Abstract
Study Objectives:
To characterize the sleep patterns of unrestrained, diurnal nonhuman primates entrained to 24-hour light-dark cycles.
Design:
EEG, EMG, and EOG were recorded continuously via implanted telemetry from 5 unrestrained male rhesus monkeys housed individually under a 16:8 light-dark cycle (LD 16:8; L = 13 lux; D = 0 lux).
Results:
In a LD 16:8 cycle, all 5 monkeys demonstrated a long period of consolidated sleep during the 8-h dark period. On average, sleep accounted for 89.2% of the 8-h dark period and 25.2% of the 16-hour light period. REM sleep occupied 23% of total sleep time over 24 h, or 10.7% of the total time. The average length of the consolidated sleep (CS) period was 10.5 h, although the time of CS onset was variable. In contrast, the end of CS, and thus the onset of consolidated wakefulness (CW) demonstrated very little variation, typically occurring within 2 min of light onset. Ultradian NREM-REM cycles with periods of approximately 60 min were also observed. EEG delta activity during NREM sleep, thought to reflect the homeostatic sleep process, peaked at 3–4 h after CS onset.
Conclusions:
The present study demonstrates the feasibility of long-term, unrestrained sleep monitoring in nonhuman primates using fully-implantable biotelemetry. With minor exceptions, most notably a delay in peak delta activity, sleep-wake architecture, regulation, and consolidation in rhesus monkeys strongly resembles that of humans. These results demonstrate that the unrestrained rhesus monkey is an excellent biomedical model for human sleep.
Citation:
Hsieh KC; Robinson EL; Fuller CA. Sleep architecture in unrestrained rhesus monkeys (Macaca mulatta) synchronized to 24-hour light-dark cycles. SLEEP 2008;31(9):1239-1250.
Keywords: NREM, nonhuman primate, telemetry, EEG, slow wave activity, REM
THE USE OF NONHUMAN PRIMATES AS A MODEL FOR HUMAN SLEEP HAS UNIQUE RELEVANCE TO HUMAN NEUROBIOLOGY. THE CLOSE PHYLOGENETIC relationship and similar diurnal habits of nonhuman primates allow them to fill a gap between sleep studies of rodents or felines and those of humans. The rhesus monkey (Macaca mulatta) was determined to be the best sleep model in a comparison of 13 nonhuman primate species,1 both for its well-defined, human-like sleep organization and for relative ease of husbandry. The rhesus monkey has also been the most extensively used nonhuman primate model for the study of human diseases, including disorders of the nervous system. An extensive knowledge of the physiology of the rhesus monkey also confers an important advantage over the use of other primate species as a model for human sleep.
Until recently, studies employing EEG for sleep recordings in rhesus monkeys were necessarily performed using methodological approaches that prevented the expression of normal sleep. For example, potentially confounding manipulations included the need to relocate animals to a novel laboratory setting for recordings, chair restraint and physical tethering of the animals during recording. Although short-term acclimation to the setting was typically (but not always) employed, the animals were nonetheless in an environment unlike their home cages and remained physically restrained for the duration of the sleep recording, forcing the animals to, at a minimum, adopt abnormal sleeping postures. The negative impact of physical restraint on sleep behaviors has been convincingly demonstrated in baboons, which displayed disrupted sleep-wake patterns, unusual drowsiness, and inattentiveness.2 Their sleep patterns also differed from those of animals with externally mounted telemetry that permitted them to move freely in their cages.3 Although no similar comparison of sleep patterns between restrained and unrestrained rhesus monkeys is available, it is known that normal behaviors of rhesus monkeys are markedly affected by chair restraint.4
Restraint induces a number of endocrine changes in rhesus monkeys, including changes in circulating renin, vasopressin, and growth hormone,5 as well as alteration of hemodynamic variables, including cardiac output, blood pressure, and total peripheral resistance.6 Perhaps unsurprisingly then, sleep in restrained rhesus monkeys has shown considerable variability, including marked fragmentation and prolonged bouts of wakefulness during the normal sleep period, suggesting that restraint, novel settings, or other experimental manipulations may have contributed to the inconsistencies seen in prior studies.7–9
Another distinct limitation of most prior rhesus sleep studies has been the relatively short duration of the recording period, which typically consisted of nighttime recordings only, possibly due in part to the limitations of paper polygraph systems. In the present study, sleep recordings of unrestrained rhesus monkeys were made in their home cages using fully implantable biotelemetry transmitters and a computer data collection system, permitting continuous recordings over multiple consecutive days, with battery life lasting up to a year of continuous operation to enable long-term sleep studies. By avoiding novel settings, restraint, backpacks, and other potentially stressful apparatus and manipulations, we anticipated obtaining a more accurate and complete characterization of normal sleep in the rhesus monkey. Specifically, we hypothesized that the rhesus in our study would demonstrate greater sleep consolidation and sleep efficiency (defined by percentage of total time in sleep) during the dark period than previously seen in restrained animals. A better characterization of the undisturbed sleep patterns of rhesus monkeys, particularly where similarities with human sleep could be established, would be an important step in validating the rhesus as a biomedical model of human sleep regulation.
METHODS
Animals and Husbandry
Five adult male rhesus monkeys (Macaca mulatta) from the California National Primate Research Center, Davis, CA were used for this study. All protocols were approved by the UC Davis IACUC, and studies were conducted in compliance with USDA and NIH guidelines for animal care and use. The animals were between 5 and 5.3 years old and weighted 6.5 to 8.6 kg immediately prior to the experiments. The animals were trained to use the Psychomotor Test System (PTS, Georgia State University) prior to final selection and surgical implantation of the biotelemetry transmitters. This fully automated microcomputer system provides both behavioral enrichment and a nutritionally complete pellet reward (P. J. Noyes, Lancaster, NH), and allows completely ad libitum feeding of trained animals.
Telemetry and Data Acquisition
The telemetry system for sleep recording consisted of a fully implantable biotelemetry transmitter (T26-D, Konigsberg Instruments Inc., Pasadena, CA) and a microcomputer-based receiving unit (TR-8 receivers and TD-14-10B demodulators, Konigsberg Instruments Inc., Pasadena, CA). The transmitter, with leads specifically tailored for rhesus monkeys, encoded 2 channels of EEG, one channel each of EOG and EMG, as well as deep brain temperature. The deep brain temperature was measured with a thermistor (Thermometrics Inc. Edison, NJ) in sealed stainless steel hypodermic tubing (data not shown in this study). An attached implanted battery pack allowed continuous operation for approximately one year. The implant can also be switched on and off remotely, using a radio-frequency signal, to extend battery life. The animals' home cages were outfitted with receiving antennas, one of which doubled as a perch for the monkey. Signals were converted to analog voltages and recorded at a sampling rate of 256 Hz using an analog to digital converter (PCI-6701, National Instruments, Inc. Austin, TX) in a Power Macintosh microcomputer. A custom LabVIEW (version 6, National Instruments, Inc. Austin, TX) program (by ELR) recorded the data continuously to an external hard drive in 30-sec epochs. This system allowed us to record for extended periods of time with only brief interruptions for animal maintenance and disk swaps.
Implant Surgery
The telemetry transmitters were implanted in fully anesthetized animals by trained veterinary surgeons (California National Primate Research Center, Davis, CA) generally following methods previously established by us.10 The inhalant anesthesia was approximately 1% isoflurane. Extradural EEG leads were placed stereotaxically to provide frontal-parietal (AP +25, ML +10 and AP −10, ML +10) and parietal-occipital EEGs parietal (AP −10, ML +10 and AP −25, ML +10) differential pairs. The EEG was recorded from wire loop electrodes secured to the cranium with bone screws in contact with the dura and secured with dental acrylic. Bilateral EMG leads were sutured to superficial dorsal neck muscles. Unilateral EOG leads were positioned at the lateral canthus and superior orbital margin of one eye and secured with bone screws. A thermistor housed in a 3-cm length of hermetically sealed hypodermic tubing was positioned at AP +14 mm, ML −10 mm and advanced vertically into white matter between the internal capsule and thalamus. The thermistor assembly was then fixed to the cranium with dental acrylic. After surgery, each animal recovered in the hospital and was given antibiotics and analgesic cream daily for one or two weeks. A recovery period of at least 2 weeks was allowed before beginning any study.
Sleep Scoring Criteria
Polysomnographic data for the first 2 days of the study were visually scored using criteria based on the standards for human sleep staging established by Rechtschaffen and Kales, 1968.11 In addition we scored an epoch as “artifact” when it consisted of >50% noise and obscured the patterns. Based on observations of simultaneous video recording, artifacts in our data most often coincided with movement of the animal and were usually associated with wakefulness.
In general, the criteria used for scoring rhesus sleep were identical to those used in human sleep scoring, except that the 3-min rule as described by Rechtschaffen and Kales11 was not employed, due to the finding that the rhesus monkey has less consolidated sleep and wake and a shorter duration for each sleep episode. This practice is also consistent with the proposals of the AASM Visual Scoring Task Force12 for revision of the Rechtschaffen and Kales scoring rules.
Automated Scoring
Data from 6 consecutive 24-h periods were scored using a custom automated scoring program (by KH). The program was developed in Matlab 5 (Mathworks Inc., Natick, MA), and consisted of a 3-layer neural network model (24 × 5 × 4) trained to recognize key features of the EEG and EMG. The input layer corresponds to 24 specific features in the EEG and EMG, and the output layer corresponds to 4 sleep stages (Wake, REM, S12, and S34). In the training phase, the program computes an error function between the network output and an expert's visual scores. Network weights are then adjusted to reduce the error function by a back-propagation algorithm. Once the network is trained, novel data are scored in a single pass consisting of data input, feature extraction, and network-output steps. Data for each individual animal were processed separately. A second data set, consisting of 48 h of raw sleep data for each animal, was used to evaluate the performance of the automated scoring program. Each 48 h of test data were also visually scored by 2 human scorers. Agreement between the visual scores of several human scorers averaged 92.3% (kappa = 0.88), and agreement between automated and visual scores was 93.7% (see Table 3). Kappa for automated to human score agreement ranged from 0.85 to 0.93.
Table 3.
Epoch-by-Epoch Comparison of Automated Sleep Scores with Visual Sleep Scores. An Average Agreement of 93.7% was Achieved Among all Sleep Stages, with the Lowest Agreement Rate for REM Sleep, about 85%. Kappa Values between 2 Sets of Human Visual Scores Averaged 0.879 (SD 0.035) in 5 Animals, and Those Between Human Visual Scores and Automated Scores Averaged 0.901 (SD 0.027).
| No. of epochs | Automated Scores |
Agreement | ||||
|---|---|---|---|---|---|---|
| Wake | REM | S12 | S34 | |||
| Human Scores |
Wake | 35584 | 690 | 511 | 75 | 96.5% |
| REM | 654 | 6288 | 414 | 0 | 85.5% | |
| S12 | 400 | 427 | 16462 | 585 | 92.1% | |
| S34 | 16 | 1 | 557 | 6084 | 91.4% | |
| Agreement | 97.1% | 84.9% | 91.7% | 90.2% | 93.7% | |
Experimental Protocol
Animals were acclimatized to the housing environment for 5 weeks. Prior to this study, animals were entrained to 24-h days consisting of 16 h of light and 8 h of darkness (LD 16:8). To minimize light masking relatively dim light was used. Light level, measured throughout the cage, averaged 13 lux at eye level during the day and was not measurable (0 lux) at night. During the day, when animals were playing PTS, they would have been exposed to levels of between 18–24 lux at the angle of gaze. Both the lights and the computer monitors for the PTS were switched on at 06:00 and off at 22:00 daily. Following the acclimation period, data were collected over 6 consecutive 24-h days.
Data Analysis
Consolidated sleep and wake episodes were defined using criteria developed for sleep in squirrel monkeys.13 A consolidated wake (CW) episode consisted of 10 min of continuous waking followed by at least 50% waking in each of the next 3 h. A consolidated sleep (CS) episode consisted of 10 min of continuous sleep, including NREM or REM sleep, followed by at least 50% occurrence of sleep in each of the next 3 h. Sleep cycle duration analysis followed criteria modified from those for humans.14 In rhesus monkeys, an arbitrary 10-min minimum was used for NREM periods to avoid classifying brief NREM intervals within REM sleep as separate NREM periods. Conversely, a 2-min minimum was adopted for defining the occurrence of discrete REM periods. Average durations for consolidated sleep and wake periods as well as for NREM/REM cycles were calculated using Excel (Microsoft, Seattle, WA).
EEG spectral analysis was carried out using a custom program developed in LabVIEW (by KH). Four-sec Hanning windows with 2-sec overlaps between windows were used in a 1024-point Fast Fourier Transform to minimize spectral leakage. The 0.25-Hz bins were then combined into 1-Hz bins between 0.125–16 Hz, 2-Hz bins between 16–32 Hz, and 4-Hz bins between 32–52 Hz. Power in each frequency bin was calculated for each 30-sec epoch. The program also tabulated sleep score, low-frequency EMG power, and a full EEG power spectrum for each 30-sec epoch. Epochs with prominent artifact were excluded from further analysis. Artifact filtering used criteria determined by examination of very low frequency (0.1–0.375 Hz) EEG power, high-frequency (72–128 Hz) EEG power, and low-frequency (0–4 Hz) EMG power. Cutoff levels for artifact filtering were determined visually. EEG spectral data were standardized by dividing by the total power (0.375–128 Hz) of all artifact-free records for each individual before they were averaged across animals.
Statistics
Statistical analysis was performed using SPSS (SPSS Inc., Chicago, IL). Means and standard deviations were calculated for each animal as well as for the group. Comparisons in sleep variables among individual animals or among various time points were made by ANOVA. An α of 0.05 was used for statistical significance.
RESULTS
Elective Sleeping Postures
The unrestrained rhesus monkeys in this study most often slept seated on either the cage floor or perch with their head and body leaning against the cage walls, or else lying prone on the cage floor with their limbs flexed. They were rarely seen sleeping either on their side or in a supine position. The mostly upright sleeping posture closely resembles their natural sleeping behavior, as reported in free-ranging rhesus monkeys, which typically huddled in clusters of 2 or more animals in the seated position when sleeping.15
EEG Patterns
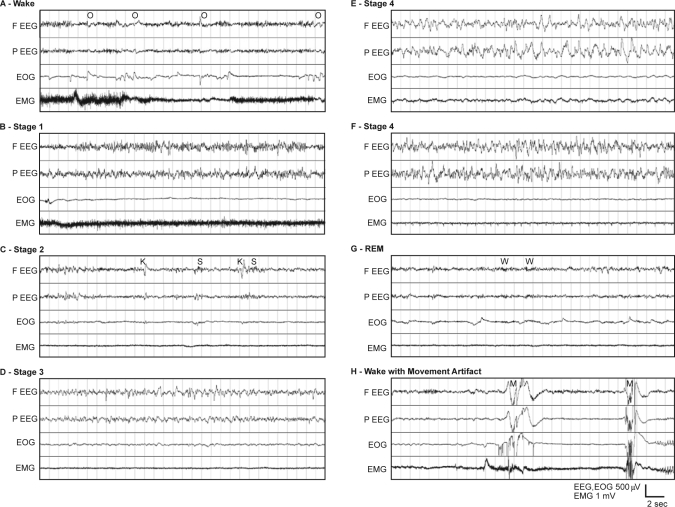
The first 48 h of continuous recordings from 5 animals were scored visually using criteria similar to those for human sleep scoring.11 Each 30-sec epoch was assigned a score corresponding to wake, sleep stage, or artifact. Examples of these stages in the data are presented in Figure 1, showing (A) a waking sample, (B–F) NREM stages 1–4, (G) REM sleep, and (H) a typical movement artifact.
Figure 1.
(A) Example 30-sec epoch polygraph record showing stage Wake in a rhesus monkey. The appearance of high-frequency, low-amplitude EEG and high tonic EMG activity define stage Wake. EOG tends to show phasic activity representing rapid saccades and blinking. Ocular artifacts (O) are common in the frontal EEG. Slow movement artifacts often are shown on top of the high tonic activity in EMG. (B) Example polygraph showing stage 1 sleep in a rhesus monkey. (C) Example polygraph of stage 2 sleep in a rhesus monkey. K-complexes (K) and spindles (S) are shown in the EEG channels with slow waves occupying less than 20% of the 30-sec epoch. (D) Example polygraph showing stage 3 sleep in a rhesus monkey. High-amplitude slow waves occupy more than 20%, but less than 50%, of the 30-sec epoch. (E) Example polygraph of Stage 4 sleep in a rhesus monkey. High-amplitude slow waves occupy more than 50% of the 30-sec epoch. The slow waves resemble those of humans. The EEG slow waves caused some slow artifacts in the EMG of this example. (F) Example polygraph of stage 4 sleep in a rhesus monkey. High-amplitude slow waves occupy more than 50% of the 30-sec epoch. In some animals, higher frequency (>5 Hz) oscillations were commonly seen mixed with slow waves in both stage 3 and stage 4. (G) Example polygraph of REM sleep in a rhesus monkey. High-frequency, low-amplitude EEG appears accompanied by minimal EMG activity. Rapid eye movements are often seen as triangular waveforms in the EOG. Spindle-like wavelets (W) of about 20 Hz are common in EEGs of REM sleep. (H) Example polygraph showing movement artifacts in a rhesus monkey. These high-amplitude artifacts (M), appearing in all channels, are very common during active wakefulness.
When awake, alpha waves (9–11 Hz), commonly occurring in relaxed wakefulness in humans, were seldom seen in the rhesus monkeys (Figure 1A). Mixed artifacts were frequently seen in multiple channels during the waking state in all animals. Most of these artifacts coincided with movement of the animal, as shown by comparison with video recordings, and seldom occurred at night when the animals were asleep. During wake, prominent eye movement potentials (O) also appeared in the frontal EEG channel, an artifact also commonly seen in human EEG recordings.
The EEG of stage 1 NREM sleep (Figure 1B) shows slower oscillations of intermediate amplitude and mixed frequency, with intermittent high-amplitude sharp waves. Sleep spindles (S) of 14–16 Hz were common in stage 2 NREM sleep (Figure 1C); however, these were often indistinguishable from the background. High-amplitude K-complexes (K) tended to be more distinctive and abundant than spindles in stage 2 NREM. Stage 3 (Figure 1D) and 4 (Figure 1E) NREM sleep are both distinguished by the presence of high-amplitude slow waves (delta). A 30-sec epoch with more than 20% delta EEG was scored as Stage 3, and epochs with greater than 50% delta EEG were scored as stage 4. In 3 of the 5 animals, the waveforms closely resembled typical stage 3 and 4 NREM EEG in humans. In 2 of the monkeys, slow waves were usually mixed with higher frequency (around 6–15 Hz) waves (Figure 1F).
The REM sleep EEGs (Figure 1G) resembled the fast, desynchronized waveforms found during waking but lacked the frequent movement artifacts. Spindle-like, 20-Hz wavelets (W), lasting approximately 1 sec also appeared in the REM sleep EEGs. Sawtooth waves in the theta band (5–8 Hz) occasionally appeared in REM sleep EEG but with low amplitude. Rapid eye movements were frequently present in the EOG. The EMG activity recorded from posterior neck muscles was at its lowest level during REM sleep. Muscle atonia was evident during most of the REM sleep episodes; however, low muscle tonus was often observed during preceding NREM sleep as well. The last 30–60 sec of REM periods were typically marked by a distinct transitional pattern of high-frequency oscillations with increased amplitude.
Distribution of Wakefulness and Sleep Stages
Undisturbed rhesus monkeys spent nearly half of the 24-h day in sleep (Table 1). Sleep accounted on average for 25.2% (SD 5.2) of the 16-h light period and for 89.2% (SD 1.3) of the 8-h dark period. The animals spent more time awake earlier in the daytime than later. Dividing the 24-h day into two 12-h periods for comparison with earlier studies showed our animals sleeping 18.4% of the time from 06:00 to 18:00.
Table 1.
Percentages of Wakefulness and Sleep Stages by Visual Scoring of a 48-h Period in 5 Animals. Undisturbed Animals Slept for 46% of the 24 h, Including 89% of the Dark Period and One-Fourth of the Light Period.
| Total time (100%) | Time in sleep (100%) |
|||||
|---|---|---|---|---|---|---|
| Asleep | S1 | S2 | S3 | S4 | REM | |
| 24-h | ||||||
| Mean | 46.4 | 15.8 | 40.2 | 11.3 | 9.7 | 23.0 |
| SD | 3.6 | 6.7 | 5.9 | 2.0 | 4.5 | 7.6 |
| Light | ||||||
| Mean | 25.2 | 26.5 | 34.5 | 8.3 | 5.5 | 25.2 |
| SD | 5.2 | 15.7 | 9.4 | 5.1 | 6.5 | 14.1 |
| Dark | ||||||
| Mean | 89.2 | 10.3 | 43.6 | 13.0 | 11.8 | 21.4 |
| SD | 1.3 | 4.4 | 8.0 | 4.3 | 5.7 | 6.2 |
REM sleep occupied 23.0% of total sleep time over 24 h, or 10.7% of total time. Most REM occurred during the dark period, accounting for 19.1% of the total time compared with 6.6% for the light period. However the percentage of sleep time in REM sleep was higher during the light period.
A similar analysis was performed on automated scores from the entire 6 days of polysomnographic recordings (Table 2). The automated scoring program generated results similar to the visual scores with only minor differences. An epoch-by-epoch comparison between automatic scores and visual scores is shown in Table 3. Scores from the program for combined NREM stages 1 and 2 (S12), NREM stages 3 and 4 (S34), REM and Wake were highly consistent with grouped visual scores.
Table 2.
Percentages of Wakefulness and Sleep Stages by Automatic Scoring of a Continuous 6-Day Period. The Automated Sleep Scoring Program Produced Very Similar Results to Hand Scoring.
| Total time (100%) | Time in sleep (100%) |
|||
|---|---|---|---|---|
| Asleep | S12 | S12 | REM | |
| 24-h | ||||
| Mean | 47.4 | 54.1 | 21.8 | 24.1 |
| SD | 1.7 | 2.1 | 1.9 | 1.1 |
| Light | ||||
| Mean | 26.5 | 56.0 | 13.7 | 30.2 |
| SD | 3.0 | 15.9 | 9.5 | 13.4 |
| Dark | ||||
| Mean | 89.2 | 53.2 | 26.4 | 20.4 |
| SD | 1.5 | 7.9 | 9.9 | 5.1 |
Individual and Day-to-Day Variation
Based on automated scores, total sleep and wake time, and sleep and wake during light and dark periods, did not differ significantly among individuals (Figure 2). However, significant differences were seen in the percentages of sleep stages (REM, S12, or S34). Day-to-day variability for individual animals was relatively small compared to differences between individuals, with each monkey displaying a characteristic distribution of sleep stages. In the light period, the total wake time was not significantly different among individual animals (P > 0.05 by ANOVA or by Kruskal-Wallis test), while all other percentages varied significantly among animals.
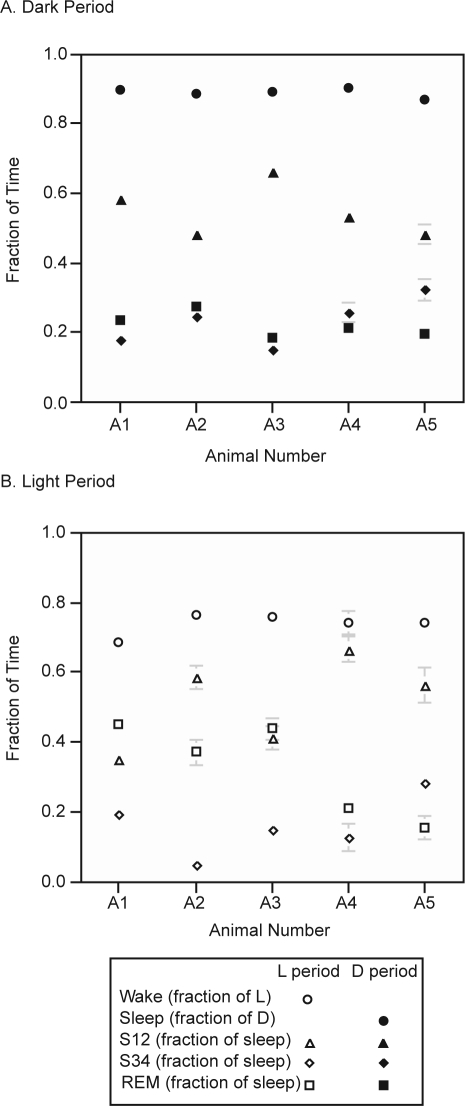
Figure 2.
Nighttime (A, filled markers) and daytime (B, open markers) distribution of sleep stages showing variability among individual animals. S12: time in stage S12 vs. total sleep time; REM: time in stage REM vs. total sleep time; S34: time in stage S34 vs. total sleep time; Sleep: total sleep time in dark period; Wake: total wake time in light period. Error bars = SEM. The sleep stage ratios vary from animal to animal, but the total time asleep is similar among animals in both dark and light periods.
Temporal Structure of Sleep-Wake Cycles
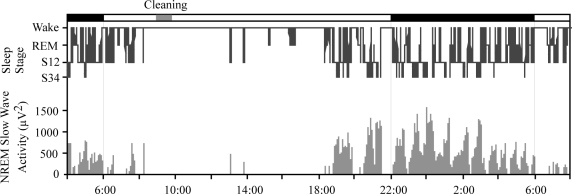
A typical hypnogram of a 28-h period is shown in Figure 3. Wake periods occurred mainly in the daytime and were interrupted by short sleep episodes, especially toward the end of the light period. The consolidated sleep period began in the evening, usually well before the end of the light period (22:00), and most commonly ended either when the lights came on at 06:00 or shortly thereafter. Brief arousals occurred throughout the night.
Figure 3.
A typical example of slow wave activity over 28 hours, and 28-h hypnogram of a representative monkey. Most sleep appears between early evening and light onset. Early occurrence of deep slow wave sleep (SWS34) and later appearance of REM sleep are among the features similar to human sleep. More frequent daytime napping is seen in the rhesus.
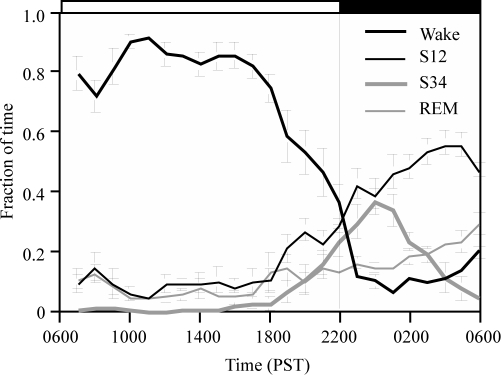
The data from all animals were evaluated in 1-h bins to show the average distribution of sleep stages by time of day. Percentage of time spent each hour in different sleep stages is shown in Figure 4. Except for a small decrease in the second hour after light onset, the animals were awake for roughly 80% of most daylight hours. Increased napping was seen later in the day, with the percentage of waking progressively decreasing between 18:00 and the time of lights off at 22:00. During the night (22:00 to 06:00), animals were awake approximately 10% of the time until the last h of darkness. Total NREM sleep (S12 and S34 combined) increased beginning at around 18:00, but did not peak until 1 to 3 h following lights off. Deep slow wave sleep (S34) increased monotonically beginning around 4 h prior to lights off, reaching a peak between 23:00 and midnight, 1 to 2 h after lights off. S34 then decreased monotonically during the latter portion of the night. Shallow slow wave sleep (S12) increased throughout most of the night. REM sleep was greatest during the second half of the dark period.
Figure 4.
Average distribution of sleep stages by time of day (n = 5). Wakefulness starts declining at around 18:00, and reaches a minimum at midnight, when deep sleep stage (S34) peaks. Both S12 and REM sleep rise across the night. Error bars = SEM.
The average length of the consolidated sleep period (CS) was 10.50 ± 1.08 (SD) h, as defined by the criteria of Wexler and Moore-Ede.13 Due to the fragmented sleep-wake pattern displayed by the rhesus monkeys during the last 4 h of the light period, the time of CS onset tended to be highly variable. Times of CS onset ranged from 18:00 to around 22:20, with the average CS onset at 20:22, preceding the time of lights-off (22:00).
In contrast, the onset of consolidated wakefulness (CW), or the end of the CS as a result, occurred in most cases within 2 min of light onset at 06:00. A very small number of CS offsets occurred from 1 to 2 h after lights on. Some of the animals awakened briefly when the lights came on but then resumed their sleep. This is reflected in the dip shown in Figure 4 for average Wake in the hours immediately after lights on.
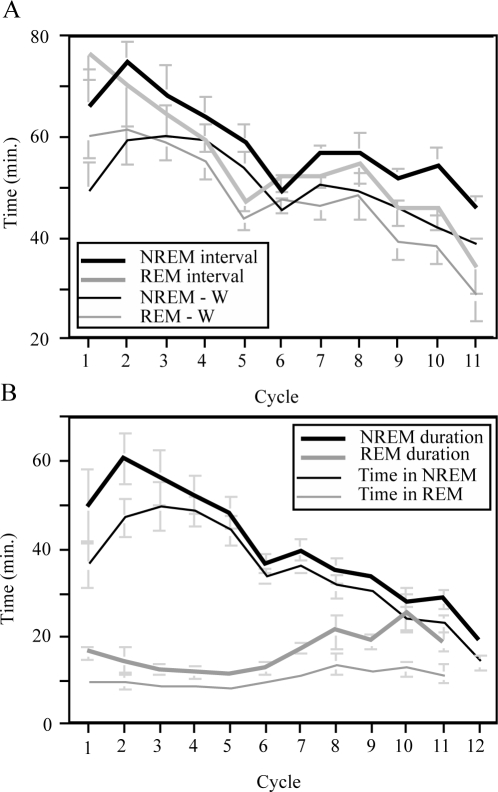
NREM and REM cycles within the consolidated sleep periods were evaluated further (Figure 5). Cycle lengths are illustrated in Figure 5A, which shows durations averaged by cycle number. NREM and REM sleep alternated with a cycle length of around one hour. The average time between NREM periods was 60.1 min (SD 4.9 min), of which 52.0 ± 4.4 min were spent in sleep, and the average time between REM periods was 58.7 min (SD 3.6 min), of which 50.4 ± 3.2 min were spent in sleep.
Figure 5.
(A) Intervals between NREM and REM periods for multiple NREM-REM cycles over the course of the night. Intervals are averages of 5 animals and are calculated both with and without the inclusion of brief waking episodes. Error bars = SEM.
The NREM interval is defined by the time lapse between the onset of one NREM period to the onset of the next NREM period within the consolidated sleep period (CS). NREM -W shows the NREM interval with short Wake periods subtracted. REM interval and REM -W are similarly defined. A general downward trend is seen in cycle length as sleep proceeds.
(B) NREM and REM episode duration, averaged by cycle. Within CS, a NREM period starts at the onset of a NREM episode that lasts longer than 10 min until the end of NREM sleep preceding the onset of a REM sleep period. Similarly, a 2-min minimum was chosen to define REM periods to avoid consideration of brief occurrences of REM within NREM. Time in NREM shows the actual time spent in NREM sleep in each NREM episode, excluding brief Wake or REM episodes. Similarly, Time in REM shows the actual time in REM sleep within each REM episode. NREM duration decreased across the night while REM duration increased. However, lengthening of REM episodes in the later part of the night was seen as a result of increased interruptions which did not demarcate separate REM episodes according to the above criteria even though they were more frequent. Error bars = SEM.
Cycle length (Figure 5A) shows a trend of decreasing duration over the course of CS, similar to what is seen in humans.14 In all adult groups studied, a shorter first cycle was seen along with a trend toward decreasing cycle length later in the night.
Similarly, the duration of NREM and REM periods differs across the CS period (Figure 5B). NREM periods decrease in length across the sleep period, while REM periods tend to increase in duration later in the night. The latter, however, is largely due to sleep interruptions (short waking), or intrusion of brief NREM sleep episodes into REM periods, either of which was too short to be considered as a separate cycle. The number of REM epochs (i.e., time in REM) within each REM period, shown in Figure 5B as the summed REM duration after Wake and NREM epochs are excluded, exhibits only a small increase later in the night.
Daily Profile of NREM Delta Activity
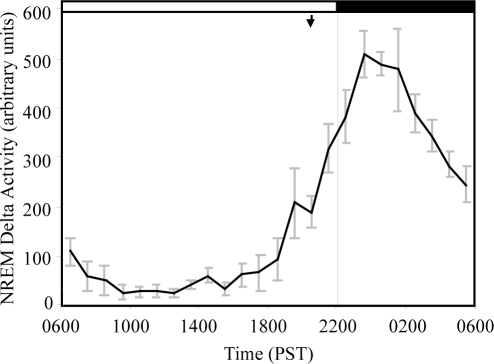
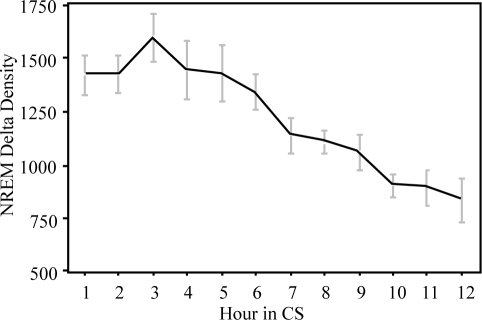
Delta EEG activity in NREM was obtained from FFT analysis and pooled into 1-h bins for each animal. After normalizing for each animal against its total spectral power, average delta activity was calculated for 5 animals. The time course of NREM delta activity (0.3–4Hz) is shown in Figure 6. As expected, the profile of NREM delta activity closely follows the time course of deep slow wave sleep (Figure 4). However, unlike humans16,17 and rats,18 whose delta activity peaks at sleep onset, delta activity in rhesus monkeys did not reach its maximum until between 23:00 and 00:00, or at least 3 h after the onset of CS. This basic pattern was seen in all of our monkeys, with the peak of delta activity occurring in the third hour of CS in 4 of the 5 animals, and in the second hour of CS in one animal. NREM delta density (power/minute NREM) plotted against the time since onset of CS is shown in Figure 7. NREM delta density, an index of the intensity of NREM sleep, peaked in the third hour of CS then declined monotonically for the rest of the CS period.
Figure 6.
Distribution of NREM delta activity by time of day, showing NREM slow wave activity (0.3–4 Hz) in the frontal EEG normalized to total EEG power and expressed in arbitrary units. The average of 5 animals is shown (error bars = SEM.). The arrow indicates the time of average onset of consolidated sleep. NREM delta activity (cf. Fig. 8) peaks at around 23:00 to 01:00, or 3 to 5 hours after CS onset.
Figure 7.
NREM delta density in relation to time in the consolidated sleep period (CS), averaged across 6 nights and 5 animals. The highest intensity of NREM sleep occurred in the third hour following the onset of CS.
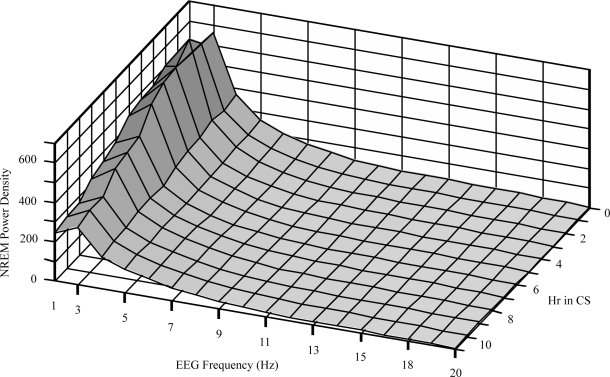
Figure 8 shows the temporal pattern of the NREM sleep EEG power spectrum over a broader frequency range. The spectrum of NREM power from 0.3 to 20 Hz was plotted against elapsed time within the CS period. The delta band exhibits the greatest changes over time. Power of the lower frequency portion of the delta band (0.3–2 Hz) peaked during the third hour of CS (Figure 7), while the power of the higher frequency portion (2-4 Hz) showed a trend of monotonic decrease over the course of sleep. The changes in other frequency bands were less pronounced.
Figure 8.
Power density spectrum of NREM EEG from 0.3 to 20 Hz shown by elapsed hour within the CS period. Spectra were obtained from 5 animals over 6 nights. EEG frequencies (in Hz) shown represent the upper bin limits beginning with 0.3 to 1 Hz (labeled 1 Hz), 1 to 2 Hz, and so on. The lowest EEG frequencies show major changes as CS progresses.
DISCUSSION
The present study showed that unrestrained rhesus monkeys spend almost half of the day in sleep, have consolidated nighttime sleep, and exhibit frequent napping, especially in the latter part of the day. In contrast to several previous studies, but consistent with our hypothesis, nighttime sleep efficiency was high in the unrestrained rhesus, with animals spending more than 89% of the 8-h dark period in sleep (Table 4). The EEG patterns of unrestrained rhesus monkeys resemble those of restrained rhesus monkeys,19 and are more similar to those of humans than are those of rodents or cats. Some modest differences between sleep in unrestrained rhesus and humans were seen, however, at the level of sleep consolidation, in NREM-REM cycle lengths, and in the time of peak NREM delta activity. Nevertheless, in general, sleep architecture, regulation and consolidation in the unrestrained rhesus monkey was highly similar to that of humans, demonstrating that the rhesus is a potentially valuable biomedical model for studying regulatory mechanisms of human sleep.
Table 4.
Summary of Previously Reported Data on Baseline Sleep in Rhesus Monkeys.
| Study | Recording session | Lighting condition | Restraint method & manipulations | % Asleep during recording | S1/TST % | S2/TST % | S3/TST % | S4/TST % | REM/TST % |
|---|---|---|---|---|---|---|---|---|---|
| Weitzman, 196519 | 7–8 h nighttime | N.R. | Restraint chair; Sleep deprivation in daytime | N.R. | 80a | 20 | |||
| Kripke et al., 196821 | 7–9 h nighttime | Recorded in light | Restraint chair; Sleep deprivation in daytime | 80 | 85a | 15 | |||
| Bert et al., 197058 | 8 h nighttime | N.R. | Restraint chair; N.R. | 88.7 | 15.0 | 33.5 | 19.5 | 16.5 | 15.5 |
| Crowley et al., 197222 | 24 h | LD 12:12 175 lux: dark | Restraint collar; Undisturbed | 51 | 69b* | 18c* | 12* | ||
| Jacoby, 19727 | 12 h in either day or night | LD 12:12 | Restraint chair; Blood sampling with catheter every 15 min. | L: 6.8* | 90.6b* | 1.3c* | 8.2* | ||
| D: 58.0* | 43.7b* | 40.9c* | 15.3* | ||||||
| David et al., 197559 | 7 h daytime or 11.3 h nighttime | LD 12:12 | Restraint chair; N.R. | L (7 h): 19.5* | 91.3b* | 6.6c* | 1.8 | ||
| D (11.3h): 64.0* | 61.0b* | 21.9c* | 10.9 | ||||||
| Balzamo et al.,199860 | 22 h | LD 16:8 | Restraint chair; Undisturbed | L (14 h): 18.7* | 97.0a* | 3.0* | |||
| D: 79.1* | 85.8a* | 14.2* | |||||||
| Benca et al., 20009 | 12 h nighttime | L:D 12:12, recorded in dim red light | Restraint chair; N.R. | 55.3 | 9.0 | 43.8 | 9.0 | 5.1 | |
| Yu et al., 200461 | 8 h nighttime for 10 nights | LD 12.5: 11.5 | Unrestrained at daytime; Restraint at nighttime | Adult: 86.5* | 69.0b* | 13.9c* | 17.1* | ||
| Adol.: 96.5* | 63.7b* | 17.7c* | 18.9* | ||||||
| Daley et al., 200620 | 12 h nighttime for 6 or 7 days | LD 12:12 | Unrestrained; daily replacement of telemetry batteries | 72.0* | 10.7* | 56.3* | 19.9c* | 12.6* | |
| Current study | Continuous for 6 days | LD 16:8 13 lux: dark | Unrestrained; Undisturbed | L: 25.2 | 26.5 | 34.5 | 8.3 | 5.5 | 25.2 |
| D: 89.2 | 10.3 | 43.6 | 13.0 | 11.8 | 21.4 | ||||
| 24 h: 46.4 | 15.8 | 40.2 | 11.3 | 9.7 | 23.0 | ||||
N.R.: Not reported.
Calculated or estimated from the reported data.
Collective Percentage of S1-S4/TST.
Collective Percentage of S1-S2/TST.
Collective Percentage of S3-S4/TST.
Comparison of Results with Literature on Sleep in Rhesus Monkeys
Comparison with prior studies of sleep in rhesus monkeys, summarized in Table 4, is constrained by varying differences in methodology. In particular, all prior reported studies except one have employed restraining devices. The remaining study used a backpack telemetry system requiring daily battery change, and recordings were not made in the animals' home cages.20 Additional manipulations that may have affected sleep in prior studies included blood sampling7 and daytime sleep deprivation.19,21 Thus, all prior studies have to some degree involved potential disturbance of sleep in the animals. Further, continuous recording over the 24-h day has been reported for only one study.22 The remaining reports cover limited periods of the day, either the entire night time period or a similarly restricted period of the day, although one study reported on 24-h sleep from combined 12-h recordings made either during 12-h L or D periods.7 The differences in sleep efficiency seen in prior studies are likely attributable to differences in both acclimation and stress due to restraint and interventions. In the present study these potentially confounding effects were minimized by the use of fully-implantable telemetry transmitters and by long-term recording in the animals' home cages.
Prior studies of restrained rhesus monkeys showed that the percentages of individual sleep stages were generally quite variable. One major difference between the current study and all prior studies of adult rhesus except one19 is in the higher percentage of REM sleep observed. As a percentage of total nighttime sleep time, rhesus monkeys in this study were in REM sleep for 21.4% of the time, close to the proportion seen in the sleep of normal young adult humans (24%23 or 20% to 25%24). Thus it is possible that undisturbed ad libitum sleep in the rhesus promotes increased REM sleep. Increased REM sleep is also seen when extended sleep is allowed in humans.25 Conversely, REM sleep is known to be significantly suppressed in humans when total time in sleep is restricted26 or during adaptation to the sleep laboratory.27
NREM Delta Activity
The cortical electroencephalogram (EEG) during NREM sleep contains multiple components, including slow oscillations (<1.0 Hz), delta waves (1–4 Hz) and sleep spindles (~7–14 Hz).28 Whereas slow oscillations are cortically generated, waves in the delta frequency band are thought to reflect bursting in hyperpolarized thalamocortical neurons. Sleep spindles are also generated in the thalamus by a simple recurrent inhibitory circuit consisting of thalamic reticular and relay neurons.29 During the NREM sleep episodes, delta waves progressively replace spindles as sleep proceeds, reflecting a progressive hyperpolarization of thalamocortical neurons during which the membrane potential oscillates first in the spindle frequency range and then in the range of delta waves.30 Delta waves are considered an important electrophysiological marker for stage 3 and 4 sleep (commonly termed slow wave sleep). Thus, total delta power and its rate of decline during sleep are considered electrophysiological correlates of sleep intensity and the sleep recovery process. While the former is supported by the correlation between slow wave activity and elevated arousal thresholds,31 the latter is based upon the observation that slow wave activity during sleep is proportional to the duration of prior wakefulness in humans17,32,33 and in rats.34 These observations have lead to the incorporation of a homeostatic “sleep drive” into models of sleep regulation including the 2-process model proposed by Borbely35 and the opponent-process model of Edgar et al.36 It is well established that NREM delta power and stage 3-4 sleep, in which delta activity occurs, decline during the course of sleep in humans37–39 and in several rodent species,40 although it is still debatable whether the decrease is linear or exponential.41 The monotonic decline of NREM delta activity during the course of sleep also makes the delta activity fit well as a correlate of the hypothetical sleep drive, which is supposedly at its peak at the end of the waking period, i.e., at the time of sleep onset.
In our study, unrestrained rhesus monkeys did not show the expected peak in delta sleep at CS onset. Instead, peak NREM delta power in the rhesus monkeys occurred 2 to 4 h after sleep onset (Figure 7). However, since CS in this study began several hours before the lights went off it is possible that light exposure inhibited deep sleep. A behavioral preference for darkness during sleep was seen in our animals, including turning away from the light source in typical sleeping postures. Prominent light masking effects on the circadian sleep-wake pattern due to intermittent light exposure were observed in another study done in this lab, using the same level of dim light (unpublished observations). However, in other studies done in this lab, peak delta power during sleep in constant dim light showed a similar delay, both with and without prior sleep deprivation (unpublished observations). In our opinion, the dim light level used in this study did not substantially modify the time course of NREM sleep.
In a prior study of restrained rhesus monkeys22 the peak of sleep stage 3–4 also occurred at around midnight. This was four hours after the scheduled lights-off time (20:00) in LD 12:12. The rhesus in our study entered sleep at about the same time as the lights off time in that study. Despite the light onset for our rhesus being earlier by 2 h, it is likely that delta activity in both studies peaked at a similar time relative to sleep onset. Similar observations of delayed peak delta (0.5–2 Hz) activity during CS have been reported in squirrel monkeys.42
However, when the delta frequency band (0.3–4 Hz) in our data is divided into low- (<2 Hz) and high- (2–4 Hz) frequency components, the high-frequency component of delta power shows a pattern of monotonic decline across CS (Figure 7). The low-frequency component comprises the majority of delta activity, which peaks 3 h after the start of CS. It has been reported that low-frequency oscillations (<1 Hz) in humans exhibit a different time course from the 2–4 Hz component and do not decline from the first sleep cycle to the second sleep cycle.43 Therefore, delayed peak delta activity in rhesus monkeys, also seen in squirrel monkeys42 does not necessarily contradict the hypothesis that delta activity reflects sleep homeostasis. Rather, the delay in peak delta activity may be due to a larger low-frequency component in NREM slow waves in nonhuman primates, with a dynamic similar to slow oscillations (<1 Hz) in humans.
Individual Variability in Sleep
There is an extensive literature demonstrating individual variability of sleep/wake measurements in humans. Evidence of variation has been shown for many aspects of sleep behaviors, for example in total sleep duration and distributions of sleep stages,44 circadian timing of sleep,45 spectral variables of sleep EEG,46–48 vulnerability to the effects of sleep loss49–52 and age-related changes in sleep.53 The magnitude of individual differences is often comparable to the effect size of many experimental manipulations and clinical interventions.44,54 A recent study also demonstrated individual characteristics in sleep and motor activity across 2 consecutive days of recording in the rat.55 Though this issue was not specifically discussed, individual variations in sleep measurements can also been found in earlier studies of rhesus monkeys.19,21,56 In the current study, the 5 animals displayed distinctive individual distributions of sleep stages with relatively little day-to-day variation. The causes underlying these individual differences are largely unknown, and although they may result in part from systematic errors of measurement, evidence throughout the literature suggests that genetic factors likely contribute to individual variability in sleep/wake behavior.
Potential Impact of Current Light-Dark Schedule on Sleep
The effects of light masking in circadian rhythms and sleep are well known. In diurnal animals including humans, bright light has immediate alerting effects whereas darkness promotes sleep. Light exposure produces the opposite effects in nocturnal animals.57 To differentiate intrinsic “clock” driven phenomena, we wished to avoid higher light levels. In the present study a low illumination level (13 lux on average) was chosen to minimize this masking effect, but still maintain entrainment of circadian rhythms. The LD schedule was primarily chosen to simulate human sleep wake schedules, and was based to some extent on prior studies in this lab comparing different length photoperiods on activity and body temperature rhythms in rhesus monkeys. In subsequent study of sleep in constant light (LL), we observed a slight decrease in the total amount of sleep, along with decreased nighttime sleep efficiency and increased daytime sleep (unpublished observation). This observation indicates that even at this low level of illumination, light exposure has a wake-promoting effect in the rhesus monkeys. Therefore we expect that shorter photoperiods, for example a LD 12:12 cycle, which has a light-dark ratio closer to the observed sleep-wake ratio, might improve evening sleep-wake consolidation.
In summary, the present study demonstrates the feasibility of long-term continuous sleep monitoring in the rhesus monkey using fully implantable biotelemetry. Under these conditions, sleep-wake architecture, regulation, and consolidation in rhesus monkeys were found to closely resemble sleep in humans with only minor exceptions, for example a delay in peak delta activity in rhesus relative to human sleep. These results demonstrate that the unrestrained rhesus monkey is an excellent biomedical model for human sleep.
ACKNOWLEDGMENTS
This work was supported by NASA grant NNJ04HF44G and by the CNPRC NIH base grant RR00169. We thank Mr. Peter Takeuchi for his invaluable technical assistance.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Balzamo E, Santucci V, Seri B, Vuillon-Cacciuttolo G, Bert J. Nonhuman primates: laboratory animals of choice for neurophysiologic studies of sleep. Lab Anim Sci. 1977;27:879–86. [PubMed] [Google Scholar]
- 2.Bouyer JJ, Dedet L, Debray O, Rougeul A. Restraint in primate chair may cause unusual behaviour in baboons; electrocorticographic correlates and corrective effects of diazepam. Electroencephalogr Clin Neurophysiol. 1978;44:562–7. doi: 10.1016/0013-4694(78)90123-2. [DOI] [PubMed] [Google Scholar]
- 3.Bert J, Balzamo E, Chase M, Pegram V. The sleep of the baboon, Papio papio, under natural conditions and in the laboratory. Electroencephalogr Clin Neurophysiol. 1975;39:657–62. doi: 10.1016/0013-4694(75)90079-6. [DOI] [PubMed] [Google Scholar]
- 4.Holcombe V, Sterman MB, Goodman SJ, Fairchild MB. The immobilization response in rhesus monkey: a behavioral and electroencephalographic study. Exp Neurol. 1979;63:420–35. doi: 10.1016/0014-4886(79)90136-5. [DOI] [PubMed] [Google Scholar]
- 5.Gauquelin-Koch G, Blanquie JP, Viso M, Florence G, Milhaud C, Gharib C. Hormonal response to restraint in rhesus monkeys. J Med Primatol. 1996;25:387–96. doi: 10.1111/j.1600-0684.1996.tb00034.x. [DOI] [PubMed] [Google Scholar]
- 6.Engel BT, Talan MI, Chew PH. Role of postural status in the nocturnal hemodynamic patterns of nonhuman primates. J Appl Physiol. 1993;74:1684–8. doi: 10.1152/jappl.1993.74.4.1684. [DOI] [PubMed] [Google Scholar]
- 7.Jacoby JH. Neuroendocrine rhythms and sleep-waking patterns in the rhesus monkey [doctoral] New York: Yeshiva University,; 1972. [Google Scholar]
- 8.Reite ML, Rhodes JM, Kavan E, Adey WR. Normal sleep patterns in macaque monkey. Arch Neurol. 1965;12:133–44. doi: 10.1001/archneur.1965.00460260023003. [DOI] [PubMed] [Google Scholar]
- 9.Benca RM, Obermeyer WH, Shelton SE, Droster J, Kalin NH. Effects of amygdala lesions on sleep in rhesus monkeys. Brain Res. 2000;879:130–8. doi: 10.1016/s0006-8993(00)02761-x. [DOI] [PubMed] [Google Scholar]
- 10.Fuller CA, Hoban-Higgins TM, Klimovitsky VY, Griffin DW, Alpatov AM. Primate circadian rhythms during spaceflight: results from Cosmos 2044 and 2229. J Appl Physiol. 1996;81:188–93. doi: 10.1152/jappl.1996.81.1.188. [DOI] [PubMed] [Google Scholar]
- 11.Rechtschaffen A, Kales A. A manual of standardized terminology, technique and scoring system for sleep stages of human subjects. Washington DC: Public Health Service, US Government Printing Office; 1968. [Google Scholar]
- 12.Silber MH, Ancoli-Israel S, Bonnet MH, et al. The visual scoring of sleep in adults. J Clin Sleep Med. 2007;3:121–31. [PubMed] [Google Scholar]
- 13.Wexler DB, Moore-Ede MC. Circadian sleep-wake cycle organization in squirrel monkeys. Am J Physiol. 1985;248:R353–62. doi: 10.1152/ajpregu.1985.248.3.R353. [DOI] [PubMed] [Google Scholar]
- 14.Feinberg I, Floyd TC. Systematic trends across the night in human sleep cycles. Psychophysiology. 1979;16:283–91. doi: 10.1111/j.1469-8986.1979.tb02991.x. [DOI] [PubMed] [Google Scholar]
- 15.Vessey SH. Night observations of free-ranging Rhesus monkeys. Am J Phys Anthropol. 1973;38:613–9. doi: 10.1002/ajpa.1330380276. [DOI] [PubMed] [Google Scholar]
- 16.Feinberg I, March JD, Fein G, Floyd TC, Walker JM, Price L. Period and amplitude analysis of 0.5-3 c/sec activity in NREM sleep of young adults. Electroencephalogr Clin Neurophysiol. 1978;44:202–13. doi: 10.1016/0013-4694(78)90266-3. [DOI] [PubMed] [Google Scholar]
- 17.Borbely AA, Baumann F, Brandeis D, Strauch I, Lehmann D. Sleep deprivation: effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol. 1981;51:483–95. doi: 10.1016/0013-4694(81)90225-x. [DOI] [PubMed] [Google Scholar]
- 18.Mistlberger R, Bergmann B, Rechtschaffen A. Period-amplitude analysis of rat electroencephalogram: effects of sleep deprivation and exercise. Sleep. 1987;10:508–22. [PubMed] [Google Scholar]
- 19.Weitzman ED, Kripke DF, Pollak C, Dominguez J. Cyclic activity in sleep of Macaca mulatta. Arch Neurol. 1965;12:463–7. doi: 10.1001/archneur.1965.00460290019003. [DOI] [PubMed] [Google Scholar]
- 20.Daley JT, Turner RS, Freeman A, Bliwise DL, Rye DB. Prolonged assessment of sleep and daytime sleepiness in unrestrained Macaca mulatta. Sleep. 2006;29:221–31. [PubMed] [Google Scholar]
- 21.Kripke DF, Reite ML, Pegram GV, Stephens LM, Lewis OF. Clinical and laboratory notes. Nocturnal sleep in rhesus monkeys. Electroencephalogr Clin Neurophysiol. 1968;24:582–6. doi: 10.1016/0013-4694(68)90047-3. [DOI] [PubMed] [Google Scholar]
- 22.Crowley TJ, Kripke DF, Halberg F, Pegram GV, Schildkraut JJ. Circadian rhythms of Macaca mulatta: sleep, EEG, body and eye movement, and temperature. Primates. 1972;13:149–68. [Google Scholar]
- 23.Siegel JM. Function of REM sleep. In: Carskadon MA, Rechtschaffen A, Richardson G, Roth T, Siegel JM, editors. Encyclopedia of sleep and dreaming. New York: Macmillan,; 1993. pp. 507–10. [Google Scholar]
- 24.Carskadon MA, Dement W. Normal human sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Philadelphia: Saunders,; 2000. pp. 15–25. [Google Scholar]
- 25.Feinberg I, Floyd TC. The regulation of human sleep. Clues from its phenomenology. Hum Neurobiol. 1982;1:185–94. [PubMed] [Google Scholar]
- 26.Brunner DP, Dijk DJ, Borbely AA. Repeated partial sleep deprivation progressively changes in EEG during sleep and wakefulness. Sleep. 1993;16:100–13. doi: 10.1093/sleep/16.2.100. [DOI] [PubMed] [Google Scholar]
- 27.Toussaint M, Luthringer R, Schaltenbrand N, et al. Changes in EEG power density during sleep laboratory adaptation. Sleep. 1997;20:1201–7. doi: 10.1093/sleep/20.12.1201. [DOI] [PubMed] [Google Scholar]
- 28.Steriade M, Contreras D, Curro Dossi R, Nunez A. The slow (< 1 Hz) oscillation in reticular thalamic and thalamocortical neurons: scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J Neurosci. 1993;13:3284–99. doi: 10.1523/JNEUROSCI.13-08-03284.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steriade M, Deschenes M, Domich L, Mulle C. Abolition of spindle oscillations in thalamic neurons disconnected from nucleus reticularis thalami. J Neurophysiol. 1985;54:1473–97. doi: 10.1152/jn.1985.54.6.1473. [DOI] [PubMed] [Google Scholar]
- 30.Dossi RC, Nunez A, Steriade M. Electrophysiology of a slow (0.5-4 Hz) intrinsic oscillation of cat thalamocortical neurones in vivo. J Physiol. 1992;447:215–34. doi: 10.1113/jphysiol.1992.sp018999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neckelmann D, Ursin R. Sleep stages and EEG power spectrum in relation to acoustical stimulus arousal threshold in the rat. Sleep. 1993;16:467–77. [PubMed] [Google Scholar]
- 32.Dijk DJ, Beersma DG, Daan S. EEG power density during nap sleep: reflection of an hourglass measuring the duration of prior wakefulness. J Biol Rhythms. 1987;2:207–19. doi: 10.1177/074873048700200304. [DOI] [PubMed] [Google Scholar]
- 33.Feinberg I, March JD, Floyd TC, Jimison R, Bossom-Demitrack L, Katz PH. Homeostatic changes during post-nap sleep maintain baseline levels of delta EEG. Electroencephalogr Clin Neurophysiol. 1985;61:134–7. doi: 10.1016/0013-4694(85)91051-x. [DOI] [PubMed] [Google Scholar]
- 34.Tobler I, Borbely AA. Sleep EEG in the rat as a function of prior waking. Electroencephalogr Clin Neurophysiol. 1986;64:74–6. doi: 10.1016/0013-4694(86)90044-1. [DOI] [PubMed] [Google Scholar]
- 35.Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 36.Edgar DM, Dement WC, Fuller CA. Effect of SCN lesions on sleep in squirrel monkeys: evidence for opponent processes in sleep-wake regulation. J Neurosci. 1993;13:1065–79. doi: 10.1523/JNEUROSCI.13-03-01065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Webb WB, Agnew HW., Jr. Stage 4 sleep: influence of time course variables. Science. 1971;174:1354–6. doi: 10.1126/science.174.4016.1354. [DOI] [PubMed] [Google Scholar]
- 38.Daan S, Beersma DG, Borbely AA. Timing of human sleep: recovery process gated by a circadian pacemaker. Am J Physiol. 1984;246:R161–83. doi: 10.1152/ajpregu.1984.246.2.R161. [DOI] [PubMed] [Google Scholar]
- 39.Dijk DJ, Brunner DP, Borbely AA. Time course of EEG power density during long sleep in humans. Am J Physiol. 1990;258:R650–61. doi: 10.1152/ajpregu.1990.258.3.R650. [DOI] [PubMed] [Google Scholar]
- 40.Tobler I. Phylogeny of sleep regulation. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 3rd ed. Philadelphia: Saunders,; 2000. pp. 72–81. [Google Scholar]
- 41.Feinberg I, Campbell IG. Kinetics of non-rapid eye movement delta production across sleep and waking in young and elderly normal subjects: theoretical implications. Sleep. 2003;26:192–200. doi: 10.1093/sleep/26.2.192. [DOI] [PubMed] [Google Scholar]
- 42.Klerman EB, Boulos Z, Edgar DM, Mistlberger RE, Moore-Ede MC. EEG delta activity during undisturbed sleep in the squirrel monkey. Sleep Res Online. 2000;3:113–9. [PubMed] [Google Scholar]
- 43.Achermann P, Borbely AA. Low-frequency (< 1 Hz) oscillations in the human sleep electroencephalogram. Neuroscience. 1997;81:213–22. doi: 10.1016/s0306-4522(97)00186-3. [DOI] [PubMed] [Google Scholar]
- 44.Tucker AM, Dinges DF, Van Dongen HP. Trait interindividual differences in the sleep physiology of healthy young adults. J Sleep Res. 2007;16:170–80. doi: 10.1111/j.1365-2869.2007.00594.x. [DOI] [PubMed] [Google Scholar]
- 45.Kerkhof GA. Inter-individual differences in the human circadian system: a review. Biol Psychol. 1985;20:83–112. doi: 10.1016/0301-0511(85)90019-5. [DOI] [PubMed] [Google Scholar]
- 46.Tan X, Campbell IG, Palagini L, Feinberg I. High internight reliability of computer-measured NREM delta, sigma, and beta: biological implications. Biol Psychiatry. 2000;48:1010–9. doi: 10.1016/s0006-3223(00)00873-8. [DOI] [PubMed] [Google Scholar]
- 47.Tan X, Campbell IG, Feinberg I. Internight reliability and benchmark values for computer analyses of non-rapid eye movement (NREM) and REM EEG in normal young adult and elderly subjects. Clin Neurophysiol. 2001;112:1540–52. doi: 10.1016/s1388-2457(01)00570-3. [DOI] [PubMed] [Google Scholar]
- 48.De Gennaro L, Ferrara M, Vecchio F, Curcio G, Bertini M. An electroencephalographic fingerprint of human sleep. Neuroimage. 2005;26:114–22. doi: 10.1016/j.neuroimage.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 49.Frey DJ, Badia P, Wright KP, Jr Inter- and intra-individual variability in performance near the circadian nadir during sleep deprivation. J Sleep Res. 2004;13:305–15. doi: 10.1111/j.1365-2869.2004.00429.x. [DOI] [PubMed] [Google Scholar]
- 50.Van Dongen HP, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 2004;27:423–33. [PubMed] [Google Scholar]
- 51.Van Dongen HP. Shift work and inter-individual differences in sleep and sleepiness. Chronobiol Int. 2006;23:1139–47. doi: 10.1080/07420520601100971. [DOI] [PubMed] [Google Scholar]
- 52.Bliese PD, Wesensten NJ, Balkin TJ. Age and individual variability in performance during sleep restriction. J Sleep Res. 2006;15:376–85. doi: 10.1111/j.1365-2869.2006.00557.x. [DOI] [PubMed] [Google Scholar]
- 53.Jenni OG, Molinari L, Caflisch JA, Largo RH. Sleep duration from ages 1 to 10 years: variability and stability in comparison with growth. Pediatrics. 2007;120:e769–76. doi: 10.1542/peds.2006-3300. [DOI] [PubMed] [Google Scholar]
- 54.Van Dongen HP, Vitellaro KM, Dinges DF. Individual differences in adult human sleep and wakefulness: Leitmotif for a research agenda. Sleep. 2005;28:479–96. doi: 10.1093/sleep/28.4.479. [DOI] [PubMed] [Google Scholar]
- 55.Tang X, Yang L, Sanford LD. Individual variation in sleep and motor activity in rats. Behav Brain Res. 2007;180:62–8. doi: 10.1016/j.bbr.2007.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaemingk K, Reite M. Social environment and nocturnal sleep: studies in peer-reared monkeys. Sleep. 1987;10:542–50. doi: 10.1093/sleep/10.6.542. [DOI] [PubMed] [Google Scholar]
- 57.Borbely AA. Effects of light on sleep and activity rhythms. Prog Neurobiol. 1978;10:1–31. doi: 10.1016/0301-0082(78)90018-7. [DOI] [PubMed] [Google Scholar]
- 58.Bert J, Pegram V, Rhodes JM, Balzano E, Naquet R. A comparative sleep study of two Cercopithecinae. Electroencephalogr Clin Neurophysiol. 1970;28:32–40. doi: 10.1016/0013-4694(70)90005-2. [DOI] [PubMed] [Google Scholar]
- 59.David J, Grewal RS, Wagle GP. Restricted sleep regime in rhesus monkeys: differential effect of one night's sleep loss and selective REM deprivation. Life Sci. 1975;16:1375–85. doi: 10.1016/0024-3205(75)90033-8. [DOI] [PubMed] [Google Scholar]
- 60.Balzamo E, Van Beers P, Lagarde D. Scoring of sleep and wakefulness by behavioral analysis from video recordings in rhesus monkeys: comparison with conventional EEG analysis. Electroencephalogr Clin Neurophysiol. 1998;106:206–12. doi: 10.1016/s0013-4694(97)00152-1. [DOI] [PubMed] [Google Scholar]
- 61.Yu S, Liu N, Zeng T, et al. Age-related effects of bilateral frontal eye fields lesions on rapid eye movements during REM sleep in rhesus monkeys. Neurosci Lett. 2004;366:58–62. doi: 10.1016/j.neulet.2004.05.011. [DOI] [PubMed] [Google Scholar]