. doi: 10.2337/diacare.26.3.702. [DOI] [PubMed] [Google Scholar]
- 69.Ohayon MM. The effects of breathing-related sleep disorders on mood disturbances in the general population. J Clin Psychiatry. 2003;64:1195–200. doi: 10.4088/jcp.v64n1009. quiz, 274–6. [DOI] [PubMed] [Google Scholar]
- 70.Sharafkhaneh A, Giray N, Richardson P, Young T, Hirshkowitz M. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep. 2005;28:1405–11. doi: 10.1093/sleep/28.11.1405. [DOI] [PubMed] [Google Scholar]
- 71.Peppard PE, Szklo-Coxe M, Hla KM, Young T. Longitudinal association of sleep-related breathing disorder and depression. Arch Intern Med. 2006;166:1709–15. doi: 10.1001/archinte.166.16.1709. [DOI] [PubMed] [Google Scholar]
- 72.Carey G. Inference about genetic correlations. Behav Genet. 1988;18:329–38. doi: 10.1007/BF01260933. [DOI] [PubMed] [Google Scholar]
- 73.Benbadis SR, Mascha E, Perry MC, Wolgamuth BR, Smolley LA, Dinner DS. Association between the Epworth sleepiness scale and the multiple sleep latency test in a clinical population. Ann Intern Med. 1999;130:289–92. doi: 10.7326/0003-4819-130-4-199902160-00014. [DOI] [PubMed] [Google Scholar]
- 74.Chervin RD, Aldrich MS. The Epworth Sleepiness Scale may not reflect objective measures of sleepiness or sleep apnea. Neurology. 1999;52:125–31. doi: 10.1212/wnl.52.1.125. [DOI] [PubMed] [Google Scholar]
- 75.Geisler P, Tracik F, Cronlein T, et al. The influence of age and sex on sleep latency in the MSLT-30--a normative study. Sleep. 2006;29:687–92. doi: 10.1093/sleep/29.5.687. [DOI] [PubMed] [Google Scholar]
- 76.Fava M. Daytime sleepiness and insomnia as correlates of depression. J Clin Psychiatry. 2004;65(Suppl 16):27–32. [PubMed] [Google Scholar]