Abstract
Study Objectives:
Previous studies have suggested that snoring and obstructive sleep apnea hypopnea syndrome may be important risk factors for the development of carotid atherosclerosis and stroke. However, it is not clear if snoring per se is independently related to the risk of developing carotid atherosclerotic plaque.
Design:
Observational cohort study.
Setting:
Volunteer sample examined in a sleep laboratory.
Participants:
One hundred ten volunteers (snorers and nonsnorers with only mild, nonhypoxic obstructive sleep apnea hypopnea syndrome) underwent polysomnography with quantification of snoring, bilateral carotid and femoral artery ultrasound with quantification of atherosclerosis, and cardiovascular risk factor assessment. Subjects were categorized into 3 snoring groups: mild (0%–25% night snoring), moderate (> 25%–50% night snoring), and heavy (> 50% night snoring).
Interventions: N/A.
Measurements and Results:
The prevalence of carotid atherosclerosis was 20% with mild snoring, 32% with moderate snoring, and 64% with heavy snoring (P < 0.04, Χ2). Logistic regression analysis was used to determine the independent effect of snoring on the prevalence of carotid and femoral atherosclerosis. After adjustment for age, sex, smoking history, and hypertension, heavy snoring was significantly associated with carotid atherosclerosis (odds ratio 10.5; 95% confidence interval 2.1–51.8; P = 0.004) but not with femoral atherosclerosis.
Conclusions:
Heavy snoring significantly increases the risk of carotid atherosclerosis, and the increase is independent of other risk factors, including measures of nocturnal hypoxia and obstructive sleep apnea severity. Considering the high prevalence of snoring in the community, these findings have substantial public health implications for the management of carotid atherosclerosis and the prevention of stroke.
Citation:
Lee SA; Amis TC; Byth K; Larcos G; Kairaitis K; RobinsonTD; Wheatley JR. Heavy snoring as a cause of carotid artery atherosclerosis. SLEEP 2008;31(9):1207-1213.
Keywords: Carotid atherosclerosis, snoring, vascular risk factors
THE OBSTRUCTIVE SLEEP APNEA HYPOPNEA SYNDROME (OSAHS) IS AMONG THE COMMONEST CHRONIC DISORDERS OF ADULTS, WITH A PREV-ALENCE in the order of 4% for middle-aged men and 2% for middle-aged women.1 Habitual snoring is associated with OSAHS but is a much more common disorder, with a prevalence in middle-aged adults between 15% and 54%.1–3 Epidemiologic data suggest that self-reported habitual snoring and OSAHS are associated with cerebrovascular disease.4,5 It is widely believed that the association of vascular disease with habitual snoring is only a marker for OSAHS.
Snores originate from the upper airway during sleep and are a result of vibration of the pharyngeal walls and associated structures.6 Hedner et al7 hypothesized that oscillatory pressure waves originating in the upper airway during snoring may be transmitted through the surrounding tissues to the carotid artery wall. The proximity of the carotid bifurcation to the lateral pharyngeal wall is such that it is likely to be exposed to these vibrations. A recent study in rabbits has confirmed that vibration from snoring can be detected at the carotid artery wall and also within the artery lumen in rabbits.8 It is well established that exposure to vibratory stimuli causes pathologic damage to arterial wall endothelial cells, which may trigger an inflammatory cascade leading to early changes of atherosclerosis.9,10 Consequently, it is plausible that exposure to chronic snoring vibrations may cause carotid artery endothelial damage, thus contributing to the pathogenesis of carotid artery atherosclerosis. Hence, the strong epidemiologic association of habitual snoring with cerebrovascular disease may reflect a direct relationship between snoring and disease pathogenesis, rather than snoring being purely a marker for OSAHS.
The aim of the present study was to establish if snoring per se constitutes a risk factor for the presence of carotid atherosclerotic plaque.
METHODS
Subjects
There were 110 participants (aged 45–80 years), consisting of 107 community volunteers and 3 patients undergoing diagnostic polysomnography for suspected sleep disordered breathing (SDB). Snorers and nonsnorers were targeted to optimize study power to detect an effect of snoring and were recruited by advertisement to either participate in an unspecified clinical study (ie, participants were initially unaware that they were volunteering for a study to investigate snoring, n = 54) or to specifically participate in a study of snoring (n = 53). Subjects were excluded if they had a past history of stroke, transient ischaemic attack, or neck surgery. Informed consent was obtained, and the protocol was approved by the Sydney West Area Health Service Human Research Ethics Committee.
Data Collection
The study protocol included measurement of anthropometric data (body mass index [BMI], waist hip ratio and neck circumference), collection of vascular risk factor data (physician-diagnosed diabetes, smoking history [current or past] and alcohol consumption), measurement of supine blood pressure in the evening and morning, and fasting serum cholesterol, HDL cholesterol, and triglycerides.
Polysomnography
Snoring and OSAHS were assessed by standard polysomnography together with sound recording using a unidirectional uncalibrated microphone suspended 60 cm above the subject while lying supine in bed. Nasal pressure was used to measure airflow for scoring hypopnoeas. Studies were scored according to standard guidelines.11–13
Carotid and Femoral Artery Doppler Ultrasound
B mode and color Doppler ultrasound was acquired with an iU-22 (Philips Ultrasound, Bothell, WA) and high-resolution, linear, broadband transducer, with standard views of both left and right internal and common carotid arteries and transverse and longitudinal views of both common femoral arteries. All ultrasound measurements were analyzed by the same experienced physician, who was blinded to the snoring status of the subject.
DATA ANALYSIS
Subject Classification
Subjects were classified as hypertensive if any blood pressure measurement was greater than 140/90 mm Hg (systolic or diastolic) or they were currently taking antihypertensive medications. Subjects were classified as hyperlipidemic if their serum cholesterol level was greater than 5.2 mmol/L, their triglyceride level was greater than 1.85 mmol/L, or they were taking lipid lowering medications.
Indexes of Hypoxemia During Sleep
The oxygen desaturation index was calculated as the number of apneas and hypopneas accompanied by a 3% or greater oxygen desaturation per hour of sleep. In addition, the percentage of sleep time spent with oxygen saturation below 91% was calculated (hypoxic sleep time).
Indexes of Snoring During Polysomnography
The recorded sound signal from the room microphone was analyzed by 2 researchers who were blinded to the snoring history of each subject. A snore sound was defined as an obvious deflection from background (with no minimum decibel threshold), which was in phase with inspiration and occurred during sleep. Each individual snore was manually scored, and a snore index (snores per hour of sleep) was calculated. Each 30-second sleep epoch that contained 3 or more snore sounds was classified as a “snoring epoch.” The total number of “snoring epochs” was then expressed as a percentage of the total number of sleep epochs (snoring sleep time). Based on snoring sleep time, subjects were classified into 3 snoring groups: 0% to 25% (absent or mild snoring group), more than 25% to 50% (moderate snoring group), and more than 50% (heavy snoring group).
Ultrasound Data
Plaque was defined as present when a focal raised lesion or protrusion into the vessel lumen was detected in both standard transverse and longitudinal planes along the intimal surface of either the common (from origin to bifurcation) or internal carotid artery (from bifurcation to skull base). The same definition was applied to the common femoral artery (from below the inguinal ligament to the bifurcation in the subsartorial canal).14 Plaque was echogenic, calcified, or hypoechoic in nature and displayed either a smooth or irregular surface. Plaque was graded as absent or an estimated stenosis degree (0%–15%, 16%–49%, 50%–69%, 70%–79%, or 80%–99% occluded) was allocated based on the reporting system described by Grant et al.15 Because plaque was mild (0%–49%) in 94% of cases and our grading system was categorical, we scored plaque as absent or present for our final analysis. The presence of plaque was interpreted as being due to atheromatous deposits and was thus considered to represent carotid atherosclerosis.
Statistical Analysis
Group data were expressed as mean (± SD) when normally distributed or median (interquartile range) for non-normally distributed data. Χ2 tests were used to test for association between categorical variables. P < 0.05 was considered significant. The prevalence of carotid atherosclerosis was examined as a function of snoring sleep time (per 10% increase) and snoring group.
Logistic regression analysis was used to investigate the association between carotid atherosclerosis status and the possible risk factors. Odds ratios with 95% confidence intervals (CI) were used to quantify the degree of association. Possible risk factors examined by the multiple logistic regression model were body habitus (BMI, waist-to-hip ratio, and neck circumference), hypoxemia, smoking history, hyperlipidemia, hypertension, and SDB (apnea-hypopnea index [AHI], arousal index [AI]). Snoring sleep time, snore index, and snoring group were then separately tested in the model after adjusting for any significant associations with the above factors. A similar analysis was used for femoral atherosclerosis.
The effect of site (carotid versus femoral) on the prevalence of arterial atherosclerosis was examined using a generalized linear mixed model for binary data. Analyses were adjusted for age, sex, hypertension, hyperlipidemia, AHI, BMI, and smoking history.
Results
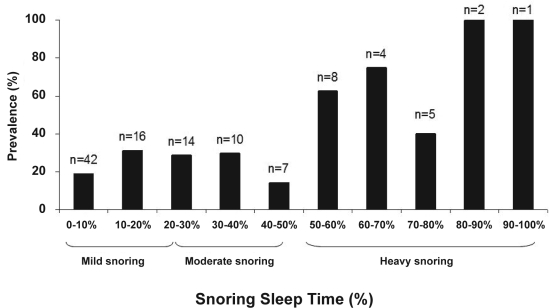
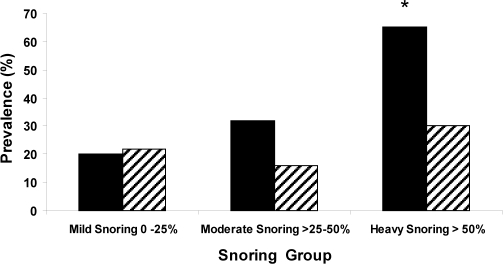
The study group characteristics are described in Table 1. Atherosclerosis prevalence was 31% for the carotid artery and 22% for the femoral artery. Carotid atherosclerosis was mostly mild with less than 50% stenosis in 32 of 34 subjects. Prevalence of atherosclerosis was related to snoring sleep time in a nonlinear fashion with a stable prevalence of atherosclerosis (less than 30%) below a snoring sleep time of 50% but increasing substantially for snoring sleep times longer than 50% (Figure 1). When the data were analyzed using the 3 snoring groups—mild, moderate, and heavy snoring, there was a significant increase in the prevalence of carotid, but not femoral, atherosclerosis across the 3 groups (mild 20%, moderate 32%, heavy 65%; P < 0.04, Χ2, Figure 2). The simple analysis between snoring and presence of carotid atherosclerosis (unadjusted) shows a significantly greater prevalence in the heavy snoring group (odds ratio [OR] 7.4, 95% confidence interval [CI] 2.5–22.3, P < 0.001) compared with the mild snoring group. This effect is not seen for the moderate snoring group (OR 1.9, 95% CI 0.7–5.3, P = 0.23). Moreover, when the prevalence of atherosclerosis for the carotid artery was compared with that for the femoral artery within the same patient after adjusting for BMI, smoking, age, sex, AHI, and hypertension, there was a significantly greater prevalence of carotid atherosclerosis, dependent upon snoring group: mild snorers (P = 0.4), moderate snorers (OR 5.9, 95% CI 1.4–25.5, P < 0.02), and heavy snorers (OR 20.9, CI 4.2–103.4, P < 0.0002).
Table 1.
Baseline Study Population Characteristicsa
| Whole group | Snoring status |
|||
|---|---|---|---|---|
| Mild | Moderate | Heavy | ||
| Age, y | 58.2 (± 7.9) | 59.5 (±8.2) | 56.6 (±4.9) | 56.2 (±9.5) |
| Sex | ||||
| Men | 59 | 29 | 15 | 15 |
| Women | 51 | 36 | 10 | 5 |
| BMI, kg/m2 | 27.5 (± 4.7) | 25.7 (±3.2) | 28.7(±4.6) | 31.8 (±5.5) |
| Neck circumference, cm | 38.2 (± 4.0) | 36.5 (±3.2) | 39.7(±3.5) | 41.8 (±3.8) |
| Smoking history, % | 27 | 20 | 40 | 35 |
| Hypertension, % | 27.3 | 18.5 | 36 | 45 |
| Hyperlipidemic, % | 69 | 61.5 | 76 | 85 |
| Index, events/h | ||||
| AHI | 10.9 (5.8 – 20.5) | 8.4 (4.3 – 14.6) | 12.7 (5.7 – 19.9) | 20.7 (15.4 – 41.6) |
| AI | 18.4 (13.3 – 25.4) | 17.7 (13.4 – 23.2) | 19.5 (14.5–25.0) | 20.8 (12.8– 32.6) |
| ODI 3% | 1.5 (0.4 – 3.7) | 0.9 (0.3 – 2.3) | 2.0 (0.5 – 3.4) | 6.9 (2.9 – 11.5) |
| Hypoxic sleep time, % TST | 0 (0 –0.7) | 0 (0 – 0.5) | 0 (0 – 1.0) | 0.8 ( 0.1 – 8.1) |
Data are provided as mean (± SD) or median (interquartile range) except, sex, which is shown as number, and smoking history, hypertension status, and hyperlipidemia status, which are shown as a percentage of the group.
BMI refers to body mass index; AHI, apnea-hypopnea index; ODI, oxygen desaturation index; AI, arousal index; TST, total sleep time.
Figure 1.
Carotid atherosclerosis prevalence stratified by snoring sleep time. Frequency histogram for the prevalence of carotid atherosclerosis grouped by percentiles of snoring sleep time. Note the substantially increased prevalence of carotid atherosclerosis for snoring sleep time longer than 50%. Snoring group classification based on snoring sleep time is shown on the x axis. Number of subjects falling within each snoring sleep time decile is shown on each column.
Figure 2.
Prevalence of carotid and femoral atherosclerosis. Prevalence of carotid atherosclerosis (black columns) and femoral atherosclerosis (shaded columns) by snoring group. Note that the prevalence of carotid atherosclerosis increased progressively across the 3 groups [*P < 0.04 (Χ2)]. However, the prevalence of femoral atherosclerosis did not differ between groups.
In the multivariate logistic regression model and when using snoring sleep time as the snoring variable, the independent predictors for the presence of carotid atherosclerosis were age, sex, smoking, hypertension, and snoring sleep time (Table 2) but not BMI or hyperlipidemia. When using snore index as the snoring variable, similar independent predictors were seen, including age, sex, smoking, hypertension, and snore index (Table 2).
Table 2.
Adjusted Odds Ratios for the Risk of Carotid Atherosclerosis
| Snoring sleep time model for carotid atherosclerosis | |||
| Covariate | OR | 95% CI | P Value |
| Age, per decade | 3.1 | 1.4–6.9 | 0.005 |
| Male sex | 4.5 | 1.4–14.8 | 0.01 |
| Positive smoking history | 3.6 | 1.1–11.6 | 0.03 |
| Positive for hypertension | 4.3 | 1.3–14.3 | 0.02 |
| Snoring sleep time (per 10% increase) | 1.4 | 1.1–1.8 | 0.02 |
| Snore Index model for carotid atherosclerosis | |||
| Age, per decade | 3.3 | 1.5–7.4 | 0.004 |
| Male sex | 5.2 | 1.6–17.2 | 0.007 |
| Positive smoking history | 3.9 | 1.2–12.7 | 0.024 |
| Positive for hypertension | 4.1 | 1.2–13.7 | 0.022 |
| Snore Index, snores/min | 1.3 | 1.1–1.6 | 0.013 |
| Snoring group model for carotid atherosclerosis | |||
| Age, per decade | 3.2 | 1.4–7.2 | 0.006 |
| Male sex | 4.6 | 1.4–15.2 | 0.013 |
| Positive smoking history | 3.9 | 1.1–13.5 | 0.032 |
| Positive for hypertension | 4.7 | 1.3–15.7 | 0.013 |
| Snoring, % of sleep time | |||
| 0–25a | |||
| 25–50 | 1.7 | 0.4–6.9 | 0.41 |
| > 50 | 10.5 | 2.1–51.8 | 0.004 |
Notes: Best-fitting multiple logistic regression model showing adjusted odds ratios (OR) for the risk of carotid atherosclerosis using snoring sleep time, snore index, or snoring group as the snoring variables. CI refers to confidence interval.
Reference group.
In the model when using snoring group as the snoring variable, the independent predictors for presence of carotid atherosclerosis were again age, sex, smoking history, hypertension, and snoring group (Table 2) but not BMI, AHI, or hyperlipidemia. The adjusted OR for the heavy snoring group was substantially increased at 10.5 (95% CI, 2.1 – 51.8), ie, an OR much higher than for any of the traditionally accepted risk factors for atherosclerosis. Importantly, when the AHI, AI, and measures of nocturnal hypoxemia were tested in any model, none showed significant associations with carotid atherosclerosis.
In contrast, the independent predictors for femoral atherosclerosis were limited to age, sex, and smoking history (Table 3). There was no significant association for BMI, hypertension, hyperlipidemia, AHI, AI, or nocturnal hypoxemia. In contrast with the model for carotid atherosclerosis, neither snoring variable was significantly associated with femoral atherosclerosis.
Table 3.
Adjusted Odds Ratios for the Risk of Femoral Atherosclerosis
| Covariate | OR | 95% CI | P value |
|---|---|---|---|
| Age, per decade | 2.5 | 1.3–4.8 | 0.004 |
| Male sex | 3.4 | 1.1–10.1 | 0.027 |
| Positive smoking history | 3.3 | 1.1–9.9 | 0.029 |
Notes: Best-fitting multiple logistic regression model showing adjusted odds ratios (OR) for the risk of femoral atherosclerosis. Note that snoring group was not associated with risk of femoral atherosclerosis.
CI refers to confidence interval.
DISCUSSION
This study is the first to describe and quantify the novel and important observation that objectively measured heavy snoring is a significant risk factor for the presence of carotid atherosclerosis. Importantly, this relationship remained after adjustment for known atherosclerosis risk factors and was independent of other measures of SDB and nocturnal hypoxia.
Limitations of the Study
The magnitude of the risk with heavy snoring in our study group (OR 10.5) is much greater than the associations found with established risk factors in published population studies. However, we believe that this is predominantly a function of the study design and the nature of the subject group. It is important to remember that this is not a population study aimed at attempting to assess overall risk factors within the general population. This is a controlled study, not designed to test for traditional risk factors, but rather designed to test for the effect of snoring while controlling for traditional risk factors. As such, this is not an unselected group, and the fact that established risk factors were less predictive of carotid vascular disease in this group is not surprising since they were either minimally (eg smoking) or almost universally (eg hyperlipidaemia) represented. Our interpretation of this result is that when the effect of traditional risk factors is minimised, heavy snoring (measured objectively) emerges as a strong risk factor for carotid atherosclerosis in this group. However, the strength of the odds ratio does not indicate the general population risk for developing carotid atherosclerosis in relation to heavy snoring.
In our study, the adjusted odds ratio for heavy vs mild snoring (10.5) is greater than the unadjusted odds ratio (7.4), contrary to usual expectation when covariates are positively correlated. For our data there was a negative correlation between age and snoring and it is this that causes the increase in the odds ratio after adjustment. This negative association between snoring and age may be explained by the decrease in slow wave NREM sleep that occurs with increasing age.
The traditional risk factors for atherosclerosis were all carefully measured in our population. Age, male sex, smoking history and hypertension were all significant independent risk factors for the presence of carotid atherosclerosis as was expected. The use of multiple logistic regression analysis has controlled for these risk factors, and resulted in heavy snoring also being recognised as an independent risk factor for carotid atherosclerosis in our study. The lack of association with hyperlipidemia was an unexpected finding, but may relate to the high use of lipid-lowering drugs by the group over a period of time, which may be a potential confounding factor. This finding should not be interpreted as hyperlipidemia not being a general risk factor for carotid atherosclerosis.
Our study group consisted of patients with mild OSAHS as well as mild to heavy snoring. We deliberately chose not to exclude OSAHS from our study, due to the sensitivity of our current diagnostic metric for OSAHS (Chicago Criteria using nasal pressure measurements 11), and the difficulty in determining what constitutes normality when using this metric. The AHI was therefore included as a marker for OSAHS in the logistic regression model, and was not found to be a significant risk factor. This lack of association was not surprising, as our study group only contained patients with relatively mild, non-hypoxic OSAHS due to a bias towards selection of snoring subjects. This finding should not be interpreted as excluding OSAHS or AHI as risk factors in a general population.
In addition, our study group consisted of patients with relatively mild degrees of nocturnal hypoxia. Again, this was not surprising due to the bias towards selection of snoring subjects over OSAHS patients. Our study was not designed or powered to test for nocturnal hypoxia as a risk factor for atherosclerosis, so the lack of association should not be interpreted as excluding hypoxia as a risk factor for atherosclerosis in other study populations (see below).
Interest in the association between snoring and carotid atherosclerosis has arisen from the recognition that SDB may be an independent risk factor for vascular diseases including stroke16, 17. However, the bulk of evidence associating SDB and stroke is derived from surrogate variables such as snoring. Furthermore the information regarding snoring itself is mostly derived from questionnaires and interviews rather than objectively quantifiable measurements. In this vein, three cohort studies using questionnaire-determined snoring status, with 3 and 8 years follow up, have demonstrated an increased relative risk of stroke in habitual snorers of 1.4 – 1.918–20. However, despite such reports, most clinical interest has focussed on the risk of vascular disease related to OSAHS, with snoring regarded as a marker for OSAHS. Recent studies have supported the association between OSAHS and carotid atherosclerosis21, 22, but have not attempted to quantify snoring. Indeed, a more recent study failed to find any association between SDB and subclinical atherosclerosis following adjustment for confounding cardiovascular disease risk factors, but also did not measure snoring23. However treatment with continuous positive airway pressure, which eliminates apneas, hypopneas and snoring, has been shown to reverse carotid intima thickening in OSAHS patients24. This finding supports a causative association between SDB and the development of carotid atherosclerosis but does not identify the relative contributions of the individual components of OSAHS, including snoring. Our study is the first to explore the issue of objectively quantified snoring per se (separate from AHI and nocturnal hypoxemia) as an independent risk factor for carotid vascular disease. Our data suggest that there is a strong association between carotid atherosclerosis and heavy snoring.
A novel feature of this study is that it is the first to objectively measure and quantify snoring on an overnight polysomnogram, rather than simply using a questionnaire, for the purpose of exploring the association between SDB and carotid atherosclerosis. Two studies18,25 that have used questionnaires to quantify snoring, and have attempted to validate the questionnaire in the sleep laboratory, have found that the prevalence of snoring was underestimated by questionnaire. Although there is a moderately strong correlation between questionnaires and objective measurements, there is still considerable variance between the 2 independent measures. Consequently, we do not believe it is possible to equate self-reported snoring data with objectively measured snoring data. Although there is bound to be some correlation, clearly the objectively measured data are superior and may be substantially different in individual cases. Thus, the precise quantification of snoring severity by polysomnography in our study has allowed more accurate assessment of the independent risk of snoring.
Heavy snoring is one of the major symptoms of OSAHS. Therefore, most patients with OSAHS included in prior studies16,17,26 are likely to have a history of heavy snoring that dates back many years. In this context, we would suggest that the AHI may actually be a surrogate variable for heavy snoring and that it is the snoring per se, rather than the apneas and hypopneas, that represents a major independent risk factor for carotid atherosclerosis. This does not exclude the possibility that other factors associated with the AHI may also be significant risk factors for carotid atherosclerosis.
Recently, much attention has been focused on the role of nocturnal hypoxia as an atherogenic factor linking OSAHS and vascular disease.27,28 In the present study, measures such as the oxygen desaturation index and hypoxic sleep time did not emerge as significant risk factors for carotid atherosclerosis. However, it should be noted that the prevalence of hypoxia during sleep in our study population was minimal (19% patients). Therefore, the lack of a relationship between nocturnal hypoxemia and carotid atherosclerosis as observed in our patient population is likely a product of the specific subject group and may underestimate the risk in a population of patients with a significant degree of hypoxemia. Nevertheless, nocturnal hypoxia is clearly not a prerequisite feature for developing carotid atherosclerosis or for establishing a link between carotid atherosclerosis and SDB.
The pathophysiologic mechanisms that link snoring, OSAHS, and stroke are not clear and remain largely unexplored. Carotid atherosclerosis is a leading cause of stroke and is a complex disease involving several highly interrelated processes, including endothelial dysfunction, inflammation, oxidative stress, vascular smooth muscle cell activation, platelet activation, and thrombosis.29 Recent studies have indicated that OSAHS is also associated with many of these processes.30–36 Experimental evidence has linked hypoxia with atherosclerosis through various pathways, all of which eventually lead to endothelial dysfunction.27 However, nocturnal hypoxia is not invariably a feature of OSAHS and is usually only present in more severe cases, whereas significant snoring is invariably present in all patients with OSAHS.
It is now more than a decade since Hedner et al7 proposed that snoring vibrations may be transmitted through surrounding tissues to the carotid artery wall. They hypothesized that these vibrations, repeated nightly over time, may result in initiation and promotion of endothelial damage leading to carotid atherosclerosis. The close proximity of the carotid bifurcation, the major site of carotid artery atherosclerotic plaques,37 to the hypopharyngeal wall adds to the plausibility of this hypothesis. Recently, we have demonstrated that, during snoring in an animal model, pressure vibrations occur in the tissues surrounding the carotid artery wall and are transmitted to the carotid artery lumen.8 In addition, the vibration of rat tail blood vessels at 60 Hz (similar to frequencies seen in human snoring) leads to initial vasoconstriction, injury of endothelial cells, and subsequent endothelial denudation.9 Taken together, these studies support the possibility of snoring vibration-induced, localized endothelial injury that may result in the development of carotid atherosclerosis, whether by direct mechanical injury to the endothelium or the local initiation of a proinflammatory response.
An important feature of our study was documentation of the prevalence of femoral atherosclerosis as a distant (from the upper airway) control artery. Our logistic regression model demonstrated that age, male sex, and smoking history (classic cardiovascular risk factors) were associated with the presence of femoral atherosclerosis. Importantly, when we included measures of snoring and OSAHS, none of these factors were found to be significant predictors of femoral atherosclerosis. This finding supports the local transmission of snoring vibrations as a major pathophysiologic mechanism leading to the development of carotid, but not femoral, atherosclerosis.
The importance of our findings is the implication that the risk of developing carotid atherosclerosis (and potentially stroke) is not confined to the population of patients with established OSAHS, but also extends to the population of heavy snorers. Heavy or habitual snoring is associated with OSAHS but is a much more common disorder, with a prevalence in middle-aged adults of between 15% and 54%.1–3 Snoring has generally been regarded as more of a social problem with minimal risk of adverse health outcomes. However, our data clearly demonstrate that heavy snoring is an independent risk factor for early carotid atherosclerosis, which may progress to be associated with the development of stroke, representing a major cause of morbidity and mortality.
In conclusion, our study provides strong evidence for heavy snoring as an independent risk factor for early carotid atherosclerosis. In addition, our study provides support for the hypothesis that snoring vibration transmission is one of the pathophysiologic mechanisms associated with the development of carotid atherosclerotic plaque. If the associations observed in this study are indeed causal, the significantly raised risk of carotid atherosclerosis in heavy snorers, coupled with the high prevalence of snoring in the community, has substantial public health implications for the prevention of stroke.
ACKNOWLEDGMENTS
The authors would like to thank Prof. S. Leeder and Prof. G. Berry for their assistance with statistical analysis. We acknowledge the staff of the Sleep Disorders Laboratory, Westmead Hospital, in particular Michael Lazaris, Melanie Madronio, Karina Falland, Pamela Johnson, Ke-ying Cao, Sein Wai Hla, and Naomi Newton for their assistance with the polysomnography studies. We would also like to thank Mr. George Bonovas and his staff for their assistance in acquiring the ultrasound studies. We are grateful to Ms. Melanie Madronio, Ms. Manisha Verma, and Mr. Jerrad Borodzicz for their assistance with data analysis.
Funding: National Health and Medical Research Council of Australia and Department of Veterans' Affairs, Commonwealth of Australia.
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. Wheatley has participated in research support funded by Boehringer Ingelheim, Astra-Zeneca, Cephalon, Takeda, and Sanofi-Aventis. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. New Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Fitzpatrick MF, Martin K, Fossey E, Shapiro CM, Elton RA, Douglas NJ. Snoring, asthma and sleep disturbance in Britain: a community-based survey. Eur Respir J. 1993;6:531–5. [PubMed] [Google Scholar]
- 3.Hiestand DM, Britz P, Goldman M, Phillips B. Prevalence of symptoms and risk of sleep apnea in the US population - Results from the National Sleep Foundation Sleep in America 2005 Poll. Chest. 2006;130:780–6. doi: 10.1378/chest.130.3.780. [DOI] [PubMed] [Google Scholar]
- 4.Neau JP, Meurice JC, Paquereau J, Chavagnat JJ, Ingrand P, Gil R. Habitual snoring as a risk factor for brain infarction. Acta Neurol Scand. 1995;92:63–8. doi: 10.1111/j.1600-0404.1995.tb00468.x. [DOI] [PubMed] [Google Scholar]
- 5.Palomaki H. Snoring and the risk of ischemic brain infarction. Stroke. 1991;22:1021–5. doi: 10.1161/01.str.22.8.1021. [DOI] [PubMed] [Google Scholar]
- 6.Liistro G, Stanescu DC, Veriter C, Rodenstein DO, Aubert-Tulkens G. Pattern of snoring in obstructive sleep apnea patients and in heavy snorers. Sleep. 1991;14:517–25. doi: 10.1093/sleep/14.6.517. [DOI] [PubMed] [Google Scholar]
- 7.Hedner J, Wilcox I, Sullivan C. Speculations on the interaction between vascular disease and obstructive sleep apnoea. In: Saunders NA, Sullivan C, editors. Sleep and Breathing. New York, NY: Dekker; 1994. pp. 823–46. [Google Scholar]
- 8.Amatoury J, Howitt L, Wheatley JR, Avolio A, Amis TC. Snoring-related energy transmission to the carotid artery in rabbits. J Appl Physiol. 2006;100:1547–53. doi: 10.1152/japplphysiol.01439.2005. [DOI] [PubMed] [Google Scholar]
- 9.Curry BD, Bain JLW, Yan J-G, et al. Vibration injury damages arterial endothelial cells. Muscle & Nerve. 2002;25:527–34. doi: 10.1002/mus.10058. [DOI] [PubMed] [Google Scholar]
- 10.Puig F, Rico F, Almendros I, Montserrat JM, Navajas D, Farre R. Vibration enhances interleukin-8 release in a cell model of snoring-induced airway inflammation. Sleep. 2005;28:1312–6. doi: 10.1093/sleep/28.10.1312. [DOI] [PubMed] [Google Scholar]
- 11.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in adults. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 12.American Sleep Disorders Association. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 13.Rechtschaffen A, Kales A. A manual of standardised terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles: UCLA Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 14.Joakimsen O, Bonaa KH, Stensland-Bugge E. Reproducibility of ultrasound assessment of carotid plaque occurrence, thickness, and morphology. The Tromso Study. Stroke. 1997;28:2201–7. doi: 10.1161/01.str.28.11.2201. [DOI] [PubMed] [Google Scholar]
- 15.Grant EG, Benson CB, Moneta GL, et al. Carotid artery stenosis: gray-scale and Doppler US diagnosis-Society of Radiologists in Ultrasound Consensus Conference. Radiology. 2003;229:340–6. doi: 10.1148/radiol.2292030516. [DOI] [PubMed] [Google Scholar]
- 16.Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172:1447–51. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. New Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 18.Koskenvuo M, Kaprio J, Telakivi T, Partinen M, Heikkila K, Sarna S. Snoring as a risk factor for ischaemic heart disease and stroke in men. Br Med J (Clin Res Ed) 1987;294:16–9. doi: 10.1136/bmj.294.6563.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jennum P, Schultz-Larsen K, Davidsen M, Christensen NJ. Snoring and risk of stroke and ischaemic heart disease in a 70 year old population. A 6-year follow-up study. Int J Epidemiol. 1994;23:1159–64. doi: 10.1093/ije/23.6.1159. [DOI] [PubMed] [Google Scholar]
- 20.Hu FB, Willett WC, Manson JE, et al. Snoring and risk of cardiovascular disease in women. J Am Coll Cardiol. 2000;35:308–13. doi: 10.1016/s0735-1097(99)00540-9. [DOI] [PubMed] [Google Scholar]
- 21.Minoguchi K, Yokoe T, Tazaki T, et al. Increased carotid intima-media thickness and serum inflammatory markers in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172:625–30. doi: 10.1164/rccm.200412-1652OC. [DOI] [PubMed] [Google Scholar]
- 22.Drager LF, Bortolotto LA, Lorenzi MC, Figueiredo AC, Krieger EM, Lorenzi-Filho G. Early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172:613–8. doi: 10.1164/rccm.200503-340OC. [DOI] [PubMed] [Google Scholar]
- 23.Wattanakit K, Boland L, Punjabi NM, Shahar E. Relation of sleep-disordered breathing to carotid plaque and intima-media thickness. Atherosclerosis. 2008;197:125–31. doi: 10.1016/j.atherosclerosis.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 24.Drager LF, Bortolotto LA, Figueiredo AC, Krieger EM, Lorenzi-Filho G. Effects of continuous positive airway pressure on early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2007;176:706–12. doi: 10.1164/rccm.200703-500OC. [DOI] [PubMed] [Google Scholar]
- 25.Gislason T, Aberg H, Taube A. Snoring and systemic hypertension-an epidemiological study. Acta Med Scand. 1987;222:415–21. doi: 10.1111/j.0954-6820.1987.tb10958.x. [DOI] [PubMed] [Google Scholar]
- 26.Yaggi H, Mohsenin V. Sleep-disordered breathing and stroke. Clin Chest Med. 2003;24:223–37. doi: 10.1016/s0272-5231(03)00027-3. [DOI] [PubMed] [Google Scholar]
- 27.Lavie L. Obstructive sleep apnoea syndrome—an oxidative stress disorder. Sleep Med Rev. 2003;7:35–51. doi: 10.1053/smrv.2002.0261. [DOI] [PubMed] [Google Scholar]
- 28.Hayashi M, Fujimoto K, Urushibata K, Takamizawa A, Kinoshita O, Kubo K. Hypoxia-sensitive molecules may modulate the development of atherosclerosis in sleep apnoea syndrome. Respirology. 2006;11:24–31. doi: 10.1111/j.1440-1843.2006.00780.x. [DOI] [PubMed] [Google Scholar]
- 29.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 30.Yaggi H, Mohsenin V. Obstructive sleep apnoea and stroke. Lancet Neurol. 2004;3:333–42. doi: 10.1016/S1474-4422(04)00766-5. [DOI] [PubMed] [Google Scholar]
- 31.Chin K, Nakamura T, Shimizu K, et al. Effects of nasal continuous positive airway pressure on soluble cell adhesion molecules in patients with obstructive sleep apnea syndrome. Am J Med. 2000;109:562–7. doi: 10.1016/s0002-9343(00)00580-5. [DOI] [PubMed] [Google Scholar]
- 32.Kato M, Roberts-Thomson P, Phillips BG, et al. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation. 2000;102:2607–10. doi: 10.1161/01.cir.102.21.2607. [DOI] [PubMed] [Google Scholar]
- 33.Shamsuzzaman ASM, Winnicki M, Lanfranchi P, et al. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105:2462–4. doi: 10.1161/01.cir.0000018948.95175.03. [DOI] [PubMed] [Google Scholar]
- 34.Schulz R, Hummel C, Heinemann S, Seeger W, Grimminger F. Serum levels of vascular endothelial growth factor are elevated in patients with obstructive sleep apnea and severe nighttime hypoxia. Am J Respir Crit Care Med. 2002;165:67–70. doi: 10.1164/ajrccm.165.1.2101062. [DOI] [PubMed] [Google Scholar]
- 35.Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, Barnes PJ. 8-Isoprostane, a marker of oxidative stress, is increased in exhaled breath condensate of patients with obstructive sleep apnea after night and is reduced by continuous positive airway pressure therapy. Chest. 2003;124:1386–92. doi: 10.1378/chest.124.4.1386. [DOI] [PubMed] [Google Scholar]
- 36.Chin K, Ohi M, Kita H, et al. Effects of NCPAP therapy on fibrinogen levels in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 1996;153:1972–6. doi: 10.1164/ajrccm.153.6.8665063. [DOI] [PubMed] [Google Scholar]
- 37.Zarins CK, Giddens DP, Bharadvaj BK, Sottiurai VS, Mabon RF, Glagov S. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res. 1983;53:502–14. doi: 10.1161/01.res.53.4.502. [DOI] [PubMed] [Google Scholar]