Abstract
Study Objectives:
Previous studies have demonstrated that as little as 18 hours of sleep deprivation can cause deleterious effects on performance. It has also been suggested that sleep deprivation can cause a “tunnel-vision” effect, in which attention is restricted to the center of the visual field. The current study aimed to replicate these behavioral effects and to examine the electrophysiological underpinnings of these changes.
Design:
Repeated-measures experimental study.
Setting:
University laboratory.
Patients or Participants:
Nineteen professional drivers (1 woman; mean age = 45.3 ± 9.1 years).
Interventions:
Two experimental sessions were performed; one following 27 hours of sleep deprivation and the other following a normal night of sleep, with control for circadian effects.
Measurements & Results:
A tunnel-vision task (central versus peripheral visual discrimination) and a standard checkerboard-viewing task were performed while 32-channel EEG was recorded. For the tunnel-vision task, sleep deprivation resulted in an overall slowing of reaction times and increased errors of omission for both peripheral and foveal stimuli (P < 0.05). These changes were related to reduced P300 amplitude (indexing cognitive processing) but not measures of early visual processing. No evidence was found for an interaction effect between sleep deprivation and visual-field position, either in terms of behavior or electrophysiological responses. Slower processing of the sustained parvocellular visual pathway was demonstrated.
Conclusions:
These findings suggest that performance deficits on visual tasks during sleep deprivation are due to higher cognitive processes rather than early visual processing. Sleep deprivation may differentially impair processing of more-detailed visual information. Features of the study design (eg, visual angle, duration of sleep deprivation) may influence whether peripheral visual-field neglect occurs.
Citation:
Jackson ML; Croft RJ; Owens K; Pierce RJ; Kennedy GA; Crewther D; Howard ME. The effect of acute sleep deprivation on visual evoked potentials in professional drivers. SLEEP 2008;31(9):1261-1269.
Keywords: Sleep deprivation, event-related potentials, visual field, driving, circadian effects
SLEEP DEPRIVATION CONTRIBUTES TO BETWEEN 15% AND 20% OF ALL MOTOR VEHICLE CRASHES WORLDWIDE AND IS THE LARGEST IDENTIFIABLE CAUSE of transport crashes.1 Sleep deprivation results in increased sleep propensity and microsleeps, but this does not fully account for the performance decrements evident in sleep-deprived drivers.2–6 This suggests that other aspects of motor, perceptual, and/or cognitive processing, such as visual perception and attention, may be impaired in sleepy drivers, accounting for the increased sleep-related accident risk. The present study was designed to assess two aspects of visual processing that may be related to such impairment: the effects of sleep deprivation on differential processing of visual stimuli presented to the foveal versus peripheral visual field, and the differential processing of the sustained (parvocellular) versus transient (magnocellular) visual pathways.
A variety of neurocognitive deficits have been reported following sleep deprivation, which may contribute to increased crash risk in drivers.7,8 A specific aspect of visual attention that may relate to increased accident risk in sleep-deprived drivers is tunnel vision. This is a restriction in the functional field of view, usually caused by sympathetic arousal.10,11 Generally, research studies have defined the useful visual field as a visual angle of 20°, and it is measured as the differential visual detection of foveal versus peripheral stimuli.12 Sleep deprivation of between 18 and 64 hours has been shown to cause peripheral-field neglect in some laboratory studies.13,14 However, a more recent replication study failed to find this effect in subjects following 40 hours of wakefulness.15 Thus, it remains unclear whether peripheral vision is impaired by sleep deprivation. The current study will discuss tunnel vision in terms of this degree of visual angle used previously.
The behavioral response in these studies (increased reaction time or missed responses to peripheral visual signals) are the summation of a number of processes—from the initial encoding of information and motor execution of the response to the later cognitive processes, such as attention and memory. An alternative explanation for the discrepancy in the behavioral findings outlined in the previous paragraph may be that only some processes are affected by sleep deprivation, and behavioral studies may not be sensitive enough to detect abnormalities in the underlying cognitive processes. One way to examine these underlying neural processes is by using visual event-related potentials (ERPs), a scalp-derived measure of neural activity. Examination of the amplitude and latency of the responses may help to determine where in the sequence of information processing sleep deprivation is influencing the behavioral response. The typical early visual components consist of P100 (a positive component peaking around 100 milliseconds after the stimulus) and N100 (a negative component around 100 milliseconds) elicited over occipital sites (eg, see Figure 1).16 The P100 is believed to reflect early activation of the primary visual cortex and is related to spatial attentive processes.17 The N100 early sensory component follows the P100 and is believed to represent activation of the ventral stream structure (eg, the lateral occipital cortex16). The N100 attention component is elicited primarily over frontal sites.18 This attention component is believed to be a general characteristic of the spatial focusing of attention to stimuli and is enhanced to attended-location stimuli.18 The P300 (a positive component around 300 milliseconds) is elicited maximally over parietal regions, with its amplitude reflecting the allocation of attention resources and its peak latency reflecting the speed of processing.19 Early visual ERP components are influenced by changes in attention and arousal levels.20,21 This is mainly manifest as progressive reductions in the early visual amplitudes, as attention is directed to locations further from the focal point, reflecting the filtering of early sensory visual information.
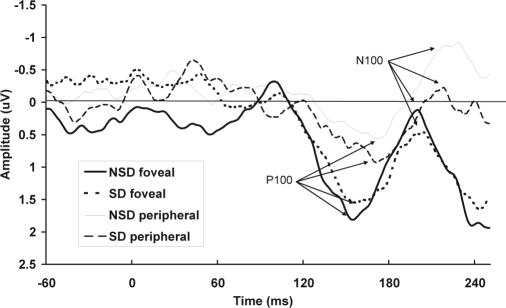
Figure 1.
Total mean event-related potentials for the early visual components in the tunnel-vision task are shown for nontarget stimuli at electrode Oz, for foveal and peripheral stimuli, and no sleep deprivation (NSD) and sleep deprivation (SD) sessions separately.
There have been mixed findings with regard to ERPs and sleep deprivation. Some studies have demonstrated effects on early (e.g., occipital N100 latency) but not late (e.g., P300) ERP components after 36 hours of sleep deprivation,22 and others have demonstrated effects on later ERP components after 40 hours of sleep deprivation, related to slower reaction times.23 The latter study employed a task that involved foveal presentations, whereas Krull et al22 utilized a task that covered the whole visual field. Hence, sleep deprivation may have different effects on foveal and peripheral processing. Given that these studies did not report the time of day of testing, circadian factors may have altered the evoked responses in addition to the effects of sleep deprivation.
Another method used to assess the integrity of the early visual system is to examine the magnocellular and the parvocellular visual pathways.24,25 The magnocellular system is responsible for the processing of transient visual stimuli,25,26 has high temporal resolution, and responds to achromatic patterns of low contrast and course detail.25 In contrast, the parvocellular pathway is a sustained system with slower processing speed and high spatial resolution and chromatic processing27 and is sensitive to pattern and fine-grained stimulus information.24 Although not an absolute measure, these pathways can be emphasized using different types of checkerboard patterns that evoke different visual ERPs.28 For example, luminance modulation biases the magnocellular pathway, whereas isoluminant chromatic modulation favors the parvocellular pathway.27 Only one study to date has examined the ERP response to magnocellular-weighted stimuli during acute sleep deprivation and found a significant reduction in the occipital N100 latency following 22 hours of sleep deprivation, with no change in amplitude.
Reductions to the P100 component during early visual processing, particularly within the magnocellular visual system, have previously been implicated in attention dysfunctions.25,29–31 However, a different effect of the magnocellular and parvocellular pathways was not examined in those studies and may provide further insight into the reported visual cognitive impairments associated with sleep deprivation.7,13,14
Thus, the aims of the study were (1) to replicate the behavioral effects of 27 hours of sleep deprivation on performance of a visual reaction-time task9; (2) to determine, using ERPs, where along the information processing chain these potential deficits are occurring; (3) to determine whether there is a tunnel-vision effect within a visual arc of 20° associated with sleep deprivation12; (4) to determine, using ERPs, where along the information processing chain these potential sleep-related tunnel-vision deficits occur; and (5) to evaluate whether sleep deprivation has a different effect on the visual system under conditions designed to emphasize the parvocellular and magnocellular pathways. To avoid potentially confounding circadian effects, time-of-day effects were controlled for to assess the independent impact of sleep deprivation alone on behavioral and visual ERPs.
METHODS
Participants
Twenty volunteer, professional truck drivers were recruited. A medical examination was carried out by a physician. Drivers who reported having medical contraindications to sleep deprivation (eg, psychiatric illness, epilepsy), having a sleep disorder, not being able to tolerate abstinence from cigarettes for up to 12 hours, or consuming 5 or more caffeinated beverages per day were excluded from the study. All participants reported having normal hearing and normal or corrected-to-normal vision and did not report having any significant daytime sleepiness according to the Epworth Sleepiness Scale scores (ESS).32 Informed consent was obtained from all participants. The study was approved by the Swinburne University and Austin Health Human Research Ethics Committees.
Procedure
Participants attended 2 sessions 1 week apart; a 27-hour sleep deprivation session, and a no sleep deprivation session (NSD). The order of sessions was counterbalanced across participants. For the sleep deprivation session, participants woke at 07:00 and attended the sleep laboratory at 22:00 following a normal 8-hour driving shift during the day. Participants stayed awake all that night until the following morning and were monitored by laboratory staff. During the night, participants watched videos, read, or played games and refrained from caffeine intake. The following morning at 07:00, participants provided a urine sample and completed a mood questionnaire. A light breakfast was provided. Participants had a manual perimetry test to assess any visual-field deficits before being fitted with the electroencephalogram (EEG) recording apparatus. No visual-field deficits were detected in any of the participants. EEG recording commenced at 10:00 after the subjects had been awake for 27 hours. Eye-movement calibration was performed prior to the 2 visual tasks.
The NSD session was identical to the sleep deprivation session, except that participants attended the laboratory at 08:00 the morning after a normal 8-hour driving shift during the day and a normal night of sleep at home. EEG commenced at the same time as in the sleep deprivation session; however, participants performed tasks in a rested state.
Stimuli
Tasks were performed in a sound-attenuated, electrically shielded, dimly lit room. Participants were seated 40 cm away from the computer screen that subtended a horizontal visual angle of 45°. During each session, participants completed 2 tasks. All stimuli and task sequences were generated with Neuroscan STIM software (Compumedics, Charlotte, NC).
Tunnel-Vision Task
This task was designed to examine visual-field processing at a 20° visual angle, as compared with a central signal.12 Participants were asked to fixate on a small cross in the center of the black screen. White squares (80%) and triangles (20%) were flashed up in 4 locations along a horizontal plane, including centrally (3.5° visual angle from fixation) and peripherally (20° visual angle from fixation) for the left and right hemifields. Stimuli were presented with equal probability at each position (5% at each position for triangles and 20% for squares). Stimuli were displayed for a duration of 100 milliseconds at random intervals between 820 milliseconds and 1180 milliseconds (mean = 1000 milliseconds). The size of the squares was 1.9 cm × 1.9 cm, with a visual angle of 2.71°. The size of the triangles was 2.1 cm × 2.1 cm, with a visual angle of 2.99°. During the task, participants were instructed to respond only to triangles. Additionally, the numbers 0 to 9 were randomly presented over the fixation cross, which was used for another version of the task that will not be described here. However, subjects were instructed to ignore the numbers and focus on the shapes. A 30-second practice trial was given prior to each test session, with a total task duration of 3.5 minutes. Two alternate forms were created and employed such that the participant did not perform the same version of the task twice. The order of administration of the forms was assigned randomly.
Pattern Reversal Task
Variants of the pattern-reversal task used previously27,33 were used to obtain measures of the parvocellular and magnocellular visual pathways.34 A black and white checkerboard pattern was used to emphasize the magnocellular pathway. There were 28 squares, with equal numbers of black and white, with a check size of 1 cm2 and a visual angle of 1.2° vertically and horizontally. The mean luminance and contrast levels of the black/white stimulation were 80 cd/m2 and 75% respectively. A red and green checkerboard was used to emphasize the parvocellular pathway. The check size was 4.5 cm × 5.5 cm, with a visual angle of 5.2° horizontally and 6.3° vertically. The mean luminance and contrast levels of the red/green stimulation were 21 cd/m2 and 38%, respectively. Each checkerboard reversed phase repeatedly at a rate of 2 Hz. Attention was directed toward the center of the screen with a blue fixation dot (8′ in diameter). Participants were instructed to ignore the flashing checkerboard background, focus on the dot, and respond quickly when it turned yellow at random intervals of between 1000 milliseconds and 4000 milliseconds (for 500 milliseconds). The total duration of the task was 4 minutes, and reaction times were recorded.
EEG Data Acquisition and ERP Indexes
EEG data were collected using tin electrodes located at 30 scalp sites plus 1 ocular site (below the left eye), referenced to the left mastoid, according to the International 10/10 System. All sites were used to ensure the validity of the artifact rejection-and-correction techniques employed on the data; however, only data from 3 sites with the strongest signal-to-noise ratios were used to assess ERP indexes (Oz for early sensory P100 and N100,18,22 Fz for the N100 attention,18 and Pz for the P30039). All data were continuously sampled at 500 Hz, with a 0.05-Hz to 100-Hz band pass. Impedances were below 10 kOhm at the start of each session.
Data were analyzed using Neuroscan Edit 4.2 software. All data were electrooculographically corrected,35 low-pass filtered at 30 Hz (zero-phase shift, 12 dB slope), and segmented (−200 milliseconds to +800 milliseconds after stimulus for the tunnel-vision task and −100 milliseconds to +460 milliseconds after stimulus for the pattern-reversal task). Segments were baseline corrected using the prestimulus interval. Nonocular artifacts were identified manually, and remaining segments were removed if the recorded signal at any EEG electrode exceeded ± 200 μV. Trials were averaged in the time domain separately for the parvocellular and magnocellular stimuli for each session in the pattern-reversal task, and correct trials were averaged separately for the target (triangle, n = 16) and nontarget (square, n = 64) stimuli for the tunnel-vision task.
Visual ERP indexes were analyzed for the tunnel-vision and pattern-reversal tasks. The early sensory P100 and N100 components were analyzed for both tasks. The attention N100 and later P300 components were assessed for only the tunnel-vision task. Amplitudes and latencies for the responses for both tasks were detected by proprietary software routines and based on previously employed latency windows.18,22,36,37
Data Analysis
Behavioral Responses
Reaction times to the first correct key press were calculated for the tunnel-vision task. Trials with reaction times shorter than 200 milliseconds or longer than the next stimulus onset (1000 milliseconds) were excluded from the reaction-time analyses. Errors of omission (number of missed responses) and errors of commission (percentage of responses outside target detection interval) were also calculated.
Event-Related Potentials
The EEG epochs of the target trials with omitted responses were not included in the ERPs. Peak-to-peak amplitudes were calculated for all analyses, as they are less susceptible to noise. The dependent measures were the N0/P100 at Oz (where N0 was the most negative peak preceding the P100) and P100/N100 sensory peak-to-peak amplitudes of nontarget responses at Oz, P100/N100 attention peak-to-peak amplitudes at Fz,18 and the N200/P300 peak-to-peak amplitudes at Pz.38 Overall, there were 60 trials for the N100 early sensory peaks, 64 trials for the P100 and N100 attention peaks, and 16 trials for the P300 peaks.
Total means were constructed across all subjects and sessions. To determine each individual magnocellular and parvocellular amplitude, the sleep deprivation and NSD sessions were averaged for each subject, and the most positive peak within the window defined by the total mean peak latency ± 30 milliseconds was selected. For each individual waveform for each subject, the P100 peak was classified as the individual total mean peak latency ± 15 milliseconds. This procedure was repeated for the N100 peaks. The dependent measures were the N0/P100 and P100/N100 peak-to-peak amplitude at Oz.22 Overall, there were 180 trials for the pattern-reversal ERPs.
Statistical Analysis
All ERP amplitude and latency data were transformed using the square root function to normalize the distributions, apart from P300 data, that was log transformed to reduce the more extreme positive skew.
Tunnel Vision Task
Two-way (Session: Sleep deprivation versus NSD; Field: Central versus Peripheral) repeated-measures analysis of variances were performed to determine the relationship between sleep deprivation and visual field and (1) behavioral responses (reaction time, errors of omission, and errors of commission); (2) early sensory processes (N0/P100 and P100/N100 visual peak-to-peak amplitudes at Oz); (3) later attention processes (P100/N100 peak-to-peak amplitude at Fz); and (4) later cognitive changes (N200/P300 peak-to-peak amplitude at Pz). Further, to determine whether there was any effect of sleep deprivation on the speed of processing of these early sensory and later cognitive processes, parallel analyses were performed using latency instead of amplitude as the dependent variable.
Pattern-Reversal Task
To determine whether there were differences in the magnocellular and parvocellular pathways following sleep deprivation, 2, 1-way (Session) repeated measures analyses of variation were performed, in which the dependent variables were (1) N0/P100 and (2) P100/N100 peak-to-peak amplitudes at Oz. To determine whether there was any effect of sleep deprivation on the speed of processing of the early sensory processes, parallel analyses were performed using latency instead of amplitude as the dependent variable.
RESULTS
Participants
One driver was found to have high levels of THC (delta-9-tetrahydrocannabinol) in his blood at the time of testing, so data from this subject were excluded from further analysis, leaving a final sample of 19 participants (1 woman), aged 23 to 62 years (mean age = 45.3 ± 9.1 years).
Tunnel-Vision Task
Behavioral Measures
Four participants were unable to correctly complete the task; therefore, these data were not included in the results of the statistical analyses for the behavioral data, leaving 15 participants overall. Mean RTs to targets and errors of omission and percentage error of commission are displayed in Table 1. Participants' responses were significantly slower to targets in the sleep-deprivation session, compared with the NSD session (F1,14 = 14.03, P < 0.01). No overall effect on RT was found for visual-field position (F1,14 = 0.03, P = 0.86), nor was there an interaction of visual-field position and sleep deprivation (F1,14 = 0.00, P = 0.97). Participants made significantly more errors of omission in the sleep deprivation session, compared with the NSD session (F1,14 = 5.84, P < 0.05); however, there was no effect of visual-field position (F1,14 = 1.46, P = 0.25) and no interaction of visual-field position and sleep deprivation on errors of omission (F1,14 = 0.35, P = 0.56). No overall effect of errors of commission was found for visual-field position (F1,14 = 1.71, P = 0.21) or sleep deprivation (F1,14 = 3.43, P = 0.09), nor was there an interaction of visual-field position and sleep deprivation (F1,14 = 0.22, P = 0.65).
Table 1.
Reaction Time to Targets and Errors of Omission and Commission in the Foveal and Peripheral Visual Field are Shown for the Sleep Deprivation and No Sleep Deprivation Sessions
| Condition and visual field | Reaction time, sec | Errors of omission, no. | Errors of commission, % |
|---|---|---|---|
| No sleep deprivation | |||
| Foveal | 455.19 ± 29.51 | 1.33 ± 1.88 | 9.07 ± 3.02 |
| Peripheral | 456.50 ± 47.17 | 2.00 ± 2.42 | 9.17 ± 2.95 |
| Sleep deprivation | |||
| Foveal | 490.91 ± 52.37 | 3.40 ± 2.7 | 7.27 ± 2.41 |
| Peripheral | 492.83 ± 48.58 | 3.67 ± 2.16 | 7.45 ± 2.83 |
Data are presented as mean ± SD.
Electrophysiological Measures
Means and standard deviations for the P100 and N100 sensory amplitudes, N100 attention amplitudes, and P300 amplitudes for foveal and peripheral evoked responses in each session are shown in Table 2. Total averages of the foveal and peripheral early visual ERPs in the sleep deprivation and NSD sessions are shown in Figure 1. Total averages of the foveal and peripheral attention components in the sleep deprivation and NSD sessions are shown in Figure 2. Total averages of the foveal and peripheral later cognitive processes, in the sleep deprivation and NSD sessions, are shown in Figure 3.
Table 2.
Early Sensory Peak-To-Peak Amplitudes and Latencies, for the Foveal and Peripheral Evoked Responses, for the Magnocellular and Parvocellular Pathways, for the Sleep Deprivation and No Sleep Deprivation Sessions Separately
| Amplitude |
Latency |
||||
|---|---|---|---|---|---|
| No. | No sleep deprivation | Sleep deprivation | No sleep deprivation | Sleep deprivation | |
| TUNNEL-VISION TASK | |||||
| P100 sensory, Oz | 19 | ||||
| Foveal | 2.48 ± 1.42 | 2.31 ± 1.64 | 117.63 ± 15.23 | 126.11 ± 21.12 | |
| Peripheral | 1.78 ± 0.94 | 1.84 ± 1.24 | 113.58 ± 21.85 | 122.74 ± 18.78 | |
| N100 sensory, Oz | 16 | ||||
| Foveal | 1.85 ± 1.32 | 1.97 ± 1.19 | 167.44 ± 11.08 | 167.00 ± 10.41 | |
| Peripheral | 1.99 ± 1.32 | 1.60 ± 1.13 | 170.44 ± 19.13 | 163.31 ± 20.41 | |
| N100 attention, Fz | 19 | ||||
| Foveal | 2.11 ± 1.81 | 2.97 ± 2.18 | 131.00 ± 17.69 | 139.53 ± 16.75 | |
| Peripheral | 4.25 ± 3.44 | 3.75 ± 2.76 | 139.58 ± 13.74 | 146.00 ± 16.62 | |
| P300, Pz | 18 | ||||
| Foveal | 5.48 ± 3.18 | 3.67 ± 3.13 | 470.94 ± 60.72 | 475.28 ± 57.96 | |
| Peripheral | 4.52 ± 3.22 | 4.07 ± 2.93 | 477.06 ± 65.99 | 480.39 ± 48.29 | |
| PR TASK | |||||
| P100, Oz | 16 | ||||
| Magnocellular | 3.43 ± 2.12 | 2.77 ± 1.58 | 101.94 ± 16.88 | 104.81 ± 15.82 | |
| Parvocellular | 5.83 ± 3.45 | 5.71 ± 2.52 | 108.06 ± 4.77 | 101.38 ± 5.43 | |
| N100, Oz | 16 | ||||
| Magnocellular | 2.35 ± 2.26 | 1.73 ± 1.55 | 148.69 ± 17.67 | 146.88 ± 20.54 | |
| Parvocellular | 3.66 ± 3.30 | 3.77 ± 2.69 | 136.69 ± 13.58 | 145.31 ± 21.59 | |
Data are presented as mean ± SD.
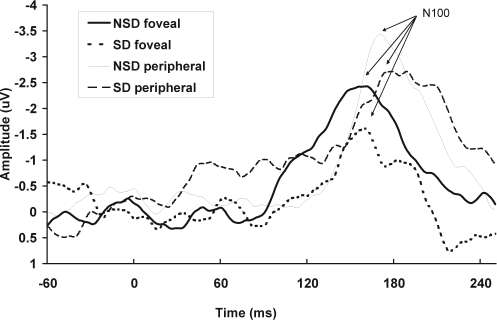
Figure 2.
Total mean event-related potentials to nontarget stimuli at Fz are shown for the tunnel-vision task, for the foveal and peripheral presentations, in the no sleep deprivation (NSD) and sleep deprivation (SD) sessions separately.
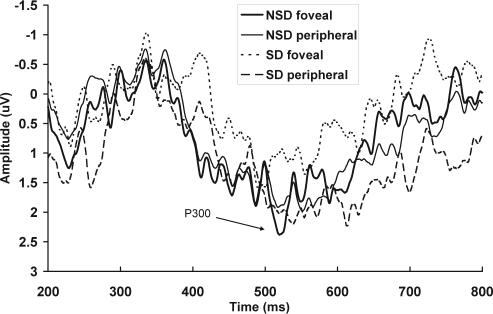
Figure 3.
Total mean event-related potentials at Pz are shown for the tunnel-vision task, for the foveal and peripheral presentations, in the no sleep deprivation (NSD) and sleep deprivation (SD) sessions separately.
Sensory Processing
Three participants' data for the N100 early sensory peaks were excluded because these peaks could not be identified in the NSD session. There was a significant visual-field effect for the amplitude of the P100 complex (F1,18 = 5.67, P < 0.05), with larger peaks at the fovea (Figure 1). No significant effect was found for sleep deprivation on the P100 complex (F1,18 = 0.33, P = 0.57), and this did not interact with visual field (F1,18 = 0.15, P = 0.70). The latency of the P100 was not significantly affected by visual-field position (F1,18 = 3.63, P = 0.07), sleep deprivation (F1,18 = 0.68, P = 0.42), or the interaction of sleep deprivation and visual field (F1,18 = 0.01, P = 0.92). There was no significant relationship between the amplitude of the N100 complex and visual-field position (F1,15 = 0.21, P = 0.65), sleep deprivation (F1,15 = 0.59, P = 0.45), or interaction between sleep deprivation and visual field (F1,15 = 1.17, P = 0.30). There was no significant effect of visual-field position (F1,15 = 0.94, P = 0.76), sleep deprivation (F1,15 = 2.08, P = 0.17), or the interaction of sleep deprivation and visual field (F1,15 = 2.54, P = 0.13) on the latency of the N100.
Attentional Processing
Larger amplitudes were evoked in response to peripheral, compared with foveal, stimuli for the attention N100 complex at Fz (F1,18 = 10.02, P < 0.01; Figure 2). No significant effect was found for sleep deprivation on the N100 amplitude (F1,18 = 0.40, P = 0.54), and this did not interact with visual-field position (F1,18 = 1.91, P = 0.18). There was a significant reduction in the N100 latency to peripheral, compared with foveal, evoked responses (F1,18 = 5.23, P < 0.05). There was no effect of sleep deprivation on the N100 latency (F1,18 = 2.83, P = 0.11), and this did not interact with visual-field position (F1,18 = 0.20, P = 0.66).
P300 Components
One participants' data for the P300 peaks were excluded because the data could not be identified for the NSD session. There was no significant effect of visual-field position on the P300 amplitude at Pz (F1,17 = 0.72, P = 0.79; Figure 3). The P300 amplitude was significantly reduced in the sleep deprivation session, compared with the NSD session (F1,17 = 5.07, P < 0.05); however, this did not interact with visual-field position (F1,17 = 2.98, P = 0.10). There was no significant effect of visual field on P300 latency (F1,17 = 0.22, P = 0.65), sleep deprivation (F1,17 = 0.20, P = 0.66), or interaction between sleep deprivation and visual-field position (F1,17 = 0.00, P = 0.98).
Pattern Reversal Task
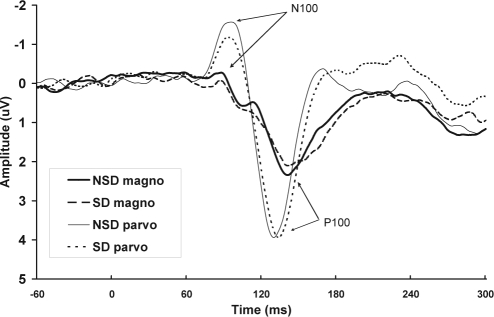
Two participants' data for both the early sensory peaks were excluded because the peaks could not be detected in the NSD session, and 1 participants' data were excluded because the peaks could not be detected in the sleep deprivation session. Means and standard deviations for the early sensory peak-to-peak amplitudes and latencies, for magnocellular and parvocellular evoked responses in each session, are shown in Table 2. Total averages of the magnocellular and parvocellular pathways, for the sleep deprivation and NSD sessions, are shown in Figure 4.
Figure 4.
Total mean event-related potentials are shown at Oz for the pattern reversal task, for pattern reversals designed to favor the magnocellular (magno) and parvocellular (parvo) pathways, for the no sleep deprivation (NSD) and sleep deprivation (SD) sessions separately.
There was no significant effect of sleep deprivation on the magnocellular N100 amplitude (F1,15 = 0.40, P = 0.54); however, there was a trend toward an effect of sleep deprivation on the P100 amplitude of the magnocellular pathway (F1,15 = 3.99, P = 0.06), with amplitude reduced with sleep deprivation. There was no significant main effect of sleep deprivation on the P100 amplitude (F1,15 = 0.03, P = 0.86), nor on the N100 amplitude (F1,15 = 0.11, P = 0.74), for the parvocellular pathway.
For the parvocellular pathway, there was a significant main effect of sleep deprivation on the P100 latency (F1,15 = 8.57, P < 0.05), with sleep deprivation prolonging latency. There was no significant main effect of sleep deprivation on the P100 latency of visual evoked responses of the magnocellular pathway (F1,15 = 0.57, P = 0.46) nor on the N100 latency (F1,15 = 0.26, P = 0.62). There was no significant effect of sleep deprivation on the N100 latency of the parvocellular pathway (F1,15 = 0.16, P = 0.69).
DISCUSSION
This study found that impaired behavioral performance following acute sleep deprivation was associated with electrophysiological evidence of altered higher cognitive processes (attention and visual information processing), whereas early processing of visual information did not appear to be affected. The reduction in P300 amplitude of the visual evoked responses suggested that the behavioral effects following sleep deprivation are related to changes to later cognitive rather than early sensory processing. Sleep deprivation was also related to slower processing of more-detailed visual information, requiring higher spatial resolution (longer P100 latency for the parvocellular pathway). In contrast with the tunnel vision hypothesis, the current study failed to find any specific central versus peripheral vision differences following 27 hours of sleep deprivation within the visual angle of 20°, either behaviorally or in terms of visual ERPs, although there was evidence of baseline differences in early sensory information processing to peripheral visual field stimuli (reduced P100 amplitude).
The present study found that sleep deprivation slows RTs9,39 and increases errors of omission,40,41 which is consistent with previous studies. These behavioral findings were not associated with changes in early visual processing ERP components elicited during the tunnel-vision task, nor with the attention components indexed by the N100. This is in contrast with the results of 1 other sleep deprivation study.22 The current study did not demonstrate any early sensory changes following 27 hours of sleep deprivation; however, it cannot be determined whether there are behavioral effects at longer periods of sleep deprivation. Significant reductions in later cognitive processing following 27 hours of sleep deprivation were found in the current study, indicating that this may be the time at which the behavioral deficits are initiated. This supports the results of previously conducted sleep deprivation studies that demonstrated a progressive and linear reduction in amplitude and lengthening of latency of later components with 40 hours of sleep deprivation, whereas the amplitude and latency of the early and middle components remained unchanged.23 This finding suggests that higher cognitive processes, such as motivation and deployment of attention resources, are affected by sleep deprivation because a reduction in P300 amplitude is believed to be associated with a decrease in attention.39 Furthermore, these deficits were also evident in the slowing of behavioral responses to stimuli in the sleep-deprived state. For instance, the reduction in P300 amplitude may reflect a reduction in vigilance, which, in turn, would prolong RTs. Similar to researchers in previous studies38–42 we did not observe any P300-latency changes with sleep deprivation. It is possible that a longer period of sleep deprivation would delay P300 latencies and that 1 night of experimental sleep loss was insufficient to elicit such changes.
The current study did not reveal any interaction between sleep deprivation and visual-field position using the behavioral data. This is in agreement with the study by Kendall et al15 in which no behavioral tunnel-vision effect was observed following 40 hours of wakefulness. However, it contrasts with recent behavioral evidence suggesting that sleep deprivation causes a tunnel vision-type effect, with deficits in responses to stimuli in the peripheral visual field.43 It is unclear whether there were problems with these previous studies. This contrary result is particularly important given that the data reported in the positive studies was derived from smaller samples (N < 10) and consisted of specific sample populations (air force pilots and younger drivers), which, together with the negative study, leaves the link between sleep deprivation and tunnel vision in some doubt. Alternatively, this tunnel-vision effect may have been too small to detect altered behavioral responses in our sample.
No significant effect of sleep deprivation on the sensory P100/N100 complex to foveal, compared with peripheral, stimuli was found. This is in contrast to the tunnel-vision hypothesis. This is also unlike the findings of neuroimaging studies that have reported decreased activation to peripheral targets when the central task had high cognitive load.44 This lack of effect in the present study does not appear to be due to a failure to achieve the experimental manipulation because a reduction in the early visual ERPs to peripheral, compared with central, stimuli was found, independent of sleep deprivation. This indicates that the position of the stimuli on the screen was appropriate to produce different sensory processing to central and peripheral stimuli. This response is expected according to the study's manipulation because neuronal firing rate is reduced in response to visual stimuli that fall outside the foveal region.
Similarly, for midlatency attention processes, sleep deprivation did not influence the visual-field position effect. The N100 attention amplitude, however, was larger for peripherally presented stimuli than for centrally presented stimuli, with a slower latency for peripheral, compared with central, stimuli, independent of sleep deprivation. Attending to stimuli in a particular location in the visual field typically elicits a larger ERP amplitude, compared with unattended locations. For example, Hoshiyama et al48 found an enhanced P100 response to foveal stimuli when attention was directed to the foveal region, and this response was suppressed to peripheral stimuli. Furthermore, Hillyard et al18 demonstrated a posterior N100 enhancement elicited by cueing to peripheral stimuli. They suggest that this enhancement may reflect orientation and engagement of attention to relevant stimuli locations. In the current study, subjects were asked to attend to stimuli presented both foveally and peripherally in a relatively demanding task. Thus, larger N100 attention amplitude to peripheral, compared with foveal, stimuli may reflect an increase in recruitment of attention resources in order to attend sufficiently to peripheral targets. Overall, this effect was not influenced by sleep deprivation, suggesting that preconscious attention is not affected by sleep deprivation or does not demonstrate differential visual field deficits induced by sleep deprivation.
Finally, although a reduced P300 amplitude was found following sleep deprivation, there was no visual-field effect, suggesting that there was not a tunnel-vision effect in terms of higher-order cognitive processes. Thus, sleep deprivation appears to be associated with a general decline in visual attention. An overall effect of visual-field position is apparent, with reduced early sensory processing and an increased attention requirement for peripheral stimuli; however, these tunnel-vision effects are not exacerbated by sleep deprivation. This suggests that sleep loss has a general effect on attention allocation to visual stimuli on a global level, with no specific effects on the location of the stimuli.
The processing speed of the parvocellular pathway was impaired by sleep deprivation in the current study, representing slow, sustained, high spatial processing of visual information. A trend level effect of sleep deprivation on the magnocellular pathway, which represents fast, transient, crude processing of visual information, was also shown. Both these findings suggest that sleep deprivation may affect visual processing at this level differently, which is potentially dangerous for driving.
The underlying mechanisms that produce these sleep deprivation-related behavioral deficits are not clear. One proposed mechanism is the “lapse” hypothesis, which suggests that short transient lapses in attention and performance occur during sleep deprivation.45 This hypothesis is supported by the increased number of errors of omission following sleep deprivation in the current study, suggesting that lapses in attention during the task lead to some stimuli being missed by participants, which is, for example, similar to failing to stop at a red light. However, this theory does not fully explain the increase in RT with sleep deprivation. In addition, the task duration was such that we expected that most participants would be able to maintain wakefulness throughout the test period. Therefore, we believe it is unlikely that the reduction in the P300 amplitude induced by sleep deprivation in the current study is due to microsleeps because, when there potentially confounding data were removed, a significant reduction was still demonstrated. Finally, it is often hard to distinguish if P300 changes are due to habituation, circadian factors, or sleep deprivation. For example, the P300 amplitude is greater in the morning, compared with the afternoon.46,47 All of the testing performed in the current study was at the same time of day, minimizing circadian influences.
There are a few possibilities as to why a peripheral visual-field effect was not found following sleep deprivation in the current study. The visual angle used for the peripheral stimulus in the current paradigm was 20° from fixation, as reported by Roge et al12; however, a wider visual angle may still induce an effect on behavioral and electrophysiological responses. It may be argued that subjects in the current study did not fixate in the center of the screen throughout the whole task, thereby reducing the foveal ERP response. However, this appears to be an unlikely explanation because ERP responses were larger to centrally presented stimuli than to those presented to the periphery. It is possible that a paradigm of 27 hours of sleep deprivation may have been insufficient to produce peripheral visual changes; however, the current authors wished to utilize a paradigm that reflected common work conditions for transport workers. Periods of sleep deprivation longer than 27 hours are not commonly experienced by professional drivers in real-life situations. The study power may have also been weakened by few number of subjects (particularly after exclusions). It is also possible that the number of trials used in the current study (ie, 64 epochs for the N100) were insufficient to produce a prominent N100 peak and that ERP waves potentially have poor reliability. Although this number of trials is not uncommon in ERP research, these factors may have limited the reliability of the findings. Despite this, the current study did demonstrate behavioral and ERP changes with 27 hours of sleep deprivation, but this was not strong enough to show peripheral visual susceptibility. Therefore, it is possible that this tunnel-vision effect might be demonstrated with longer periods of extended wakefulness or if testing were performed during the circadian nadir period. Additionally, the length of the tunnel-vision task may have been too short to elicit such changes because our participants may have been able to maintain their performance at a relatively high level for that short period when sleep deprived. Performance deficits on RT tasks have been reported with task lengths of 10 minutes.9 However, it would be expected that the underlying neural mechanisms would still be apparent in the ERP data, despite the ability of participants to compensate for their sleepiness, which was not demonstrated in the current study.
It is difficult to generalize the findings of the current study to more complex tasks such as driving. We assessed a simple RT task, and these findings cannot be extrapolated across all the other neurocognitive components of the complex integrated task of driving a motor vehicle. These findings do, however, allow insight into the specific effects of sleep deprivation on both the visual behavioral responses, and smaller electrophysiological brain changes associated with a visual task, which is an important aspect of driving. This research has implications for understanding the possible mechanisms that may be related to countermeasures, such as the ingestion of caffeine or stimulant drugs (eg, amphetamine or modafinil) or napping, and their effectiveness in reversing the changes that occur with sleep deprivation. It would be beneficial for future studies to examine the effects of sleep deprivation on a wider field of view, perhaps using a longer, more-complex task, and following a longer period of sleep loss.
In conclusion, this study found that 27 hours of sleep deprivation caused a decline in performance on a visual RT task, in terms of both slowing RTs and increased errors of omission. This sleep deprivation-related behavioral change was not explained by alterations in early visual processing but appears to be related to effects on later, higher-order, cognitive processing. These effects were found to be specific to sleep deprivation rather than due to circadian influences. Sleep deprivation was also related to slower processing of the parvocellular visual pathway, which involved more-sustained processing of detailed visual information. In addition, we failed to find a sleep deprivation tunnel-vision effect within the visual range (20°) employed in this study, either in the behavioral or ERP results. This suggests that sleep deprivation of 27 hours affects attention across the entire visual field assessed in this study, rather than specifically causing peripheral deficits.
ACKNOWLEDGMENTS
This study was performed at the Brain Sciences Institute, Swinburne University of Technology, Australia
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. Pierce has participated in research supported by Compumedics and ResMed and has consulted for Compumedics. Dr. Howard has had free use of equipment provided by Optalert for investigative studies. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Akerstedt T. Consensus statement: fatigue and accidents in transport operations. J Sleep Res. 2000;9:395. doi: 10.1046/j.1365-2869.2000.00228.x. [DOI] [PubMed] [Google Scholar]
- 2.Welsh A, Thomas M, Thorne D. Effects of 64 hours of sleep deprivation on accidents and sleep events during a driving simulator test. Sleep. 1998;21:S234. [Google Scholar]
- 3.Russo M, Thorne D, Thomas M, et al. Sleep deprivation-induced Balient's syndrome (Peripheral Visual Field Neglect): A hypothesis for explaining driving simulator accidents in awake but sleepy drivers. Sleep. 1999;22:S327. [Google Scholar]
- 4.Thorne D, Thomas M, Sing H. Accident rate, attention and performance in a driving simulator during 64 hours of progressive sleep deprivation. Sleep Res. 1997;26:S634. [Google Scholar]
- 5.Thorne D, Thomas M, Russo M, et al. Performance on a driving simulator divided attention task during one week of restricted nightly sleep. Sleep. 1999;22:S301. [Google Scholar]
- 6.Akerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 7.Rogers NL, Dorrian J, Dinges DF. Sleep, waking and neurobehavioural performance. Front Biosci. 2003;8:S1056–67. doi: 10.2741/1174. [DOI] [PubMed] [Google Scholar]
- 8.Koslowsky M, Babkoff H. Meta-analysis of the relationship between total sleep deprivation and performance. Chronobiol Int. 1992;9:132–136. doi: 10.3109/07420529209064524. [DOI] [PubMed] [Google Scholar]
- 9.Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20:267–267. [PubMed] [Google Scholar]
- 10.Mackworth NH. Visual noise causes tunnel vision. Psychonom Sci. 1965;3:67–68. [Google Scholar]
- 11.Easterbrook JA. The effect of emotion on cue utilisation and the organisation of behaviour. Psychol Rev. 1959;66:183–201. doi: 10.1037/h0047707. [DOI] [PubMed] [Google Scholar]
- 12.Roge J, Pebayle T, Kiehn L, Muzet A. Effect of sleep deprivation and driving duration on the useful visual field in younger and older subjects during simulator driving. Vision Res. 2003;43:1465–72. doi: 10.1016/s0042-6989(03)00143-3. [DOI] [PubMed] [Google Scholar]
- 13.Mills KC, Spruill SE, Kanne RW, Parkman KM, Zhang Y. The influence of stimulants, sedatives, and fatigue on tunnel vision: Risk factors for driving and piloting. Hum Factors. 2001;43:310–27. doi: 10.1518/001872001775900878. [DOI] [PubMed] [Google Scholar]
- 14.Russo M, Escolas S, Thomas M, et al. Visual neglect in sleep deprived air force pilots in a simulated 12-hour flight. Sleep. 2002;25:A88. [Google Scholar]
- 15.Kendall AP, Kautz MA, Russo M, Killgore WDS. Effects of sleep deprivation on lateral visual field. Int J Neurosci. 2006;116:1125–38. doi: 10.1080/00207450500513922. [DOI] [PubMed] [Google Scholar]
- 16.Schechter I, Butler PD, Zemon VM, et al. Impairments in generation of early-stage transient visual evoked potentials to magno- and parvocellular-selective stimuli in schizophrenia. Clin Neurophysiol. 2005;116:2204–15. doi: 10.1016/j.clinph.2005.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mangun GR, Hillyard SA. Modulations of sensory-evoked brain potentials provide evidence for changes in perceptual processing during visual-spatial priming. J Exper Psychol Hum Percept Perform. 1991;17:1057–74. doi: 10.1037//0096-1523.17.4.1057. [DOI] [PubMed] [Google Scholar]
- 18.Hillyard SA, Anllo-Vento L. Event-related brain potentials in the study of visual selective attention. Proc Natl Acad Sci. 1998;95:781–7. doi: 10.1073/pnas.95.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donchin E, Coles MGH. Is the P300 component a manifestation of context-updating? Behav Brain Sci. 1988;11:357–74. [Google Scholar]
- 20.Mangun GR, Hillyard SA. Spatial gradients of visual attention: behavioral and electrophyisological evidence. Electroencephalogr Clin Neurophysiol. 1988;70:417–28. doi: 10.1016/0013-4694(88)90019-3. [DOI] [PubMed] [Google Scholar]
- 21.Morris AM, So Y, Lee KA, Lash AA, Becker CE. The P300 event-related potential. The effects of sleep deprivation. J Occup Med. 1992;34:1143–52. [PubMed] [Google Scholar]
- 22.Krull KR, Smith LT, Sinha R, Parsons OA. Simple reaction time event-related potentials: effects of alcohol and sleep deprivation. Alcohol Clin Exp Res. 1993;17:771–7. doi: 10.1111/j.1530-0277.1993.tb00839.x. [DOI] [PubMed] [Google Scholar]
- 23.Corsi-Cabrera M, Arce C, Del Rio-Portilla IY, Perez-Garci E, Guevara MA. Amplitude reduction in visual event-related potentials as a function of sleep deprivation. Sleep. 1999;22:181–9. [PubMed] [Google Scholar]
- 24.Farrag AF, Khedr EM, Abel-Naser W. Impaired parvocellular pathway in dyslexic children. Eur J Neurol. 2002;9:359–63. doi: 10.1046/j.1468-1331.2002.00410.x. [DOI] [PubMed] [Google Scholar]
- 25.Schechter I, Butler PD, Zemon VM, et al. Impairments in generation of early-stage transient visual evoked potentials to magno- and parvocellular-selective stimuli in schizophrenia. Clin Neurophysiol. 2005;116:2204–15. doi: 10.1016/j.clinph.2005.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samar VJ, Parasnis I, Berent GP. Deaf poor readers' pattern reversal visual evoked potentials suggest magnocellular system deficits: implications for diagnostic neuroimaging of dyslexia in deaf individuals. Brain Language. 2002;80:21–44. doi: 10.1006/brln.2001.2498. [DOI] [PubMed] [Google Scholar]
- 27.Alexander K, Rajagopalan AS, Seiple W, Zemon V, Fishman G. Contrast response properties of magnocellular and parvocellular pathways in retinitis pigmentosa assessed by the visual evoked potential. Invest Opthamol Visual Sci. 2005;46:2967–73. doi: 10.1167/iovs.05-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mezer E, Bahir Y, Leibu R, Perlman I. Effect of defocusing and of distracted attention upon recordings of the visual evoked potential. Documenta Ophthalmol. 2004;109:229–38. doi: 10.1007/s10633-004-8055-5. [DOI] [PubMed] [Google Scholar]
- 29.Doniger GM, Foxe JJ, Murray MM, H B. A., Javitt DC. Impaired visual object recognition and dorsal/ventral stream interaction in schizophrenia. Arch Gen Psychiatry. 2002;59:1011–20. doi: 10.1001/archpsyc.59.11.1011. [DOI] [PubMed] [Google Scholar]
- 30.Keri S, Kelemen O, Benedek G, Janka Z. Vernier threshold in patients with schizophrenia and in their unaffected siblings. Neuropsychology. 2004;18:537–42. doi: 10.1037/0894-4105.18.3.537. [DOI] [PubMed] [Google Scholar]
- 31.Kim D, Wylie G, Pasternak R, Butler PD, Javitt DC. Magnocellular contributions to impaired motion processing in schizophrenia. Schizophrenia Res. 2006;82:1–8. doi: 10.1016/j.schres.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea. The Epworth Sleepiness Scale. Chest. 1993;103:30–6. doi: 10.1378/chest.103.1.30. [DOI] [PubMed] [Google Scholar]
- 33.Butler PD, Schechter I, Zemon V, et al. Dysfunction of early-stage visual processing in schizophrenia. Am J Psychiatry. 2001;158:1126–33. doi: 10.1176/appi.ajp.158.7.1126. [DOI] [PubMed] [Google Scholar]
- 34.Valberg A, Rudvin I. Possible contributions of magnocellular- and parvocellular-pathway cells to transient VEPs. Visual Neurosci. 1997;14:1–11. doi: 10.1017/s0952523800008701. [DOI] [PubMed] [Google Scholar]
- 35.Croft R, Barry R. EOG correction of blinks with saccade coefficients: a test and revision of the aligned-artefact average solution. Clin Neurophysiol. 2000;111:444–51. doi: 10.1016/s1388-2457(99)00296-5. [DOI] [PubMed] [Google Scholar]
- 36.Han S, Lui W, Yund EW, Woods DL. Interactions between spatial attention and global/local feature selection: an ERP study. NeuroReport. 2000;11:2753–8. doi: 10.1097/00001756-200008210-00029. [DOI] [PubMed] [Google Scholar]
- 37.Polich J, Bondurant T. P300 sequence effects, probability, and interstimulus interval. Physiol Behav. 1997;61:843–9. doi: 10.1016/s0031-9384(96)00564-1. [DOI] [PubMed] [Google Scholar]
- 38.Tsai L, Young H, Hsieh S, Lee C. Impairment of error monitoring following sleep deprivation. Sleep. 2005;28:707–13. doi: 10.1093/sleep/28.6.707. [DOI] [PubMed] [Google Scholar]
- 39.Lisper HO, Kjellberg A. Effects of 24-hour sleep deprivation on rate of decrement in a 10-minute auditory reaction time task. J Experiment Psychol. 1972;96:287–90. doi: 10.1037/h0033615. [DOI] [PubMed] [Google Scholar]
- 40.Doran SM, Van Dongen HP, Dinges DF. Sustained attention performance during sleep deprivation: evidence of state instability. Arch Ital Biol. 2001;139:253–67. [PubMed] [Google Scholar]
- 41.May J, Kline P. Measuring the effects upon cognitive abilities of sleep loss during continuous operations. Br J Psychol. 1987;78:443–55. doi: 10.1111/j.2044-8295.1987.tb02261.x. [DOI] [PubMed] [Google Scholar]
- 42.Kingshott R N, Cosway RJ, Deary IJ, Douglas NJ. The effect of sleep fragmentation on cognitive processing using computerized topographic brain mapping. J Sleep Res. 2000;9:353–7. doi: 10.1046/j.1365-2869.2000.00223.x. [DOI] [PubMed] [Google Scholar]
- 43.Russo M, Thomas M, Thorne D, et al. Oculomotor impairment during chronic partial sleep deprivation. Clin Neurophysiol. 2003;114:723–36. doi: 10.1016/s1388-2457(03)00008-7. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz S, Veilleumier P, Hutton C, Maravita A, Dolan RJ, Driver J. Attentional load and sensory competition in human vision: Modulation of fMRI responses by load fixation during task-irrelevant stimulation in the peripheral visual field. Cerebral Cortex. 2005;15:770–86. doi: 10.1093/cercor/bhh178. [DOI] [PubMed] [Google Scholar]
- 45.Broadbent DE. Noise, paced performance, and vigilance tasks. Br J Psychol. 1953;44:295–303. doi: 10.1111/j.2044-8295.1953.tb01210.x. [DOI] [PubMed] [Google Scholar]
- 46.Wesensten NJ, Belenky G, Kautz MA, Thorne DR, Reichardt RM, Balkin TJ. Maintaining alertness and performance during sleep deprivation: modafinil versus caffeine. Psychopharmacology. 2002;159:238–47. doi: 10.1007/s002130100916. [DOI] [PubMed] [Google Scholar]
- 47.Geisler MW, Polich J. P300 and time of day: circadian rhythms, food intake, and body temperature. Biol Psychol. 1990;31:117–36. doi: 10.1016/0301-0511(90)90012-l. [DOI] [PubMed] [Google Scholar]
- 48.Hoshiyama M, Kakigi R. Effects of attention on pattern-reversal visual evoked potentials: foveal field stimulation versus peripheral field stimulation. Brain Topography. 2001;13:293–8. doi: 10.1023/a:1011132830123. [DOI] [PubMed] [Google Scholar]