Abstract
Study Objectives:
Epidemiologic studies have shown a high frequency of major cardiac events at night in patients with coronary artery disease. This has been attributed to the sympathetic surges accompanying rapid eye movement (REM) sleep; the role of non-REM sleep, which comprises 80% of total sleep duration, has been largely neglected. Accordingly, we evaluated the effect of non-REM sleep on contractile function in a region of the left ventricular wall supplied by a flow-limiting coronary stenosis.
Design:
Eight domestic pigs were chronically instrumented to measure regional left ventricular contractile function (wall thickening), coronary blood flow, and systemic hemodynamic variables. Measurements were obtained: (1) during wakefulness, i.e., conscious condition, prior to imposition of coronary stenosis; (2) during wakefulness following imposition of coronary stenosis (30% reduction of baseline coronary blood flow from 40 ± 4 to 27 ± 3 mL/min); and (3) during non-REM sleep with coronary stenosis maintained.
Results:
During wakefulness, coronary stenosis reduced wall thickening (from 23.3 ± 3.4% to 15.7 ± 2.0%), whereas mean arterial pressure and heart rate were unchanged. With coronary stenosis maintained, the onset of non-REM sleep caused 20% decreases in mean arterial pressure and coronary blood flow, accompanied by a cessation of regional wall thickening, i.e., akinesis (wall thickening = 0.2 ± 2.8%), indicating severe myocardial ischemia.
Conclusions:
The arterial hypotension, and associated reduction in coronary blood flow, during non-REM sleep precipitated severe myocardial ischemia in a region of the left ventricular wall supplied by flow-limiting coronary stenosis. Such episodes would occur repeatedly during the sleep cycle and could potentially set the stage for a major cardiac event during the sympathetic activation accompanying REM sleep or morning activities.
Citation:
Kim SJ; Kuklov A; Kehoe RF; Crystal GJ. Sleep-induced hypotension precipitates severe myocardial ischemia. SLEEP 2008;31(9):1215-1220.
Keywords: Myocardial ischemia, sleep, coronary stenosis, myocardial contractility, coronary blood flow
RETROSPECTIVE STUDIES IN PATIENTS WITH CORONARY ARTERY DISEASE HAVE SHOWN A HIGH INCIDENCE OF ANGINAL PAIN, ACUTE MYOCARDIAL infarction, and sudden cardiac death at night.1,2 Previous reports addressing this issue have emphasized a role for the intense increase in sympathetic activation during rapid eye movement (REM) sleep, which occurs four to six times per night with a total duration of 90 minutes. According to this widely accepted hypothesis, the sympathetic surges precipitate myocardial ischemia by increasing myocardial oxygen demand in the presence of restricted coronary blood flow (CBF) and by promoting thrombotic processes and plague rupture, leading ultimately to electrical instability and ventricular fibrillation.3–7
The possibility that hemodynamic changes during non-REM (NREM) sleep, e.g., slow-wave sleep, may play a role in nocturnal or early morning cardiac events has received minimal attention. This stage of sleep (which accounts for 80% of total sleep duration) is usually accompanied by hypotension and bradycardia, which is mainly attributed to reduced sympathetic vasoconstrictor tone and increased cardiac vagal discharge.8,9 Because these responses would be expected to reduce the cardiac workload, most investigators have considered NREM sleep to be cardioprotective. However, this analysis ignores the potentially countervailing influence of reduced CBF secondary to arterial hypotension, i.e., reduced perfusion pressure, when coronary vasodilator reserve has been compromised by an upstream stenosis. Although this mechanism has been hypothesized for many years to occur in subjects with coronary artery disease,10–12 it has never been confirmed either in humans or in a clinically relevant animal model.
This report contains observations made in pigs that were chronically instrumented for measurements of left ventricular (LV) and systemic hemodynamics, CBF, and global and regional myocardial contractile function.13 These animals were subjects in a study in which persistent myocardial stunning leading to hibernation was induced by six repetitive episodes of 90-minute coronary stenosis (30% reduction in baseline CBF) followed by full reperfusion every 12 hours. The findings presented herein describe the effects of sleep during one of these episodes of CS on regional myocardial contractile function. The present study demonstrates that NREM sleep was not cardioprotective in myocardium in the presence of a flow-limiting stenosis. In fact, it precipitated severe ischemia, as reflected by akinesis, in this region of the LV wall. Interestingly, in contradiction to the prevailing view, this impairment to regional contractile function (and degree of ischemia) was worse than the impairment observed during REM sleep.
METHODS
The study was conducted after approval from the Institutional Animal Care Committee. Animals used in this study were maintained in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, revised 1996).
Animal Preparation
Domestic swine (25-30 kg, female, N = 8) were sedated with ketamine (10-20 mg/kg, intramusclar) and xylazine (2.2 mg/kg, intramusclar), and then general anesthesia was maintained with isoflurane (0.5%-2.0% vol). The animals were instrumented chronically to measure hemodynamic variables, including CBF and regional wall thickening in the stenotic region (anterior wall) and the nonstenotic control region (posterior wall), as described previously.13 After the pig was allowed to recover for one to two weeks postoperatively, experiments were initiated with the pig resting comfortably and quietly in a Panepinto sling (Lomir Biomedical, Inc., Malone, NY) under conscious condition. A coronary stenosis was induced by introducing air into a hydraulic occluder to reduce baseline CBF by approximately 30% for 90 minutes, followed by full reperfusion for 12 hours. Myocardial stunning was maintained in the anterior region of the LV by six of these coronary stenosis-reperfusion cycles sequentially. This protocol for persistent myocardial stunning caused no necrosis, and produced the phenotype for hibernating myocardium, e.g., glycogen deposition.13
Systemic hemodynamic parameters (including arterial pressure and heart rate), LV global and regional contractile function, and CBF were monitored continuously during wakefulness and sleep. All hemodynamic measurements were recorded on a digital multiple recorder (PC216Ax, Sony Precision Technology Inc., Tokyo, Japan), digitized (1000 Hz), and analyzed with a computer-based system (Notocord, Croissy, France).
Statistical Analysis
Data are presented as mean ± SEM. Comparisons between values during wakefulness and sleep were performed using the Student t-test for paired samples. A P value less than 0.05 was considered statistically significant.
RESULTS
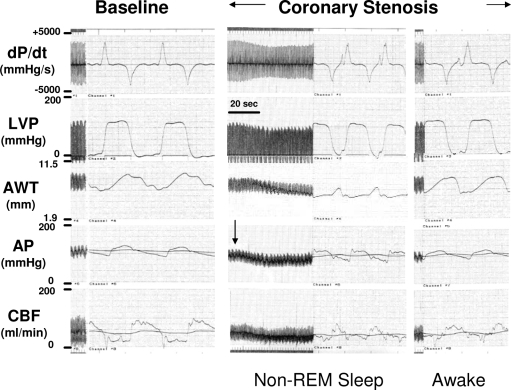
The reported data were obtained during the initial NREM phase of sleep, which occurred spontaneously following imposition of the coronary stenosis. This behavior occurred frequently and was observed in all six episodes of coronary stenosis. The systemic hemodynamic and myocardial responses for the multiple periods of sleep in a given animal were identical. Sleep in the presence of a coronary stenosis consistently caused hypotension (reduced perfusion pressure) and converted regional dyskinesis to akinesis, indicating severe myocardial ischemia. In order to prevent a prolonged period of akinesis during NREM sleep, which could lead to myocardial infarction, hemodynamics had to be returned to the pre-NREM values by purposely awakening the animal (Figure 1). A representative response for each animal was used for the statistical analysis (Table 1).
Figure 1.
Representative tracings of hemodynamic variables at baseline (prior to coronary stenosis) and during a coronary stenosis. Induction of a coronary stenosis caused decreases in coronary blood flow (CBF) and anterior wall thickness (AWT). Under this condition, the onset of non-rapid eye movement (REM) sleep was associated with a decrease in arterial pressure (AP), which reduced CBF further, resulting in akinesis in the anterior wall (stenotic region). Intentionally reawakening the animal caused a rapid reversal of these hemodynamic responses. dP/dt refers to the rate of change of left ventricular pressure; LVP, left ventricular pressure.
Table 1.
Hemodynamic Variables at Baseline and During Sleep in the Presence of Coronary Stenosis
| Baseline | CS | CS-Sleep | CS-Recovery | |
|---|---|---|---|---|
| MAP, mmHg | 92 ± 3 | 95 ± 3 | 77 ± 3a,b | 92 ± 4 |
| LVP, mmHg | 116 ± 3 | 118 ± 3 | 100 ± 3a,b | 116 ± 5 |
| LV EDP, mmHg | 12 ± 1 | 10 ± 1 | 9 ± 1 | 12 ± 1 |
| dP/dtmax, mmHg/s | 3400 ± 113 | 3125 ± 164 | 2575 ± 129a,b | 3050 ± 124a |
| HR, beats/min | 133 ± 6 | 137 ± 5 | 152 ± 11a | 134 ± 4 |
| CBF, ml/min | 40 ± 4 | 27 ± 3a | 22 ± 2a,b | 27 ± 3a |
| AWT, % | 23.3 ± 3.4 | 15.7 ± 2.0a | 0.2 ± 2.8a,b | 14.1 ± 1.8a |
| PWT, % | 20.9 ± 2.9 | 18.3 ± 2.0 | 18.3 ± 2.0 | 19.9 ± 2.2 |
Values are mean ± SEM. N = 8, except posterior wall thickness (PWT, non- coronary stenosis [CS] region, n = 4). Baseline values were obtained prior to the first CS. The values for CS, CS-sleep, and CS recovery were obtained in the second CS in 2 animals, in the third CS in 2 animals, in the fourth CS in 1 animal, and in the sixth CS in 2 animals. Abbreviations: MAP, mean arterial pressure; LVP, left ventricular pressure; LV EDP, LV end-diastolic pressure; dP/dtmax, maximal rate of change of left ventricular pressure; HR, heart rate; CBF, coronary blood flow, AWT, anterior wall thickness (CS region).
p < 0.05 vs. baseline.
;p < 0.05 vs CS
Table 1 summarizes the hemodynamic variables at baseline (prior to the imposition of the coronary stenosis), following imposition of the coronary stenosis, and with the coronary stenosis maintained, during and following NREM sleep. The coronary stenosis caused a 30% reduction in CBF (from 40 ± 4 mL/min to 27 ± 3 mL/min) accompanied by a parallel reduction in anterior wall thickening (from 23.3 ± 3.4% to 15.7 ± 2.0%), whereas posterior wall thickening, i.e., the nonstenotic region, was not affected. There were no changes in mean arterial pressure, LV systolic and diastolic pressures, LV dP/dtmax, or heart rate. Sleep in the presence of the coronary stenosis caused essentially parallel, approximately 20%, reductions in mean arterial pressure pressure, CBF, and LV dP/dtmax, and akinesis in the stenotic anterior region; posterior wall thickening did not change. Heart rate increased (+11%). Figure 1 is a representative tracing demonstrating the strong temporal relationship consistently observed between the decreases in arterial blood pressure and CBF and the appearance of akinesis in the stenotic region, i.e., the decreases in CBF and anterior wall thickening closely followed the decrease in arterial blood pressure. Noteworthy is the rapidity with which these changes were reversed by awakening the animal.
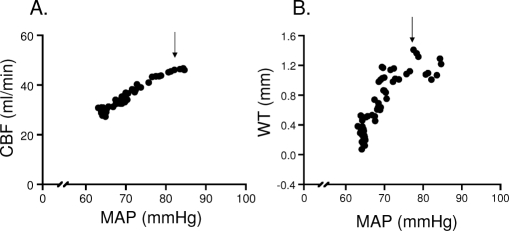
Figure 2 is a plot of the pressure-flow relationship (A) and the pressure-wall thickening relationship (B) for findings obtained in one animal during wakefulness and NREM sleep in the presence of the coronary stenosis. The figure shows (1) that sleep caused progressive decreases in mean arterial pressure, CBF, and myocardial wall thickening; (2) that the pressure-related decreases in CBF were more gradual than those in wall thickening; and (3) that wall thickening was abolished (indicating akinesis) at a time that CBF remained approximately 50% of baseline.
Figure 2.
A representative pressure-flow (A) and pressure-function (B) relationship during sleep in the presence of coronary stenosis (CS) in 1 animal. Each point represents an average of 3 beats. Sleep, as indicated by the arrow, initiated a decrease in mean arterial pressure (MAP), which caused a proportional reduction in coronary blood flow (CBF), indicating a lack of coronary autoregulation. This leads to a complete loss of contractile function (WT: wall thickening), i.e., akinesis in the CS region.
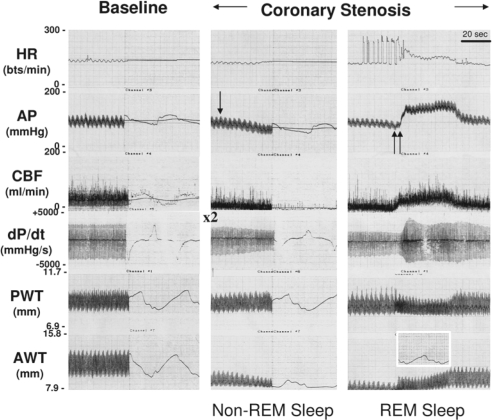
Figure 3 presents a representative hemodynamic tracing from one pig with a coronary stenosis during NREM sleep followed by REM sleep. This figure demonstrates that the onset of REM sleep (indicated by an abrupt marked increase in heart rate, arterial blood pressure, and dP/dtmax) eliminated the akinesis that was evident in the stenotic region during NREM sleep. This was associated with an increase in regional CBF.
Figure 3.
Representative tracings for hemodynamic variables at baseline (prior to stenosis) and during non-rapid eye movement (REM) (single arrow) and REM sleep (double arrow) in the presence of coronary stenosis. Non-REM sleep initiated a decrease in arterial pressure (AP), resulting in akinesis in the anterior wall (stenotic region). REM sleep induced a rapid increase in heart rate, arterial pressure, and dP/dtmax (rate of change of left ventricular pressure). The onset of REM sleep increased coronary blood flow, which returned anterior wall function to the post-stenotic condition prior to the onset of NREM sleep (see expanded tracing in box). HR refers to heart rate; AP, arterial pressure; CBF, coronary blood flow; PWT, posterior wall thickness; AWT, anterior wall thickness.
DISCUSSION
We present herein the first direct evidence that NREM sleep-induced hypotension can lead to myocardial akinesis (implying severe ischemia) in the presence of a flow-limiting coronary stenosis. This phenomenon occurred consistently and reproducibly in our swine model and was rapidly reversed by awakening. These findings are important because they may help to explain why adverse cardiac events occur at a high rate during the nocturnal period, with a second peak in early morning hours.1,2,14–17
Previous investigators have focused on the role of the sympathetic surges during REM sleep in the precipitation of nocturnal cardiac events.5,7,18 It is well established that enhanced sympathetic activity can promote myocardial ischemia, angina pectoris, and arrhythmias in patients with coronary insufficiency.5,7,18 The underlying mechanism for these effects is an imbalance between myocardial oxygen demand and oxygen supply caused by increases in cardiac work, i.e., arterial pressure and heart rate, in the absence of adequate increases in CBF.4,19 Slow-wave (NREM) sleep has been assumed by most previous investigators to be free of cardiac risk because it is associated with hypotension, bradycardia, and a reduction in cardiac output and systemic vascular resistance. This is largely ascribed to a reduction in sympathetic activity and, in part, to an increase in vagal activation.20 This view has failed to take into account the possibility that the reductions in arterial pressure during NREM sleep could lead to myocardial ischemia in patients with coronary artery disease, e.g., if the induced decreases in CBF were disproportionate to those in cardiac work.
Our findings are in keeping with the results of human and animal studies, indicating that arterial pressure decreases 10 to 20 mm Hg during the NREM phase of sleep.9,11,21–24 We observed that this hypotension was associated with decreases in CBF (and contractile function) in the region supplied by the stenotic coronary artery. Hypotension was accompanied by a modest increase in heart rate, presumably via a baroreflex-mediated activation of the cardiac sympathetic nerves sufficient to override the presumed increase in cardiac vagal activity. This reflex pathway has been shown to have heightened sensitivity during sleep.25 The increases in heart rate would have the effect of blunting the decrease in cardiac work caused by the reductions in afterload, while also reducing the duration of diastole, the phase during which most coronary perfusion occurs.26,27 The net effect of the hemodynamic changes during NREM sleep was severe myocardial ischemia, as reflected in akinesis in the stenotic region (Figure 1 and Table 1). The complete cessation of transmural contractile activity in the face of a relatively modest, 50%, decrease in CBF is noteworthy. It likely reflects a disproportionate decrease in subendocardial versus subepicardial flow because of more limited autoregulatory capability in the deep layers of the LV wall.28–30
Conventional thought has been that REM sleep, not NREM sleep, is harmful in myocardium with limited coronary reserve because the marked sympathetic activation, i.e., increases in heart rate and arterial pressure (afterload), results in an imbalance between myocardial oxygen supply and demand.9 However, in the current study (Figure 3), we demonstrated that REM sleep reversed the severe ischemia and resulting akinesis during NREM sleep. Apparently, the ability of the REM-related increase in arterial pressure, i.e., perfusion pressure, to augment CBF (presumably preferentially in the subendocardium), more than offset the effect of the increases in determinants of myocardial oxygen demand (afterload, heart rate, and LV contractility).
Two previous studies have examined the hemodynamic and CBF responses during sleep in normal animals and in animals with an experimentally-induced coronary stenosis,4,31 although neither obtained measurements of global or regional contractile function. In one of the studies conducted in pigs with a normal coronary circulation, Zinkovska et al31 found that NREM sleep caused no change in mean arterial pressure, heart rate, or CBF and that REM sleep caused a decrease in mean arterial pressure, a decrease in heart rate, and no change in CBF. In the other study performed in dogs fitted with an occluder to induce a 60% decrease in baseline CBF,4 it was observed that NREM sleep had no effect on mean arterial pressure, heart rate, or CBF, whereas REM sleep, during apparent sympathetic activation, caused an increase in heart rate and a decrease in CBF; mean arterial pressure was not affected. It is difficult to compare the present findings to those from these aforementioned studies because of differences in species or severity of the coronary stenosis. For example, the more severe coronary stenosis cited above 4 would have (1) rendered the subendocardial vasculature more vulnerable to mechanical closure because of increased extravascular compression secondary to tachycardia32 and (2) caused a more marked decrease in regional myocardial contractile function, which would have limited the ability of the heart to respond to stresses, including sympathetic activation.
It is important to recognize that the current findings apply specifically to the conditions of the present study, ie, a 30% decrease in baseline CBF. These conditions would have eliminated vasodilator reserve in the stenotic region, thus rendering CBF pressure-dependent and increasing the risk for myocardial ischemia. It would be expected that the heart with a less severe coronary stenosis would have a better ability to maintain perfusion in the presence of the drop in perfusion pressure during NREM sleep. It must be emphasized that our findings apply to a model of acute ischemia and cannot be extended to conditions of chronically stenotic arteries, such as occurs in atherosclerotic coronary artery disease.
The animals, which provided the findings during sleep, were subjects in a broader study evaluating the ability of chronic stunning, caused by repeated episodes of coronary stenosis, to recapitulate the phenotype of hibernating myocardium.13 Noteworthy is (1) that NREM sleep converted dyskinesis to akinesis regardless of the coronary stenosis episode and (2) that waking the animal reversed the effect immediately. These observations are evidence that the akinesis responses were not due to a cumulative effect of the repeated periods of ischemia but were undoubtedly the effect of the acute hemodynamic responses induced by sleep.
The animals that we studied were not equipped with electrodes for monitoring the electroencephalogram. Thus, states of wakefulness and sleep were surmised on the basis of the monitored hemodynamic responses (Figure 1 and 3) and observed behavior state. The use of a sling, which allowed the pig to rest comfortably and quietly for a long period of study while constrained, facilitated direct and uninterrupted observations of behavior.
The chronically instrumented pig has been used extensively by us13,33,34 and others31,35 for studies of cardiovascular physiology and pharmacology. An advantage of the pig is its size, which permits extensive hemodynamic monitoring, and a coronary circulation that, like that of the human, is free of intrinsic collateral vessels.36,37 Limitations of the pig are a weak vagal tone and a respiratory sinus arrhythmia that was intermediate in size between that observed in the dog and human.31
Clinical Implications
The conventional wisdom has been that sudden cardiac death during the nocturnal period occurs predominantly during REM sleep because of a surge in sympathetic activity, which increases the susceptibility to ventricular fibrillation and causes unfavorable changes in oxygen supply-demand balance.26,27 The period of NREM sleep, with its heightened cardiac vagal tone and reduced sympathetic activity, has been looked upon as a period of cardiac protection. The present study dispels this notion in demonstrating that NREM sleep can precipitate severe myocardial ischemia in a region of the left ventricle supplied by a coronary stenosis. This was due to the inability of the coronary circulation to autoregulate its flow in response to the sleep-induced decrease in arterial pressure (perfusion pressure). This observation has important clinical implications in relation to patients with limited coronary vasodilator reserve (and impaired autoregulatory capability), whether it be due to coronary artery disease, LV hypertrophy, or anemia.28–30 Episodes of myocardial ischemia during NREM sleep in these patients would increase their susceptibility to arrhythmias and myocardial infarction during the enhanced sympathetic activity accompanying REM sleep or early morning activities, both mental and physical, following awakening.38–40
An additional clinical implication of our findings relates to patients with either coronary artery disease or LV hypertrophy, or both these conditions, who may be taking antihypertensive drugs, or using nitroglycerin or long-acting nitrates to relieve acute anginal pain, during nighttime hours. The presence of such drugs could potentiate the hypotensive responses during NREM sleep leading to more pronounced decreases in CBF and an exacerbation of myocardial ischemia. The likelihood of this effect is increased by an enhanced sensitivity of the circulation to vasodilators during sleep.9 In light of our findings, it seems prudent to reevaluate the use of vasodilators at bedtime in the patient in whom impaired coronary autoregulation has been demonstrated or is suspected.
ACKNOWLEDGMENTS
This work was supported in part by National Institutes of Health Grants HL62442 and AHA Grant #0030125N and by the Joan and Norman Chapman Family Foundation.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Lavery CE, Mittleman MA, Cohen MC, Muller JE, Verrier RL. Nonuniform nighttime distribution of acute cardiac events: a possible effect of sleep states. Circulation. 1997;96:3321–7. doi: 10.1161/01.cir.96.10.3321. [DOI] [PubMed] [Google Scholar]
- 2.Thompson DR, Sutton TW, Jowett NI, Pohl JE. Circadian variation in the frequency of onset of chest pain in acute myocardial infarction. Br Heart J. 1991;65:177–8. doi: 10.1136/hrt.65.4.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King MJ, Zir LM, Kaltman AJ, Fox AC. Variant angina associated with angiographically demonstrated coronary artery spasm and REM sleep. Am J Med Sci. 1973;265:419–22. doi: 10.1097/00000441-197305000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Kirby DA, Verrier RL. Differential effects of sleep stage on coronary hemodynamic function during stenosis. Physiol Behav. 1989;45:1017–20. doi: 10.1016/0031-9384(89)90231-x. [DOI] [PubMed] [Google Scholar]
- 5.Nowlin JB, Troyer WG, Jr., Collins WS, et al. The association of nocturnal angina pectoris with dreaming. Ann Intern Med. 1965;63:1040–6. doi: 10.7326/0003-4819-63-6-1040. [DOI] [PubMed] [Google Scholar]
- 6.Tofler GH, Brezinski D, Schafer AI, et al. Concurrent morning increase in platelet aggregability and the risk of myocardial infarction and sudden cardiac death. N Engl J Med. 1987;316:1514–8. doi: 10.1056/NEJM198706113162405. [DOI] [PubMed] [Google Scholar]
- 7.Verrier RL, Muller JE, Hobson JA. Sleep, dreams, and sudden death: the case for sleep as an autonomic stress test for the heart. Cardiovasc Res. 1996;31:181–211. [PubMed] [Google Scholar]
- 8.Mancia G A Z. Cardiovascular regulation during sleep. New York, NY: Academic Press; 1980. [Google Scholar]
- 9.Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med. 1993;328:303–7. doi: 10.1056/NEJM199302043280502. [DOI] [PubMed] [Google Scholar]
- 10.Bonsignore MR, Smirne S, Marrone O, Insalaco G, Salvaggio A, Bonsignore G. Myocardial ischemia during sleep. Sleep Med Rev. 1999;3:241–55. doi: 10.1016/s1087-0792(99)90005-9. [DOI] [PubMed] [Google Scholar]
- 11.Floras JS. Antihypertensive treatment, myocardial infarction, and nocturnal myocardial ischaemia. Lancet. 1988;2:994–6. doi: 10.1016/s0140-6736(88)90745-3. [DOI] [PubMed] [Google Scholar]
- 12.Mancia G. Autonomic modulation of the cardiovascular system during sleep. N Engl J Med. 1993;328:347–9. doi: 10.1056/NEJM199302043280511. [DOI] [PubMed] [Google Scholar]
- 13.Kim SJ, Peppas A, Hong SK, et al. Persistent stunning induces myocardial hibernation and protection: flow/function and metabolic mechanisms. Circ Res. 2003;92:1233–9. doi: 10.1161/01.RES.0000076892.18394.B6. [DOI] [PubMed] [Google Scholar]
- 14.Muller JE, Stone PH, Turi ZG, et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med. 1985;313:1315–22. doi: 10.1056/NEJM198511213132103. [DOI] [PubMed] [Google Scholar]
- 15.Cannon CP, McCabe CH, Stone PH, et al. Circadian variation in the onset of unstable angina and non-Q-wave acute myocardial infarction (the TIMI III Registry and TIMI IIIB) Am J Cardiol. 1997;79:253–8. doi: 10.1016/s0002-9149(97)00743-1. [DOI] [PubMed] [Google Scholar]
- 16.Figueras J, Lidon RM. Circadian rhythm of angina in patients with unstable angina: relationship with extent of coronary disease, coronary reserve and ECG changes during pain. Eur Heart J. 1994;15:753–60. doi: 10.1093/oxfordjournals.eurheartj.a060582. [DOI] [PubMed] [Google Scholar]
- 17.Willich SN, Kulig M, Muller-Nordhorn J. European survey on circadian variation of angina pectoris (ESCVA) in treated patients. Herz. 2004;29:665–72. doi: 10.1007/s00059-004-2536-x. [DOI] [PubMed] [Google Scholar]
- 18.Brodsky M, Wu D, Denes P, Kanakis C, Rosen KM. Arrhythmias documented by 24 hour continuous electrocardiographic monitoring in 50 male medical students without apparent heart disease. Am J Cardiol. 1977;39:390–5. doi: 10.1016/s0002-9149(77)80094-5. [DOI] [PubMed] [Google Scholar]
- 19.Rowe K, Moreno R, Lau TR, et al. Heart rate surges during REM sleep are associated with theta rhythm and PGO activity in cats. Am J Physiol. 1999;277:R843–9. doi: 10.1152/ajpregu.1999.277.3.R843. [DOI] [PubMed] [Google Scholar]
- 20.Baccelli G, Guazzi M, Mancia G, Zanchetti A. Neural and non-neural mechanisms influencing circulation during sleep. Nature. 1969;223:184–5. doi: 10.1038/223184a0. [DOI] [PubMed] [Google Scholar]
- 21.Staessen JA, Bieniaszewski L, O'Brien E, et al. Nocturnal blood pressure fall on ambulatory monitoring in a large international database. The «Ad Hoc' Working Group. Hypertension. 1997;29:30–9. doi: 10.1161/01.hyp.29.1.30. [DOI] [PubMed] [Google Scholar]
- 22.Van de Borne P, Nguyen H, Biston P, Linkowski P, Degaute JP. Effects of wake and sleep stages on the 24-h autonomic control of blood pressure and heart rate in recumbent men. Am J Physiol. 1994;266:H548–54. doi: 10.1152/ajpheart.1994.266.2.H548. [DOI] [PubMed] [Google Scholar]
- 23.Mancia G, Baccelli G, Adams DB, Zanchetti A. Vasomotor regulation during sleep in the cat. Am J Physiol. 1971;220:1086–93. doi: 10.1152/ajplegacy.1971.220.4.1086. [DOI] [PubMed] [Google Scholar]
- 24.Schaub CD, Tankersley C, Schwartz AR, Smith PL, Robotham JL, O'Donnell CP. Effect of sleep/wake state on arterial blood pressure in genetically identical mice. J Appl Physiol. 1998;85:366–71. doi: 10.1152/jappl.1998.85.1.366. [DOI] [PubMed] [Google Scholar]
- 25.Conway J, Boon N, Jones JV, Sleight P. Involvement of the baroreceptor reflexes in the changes in blood pressure with sleep and mental arousal. Hypertension. 1983;5:746–8. doi: 10.1161/01.hyp.5.5.746. [DOI] [PubMed] [Google Scholar]
- 26.Berne RM, Rubio R. Regulation of coronary blood flow. Adv Cardiol. 1974;12:303–17. doi: 10.1159/000395474. [DOI] [PubMed] [Google Scholar]
- 27.Feigl EO. Coronary physiology. Physiol Rev. 1983;63:1–205. doi: 10.1152/physrev.1983.63.1.1. [DOI] [PubMed] [Google Scholar]
- 28.Hittinger L, Shannon RP, Bishop SP, Gelpi RJ, Vatner SF. Subendomyocardial exhaustion of blood flow reserve and increased fibrosis in conscious dogs with heart failure. Circ Res. 1989;65:971–80. doi: 10.1161/01.res.65.4.971. [DOI] [PubMed] [Google Scholar]
- 29.Hittinger L, Shen YT, Patrick TA, et al. Mechanisms of subendocardial dysfunction in response to exercise in dogs with severe left ventricular hypertrophy. Circ Res. 1992;71:423–34. doi: 10.1161/01.res.71.2.423. [DOI] [PubMed] [Google Scholar]
- 30.Vatner SF, Shannon R, Hittinger L. Reduced subendocardial coronary reserve. A potential mechanism for impaired diastolic function in the hypertrophied and failing heart. Circulation. 1990;81:III8–14. [PubMed] [Google Scholar]
- 31.Zinkovska SM, Rodriguez EK, Kirby DA. Coronary and total peripheral resistance changes during sleep in a porcine model. Am J Physiol. 1996;270:H723–9. doi: 10.1152/ajpheart.1996.270.2.H723. [DOI] [PubMed] [Google Scholar]
- 32.Neill WA, Oxendine J, Phelps N, Anderson RP. Subendocardial ischemia provoked by tachycardia in conscious dogs with coronary stenosis. Am J Cardiol. 1975;35:30–6. doi: 10.1016/0002-9149(75)90555-x. [DOI] [PubMed] [Google Scholar]
- 33.Kim SJ, Depre C, Vatner SF. Novel mechanisms mediating stunned myocardium. Heart Fail Rev. 2003;8:143–53. doi: 10.1023/a:1023040718319. [DOI] [PubMed] [Google Scholar]
- 34.Kudej RK, Kim SJ, Shen YT, et al. Nitric oxide, an important regulator of perfusion-contraction matching in conscious pigs. Am J Physiol Heart Circ Physiol. 2000;279:H451–6. doi: 10.1152/ajpheart.2000.279.1.H451. [DOI] [PubMed] [Google Scholar]
- 35.Fallavollita JA, Perry BJ, Canty JM., Jr. 18F-2-deoxyglucose deposition and regional flow in pigs with chronically dysfunctional myocardium. Evidence for transmural variations in chronic hibernating myocardium. Circulation. 1997;95:1900–9. doi: 10.1161/01.cir.95.7.1900. [DOI] [PubMed] [Google Scholar]
- 36.Eckstein RW. Coronary interarterial anastomoses in young pigs and mongrel dogs. Circ Res. 1954;2:460–5. doi: 10.1161/01.res.2.5.460. [DOI] [PubMed] [Google Scholar]
- 37.Patterson RE, Kirk ES. Analysis of coronary collateral structure, function, and ischemic border zones in pigs. Am J Physiol. 1983;244:H23–31. doi: 10.1152/ajpheart.1983.244.1.H23. [DOI] [PubMed] [Google Scholar]
- 38.Cohen MC, Rohtla KM, Lavery CE, Muller JE, Mittleman MA. Meta-analysis of the morning excess of acute myocardial infarction and sudden cardiac death. Am J Cardiol. 1997;79:1512–6. doi: 10.1016/s0002-9149(97)00181-1. [DOI] [PubMed] [Google Scholar]
- 39.Willich SN, Linderer T, Wegscheider K, Leizorovicz A, Alamercery I, Schroder R ISAM Study Group. Increased morning incidence of myocardial infarction in the ISAM Study: absence with prior beta-adrenergic blockade. Circulation. 1989;80:853–8. doi: 10.1161/01.cir.80.4.853. [DOI] [PubMed] [Google Scholar]
- 40.Willich SN, Lowel H, Lewis M, et al. TRIMM Study Group. Association of wake time and the onset of myocardial infarction. Triggers and mechanisms of myocardial infarction (TRIMM) pilot study. Circulation. 1991;84:VI62–7. [PubMed] [Google Scholar]