Abstract
Study Objectives:
To investigate polysomnographic (PSG) sleep and NREM sleep characteristics, including sleep spindles and spectral activity involved in offline consolidation of a motor sequence learning task.
Design:
Counterbalanced within-subject design.
Setting:
Three weekly visits to the sleep laboratory.
Participants:
Fourteen healthy participants aged between 20 and 30 years (8 women).
Interventions:
Motor sequence learning (MSL) task or motor control (CTRL) task before sleep.
Measurements and Results:
Subjects were trained on either the MSL or CTRL task in the evening and retested 12 hours later the following morning on the same task after a night of PSG sleep recording. Total number and duration of sleep spindles and spectral power between 0.5 and 24 Hz were quantified during NREM sleep. After performing the MSL task, subjects exhibited a large increase in number and duration of sleep spindles compared to after the CTRL task. Higher sigma (σ; 13 Hz) and beta (β; 18–20 Hz) spectral power during the post-training night's sleep were also observed after the MSL task.
Conclusions:
These results provide evidence that sleep spindles are involved in the offline consolidation of a new sequence of finger movements known to be sleep dependent. Moreover, they expand on prior findings by showing that changes in NREM sleep following motor learning are specific to consolidation (and learning), and not to nonspecific motor activity. Finally, these data demonstrate, for the first time, higher fast rhythms (β frequencies) during sleep after motor learning.
Citation:
Morin A; Doyon J; Dostie V; Barakat M; Tahar AH; Korman M; Benali H; Karni A; Ungerleider LG; Carrier J. Motor sequence learning increases sleep spindles and fast frequencies in post-training sleep. SLEEP 2008;31(8):1149-1156.
Keywords: Motor sequence learning, memory consolidation, sleep, sleep spindles, spectral analysis, memory
THE BENEFICIAL ROLE OF SLEEP IN MOTOR MEMORY CONSOLIDATION IS NOW WELL DOCUMENTED. FOR EXAMPLE, AMPLE EVIDENCE INDICATES THAT consolidation of a newly learned sequence of movements, defined as a spontaneous (offline) improvement in performance that emerges in the absence of any further practice, is sleep dependent.1–3 Indeed, our group4 and others have demonstrated that delayed gains on a sequential finger-tapping task are triggered only after a night of sleep, whereas a comparable interval without sleep provides no additional benefit.
Despite the accumulating evidence in support of sleep-dependent motor skill consolidation, there is still no consensus with respect to the sleep stages that are preferentially involved in this memory phase.5 Several studies support the hypothesis that post-training REM sleep is required for efficient motor skill consolidation.1,7–10 Yet other recent investigations have emphasized the importance of NREM sleep, including delta11 and stage 2 sleep.12 For example, Huber and colleagues11 have shown that slow wave activity (< 4 Hz) in the right parietal area during post-training sleep increases following practice on a visuomotor adaptation task, while others have reported that memory on a simple motor skill (i.e., the rotary pursuit task) is vulnerable to stage 2 sleep deprivation but not to REM sleep loss.13 Finally, Walker and colleagues3 demonstrated that improved overnight performance on a motor sequence learning task is associated with the amount of stage 2 sleep in the last quarter of the night.
There has been increasing attention to the possible contribution of sleep spindles to memory processes.14 Spindles are an essential feature of stage 2 sleep that also appear throughout the depolarizing phase of slow wave sleep oscillation (<1 Hz). Generated by reticular thalamic neurons, they constitute synchronous waveforms between 12–16 Hz that propagate in the thalamocortical loop.20 They are therefore thought to provide proper conditions for synaptic changes21 and to elicit long-term potentiation,22 a cellular mechanism known to be involved in learning.23 Recently, in a study on motor procedural learning, increased density of sleep spindles and duration of stage 2 sleep following intensive training on several motor skills (including ball-n-cup, rotor pursuit, direct tracing, and logic operation game) were reported.24 Interestingly, the authors found a correlation between increased spindle density and overall task performance improvement. Despite these findings, however, sleep spindle activity could not be related to the nighttime offline consolidation process per se, because the training and retest sessions were conducted one week apart. Moreover, as no motor control condition was presented, changes in sleep architecture following motor skills acquisition could be associated with nonspecific motor activity generated by the tasks themselves.
In summary, the electroencephalographic (EEG) characteristics of post-training NREM sleep involved in sleep-dependent motor consolidation remain largely unknown. Thus, the present study aimed to identify the sleep characteristics underlying offline motor memory consolidation. In particular, we sought to compare NREM sleep—including sleep spindles and spectral power activity—following a motor sequence learning task known to result in significant overnight delayed gains4 and after training on a motor control task involving equivalent motor activity but with no expected learning or consolidation. We predicted that sleep following motor sequence learning (MSL) would show higher spectral activity—especially in δ (< 4 Hz) and σ frequency bins (12–15 Hz)—and greater number of sleep spindles than in sleep after the motor control task (CTRL).
METHODS
Participants
A total of 14 healthy participants aged between 20 and 30 years (mean age: 23.6 ± 3.2 years; 8 women) took part in this study. All subjects were strongly right-handed, as assessed by the Edinburgh Handedness Inventory.25 All subjects also reported sleeping regularly for 7–9 hours per night. Participants reported using no medications, had no significant medical or sleep complaints, and no psychiatric or neurological illness. All subjects had a body mass index < 27. Extreme evening- and morning-type individuals, regular nappers, and smokers were excluded. Each participant underwent a PSG screening night at the sleep laboratory 7 days prior to testing to confirm the absence of sleep disorders. Presence of sleep disturbances such as < 85% sleep efficiency, > 30 min sleep latency, sleep apnea, hypopnea, or periodic leg movements (> 5 events per hour) were also used as exclusion criteria. All subjects scored below 4 on the short version of the Beck Depression Scale,26 and all women were tested in the follicular phase of the menstrual cycle. Subjects who worked night shifts or who had gone on a transmeridian trip within 3 months prior to the study were excluded. Finally, musicians were excluded to avoid subjects with previous experience on motor sequence tasks. Subjects were required not to consume alcohol or caffeine for ≥ 12 h prior to each testing. This study was approved by the Ethics Committee at the Hôpital du Sacré-Coeur de Montréal, and all subjects signed an informed consent form. Subjects received financial compensation for their participation.
Procedure
Subjects were instructed to maintain a regular sleep schedule and adhere to their preferred bedtime and wake time (± 30 min) for 7 days prior to and during the study. Compliance was verified by sleep diaries. The research protocol was then scheduled according to the subject's habitual sleep-wake cycle. After an adaptation/screening night, subjects came to the sleep laboratory for 2 visits, one week apart. The 2 motor tasks (MSL and CTRL) were administrated in counterbalanced order across the 2 visits. Subjects were required to arrive at the laboratory by 19:00 for application of EEG recording electrodes. At around 21:00 (mean time: 21:39 ± 0:48), subjects were trained on either the MSL or CTRL task. A retest session on the respective motor task was conducted 12 h later, on the morning following a night of PSG sleep recording. Morning sessions began 2 h after the subject's habitual wake time, as determined by the sleep diaries (mean time: 09:39 ± 0:48).
Motor Tasks
A computerized version of the sequential finger-tapping task initially developed by Karni et al.27 was used in the present study to measure motor sequence learning. This task was chosen because it shows robust sleep-dependent consolidation effects.2,3 Four numeric keys arranged ergonomically on a standard computer keyboard were used (key-to-number assignment: B[1], F[2], D[3], Z[4]). Subjects were asked to repeat an explicitly known sequence of 5 finger movements using the left (nondominant) hand. Introductory trials were used to familiarize subjects with the sequence (4–1–3–2–4), which was displayed on a computer screen. Visual feedback was provided after each response using green and red dots to indicate whether subjects had respectively produced “correct” or “wrong” responses. Once 3 consecutive correct sequences were executed, the introductory block ended, and subjects got ready for the training session. The latter session consisted of 12 trial blocks of 30-sec each. With no visual feedback, subjects had to repeat the sequence as quickly and accurately as possible. Trial blocks were separated by 30-sec rest periods, for a total training session duration of 12 min. In the retest session, subjects were required to perform five 30-sec trial blocks of the same sequence with 30-sec rest periods between blocks. For each block, subjects were given an audible “Start” signal. They then continuously tapped the sequence until they heard the “Stop” signal. No visual feedback was provided during the retest session.
A CTRL task was also administered to control for nonspecific motor activity, using the same 4 numeric keys placed ergonomically on a standard computer keyboard. Unlike the sequence task, subjects were instructed to press the response key corresponding to a number (1 to 4) displayed on the computer screen. Numbers were presented in random order. Subjects had to respond as quickly and accurately as possible using the fingers of the left (nondominant) hand. Once the subject responded (correctly or not), the screen stimulus was replaced by the next one. No practice session was provided prior to training. The training session in this condition consisted again of twelve 30-sec trial blocks with 30-sec rest periods between blocks (12 min duration total), while the retest comprised five 30-sec trial blocks with 30-sec rest periods between them.
Key-press responses were recorded on both tasks. The number of correctly typed sequences (or the number of correct responses on the CTRL task) per 30-sec trial block and average speed per block were calculated. Proportion of errors to correct sequences (or correct responses) per 30-sec block (accuracy) was also recorded and used as a dependent variable.
Polysomnographic Recording
EEG electrodes were applied to the subject's head according to the International 10–20 System, using a referential montage with linked ears, a right and left electrooculogram (EOG), and a bipolar montage EMG recording (to which we added a third chin electrode, in case one of the originals was lost). Signals were recorded using a digital ambulatory sleep recorder (Vitaport-3 System; TEMEC Instruments, Kerkrade, Netherlands). EEG signals were filtered at 70 Hz (low pass) with 1-s time constant and digitized at a sampling rate of 256 Hz using commercial software (Columbus).
Sleep Data Analysis
Sleep stages were visually scored according to standard criteria and modified to 20-sec epochs28 using an EEG layout (C3 derivation) displayed on a computer screen (Luna, Stellate System, Montreal, Canada). Sleep spindles were visually identified in epochs scored as NREM sleep (stages 1, 2, 3, and 4) at sites FZ, CZ, and PZ by a single experienced technician who was unaware of the nature of the study. Criteria for sleep spindle detection included 12–16 Hz frequency, 0.5–2.5 sec duration, and fusiform (waxing and waning amplitude) with typical spindle morphology. Total number and duration (sec) of sleep spindles were calculated per third of night of sleep.
Spectral (μV2/Hz) analyses were performed on Fp1, Fp2, F3, F4, FZ, C3, C4, CZ, P3, P4, PZ, O1, O2, and OZ (linked ears) during NREM sleep (excluding stage 1) using a commercial software package (Harmonie, Stellate System, Montreal, Canada) to compute fast Fourier transforms on 4-sec epochs with a cosine-window tapering and a spectral resolution of 0.25 Hz. EMG artifacts were automatically detected and excluded from analysis. Further artifacts were eliminated by visual inspection. Artifact-containing epochs were regarded as missing data in order to preserve sleep continuity. Five 4-sec spectral epochs were averaged to maintain correspondence with the 20-sec sleep scoring windows. Spectral activity was then averaged for all NREM epochs during the night,29 and analyses were performed per 1 Hz frequency bin ranging from 1.0 to 24.0 Hz (identified by their lower boundary value), and between 0.5 and 1.0 Hz.
Statistical Analysis
For each motor task, the amount of learning during the initial training session was evaluated using paired t-tests to compare performances between the first and last blocks of practice. In order to assess when asymptotic performance was reached during training for each motor task, one-way analyses of variance (ANOVAs) with repeated measures (blocks) on performance were used, starting with the last pair of blocks, and going backward by adding one block at a time to the ANOVA until the effect of block became significant. The final 3 trials of the training session and the first 3 trials of the retest session were used to evaluate memory consolidation for each motor task using a 2-way ANOVA (2 moments [post-training, retest] × 3 blocks). A P value < 0.05 was considered significant.
PSG parameters (including sleep spindles) and spectral data that did not distribute normally (Shapiro-Wilks W test) were log transformed for statistical analysis. Paired t-tests were used to compare differences in PSG sleep parameters after practicing the MSL and CTRL tasks. Total number and duration of sleep spindles per third of night were analyzed using 3-way ANOVAs for repeated measures (task × third of night × derivation). Simple effect analysis was used to decompose significant interactions. Degrees of freedom were corrected (Huynh-Feldt) for sphericity of variables with more than 2 levels, although original degrees of freedom are reported. All-night NREM spectral EEG activity was assessed using 2-way ANOVAs for repeated measures (task × midline derivation) on each 1-Hz bin. In view of the multiple comparisons, significance level was set at 0.01 for these analyses. Two-way ANOVAs for repeated measures (task × hemisphere) were also carried out for all-night NREM sleep to examine hemispheric differences across topographic regions. To explore possible relationships between delayed performance gains and sleep, Pearson product-moment correlations were carried out between overnight gains in the MSL task and sleep changes (spindles at each derivation, spectral power at 13 Hz, and β band [18–20 Hz] at each derivation) between the MSL and CTRL tasks. One participant in the MSL task showed no learning progress in the initial training session and was excluded from all statistical analyses. Another subject was excluded from the sleep analyses because he did not attend the second visit (CTRL task) at the laboratory.
RESULTS
Behavioral Measures
Initial Training Session
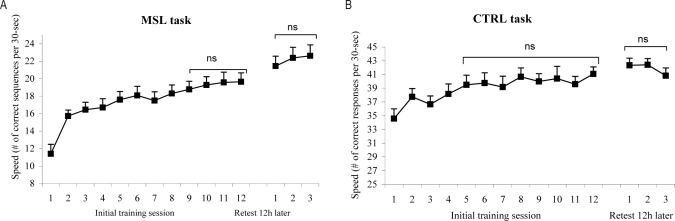
Learning curves for the MSL and the CTRL tasks are illustrated in Figures 1A and 1B. In the initial learning session, a 72% improvement in performance speed was seen from block 1 to block 12 in the MSL task (11.4 to 19.6 sequences/block; t(12) = −6.02, P < 0.0001), while a small yet significant 19% improvement was observed in the CTRL task (35 to 41 correct responses/block; t(11) = −4.64, P < 0.001). It should be noted, however, that asymptotic performance at the end of the training session was reached faster in the CTRL than the MSL task, as demonstrated by the absence of performance speed improvement in the last 8 practice blocks of the CTRL task (F(7, 77) = 1.13, P = 0.35), in contrast to the last 4 blocks of the MSL task (F(3, 36) = 0.67, P = 0.58).
Figure 1.
Learning curves expressed in (A) as speed (= number of correct sequences per 30-sec trial block on the MSL task) and in (B) as speed (= number of correct response per 30-sec trial block on the CTRL task). Performance levels are shown across the initial training session and the retest session, which was performed 12 hours later, after sleep. (A) In the MSL task, a 72% improvement in performance speed was seen from block 1 to block 12, with asymptotic level reached in the last 4 blocks. No within-session improvement was found in the retest session. (B) In the CTRL task, a 19% improvement was observed and asymptotic level was reached by the end of the training, as measured by the absence of performance speed improvement in the last 8 practice blocks. Again, no learning effect was observed between trials in the retest session. Error bars, SEM; NS, nonsignificant.
Consolidation Effects
When subjects were retested on the MSL task following a night of sleep, performance speed (number of correct sequences per 30-sec trial block) spontaneously improved by a significant 14% ± 2.2% (last 3 blocks in post-training: 19.50, SD = 3.61 vs. first 3 blocks in retest: 22.15, SD = 4.07). Indeed, a 2-way ANOVA (2 moments [post-training; retest] × 3 blocks) showed a significant effect of moment on the MSL task, as measured by the number of correct sequences in 30-sec blocks (F(1,12) = 41,381, P < 0.0001). A 4% ± 1.8% gain in the number of correct responses per 30 sec was also observed at retest on the CTRL task (last 3 blocks in post-training: 40.4 correct answers in 30 sec, SD = 4.2 vs. first 3 blocks in retest: 41.9 correct answers in 30 sec, SD 2.9). A 2-way ANOVA (2 moments [post-training; retest] × 3 blocks) showed a significant effect of moment on the CTRL task, as measured by the number of correct responses per 30 sec blocks (F(1,11) = 7.21, p = 0.02). No significant effect of block or interaction between block and moment was found for the MSL task and the CTRL task. Finally, effect size analyses revealed greater significance of delayed improvement in performance speed in the MSL (d = 2.54) than the CTRL (d = 1.39) task.
Sleep Quality
As depicted in Table 1, PSG parameters—sleep latency, sleep efficiency, total sleep time, and amount of sleep stages—showed no significant differences following training on either MSL or CTRL tasks.
Table 1.
Means (SD) of EEG Sleep Parameters
| Following motor sequence learning task | Following motor control task | |
|---|---|---|
| Sleep latency (min)* | 9.51 (7.7) | 11.08 (10.08) |
| Total sleep time (min) | 449.4 (26.1) | 444.3 (27.5) |
| Sleep efficiency (%)* | 93.9 (2.7) | 93.8 (5.4) |
| Stage 1 (min)* | 24.7 (11.4) | 28.3 (21.2) |
| Stage 2 (min) | 279.3 (29.3) | 273.3 (25.7) |
| Stage 3 (min) | 33.5 (20.6) | 32.7 (19.7) |
| Stage 4 (min)* | 5.28 (5.4) | 5.28 (6.5) |
| REM sleep (min) | 106.6 (22.9) | 104.7 (28.3) |
Indicates Log transformation [Log 10(variable)] performed before analysis. All P values from t-tests are nonsignificant (P > 0.4).
Sleep Spindles in Post-Training NREM Sleep
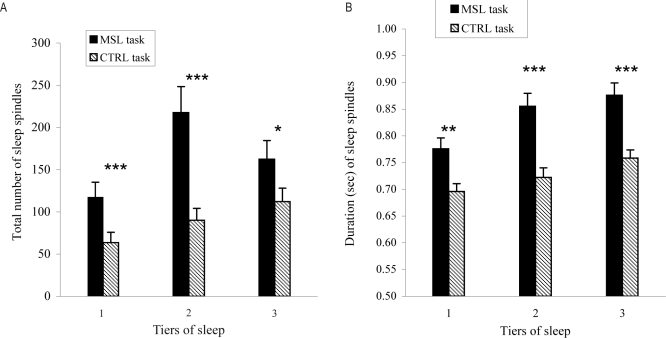
A highly significant task × third of night interaction was observed for both the total number (F(2,66) = 25.64, P < 0.0001) and duration (F(2,64) = 14.47, P < 0.0001) of sleep spindles. Contrast analyses revealed that, compared to the CTRL task, number and duration of sleep spindles after training on the MSL task were significantly higher in all 3 thirds of night (all P values < 0.001). As shown in Figure 2A, the difference between MSL and CTRL nights in the total number of sleep spindles was smaller in the last third of night than in the first and second third of night. As illustrated in Figure 2B, the difference between MSL and CTRL nights in the duration of sleep spindles was larger in the second tier, followed by the third and first third of night, respectively. Total number and duration of sleep spindles increased across all 3 sleep tiers (F(2,66) = 41.85 for total number, F(2,66) = 88.44 for duration; all P values < 0.0001 for the main effect of third of night of sleep). Finally, the total number of sleep spindles presented a centroparietal prevalence (F(2,33) = 4.1, P = 0.03 for the main derivation effect). No significant interaction (derivation × task) was found. No significant correlation between spindles increases at each derivation and overnight gains were found.
Figure 2.
(A) Total number and (B) duration (sec) of sleep spindles averaged by third of night of sleep. Filled bars indicate sleep following the MSL task and hatched bars represent sleep after the CTRL task. Total number and duration of sleep spindles were significantly higher across all sleep thirds of night (***P < 0.000001; **P < 0.00001; *P < 0.001).
Spindle density in stage 2 sleep (number of spindles per minute) for the entire sleep episode was also calculated. A paired-samples t-test showed an average increase of 0.814 per minute from CTRL (M = 1.11, SD = 0.94) to SEQ (M =1.93, SD = 1.44) (t(11) = 3.85, P < 0.05).
EEG Spectral Power in Post-Training NREM Sleep
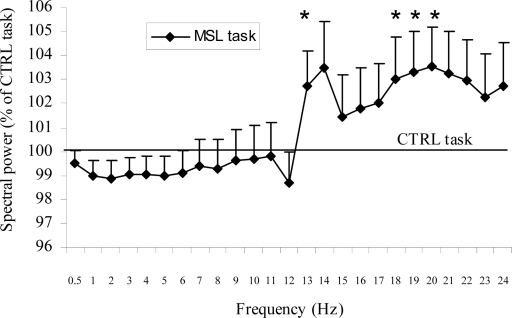
As illustrated in Figure 3, spectral power during post-training sleep was significantly higher after the MSL than the CTRL task at 13 Hz and in the 18–20 Hz range (Task main effects; F(1,43) ≤ 8.31, all P values ≤ 0.01). No significant interaction (task × midline derivation) was found for any of the frequency bins.
Figure 3.
Mean (FZ, CZ, PZ, OZ derivations) all-night spectral power in NREM sleep following the MSL task expressed relative to the values of the CTRL task. Data are averaged within each 1-Hz frequency bin identified by their lower boundary value. *Significant task effects. Error bars, SEM.
As tasks were executed with the left hand, we also explored potential hemispheric differences (HEM: right side versus left side) at each topographic region (prefrontal, frontal, central, parietal, and occipital) for separate frequency bins that previously showed significant task effect (13 Hz, 18 to 20 Hz). Activity between 18 and 20 Hz was summed and defined as the β band. Significant ANOVA results (HEM × task) are summarized in Table 2. Only one significant task × HEM interaction was found for the 13-Hz bin (F(1,22) = 4.74, P = 0.04). In contrast to the CTRL task, higher spectral power in the parietal area in the MSL task was observed on the right side only (P4 derivation: P = 0.003). Higher spectral power in the 13-Hz bin was also found in the MSL compared to the CTRL task in the prefrontal (F(1,22) = 7.54, P = 0.01), frontal (F(1,22) = 9.54, P = 0.005), central (F(1,22) = 8.62, P = 0.008), and parietal (F(1,22) = 6.12, P = 0.02) areas, but not in the occipital region. For the β band, the main effect of task reached significance in the central (F(1,22) = 4.45, P = 0.047), parietal (F(1,22) = 12.68, P = 0.002), and occipital (F(1,22) = 4.54, P = 0.046) regions. A trend toward higher spectral power in the prefrontal region was also found (F(1,22) = 4.28, P = 0.05). No correlations emerged between σ (13 Hz) or β power increases (18–20 Hz) at each derivation and overnight gains in the MSL task.
Table 2.
Significant effects of Task × Hemisphere ANOVAs for Spectral Power in 13-Hz bin and β Band in Each Topographic Region
| Topographic region | 13-Hz |
Beta band (18–20 Hz) |
||||||
|---|---|---|---|---|---|---|---|---|
| Task effect |
Task × HEM interaction |
Task effect |
Task × HEM interaction |
|||||
| F1,22 | P | F1,22 | P | F1,22 | P | F1,22 | P | |
| Prefrontal (FP1/FP2) | 7.5 | 0.01 | - | NS | 4.28 | 0.05 | - | NS |
| Frontal (F3/F4) | 9.54 | 0.005 | - | NS | - | NS | - | NS |
| Central (C3/C4) | 8.62 | 0.008 | - | NS | 4.45 | 0.047 | - | NS |
| Parietal (P3/P4) | 6.12 | 0.02 | 4.74 | 0.04 | 12.68 | 0.002 | - | NS |
| Occipital (O1/O2) | - | NS | - | NS | 4.54 | 0.046 | - | NS |
NS indicates nonsignificant.
DISCUSSION
Compared to the CTRL task, the MSL task showed more sleep spindles of longer duration in post-training sleep. Moreover, σ (13 Hz) and β (18–20 Hz) spectral power were higher in NREM sleep following the MSL task than the control task. Importantly, these changes cannot be explained by differences in time spent in specific sleep stages, as the sleep architecture was similar after both tasks. Likewise, since we used a counterbalanced within-subject design, sleep modifications are unlikely to be attributable to inter-individual differences or a night effect. Overall, these results thus provide evidence that sleep spindles are involved in offline consolidation of a new sequence of finger movements, which is known to be sleep-dependent.2–4 Furthermore, our data extend prior findings by demonstrating that changes in NREM sleep following motor learning are specific to the consolidation process and not to nonspecific motor activity. Finally, these results demonstrate that β frequencies (18–20 Hz) during sleep are greater after motor learning, although they are not significantly correlated with motor memory consolidation.
Our aim was to compare sleep after 2 highly comparable motor tasks involving similar motor outputs. We hypothesized that, in contrast to the MSL task, the CTRL task would not elicit an overnight consolidation effect. Surprisingly, the results revealed slow learning improvement on the CTRL task, as well as small but significant offline gains in the retest session. Due to the higher gain effect on the MSL compared to the CTRL task, however, it seems reasonable to assume that the MSL task showed superior offline consolidation compared with the CTRL task. As some learning (and consolidation) in the present study took place in the CTRL task, we cannot exclude the fact that other factors linked to the nature of the 2 motor tasks, e.g., the level of complexity, might have contributed to the overall changes in the physiological characteristics of sleep. For example, a study by Kuriyama et al.19 showed a relationship between difficulty of a learned task and improvement of performance after a night of sleep, suggesting that the more difficult is a task, the more it will benefit from sleep. Another study by Schmidt et al.18 showed that the level of difficulty in encoding verbal material is critical for eliciting sleep spindle changes in post-training sleep. Because asymptotic performance was reached after only 4 blocks of practice in the CTRL task, while learning progress persisted until the ninth block in the MSL task, this would suggest that the CTRL task was easier (i.e., less complex) than the MSL task. Accordingly, it is possible that the higher complexity of the MSL task influenced subsequent sleep features, which would explain why consolidation-related sleep spindle changes were seen in greater numbers and of longer duration after motor sequence learning than following the random production of finger movements.
Interestingly, the large increase in the number and duration of sleep spindles was combined with increased EEG power in the σ frequency (13 Hz) on the MSL task, but not on the CTRL task. However, no significant correlation emerged between overnight gains in the MSL task and the spindles or σ power changes. In contrast, Milner et al.30 recently reported correlations between high σ activity (13.5–15 Hz) and motor performance on a ball-n-cup task performed after a short nap. However, the authors only used 2-min samples from each sleep stage, a measure that is not representative of overall sleep activity. Alternatively, the absence of correlation in the present study can be attributed to the small number of subjects as well as intersubject variability in spindles. Part of this variability might be due to visual scoring of spindles. Completely reproducible detection could be reach in future studies with the use of programmed algorithms.
Our finding of increased sleep spindles density after the MSL task is consistent with previous observations in humans which demonstrated that spindle density increases following intensive training on simple motor tasks6,24 and as a consequence of spatial31 or verbal16,18 learning. Moreover, the relationship between memory processing and spindle density has recently been demonstrated in animals as increased learning-related spindles have been reported after an odor-reward association task in rodents.32 Together, these findings support the hypothesis that learning-related activity before sleep can selectively modulate the brain activity involved in sleep spindle generation. It has been demonstrated that spindle-related spike discharges can induce long-term potentiation in neocortical cells.22 Based on our findings, sleep spindles would be the ideal physiological mechanism to facilitate the neuronal plasticity related to motor memory consolidation processes per se. Some authors have suggested that slow spindles (<13 Hz) and fast spindles (>13 Hz) may play a different role in learning/cognition.17,38 Future studies should evaluate possible differential impact of learning on fast and slow spindles.
Compared to the CTRL task, sleep after the MSL task also revealed higher NREM spectral power in the β band. Importantly, and similar to spindles, fast rhythmic activity is known to occur selectively over the depolarizing phase of the slow oscillations20 and to modulate plasticity.21 Increased β power during post-training NREM sleep might therefore be related to similar synaptic processes than spindles. Yet the β augmentation could also reflect general cognitive processes related to the MSL task, since in humans, an association has been reported between fast oscillations (β/γ frequencies) and cognitive activity,33,34 and a relation has been proposed between β EEG activity during sleep in patients with insomnia and cognition (e.g., attention, sensory process).35 Finally, with respect to motor sequence learning, β activity (21–23 Hz) during wakefulness has been linked to difficulty of transitions (i.e., level of complexity) between sequential finger movements.36 As the latter study demonstrated that β rhythm changes in EEGs occurred in the absence of motor memory load, our present findings on β frequencies might also reflect the complexity of movements in the MSL task rather than memory process-related changes.
The absence of effect on slow wave activity in the MSL task observed in the present study is in line with a recent study by Marshall and colleagues,37 who demonstrated that sleep-associated offline performance gains in a similar procedural finger-sequence task were not increased by slow oscillation stimulation. However, it argues against the results of Huber et al.,11 who found a selective increase in slow wave activity during the night following learning of a motor adaptation skill. Although it is also possible that the small overnight gains seen after practicing the CTRL task masked changes in slow wave activity, these discrepancies between studies suggest that the specific role of sleep stages in motor learning consolidation may be influenced by the nature (sequence vs adaptation task) of the newly learned skill.
In the present study, a spectral power increase in σ activity (13 Hz) in both hemispheres was observed in the prefrontal, frontal, central, and parietal regions, but not in occipital areas. These results are in accord with previous imaging studies that demonstrate the contribution of the premotor cortex, primary motor region,39 and superior parietal area40 to sleep-dependent consolidation of motor sequence learning. Furthermore, our results reveal that higher σ power (13 Hz) changes were observed in the contralateral right parietal region after the MSL task. This pattern was also expected for the right central region, but was not significant. Nevertheless, our findings are in line with those of Huber et al.,11 who demonstrated sleep changes located exclusively within the parietal region following motor adaptation. They are also in accord with more recent works demonstrating a local association between sleep spindles and the type of memory task.9,14,15
In conclusion, our findings expand on the understanding of offline consolidation of motor learning and demonstrate that sleep spindle activity plays a possible role in motor memory consolidation. Beta oscillations also appear to be a relevant marker of the synaptic plasticity that underlies cognitive processes. However, further studies are needed to investigate whether this rhythm is related to motor memory processes as well.
ACKNOWLEDGMENTS
Support for this research was provided by a grant from the Canadian Institutes of Health Research to JD, JC, AHT, AK, HB, and LGU, a FRSQ scholarship to JC, and a fellowship from the Natural Sciences and Engineering Research Council of Canada to AM. The authors are grateful to Sonia Frenette and Maryse Parenteau for technical assistance and to our research assistants for day-to-day study management.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Fischer S, Hallschmid M, Elsner AL, Born J. Sleep forms memory for finger skills. Proc Natl Acad Sci USA. 2002;99:11987–91. doi: 10.1073/pnas.182178199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korman M, Raz N, Flash T, Karni A. Multiple shifts in the representation of a motor sequence during the acquisition of skilled performance. Proc Natl Acad Sci USA. 2003;100:12492–97. doi: 10.1073/pnas.2035019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker MP, Brakefield T, Morgan A, Hobson A, Stickgold R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002;35:205–11. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- 4.Doyon J., Korman M, Morin A, Dostie V, et al. Distinctive roles of sleep and simple passage of daytime on consolidation of motor sequence and visuomotor adaptation learning. Submitted. [DOI] [PMC free article] [PubMed]
- 5.Rauchs G, Desgranges B, Foret J, Eustache F. The relationship between memory systems and sleep stages. J Sleep Res. 2005;14:123–40. doi: 10.1111/j.1365-2869.2005.00450.x. [DOI] [PubMed] [Google Scholar]
- 6.Fogel SM, Smith C, Cote K. Dissociable learning-dependent changes in REM and non-REM sleep in declarative and procedural memory systems. Behav Brain Res. 2007;180:48–61. doi: 10.1016/j.bbr.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 7.Cohen DA, Pascual-Leone A, Press DZ, Robertson EM. Off-line learning of motor skill memory: a double dissociation of goal and movement. Proc Natl Acad Sci USA. 2005;102:18237–41. doi: 10.1073/pnas.0506072102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maquet P, Peigneux P, Fuchs S, et al. Experience-dependent changes in cerebral activation during human REM sleep. Nat Neurosci. 2000;3:831–36. doi: 10.1038/77744. [DOI] [PubMed] [Google Scholar]
- 9.Nishida M, Walker MP. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS ONE. 2007;4(2):e341. doi: 10.1371/journal.pone.0000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith C, Nixon M, Nader RS. Posttraining increases in REM sleep intensity implicate REM sleep in memory processing and provide a biological marker of learning potential. Learn Mem. 2004;11:714–19. doi: 10.1101/lm.74904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huber R, Ghilardi MF, Massimini M, Tonini G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 12.Nader R, Smith C. A role for stage 2 sleep in memory processing. In: Maquet P, Smith C, Stickgold R, editors. Sleep and brain plasticity. New York: Oxford University Press; 2003. pp. 87–98. [Google Scholar]
- 13.Smith C, MacNeill C. Impaired motor memory for a pursuit rotor task following stage 2 sleep loss in college students. J Sleep Res. 1994;3:206–13. doi: 10.1111/j.1365-2869.1994.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 14.Clemens Z, Fabo D, Halasz P. Overnight verbal memory retention correlates with the number of sleep spindles. Neuroscience. 2005;132:529–35. doi: 10.1016/j.neuroscience.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Clemens Z, Fabo D, Halasz P. Twenty-four hours retention of visuospatial memory correlates with the number of parietal sleep spindles. Neurosci Lett. 2006;403:52–6. doi: 10.1016/j.neulet.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 16.Gais S, Molle M, Helms K, Born J. Learning-dependent increases in sleep spindle density. J Neurosci. 2002;22:6830–4. doi: 10.1523/JNEUROSCI.22-15-06830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schabus M, Gruber G, Parapatics S, et al. Sleep spindle-related activity in the human EEG and its relation to general cognitive and learning abilities. Eur J Neurosci. 2006;23:1738–46. doi: 10.1111/j.1460-9568.2006.04694.x. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt C, Peigneux P, Muto V, et al. Encoding difficulty promotes postlearning changes in sleep spindle activity during napping. J Neurosci. 2006;26:8976–82. doi: 10.1523/JNEUROSCI.2464-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuriyama K, Stickgold R, Walker M. Sleep-dependent learning and motor-skill complexity. Learn Mem. 2004;11:705–13. doi: 10.1101/lm.76304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steriade M. Grouping of brain rhythms in corticothalamic systems. Neurosci. 2006;137:1087–106. doi: 10.1016/j.neuroscience.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 21.Steriade M, Timofeev I. Neuronal plasticity in thalamocortical networks during sleep and waking oscillations. Neuron. 2003;37:563–76. doi: 10.1016/s0896-6273(03)00065-5. [DOI] [PubMed] [Google Scholar]
- 22.Rosanova M, Ulrich D. Pattern-specific associative long-term potentiation induced by a sleep spindle-related spike train. J Neurosci. 2005;25:9398–405. doi: 10.1523/JNEUROSCI.2149-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290:533–6. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- 24.Fogel SM, Smith C. Learning-dependent changes in sleep spindles and stage 2 sleep. J Sleep Res. 2006;15:250–55. doi: 10.1111/j.1365-2869.2006.00522.x. [DOI] [PubMed] [Google Scholar]
- 25.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 26.Beck AT, Steer RA. The Beck Depression Inventory. Psychological Corporation; 1987. [Google Scholar]
- 27.Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995;377:155–8. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- 28.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles: Brain Information Service/Brain Research Institute, UCLA; 1968. [Google Scholar]
- 29.Feinberg I, Floyd CT. Systematic trends across the night in human sleep cycles. Psychophysiology. 1979;16:283–91. doi: 10.1111/j.1469-8986.1979.tb02991.x. [DOI] [PubMed] [Google Scholar]
- 30.Milner CE, Fogel SM, Cote KA. Habitual napping moderates motor performance improvements following a short daytime nap. Biol Psychol. 2006;73:141–56. doi: 10.1016/j.biopsycho.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 31.Meier-Koll A, Bussmann B, Schmidt C, Neuschwander D. Walking through a maze alters the architecture of sleep. Percept Mot Skill. 1999;88:1141–59. doi: 10.2466/pms.1999.88.3c.1141. [DOI] [PubMed] [Google Scholar]
- 32.Eschenko O, Mölle M, Born J, Sara SJ. Elevated sleep spindles density after learning or after retrieval in rats. J Neurosci. 2006;26:12914–20. doi: 10.1523/JNEUROSCI.3175-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gross DW, Gotman J. Correlation of high-frequency oscillations with the sleep-wake cycle and cognitive activity in humans. Neuroscience. 1999;94:1005–8. doi: 10.1016/s0306-4522(99)00343-7. [DOI] [PubMed] [Google Scholar]
- 34.Pulvermuller F, Lutzenberger W, Preissl H, Birbamer N. Spectral responses in the gamma-band: physiological signs of higher cognitive processes? Neuroreport. 1995;6:2059–64. doi: 10.1097/00001756-199510010-00025. [DOI] [PubMed] [Google Scholar]
- 35.Perlis ML, Merica H, Smith MT, Giles DE. Beta EEG activity and insomnia. Sleep Med Rev. 2001;5:363–74. doi: 10.1053/smrv.2001.0151. [DOI] [PubMed] [Google Scholar]
- 36.Hummel F, Kirsammer R, Gerloff C. Ipsilateral cortical activation during finger sequences of increasing complexity: representation of movement difficulty. Clin Neurophysiol. 2003;114:605–13. doi: 10.1016/s1388-2457(02)00417-0. [DOI] [PubMed] [Google Scholar]
- 37.Marshall L, Helgadottir H, Mölle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;44:610–3. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 38.Bòdizs R, Kis T, Lázár AS, Havrán L, Rigo P, Clemens Z, Halász P. Prediction of general mental ability based on neural oscillation measures of sleep. J Sleep Res. 2005;14:285–92. doi: 10.1111/j.1365-2869.2005.00472.x. [DOI] [PubMed] [Google Scholar]
- 39.Walker MP, Stickgold R, Alsop D, Gaab N, Schlaug G. Sleep-dependent motor memory plasticity in the human brain. Neuroscience. 2005;133:911–7. doi: 10.1016/j.neuroscience.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 40.Fischer S, Nitschke M, Melchert UH, Erdmann C, Born J. Motor memory consolidation in sleep shapes more effective neuronal representations. J Neurosci. 2005;25:11248–55. doi: 10.1523/JNEUROSCI.1743-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]