Abstract
Study Objectives:
To determine the effects of sleeping position on development of circulatory control in infants over the first 6 months of postnatal age (PNA).
Design:
Effects of sleeping position, sleep state and PNA on beat-beat heart rate (HR) and mean arterial pressure (MAP) responses to a head-up tilt (HUT) were assessed during sleep in infants at 2–4 wks, 2–3 mo and 5–6 mo PNA.
Measurements:
Daytime polysomnography was performed on 20 full-term infants (12 F/8 M) and MAP was recorded continuously and noninvasively (Finometer™). HUTs of 15° were performed during active sleep (AS) and quiet sleep (QS) in both the prone and supine sleeping positions. MAP and HR data were expressed as the percentage change from baseline, and responses were divided into initial, middle and late phases.
Results:
In the supine position HUT usually resulted in an initial increase (P < 0.05) in HR and MAP, followed by decreases (P < 0.05) in HR and MAP in the middle phase; subsequently HR and MAP returned to baseline in the late phase. By contrast, in the prone position the initial HUT-induced rises in HR and MAP were usually absent, and at 2–3 mo MAP actually decreased (P < 0.05); subsequently HR but not MAP returned to baseline. At 2–3 mo, MAP was lower (P < 0.05) in prone than supine sleeping throughout the HUT.
Conclusions:
Prone sleeping alters MAP responses to a HUT during QS at 2–3 mo PNA. Decreased autonomic responsiveness may contribute to the increased risk for SIDS of infants sleeping in the prone position.
Citation:
Yiallourou SR, Walker AM; Horne RSC. Prone sleeping impairs circulatory control during sleep in healthy term infants: implications for SIDS. SLEEP 2008;31(8):1139-1146.
Keywords: Sudden Infant Death Syndrome, blood pressure, development, prone sleeping, cardiovascular control
EPIDEMIOLOGICAL STUDIES HAVE REVEALED THAT THE PRONE SLEEPING POSITION IS A MAJOR RISK FACTOR FOR SUDDEN INFANT DEATH SYNDROME (SIDS), the incidence of which peaks between 2 and 3 mo postnatal age (PNA).1–3 Despite the identification of risk factors which significantly increase the chance of an infant dying from SIDS, the underlying mechanism for SIDS still remains unknown. However, the pattern of cardiovascular changes seen in physiological recordings of SIDS deaths suggest that an impairment of protective autonomic cardiovascular control mechanisms are involved.4–8 It has been further hypothesized that a failure of integrative cerebellar mechanisms that act via motor actions to overcome profound hypotension may underlie the fatal event.8 In particular, cerebellar outputs to vestibular pathways that contribute to blood pressure (BP) regulation may be responsible for the position-dependent risk for SIDS.9
Currently, there are few data available on the development of BP control during sleep in infants, due primarily to an inability to measure BP continuously and noninvasively. However, new technology has now become available allowing noninvasive beat-beat measurement of BP in infants during sleep.10
Previous studies assessing cardiovascular control in infants have used the head-up tilt (HUT).11–17 Studies which have used the HUT to examine the effects of sleeping position at 2–3 mo have suggested a decreased vasomotor tone in the prone position,14,15 however, these studies did not use continuous measures of BP. Studies which have used noninvasive beat-beat BP measurement have not studied infants in the prone position and have not investigated developmental changes.11,18 Notably, there is no information on the development of cardiovascular control during the period of greatest SIDS risk, nor on the effects of sleeping position.
Using noninvasive beat-beat BP measurement we aimed to determine the effects of sleeping position on the development of HR and BP responses to the HUT in healthy term infants over the first 6 mo of life.
METHODS
This study was approved by the Southern Health and Monash University Human Research Ethics Committees. Prior to commencement of the study, written parental consent was obtained for all subjects. No monetary incentive was provided for participation.
Subjects
Twenty full-term infants (12 F/8 M) born at 38–42 wks of gestational age with normal birth weights ranging from 2970 g to 4250 g (mean 3590 ± 100 g, mean ± SEM) and Apgar scores of 9–10 (median 9) at 5 min were recruited for the study. Each infant was of appropriate birth weight for gestation and had no congenital abnormalities. All infants enrolled in the study routinely slept supine at home and were born to nonsmoking mothers, with only 1 infant having a father who smoked. We have previously reported the effects of sleeping position on basal BP and HR during development of these infants.19
Each infant was studied on three occasions over the first 6 mo of life, at 2–4 wks PNA (mean age 3 ± 0.1 wks); at 2–3 mo PNA (mean age 10 ± 0.1 wks); and at 5–6 mo PNA (mean age 22 ± 0.3 wks).
Polysomnography
At each study infants underwent daytime polysomnography at the Melbourne Children's Sleep Unit, Monash Medical Centre. All electrodes and measuring devices for polysomnography were attached during the infant's morning feed. Following the infant's normal sleep patterns, infants were placed in a pram and allowed to sleep naturally in a darkened room at constant temperature (22–23°C). Infants were slept in both the prone (P) and supine (S) sleeping positions, with the initial starting position randomized. Sleeping position was changed between morning and afternoon sleep periods that were interrupted by a midday feed.
The polysomnographic recordings consisted of continuous monitoring of electroencephalogram (EEG); electrooculogram (EOG), submental electromyogram (EMG), electrocardiogram (ECG), thoracic and abdominal breathing movements (Resp-ez piezoelectric sensor, EPM Systems, Midlothian VA, USA), arterial blood oxygen saturation (SpO2) (Biox 3700e Pulse Oximeter, Ohmeda, Louisville CO, USA), and abdominal skin temperature (YSI 400 series thermistor, Yellow Springs Instruments, Yellow Springs OH, USA).
Blood Pressure Measurement
In addition to standard polysomnographic measurements, noninvasive continuous BP was measured with a Finometer (FMS, Finapres Medical Systems, The Netherlands), with the cuff placed around the infant's left wrist and zeroed at heart level.10
Polygraphic data recorded at a frequency of 400 Hz were amplified via a polygraph (Grass Instrument Co, Quincy MA, USA) digitized via a 16-channel Powerlab system (ADInstruments, Sydney, Australia) and recorded onto a computer program for data storage, analysis and visualization (Chart 5.2, ADInstruments, Sydney Australia).
Sleep State
Infants were visually monitored continuously via an infrared camera placed above the pram. Sleep state was assessed as quiet sleep (QS), active sleep (AS), or indeterminate sleep using EEG, behavioral, heart rate, and breathing pattern criteria.20 Data from indeterminant sleep were excluded from subsequent analysis.
Head-Up Tilt
In both sleep states and sleeping positions repeated 15° HUTs (n = 3) were performed manually over 2–3 s in each infant. The angle of 15° was chosen to limit arousal in response to the HUT. Each tilt consisted of a baseline period and a tilt period, each of 1 min duration. All HUTs were commenced when the infants were physiologically stable and no movement was present. HUTs that were interrupted by movement, arousal, sigh or apnea pauses within the pre-tilt or tilt phases were excluded from cardiovascular analysis.
Data Analysis
Arousal Responses
Arousal responses to the HUT were scored according to the recommendations of the International Paediatric Work Group on Arousals,21 and expressed as a percentage of the total HUT tests.
Head-Up Tilt
For each HUT beat-beat values of mean arterial pressure (MAP) and HR were obtained by peak detection (Chart 5.2, ADInstruments, Sydney, Australia) for the 30 beats prior to the HUT and the 90 beats following the HUT. Data were expressed as the percentage change (Δ%) from baseline values, averaged over 30 beats prior to the tilt. HUT responses were assessed using methods adapted from Harrington et al,11 and Kirjavainen et al,18 who reported that a typical infant response to a HUT was biphasic, consisting of: (1) an initial increase in HR and BP, rising rapidly to a peak; (2) a reflex decrease in BP and HR; and (3) a subsequent return to baseline HR accompanied by a sustained decrease in BP. To quantify the response, the HUT was divided into 3 phases: an initial phase (the average of the 3 beats at the peak of the rise in HR and BP usually occurring within 15 beats following the HUT); a middle phase (the average of the 3 beats at the nadir in HR and BP, usually occurring between 15–30 beats post-tilt); and a late phase (an average of the 40–70 beats post HUT).
Statistical Analysis
Initial, middle and late phases of the HUT response were compared to pre-HUT baseline values using 1-way RM ANOVA with Dunnett post hoc analysis. Sleep state and sleeping position data were compared for the % arousal to the HUT, pre-HUT baseline, initial, middle, and late phases of the HUT using 2-way RM ANOVA, with Student Newman Kuels post hoc analysis. PNA effects were compared using 2-way ANOVA, with Student Newman Kuels post hoc analysis. Statistical significance was taken at the P < 0.05 level.
RESULTS
Of the 20 infants studied, successful HUT tests in both sleep positions and sleep states were obtained in 9 infants at 2–4 wks PNA, 16 infants at 2–3 mo PNA, and 18 infants at 5–6 mo PNA.
Arousal Responses
There were significantly fewer arousal responses to the HUT in QS than AS in both sleeping positions at 2–3 mo (supine P < 0.001; prone P < 0.01) and in the supine sleeping position at 5–6 mo (P < 0.01) (Table 1). However, there was no effects of sleeping position on the % arousal responses to the HUT in either of the sleep states, nor any effect of PNA in either sleep state or position.
Table 1.
Arousal Responses (% Tests) to the HUT in the Supine (S) and Prone (P) Sleeping Positions During AS and QS at 2–4 wks, 2–3 mo, and 5–6 mo PNA
| AROUSAL (%) 2–4 WKS | AROUSAL (%) 2–3 MO | AROUSAL (%) 5–6 MO | |
|---|---|---|---|
| QS-S | 27 | 12 | 18 |
| AS-S | 53 | 59*** | 53** |
| QS-P | 30 | 24 | 29 |
| AS-P | 51 | 54** | 24 |
**P < 0.01, ***P < 0.001, QS versus AS
Pre-HUT Baseline HR and MAP
Pre-HUT baseline values for HR and MAP are presented in Table 2.
Table 2.
Pre-Tilt Baseline Values of HR and MAP (mean ± SEM) During QS and AS in the Prone (P) and Supine (S) Sleeping Positions at 2–4 wks, 2–3 mo and 5–6 mo PNA
| Sleep state/ position | 2–4 WKS (n = 9) |
2–3 MO (n = 16) |
5–6 MO (n = 18) |
|||
|---|---|---|---|---|---|---|
| HR | MAP | HR | MAP | HR | MAP | |
| QS-S | 134 ± 4##‡ | 68 ± 3 | 129 ± 2## | 72 ± 4 | 120 ± 2 | 77 ± 4 |
| AS-S | 135 ± 3# | 80 ± 7 | 130 ± 2 | 76 ± 4 | 127 ± 2†† | 84 ± 5 |
| QS-P | 137 ± 3## | 66 ± 5 | 134 ± 2*## | 70 ± 3 | 122 ± 2 | 74 ± 4 |
| AS-P | 135 ± 3 | 80 ± 7† | 133 ± 3 | 79 ± 6 | 127 ± 2† | 79 ± 5 |
*P < 0.05, **P < 0.01 Prone Versus Supine; † P < 0.05, †† P < 0.01 QS Versus AS
PNA Differences: Compared with 2–3 mo ‡ P < 0.05, ‡‡ P < 0.001; Compared with 5–6 mo #P < 0.05, ##P < 0.001
At 2–3 mo, sleeping position had an effect on HR, which was higher in the prone position during QS (P < 0.05). There was no effect of sleeping position on MAP at any of the ages studied. Sleep state had no effect on HR at 2–4 wks or 2–3 mo PNA; however at 5–6 mo, HR was higher in AS than QS in both the supine (P < 0.01) and prone positions (P < 0.05). Sleep state had no effect on MAP at 2–3 mo or 5–6 mo; however at 2–4 wks, MAP was higher in AS than QS in the prone position (P < 0.05).
PNA had a significant effect on HR, which decreased with increasing PNA in both sleep positions in QS and in the supine position in AS. PNA had no effect on MAP in either sleep state or sleeping position.
Head-up Tilt HR and MAP Profiles
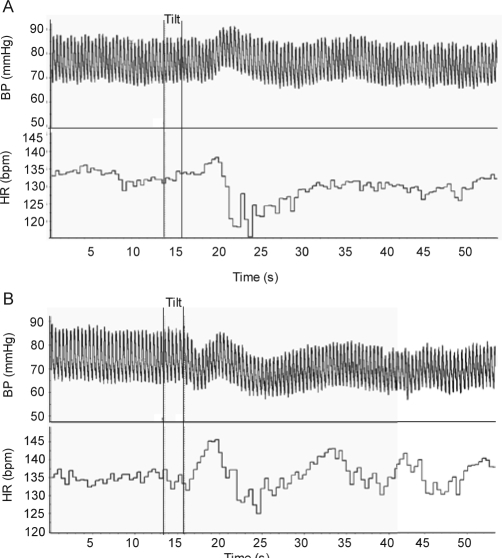
Typical HR and MAP profiles to a HUT during QS in a 2–3 mo old infant in the supine and prone positions are presented in Figure 1.
Figure 1.
Typical recording of the BP and HR response to a HUT in the supine (a) and prone (b) sleeping positions in a 2–3 mo infant during QS. Note the biphasic response of BP in the supine position characterized by an initial increase, then a return to baseline, is substantially altered in the prone position, in which BP falls and remains depressed throughout the HUT.
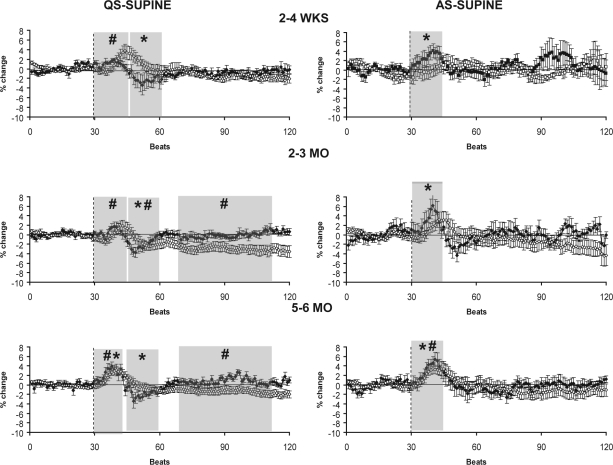
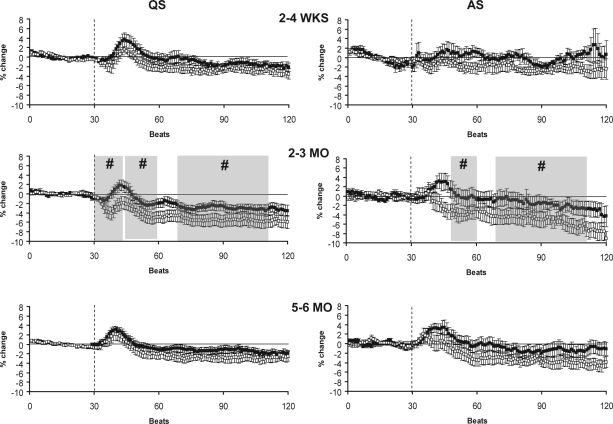
HR and MAP responses to the HUT at 2–4 wks, 2–3 mo, and 5–6 mo are presented for the supine position in Figure 2A and in the prone position in Figure 2B. The overall HR response to the HUT in both positions and sleep states followed a characteristic pattern with an initial increase above baseline, followed by a decrease below baseline, and then a return to baseline in the late phase. HR was significantly increased (P < 0.05) above baseline levels in the initial phase of the response in AS-S at 2–4 wks, AS-S and AS-P at 2–3 mo, and in both sleep states and positions at 5–6 mo. In the middle phase, HR fell below baseline levels (P < 0.05) in QS-S and QS-P at 2–4 wks, 2–3 mo, and 5–6 mo. HR returned to baseline levels in the late phase of the tilt in each sleep state and position at all 3 ages studied.
Figure 2a.
HR (closed circles) and MAP (open circles) responses to HUT in the supine (S) sleeping position during QS (left) and AS (right) at 2–4 wks (n = 9 infants), 2–3 mo (n = 16 infants), and 5–6 mo (n = 18 infants). The tilt is indicated by the dotted line. Shaded areas represent the initial, middle, and late phases of the HUT, shown only when the changes in HR and MAP were significantly different from pre-tilt baseline. * P < 0.05 HR versus pre-tilt baseline; # P < 0.05 MAP versus pre-tilt baseline.
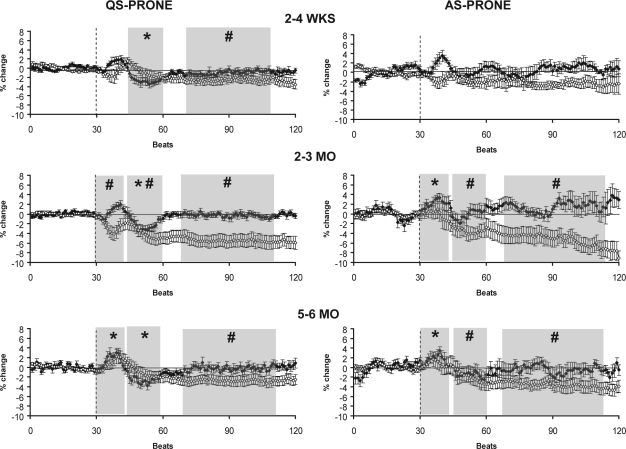
Figure 2b.
HR (closed circles) and MAP (open circles) responses to HUT in the prone (P) sleeping position during QS (left) and AS (right) at 2–4 wks (n = 11 infants), 2–3 mo (n = 17 infants), and 5–6 mo (n = 20 infants). The tilt is indicated by the dotted line. Shaded areas represent the initial, middle and late phases of the HUT, shown only when the changes in HR and MAP were significantly different from pre-tilt baseline. *P < 0.05 HR versus pre-tilt baseline; #P < 0.05 MAP versus pre-tilt baseline.
In the supine sleeping position (Figure 2A) HUT resulted in an increase in MAP in the initial phase, a fall in MAP to or below baseline in the middle phase, and a sustained decrease or return to baseline in the late phase. MAP was significantly increased in the initial phase in QS-S at 2–4 wks, QS-S at 2–3 mo, and in both QS-S and AS-S at 5–6 mo (P < 0.05). In the middle phase MAP fell below baseline levels in QS-S at 2–3 mo (P < 0.05). In the late phase MAP fell significantly below baseline levels in QS-S at 2–3 mo and 5–6 mo (P < 0.05).
In the prone sleeping position (Figure 2B) the biphasic response of MAP to HUT seen in the supine position was absent during both AS and QS. In this position, MAP did not increase above baseline in the initial phase at any of the ages studied. Rather, MAP decreased in the initial phase during QS-P at 2–3 mo (P < 0.05). In the middle phase MAP fell below baseline in both QS-P and AS-P at 2–3 mo, and in AS-P at 5–6 mo (P < 0.05). In the late phase, MAP remained below baseline levels in QS-P at all ages, and in AS-P at both 2–3 mo and 5–6 mo (P < 0.05).
Effects of Sleeping Position on HUT Responses
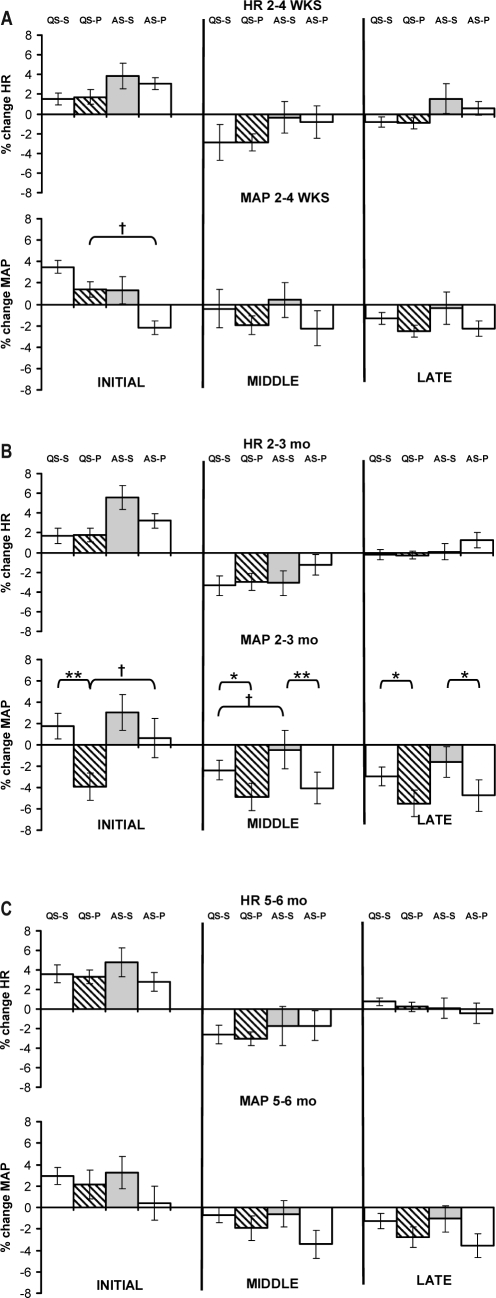
The effects of sleeping position on the response of HR and MAP to HUT at each age studied are presented in Figure 3. There was no effect of sleeping position on the HR response to HUT in either sleep state at any of the 3 ages studied.
Figure 3.
Maximum % change for HR and MAP responses during the initial, middle and late phases of the HUT during QS and AS in the supine and prone sleeping positions at 2–4 wks (a), 2–3 mo (b), and 5–6 mo (c) PNA. *P < 0.05 **P < 0.01 prone versus supine; †P < 0.05 QS versus AS.
There was no effect of sleeping position on the MAP response to HUT in either sleep state at either 2–4 wks or 5–6 mo. However, at 2–3 mo in the initial phase of the HUT there was a fall in MAP in the prone position during QS compared to the supine position, with a mean difference of 6% (P < 0.01). There was also a larger fall in MAP in the prone position than the supine position in both sleep states in both the middle phase (QS-S: −2% ± 1% vs QS-P: −5% ± 1%, P < 0.05; AS-S 0% ± 2% vs AS-P vs −4% ± 1% (P < 0.01) and late phase (QS-S −3% ± 1% vs QS-P −5% ± 1%, P < 0.05; AS-S −2% ± 1% vs AS-P −5% ± 1%, P < 0.05) of the HUT.
The effect of sleeping position on the beat-beat MAP responses to HUT is also presented in Figure 4 for each sleep state and PNA to highlight the marked positional effects.
Figure 4.
MAP responses to HUT in the supine (black boxes) and prone (open boxes) position during QS and AS at 2–4 wks, 2–3 mo, and 5–6 mo PNA. Shaded areas represent the initial, middle and late phases of the HUT where the MAP was significantly lower in the prone versus supine sleeping position. The tilt is indicated by the dotted line. #P < 0.05 MAP prone versus supine.
Effect of Sleep State on HUT Responses
Effects of sleep state on HUT responses for HR and MAP at each age studied are also presented in Figure 3. There was no effect of sleep state on HR responses to the HUT in either sleeping position at any of the 3 ages studied. However, at 2–4 wks MAP was higher in the initial phase in QS-P compared to AS-P (QS-P 1% ± 1% vs AS-P −2% ± 1%, P < 0.05). At 2–3 mo MAP was lower in QS-P compared to AS-P (QS-P −4% ± 1% vs AS-P 1% ± 2%, P < 0.05) in the initial phase and in the middle phase QS-S showed a greater decrease in MAP than AS-S (QS-S −2% ± 1% vs AS-S 0% ± 2%, P < 0.05). At 5–6 mo, there was no effect of sleep state on MAP responses to HUT in either sleeping position.
Effect of PNA on HUT Responses
The HR response to the HUT was not affected by PNA in either sleep state or sleeping position. However, in QS-P the MAP response was altered by PNA, being lower at 2–3 mo (−4% ± 1%) compared with both 2–4 wks (1% ± 1%, P < 0.01) and 5–6 mo (2% ± 1%, P < 0.01). There were no effects of PNA in QS-S or in either AS-S or AS-P.
DISCUSSION
To our knowledge this was the first study to longitudinally assess the effects of sleeping position on autonomic control of the cardiovascular system within the first 6 mo of life. Using noninvasive, continuous measurements of BP we found that there were marked positional differences on the control of BP and HR, which were most prominent at 2–3 mo, the age of greatest SIDS risk.
Our study of infants over the first 6 mo of life has revealed that in infants sleeping supine there is a biphasic response of BP and HR to HUT that differs from the mature, adult response. Our data extend previous studies that demonstrated this pattern in sleeping infants only at 3 mo PNA.11,15,17,18 In adults, the HUT induces a typical baroreflex response, with a postural change from supine to upright causing venous pooling in the lower extremities, which in turn decreases venous return and results in a fall in arterial BP. This fall in BP induces a compensatory baroreflex rise in HR and total peripheral resistance, causing BP to return to normal levels. In contrast, the infant response to the HUT does not involve an initial fall in BP, but rather a rapid increase within the first 15 beats of the tilt. Our findings are in keeping with the suggestion of Harrington et al., that the response to the HUT may be mediated firstly by vestibular mechanisms in the initial phase, followed by secondary baroreflex mechanisms in the middle and late phases.11 In support of this, a substantial body of evidence in both animal and human subjects has demonstrated that the vestibular system contributes to the adjustment of vascular resistance and BP during voluntary movement and passive changes in posture.22–24 Alternatively, the infant response to the HUT may be different to that of the adult due to the substantial differences in body conformation and blood volume distribution. In infants, the distribution of blood volume is predominantly in the upper body, as the legs and head account for 8% and 30%, respectively, of the total body volume, compared to 18% and 10% in the adult.12 Therefore, in contrast to the adult, where redistribution of blood to the lower body reduces venous return and BP with HUT, in infants a hydrostatic shift in blood volume may occur towards the heart, thus increasing venous return and BP. These alternative mechanisms suggest that the rise in BP in the initial phase of the HUT in the supine position may be due to a vestibular-mediated reflex increase, with an additional hydrostatic component. In response to this increase in BP, a baroreflex fall in HR appears to contribute to returning BP to baseline levels in the middle phase of the infant response, rather than the initial phase of the tilt as is typical in the adult.
We found that in the prone sleeping position, the MAP profile during HUT was significantly altered, most prominently at 2–3 mo, the age of greatest SIDS risk. At this age the initial rise in MAP was abolished, and in contrast MAP decreased from baseline throughout all phases of the tilt in QS. In addition, when the effects of sleeping position were compared, prone sleeping produced a larger fall in MAP in all phases of the HUT. Despite the larger MAP fall when prone, there was no difference in the HR response between prone and supine positions, suggesting that compensatory vestibular or baroreflex effects may be less efficient at this age. This finding of an exaggerated BP fall in the prone position could be due to a number of different mechanisms. Prone sleeping may alter vestibular influences on BP regulation, abolishing the sympathetic rise in BP in the initial phase of the HUT present in the supine position. In support of this, animal studies have shown that removal of vestibular inputs via vestibular nuclei lesions, produces a larger fall in BP in response to the HUT compared to the response measured in intact animals.22 Alternatively, the larger BP response in the prone position could be due to a redistribution of blood volumes in this sleeping position. CT scans in adults have shown that the prone position significantly alters the position of organs in the thorax, with the heart and great vessels moving ventrally and caudally, a finding which could potentially alter blood volume distribution.25 Alternatively, changes in vascular tone in the prone position could also be involved. Previous studies in infants have shown that systolic arterial pressure is reduced in the prone sleeping position with only a partially compensating increase in HR, a finding which has been attributed to thermoregulatory–induced peripheral vasodilatation mechanisms.14,19 In addition, other studies in infants have demonstrated that vasoconstriction in response to a HUT, as indicated by cutaneous blood flow, is significantly reduced in the prone position.15 Taken together, these responses suggest that altered vestibular inputs, a redistribution of blood volume, or compensatory mechanisms to overcome reduced temperature loss in the prone position may explain the altered responses to the HUT in this sleep position.
We also assessed arousal responses from sleep to the HUT. We found that the probability of arousal was significantly decreased during QS compared to AS at both 2–3 mo and 5–6 mo PNA; these changes are consistent with previously reported studies using a range of stimuli to induce arousal such as the HUT,26 trigeminal stimulation,27 and hypoxia.28 It has been well established that prone sleeping decreases arousability at an age when SIDS incidence is highest.27,29,30 However, the arousal response to the HUT was not altered by sleeping position at any of the ages studied, as has been previously reported.15 The absence of a positional difference in arousal responses to the HUT may be due to the small magnitude of the BP change (−6%) induced by the HUT in this study. Previous studies in the newborn lamb have shown that large falls in BP (∼35%) that are mediated via arterial baroreceptors, are a potent stimulus for arousal from sleep.31 By design, we limited the angle of the HUT to limit arousal responses; with an increased angle of tilt we may have observed larger falls in BP and positional differences in the arousal response. By increasing HR, BP, and ventilation,32 the arousal response has been suggested to be a vital survival response to a life-threatening event such as hypotension or prolonged apnea.33 Therefore, the prone position could potentially induce a condition in which the arousal mechanism is blunted in combination with altered BP regulation, each increasing the risk for SIDS.
In summary, our study has shown that in normal healthy term infants, the prone sleeping position alters autonomic control of the infant cardiovascular system during sleep, possibly due to alterations in vestibular influences, redistribution of blood volume, or changes in vasomotor tone. Notably, alteration in autonomic control was most marked at 2–3 mo when the risk of SIDS is greatest. Though the changes are small in the normal infant, they may be exaggerated in SIDS infants, who are known to have preexisting deficits in medullary signaling pathways critical for cardiovascular control.34 Failure of cerebellar mechanisms that normally protect against profound hypotension may further amplify the normal BP changes associated with the prone sleeping position in SIDS infants.
ACKNOWLEDGMENTS
The authors thank the parents and infants who participated in this study and the staff of the maternity wards at Monash Medical Centre and Jessie McPherson Private Hospital. This project was supported by the National Health and Medical Research Council of Australia.
ABBREVIATIONS
- SIDS
sudden infant death syndrome
- PNA
postnatal age
- BP
blood pressure
- HR
heart rate
- MAP
mean arterial pressure
- HUT
head-up tilt
- AS
active sleep
- QS
quiet sleep
- EEG
electroencephalogram
- EOG
electrooculogram
- EMG
submental electromyogram
- ECG
electrocardiogram
- SpO2
arterial blood oxygen saturation, measured by pulse oximeter
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Mitchell EA, Scragg R, Stewart AW, et al. Results from the first year of the New Zealand cot death study. N Z Med J. 1991;104:71–6. [PubMed] [Google Scholar]
- 2.Australian Bureau of Statistics A. SIDS Review: 1981–2000, A statistical review. 2000 [cited; Available from: http://www.sidsandkids.org/research-statistics.html]
- 3.Blair PS, Sidebotham P, Berry PJ, Evans M, Fleming PJ. Major epidemiological changes in sudden infant death syndrome: a 20-year population-based study in the UK. Lancet. 2006;367:314–9. doi: 10.1016/S0140-6736(06)67968-3. [DOI] [PubMed] [Google Scholar]
- 4.Schechtman VL, Harper RM, Kluge KA, Wilson AJ, Hoffman HJ, Southall DP. Heart rate variation in normal infants and victims of the sudden infant death syndrome. Early Hum Dev. 1989;19:167–81. doi: 10.1016/0378-3782(89)90077-7. [DOI] [PubMed] [Google Scholar]
- 5.Schechtman VL, Harper RM, Kluge KA, Wilson AJ, Hoffman HJ, Southall DP. Cardiac and respiratory patterns in normal infants and victims of the sudden infant death syndrome. Sleep. 1988;11:413–24. doi: 10.1093/sleep/11.5.413. [DOI] [PubMed] [Google Scholar]
- 6.Kahn A, Groswasser J, Rebuffat E, et al. Sleep and cardiorespiratory characteristics of infant victims of sudden death: a prospective case-control study. Sleep. 1992;15:287–92. doi: 10.1093/sleep/15.4.287. [DOI] [PubMed] [Google Scholar]
- 7.Kato I, Franco P, Groswasser J, et al. Incomplete arousal processes in infants who were victims of sudden death. Am J Respir Crit Care Med. 2003;168:1298–303. doi: 10.1164/rccm.200301-134OC. [DOI] [PubMed] [Google Scholar]
- 8.Harper RM. Sudden infant death syndrome: a failure of compensatory cerebellar mechanisms? Pediatr Res. 2000;48:140–2. doi: 10.1203/00006450-200008000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Harper RM, Kinney HC, Fleming PJ, Thach BT. Sleep influences on homeostatic functions: implications for sudden infant death syndrome. Respir Physiol. 2000;119:123–32. doi: 10.1016/s0034-5687(99)00107-3. [DOI] [PubMed] [Google Scholar]
- 10.Yiallourou SR, Walker AM, Horne RSC. Validation of a new noninvasive method to measure blood pressure and assess baroreflex sensitivity in preterm infants during sleep. Sleep. 2006;29:1083–8. doi: 10.1093/sleep/29.8.1083. [DOI] [PubMed] [Google Scholar]
- 11.Harrington C, Kirjavainen T, Teng A, Sullivan CE. Cardiovascular responses to three simple, provocative tests of autonomic activity in sleeping infants. J Appl Physiol. 2001;91:561–8. doi: 10.1152/jappl.2001.91.2.561. [DOI] [PubMed] [Google Scholar]
- 12.Thoresen M, Cowan F, Walloe L. Cardiovascular responses to tilting in healthy newborn babies. Early Hum Dev. 1991;26:213–22. doi: 10.1016/0378-3782(91)90161-u. [DOI] [PubMed] [Google Scholar]
- 13.Andrasyova D, Kellerova E. Blood pressure and heart rate response to head-up position in full-term newborns. Early Hum Dev. 1996;44:169–78. doi: 10.1016/0378-3782(95)01706-2. [DOI] [PubMed] [Google Scholar]
- 14.Chong A, Murphy N, Matthews T. Effect of prone sleeping on circulatory control in infants. Arch Dis Child. 2000;82:253–6. doi: 10.1136/adc.82.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galland BC, Taylor BJ, Bolton DPG, Sayers RM. Vasoconstriction following spontaneous sighs and head-up tilts in infants sleeping prone and supine. Early Hum Dev. 2000;58:119–32. doi: 10.1016/s0378-3782(00)00070-0. [DOI] [PubMed] [Google Scholar]
- 16.Edner A, Katz-Salamon M, Lagercrantz H, Milerad J. Heart rate response profiles during head upright tilt test in infants with apparent life threatening events. Arch Dis Child. 1997;76:27–30. doi: 10.1136/adc.76.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fifer WP, Greene M, Hurtado A, Myers MM. Cardiorespiratory responses to bidirectional tilts in infants. Early Hum Dev. 1999;55:265–79. doi: 10.1016/s0378-3782(99)00026-2. [DOI] [PubMed] [Google Scholar]
- 18.Kirjavainen T, Viskari S, Pitkanen O, Jokinen E. Infants with univentricular heart have reduced heart rate and blood pressure responses to side motion and altered responses to head-up tilt. J Appl Physiol. 2005;228:518–25. doi: 10.1152/japplphysiol.00248.2004. [DOI] [PubMed] [Google Scholar]
- 19.Yiallourou SR, Walker AM, Horne RS. Effects of sleeping position on development of infant cardiovascular control. Arch Dis Child. 2008 May 2; doi: 10.1136/adc.2007.132860. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 20.Anders T, Emde R, Parmalee A. A manual of standardized terminology, techniques and criteria for scoring states of sleep and wakefulness in newborn infants. Los Angeles, California: UCLA Brain Information Service/BRI; 1971. [Google Scholar]
- 21.International Paediatric Work Group on arousals. The scoring of arousals in healthy term infants (between the ages of 1 and 6 months) J Sleep Res. 2005;14:37–41. doi: 10.1111/j.1365-2869.2004.00426.x. [DOI] [PubMed] [Google Scholar]
- 22.Mori RL, Cotter LA, Arendt HE, Olsheski CJ, Yates BJ. Effects of bilateral vestibular nucleus lesions on cardiovascular regulation in conscious cats. J Appl Physiol. 2005;98:526–33. doi: 10.1152/japplphysiol.00970.2004. [DOI] [PubMed] [Google Scholar]
- 23.Wilson TD, Cotter LA, Draper JA, et al. Effects of postural changes and removal of vestibular inputs on blood flow to the head of conscious felines. J Appl Physiol. 2006;100:1475–82. doi: 10.1152/japplphysiol.01585.2005. [DOI] [PubMed] [Google Scholar]
- 24.Kaufmann H, Biaggioni I, Voustianiouk A, et al. Vestibular control of sympathetic activity. An otolith-sympathetic reflex in humans. Experimental brain research. Experimentelle Hirnforschung. 2002;143:463–9. doi: 10.1007/s00221-002-1002-3. [DOI] [PubMed] [Google Scholar]
- 25.Ball WS, Wicks JD, Mettler FA., Jr Prone-supine change in organ position: CT demonstration. AJR Am J Roentgenol. 1980;135:815–20. doi: 10.2214/ajr.135.4.815. [DOI] [PubMed] [Google Scholar]
- 26.Galland BC, Hayman RM, Taylor BJ, Bolton DP, Sayers RM, Williams SM. Factors affecting heart rate variability and heart rate responses to tilting in infants aged 1 and 3 months. Pediatr Res. 2000;48:360–8. doi: 10.1203/00006450-200009000-00017. [DOI] [PubMed] [Google Scholar]
- 27.Horne RS, Ferens D, Watts AM, et al. The prone sleeping position impairs arousability in term infants. J Pediatr. 2001;138:811–6. doi: 10.1067/mpd.2001.114475. [DOI] [PubMed] [Google Scholar]
- 28.Parslow PM, Cranage SM, Adamson TM, Harding R, Horne RS. Arousal and ventilatory responses to hypoxia in sleeping infants: effects of maternal smoking. Respir Physiol Neurobiol. 2004;140:77–87. doi: 10.1016/j.resp.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Galland BC, Reeves G, Taylor BJ, Bolton DP. Sleep position, autonomic function, and arousal. Arch Dis Child Fetal Neonatal Ed. 1998;78:F189–94. doi: 10.1136/fn.78.3.f189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franco P, Pardou A, Hassid S, Lurquin P, Groswasser J, Kahn A. Auditory arousal thresholds are higher when infants sleep in the prone position. J Pediatr. 1998;132:240–3. doi: 10.1016/s0022-3476(98)70438-x. [DOI] [PubMed] [Google Scholar]
- 31.Horne RS, Berger PJ, Bowes G, Walker AM. Effect of sinoaortic denervation on arousal responses to hypotension in newborn lambs. Am J Physiol. 1989;256:H434–40. doi: 10.1152/ajpheart.1989.256.2.H434. [DOI] [PubMed] [Google Scholar]
- 32.Horner RL. Autonomic consequences of arousal from sleep: mechanisms and implications. Sleep. 1996;19:S193–5. doi: 10.1093/sleep/19.suppl_10.s193. [DOI] [PubMed] [Google Scholar]
- 33.Phillipson EA, Sullivan CE. Arousal: the forgotten response to respiratory stimuli. Am Rev Respir Dis. 1978;118:807–9. doi: 10.1164/arrd.1978.118.5.807. [DOI] [PubMed] [Google Scholar]
- 34.Kinney HC. Abnormalities of the brainstem serotonergic system in the sudden infant death syndrome: a review. Pediatr Dev Pathol. 2005;8:507–24. doi: 10.1007/s10024-005-0067-y. [DOI] [PubMed] [Google Scholar]