Abstract
Study Objectives:
Although subjective complaints about daytime cognitive functioning are an essential symptom of chronic insomnia, abnormalities in functional brain activation have not previously been investigated. This study was designed to investigate functional brain activation differences as a possible result of chronic insomnia, and the reversibility of these differences after nonmedicated sleep therapy.
Design:
Insomniacs and carefully matched controls underwent functional magnetic resonance imaging (fMRI) scanning during the performance of a category and a letter fluency task. Insomniacs were randomly assigned to either a 6-week period of nonpharmacological sleep therapy or a wait list period, after which fMRI scanning was repeated using parallel tasks. Task-related brain activation and number of generated words were considered as outcome measures.
Setting:
The outpatient sleep clinic of the VU University Medical Center, Department of Clinical Neurophysiology; fMRI was performed at the Department of Radiology.
Participants:
Twenty-one patients suffering from chronic insomnia and 12 matched controls.
Interventions:
Nonpharmacological sleep therapy for 6 weeks, consisting of cognitive behavioral therapy, body temperature and bright light interventions, sleep hygiene, and physical activity counseling.
Measurement and Results:
Compared to controls, insomnia patients showed hypoactivation of the medial and inferior prefrontal cortical areas (Brodmann Area 9, 44–45), which recovered after sleep therapy but not after a wait list period.
Conclusions:
Insomnia interferes in a reversible fashion with activation of the prefrontal cortical system during daytime task performance.
Citation:
Altena E; Van Der Werf YD; Sanz-Arigita EJ; Voorn TA; Rombouts SARB; Kuijer JPA; Van Someren EJW. Prefrontal hypoactivation and recovery in insomnia. SLEEP 2008;31(9):1271-1276.
Keywords: Insomnia, brain imaging, sleep therapy
EXPERIMENTAL ACUTE SLEEP DEPRIVATION INDUCES COGNITIVE DEFICITS, PARTICULARLY IN TASKS THAT RELY ON THE INTEGRITY OF THE PREFRONTAL CORTICAL system.1 An example of such a prefrontally dependent task is the verbal fluency task.2,3 Performance on this task is known to be affected by experimental sleep deprivation.4 A naturalistic form of chronically disturbed sleep is primary insomnia, occurring in a large proportion of the population and increasingly so with aging.5 Insomnia can be treated with non-pharmacological therapy, which has proved its long-term effectiveness on sleep measures and daytime functioning in numerous previous studies.6,7 Sleep restriction and multifaceted cognitive-behavior therapy have been reported to be particularly effective for treating insomnia in the elderly.8
Cognitive dysfunction in insomnia has been suggested, but few behavioral studies found abnormal performance in this condition, and no patterns of consistent results have emerged across studies.9 A possible reason for this inconsistency is that two opposing factors could result in masking of performance differences between insomniacs and well-sleeping participants: the high level of perfectionism and arousal that characterizes insomnia may mask performance decreases due to poor sleep.10,11 As a result, the net performance could become indistinguishable from that of well-sleeping controls. Cognitive neuroimaging studies could instead be used to elucidate alterations in cognitive processes that might not readily emerge in behavioral performance measures. These have, up till now, not been reported in insomnia.
We therefore undertook a functional imaging study to test the hypothesis that chronic insomnia would interfere with prefrontal activation during verbal fluency, and to investigate whether this possible interference would be reversible upon successful sleep therapy.
METHODS AND MATERIALS
All procedures complied with the declaration of Helsinki and medical ethical approval was obtained from the medical ethical committee of the VU University medical center. Informed consent was obtained from all participants.
Participants
The data for 21 chronic insomnia patients, off medication for at least two months, and 12 healthy, age, sex, and education matched controls are reported (see Table 1 for details). Of the 25 insomnia patients and 14 healthy controls initially included, scans of 3 patients could not be analyzed due to technical failure. Moreover, one patient and one control found their native non-Dutch language to interfere with word generation in Dutch and one control participant proved to have a right-lateralized language dominance and was therefore excluded from analysis.
Table 1.
Age, Sex, Cognitive Status and Intellectual Functioning Scores for Both Groups of Participants
| control mean ± sd | insomnia mean ± sd | P | |
|---|---|---|---|
| N | 12 | 21 | |
| M/F | 3/9 | 4/17 | |
| age | 60 ± 8.2 | 61 ± 6.2 | 0.82 |
| MMSE | 29 ± 1.2 | 29 ± 1.3 | 0.75 |
| DART | 105 ± 10.3 | 111 ± 10.3 | 0.12 |
| GIT | 120 ± 15.5 | 124 ± 12.1 | 0.42 |
N = number of participants; M/F = male/female; MMSE = Mini Mental State Examination, with scores of 26-30 indicating absence of dementia.15 Scores on the Dutch Adult Reading Test (DART)16–18 and the Groninger Intelligence Test (GIT)19 are expressed in an IQ-score and are indicative of general intellectual functioning. The rightmost column shows P-values according to unpaired t-tests.
Insomnia patients were randomly assigned to a sleep therapy group, receiving 6 weeks of sleep therapy between 2 measurement sessions, and a wait list group. One patient participating in the baseline session did not participate in the session after sleep therapy due to personal circumstances. Consequently, the time-by-treatment group analyses were restricted to 10 participants in the sleep therapy group and 10 participants in the wait list group. For ethical reasons, we offered sleep therapy to the wait list group after completion of the experiment.
Chronic insomnia, lasting for at least 2.5 years, was diagnosed according to established qualitative and quantitative criteria.12–14 Sleep-related exclusion criteria were sleep apnea syndrome and severe restless legs or periodic leg movement syndrome (measured on intake polysomnography [PSG]). A clinical interview by a specialized neurologist and a standardized screening were administered to determine other factors for exclusion such as a somatic disorder that might affect sleep and use of sedative medication. Matching of insomniacs and controls was furthermore evaluated using (1) the Mini Mental State Examination (MMSE)15 to screen for dementia-related cognitive impairments; (2) the Dutch Adult Reading Test (DART)16–18 and the (3) Groninger Intelligentie Test (GIT)19 to match groups for levels of intellectual functioning (see table 1); (4) the geriatric depression scale (GDS)20 to determine the presence and severity of depressive symptoms; (5) the symptoms check list for psychological problems and psychopathology (SCL-90)21 to determine the presence and severity of psychological problems and (6) the Short Form-36 health survey (SF-36)22 to determine quality of life (see Table 2). No differences between insomniacs and controls were present on the MMSE, DART, GIT or GDS (all P > 0.12). Insomniacs also did not differ on any but the sleep related subscales of the SCL-90 (t-tests: subscale Sleep P = 0.000, all other subscales P > 0.08) and the SF-36 (sleep-related subscale Vitality P = 0.03; all other subscales P > 0.17).
Table 2.
Groups Only Differ on Sleep Related (Sub)Scales of Sleep and Health Questionnaires
| control mean ± sd | insomnia mean ± sd | P | |
|---|---|---|---|
| PSQI | 4.2 ± 3.0 | 12.1 ± 3.1 | 0.00 |
| SDQ | |||
| insomnia | 1.8 ± 0.5 | 3.3 ± 0.3 | 0.00 |
| PLMS/RL | 1.5 ± 0.5 | 1.2 ± 0.3 | 0.13 |
| EDS | 1.6 ± 0.5 | 1.8 ± 0.7 | 0.39 |
| narcolepsy | 1.3 ± 0.3 | 1.1 ± 0.2 | 0.09 |
| apnea | 1.8 ± 0.6 | 1.6 ± 0.5 | 0.43 |
| psychiatry | 1.7 ± 0.4 | 2.0 ± 0.5 | 0.12 |
| SCL-90 | |||
| anx | 12.3 ± 2.5 | 12.9 ± 3.4 | 0.54 |
| ago | 7.3 ± 0.9 | 7.9 ± 1.6 | 0.14 |
| dep | 20.3 ± 5.4 | 22.0 ± 6.5 | 0.43 |
| som | 15.5 ± 2.3 | 17.6 ± 4.3 | 0.08 |
| inad | 13.5 ± 3.3 | 14.6 ± 3.8 | 0.40 |
| sen | 23.3 ± 7.8 | 25.3 ± 7.3 | 0.49 |
| hos | 6.8 ± 0.9 | 7.5 ± 2.6 | 0.34 |
| sle | 4.0 ± 1.3 | 10.2 ± 2.5 | 0.00 |
| oth | 10.5 ± 1.5 | 11.1 ± 2.5 | 0.40 |
| total | 113.4 ± 19.5 | 129.0 ± 25.4 | 0.06 |
| SF-36 | |||
| PF | 92.1 ± 8.6 | 90.6 ± 10.1 | 0.66 |
| RP | 95.0 ± 15.8 | 86.1 ± 27.4 | 0.29 |
| BP | 83.0 ± 13.6 | 74.8 ± 16.3 | 0.17 |
| GH | 71.1 ± 25.0 | 76.9 ± 11.5 | 0.46 |
| VI | 76.4 ± 12.1 | 64.4 ± 15.9 | 0.03 |
| SF | 90.9 ± 13.8 | 82.6 ± 18.3 | 0.18 |
| RE | 90.0 ± 22.5 | 88.9 ± 22.9 | 0.90 |
| MH | 80.7 ± 10.2 | 79.9 ± 13.6 | 0.85 |
| GDS | 5.2 ± 6.0 | 5.0 ± 4.2 | 0.93 |
Insomnia patients (n=21) compared to control patients (n=12). Insomnia was diagnosed on the basis of the Pittsburgh Sleep Questionnaire index (PSQI)23 and the Sleep Diagnosis Questionnaire (SDQ),24,25 which include subscales for rates of insomnia, (periodic) leg movements (PLMS/RL), excessive daytime sleepiness (EDS), narcolepsy, apnea and psychiatric symptoms (psychiatry) (see text for details on scores). Subscale and total scores of the Symptoms Check List (SCL-90),21 measuring psychiatric symptoms such as anxiety (anx), agoraphobia (ago), depression (dep), somatic complaints (som), feelings of inadequacy (inad), sensitivity to critics (sen), hostility (hos), sleeping problems (sle) and other (oth). For the Short Form-36 health survey (SF-36,)22 measuring quality of life, subscores for physical functioning (PF), how physical functioning limits social interaction (RP), bodily pain (BP), general health (GH), vitality (VI), social functioning (SF), how emotional functioning limits social interaction (RE) and general mental health (MH) are included. The Geriatric Depression Scale (GDS)20 shows depression scores for both groups. The rightmost column shows P-values according to unpaired t-tests; significant differences are indicated in bold.
Functional imaging
Imaging was performed with a 1.5T MRI scanner (Magnetom Sonata, Siemens, Erlangen, Germany) using a standard circularly polarized head coil with foam padding to restrict head motion. A high-resolution T1-weighted magnetization prepared rapid acquisition (MPRAGE; TR = 27 ms; TE = 3.97 ms; flip angle = 8°; 160 coronal slices, 1 × 1 × 1.5 mm voxels) MRI was collected for anatomic co-registration with the fMRI data set. For the functional scans, the T2*-weighted echo planar images (EPI) sequence acquisition parameters were TR = 3.5 sec, TE = 60 msec, flip angle = 90°, scanning 35 slices with 3-mm isotropic voxels and an interslice gap of 20%. The functional data were convolved with a double-gamma hemodynamic response function, after motion correction and preprocessing using FSL software (FEAT analysis, FSL 3.3, FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl). Activation patterns during letter and category fluency were contrasted separately against a baseline task of counting backwards (for a detailed description of the task, see below). Results were transformed to standard stereotactic space using a linear warping procedure in a 2-step fashion: EPI scans were registered to the high-resolution T1 scan and subsequently to the MNI template (MNI-152 average brain). A higher-level analysis was then performed, using a mixed-effects model. Main effects of task in both the patient and control groups were considered significant at z > 2.3, cluster-corrected. The analysis of the group-effect (insomnia, n = 21 vs. controls, n = 12) was restricted to the areas significantly activated by each task in the main effects across all patients and control subjects and results were considered significant at z > 3.1, uncorrected. The treatment effect (pre versus post for the therapy and waitlist groups) was evaluated within the region that showed reduced activation in the patient group in the group-effect analysis, using an uncorrected threshold of z > 2.3, in order to maximize sensitivity to small changes within the affected areas.
Task and Procedure
The letter and category fluency tasks were both administered within the same blocked design. Three letter fluency blocks and 3 category fluency blocks, of 30 sec each, alternated with 7 blocks of a baseline task (counting backwards) of 15 sec each. During the fluency blocks, participants were asked to press a button for every covertly generated word either belonging to the category or starting with the letter shown on the screen. Participants were informed about the task instructions before going into the scanner and examples of each of both tasks not used in the administered tasks, were given. No names (e.g., cities, persons) and no consecutive words starting with the same preposition (e.g., “conflict,” “contact”) were allowed as responses. During the baseline task, participants pressed a button for every number counted backwards. Performance was measured as the total number of button presses.
Parallel versions were designed for both the letter and category fluency tasks. One version contained the letters K, O, and M with the categories “animals,” “tools,” and “professions”; the other D, A, and T with “clothes,” “vegetables/fruit,” and “herbs.” The order of administration of the parallel versions was counterbalanced across both testing sessions. All measurements were performed within a fixed time window between 17:00 and 20:30.
Sleep Therapy
Therapy consisted of 6 weeks of intensive nonpharmacological sleep therapy combining sleep restriction, multifaceted cognitive-behavior therapy,6 morning and late afternoon bright light exposure, and body temperature manipulations. After the intake interview, a “treatment contract” was established. Weekly follow-ups by telephone were administered to confirm or adjust the treatment on the basis of patients' reports.
Sleep hygiene advice and cognitive behavioral therapy were given according to established procedures.8,26 For the sleep restriction component, participants were initially allowed to be in bed for no more than the total sleep time at intake PSG, with a minimum of 6 h. A time frame was set in agreement with the participant to approach his or her preferred sleep period closely (e.g., from 23:30 to 05:30). As soon as the sleep efficiency within this limited time window reached ≥ 90% (according to the participant's subjective experience), the allowed time in bed was increased by half an hour; this procedure was repeated if 90% was reached again.
For the bright light component, a light box (Zeus-Max, Outside In, UK; or dedicated comparable fixture from Philips Lighting, The Netherlands) was suspended from the ceiling above the dining table at a 45° angle, resulting in light intensity >10 kLux measured at eye level in gaze direction. This approach allowed participants to engage in normal activities, such as having breakfast or dinner or reading a newspaper. In agreement with the participant, 2 fixed half-hour time windows were chosen: the first as soon as possible after getting up in the morning and the second around evening dinner time.
Body temperature manipulations were applied by asking the participant, on alternating days, to either exercise vigorously or take a hot bath (39°C) for half an hour, finishing 2 hours before desired bedtime. These treatments, including the 2-h interval, aimed at optimizing heat dissipation to induce sleep: a normalized core temperature in combination with the after-effect of increased skin temperature promotes sleep onset.27,28
The effectiveness of the therapy was evaluated using sleep diaries in all 10 patients receiving therapy. One week prior to the treatment and one week in the end of the treatment or wait list period, participants kept a diary of lights-off and wake-up times, subjective sleep latency, and subjective duration of nocturnal wakefulness. Sleep latency and sleep efficiency were regarded as the 2 primary treatment outcome measures of interest. The number of entries per subject and per measurement occasion varied due to postal delays in sending out and receiving the diaries, holidays, omissions, etc. On average, 6.2 ± 1.7 nights per assessment occasion per subject were available for analysis.
Statistical Analyses of Sleep and Performance Data
Subjective sleep data were analyzed using linear mixed-effects regression modeling (MLwiN software package version 2.0, Centre for Multilevel Modelling, Institute of Education, London, UK). This approach takes the hierarchical nature of the data into account; e.g., sleep was assessed from a variable number of diary entries (level 1) on 2 occasions (level 2, pre and post therapy or wait list) for each patient (level 3). Data were analyzed with full factorial models, i.e. a 2-level within-subject factor time (pre and post), a 2-level between-subject factor group (therapy or wait list) and their interaction as the major focus of interest. The same approach was taken for comparisons of task performance data between patients and controls and, within patients, to evaluate treatment-by-time interaction effects.
RESULTS
Behavioral Results
Sleep
Subjective sleep latency decreased by 25.1 ± 6.5 minutes after therapy versus an increase of 2.6 ± 7.1 minutes after the wait list period, resulting in a highly significant group by time interaction effect (P = 0.004). Subjective sleep efficiency increased by 14.5% ± 2.6% after therapy, versus an increase of 0.7% ± 2.4% after the wait list period, resulting in a highly significant group by time interaction (P < 0.001).
Fluency
Insomniacs did not show any pre-treatment performance deficit in fluency as compared to controls; in fact, insomniacs produced more words regardless of task version. On the category fluency task, insomnia patients generated 46.4 ± 9.5 (average ± standard deviations) words and controls 38.7 ± 5.8 words (P = 0.008) while on the letter fluency task, insomnia patients generated 40.1 ± 10.2 words and control subjects generated 32.7 ± 10.4 words (P = 0.038). Category fluency resulted in a significantly higher number of responses than letter fluency for insomniacs and healthy control subjects alike (P = 0.034); this ensured that all subjects had complied with the task instructions, as the difference between the 2 types of fluency are compatible with those reported in the literature.29 Separate regression models for the 2 fluency tasks showed that sleep therapy appeared to improve performance specifically on the more demanding letter version, relative to the wait list group. The therapy group generated 38.0 ± 11.7 words on the letter fluency task before treatment, which increased to 47±13.1 words after treatment. In comparison, the wait list group generated 39.4 ± 5.1 words before and 42.9 ± 8.8 words after the 6 week interval on the letter fluency task, leading to a near-significant treatment by time interaction (P = 0.064). On the category fluency task, the therapy group generated 44.4 ± 12.9 words before and 49.7 ± 8.0 words after treatment while the wait list group produced 45.9 ± 9.3 words before and 47.9 ± 4.8 words after the 6-week interval, leading to a nonsignificant treatment by time interaction (P = 0.342).
Neuroimaging Results
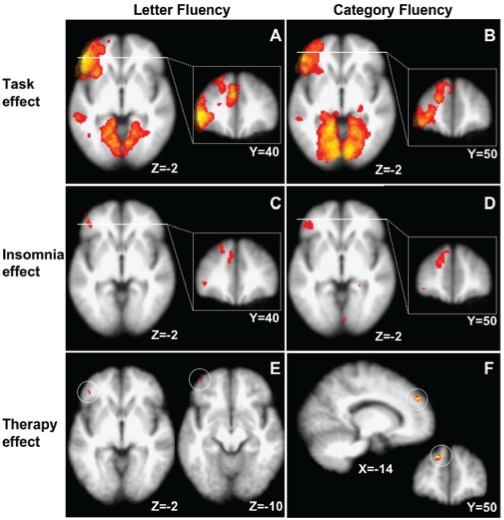
For the baseline group comparisons, the initial measurements of 21 insomniacs and 12 controls were included in the analysis. Because one of the insomniacs did not participate on the post-treatment assessment, the within-subject pre- vs. post-therapy analyses were based on 10 insomniacs in the active treatment condition and 10 insomniacs in the wait list condition. Whole-brain analysis of the functional activation data across all participants at their baseline assessment showed the characteristic fluency-specific activation of clusters of brain regions, including occipital, medial temporal, inferior frontal, dorsal lateral prefrontal, and anterior cingulate cortices (Figure 1 a and b and Table 3 and 4).
Figure 1.
BOLD effects for insomnia patients and controls on 2 verbal fluency tasks (FEAT analysis, FSL software (FMRIB, Oxford, UK)) A and B: Task-related activation. Both letter and category fluency tasks activate left inferior frontal gyrus, medial prefrontal, visual, and medial temporal cortex across all insomnia patients and control participants. Note the higher intensity in the left inferior frontal gyrus for the letter fluency task (z-threshold 2.3, cluster threshold P = 0.05). C and D: Patients show reduced activation.Lower activation in the left inferior frontal gyrus and medial prefrontal cortex in both tasks for insomniacs relative to controls (z >3.1, uncorrected). Images are thresholded at Z >2.3 to show the extent of the activated clusters. E and F: Task-specific recovery upon therapy. Sleep therapy reinstates activation in the left inferior frontal gyrus for letter fluency and in the medial prefrontal cortex for the category fluency task (indicated by white circles). The activation differences shown correspond to a higher activation in the post-therapy as compared to pre-therapy within the group of treated patients (n =10). For both tasks, treatment effects were regarded significant at z >2.3, uncorrected.
Table 3.
Areas Activated During the Letter Fluency Task as Obtained from Baseline Assessments of All Participants (21 Insomnia Patients vs. 12 Controls) from Anterior to Posterior Brain Regions
| anatomical region | Z value | x | y | z |
|---|---|---|---|---|
| left inferior frontal gyrus (BA 44,45) | 6.68 | −46 | 38 | 2 |
| left inferior frontal gyrus (BA 44,45) | 6.56 | −50 | 36 | 0 |
| left frontal polar cortex (BA 10) | 6.61 | −40 | 34 | −18 |
| left anterior cingulate cortex (BA 24, 32) | 6.07 | −4 | 28 | 38 |
| left dorsolateral prefrontal cortex (BA 9, 46) | 6.61 | −48 | 20 | 22 |
| left supplementary motor area (BA 6) | 6.22 | −44 | 14 | 14 |
| left parahippocampal region | 4.85 | −30 | −36 | −24 |
| right visual cortex | 5.26 | 8 | −66 | 4 |
| right visual cortex | 5.58 | 8 | −72 | 0 |
| left visual cortex | 5.47 | −6 | −72 | 6 |
| left visual cortex | 5.47 | −8 | −72 | 12 |
| right visual cortex | 5.92 | 4 | −84 | 10 |
| left visual cortex | 5.34 | −8 | −84 | 12 |
X, y, and z coordinates (in MNI space) refer to the voxel with the highest level of activation within the activated cluster of voxels, with corresponding Z-value.
Table 4.
Areas Activated During the Category Fluency Task as Obtained from Baseline Assessments of All Participants (21 Insomnia Patients vs. 12 Controls) from Anterior to Posterior Brain Regions
| anatomical region | Z value | x | y | z |
|---|---|---|---|---|
| left inferior frontal gyrus (BA 44,45) | 6.42 | −52 | 38 | 0 |
| left inferior frontal gyrus (BA 44,45) | 6.14 | −42 | 36 | 2 |
| left frontal polar cortex (BA 10) | 6.28 | −38 | 30 | −18 |
| left anterior cingulate gyrus (BA 24, 32) | 6.23 | −2 | 28 | 36 |
| left dorsolateral prefrontal cortex (BA 9, 46) | 5.85 | −48 | 20 | 20 |
| left dorsolateral prefrontal cortex (BA 9, 46) | 5.87 | −44 | 18 | 22 |
| left hippocampus | 4.11 | −24 | −26 | −20 |
| left parahippocampal region | 5.88 | −28 | −36 | −24 |
| left visual cortex | 6.69 | −14 | −54 | 0 |
| left visual cortex | 6.74 | −8 | −68 | 10 |
| right visual cortex | 7.16 | 12 | −82 | 0 |
| right visual cortex | 6.68 | 2 | −84 | 26 |
| right visual cortex | 7.28 | 10 | −86 | 2 |
| left visual cortex | 6.70 | −12 | −88 | 6 |
X, y, and z coordinates (in MNI space) refer to the voxel with the highest level of activation within the activated cluster of voxels, with corresponding Z-value.
Within this network defined as a region-of-interest, insomnia patients showed less activation than controls in the left medial prefrontal cortex (mPFC) and left inferior frontal gyrus (IFG) for both let9ter fluency (mPFC Z = 3.73; IFG Z = 4.17, Figure 1 c and Table 5) and category fluency (mPFC Z = 4.18; IFG Z = 3.3, 9Figure 1d and Table 6). The reverse contrast revealed no areas that were significantly activated more by patients than by controls.
Table 5.
Insomnia Patients Show Less Activation than Controls: Letter Fluency Related Brain Activation
| anatomical region | Z value | x | y | z |
|---|---|---|---|---|
| left polar frontal cortex (BA 9,10) | 4.20 | −6 | 62 | 22 |
| left superior frontal gyrus (BA 9) | 3.44 | −4 | 56 | 34 |
| left inferior frontal gyrus (BA 45, 10) | 4.17 | −36 | 52 | −12 |
| left superior frontal gyrus (BA 9) | 3.49 | −14 | 46 | 36 |
| left superior frontal gyrus (BA 9) | 3.73 | −6 | 44 | 38 |
| left middle frontal gyrus | 3.15 | −40 | 24 | 44 |
Brain regions are listed from anterior to posterior. X, y, and z coordinates (in MNI space) refer to the voxel with the highest level of activation within the activated cluster of voxels, with corresponding Z-value.
Table 6.
Insomnia Patients Show Less Activation than Controls: Category Fluency Related Brain Activation
| anatomical region | Z value | x | y | z |
|---|---|---|---|---|
| left superior frontal gyrus (BA 10) | 3.66 | −38 | 56 | −8 |
| left superior frontal gyrus (BA 9–10) | 3.95 | −18 | 50 | 26 |
| left superior frontal gyrus (BA 9–10) | 4.18 | −14 | 46 | 36 |
| left inferior frontal gyrus (BA 45) | 3.30 | −42 | 38 | −2 |
| left middle frontal gyrus | 3.11 | −38 | 20 | 42 |
| left anterior temporal cortex | 3.21 | −34 | 8 | −44 |
Brain regions are listed from anterior to posterior. X, y, and z coordinates (in MNI space) refer to the voxel with the highest level of activation within the activated cluster of voxels, with corresponding Z-value.
Within the affected areas defined as a second region-of-interest, therapy—but not wait list control—partly restored the letter fluency activation in 2 regions in the IFG (voxel of maximum activation at x = −44, y = 40, z = −2, Z-value = 2.70, and voxel of maximum activation at x = −40, y = 56, z = −12, Z-value = 2.92) but not in the mPFC. Therapy also partly restored the category fluency activation in the mPFC (voxel of maximum activation at x = −14, y = 50, z = 34, Z-value = 2.84) but not in the IFG. This task-specific recovery corresponds with the relative preference of area activation for the 2 tasks, su9ggestive of restoration of compromised activation (Figure 1 a and b and Tables 3 and 4).
DISCUSSION
The present findings are the first to demonstrate that verbal fluency-related prefrontal brain activation is compromised in insomnia in the absence of a behavioral deficit.
The set of behavioral and activation results is compatible with our hypothesis that the behavioral performance level of insomniacs can be regarded as one of high achievers, albeit attenuated by chronic suboptimal sleep. The careful selection in the present study of a considerable group of elderly insomnia patients, suffering from chronic primary insomnia only, excludes the possibility of ascribing the task-related prefrontal hypoactivation to other comorbid or primary disorders such as depression or OSAS.
Recovery after sleep therapy suggests that at least this part of brain function alterations in insomniacs is reversible. This further confirms the efficacy of this easily applicable and low-cost intervention and encourages its application in clinical practice, also in the elderly.
Prefrontal hypoactivation in the absence of behavioral differences may be related to individually differential recruitment of other brain regions for successful task completion. A similar lack of prefrontal activation in spite of non-significant changes in fluency performance has been reported in a near infrared spectroscopy study in depressed patients.30
Because the BOLD MRI signal is not an absolute measure of activation, but a measure of the activation during the task (here fluency) relative to the activation during a baseline task (here counting backwards), the results might equally well be interpreted as (1) attenuated activation during fluency relative to a normal baseline activation (as suggested in the above) or (2) normal activation during fluency relative to an increased baseline activation. We have therefore initiated an arterial spin labeling study to obtain absolute, rather than relative, measures of cerebral perfusion in insomnia. Preliminary results, in fact, demonstrate a lower baseline daytime perfusion of a corresponding left inferior frontal cortical region that shows attenuated activation in the current study (Van Der Werf et al., in prep). This finding strongly argues against the second, alternative interpretation described above.
An additional argument against the second interpretation of an increased prefrontal baseline activation in insomnia is provided by a recent [18F]Fluorodeoxyglucose positron emission tomography (PET) study31: although insomniacs showed a global increase in cerebral glucose metabolism, the relative regional glucose metabolism in the prefrontal areas was decreased relative to controls.
In conclusion, the current results show, for the first time, that fMRI can reveal prefrontal hypoactivation in a group of carefully selected elderly patients suffering from primary chronic insomnia only. We furthermore revealed recovery of this prefrontal hypocactivation after nonpharmacological sleep therapy.
ACKNOWLEDGMENTS
Financial support by the Netherlands Organization of Scientific Research (NWO), The Hague (VIDI 016.025.041, NWOCOG/04-07 and VICI 453-07-001), Philips Lighting, Eindhoven and the Amsterdam Brain Imaging Platform, Amsterdam, The Netherlands. We would like to acknowledge the help of Ms. Karin Plugge, Ms. Iet Beckmann, Dr. Rob Strijers, Ms. Rebecca Schutte, and Dr. José Vis for recruiting and screening patients, Dr. Ben Schmand for supplying us with the normative data for the letter fluency task and Dr. Dé Waterman for training in administering sleep therapy.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Harrison Y, Horne JA. The impact of sleep deprivation on decision making: a review. J Exp Psychol Appl. 2000;6:236–249. doi: 10.1037//1076-898x.6.3.236. [DOI] [PubMed] [Google Scholar]
- 2.Troyer AK, Moscovitch M, Winocur G, Alexander MP, Stuss D. Clustering and switching on verbal fluency: the effects of focal frontal- and temporal-lobe lesions. Neuropsychologia. 1998;36:499–504. doi: 10.1016/s0028-3932(97)00152-8. [DOI] [PubMed] [Google Scholar]
- 3.Cuenod CA, Bookheimer SY, Hertz-Pannier L, Zeffiro TA, Theodore WH, Le Bihan D. Functional MRI during word generation, using conventional equipment: a potential tool for language localization in the clinical environment. Neurology. 1995;45:1821–7. doi: 10.1212/wnl.45.10.1821. [DOI] [PubMed] [Google Scholar]
- 4.Horne JA. Sleep loss and “divergent” thinking ability. Sleep. 1988;11:528–36. doi: 10.1093/sleep/11.6.528. [DOI] [PubMed] [Google Scholar]
- 5.Van Someren EJ. Circadian and sleep disturbances in the elderly. Exp Gerontol. 2000;35:1229–37. doi: 10.1016/s0531-5565(00)00191-1. [DOI] [PubMed] [Google Scholar]
- 6.Morin CM, Hauri PJ, Espie CA, Spielman AJ, Buysse DJ, Bootzin RR. Nonpharmacologic treatment of chronic insomnia. An American Academy of Sleep Medicine review. Sleep. 1999;22:1134–56. doi: 10.1093/sleep/22.8.1134. [DOI] [PubMed] [Google Scholar]
- 7.Benca RM. Diagnosis and treatment of chronic insomnia: a review. Psychiatr Serv. 2005;56:332–43. doi: 10.1176/appi.ps.56.3.332. [DOI] [PubMed] [Google Scholar]
- 8.Morin CM, Mimeault V, Gagne A. Nonpharmacological treatment of late-life insomnia. J Psychosom Res. 1999;46:103–16. doi: 10.1016/s0022-3999(98)00077-4. [DOI] [PubMed] [Google Scholar]
- 9.Fulda S, Schulz H. Cognitive dysfunction in sleep disorders. Sleep Med Rev. 2001;5:423–45. doi: 10.1053/smrv.2001.0157. [DOI] [PubMed] [Google Scholar]
- 10.Vincent NK, Walker JR. Perfectionism and chronic insomnia. J Psychosom Res. 2000;49:349–54. doi: 10.1016/s0022-3999(00)00175-6. [DOI] [PubMed] [Google Scholar]
- 11.Drummond SP, Smith MT, Orff HJ, Chengazi V, Perlis ML. Functional imaging of the sleeping brain: review of findings and implications for the study of insomnia. Sleep Med Rev. 2004;8:227–42. doi: 10.1016/j.smrv.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155–73. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 13.Edinger JD, Bonnet MH, Bootzin RR, et al. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27:1567–96. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 14.Lichstein KL, Durrence HH, Taylor DJ, Bush AJ, Riedel BW. Quantitative criteria for insomnia. Behav Res Ther. 2003;41:427–45. doi: 10.1016/s0005-7967(02)00023-2. [DOI] [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 16.Nelson HE. National Adult Reading Test. Manual. Windsor: NFER-Nelson Publishing Company; 1982. [Google Scholar]
- 17.Nelson HE. The Revised National Adult Reading Test - Test Manual. Windsor: NFER-Nelson Publishing Company; 1991. [Google Scholar]
- 18.Schmand B, Lindeboom J, Van Harskamp F. De Nederlandse leestest voor volwassenen. [The Dutch Adult Reading Test.] Lisse: Swets and Zeitlinger; 1992. [Google Scholar]
- 19.Luteijn F, Van der Ploeg FAE. Groninger Intelligentie test. Lisse: Swets and Zeitlinger; 1983. [Google Scholar]
- 20.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 21.Derogatis LR, Lipman RS, Covi L. SCL-90: an outpatient psychiatric rating scale--preliminary report. Psychopharmacol Bull. 1973;9:13–28. [PubMed] [Google Scholar]
- 22.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36) I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 23.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 24.Douglass AB, Bornstein R, Nino-Murcia G, et al. The Sleep Disorders Questionnaire. I: Creation and multivariate structure of SDQ. Sleep. 1994;17:160–7. doi: 10.1093/sleep/17.2.160. [DOI] [PubMed] [Google Scholar]
- 25.Sweere Y, Kerkhof GA, De Weerd AW, Kamphuisen HA, Kemp B, Schimsheimer RJ. The validity of the Dutch Sleep Disorders Questionnaire (SDQ) J Psychosom Res. 1998;45:549–55. doi: 10.1016/s0022-3999(98)00030-0. [DOI] [PubMed] [Google Scholar]
- 26.Woodward M. Insomnia in the elderly. Aust Fam Physician. 1999;28:653–8. [PubMed] [Google Scholar]
- 27.Van Someren EJW. Sleep propensity is modulated by circadian and behavior-induced changes in cutaneous temperature. J Therm Biol. 2004;29:437–444. [Google Scholar]
- 28.Raymann RJ, Swaab DF, Van Someren EJ. Skin deep: enhanced sleep depth by cutaneous temperature manipulation. Brain. 2008;131:500–13. doi: 10.1093/brain/awm315. [DOI] [PubMed] [Google Scholar]
- 29.Auriacombe S, Fabrigoule C, Lafont S, Amieva H, Jacqmin-Gadda H, Dartigues JF. Letter and category fluency in normal elderly participants: a population-based study. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2001;8:98–108. [Google Scholar]
- 30.Herrmann MJ, Ehlis AC, Fallgatter AJ. Bilaterally reduced frontal activation during a verbal fluency task in depressed patients as measured by near-infrared spectroscopy. J Neuropsychiatry Clin Neurosci. 2004;16:170–5. doi: 10.1176/jnp.16.2.170. [DOI] [PubMed] [Google Scholar]
- 31.Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161:2126–8. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]