Abstract
Study Objectives:
To study the association between sleep/wake patterns among older adults during inpatient post-acute rehabilitation and their immediate and long-term functional recovery
Design:
Prospective, observational cohort study
Setting:
Two inpatient post-acute rehabilitation sites (one community and one Veterans Administration)
Participants:
Older patients (aged ≥ 65 years, N = 245) admitted for inpatient post-acute rehabilitation Interventions: None
Measurements and Results:
Based on 7-day wrist actigraphy during the rehabilitation stay, mean nighttime percent sleep was only 52.2% and mean daytime percent sleep was 15.8% (16.3% based on structured behavioral observations). Using the Pittsburgh Sleep Quality Index (PSQI), participants reported their sleep was worse during rehabilitation compared to their premorbid sleep. Functional recovery between admission and discharge from rehabilitation (measured by the motor component of the Functional Independence Measure) was not significantly associated with reported sleep quality (PSQI scores) or actigraphically measured nighttime sleep. However, more daytime percent sleep (estimated by actigraphy and observations) during the rehabilitation stay was associated with less functional recovery from admission to discharge, even after adjusting for other significant predictors of functional recovery (mental status, hours of rehabilitation therapy received, rehospitalization, and reason for admission; adjusted R2 = 0.267, P < 0.0001). More daytime sleeping during rehabilitation remained a significant predictor of less functional recovery in adjusted analyses at 3-month follow-up.
Conclusions:
Sleep disturbance is common among older people undergoing inpatient post-acute rehabilitation. These data suggest that more daytime sleeping during the rehabilitation stay is associated with less functional recovery for up to three months after admission for rehabilitation.
Citation:
Alessi CA; Martin JL; Webber AP; Alam T; Littner MR; Harker JO; Josephson KR. More Daytime Sleeping Predicts Less Functional Recovery Among Older People Undergoing Inpatient Post-Acute Rehabilitation. SLEEP 2008;31(9):1291-1300.
Keywords: Sleep, aged, rehabilitation, recovery of function
OLDER PEOPLE ARE AT HIGH RISK FOR FUNCTIONAL DECLINE (I.E., LOSS OF INDEPENDENCE IN PERSONAL CARE ACTIVITIES) DURING ACUTE HOSPITALIZATION. This functional decline often leads to increased caregiving needs at home, nursing home placement, or death.1 For older persons, rehabilitation (i.e., physical and occupational therapies) is increasingly provided outside of the acute care hospital in post-acute facilities, such as nursing homes and intermediate care facilities. The goal of post-acute rehabilitation is to facilitate return home by assisting patients in achieving independence in personal care activities. Prior research has identified specific factors that predict poorer outcomes of rehabilitation among patients recovering from hip fracture, stroke, medical conditions and surgery.2–11 These predictors include older age, cognitive impairment, dependence in personal care activities before the health event, depression, pain, lack of family involvement, and fewer hours of physical therapy during rehabilitation.
There has been some prior research addressing the potential importance of sleep in rehabilitation settings. Studies among older patients admitted for rehabilitation after a stroke show that sleep apnea is common in this situation,12 is associated with worse functional impairment on admission and predicts less recovery of functional abilities.12,13 To date, however, disturbance of sleep/wake patterns has not been explored as a potential factor impacting functional outcomes among older adults admitted to post-acute rehabilitation settings.
Evidence suggests that sleep disturbance is associated with both poor health,14–16 and worse health-related quality of life among older people.17,18 Reports of poor sleep among older people correlate strongly with health complaints and depressive symptoms.19 Prior research also demonstrates a relationship between long sleep duration and mortality.20,21 In contrast, healthy older adults have few sleep complaints.22,23
Studies in other institutional settings (e.g., nursing homes, acute care hospitals) suggest that sleep problems are associated with functional impairment, social isolation and poor health in older people. In our prior work, we found that nursing home residents with excessive daytime sleeping required more assistance with self-care activities and engaged in fewer social interactions.24,25 In the acute care hospital setting, Redeker et al. found that both better sleep efficiency (by wrist actigraphy) and better self-reported sleep predicted improved physical function and emotional well-being among older men and women 4 and 8 weeks after cardiac surgery.26 These same investigators also found that women with more normal circadian activity-rest patterns (measured by wrist actigraphy) have shorter hospital stays and better functioning after coronary artery bypass surgery.27 Many of these studies have been correlational or cross-sectional in nature, limiting the ability to make causal inferences about the relationship between sleep and health.
The development of poor sleep during rehabilitation may be particularly important, since once established, abnormal sleep/wake patterns may persist long after inciting events have resolved. In addition, abnormal sleep/wake patterns might affect a patient's motivation, level of fatigue and participation in rehabilitation, which, in turn, may impact functional recovery of personal care activities.
The purpose of the current study was to describe sleep patterns among older people in the post-acute rehabilitation setting and to examine sleep/wake patterns as a potential predictor of recovery of functional abilities. We performed a prospective, observational cohort study among older people admitted for inpatient post-acute rehabilitation. We hypothesized that (1) disrupted nighttime sleep and daytime sleeping would be common in post-acute rehabilitation settings, (2) greater nighttime sleep disruption and more daytime sleeping would be associated with less recovery of functional abilities during the rehabilitation stay (from admission to discharge), and (3) the effect of sleep disturbance during the rehabilitation stay on recovery of functional abilities would persist after discharge from the rehabilitation setting.
METHODS
Study Design and Setting
The study design was a prospective, observational cohort study among older people admitted to 2 post-acute rehabilitation sites in the Los Angeles area. The first site (Site A) was a freestanding, for-profit, community nursing home with 130 Medicare-certified beds, which focused on short-term rehabilitation. This site was included to provide an adequate representation of women in the final sample. The second site (Site B) was an inpatient rehabilitation unit located within a Veterans Administration Medical Center. Inclusion of this site provided greater diversity of race/ethnicity in the final sample. Participant recruitment was performed at one site at a time, between September 2002 and March 2004. All research methods were approved by the Veterans Administration Greater Los Angeles Healthcare System Institutional Review Board. Written informed consent was obtained from all participants. For individuals who were unable to provide self-consent, written informed consent was obtained from their responsible party, with the assent of the participant.
Participants
All patients admitted to the study sites were approached for screening as soon as possible after admission to the rehabilitation unit. The mean number of days between admission to the rehabilitation unit and participant consent and enrollment was 3.8 (SD 2.6) days. Inclusion criteria were: (1) aged 65 years or older, and (2) admitted for rehabilitation (i.e., receiving physical or occupational therapy). Exclusion criteria were: (1) resided in a nursing home prior to admission; (2) transferred, died, discharged, or were not identified within one week of admission; and (3) judged to be unable to participate in the study due to a severe medical illness (e.g., end of life care) or severe behavioral disorder (e.g., dementia with severe agitation identified on9 screening interview).
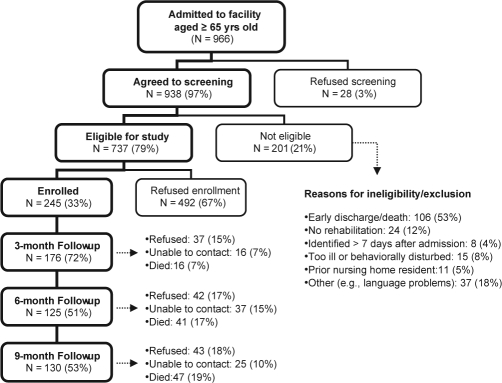
As shown in Figure 1, 97% of patients admitted to the 2 study sites during the enrollment period were screened for the study; 79% were eligible based on inclusion and exclusion criteria. Of these eligible patients, 33% consented to participate in the study (158 from Facility A and 87 from Facility B).
Figure 1.
Participant screening, enrollment and follow-up
Sleep Measures
Subjective sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI).28 The PSQI is an 18-item questionnaire that includes subscales to estimate subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction (score range 0-21; score >5 indicates sleep disturbance).
The PSQI was administered first in a format that asked participants to answer questions regarding their sleep “before their recent illness.” This was intended to reflect their premorbid sleep patterns in the community prior to their acute hospitalization or rehabilitation admission. The PSQI was administered again after the participant had been in the rehabilitation facility for at least 7 days. Questions were modified to query participants about their sleep over the past week (i.e., while in the post-acute rehabilitation facility).
To obtain an objective measure of sleep during the rehabilitation admission, participants wore a wrist actigraph (Octagonal Sleep Watch-L, Ambulatory Monitoring, Inc, AMI, Ardsley, NY) on their dominant arm (unless paralyzed or otherwise unable) for 7 consecutive days and nights. Raw actigraphy data (1-min epoch length) were reviewed visually to eliminate technical (i.e., device failure) and situational (i.e., device was removed) artifact, prior to scoring sleep using a validated algorithm within commercially available software (ACT software, AMI). Here we report variables from automatic sleep scoring using time above threshold (TAT; default algorithms), based on prior literature and our own data comparing actigraphy with standardized observations of sleep/wake in a portion of this sample.29,30 Participants were queried daily for their bedtime and wake-up times to determine nighttime and daytime periods for analysis of actigraphy data.
Because of limited prior data on the validity of actigraphy as a measure of daytime sleeping, participants also had 2 days (09:00–17:00) of structured behavioral observations of sleep versus wakefulness and whether the participants were in or out of bed. The behavioral observations were performed by trained research staff every 15 min; sleep was defined as eyes closed with no purposeful activity.24,29
Participants had one night of attended (i.e., research staff present all night) bedside multichannel respiratory sleep recording using the EdenTraceII Digital Recorder (NellCor Puritan Bennett, Ottawa, Ontario), with simultaneous measurement of oxygen saturation, heart rate, respiratory effort, nasal airflow, and snoring. Recordings were hand scored, using available software (Sandman EASy). A respiratory disturbance index (number of apneas and hypopneas per hour of sleep) was calculated to quantify evidence of sleep apnea. An apnea was defined as a ≥ 10 sec decrease in tidal volume to less than 10%, associated with significant desaturation. A hypopnea was defined as ≥ 10 sec decrease in tidal volume to less than 50%, with significant desaturation. Significant desaturation on pulse oximetry was defined as a drop in arterial blood oxygen saturation of ≥ 4% and to a value of ≤ 90%.
Measure of Functional Independence
Functional independence in personal care activities was measured using the motor subscale of the Functional Independence Measure (mFIM),31 which is widely used in rehabilitation settings to assess functional limitations and change in functional status with rehabilitation therapy. The mFIM is an ordinal scale that measures level of disability based on the need for assistance and/or assistive devices or aids during the performance of activities of daily living (eating, grooming, transfer in/out of the bath, bathing, dressing upper body, dressing lower body, toileting, transferring in/out of a bed/chair/wheelchair, bladder control, bowel control, transferring in/out of a bed/chair/wheelchair, walking and taking stairs). Each of the 13 items of the mFIM has 7 possible levels, ranging from 1 (total dependence) to 7 (total independence) with a possible range in total mFIM score from 13 to 91, where higher scores indicate greater independence. Admission and discharge mFIM scores were obtained by mFIM-trained research staff from medical record review. At facility A (which did not use the mFIM scoring system in patients but did evaluate the functional areas assessed by the mFIM), a study physician (AW) used therapists' records and nursing documentation to complete a structured scoring algorithm, based on the mFIM manual, to obtain a mFIM score. At Facility B, therapists documented mFIM scores in the medical record. The therapists' scores were abstracted by a study research nurse trained in use of the mFIM. For the follow-up assessments, since no therapists' notes were available, trained research staff asked participants a series of questions to assess level of assistance required for each of the 13 mFIM items. This information was then scored by the research nurse to obtain the mFIM score.
Other Measures
Basic demographic information was recorded for all participants, including age, gender, and ethnicity. Reason for admission to post-acute rehabilitation was recorded from the transferring hospital discharge records and/or review of the rehabilitation facility medical record. The total number of hours of rehabilitation therapy (i.e., physical therapy, occupational therapy, kinesiotherapy) received were calculated from therapists' notes in the medical record.
Questionnaire assessments included the Mini-Mental State Examination32 (MMSE; a 20-item measure of general cognitive functioning, assessing 5 cognitive domains; score range = 0-30; scores < 24 suggest cognitive impairment) and the 15-item version of the Geriatric Depression Scale33 (GDS-15; this abbreviated version assesses symptoms of depression; score range = 0-15; scores > 5 suggest depression). Pain was assessed using the Geriatric Pain Measure34 (GPM; a 24-item measure that assesses pain intensity, disengagement due to pain, pain with ambulation, and pain with strenuous activities and other activities; adjusted score range 0-100, with higher scores suggesting more pain).
The Cumulative Illness Rating Scale for Geriatrics35 (CIRS-G) was used to assess baseline illness severity and comorbidity.26 The CIRS-G was completed by a highly trained research registered nurse, using data collected from a structured medical record review and a brief physical examination by a study physician. All medications received and transfers to an acute care hospital during the rehabilitation stay were recorded from facility records. An acute care hospital transfer (e.g., due to an acute illness occurring during the rehabilitation stay) was defined as spending one or more days in an acute setting between the initial admission and final discharge from the rehabilitation facility. Participants who did not return to the rehabilitation facility after transfer to an acute care hospital were considered discharged from the facility at the time of transfer.
Procedures
After enrollment, participants completed a baseline assessment. This assessment included one night of attended bedside multichannel respiratory sleep recording, followed by 7 days and nights of wrist actigraphy, and a battery of self-report questionnaires (described above). On 2 days of wrist actigraph recordings, participants were observed every 15 min from 09:00 to 17:00. After discharge from the rehabilitation facility, a structured medical record review was completed. All data were collected by trained research personnel.
The actigraphy, PSQI, and behavioral observations were collected several days after admission to the rehabilitation unit to allow for a period of adjustment to the new setting to reflect more persistent, rather than transient measures of sleep. The PSQI was collected, on average, 9.1 (SD 4.3) days after admission to the rehabilitation unit. The first day of actigraphy began, on average, 5.9 (SD 3.1) days after admission, and the first day of behavioral observations was performed 13.3 (SD 7.4) days after admission to the rehabilitation unit.
Follow-up assessments were conducted 3, 6, and 9 months from the date of admission to the rehabilitation facility. Follow-up assessments were conducted at the participant's home (or other living location) by research staff. When an in-person visit was not possible (e.g., participant had moved out of the area), the assessment was performed by telephone (33% of all follow-up interviews). The follow-up assessment included all of the components of the baseline assessment except the multichannel respiratory sleep recording and behavioral observations, which were not repeated. Actigraphy was included in the follow-up assessments but is not reported here. Participants (or their proxies) were queried about use of medications, emergency room visits, and hospital and nursing home admissions since discharge from the rehabilitation facility (at 3-month follow-up) or since their last follow-up assessment (at 6 and 9 months).
Statistical Analysis
Data were analyzed with SPSS 13.0 (SPSS Inc., Chicago, IL). As expected, comparison of demographic and other descriptive characteristics (listed in Table 1) of participants between the 2 study facilities found many significant differences (e.g., participants in Site B were younger, more likely to be male, and had a shorter length of stay than participants in Site A). However, there were few significant differences in sleep measures between the 2 sites (see Tables 2 and 3), so analyses were conducted with the combined sample.
Table 1.
Participant Characteristics at Baseline and Characteristics of their Inpatient Post-Acute Rehabilitation Stay (N = 245)
| Variable | Site A | Site B | Total sample |
|---|---|---|---|
| Mean (SD) or n (%) | Mean (SD) or n (%) | Mean (SD) or n (%) | |
| Age, in yearsa | 82.0 (7.1) | 78.1 (6.7) | 80.6 (7.2) |
| Gender, femalea | 90 (57.0%) | 3 (3.4%) | 93 (38.0%) |
| Ethnicity, non-Hispanic whitea | 145 (91.8%) | 50 (57.5%) | 195 (79.6%) |
| Reason for admission to rehabilitation | |||
| Orthopedic problem | 72 (45.6%) | 33 (37.9%) | 103 (42.1%) |
| Cardiac problem | 16 (10.1%) | 15 (17.2%) | 32 (13.2%) |
| Stroke/neurological disorder | 13 (8.2%) | 12 (13.8%) | 26 (10.6%) |
| Debility, general weakness | 18 (11.4%) | 5 (5.7%) | 23 (9.4%) |
| Pulmonary problem | 4 (2.5%) | 2 (2.3%) | 6 (2.6%) |
| Other | 35 (22.2%) | 20 (23.0%) | 54 (22.1%) |
| Rehabilitation therapy received, total hours | 24.8 (10.9) | 12.8 (7.6) | 20.5 (11.4) |
| Length of stay on rehabilitation unit, days | 24.3 (12.0) | 15.1 (8.5) | 21.1 (11.75) |
| Cumulative Illness Rating Scale-Geriatrics, total score | 22.6 (6.0) | 22.7 (5.5) | 22.6 (5.9) |
| Number of routine medicationsa | 9.0 (4.0) | 12.1 (4.2) | 10.1 (4.3) |
| Number of “as needed” medicationsa | 1.5 (1.5) | 3.5 (2.5) | 2.2 (2.1) |
| Motor component Functional Independence Measure, total scorea | 42.1 (10.9) | 49.5 (13.8) | 44.7 (12.5) |
| Mini-Mental State Examination, total scorea | 22.6 (6.9) | 25.1 (4.4) | 23.5 (6.2) |
| Geriatric Depression Scale-Short Form, total score | 4.2 (3.4) | 4.0 (3.1) | 4.1 (3.3) |
| Geriatric Pain Measure, total score | 47.2 (27.4) | 44.8 (30.4) | 46.3 (28.5) |
aP-value < 0.05 for comparison between sites A and B (independent samples t-test or chi-square, as appropriate)
Table 2.
Self-Reported Sleep Quality Measures: Premorbid Sleep Versus Sleep During the Inpatient Post-Acute Rehabilitation Stay
| Premorbid sleep a | Sleep during rehab stay b | P-valuec | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Pittsburgh Sleep Quality Index (PSQI), total score | 5.2 (3.8) | 8.3 (4.4) | <0.0001 |
| Pittsburgh Sleep Quality Index subscales: | |||
| Sleep quality | 0.8 (0.8) | 1.2 (0.9) | <0.0001 |
| Sleep latency | 1.2 (1.1) | 1.6 (1.2) | <0.0001 |
| Sleep duration | 0.7 (1.0) | 1.3 (1.3) | <0.0001 |
| Habitual sleep efficiency | 0.9 (1.1) | 1.4 (1.3) | <0.0001 |
| Use of sleeping medications | 0.5 (1.1) | 1.2 (1.4) | <0.0001 |
| Sleep disturbance | 0.8 (0.6) | 1.0 (0.6) | <0.0001 |
| Daytime dysfunction | 0.4 (0.7) | 0.6 (0.8) | <0.0001 |
PSQI administered after enrollment, modified to query participants about their sleep “before their recent illness,” to reflect their premorbid sleep patterns
PSQI administered after the participant had been in the facility for at least 7 days, modified to query participants about their sleep over the past week since admission to the inpatient post-acute rehabilitation setting. This PSQI was collected, on average, 9.1 (SD 4.3) days after admission to the rehabilitation unit.
T-test comparing premorbid PSQI versus PSQI values during the inpatient post-acute rehabilitation stay in the combined sample
Table 3.
Objective Sleep Measures During the Inpatient Post-Acute Rehabilitation Stay
| Variable | Site A | Site B | Total sample |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Multi-channel respiratory sleep recording (n = 122): | |||
| Respiratory disturbance indexa | 11.6 (13.5) | 10.3 (12.1) | 11.2 (13.0) |
| Sleep schedule (based on daily sleep diary; n = 242): | |||
| Bedtime (self-report, 24-hr clock) | 21:43 (01:04) | 21:47 (01:07) | 21:45 (1:05) |
| Wakeup time (self-report, 24-hr clock)d | 07:20 (00:46) | 06:34 (00:38) | 7:04 (0:49) |
| Wrist actigraphy variablesb (n = 241): | |||
| Nighttime total sleep time, hoursd | 5.3 (2.1) | 4.6 (2.1) | 5.1 (2.1) |
| Nighttime percent sleep (time asleep over time between bedtime and wake-up time) | 56.0 (20.7) | 52.9 (22.7) | 54.9 (21.5) |
| Nighttime number of awakenings | 16.1 (7.0) | 14.8 (6.4) | 15.6 (6.8) |
| Daytime total sleep time, hours | 1.9 (1.4) | 2.3 (1.8) | 2.1 (1.6) |
| Daytime percent sleep (time asleep over time between wake-up time and bedtime) | 14.8 (11.4) | 17.6 (13.1) | 15.8 (12.1) |
| Daytime minutes of light >1000 luxd | 18.6 (26.0) | 9.7 (24.0) | 15.4 (25.6) |
| Daytime behavioral observationsc (n = 241): | |||
| Daytime percent sleep | 16.2 (12.5) | 16.5 (14.7) | 16.3 (13.3) |
| Daytime percent of observations in bedd | 52.7 (28.0) | 61.5 (25.3) | 55.8 (27.4) |
Nighttime respiratory monitoring data available in 47% of participants
Based on 7 consecutive days and nights of wrist actigraphy data where nighttime and daytime hours were determined based on reported bedtimes and wake-up times
Performed every 15 min from 09:00 to 17:00 for 2 days
P-value < 0.05 for comparison between sites A and B (independent samples t-test)
Actigraphy variables were averaged over the 7 nights of recording, including nighttime hours of sleep, nighttime percent sleep (hours asleep/hours between bedtime and wake-up time), and nighttime number of awakenings. Nighttime was defined as the period between reported bedtime and reported wake-up time. Daytime hours of sleep, daytime percent sleep, and daytime minutes of exposure to light greater than 1,000 lux were also examined. Daytime was defined as the period between reported wake-up time and reported bedtime for actigraphy measures. Daytime percent sleeping by observation was also included as a key sleep measure, based on the percent of observations asleep from 09:00 to 17:00 averaged over 2 days. We used the observational data as the measure of daytime percent sleep (rather than daytime sleeping estimated by actigraphy) in regression analyses because observational data were available in all participants, and these daytime observational techniques have been validated in prior research,29 while actigraphy has not been adequately validated as a measure of daytime sleep.
To address our first hypothesis, we compared premorbid sleep quality (premorbid PSQI) to sleep quality during rehabilitation (7-day PSQI, i.e., performed at least 7 days after admission to reflect subject sleep while in the rehabilitation unit). We also summarized actigraphy-measured sleep variables and behaviorally observed daytime sleep.
To address our second hypothesis, we examined sleep variables, demographic variables, and clinical measures as potential predictors of immediate functional recovery, defined as change in mFIM score between admission and discharge (i.e., discharge mFIM score minus admission mFIM) with univariate analyses (Pearson correlation coefficients). We then entered the variables with the strongest relationship with immediate functional recovery (using P < 0.01) in regression models to determine whether sleep variables that were associated with immediate functional recovery in univariate analyses remained significant independent predictors when adjusted for other significant predictors of functional change. These potential independent predictors were checked for colinearity (nonindependence) based on high inter-correlation prior to inclusion in the regression models. When there were pairs of highly correlated variables, we chose the better variable (in terms of data characteristics and relationship with the outcome of interest) to include in the regression models. In addition, each regression model was checked for evidence of multicolinearity among the variables prior to acceptance of the final models. Regression analyses were repeated to predict change in mFIM score from baseline to each follow-up time point (i.e., 3-month mFIM minus baseline mFIM, 6-month mFIM minus baseline mFIM, 9-month mFIM minus baseline mFIM) to determine whether relationships identified between sleep during the rehabilitation stay and functional recovery persisted after discharge from the rehabilitation facility. We attempted to test separate regression models for 3-month, 6-month, and 9-month follow-up; however, significant loss to follow-up by 6 months prevented appropriate testing of the models using the 6-month and 9-month follow-up data. In all tests, P < 0.05 was used for statistical significance.
RESULTS
Participants
Characteristics of participants on admission to the post-acute rehabilitation facility and selected characteristics of their stay are summarized in Table 1. Only 17 (7%) participants were receiving rehabilitation after a cerebrovascular accident. One-third of participants had an MMSE score that indicated cognitive impairment (score < 24), and 28% had significant symptoms of depression (GDS-15 > 5). As expected, there were several significant differences in descriptive characteristics between participants admitted to the 2 study sites. Compared to Site B (Veterans Administration), participants at Site A (community facility) were more likely to be older, female, non-Hispanic white, use fewer medications, have worse functional status on admission to the rehabilitation facility, and more cognitive impairment (see Table 1). Death and loss to follow-up rates over the course of the 9study are shown in Figure 1.
Characteristics of Sleep/Wake Disturbance
Respiratory sleep monitoring data were collected in 115 (47%) participants (53% refused monitoring or provided inadequate data for analysis). Based on the available data, 26 (23%) participants had evidence of mild-to-moderate sleep apnea (i.e., ≥ 15 respiratory events per hour of recording), and 9 (8%) had evidence of severe sleep disordered breathing (i.e., ≥ 30 respiratory events per hour of recording).
In comparing PSQI scores between the 2 sites, there were no significant differences in PSQI total scores or subscales between sites except in 2 subscales of the premorbid PSQI (premorbid sleep quality: Site A 0.87 [SD 0.83], Site B 0.60 [SD 0.85], P = 0.021; premorbid sleep disturbance: Site A 0.90 [SD 0.63], Site B 0.70 [SD 0.56], P = 0.014 ). There were no other differences between sites in PSQI scores, so data for the combined sample are provided in Table 2. Participants reported worse sleep during their rehabilitation admission compared to their premorbid sleep (PSQI scores obtained at least 7 days after admission compared to premorbid PSQI scores). Twenty-six percent of participants reported clinically significant sleep disturbance (PSQI > 5) prior to the onset of their recent illness, while 50% of participants reported clinically significant sleep disturbance during their rehabilitation stay (chi-square = 23.3, P < 0.001).
Table 3 shows objective sleep measures during post-acute rehabilitation. There were some differences between sites in these variables. Compared to Site B, participants at Site A had more nighttime total sleep by actigraphy (P = 0.007), an earlier wake-up time by sleep log (P < 0.0001), more minutes per day of exposure to light > 1000 lux (P = 0.008), and spent less time in bed during the daytime based on behavioral observations (P = 0.014). However these and the remaining sleep measures demonstrated marked sleep disturbance among participants at each site. For the combined sample (based on wrist actigraphy), participants slept 54.9% of nighttime hours (bedtime to wake-up time) and 15.8% of daytime hours (wake-up time to bedtime). Structured observations also found that participants were asleep on 16.3% of observations and in bed on 55.8% of observations during the hours of 09:00 to 17:00.
Predictors of Functional Recovery
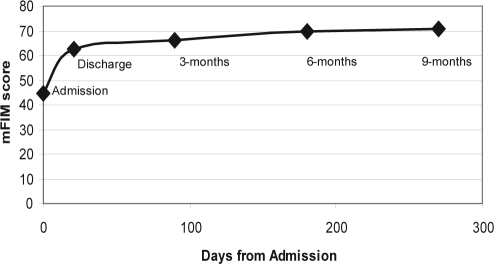
Participant mFIM scores (mean ± SD) showed the greatest improvement between admission (44.7 ± 12.5) and discharge (62.6 ± 16.5) from the rehabilitation facility, with evidence of a relative9 plateau in functional recovery at later follow-up (see Figure 2).
Figure 2.
Trajectory of improvement in the motor component of the Functional Independence Measure (mFIM) score from admission to the inpatient post-acute rehabilitation setting through 9-months follow-up
Our main interest was in identifying predictors of immediate functional recovery (i.e., discharge mFIM minus admission mFIM). To do this, we tested whether participant characteristics (listed in Table 1) and sleep variables (listed in Tables 2 and 3) were associated with immediate functional recovery using univariate analyses (Table 4). Several participant characteristics were significantly correlated with immediate functional recovery (see Table 4). Among the key sleep variables, more daytime sleeping during the rehabilitation stay (both by observation and wrist actigraphy) and earlier wake-up time were associated with less immediate functional recovery. Nighttime sleep percent (by actigraphy) and self-reported sleep (by PSQI total score and subscale scores) were not associated with immediate functional recovery.
Table 4.
Univariate Associations Between Descriptive Characteristics and Immediate Functional Recovery (i.e., Discharge mFIM Score Minus Baseline mFIM Score) During Inpatient Post-Acute Rehabilitation
| Variable | Correlation (r) with immediate functional recovery a | P-value |
|---|---|---|
| Demographics: | ||
| Age, in years | −0.16 | 0.01 |
| Gender, % female | −0.12 | 0.07 |
| Ethnicity, % non-Hispanic white | 0.02 | 0.76 |
| Clinical measures and characteristics: | ||
| Reason for admission: orthopedic problem | 0.26 | <0.0001 |
| Cumulative Illness Rating Scale-Geriatrics, total score | −0.16 | 0.01 |
| Number of routine medications | 0.03 | 0.65 |
| Number of “as needed” medications | 0.18 | 0.01 |
| Mini-Mental State Examination | 0.35 | < 0.0001 |
| Geriatric Depression Scale-Short Form, total score | 0.02 | 0.77 |
| Geriatric Pain Measure, total score | 0.06 | 0.38 |
| Hours of rehabilitation therapy received | 0.19 | 0.004 |
| Acute hospitalization during rehabilitation stay | −0.27 | < 0.0001 |
| Sleep measures: | ||
| Nighttime total sleep time, hours (by actigraphy) | −0.09 | 0.16 |
| Nighttime percent sleep (time asleep over time between bedtime and wake-up time; by actigraphy) | −0.02 | 0.72 |
| Nighttime number of awakenings (by actigraphy) | −0.01 | 0.89 |
| Daytime percent sleep (by actigraphy) | −0.25 | <0.0001 |
| Respiratory disturbance index | 0.15 | 0.104 |
| Pittsburgh Sleep Quality Index, total score | 0.10 | 0.18 |
| Wake-up time (sleep diary) | −0.27 | < 0.0001 |
| Bedtime (sleep diary) | 0.03 | 0.627 |
| Daytime percent sleep (by observation) | −0.27 | < 0.0001 |
| Daytime percent time in bed (by observation) | −0.09 | 0.19 |
Immediate functional recovery = mFIM score at discharge from the inpatient rehabilitation setting minus mFIM score at admission to the inpatient rehabilitation setting; r = Pearson correlation coefficient
We then tested whether the association between less daytime sleeping (by observation) during the rehabilitation stay and greater immediate functional recovery persisted in regression analyses, after adjusting for other significant predictors of mFIM change. Earlier wake-up time had evidence of colinearity (high inter-correlation) with daytime sleeping (by observation), so earlier wake-up time was not included as a possible independent variable. In the regression analysis predicting immediate functional recovery (i.e., discharge mFIM score minus admission mFIM score), we found that lower daytime percent sleep during the rehabilitation stay remained a significant independent predictor of greater immediate functional recovery (adjusted R2 = 0.267, model P < 0.0001) (Table 5, columns 2-4) even after adjusting for other significant independent predictors of greater immediate functional recovery including better cognitive functioning (by MMSE score), more total hours of rehabilitation therapy received, lack of transfer to an acute care hospital during rehabilitation, and admission for an orthopedic problem (versus other reasons for admission). Of note, participants admitted for an orthopedic problem had lower observed daytime sleeping (13.8%, SD 10.7%) and lower daytime percent sleeping by actigraphy (11.5%, SD 9.5%) compared to participants admitted for all other reasons (daytime percent sleeping by observation and actigraphy 17.5% [SD 14.3%] and 18.0% [SD 12.7%], respectively; independent sample t-test P = 0.024 and P < 0.0001, respectively). However, daytime sleeping remained a significant independent predictor of immediate functional recovery in the adjusted analysis. Other predictors that were significant in univariate analysis (e.g., age, gender, CIRS-G score, and number of “as needed” medications) were not significant independent predictors of immediate functional recovery in regression analysis.
Table 5.
Predictors of Functional Recovery from Admission to Discharge from Post-Acute Rehabilitation, and from Admission to 3-Month Follow-Up (in Regression Analyses)
| Discharge mFIM minus admission mFIMa |
3-month mFIM minus admission mFIMb |
|||||
|---|---|---|---|---|---|---|
| B (SE) | T | P-value | B (SE) | T | P-value | |
| Constant | −1.64 (3.30) | 0.49 | 0.620 | 2.65 (6.25) | 0.42 | 0.672 |
| MMSE, total score | 0.60 (0.11) | 5.48 | <0.001 | 0.82 (0.21) | 3.96 | 0.000 |
| Daytime percent sleep | −0.14 (0.05) | −2.87 | 0.005 | −0.31 (0.09) | −3.27 | 0.001 |
| Total hours of rehabilitation therapy received | 0.19 (0.06) | 3.36 | 0.001 | 0.007 (0.105) | 0.07 | 0.945 |
| Acute care hospital transfer during rehabilitation (yes/no) | −10.04 (2.82) | −3.57 | < 0.001 | −8.12 (5.79) | −1.40 | 0.163 |
| Admitted for orthopedic problem | 3.87 (1.35) | 2.86 | 0.005 | 9.75 (2.54) | 3.84 | 0.000 |
Daytime percent sleep based on structured behavioral observations
Adjusted R2 = 0.267, P < 0.0001; df = 223
Adjusted R2 = 0.249, P < 0.0001; df = 171
In order to determine if the relationship between daytime sleeping during rehabilitation and functional recovery persisted in long-term follow-up, we repeated regression analyses using the same independent variables to predict functional recovery from admission to 3-month, 6-month, and 9-month time points. Using only participants with available data for each follow-up time point, less daytime sleeping during rehabilitation was a significant predictor of greater functional recovery between admission and 3-month follow-up (adjusted R2 = 0.249, model P < 0.0001; n = 171 participants included in analysis). Daytime sleeping during rehabilitation was no longer a significant independent predictor in regression analyses predicting 6-month (i.e., admission mFIM minus 6-month mFIM; n = 120 available for analysis) and 9-month (admission mFIM minus 9-month mFIM) functional recovery. Because of signific9ant loss to follow-up by the 6-month follow-up (see Figure 1), we were unable to adequately test whether the relationship between daytime sleeping and functional recovery persisted at 6-month and 9-month follow-up. Based on the9 plateau of mFIM scores among available follow-up data (see Figure 2) and the high correlation between each follow-up score with the immediately prior score, we chose last observation carried forward (LOCF) as a simple imputation method to replace missing data. When the last mFIM score available was used for participants with missing data who survived 6 months (i.e., last value carried forward for survivors; n = 190 for analysis), less daytime sleeping remained a significant predictor of better functional recovery at 6-month follow-up (adjusted R2 = 0.212, model P < 0.0001; n = 190); however, there is significant concern about the validity of this imputed data.
DISCUSSION
To our knowledge, this is the first prospective descriptive study of sleep among older people undergoing inpatient post-acute rehabilitation. Similar to studies in other institutional settings,24,37 we found high rates of both objectively measured and subjectively reported sleep disturbance. According to self-report, sleep was more disturbed during rehabilitation than at home prior to the onset of the current illness. While self-report may be biased, the high degree of sleep disruption was also reflected in the actigraphy recordings. Although there has been some prior work to identify factors contributing to disturbed sleep during acute hospitalization,38,39 factors that contribute to this sleep disruption in the rehabilitation setting remain to be explored. Medical comorbidities, environmental factors (e.g., noise at night), and sleep disorders are likely important considerations.
Given the prior literature on stroke, sleep disordered breathing (SDB), and rehabilitation outcomes,12,13 we planned to assess SDB as a predictor of functional recovery among participants in this study. To enhance compliance, we chose a recording system that was minimally cumbersome. Despite these efforts, we had a very high refusal rate for overnight respiratory monitoring (53%). In participants who agreed to the monitoring, 23% of participants had evidence of mild to moderate SDB, and 8% had evidence of severe SDB; this prevalence is similar to community samples of older people. For example, in a large study of community-dwelling adults aged 45 years and older participating in the Sleep Heart Health Study (mean age = 63 years),40 24% of men and 11% of women had evidence of mild-to-moderate SDB (i.e., ≥ 15 respiratory events per hour of recording), and 9% of men and 3% of women had evidence of severe SDB (i.e., ≥ 30 respiratory events per hour of recording). Rates of SDB in our sample were similar to this community-based study of a slightly younger cohort.
Among participants with respiratory sleep recordings, there was not a significant relationship between their respiratory disturbance index and immediate functional recovery. Stroke or other neurological conditions were uncommonly the reason for admission to rehabilitation in this study. Therefore, our study may not be comparable to prior research suggesting that sleep apnea is related to functional recovery with rehabilitation after a stroke.41 However, the high rate of missing respiratory sleep recordings makes it difficult for us to adequately test for a possible relationship between SDB and functional recovery in this sample.
Perhaps the most important finding from this study is that daytime sleeping is associated with functional improvement, which is the main goal of post-acute rehabilitation. We found that more daytime sleeping during the rehabilitation stay was associated with less functional recovery between admission and discharge. This association persisted when analyses were adjusted for other significant independent predictors of functional recovery, including total hours of therapy received, mental status, acute hospital transfer during the rehabilitation stay, and reason for admission to rehabilitation. In addition, the relationship between daytime sleeping during rehabilitation and functional recovery persisted up to 3 months after admission. Unfortunately, because of significant loss to follow-up by 6 months, we were unable to adequately test whether the relationship between more daytime sleeping during the rehabilitation stay and functional recovery persisted at 6- and 9-month follow-up.
Counter to our hypotheses, nighttime sleep was not related to functional recovery. Actigraphy data revealed that most participants showed a low percentage of sleep at nighttime, perhaps making it difficult to identify an association. Alternatively, daytime sleeping may play a more direct role in attenuating functional gains due to decreased motivation and effort expended during therapy sessions. Unfortunately, quantifying these constructs is difficult and we can only speculate about these potential mediators of the relationship between daytime sleeping and functional recovery.
Our findings are particularly relevant because, while many other predictors of rehabilitation outcomes (e.g., cognitive functioning, hospital readmission) are difficult or impossible to change, sleep disturbance may represent a modifiable predictor of rehabilitation outcomes. Interventions to improve abnormal sleep/wake patterns during rehabilitation (particularly excessive daytime sleeping) may result in better functional recovery among older people. The potential impact of addressing sleep problems in this setting is not trivial. Prior work examining the clinical significance of change in mFIM scores suggests that a 1-point improvement in mFIM score is associated with 2.2 fewer min of help required from another person each day.42 This translates into 15 min of caregiving per week for every 1-point change in mFIM. In our study, the mean improvement between admission and discharge mFIM (17.9 points) translates to a reduction of 39.4 min of personal care needed each day, which represents a clinically significant amount of personal care. Further, in the context of our study, a 10% reduction in daytime sleeping would be associated with a 1-point additional improvement in mFIM during rehabilitation. This degree of change in daytime sleeping is attainable, since in our prior work with nursing home residents we were able to reduce daytime sleeping by 11% using a multifactorial nonpharmacological intervention to improve sleep patterns (which was also associated with increased participation in social and physical activities).24
There are important limitations of this study. First, as expected, there were several significant differences in descriptive characteristics between participants admitted to the 2 study sites. As expected, Site A had more women and Site B had greater diversity in race/ethnicity. However, there were many other differences in participant characteristics between the 2 sites. Although the combination of these 2 sites provided greater diversity (and perhaps more generalizability) in the final sample, caution is warranted in the interpretation of these findings. Second, as previously mentioned our ability to determine whether sleep apnea played a significant role in the observed relationship between daytime sleeping and functional recovery was hindered by a high refusal rate for respiratory monitoring. Third, we did not perform polysomnography and are unable to test for a relationship between differences in sleep architecture and functional recovery. Finally, the significant loss to follow-up over time, due to death and other loss (among these older people with significant comorbidity) made it difficult to know with certainty the duration of the relationship between daytime sleeping during the rehabilitation stay and functional recovery beyond discharge from the rehabilitation setting. Newer methods to address missing data have been described,43 but the significant loss in our sample by the 6-month follow-up makes it unlikely that our data meet the assumptions of missing data incorporated in most of these methods. We performed a simple imputation method (last value carried forward for survivors) to begin to address whether the relationship between daytime sleeping and functional recovery persists beyond 3 months, but we are not confident that our data meet the assumptions of this method.43 Further research is needed to address this issue.
This prospective study suggests that sleep disruption is common and severe among older people receiving inpatient post-acute rehabilitation after illness or injury. The relationship between more daytime sleeping and less favorable immediate and long-term functional recovery suggests sleep is an important modifiable predictor of rehabilitation outcomes for this vulnerable patient population. Interventions to improve sleep patterns (particularly to reduce daytime sleeping) in this setting are needed to further test for a causal relationship between sleep and functional recovery, and to test whether such interventions lead to clinically relevant improvements, such as increased independence and reduced caregiving needs.
ACKNOWLEDGMENTS
The authors thank the participating facilities and their staff, and the research staff who made this study possible. In particular, we wish to thank Terry Vandenberg, MA, Sergio Martinez, Maryanne Devereaux, Christina Kurtz, RN, Rebecca Saia, and Crystal Barker, RN.
Institutions at which the work was performed: Veterans Administration Greater Los Angeles Healthcare System and University of California, Los Angeles, David Geffen School of Medicine
Financial support: Veterans Administration Health Services Research and Development Service (IIR 01-053-1; AIA 03-047); Veterans Administration Greater Los Angeles Healthcare System Geriatric Research, Education and Clinical Center (GRECC); and UCLA Claude Pepper Older Americans Independence Center (AG-10415)
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. Alessi has received research support from Sepracor and has consulted for Prescription Solutions. Dr. Martin has consulted for and participated in speaking engagements for Sepracor. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Hoogerduijnles JG, Schuurmans MJ, Duijnstee MSH, deRooij SE, Grypdonck MFH. A systematic review of predictors and screening instruments to identify older hospitalized patients at risk for functional decline. J Clin Nurs. 2006;16:46–57. doi: 10.1111/j.1365-2702.2006.01579.x. [DOI] [PubMed] [Google Scholar]
- 2.Bonar SK, Tinetti ME, Speechley M, Cooney LM. Factors associated with short-versus long-term skilled nursing facility placement among community-living hip fracture patients. J Am Geriatr Soc. 1990;38:1139–44. doi: 10.1111/j.1532-5415.1990.tb01378.x. [DOI] [PubMed] [Google Scholar]
- 3.Kramer AM, Steinter JF, Schlenker RE, et al. Outcomes and costs after hip fracture and stroke. JAMA. 1997;277:396–404. [PubMed] [Google Scholar]
- 4.Johnson MF, Kramer AM, Lin MK, Kowalsky JC, Steiner JF. Outcomes of older persons receiving rehabilitation for medical and surgical conditions compared with hip fracture and stroke. J Am Geriatr Soc. 2000;48:1389–97. doi: 10.1111/j.1532-5415.2000.tb02627.x. [DOI] [PubMed] [Google Scholar]
- 5.Keith RA, Wilson DB, Gutierrez P. Acute and subacute rehabilitation for stroke: A comparison. Arch Phys Med Rehabil. 1995;76:495–500. doi: 10.1016/s0003-9993(95)80501-x. [DOI] [PubMed] [Google Scholar]
- 6.Cummings SR, Phillips SL, What ME, et al. Recovery of functional after hip fracture. The role of social supports. J Am Geriatr Soc. 1988;36:801–6. doi: 10.1111/j.1532-5415.1988.tb04263.x. [DOI] [PubMed] [Google Scholar]
- 7.Ceder L, Thorngren KG, Wallden B. Prognostic indicators and early home rehabilitation in elderly patients with hip fractures. Clin Orthop Rel Res. 1980;152:173–84. [PubMed] [Google Scholar]
- 8.Nickens HW. A review of factors affecting the occurrence and outcomes of hip fracture, with special reference to psychosocial issues. J Am Geriatr Soc. 1983;31:166–70. doi: 10.1111/j.1532-5415.1983.tb04857.x. [DOI] [PubMed] [Google Scholar]
- 9.Magaziner J, Simonsick EM, Kahsner TM, et al. Predictors of functional recovery on year following hospital discharge for hip fracture: a prospective sty. J Gerontol Med Sci. 1990;45:M101–107. doi: 10.1093/geronj/45.3.m101. [DOI] [PubMed] [Google Scholar]
- 10.Granger C, Hamilton BB, Fiedler RD. Discharge outcome after stroke rehabilitation. Stroke. 1992;23:978–82. doi: 10.1161/01.str.23.7.978. [DOI] [PubMed] [Google Scholar]
- 11.Lorish TR. Stroke rehabilitation. Clin Geriatr Med. 1993;9:705–716. [PubMed] [Google Scholar]
- 12.Kaneko Y, Hajek VE, Zivanovic V, Raboud J, Bradley TD. Relationship of sleep apnea to functional capacity and length of hospitalization following stroke. Sleep. 2003;26:293–7. doi: 10.1093/sleep/26.3.293. [DOI] [PubMed] [Google Scholar]
- 13.Wessendorf TE, Teschler H, Wang YM, Konietzko N, Thilmann AF. Sleep-disordered breathing among patients with first-ever stroke. J Neurol. 2000;247:41–7. doi: 10.1007/pl00007787. [DOI] [PubMed] [Google Scholar]
- 14.Barbar SI, Enright PL, Boyle P, et al. Sleep disturbances and their correlates in elderly Japanese American men residing in Hawaii. J Gerontol A Biol Sci Med Sci. 2000;55:M406. doi: 10.1093/gerona/55.7.m406. [DOI] [PubMed] [Google Scholar]
- 15.Kutner NG, Schechtman KB, Ory MG, Baker DI. Older adults' perceptions of their health and functioning in relation to sleep disturbance, falling, and urinary incontinence. J Am Geriatr Soc. 1994;42:757–62. doi: 10.1111/j.1532-5415.1994.tb06537.x. [DOI] [PubMed] [Google Scholar]
- 16.Schmitt FA, Phillips BA, Cook YR, Berry DTR, Wekstein DR. Self report of sleep symptoms in older adults: correlates of daytime sleepiness and health. Sleep. 1996;19:59–64. [PubMed] [Google Scholar]
- 17.Khan-Hudson A, Alessi CA. Sleep and quality of life in older people. In: Verster JC, Pandi-Perumal SR, Streiner DL, editors. Sleep and Quality of Life in Clinical Medicine. Totowa, NJ: Humana Press; 2008. pp. 131–8. [Google Scholar]
- 18.Ohayon MM, Vecchierini MF. Normative sleep data, cognitive function and daily living activities in older adults in the community. Sleep. 2005;28:981–9. [PubMed] [Google Scholar]
- 19.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18:425–32. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 20.Patel SR, Malhotra A, Gottlieb DJ, et al. Correlates of long sleep duration. Sleep. 2006;29:881–9. doi: 10.1093/sleep/29.7.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Youngstedt SD, Kripke DF. Long sleep and mortality: rationale for sleep restriction. Sleep Med Rev. 2004;8:159–74. doi: 10.1016/j.smrv.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Dew MA, Hoch CC, Buysse DJ, et al. Healthy older adults' sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom Med. 2003;65:63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- 23.Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbance and chronic disease in older adults: Results of the 2003, National Sleep Foundation Sleep in America survey. J Psychosom Res. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Alessi CA, Martin JL, Webber AP, Kim EC, Harker JO, Josephson KR. Randomized controlled trial of a nonpharmacological intervention to improve abnormal sleep/wake patterns in nursing home residents. J Am Geriatr Soc. 2005;53:619–626. doi: 10.1111/j.1532-5415.2005.53251.x. [DOI] [PubMed] [Google Scholar]
- 25.Martin JL, Webber AP, Alam T, Harker JO, Josephson KR, Alessi CA. Daytime sleeping, sleep disturbance and circadian rhythms in nursing home residents. Am J Geriatr Psychiatry. 2006;14:121–129. doi: 10.1097/01.JGP.0000192483.35555.a3. [DOI] [PubMed] [Google Scholar]
- 26.Redeker NS, Ruggiero JS, Hedges C. Sleep is related to physical function and emotional well-being after cardiac surgery. Nurs Res. 2004;53:154–62. doi: 10.1097/00006199-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Redeker NS, Mason DJ, Wykpisz E, Glica B, Miner C. First postoperative week activity patterns and recovery in women after coronary artery bypass surgery. Nurs Res. 1994;49:168–73. [PubMed] [Google Scholar]
- 28.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 29.Bliwise DL, Beview WC, Blisise NG, Edgar DM, Dement WC. Systematic 24-hour behavioral observations of sleep and wakefulness in a skilled nursing facility. Psychol Aging. 1990;5:16–24. doi: 10.1037//0882-7974.5.1.16. [DOI] [PubMed] [Google Scholar]
- 30.Martin JL, Webber AP, Josehpson KR, Harker JO, Alessi CA. Observed versus actigraphically estimated daytime sleep in older adults undergoing subacute rehabilitation in a nursing home. Sleep. 2004;27:A353. [Google Scholar]
- 31.Hamilton BB, Granger CV, Sherwin FS, et al. A uniform national data system for medical rehabilitation. In: Fuhrer JF, editor. Rehabilitation outcomes: analysis and measurement. Baltimore, MD: 1987. pp. 137–47. [Google Scholar]
- 32.Folstein MF, Folstein SF, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 33.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–72. [Google Scholar]
- 34.Ferrell BA, Stein WM, Beck JC. The Geriatric Pain Measure: validity, reliability and factor analysis. J Am Geriatr Soc. 2000;48:1669–73. doi: 10.1111/j.1532-5415.2000.tb03881.x. [DOI] [PubMed] [Google Scholar]
- 35.Miller MD, Paradis CF, Houck PR, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41:237–48. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 36.Parmelee PA, Thuras PD, Katz IR, Lawton MP. Validation of the Cumulative Illness Rating Scale in a geriatric residential population. J Am Geriatr Soc. 1995;43:130–7. doi: 10.1111/j.1532-5415.1995.tb06377.x. [DOI] [PubMed] [Google Scholar]
- 37.Ancoli-Israel S, Klauber MR, Jones DW, et al. Variations in circadian rhythms of activity, sleep and light exposure related to dementia in nursing home patients. Sleep. 1997;20:18–23. [PubMed] [Google Scholar]
- 38.Griffiths MF, Peerson A. Risk factors for chronic insomnia following hospitalization. J Adv Nurs. 2005;49:245–53. doi: 10.1111/j.1365-2648.2004.03283.x. [DOI] [PubMed] [Google Scholar]
- 39.Frighetto L, Marra C, Bandall S, et al. An assessment of quality of sleep and the use of drugs with sedating properties in hospitalized adult patients. Health Qual Life Outcomes. 2004;17:17. doi: 10.1186/1477-7525-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baldwin CM, Griffith KA, Nieto FJ, O'Connor GT, Walsleben JA, Redline S. The association of sleep-disordered breathing and sleep symptoms with quality of life in the Sleep Heart Health Study. Sleep. 2001;24:96–105. doi: 10.1093/sleep/24.1.96. [DOI] [PubMed] [Google Scholar]
- 41.Good DC, Henkle JQ, Gelber D, Welsh J, Verhulst S. Sleep-disordered breathing and poor functional outcome after stroke. Stroke. 1996;27:252–9. doi: 10.1161/01.str.27.2.252. [DOI] [PubMed] [Google Scholar]
- 42.Granger CV, Cotter AC, Hamilton BB, Fiedler RC. Functional assessment scales: a study of persons after stroke. Arch Physical Med Rehab. 1993;74:133–8. [PubMed] [Google Scholar]
- 43.Enders CK. A primer on the use of modern missing-data methods in psychosomatic medicine research. Psychosom Med. 2006;68:427–36. doi: 10.1097/01.psy.0000221275.75056.d8. [DOI] [PubMed] [Google Scholar]