Abstract
A previously described microbroth kinetic system (J. Meletiadis, J. F. Meis, J. W. Mouton, and P. E. Verweij, J. Clin. Microbiol. 39:478-484, 2001) based on continuous monitoring of changes in the optical density of fungal growth was used to describe turbidimetric growth curves of different filamentous fungi in the presence of increasing concentrations of antifungal drugs. Therefore, 24 clinical mold isolates, including Rhizopus oryzae, Aspergillus fumigatus, Aspergillus flavus, and Scedosporium prolificans, were tested against itraconazole, terbinafine, and amphotericin B according to NCCLS guidelines. Among various parameters of the growth curves, the duration of the lag phase was strongly affected by the presence of antifungal drugs. Exposure to increasing drug concentrations resulted in prolonged lag phases of the turbidimetric growth curves. The lag phases of the growth curves at drug concentrations which resulted in more than 50% growth (for itraconazole and terbinafine) and more than 75% growth (for amphotericin B) after 24 h of incubation for R. oryzae, 48 h for Aspergillus spp., and 72 h for S. prolificans were 4 h longer than the lag phases of the growth curves at the corresponding drug-free growth controls which varied from 4.4 h for R. oryzae, 6.5 h for A. flavus, 7.9 h for A. fumigatus, and 11.6 h for S. prolificans. The duration of the lag phases showed small experimental and interstrain variability, with differences of less than 2 h in most of the cases. Using this system, itraconazole and terbinafine resistance (presence of >50% growth) as well as amphotericin B resistance (presence of >75% growth) was determined within incubation periods of 5.0 to 7.7 h for R. oryzae (for amphotericin B resistance incubation for up to 12 h was required), 8.8 to 11.4 h for A. fumigatus, 6.7 to 8.5 h for A. flavus, and 13 to 15.6 h for S. prolificans while awaiting formal MIC determination by the NCCLS reference method.
Filamentous fungi can cause invasive infections, which are associated with high morbidity and mortality, particularly in immunocompromised patients, despite antifungal chemotherapy (7, 19, 27, 30). Although many factors can influence the clinical outcome of an infection (36), effective antifungal treatment initiated at an early stage of infection may contribute substantially to successful outcome (2, 9). Since different strains of filamentous fungi are variably susceptible to drugs even within the same species (5, 11, 21) and certain strains can develop resistance to antifungal drugs (4, 10), in vitro susceptibility testing has become increasingly important. Furthermore, the spectrum of molds that cause invasive fungal infections has broadened with genera and species of molds causing infection of which the susceptibility to antifungal agents is unknown. However, even though low MICs do not guarantee successful outcomes and high MICs do not guarantee clinical failure, the prediction of resistance may be far more reliable clinically than the prediction of susceptibility (15, 33). Therefore, it was suggested that in vitro antifungal susceptibility testing methods should be able to detect and monitor the development of antifungal resistance rather than determine susceptibility (16). To be of value in clinical decision making, the results of in vitro susceptibility testing need to be available rapidly, allowing the treating physician to switch to a more potent antifungal drug before the infection has progressed due to inefficiency of the empirical antifungal regimen. Finally, the methods for in vitro susceptibility testing should ideally be reproducible, comprehensive, objective, easy, and cost effective (16, 32).
Considerable progress has been made in the in vitro antifungal susceptibility testing of conidium-forming fungi with the publication of the NCCLS standard M-38A and the achievement of high levels of reproducibility (29). However, the latter methodology is based on visual MIC reading and requires long incubation periods ranging from 24 h for fast-growing fungi, including Rhizopus species, and up to 72 h for slow-growing fungi, such as Scedosporium species, before results are obtained. More objective methods (32) have been developed based on spectrophotometric readings (6, 24), colorimetric assays (20, 23, 25), glucose consumption (13), glucan synthase inhibition (17), or quantification of fungal products (37), but with these methods, the time required to generate the results was not reduced. Although rapid susceptibility testing methods have been developed based on radiometric (26) and flow cytometric (1) assays generating results within 3 h, their use in routine clinical laboratories may be restricted due to the expensive equipment required (flow cytometer) or to the use of potentially hazardous substances. Furthermore, these methods may not be applicable for different mold species and antifungal drugs.
Spectrophotometric assays based on turbidimetric measurements, where fungal biomass is correlated with optical density, were applied to in vitro susceptibility testing of filamentous fungi, resulting in reproducible and objective results showing excellent agreement with the NCCLS standard M-38P (6, 24). Turbidimetry was also used in a recently developed microbroth kinetic system to monitor fungal growth over time (22). With this system, detailed and reproducible growth curves for different fungal species can be obtained and different growth stages during the fungal growth can be distinguished. The system was also used to study post-antifungal treatment effects in molds by comparing changes in the growth curves following drug exposure (35). Monitoring of fungal growth in the microbroth kinetic system showed a delay before the optical density increased due to fungal growth. This delay, referred to as the lag phase, was shown to be due to germination of the spores and initial hyphal formation (22). The lag phases were species dependent, with small interstrain variation, and highly reproducible.
The microbroth kinetic system was used in the present study to describe the growth curves of different species in the presence of various antifungal drugs at different concentrations. For this purpose, drugs belonging to different classes of antifungal agents, such as amphotericin B (AB), itraconazole (IT), and terbinafine (TB), and filamentous fungi characterized by different growth rates, such as Rhizopus spp., Aspergillus spp., and Scedosporium spp., were included. Based on the effects of drug concentrations, particularly on the duration of the lag phase (the time until the first significant increase in optical density) of each strain, a microdilution broth susceptibility testing method based on growth curves was developed, enabling the early determination of antifungal drug resistance.
MATERIALS AND METHODS
Isolates.
Twenty-four clinical isolates of filamentous fungi belonging to three different genera, which are characterized by different growth rates, were tested in this study. These included five strains of each of the following species: Rhizopus oryzae (AZN23, AZN190, AZN185, AZN5005, AZN5816, and AZNA551), Aspergillus fumigatus (AZN5241, AZN7151, AZN7275, AZN8248, AZN5161, and AZN5242), Aspergillus flavus (AZN2865, AZN4094, AZN137, AZN510, AZN2865, and AZN4132), and Scedosporium prolificans (AZN7890, AZN7921, AZN7886, AZN7902, AZN7903, and AZN7930). Candida parapsilosis (ATCC 22019) and Candida krusei (ATCC 6258) were used for quality control.
Isolates had been frozen in 50% glycerol at −70°C and were revived by subculture on Sabouraud glucose agar (SGA) tubes supplemented with 0.5% chloramphenicol and incubated at 29°C for 7 days. The isolates were subcultured again on SGA tubes and incubated for another 5 to 7 days at 37°C.
Medium.
RPMI 1640 medium (with l-glutamine but without bicarbonate) (GIBCO BRL, Life Technologies, Woerden, The Netherlands) buffered to pH 7.0 with 0.165 M 3-N-morpholinopropanesulfonic acid (MOPS) (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) was used as the assay medium.
Inoculum.
Spores were collected with a cotton swab and suspended in sterile saline containing 0.1% Tween 80. After heavy particles were allowed to settle, conidia were counted with a counting chamber and inocula were adjusted by dilution to an initial inoculum of 4 × 104 conidia/ml, which, after the inoculation of the wells, resulted in a final inoculum of 2 × 104 conidia/ml. The inoculum size was confirmed by plating serial dilutions on SGA plates, with initial inocula ranging from 1.1 × 104 to 6.5 × 104 CFU/ml (average ± 95% confidence interval [95% CI], 3 × 104 ± 1.1 × 104 CFU/ml).
Antifungal drugs.
Three antifungal drugs belonging to different classes of antifungal agents were tested. These included the azole IT (Janssen-Cilag, Beerse, Belgium), the allylamine TB (Novartis, Basel, Switzerland), and the polyene AB (Bristol-Myers Squibb, Woerden, The Netherlands). All drugs were dissolved in dimethyl sulfoxide at final concentrations of 3,200 mg/liter for IT and TB and 1,600 mg/liter for AB.
Antifungal susceptibility testing method.
A broth microdilution method was performed according to NCCLS guidelines (standard M38-P) in 96-well flat-bottom microtiter plates. Briefly, twofold serial dilutions of the drugs were performed in 100 μl of the medium to obtain two times the final drug concentrations, which ranged from 32 to 0.015 mg/liter for IT and TB and from 16 to 0.007 mg/liter for AB. A drug-free well containing 1% dimethyl sulfoxide in the medium served as the growth control. Each well was inoculated with 100 μl of the inoculum.
Growth curves.
After inoculation, the microtiter plates were agitated for 15 s and incubated at 37°C inside a plate reader (Rosys Anthos ht3; Anthos Labtec Instruments GmbH, Salzburg, Austria) for 24 h in ambient conditions. The optical density at 405 nm (OD405) was automatically recorded for each well every 10 min without shaking. The changes in OD over time were used to generate growth curves at each drug concentration and for the drug-free growth control.
Quantification of fungal growth.
After the first 24 h of incubation inside the plate reader, incubation of the microtiter plates was continued under the same conditions in a conventional incubator. The OD405 of each well after 24 h (R. oryzae), 48 h (A. fumigatus and A. flavus), and 72 h (S. prolificans) was measured spectrophotometrically. After the background ODs (ODs of microorganism-free wells under the same conditions as the inoculated wells) were subtracted, the percentage of growth at each drug concentration was calculated with the following equation: % growth = (OD405 of wells containing the drug/OD405 of the drug-free well) × 100.
MIC determination.
The MICs were determined visually and spectrophotometrically after the above mentioned incubation periods for each species according to the NCCLS guidelines. For AB, IT, and TB, the MIC-0 was determined as the lowest drug concentration showing absence of visual growth or 90% growth inhibition compared with the growth in the drug-free well. In addition, for IT and TB, the MIC-2 was determined as the lowest drug concentration showing prominent reduction in growth or 50% growth inhibition compared to the growth in the drug-free well.
Pilot study.
In preliminary studies, one strain each of R. oryzae, A. fumigatus, and S. prolificans was tested against IT, TB, and AB as described above. Using the MicroWin, version 3, software (Mikrotek Laborsysteme GmbH, Overath, Germany), various parameters of the growth curves were calculated, such as the highest OD (ODmax), the changes in OD (ΔOD) per minute (ΔOD/min, where ΔOD = ODfinal − ODinitial in an interval of 10 min), the average ΔOD per minute (mean ΔOD/min), the slope (S) of the curve calculated with linear regression analysis, the maximal slope (Smax, which was the largest increase rate in OD repeated for 25 consecutive time points), the duration of lag phases (Tlag), and the area under the growth curve (AUC). All of these parameters were correlated by using Spearman rank correlation analysis with the percentages of growth in each well estimated as described above to find which of these parameters could best predict the percentage of growth. Since the highest correlation was found for the Tlag, this parameter was used for further analysis with the collection of the 24 isolates.
Furthermore, replicate experiments were performed in order to study the reproducibility of the Tlag of the growth curves. In addition, the Tlag of the growth curves of the drug-free controls was determined by using the following 10-fold serially diluted inocula ranging from 103 to 106 CFU/ml.
Tlag determination.
For each growth curve, the duration of the lag phase, Tlag, was determined based on the change in the average OD (ΔMOD). The ΔMOD was calculated for each time point t of the growth curve with the following equation:
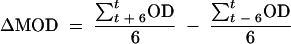 |
For each time point t, the average OD of the next 6 measurements until the t + 6 time point is subtracted from the average OD of the previous 6 measurements from the t − 6 time point. The first significant increase in OD, which indicates the end of the lag phase, was determined as the time point t when ΔMOD is equal to or greater than 0.001 for the next 6 consecutive time points. The Tlags were determined for each drug concentration and for the growth control. For the drug concentration at which fungal growth was absent, the Tlag was considered to be >24 h.
Tlag endpoints.
In accordance with the MIC endpoints, the following Tlag endpoints were determined: Tlag10, Tlag50, and Tlag75, which were defined as the Tlags of the growth curve at the highest drug concentration that showed more than 10, 50, and 75% growth compared to that of the growth controls after 24 h (for R. oryzae), 48 h (for Aspergillus spp.), and 72 h (for S. prolificans) of incubation. The Tlag50 and Tlag10 were actually the Tlags of the growth curves at the next lower doubling drug concentration of the MIC-2 and MIC-0, respectively. The Tlag endpoints were determined for each strain and drug, and the average Tlag50, Tlag75, and Tlag10 and their 95% CIs among the strains of each species were calculated for TB, IT, and AB. Furthermore, the differences between the Tlag endpoints and the Tlaggc (duration of the lag phase growth curve of the growth control) were calculated for each strain and drug, and the average and 95% CI among the strains were reported for each drug and species.
RESULTS
Growth curves.
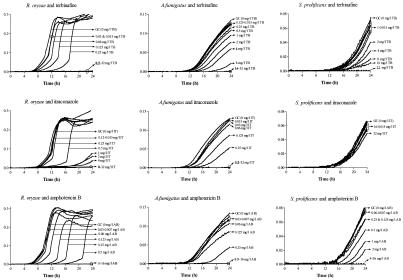
Growth curves that differed in shape and growth rate were obtained for each species and drug concentration. A representative example is shown in Fig. 1, where the growth curves of strains of R. oryzae, A. fumigatus, and S. prolificans in the presence of increasing concentrations of TB, IT, and AB are presented. Although each growth phase is affected by each drug in different ways, the most obvious change induced by exposure of the mold to the antifungal agent is that with increasing drug concentration the growth curve is shifted to the right. The latter is due to longer lag phases.
FIG. 1.
Growth curves of an R. oryzae, A. fumigatus, and S. prolificans strains in the presence of twofold-increasing concentrations of TB, IT, and AB, including those in the drug-free growth controls (GC) indicated by arrows. ODs were normalized for each growth curve by subtracting the background OD at each time point (y axes show optical densities at 450 nm). The bold and underlined drug concentrations correspond to MIC-0 and MIC-2, respectively. ICZ, itraconazole.
Microscopic examination of the wells revealed that the delay of the spectrophotometer in detecting the first significant increase in OD (i.e., longer lag phases) at sub-MIC drug concentrations was associated with lower germination rates of conidia and elongation rates of the formed hyphae than those of the growth control. These effects were more pronounced with TB and IT than with AB. For AB, the germination was delayed but the elongation rates of the formed hyphae were similar to that of the growth control.
Pilot study.
The Spearman rank correlation coefficients between the various parameters calculated from the growth curves of R. oryzae, A. fumigatus, and S. prolificans and the percentage of growth after 24, 48, and 72 h of incubation, respectively, are shown in Table 1. A statistically significant correlation was found for all parameters (rs> 0.77, P < 0.05), with the Tlag showing the highest correlation (0.86 to 0.99) for all drug-species combinations tested. The Tlag was highly correlated with the percentage of growth (rs> 0.87, P < 0.001) for all strains and drugs tested in the present study (data not shown). With increasing concentrations of antifungal drugs, most of the growth curve parameters decreased, with the exception of the Tlag. For the latter, the higher the drug concentration, the longer the Tlag (Fig. 1), which is also indicated by the negative correlation coefficients in Table 1.
TABLE 1.
Correlation coefficients obtained by Spearman rank correlation analysis between various parameters of the growth curves and the percentages of growth estimated for each drug concentration after 24 h (R. oryzae), 48 h (A. fumigatus), and 72 h (S. prolificans) of incubationa
| Species | Drug | Result for growth curve parameterc
|
||||||
|---|---|---|---|---|---|---|---|---|
| Tlag (h) | S (OD/ min) | Smax (ODX/ min) | AUC (ODX/ min) | ΔOD/ min | Mean ΔOD/ min | ODmax | ||
| A. fumigatus | TB | −0.99 | 0.96 | 0.92 | 0.82 | 0.97 | 0.97 | 0.90 |
| IT | −0.93 | 0.82 | 0.82 | 0.72 | 0.82 | 0.82 | 0.82 | |
| AB | −0.91 | 0.87 | 0.87 | 0.87 | 0.87 | 0.87 | 0.87 | |
| R. oryzae | TB | NCa | NC | NC | NC | NC | NC | NC |
| IT | −0.89 | 0.86 | 0.87 | 0.87 | 0.77 | 0.88 | 0.82 | |
| AB | −0.98 | 0.86 | 0.87 | 0.86 | 0.84 | 0.87 | 0.83 | |
| S. prolificans | TB | −0.95 | 0.94 | 0.92 | 0.91 | 0.94 | 0.94 | 0.94 |
| IT | NC | NC | NC | NC | NC | NC | NC | |
| AB | −0.86 | 0.85 | 0.86 | 0.81 | 0.86 | 0.86 | 0.86 | |
NC, not calculated.
All coefficients were statistically significant (P < 0.01).
S, slope of the growth curve; Smax, maximal slope; AUC, area under the concentration-time curve.
The Tlags were reproducible, since the within-day differences were less than 1 h and the between-day differences, although larger, were less than 2 h for most of the strains. This variability had little effect on Tlag endpoints, particularly on Tlag75 and Tlag50. For some strains where larger differences were found, these were mainly dependent on the size and quality of the inoculum, with lower inocula and those obtained from old cultures resulting in larger Tlags. The same effect was also found when Tlags were determined with increasing inocula. Inocula ranging from 106 to 103 CFU/ml resulted in Tlags ranging from 2 to 10 h for R. oryzae, from 4 to 14 h for A. fumigatus, and from 6 to 23 h for S. prolificans, with an increase in the Tlag rate of 2 to 3 h for R. oryzae, 3 to 4 h for A. fumigatus, and 4 to 5 h for S. prolificans for every 10-fold decrease in inoculum size.
MICs.
The MICs of the drugs tested in this study are shown for each species in Table 2. The MICs covered the whole range of drug concentrations tested, with TB showing the highest growth inhibitory activity against A. flavus (median MIC, 0.06 mg/liter) and with very low growth inhibitory activities for all tested drugs against S. prolificans (median MICs, >16 mg/liter). Low growth inhibitory activities were also found for IT against two A. fumigatus strains which were previously proven to be resistant in vivo (8).
TABLE 2.
MICs and Tlag endpointsa of each drug for each species
| Species (no. of strains) | Drug | Median MIC-0 (mg/liters) (range) | Median MIC-2 (mg/liters) (range) | Tlag10 | Tlag50 | Tlag75 | Tlaggcc | Tlag10 − Tlaggc | Tlag50 − Tlaggc | Tlag75 − Tlaggc |
|---|---|---|---|---|---|---|---|---|---|---|
| R. oryzae (6) | TB | 0.5 (0.25-1) | 0.5 (0.25-1) | 11.1 ± 3.8 | 7.7 ± 2.0 | 7.7 ± 2.2 | 6.7 ± 3.4 | 3.4 ± 2.3 | 3.4 ± 2.3 | |
| IT | 32 (2->32) | 3 (1->32) | ND | 5.3 ± 0.8 | 5.0 ± 1.0 | 4.4 ± 0.9 | ND | 0.9 ± 0.4 | 0.6 ± 0.3 | |
| AB | 1 (0.5-1) | NDb | 17.4 ± 1.6 | 15.1 ± 1.6 | 12.0 ± 2.0 | 13.0 ± 1.3 | 10.7 ± 1.8 | 7.5 ± 2.6 | ||
| A. fumigatus (6) | TB | 16 (8->32) | 3 (1-16) | 21.9 ± 2.6 | 11.0 ± 1.8 | 8.8 ± 0.8 | 13.9 ± 3.4 | 3.1 ± 2.4 | 0.9 ± 1.1 | |
| IT | 1 (0.5->32) | 0.5 (0.25->32) | 13.5 ± 3.6 | 11.4 ± 1.8 | 9.4 ± 0.6 | 7.9 ± 1.1 | 5.6 ± 3.5 | 3.5 ± 1.2 | 1.5 ± 1.0 | |
| AB | 1 (0.5-4) | ND | 21.8 ± 4.1 | 14.9 ± 3.2 | 10.5 ± 1.9 | 13.9 ± 4.6 | 7.0 ± 2.9 | 2.5 ± 2.2 | ||
| A. flavus (6) | TB | 0.125 (0.125-0.5) | 0.06 (0.03-0.125) | 14.3 ± 3.0 | 8.4 ± 1.2 | 6.6 ± 0.7 | 7.9 ± 3.2 | 2.0 ± 0.9 | 0.2 ± 0.4 | |
| IT | 0.5 (0.5) | 0.5 (0.25-0.5) | 8.6 ± 1.1 | 8.2 ± 0.4 | 6.7 ± 0.5 | 6.5 ± 0.8 | 2.1 ± 0.7 | 1.8 ± 0.7 | 0.3 ± 0.4 | |
| AB | 2 (1-4) | ND | 21.0 ± 3.0 | 15.9 ± 4.6 | 8.5 ± 1.0 | 14.5 ± 3.2 | 9.4 ± 4.1 | 2.0 ± 0.8 | ||
| S. prolificans (6) | TB | >32 | 32 (32->32) | ND | ND | 15.6 ± 1.5 | ND | ND | 4.0 ± 1.8 | |
| IT | >32 | >32 | ND | ND | 13 ± 1.7 | 11.6 ± 1.5 | ND | ND | 1.4 ± 1.0 | |
| AB | >16 (4->16) | ND | 19.1 ± 2.9 | 15.2 ± 2.4 | ND | 7.5 ± 3.2 | 3.6 ± 2.5 |
Tlag10, Tlag50, and Tlag75 are the mean Tlag values of the growth curves at the highest drug concentration showing more than 10, 50, and 75% growth, respectively, compared to the growth control after 24 h (R. oryzae), 48 h (Aspergillus spp.), and 72 h (S. polificans) of incubation. Tlag values are shown ±95% CIs.
ND, not determined.
Values correspond with fungal species, regardless of drug used.
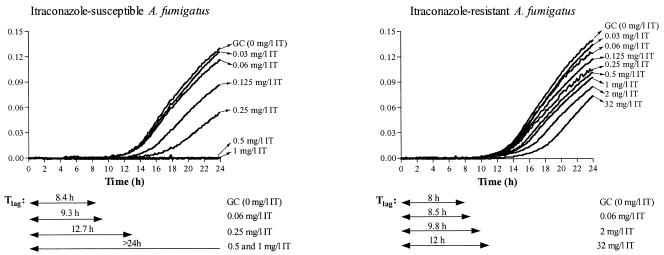
In Fig. 2, the growth curves of an IT-susceptible and -resistant A. fumigatus strain at different drug concentrations are shown. Note that, for the IT-resistant strain, the Tlag was shorter than 12 h for all drug concentrations, whereas for the susceptible strain, the same holds only for sub-MIC concentrations.
FIG. 2.
Growth curves of IT-susceptible and -resistant A. fumigatus strains in the presence of increasing concentrations of IT. ODs were normalized for each growth curve by subtracting the background OD at each time point. The Tlags for the growth control (GC) and some drug concentrations are presented. The MIC-2 after 48 h of incubation was 0.25 mg/liter for the susceptible strain and >32 mg/liter for the resistant strain. ICZ, itraconazole.
Tlags.
The Tlag endpoints are shown in Table 2 for each drug and species. Overall, very little variation among the strains was found for all endpoints, independent of the corresponding MIC, since the 95% CIs were always lower than 4.6 h. The Tlaggcs were different for each species, with R. oryzae showing the shortest (4.4 h) and S. prolificans showing the longest (11.6 h) Tlaggc. The Tlaggcs of the A. flavus strains (6.5 h) were closer to those of A. fumigatus (7.9 h). Overall, shorter Tlags were found for higher levels of growth, since Tlag10 > Tlag50 > Tlag75. The Tlag10 and Tlag50 of all drugs for S. prolificans and the Tlag10 of IT for R. oryzae could not be determined, since the drug concentrations showing 10 and 50% growth were higher than the highest tested concentration. When the Tlags were normalized by calculating the differences (Tlag10,50,75 − Tlaggc) for each drug, the Tlag75 − Tlaggc was less than 4 h in all cases except for R. oryzae when tested with AB (7.5 h). Less than 4 h was also the difference of Tlag50 − Tlaggc for all drugs except for AB. For AB, however, the 4-h difference was found for 90% of growth (data not shown).
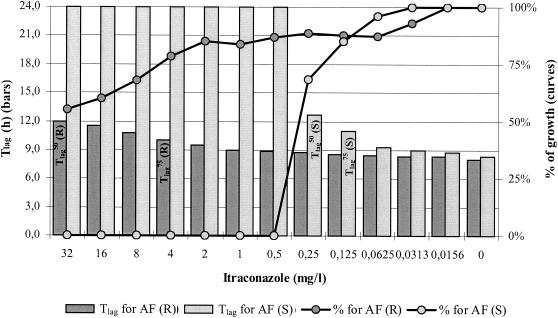
The relation of Tlags with the percentage of growth after 48 h of incubation is depicted in Fig. 3, where the concentration-effect curves for an IT-susceptible and an IT-resistant A. fumigatus strain were plotted together with the Tlag at each drug concentration.
FIG. 3.
Graphical representation of the correlation between Tlag (bars) and percentages of growth (curves) after 48 h of incubation for IT-susceptible [AF (S)] (light gray) and -resistant [AF (R)] (dark gray) A. fumigatus strains for which the MIC-2s were 0.5 and >32 mg/liter, respectively. Note that while Tlags increased with increasing drug concentrations, the percentages of growth decreased. For the resistant strain, the Tlags at any drug concentration did not exceed 12 h and were within a 4-h difference compared with the Tlag of growth control (8 h). The Tlag endpoints are also shown inside the corresponding bars for the IT-susceptible [AF (S)] and -resistant [AF (R)] A. fumigatus strains.
DISCUSSION
The early detection of TB-, IT-, AB-resistant strains of different filamentous fungi was achieved by using a microbroth kinetic system which is based on continuous monitoring of the OD of fungal growth over time. In vitro detection of resistance to IT and TB (presence of >50% growth) and to AB (presence of >75% growth), including statistical significance levels, was achieved within 4 h after growth in the drug-free control was detected, i.e., within incubation periods of 5.0 to 7.7 h for R. oryzae (for AB resistance, incubation for up to 12 h was required), 8.8 to 11.4 h for A. fumigatus, 6.7 to 8.5 h for A. flavus, and 13 to 15.6 h for S. prolificans, while awaiting formal MIC determination by the NCCLS reference method.
Continuous monitoring of the changes in the OD of fungal growth over time was previously used to analyze the growth characteristics of different filamentous fungi with high reproducibility and accuracy (22). Among the different growth phases of the turbidimetric growth curves determined with this system, the lag phase (the period until the first detectable change in OD) showed high reproducibility and small interstrain variation (22, 35). This was also confirmed in the present study, since the differences in duration of the lag phases (Tlag) of the drug-free wells among the strains of each species were less than 3 h. Since the Tlags of the different species were statistically significantly different (P < 0.05), except between A. fumigatus and A. flavus, the Tlag may be genus dependent. However, larger collections of filamentous fungi, including more different species, may be required in order to check whether the small overlapping between the Tlags of A. fumigatus and A. flavus is significant and if the Tlag could be used for genus or even species identification. Although the Tlag was very reproducible, it was strongly dependent on the size and the quality of the inoculum, and therefore, these factors should be always checked and kept constant.
It was found previously that the end of the lag phase or first increase in OD occurred when the germ tubes of an inoculum of 2 × 104 CFU/ml in 200 μl, i.e., 4,000 spores, reached the length of 70 μm (22). Using increasing inocula in the present study, shorter lag phases of the turbidimetric growth curves were found. Assuming that germination and elongation rates are not affected by population factors and that they are intrinsic characteristics of inocula, independent of inoculum size, the longer lag phases found with smaller inocula are not due to lower germination or elongation rates but are likely due to the fact that the critical turbidity (similar to that of 4,000 70-μm-long germinated spores) is reached later by smaller inocula than by larger ones. Therefore, at the end of the lag phases, the germ tubes of spores in small inocula should be longer than the corresponding germ tubes of spores in larger inocula given that the OD is a function of the number of spores or hyphae as well as of their size (12, 31).
When curves of growth in the presence of increasing concentrations of antifungal drugs were studied, various parameters of the growth curves were affected compared with those of the drug-free controls, including the Tlag, as is illustrated in Fig. 1. The higher the drug concentrations, the longer the Tlag of the growth curves (Fig. 2). This effect was previously observed for Candida spp. when growth curves based on capacitance were obtained (3). The presence of drugs decreased the germination and elongation rates of spores and germinated spores, respectively, and therefore, the critical turbidity is reached later, resulting in longer lag phases. Based on this, the critical OD, which is detected by the spectrophotometer, can be lowered by using wells with smaller surfaces, e.g., the 144-well microtiter plates, thus generating the same NCCLS MIC results even faster, since earlier detection of fungal growth might be achieved. The same could be achieved by increasing the sensitivity of the system by using colorimetric or fluorescence assays instead of turbidimetry.
Given that, for a certain strain and drug, the Tlag is dependent on the drug concentration and is very reproducible, the determination of the Tlag could be used to check whether the final drug concentrations inside the wells are correct. For instance, in quality control experiments, the quality of microtiter plates is checked by determining the MIC for a quality control strain and then comparing it with the acceptable reference MIC ranges, a procedure which gives information for only a small range of drug concentrations. Using the present microbroth kinetic system, each drug concentration could be checked by comparing the Tlag at a certain drug concentration with the acceptable reference Tlag ranges for that specific concentration established after numerous experiments with quality control strains.
There was a strong, statistically significant correlation between the Tlags of the growth curves at a certain drug concentration with the percentage of growth at that concentration after 72 h of incubation. Although in the present study the recommended incubation periods for each species were used (NCCLS guidelines), the Tlag could predict the percentage of growth after any amount of time of incubation, since the smaller the Tlag, the higher the percentage of growth. Based on this finding, resistant strains for a specific drug concentration can be detected earlier than the recommended incubation periods for each species, since the Tlags of the latter would be smaller than the Tlags of the susceptible strains, as is illustrated in Fig. 3.
The Tlags of the growth curves at drug concentrations which showed similar levels of growth after a certain period of time of incubation were similar among the strains of each species, and the differences were less than 4.6 h. Since, for a certain inoculum, Tlags are dependent both on germination and elongation rates of germ tubes and given that different Tlags were obtained for each species and drug, Tlags might describe the effect of antifungal drugs on germination and elongation of germ tubes of each species. This is supported by the fact that the Tlags for IT and TB, two fungistatic drugs acting in similar manners, were similar to each other but different from the Tlags for AB, which has a different mechanism of action, in most of the cases. However, more studies are required to investigate in details the effect of antifungal drugs on the germination of spores and the elongation of hyphae of each species.
An interesting thing was found when the Tlags of each species were normalized by subtracting from them the Tlags of the corresponding growth controls. For all strains and species, these differences were less than 4 h for all drug concentrations that showed more than 50% growth for IT and TB and more than 75% growth for AB after the recommended incubation periods. In other words, within 4 h after the growth was detected at the drug-free control, we were able to infer conclusions about the resistance of the strain tested for a specific drug concentration. The larger differences found overall with AB could be due to the delayed detection of fungal growth by the spectrophotometer. Given that absorbance is a function of both the number and size of germinated spores and that at concentrations near the MIC only a few conidia escape the killing of AB, longer germ tubes (i.e., prolonged incubation) are required to reach the critical OD. Since these few germ tubes elongate at the same rate as in the growth control, higher levels of growth result after the recommended incubation periods.
We could also determine the susceptibility of strains at the level of 50% growth by using one concentration rather than serial dilutions of the drug, since at sub-MIC concentrations, the Tlags will be within 4 h of the Tlags of the growth control while at supra-MIC concentrations, the Tlags would be longer. The same holds also for AB, despite the fact that higher levels of growth (i.e., 75% rather than 50% growth) resulted with an additional 4 h of incubation than for the Tlags at the growth control. Even 25% growth inhibition at a certain drug concentration would result in the absence of growth at the next highest doubling concentration, given the steepness that characterized the dose-response curves of AB (23). Furthermore, because acquired resistance to AB is infrequent in filamentous fungi (28), the use of 75% growth instead of 50% would not affect the detection of AB-resistant strains.
However, a problem encountered when determining the susceptibility of strains with the model described in the present study could be a phenomenon related to different patterns of resistance, which were described previously for bacteria (34), such as delayed resistance or heteroresistance. Such phenomena, which were also suggested for filamentous fungi (14), would require extended incubation periods before conclusions about the susceptibility of the strains can be inferred. Delayed regrowth of Aspergillus spp. after azole treatment was previously reported (18). Elaboration of the MICs after prolonged incubation periods might be another problem encountered when determining the susceptibility with this model. This is the case when R. oryzae tested against IT, the MICs of which increased dramatically at 48 h of incubation, as was found previously (5) and is presented in Fig. 1, with the two distinct groups of growth curves at concentrations lower and higher than 0.5 mg/liter. This problem, however, can be avoided by standardizing the incubation periods and thereafter adjusting the present model based on the results after these periods. Nevertheless, many commercial systems for identification and in vitro susceptibility testing of bacteria and yeasts rely on the principles of continuous turbidity measurements.
Although the use of the above-mentioned microbroth kinetic system in its present form is too cumbersome to be widely applicable in the clinical microbiology laboratory, the same concept is already used in automated systems for in vitro susceptibility testing of bacteria and yeasts. Additional studies are required to simplify the method, including reducing the concentration test range of antifungal agents. When breakpoints for different antifungal agents and pathogenic molds are selected, indicator drug concentrations can be selected.
In conclusion, turbidimetry is a rapid, nondestructive and inexpensive method for monitoring the growth of filamentous fungi. Besides the early detection of resistant strains, it enables the modeling of fungal growth under different in vitro conditions. The use of turbidimetric growth curves may facilitate the study of antifungal effects of drugs administered alone or in combination and simultaneously or sequentially at different phases of fungal growth, introducing a new field of fungal dynamics for filamentous fungi.
REFERENCES
- 1.Balajee, S. A., and K. A. Marr. 2002. Conidial viability assay for rapid susceptibility testing of Aspergillus species. J. Clin. Microbiol. 40:2741-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caillot, D., L. Mannone, B. Cuisenier, and J. F. Couaillier. 2001. Role of early diagnosis and aggressive surgery in the management of invasive pulmonary aspergillosis in neutropenic patients. Clin. Microbiol. Infect. 7:54-61. [DOI] [PubMed] [Google Scholar]
- 3.Chang, H. C., J. J. Chang, A. H. Huang, and T. C. Chang. 2000. Evaluation of a capacitance method for direct antifungal susceptibility testing of yeasts in positive blood cultures. J. Clin. Microbiol. 38:971-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dannaoui, E., E. Borel, M. F. Monier, M. A. Piens, S. Picot, and F. Persat. 2001. Acquired itraconazole resistance in Aspergillus fumigatus. J. Antimicrob. Chemother. 47:333-340. [DOI] [PubMed] [Google Scholar]
- 5.Dannaoui, E., J. Meletiadis, J. W. Mouton, J. F. Meis, and P. E. Verweij. 2003. In vitro susceptibilities of zygomycetes to conventional and new antifungals. J. Antimicrob. Chemother. 51:45-52. [DOI] [PubMed] [Google Scholar]
- 6.Dannaoui, E., F. Persat, M. F. Monier, E. Borel, M. A. Piens, and S. Picot. 1999. Use of spectrophotometric reading for in vitro antifungal susceptibility testing of Aspergillus spp. Can. J. Microbiol. 45:871-874. [PubMed] [Google Scholar]
- 7.Denning, D. W. 1996. Therapeutic outcome in invasive aspergillosis. Clin. Infect. Dis. 23:608-615. [DOI] [PubMed] [Google Scholar]
- 8.Denning, D. W., S. A. Radford, K. L. Oakley, L. Hall, E. M. Johnson, and D. W. Warnock. 1997. Correlation between in-vitro susceptibility testing to itraconazole and in-vivo outcome of Aspergillus fumigatus infection. J. Antimicrob. Chemother. 40:401-414. [DOI] [PubMed] [Google Scholar]
- 9.Denning, D. W., and D. A. Stevens. 1990. Antifungal and surgical treatment of invasive aspergillosis: review of 2,121 published cases. Rev. Infect. Dis. 12:1147-1201. (Erratum, 13:345, 1991.) [DOI] [PubMed] [Google Scholar]
- 10.Denning, D. W., K. Venkateswarlu, K. L. Oakley, M. J. Anderson, N. J. Manning, D. A. Stevens, D. W. Warnock, and S. L. Kelly. 1997. Itraconazole resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 41:1364-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espinel-Ingroff, A. 2001. In vitro fungicidal activities of voriconazole, itraconazole, and amphotericin B against opportunistic moniliaceous and dematiaceous fungi. J. Clin. Microbiol. 39:954-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espinel-Ingroff, A., and T. M. Kerkering. 1991. Spectrophotometric method of inoculum preparation for the in vitro susceptibility testing of filamentous fungi. J. Clin. Microbiol. 29:393-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garrigues, J. C., G. Cadet de Fontenay, M. D. Linas, M. Lagente, and J. P. Seguela. 1994. New in vitro assay based on glucose consumption for determining intraconazole [sic] and amphotericin B activities against Aspergillus fumigatus. Antimicrob. Agents Chemother. 38:2857-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gehrt, A., J. Peter, P. A. Pizzo, and T. J. Walsh. 1995. Effect of increasing inoculum sizes of pathogenic filamentous fungi on MICs of antifungal agents by broth microdilution method. J. Clin. Microbiol. 33:1302-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghannoum, M. A., J. H. Rex, and J. N. Galgiani. 1996. Susceptibility testing of fungi: current status of correlation of in vitro data with clinical outcome. J. Clin. Microbiol. 34:489-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kontoyiannis, D. P., and R. E. Lewis. 2002. Antifungal drug resistance of pathogenic fungi. Lancet 359:1135-1144. [DOI] [PubMed] [Google Scholar]
- 17.Kurtz, M. B., I. B. Heath, J. Marrinan, S. Dreikorn, J. Onishi, and C. Douglas. 1994. Morphological effects of lipopeptides against Aspergillus fumigatus correlate with activities against (1,3)-β-d-glucan synthase. Antimicrob. Agents Chemother. 38:1480-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lass-Florl, C., M. Nagl, C. Speth, H. Ulmer, M. P. Dierich, and R. Wurzner. 2001. Studies of in vitro activities of voriconazole and itraconazole against Aspergillus hyphae using viability staining. Antimicrob. Agents Chemother. 45:124-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marr, K. A., R. A. Carter, F. Crippa, A. Wald, and L. Corey. 2002. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 34:909-917. [DOI] [PubMed] [Google Scholar]
- 20.Meletiadis, J., J. F. Meis, J. W. Mouton, J. P. Donnelly, and P. E. Verweij. 2000. Comparison of NCCLS and 3-(4,5-dimethyl-2-thiazyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) methods of in vitro susceptibility testing of filamentous fungi and development of a new simplified method. J. Clin. Microbiol. 38:2949-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meletiadis, J., J. F. Meis, J. W. Mouton, J. L. Rodriquez-Tudela, J. P. Donnelly, and P. E. Verweij. 2002. In vitro activities of new and conventional antifungal agents against clinical Scedosporium isolates. Antimicrob. Agents Chemother. 46:62-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meletiadis, J., J. F. Meis, J. W. Mouton, and P. E. Verweij. 2001. Analysis of growth characteristics of filamentous fungi in different nutrient media. J. Clin. Microbiol. 39:478-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meletiadis, J., J. W. Mouton, J. F. Meis, B. A. Bouman, J. P. Donnelly, and P. E. Verweij. 2001. Colorimetric assay for antifungal susceptibility testing of Aspergillus species. J. Clin. Microbiol. 39:3402-3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meletiadis, J., J. W. Mouton, J. F. Meis, B. A. Bouman, P. J. Donnelly, and P. E. Verweij. 2001. Comparison of spectrophotometric and visual readings of NCCLS method and evaluation of a colorimetric method based on reduction of a soluble tetrazolium salt, 2,3-bis{2-methoxy-4-nitro-5-[(sulfenylamino) carbonyl]-2H-tetrazolium-hydroxide}, for antifungal susceptibility testing of Aspergillus species. J. Clin. Microbiol. 39:4256-4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meletiadis, J., J. W. Mouton, J. F. Meis, B. A. Bouman, and P. E. Verweij. 2002. Comparison of the Etest and the sensititre colorimetric methods with the NCCLS proposed standard for antifungal susceptibility testing of Aspergillus species. J. Clin. Microbiol. 40:2876-2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merz, W. G., D. Fay, B. Thumar, and D. Dixon. 1984. Susceptibility testing of filamentous fungi to amphotericin B by a rapid radiometric method. J. Clin. Microbiol. 19:54-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyers, J. D. 1990. Fungal infections in bone marrow transplant patients. Semin. Oncol. 17:10-13. [PubMed] [Google Scholar]
- 28.Moore, C. B., N. Sayers, J. Mosquera, J. Slaven, and D. W. Denning. 2000. Antifungal drug resistance in Aspergillus. J. Infect. 41:203-220. [DOI] [PubMed] [Google Scholar]
- 29.NCCLS. 1998. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi, proposed standard. Document M-38P. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 30.Patterson, T. F., W. R. Kirkpatrick, M. White, J. W. Hiemenz, J. R. Wingard, B. Dupont, M. G. Rinaldi, D. A. Stevens, and J. R. Graybill. 2000. Invasive aspergillosis. Disease spectrum, treatment practices, and outcomes. I3 Aspergillus Study Group Medicine (Baltimore). 79:250-260. [DOI] [PubMed] [Google Scholar]
- 31.Petrikkou, E., J. L. Rodriguez-Tudela, M. Cuenca-Estrella, A. Gomez, A. Molleja, and E. Mellado. 2001. Inoculum standardization for antifungal susceptibility testing of filamentous fungi pathogenic for humans. J. Clin. Microbiol. 39:1345-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rex, J. H., M. A. Pfaller, T. J. Walsh, V. Chaturvedi, A. Espinel-Ingroff, M. A. Ghannoum, L. L. Gosey, F. C. Odds, M. G. Rinaldi, D. J. Sheehan, and D. W. Warnock. 2001. Antifungal susceptibility testing: practical aspects and current challenges. Clin. Microbiol. Rev. 14:643-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanders, C. C. 1991. ARTs versus ASTs: where are we going? J. Antimicrob. Chemother. 28:621-623. [DOI] [PubMed] [Google Scholar]
- 34.Stotler, R. W., and M. C. Meyer. 1983. Detection of oxacillin-resistant staphylococci by the AutoMicrobic system. J. Clin. Microbiol. 18:1205-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vitale, R. G., J. W. Mouton, J. Afeltra, J. F. Meis, and P. E. Verweij. 2002. Method for measuring postantifungal effect in Aspergillus species. Antimicrob. Agents Chemother. 46:1960-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White, T. C., K. A. Marr, and R. A. Bowden. 1998. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin. Microbiol. Rev. 11:382-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamada, H., S. Kohno, S. Maesaki, H. Koga, M. Kaku, K. Hara, and H. Tanaka. 1993. Rapid and highly reproducible method for antifungal susceptibility testing of Aspergillus species. J. Clin. Microbiol. 31:1009-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]