Abstract
Study Objectives:
To define which leg movements (LM) associated with restless legs syndrome (RLS) respond to dopamine-agonist treatment and verify if they fall within current diagnostic criteria for periodic LM during sleep (PLMS).
Design:
Single-blind placebo-controlled study.
Settings:
Sleep laboratory.
Patients:
43 consecutive untreated patients with idiopathic restless legs syndrome.
Interventions:
Patients underwent clinical and neurophysiological evaluation, hematological screening, and 2 consecutive full-night polysomnographic studies. Before the second polysomnographic study, all patients were randomized to receive 0.25 mg of pramipexole or placebo.
Measurements and results:
LM parameters such as duration, amplitude, interval, and periodicity were analyzed. Compared to placebo, pramipexole significantly (P < 0.01) reduced PLMS while increasing sleep efficiency. Specifically we observed a significant (P < 0.01) reduction in LM ranging 2-4 s in duration and with intermovement interval of 6-46 s and a significant decrease in the periodicity of motor events. No effect of pramipexole was observed on isolated LM.
Conclusions:
These results support a heterogeneous basis for LM in RLS patients; while isolated LM do not respond to pramipexole treatment, most, but not all, PLMS classified by means of the current criteria do. Further studies with different pramipexole doses or dopamine agonists with different receptor-binding preference are warranted to better define the borders of dopamine response of PLMS.
Citation:
Manconi M; Ferri R; Feroah TR; Zucconi M; Ferini-Strambi L. Defining the boundaries of the response of sleep leg movements to a single dose of dopamine agonist. SLEEP 2008;31(9):1229-1237.
Keywords: Periodic leg movements, restless legs syndrome, dopamine
PERIODIC LEG MOVEMENTS DURING SLEEP (PLMS) ARE ONE OF THE MOST INTRIGUING AND STILL INCOMPLETELY UNDERSTOOD MOTOR PHENOMENA affecting millions of people worldwide. However, the present definitions for PLMS are fundamentally based on observations, the scope of which has not been explicitly defined.
PLMS were first observed by Symmonds in 1953,1 but clearly identified with electromyography (EMG) by Lugaresi et al.2 when 2 electrodes were placed over each tibialis anterior (TA) muscle during a standard polysomnographic recording. The initial term “nocturnal myoclonus,” proposed by Symmonds,1 was changed to “PLMS” because of the length of the contractions (longer than 0.5 s) and their pseudoperiodic occurrence.
PLMS are repetitive leg jerks characterized by a flexion movement at ankle, knee, and hip, which arise from sleep, especially during NREM stages 1 and 2, and from relaxing wakefulness (periodic leg movements during wakefulness, PLMW).3 Since arms may be also involved, the expression of “periodic limb movements” is considered to be more appropriate.4 PLMS represent the only diagnostic objective marker in restless legs syndrome (RLS), occurring in more than 80% of these patients.5 Although the diagnostic specificity of the PLMS is notably lowered by their frequent occurrence in other sleep or neurological disorders,6 as well as in healthy elderly subjects,7 the detection of PLMS is a standard procedure in accredited sleep laboratories. Although the clinical significance of PLMS is still debated, the methodology for the detection and the criteria for the scoring of PLMS have improved considerably.
PLMS are often associated with cortical arousals, insomnia, and excessive daytime sleepiness, but the direct effects of PLMS, as well as the nosological entity of the so-called periodic leg movement disorder (PLMD) remain controversial.8,9 Although the etiopathogenesis of PLMD and RLS is still unknown, several lines of evidence suggest dopaminergic system dysfunction as their basic mechanism.10 Usually, low doses of dopamine agonists are immediately effective in RLS and PLMS11–13; PLMS can be associated with Parkinson disease14 and the circadian PLMS distribution is inversely related to the levels of blood and cerebrospinal fluid dopamine.15
PLMS Scoring Criteria
Based on the work of Coleman,16,17 the American Sleep Disorders Association (ASDA) established the standard criteria in 1993 to recognize and quantify PLMS.18 The ASDA rules defined PLMS as EMG activity of TA during sleep ranging between 0.5 and 5 s in duration, exceeding 25% of the amplitude of the pre-recording voluntary contraction of TA, separated from the following and the previous leg movements (LM) by an interval ranging from 4 to 90 s, and included in a series of ≥ 4 LM in a row. When the interval between the end of a LM in one leg and the onset of a LM in the opposite leg is < 4 s, both LM should be classified as a single bilateral PLMS and as 2 distinct monolateral PLMS when the separation is ≥ 4 s. Based on new reliable automatic computed detection19 and a more sophisticated statistic analysis,20 new criteria have been recently proposed by a task force of the IRLSSG (International RLS Study Group) and endorsed by the World Association of Sleep Medicine.21 The main changes included: increase in the maximum duration of a PLM to 10 seconds; reduction of the minimum duration needed to separate 2 EMG bursts into separate LM to 0.5 s—with bursts either in the same or separate legs; and revision of the minimum amplitude to 8μV above baseline EMG level. Moreover, a new periodicity index (number of intervals in sequences of ≥ 3 inter-LM intervals 10 < i ≤ 90 s / total number of inter-LM intervals) has been proposed to quantify the time structure of the whole EMG TA activity during sleep.20
Also for these new criteria, the validation was based on the statistic evaluation of the significant discrepancies between RLS and control subjects in whole-sleep TA activity. In other words, all LM that, in terms of amplitude, duration, and intermovement interval, were significantly greater in RLS patients than healthy subjects, were considered to be part of the pathological phenomenon and classified as PLMS. Such criteria are therefore the fruit of a statistically driven approach to a biological phenomenon.
The aim of the present study was to delineate the boundaries of PLMS, in terms of amplitude, duration, and periodicity by applying a new pharmacological approach to identify a possible LM subgroup with a common responsiveness to dopamine agonist treatment. We also applied statistical methods to redefine the PLMS boundaries, by moving from a correlational-observational research where variables are not influenced but only measured for their relations, toward an experimental research method where independent variables are manipulated to measure their effects on the dependent variables.
METHODS
Subjects
A prospective single-blind placebo-controlled study with a consecutive enrolment of subjects affected by idiopathic RLS was carried out on patients admitted to the sleep center. The diagnosis of RLS was established following the IRLSSG criteria.22 In addition, for inclusion into the study, the mean frequency of symptoms during the last 6 months had to be > 2 times per week, with a score ≥ 20 on the IRLSSG rating scale (corresponding to severe RLS).23 Only patients between the ages 35 and 85 years, free of medication at the time of the study, and never treated before for RLS (including dopaminergic agents, benzodiazepines, opioids, and anticonvulsants) were included in the study. Subjects suffering from known causes of secondary RLS (renal failure, anemia with iron deficiency, pregnancy, rheumatoid arthritis, recent anesthesia, or clinical myelopathy and peripheral neuropathy), other sleep disorders (e.g., narcolepsy, sleep terrors, sleepwalking, sleep disordered breathing), other movement disorders, or any other medical conditions that would affect the assessment of RLS were excluded from the study.
All patients underwent a neurological examination, routine blood tests (including serum iron and ferritin, vitamin B12, and folate), electromyography (EMG) and electroneurography of the lower limbs. Patients with any abnormality at the above-mentioned tests and with an apnea/hypopnea index > 5 were also excluded.
All subjects underwent 2 nocturnal polysomnographic recordings, after one adaptation night, and were randomly subdivided into 2 subgroups: pramipexole and placebo groups. Before the 2nd night recording, one group received a single oral dose of 0.25 mg pramipexole at 21:00, while the other group received a placebo (single blind procedure). No medication was administered before the first night recording (baseline). All patients gave written consent for these procedures and were unaware of the content (drug or placebo) of the medication. The local ethics committee approved the study.
Nocturnal Polysomnography
Nocturnal polysomnography was carried out after one adaptation night in a standard sound-attenuated (noise level to a maximum of 30 dB nHL) sleep laboratory room. Subjects were not allowed caffeinated beverages the afternoon preceding the recordings and were allowed to sleep until their spontaneous awakening in the morning. Lights-out time was based on individual habitual bedtime and ranged between 22:30 and 23:30. The following signals were recorded: EEG (at least 6 channels, including C3 or C4 and O1 or O2, referred to the contralateral mastoid); electrooculogram (electrodes placed 1 cm above the right outer cantus and 1 cm below the left outer cantus and referred to the left mastoid), electromyogram (EMG) of the submentalis muscle, EMG of the right and left TA muscles (bipolar derivations with two electrodes placed 3 cm apart on the belly of the TA muscle of each leg, impedance was kept less than 10 KΩ, and ECG (CM4 derivation: anode in position V4 and cathode attached to the manubrium of the sternum). Sleep signals were sampled at 200 Hz and stored on hard disk in European data format24 for further analysis. EMG signals, in particular, were digitally band-pass filtered at 10–100 Hz, with a notch filter at 50 Hz. The sleep respiratory pattern of each patient was monitored using oral and nasal airflow thermistors and/or nasal pressure cannula, thoracic and abdominal respiratory effort strain gauge, and by monitoring oxygen saturation (pulse oximetry). This was performed in all subjects in a previous recording (within one week) or during the study recording.
Sleep Scoring and Detection of Leg Movements
Prior to any recording, we verified that the rectified EMG amplitude recorded from the 2 TA muscles was < 2 μV at rest and exceeded 7–10 μV for small voluntary flexions of the foot. Sleep stages were visually scored following standard criteria on 30-s epochs using the sleep analysis software Hypnolab 1.2 (SWS Soft, Italy; http://www.sws-soft.com). LM during sleep were first detected by the same software which allows their computer-assisted detection.19 With this software, the detection is performed on the rectified EMG signal, using a human-supervised automatic approach controlled by the scorer (blind to the treatment assignment) that used the newly introduced WASM-IRLSSG criteria.21 The performance of this system has been evaluated and validated,19 but in this study one scorer, blind to treatment assignment, visually edited the detections proposed by the automatic analysis, before computing the final results. In particular, the total LM Index was calculated to represent the total number of leg movements per hour of sleep while the PLMS index was calculated as the number of LM included in a series ≥ 4, separated by > 5 and < 90 s per hour of sleep.
Further Analysis of Leg Movement Structure
In this analysis, we included, in line with the new WASM-IRLSSG rules,21 all LM (isolated and periodic) with the following features: (a) Duration—All movements lasting 0.5–10 s; (b) Amplitude—the amplitude of the EMG signal from the 2 TA muscles was < 2 μV at rest and exceeded 7–10 μV for small voluntary flexions of the foot. LM were included when the EMG increased to ≥ 8 μV above the resting baseline (e.g., 10 μV for a baseline of 2 μV, for our rectified signal), the ending point was when the EMG decreased to < 2 μV above the resting level and remained below that value for ≥ 0.5 s All these values were calculated on the rectified EMG signals. The analysis also took in consideration the following characteristics: (c) Sleep stage—sleep stage in which each LM starts; (d) Side—Right or left leg; (e) Start and ending time—these 2 values were used for the calculation of the 2 intervals described below; (f) Interval 1—this interval was defined as the time between the onset of 2 subsequent LM and was used for the evaluation of their periodicity (see below); (g) Interval 2—defined as the time between the end of one LM and the onset of the following LM; this interval was used for the separation of different LM occurring in the same leg on or the contralateral leg; (h) Minimum interval between two different LM—we applied a time resolution of 0.5 s for the detection of the presence of movement (see above), then we applied the same time resolution for the detection of the absence of movement. For this reason, the minimum interval between different LM (Interval 2) was set to 0.5 s; (i) Bilateral/monolateral movements—bilateral LM were defined as 2 EMG bursts on the 2 legs separated (Interval 2) by < 0.5 s; monolateral LM were defined as EMG bursts involving only one leg and separated by ≥ 0.5 s from any other LM in the opposite leg; (j) Periodicity Index (PI)—number of intervals belonging to sequences of ≥ 3 inter-LM intervals 10 < i ≤ 90 s / total number of inter-LM intervals. This index can vary between 0 (absence of periodicity) to 1 (all intervals with length 10 < i ≤ 90 s).20 PI is independent on the absolute number of LM recorded and was calculated for all the subjects included in this study.
Statistical Analysis
The comparison between the different sleep scoring and LM parameters obtained before and after the administration of pramipexole or placebo was carried out by means of the factorial analysis of variance (ANOVA), with group (pramipexole or placebo) and night (baseline or treatment) as factors; we only considered parameters with a significant ANOVA result (P < 0.05) for further analysis, then we reported the univariate analysis results only for such significant parameters and, only when they were significant, we proceeded with the LSD post hoc test for multiple comparisons. For this analysis the commercially available Statistica software package (StatSoft, Inc., 2001. STATISTICA data analysis software system, version 6. www.statsoft.com) was used. All values (unless otherwise stated) are expressed as mean values ± standard deviation. The statistical comparison between the distribution of inter-LM intervals, the distribution of LM duration, the distribution of LM area-under-curve, and the night distribution of number of PLMS, isolated LM, and total LM, before and after treatment with pramipexole or placebo was performed by means of the Student t-test; to take into account the multiple comparisons performed, the Bonferroni correction was applied; differences were considered significant when they reached the P < 0.05 level after this correction.
RESULTS
Forty-three consecutive untreated patients were included in this study (19 males and 24 females); 25 patients (11 males and 14 females, mean age 59.3 years, ± 10.87, range 37–75) received pramipexole and 18 (8 males and 10 females, mean age 56.4 years, ± 11.27, range 41–83) were given placebo. Two patients treated with pramipexole and another one who was administered placebo reported mild morning nausea; the rest of the patients did not report significant side effects. The mean RLS symptom severity, measured by means of the International RLS Rating Scale, was similar in both groups of patients (pramipexole treated group 27.3 ± 5.2; placebo treated group 26.7 ± 4.6).
Table 1 shows the sleep scoring parameters obtained before and after the administration of pramipexole or placebo. A significant increase (P < 0.01) in sleep efficiency and the percentage of sleep stage 2 were observed in the pramipexole treatment night compared to the baseline study. Only the percentage of REM sleep was found to be lower in the pramipexole group than the placebo group during the baseline study. The remaining sleep scoring parameters did not show significant differences between the 2 groups at baseline or after treatment.
Table 1.
Comparison Between the Sleep Scoring Parameters Found Before (1st Night = Baseline) and After (2nd Night) Treatment with Pramipexole or placebo
| Baseline |
Treatment |
ANOVA |
Post-hoc comparisons |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pramipexole |
Placebo |
Pramipexole |
Placebo |
Baseline vs. Treatment |
Pramipexole vs. Placebo |
||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Night | Group | Night×Group | Pramipexole | Placebo | Baseline | Treatment | |
| TIB, min | 504.2 | 96.16 | 514.5 | 83.99 | 518.4 | 43.40 | 481.2 | 91.21 | N.S. | N.S. | N.S. | ||||
| SPT, min | 466.7 | 98.82 | 480.3 | 82.86 | 490.7 | 48.43 | 453.4 | 88.63 | N.S. | N.S. | N.S. | ||||
| TST, min | 332.4 | 118.99 | 397.3 | 92.01 | 407.4 | 62.14 | 365.8 | 79.39 | N.S. | N.S. | 0.009 | ||||
| SL, min | 26.0 | 30.76 | 21.9 | 22.38 | 17.6 | 22.64 | 17.5 | 20.62 | N.S. | N.S. | N.S. | ||||
| RL, min | 117.2 | 89.95 | 96.8 | 39.26 | 133.8 | 88.29 | 120.6 | 63.38 | N.S. | N.S. | N.S. | ||||
| SS/hour | 11.2 | 4.58 | 12.6 | 4.10 | 13.4 | 4.28 | 12.2 | 4.09 | N.S. | N.S. | N.S. | ||||
| AWN/hour | 4.9 | 2.68 | 4.3 | 2.55 | 5.7 | 3.26 | 4.2 | 2.38 | N.S. | N.S. | N.S. | ||||
| SE% | 65.2 | 19.36 | 77.1 | 11.10 | 78.7 | 10.41 | 76.6 | 11.53 | 0.035 | N.S. | 0.025 | 0.0008 | N.S. | ||
| WASO, % | 30.0 | 18.84 | 17.3 | 12.38 | 17.0 | 9.05 | 18.6 | 12.27 | 0.1>P>0.05 | N.S. | 0.02 | ||||
| S1, % | 6.3 | 6.02 | 6.7 | 4.04 | 5.3 | 4.74 | 5.9 | 4.57 | N.S. | N.S. | |||||
| S2, % | 38.0 | 10.79 | 43.6 | 10.65 | 49.9 | 12.00 | 44.7 | 10.32 | 0.008 | N.S. | 0.03 | 0.0002 | N.S. | ||
| SWS, % | 13.7 | 8.32 | 15.3 | 8.92 | 15.6 | 7.50 | 16.2 | 5.94 | N.S. | N.S. | N.S. | ||||
| REM, % | 12.1 | 7.91 | 17.1 | 5.23 | 12.2 | 4.82 | 14.6 | 4.54 | N.S. | 0.005 | N.S. | 0.007 | N.S. | ||
TIB, time in bed; SPT, sleep period time; TST, total sleep time; SL, sleep latency; RL, REM latency; SS, stage shifts; AWN, awakenings number; SE, sleep efficiency; WASO, wakefulness after sleep onset; S1, stage 1; S2, stage 2; SWS, slow wave sleep; REM, REM sleep.
Table 2 presents the LM scoring parameters obtained before and after the administration of pramipexole or placebo. In the group treated with pramipexole the total LM index, PLMS index, and the number of PLMS sequences were significantly lower during the pramipexole treatment night compared to the baseline study. In contrast, the PLMS mean duration was slightly but significantly increased in this same group. The number of PLMS sequences and PI were significantly lower in the pramipexole group compared to the placebo group after treatment but not at baseline. The index and the duration of the isolated LM were unchanged after pramipexole administration. No detectable changes in LM scoring parameters were observed following placebo treatment.
Table 2.
Comparison between the WASM PLMS parameters found during sleep before (1st night = baseline) and after (2nd night) treatment with pramipexole or placebo
| Baseline |
Treatment |
ANOVA |
Post-hoc comparisons |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pramipexole |
Placebo |
Pramipexole |
Placebo |
Baseline vs. Treatment |
Pramipexole vs. Placebo |
||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Night | Group | Night×Group | Pramipex. | Placebo | Baseline | Treatment | |
| Total LM, index | 70.2 | 58.37 | 49.1 | 24.22 | 21.0 | 16.81 | 57.4 | 34.93 | 0.016 | N.S. | 0.00085 | 0.00001 | N.S. | ||
| PLMS, index | 61.9 | 58.06 | 40.1 | 24.95 | 11.1 | 17.38 | 47.9 | 33.96 | 0.011 | N.S. | 0.00065 | 0.00001 | N.S. | ||
| Isolated LM, index | 8.2 | 3.29 | 9.0 | 2.33 | 9.9 | 3.77 | 9.6 | 4.41 | N.S. | N.S. | N.S. | ||||
| PLMS sequences, n | 11.4 | 5.14 | 13.4 | 6.03 | 7.1 | 6.95 | 10.7 | 4.42 | 0.006 | 0.030 | N.S. | 0.008 | N.S. | N.S. | 0.046 |
| PLMS sequence duration, s | 119.7 | 353.43 | 161.7 | 209.63 | 23.7 | 60.52 | 153.7 | 151.02 | N.S. | N.S. | N.S. | ||||
| PLMS duration, s | 3.0 | 0.80 | 2.8 | 0.58 | 3.7 | 0.98 | 2.8 | 0.60 | 0.039 | 0.001 | 0.04 | 0.001 | N.S. | N.S. | 0.0002 |
| Isolated LM duration, s | 2.9 | 1.12 | 3.1 | 1.02 | 3.4 | 0.93 | 3.1 | 0.91 | N.S. | N.S. | N.S. | ||||
| Periodicity Index | 0.726 | 0.132 | 0.723 | 0.135 | 0.564 | 0.193 | 0.770 | 0.118 | N.S. | 0.003 | 0.002 | N.S. | 0.00003 | ||
LM leg movements, PLMS periodic leg movements during sleep (parameters further defined in Results section).
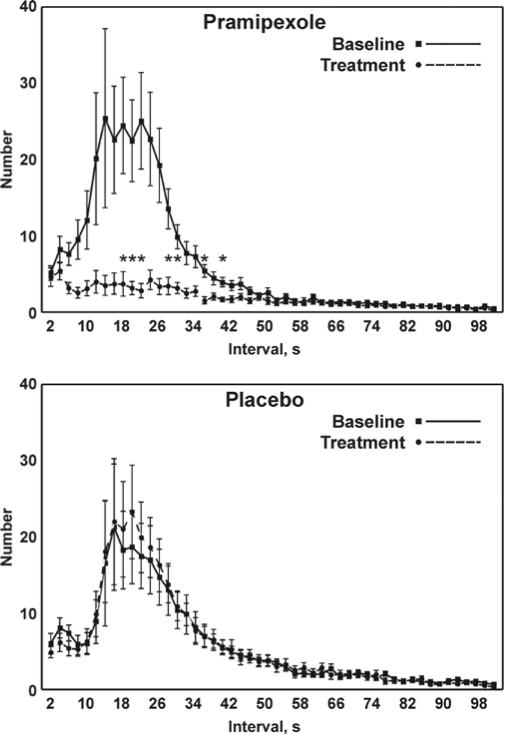
Figure 1 shows the distribution of inter-LM intervals before (baseline) and after treatment with pramipexole (panel A) or placebo (panel B). In both groups the inter-LM intervals during the baseline follows a bimodal distribution with a smaller peak at ∼3 s and a larger peak close to 20 s.
Figure 1.
Comparison between the distribution of inter-LM intervals before (baseline) and after treatment with pramipexole (top panel) or placebo (bottom panel); values are shown as mean and SEM (whiskers). Asterisks indicate the points for which a significant difference was found between the graphs in each panel at statistical analysis (Student t-test followed by the Bonferroni correction for 50 comparisons; P < 0.001*50 = P < 0.05).
While the first peak remained almost unchanged, the second peak was considerably flattened after pramipexole treatment, reaching statistical significance (P < 0.01) between 18 and 40 s; however, some statistically nonsignificant changes were evident in a larger interval range (6 < i ≤ 46 s). No statistically significant differences were observed for the curves obtained in the placebo treatment group.
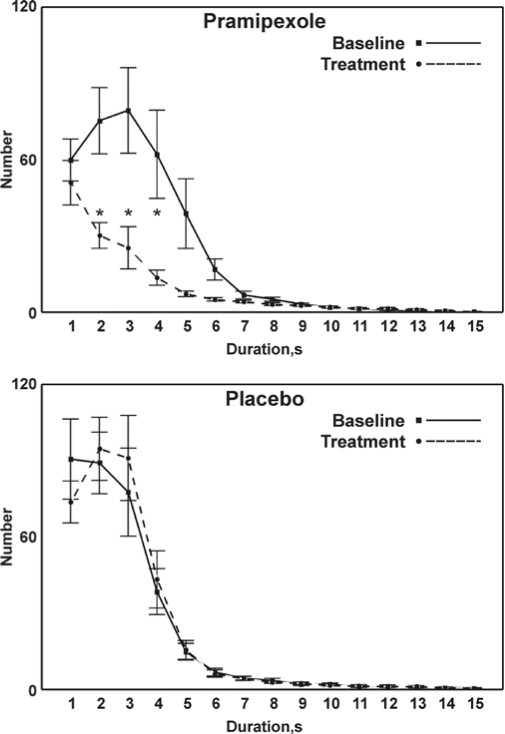
The distribution of LM duration during the baseline study and after treatment is shown in Figure 2. A significant reduction (P < 0.01) is seen in LM durations following pramipexole treatment (panel A) compared to the pramipexole baseline study. Also in this case, only the two curves related to the pramipexole group show clear differences reaching statistical significance for values approximately between 2 and 4 s.
Figure 2.
Comparison between the distribution of LM duration before (baseline) and after treatment with pramipexole (top panel) or placebo (bottom panel); values are shown as mean and SEM (whiskers). Asterisks indicate the points for which a significant difference was found between the graphs in each panel at statistical analysis (Student t-test followed by the Bonferroni correction for 15 comparisons; P < 0.0033*15 = P < 0.05).
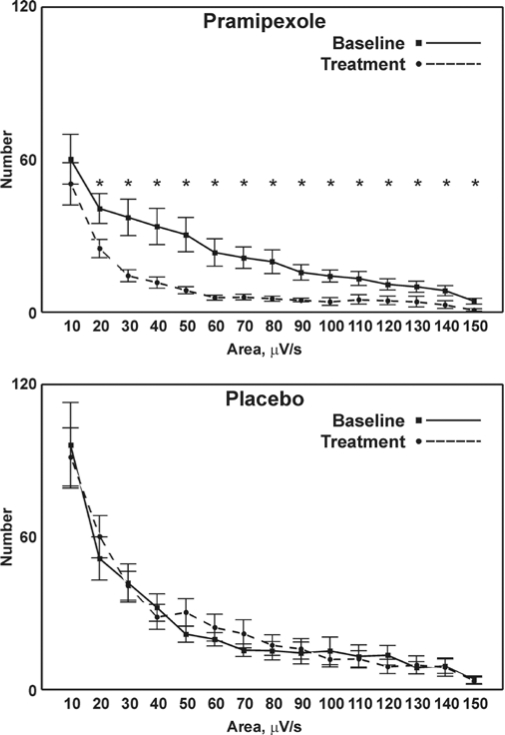
The distribution of LM area-under-curve (Figure 3, panel A) further demonstrates a significant reduction (P < 0.01) in the pramipexole treatment group compared to the values found during the baseline study. This reduction was significant for values of 20 μV/s and greater. No differences were observed for either LM duration or LM area-under-curves in the placebo groups (Figure 2 and Figure 3).
Figure 3.
Comparison between the distribution of LM area-under-curve before (baseline) and after treatment with pramipexole (top panel) or placebo (bottom panel); values are shown as mean and SEM (whiskers). Asterisks indicate the points for which a significant difference was found between the graphs in each panel at statistical analysis (Student t-test followed by the Bonferroni correction for 15 comparisons; P < 0.00333*15 = P < 0.05).
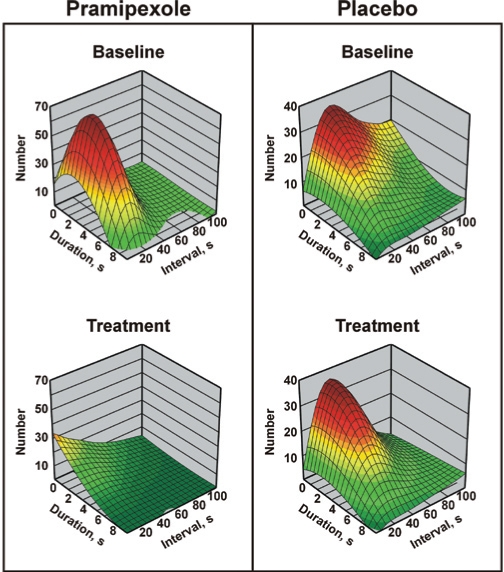
In Figure 4, the 3-D graph depicts the distribution of the total number of LM classified per duration and interval in the pramipexole (left panels) and placebo (right panels) groups. With this graphical representation it is possible to see that within the limits allowed by the current criteria for PLMS, only LM characterized by a slightly narrower range of duration (approximately 2–7 s) and interval values (approximately 10–50 s) were dramatically decreased after pramipexole treatment (bottom left panel) compared to baseline results (top left panel). Placebo treatment group values remained unaffected (right panels).
Figure 4.
3-D graph of the distribution of the total number of LM classified per duration and interval in the pramipexole (left panels) and placebo (right panels) groups, at baseline (top panels) and after treatment (bottom panels). Please note that this graph was drawn by fitting a distance-weighted least-square function to the measured values.
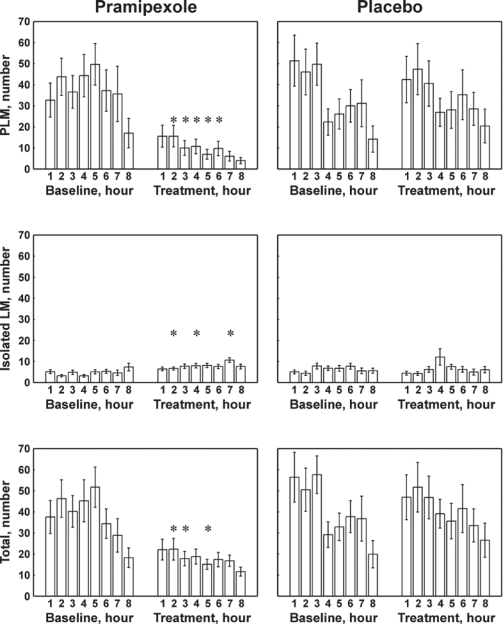
In Figure 5, the results of the distribution of the number of PLMS, isolated LM, and total LM for the baseline and treatment studies with pramipexole or placebo are shown. During the baseline studies the number of PLMS and the total LM progressively decreased throughout the night in both groups of patients, while isolated LM resulted equally distributed across the night. However, clear and significant differences (P < 0.01) were induced by pramipexole treatment with a decrease in PLMS and of total LM throughout the whole night, paralleled by a small, but sometimes significant, increase in isolated LM.
Figure 5.
Comparison between the distribution of number of PLMS (top panel), isolated LM (middle panel), and total LM (bottom panel) per hour of sleep (first 8 h shown) before (1st night - baseline) and after (2nd night) treatment with pramipexole (left panels) or placebo (right panels). All values are shown as mean and SEM (whiskers). Asterisks indicate the points for which a significant difference was found between the graphs in each panel at statistical analysis (Student t-test followed by the Bonferroni correction for 8 comparisons; P < 0.00625*8 = P < 0.05).
DISCUSSION
The significant findings of this study are based on the use of new analytical tools and on the application of the most recent criteria for recording and scoring PLMS; in this way, we quantified and characterized in details the LM activity of our patients and then evaluated the usefulness of these new measures in the description of changes following a single low dose of a preferential D3-dopamine agonist or placebo. The major result of this experimental pharmacological intervention is the differential response of distinct LM ranges to the acute treatment with pramipexole. Secondary findings of this study include the efficacy of pramipexole even during the initial night of use and the night-to-night variability of the temporal features of PLMS seen in the placebo group.
Pramipexole produced an important effect in the primary measures of PLMS in RLS patients. The significant improvement in PLMS in the first night of treatment with a low dose of pramipexole observed in this study was recently suggested by our group, in an essentially clinical study, which demonstrated an acute and significant effect of pramipexole also on symptoms subjectively reported by RLS patients.13 In the present study, the treatment effect is also associated with a modest but significant increase in the percentage of sleep stage 2 NREM. Similar results have been obtained in the past also after acute administration of ropinirole.11,25 These findings may also have clinical implications for the use of a low dose of dopamine agonists as on-demand therapy for mild or intermittent RLS13 and as a pharmacological test for the diagnosis of uncertain RLS cases.26
While the single-dose of pramipexole inhibited PLMS across the whole night, their reduction was most evident between the second and the sixth hour of recording; this time-dependent response might be explained by the molecular pharmacokinetics of pramipexole. Pramipexole is a preferential D3-agonist with an oral biodisponibility > 90%, which takes about 1 hour to exert its effect, and with a t1/2 of 8–12 h.27
PLMS are the primary objective marker for RLS, and, as demonstrated by this study, are not influenced by the placebo effect. Thus, the reliability of this polysomnographic marker depends to a large degree on its night-to-night variability/stability. This topic has been previously evaluated by two studies.6,28 Sforza et al. observed that the PLMS index and their nocturnal pattern of occurrence are reliable across nights in RLS patients;28 while Hornyak and coworkers found a variability of the PLMS index between 2 consecutive nights.6 In our study, no significant night-to-night variability of the PLMS index was observed between the baseline study and the placebo treatment study. However, we did find a small nonsignificant night-to-night variability in other measures of PLMS such as duration, intermovement interval, area under the curve, and periodicity.
While we found a significant effect of pramipexole on PLMS, we did not find a similar effect on isolated LMs. The first dose effect of pramipexole on PLMS and the related decrease in the periodicity index during sleep support the dopaminergic hypothesis for the origin of PLMS. However, it also suggests separate neurotransmitter for the regulation of non-periodic LM. The view that a different neurotransmitter is involved in periodic and non-periodic TA activity is also supported by the dissimilar pattern of these 2 motor phenomena observed during the baseline night. In this study, the PLMS index progressively declined across the night, while isolated LM activity showed no particular pattern with stable levels during the night (Figure 5).
Even though the current criteria18,21 for PLMS scoring consider intervals between 5 and 90 s, our analysis shows that a narrower range of PLMS intervals seem to be influenced by pramipexole treatment, i.e., 6 ≤ i ≤ 46 s. At this point, it should be noted that the use of intervals as short as 0.5 s is of fundamental importance to reach the conclusion that the real periodic phenomenon lies between narrower boundaries and only including as many different intervals as possible it is possible to pick up the characteristics of a different significant range of events. Similarly, we found an effect of pramipexole restricted to PLMS with a duration between 2 and 4 s, while the proposed PLMS scoring criteria include a duration ranging between 0.5 and 5 or 10 s.21 However, we should admit that the lack of a statistical significant decrease of PLMS longer than 7 s in duration and of PLMS with an intermovement interval longer than 46 s may also depend on the scarcity of events with these features.
The decreased effect on PLMS longer than 4–5 s is also responsible for the mild but significant increase in PLMS mean duration observed in the pramipexole treatment night. PLMS with a long duration (> 5 s) are frequently associated with a EEG desynchronization or with a brief awakening, which may prolong the burst by means of an arousal-dependent input.29 In this respect, the EMG burst may be the final event of the action of different neurotransmitter systems. The first duration class (≤ 1 s) was not influenced by pramipexole; this possibly suggests, again, that at least some of these brief movements are not influenced by dopamine. Concerning the effect on the area under curve (EMG amplitude/duration), pramipexole seems to be able to reduce significantly all PLMS ≥ 2 μV/s, without any effect on those < 2 μV/s, which correspond to the previously mentioned PLMS in the brief duration class (≤ 1 s) also not influenced by pramipexole.
The acute effect, the preferential D3-receptor selectivity, and the single dose of pramipexole probably represent limitations of this study. The LM response following a longer period of treatment, or a different pramipexole dose, or a dopamine agonist with a different receptor-binding preference may be different from that observed. Therefore, further similar studies on the chronic effect of pramipexole and on the consequences on other dopamine agonists with different receptor selectivity are probably needed to complete the picture of dopaminergic influence on nocturnal LM.
The application of the current PLMS scoring criteria18,21 resulted in the inclusion of all LM significantly suppressed by the pramipexole treatment. This result supports the hypothesis that dopamine influences LM that can be categorized as PLMS. In conclusion, the analysis of the response of LM activity during sleep to a single dose of pramipexole suggests three important findings: first, the acute therapeutic effect of pramipexole on PLMS in RLS; second, a possible heterogeneous pathogenesis of the leg motor pattern during sleep in RLS patients; and third, the existing criteria for PLMS may be too generic and potentially include some motor phenomena with different neurophysiological origins. The large array of LM measures suggests that a clear phenotypic description can be developed along the continuum of LM expression, to differentiate the neurophysiological basis of LM and their possible pathological impact.
ACKNOWLEDGMENTS
The work was performed at: Sleep Disorders Center, Department of Neurology, Scientific Institute and University Ospedale San Raffaele, Vita-Salute University, Milan, Italy.
There is no off-label or investigational use.
APPENDIX
Markov Chain Analysis of Leg Movement Occurrence
The study of LM occurrence across time was also studied using a Markov chain analysis. This analysis characterizes the time dependency (also a feature of periodicity) of time series. A Markov chain is a sequence of values (states) whose probabilities at a time interval depends upon the value at the previous time. The controlling factor in a Markov chain is the transition probability (a conditional probability value for the system to go to a particular new state, given the current state of the system). From these probabilities, the entropy of the system can be computed. The basic concept of entropy in Information Theory deals with how much randomness is present in a signal or in a sequence of events. It also gives a measure of how much information is carried by the signal. In this study, for each subject included, the length of each interval between subsequent LM was assigned to 3 states as follows: State 1 (i ≤ 10 s), State 2 (10 < i ≤ 90 s), and State 3 (i > 90 s).
The unconditional probability of occurrence of each state from which we obtained the zero-memory Markov model entropy (H0) was first calculated; in other words, we considered each state as occurring following its own intrinsic probability, unconditioned by the previous state. A 3×3 cell matrix was next obtained; the 9 entries in this matrix are the probabilities of transition from a given state to the next state, in successive interval occurrences. For example, if the transition from State 1 to State 3 occurred 7 times in N possible transitions from State 1 to any other State (including State 1), then the transition probability in cell [1,3] of the matrix (i.e., cell in the first row and third column of the matrix) was given the value 7/N. In this model, the sum of all probability entries in a row of the matrix (e.g., the contents of cells [2,1], [2,2], and [2,3]) is 1. This transition probability (or conditional probability) matrix (TPM), a state transition probability matrix of the Markov chain theory, can be used to quantify the time structure of any other sequences of events recorded during sleep. For a reliable estimation of transition probabilities, a number of transitions equal to at least 8 times the number of matrix entries is needed. Since the average total number of LM available was more than 90 for all RLS patients at baseline and for those taking placebo, after treatment, the use of 3×3 matrices (9 matrix entries) can be considered reliable in these groups. Of note, however, the total number of LM dropped below 72 in 5 patients who were treated with pramipexole. The first-order Markov model entropy (H1) was computed from each matrix; if H1 is lower than H0, the result indicates that a first-order relationship exist between the states of the system, i.e. that each state does not occur randomly according to its own probability of occurrence but is also influenced by the value of the state preceding it. The degree of this influence can be described by the Dependency Index (DI = H0-H1/H0). This index can range from 0 (no first-order interdependencies) to 1 (complete first-order dependency).
Results obtained from the Markov chain analysis of intervals between consecutive LM during the baseline study and after treatment with pramipexole or placebo are presented in Table 1.1. Both values of entropy (H0 and H1) tend to increase while the Dependency index tends to decrease after pramipexole; at statistical analysis a significant difference was found for H0 and H1 between the pramipexole and placebo groups, only after treatment.
Table 1.1.
Comparison Between the Results Obtained from the Markov Chain Analysis of Intervals Between Consecutive LM Before (Baseline) and After Treatment With Pramipexole or Placebo
| Baseline |
Treatment |
ANOVA |
Post-hoc comparisons |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pramipexole |
Placebo |
Pramipexole |
Placebo |
Baseline vs. Treatment |
Pramipexole vs. Placebo |
||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Night | Group | Night×Group | Pramipexole | Placebo | Baseline | Treatment | |
| H0 | 0.970 | 0.240 | 1.034 | 0.248 | 1.213 | 0.240 | 0.931 | 0.290 | N.S. | 0.050 | 0.0022 | N.S. | 0.0005 | ||
| H1 | 0.885 | 0.226 | 0.952 | 0.243 | 1.160 | 0.236 | 0.853 | 0.285 | N.S. | 0.027 | 0.0007 | N.S. | 0.0001 | ||
| Dependency Index | 0.087 | 0.075 | 0.079 | 0.053 | 0.042 | 0.062 | 0.089 | 0.045 | N.S. | N.S. | NS | ||||
H0, the zero-memory Markov model entropy; H1, first-order Markov model entropy; Dependency Index, = H0-H1/H0.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Symmonds C. Nocturnal myoclonus. J Neurol Neurosurg Psychiatry. 1953;16:166–71. doi: 10.1136/jnnp.16.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lugaresi E, Coccagna G, Tassinari CA, Ambrosetto C. [Polygraphic data on motor phenomena in the restless legs syndrome] Riv Neurol. 1965;35:550–61. [PubMed] [Google Scholar]
- 3.Trenkwalder C, Walters AS, Hening W. Periodic limb movements and restless legs syndrome. Neurol Clin. 1996;14:629–50. doi: 10.1016/s0733-8619(05)70277-2. [DOI] [PubMed] [Google Scholar]
- 4.Chabli A, Michaud M, Montplaisir J. Periodic arm movements in patients with the restless legs syndrome. Eur Neurol. 2000;44:133–8. doi: 10.1159/000008221. [DOI] [PubMed] [Google Scholar]
- 5.Montplaisir J, Boucher S, Poirier G, et al. Clinical, polysomnographic, and genetic characteristics of restless legs syndrome: a study of 133 patients diagnosed with new standard criteria. Mov Disord. 1997;12:61–5. doi: 10.1002/mds.870120111. [DOI] [PubMed] [Google Scholar]
- 6.Hornyak M, Kopasz M, Feige B, Riemann D, Voderholzer U. Variability of periodic leg movements in various sleep disorders: implications for clinical and pathophysiologic studies. Sleep. 2005;28:331–5. [PubMed] [Google Scholar]
- 7.Bliwise D, Petta D, Seidel W, Dement W. Periodic leg movements during sleep in the elderly. Arch Gerontol Geriatr. 1985;4:273–81. doi: 10.1016/0167-4943(85)90009-3. [DOI] [PubMed] [Google Scholar]
- 8.Hornyak M, Riemann D, Voderholzer U. Do periodic leg movements influence patients' perception of sleep quality? Sleep Med. 2004;5:597–600. doi: 10.1016/j.sleep.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Nicolas A, Lesperance P, Montplaisir J. Is excessive daytime sleepiness with periodic leg movements during sleep a specific diagnostic category? Eur Neurol. 1998;40:22–6. doi: 10.1159/000007951. [DOI] [PubMed] [Google Scholar]
- 10.Trenkwalder C, Paulus W. Why do restless legs occur at rest?—pathophysiology of neuronal structures in RLS. Neurophysiology of RLS (part 2) Clin Neurophysiol. 2004;115:1975–88. doi: 10.1016/j.clinph.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 11.Saletu B, Gruber G, Saletu M, et al. Sleep laboratory studies in restless legs syndrome patients as compared with normals and acute effects of ropinirole. 1. Findings on objective and subjective sleep and awakening quality. Neuropsychobiology. 2000;41:181–9. doi: 10.1159/000026658. [DOI] [PubMed] [Google Scholar]
- 12.Saletu M, Anderer P, Saletu-Zyhlarz G, Hauer C, Saletu B. Acute placebo-controlled sleep laboratory studies and clinical follow-up with pramipexole in restless legs syndrome. Eur Arch Psychiatry Clin Neurosci. 2002;252:185–94. doi: 10.1007/s00406-002-0380-7. [DOI] [PubMed] [Google Scholar]
- 13.Manconi M, Ferri R, Zucconi M, et al. First night efficacy of pramipexole in restless legs syndrome and periodic leg movements. Sleep Med. 2007;8:491–7. doi: 10.1016/j.sleep.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Poewe W, Högl B. Akathisia, restless legs and periodic limb movements in sleep in Parkinson's disease. Neurology. 2004;63:S12–S16. doi: 10.1212/wnl.63.8_suppl_3.s12. [DOI] [PubMed] [Google Scholar]
- 15.Michaud M, Dumont M, Selmaoui B, Paquet J, Fantini ML, Montplaisir J. Circadian rhythm of restless legs syndrome: relationship with biological markers. Ann Neurol. 2004;55:372–80. doi: 10.1002/ana.10843. [DOI] [PubMed] [Google Scholar]
- 16.Coleman RM, Pollak CP, Weitzman ED. Periodic movements in sleep (nocturnal myoclonus): relation to sleep disorders. Ann Neurol. 1980;8:416–21. doi: 10.1002/ana.410080413. [DOI] [PubMed] [Google Scholar]
- 17.Coleman RM, Pollack CP, Weitzman ED. Periodic nocturnal myoclonus in a wide variety of sleep-wake disorders. Trans Am Neurol Assoc. 1978;103:230–3. [PubMed] [Google Scholar]
- 18.The Atlas Task Force. Recording and scoring leg movements. Sleep. 1993;16:748–59. [PubMed] [Google Scholar]
- 19.Ferri R, Zucconi M, Manconi M, et al. Computer-assisted detection of nocturnal leg motor activity in patients with restless legs syndrome and periodic leg movements during sleep. Sleep. 2005;28:998–1004. doi: 10.1093/sleep/28.8.998. [DOI] [PubMed] [Google Scholar]
- 20.Ferri R, Zucconi M, Manconi M, Plazzi G, Bruni O, Ferini-Strambi L. New approaches to the study of periodic leg movements during sleep in restless legs syndrome. Sleep. 2006;29:759–69. [PubMed] [Google Scholar]
- 21.Zucconi M, Ferri R, Allen R, et al. The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG) Sleep Med. 2006;7:175–83. doi: 10.1016/j.sleep.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 23.Walters AS, LeBrocq C, Dhar A, et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4:121–32. doi: 10.1016/s1389-9457(02)00258-7. [DOI] [PubMed] [Google Scholar]
- 24.Kemp B, Varri A, Rosa AC, Nielsen KD, Gade J. A simple format for exchange of digitized polygraphic recordings. Electroencephalogr Clin Neurophysiol. 1992;82:391–3. doi: 10.1016/0013-4694(92)90009-7. [DOI] [PubMed] [Google Scholar]
- 25.Saletu M, Anderer P, Saletu B, et al. Sleep laboratory studies in restless legs syndrome patients as compared with normals and acute effects of ropinirole. 2. Findings on periodic leg movements, arousals and respiratory variables. Neuropsychobiology. 2000;41:190–9. doi: 10.1159/000026659. [DOI] [PubMed] [Google Scholar]
- 26.Stiasny-Kolster K, Kohnen R, Carsten MJ, Trenkwalder C, Oertel WH. Validation of the “L-DOPA test” for diagnosis of restless legs syndrome. Mov Disord. 2006;21:1333–9. doi: 10.1002/mds.20969. [DOI] [PubMed] [Google Scholar]
- 27.Kvernmo T, Hartter S, Burger E. A review of the receptor-binding and pharmacokinetic properties of dopamine agonists. Clin Ther. 2006;28:1065–78. doi: 10.1016/j.clinthera.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Sforza E, Haba-Rubio J. Night-to-night variability in periodic leg movements in patients with restless legs syndrome. Sleep Med. 2005;6:259–67. doi: 10.1016/j.sleep.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Michaud M, Poirier G, Lavigne G, Montplaisir J. Restless legs syndrome: scoring criteria for leg movements recorded during the suggested immobilization test. Sleep Med. 2001;2:317–21. doi: 10.1016/s1389-9457(00)00072-1. [DOI] [PubMed] [Google Scholar]