Abstract
Objective:
This study examines the association of walking with mortality in persons with type 2 diabetes compared to those with normal glucose tolerance.
Study Design and Setting:
This prospective study included community-dwelling adults from the Rancho Bernardo Study aged 50-90 in 1984-86 who had type 2 diabetes (n=347) or normal glucose tolerance (n=1,317). During the 10-year follow up, Cox proportional hazards modeling was used to model time until death from all-causes (n=538), coronary heart disease (CHD, n=143), other cardiovascular disease (non-CHD CVD, n=138), and other causes (n=257) while adjusting for multiple potential confounders.
Results:
After adjusting for sex, age, smoking, body mass index, alcohol, exercise, CHD history, and other covariates, adults with diabetes who walked ≥1 mile/day were half as likely to die from all-causes combined (HR=0.54; 95% CI=0.33, 0.88), and less than one fifth as likely to die from non-CHD CVD (HR=0.19; 95% CI=0.04, 0.86) compared to adults with diabetes who did not walk. Walking was also protective among adults with normal glucose tolerance (HR=0.55; 95% CI=0.32, 0.96), but with a smaller effect size.
Conclusion:
Results suggest walking ≥1 mile per day may provide strong protection from all-cause and non-CHD CVD mortality in older adults with diabetes.
Keywords: diabetes mellitus, cardiovascular diseases, walking, mortality, public health, prospective studies
Type 2 diabetes is epidemic, projected to affect 5.4 percent of the world's adult population by 2025 [1]. This increase is especially alarming because diabetes is a strong risk factor for premature coronary heart disease (CHD) and cardiovascular disease (CVD) mortality [2-6]. The benefits of regular physical exercise to lower the risk of all-cause CHD and CVD death have been demonstrated [7-11], with some studies suggesting that even relatively low levels of physical activity, such as regular brisk walking, can lower the risk of CHD and CVD [12,13]. Exercise has been shown in clinical trials to prevent diabetes [14] and to be of benefit for those who already have diabetes [15-23]. Some individuals with diabetes may lack the ability to exercise at moderate to strenuous levels but can participate in low stress exercise such as walking. Recently, Gregg and colleagues [24] reported that walking is associated with lower rates of all-cause mortality and CVD mortality among adults with self-reported diabetes. Such persons could have been prompted to walk after diagnosis of diabetes. The purpose of the present study was to examine whether regular walking is associated with all-cause, CHD, and other CVD mortality among older community-dwelling adults with and without known diabetes or previously undiagnosed diabetes identified through an oral glucose tolerance test.
MATERIALS AND METHODS
Study population
Between 1972-1974, 82 percent of all adult residents of a southern California community, Rancho Bernardo, participated in a study of heart disease risk factors [25]. All were Caucasian, middle-class, and community-dwelling. They have been followed since initial enrollment with periodic clinic visits and yearly mailed questionnaires. Between 1984-1987, 80 percent of survivors (n = 2,854) who were 40 years or older at baseline participated in a follow-up clinic visit [26]. Participants younger than age 50 or older than 90 years in 1984-1987 (n = 98) and those completing only a mail or telephone interview (n = 374) were excluded from the present analyses. Participants without information on diabetes or anti-diabetic medication use, or who were missing plasma glucose levels, were excluded (n = 25), as were participants with type 1 (currently using insulin and fasting C-peptides<0.60) diabetes mellitus (n = 8) or without walking data (n = 1). Additionally, to provide two distinct populations for study, those with diabetes and those with normal glucose tolerance levels were compared; persons with impaired fasting glucose (n=105), impaired glucose tolerance (n = 478), or both (n = 101) were excluded leaving a cohort of 1,664 for this report. Participants (n=731) who also attended a follow-up visit in 1992-1996 provided information regarding change in exercising ≥ 3 times per week and number of blocks walked, and had a repeated measure of body mass index (BMI). The study was approved by the Institutional Review Board of the University of California, San Diego; all participants gave written informed consent prior to participation.
At the 1984-87 and the 1992-96 clinic visits, a standard interview was used to obtain information regarding age, smoking (never/past/current), average number of alcoholic drinks per day (none, 1-2, and 3 or more), use of selected medications, and exercise ≥ 3 times per week (no/yes). Walking intensity information was obtained using a single question “How many city blocks or their equivalent do you walk each day? (12 blocks = 1 mile)…must be associated with a walking or activity program, not jogging”. Participants were categorized into non-walkers, light walkers (those walking less than a mile each day), and moderate walkers (those walking a mile or more each day). Height and weight were measured with participants wearing light clothing without shoes; BMI (kg/m2) was used as an estimate of obesity. Systolic (SBP) and diastolic blood pressures (DBP) were measured according to the Hypertension Detection and Follow-up Program Protocol [27] after the participant had been seated quietly for five minutes; the average of two measurements was used. Current medication use was validated with prescriptions and containers brought to the clinic for that purpose. At the 1984-87 clinic visit a standard 75-gram oral glucose load was administered between 0700 and 1100 after a 12-16 hour fast. Blood was drawn before and two hours after the glucose challenge for measurement of plasma glucose.
Fasting plasma cholesterol and triglycerides were measured in a Centers for Disease Control-certified lipid research clinic laboratory, using an Auto Analyzer I (Technicon Instruments, Tarrytown, New York). Fasting plasma cholesterol was measured by a hexokinase method in a clinical laboratory. Plasma glucose levels were measured in a research laboratory using a glucose oxidase. Type 2 diabetes was defined using the 1999 World Health Organization Diabetes criteria [28] of fasting plasma glucose of 126 mg/dl or higher, post-challenge glucose of 200 mg/dl or higher, a history of diabetes diagnosed by a physician, or medication for diabetes. Investigations of the severity of the diabetes in this population found prevalence of CVD and renal disease [29] suggestive of a population with less severe diabetes than what may be found in clinic based studies.
Death outcome
Vital status was ascertained by annual mailings and telephone calls to participants or their contacts. Death certificates were obtained for decedents and were coded by a certified nosologist for underlying cause of death (CHD, 410 – 414; CVD, 400 – 438) using the ninth revision of the International Classification of Diseases, Clinical Modification (ICD-9-CM) [30]. In this report death certificate data were used for the years 1984 through 1996, available for 97.9 percent decedents.
Statistical analyses
Analyses compared persons with type 2 diabetes and those with normal glucose tolerance. Age was investigated as a continuous variable and by decade. Hypertension was defined by use of antihypertensive medication in the past two weeks, or SBP greater than or equal to 130 mmHg, or DBP greater than or equal to 85 mm Hg [27]. High triglyceride levels were defined by fasting triglycerides greater than or equal to 150 mg/dl; [31] low HDL (fasting HDL < 40 mg/dl for men and < 50 mg/dl for women. BMI was categorized into underweight (< 18.5 kg/m2), normal (18.5-24.9 kg/m2), overweight (25.0-29.9 kg/m2), or obese (> 30.0 kg/m2). Coronary heart disease was based on the presence of angina or possible myocardial infarction according to the Rose chest pain questionnaire [32,33], or a history of a medical diagnosis of myocardial infarction or coronary heart disease. Self reported heart disease was validated by record review in 85 percent for whom records were requested [34].
Based on a combination of diabetes and walking status, individuals were categorized into six groups: non-walkers, light walkers, and moderate walkers with diabetes; non-walkers, light walkers, and moderate walkers without diabetes. Comparisons of demographic and lifestyle variables, and death outcomes by diabetes and walking status were performed with t-tests and chi-square analyses. Age-adjusted death rates from all-cause, CHD, other CVD, and other causes were computed for the six subgroups. Univariate analyses were performed to compare those with missing to those with complete data. An exploratory model analysis was completed to first assess multicollinearity and then to assess regression diagnostics, significant associations, and possible confounding, while simultaneously adjusting for all other variables in the model. Both stratified analyses and investigation of interaction terms were completed to investigate for varying effects over gender of walking and diabetes with mortality.
Multivariable Cox proportional hazards time-to-event modeling was conducted to compare death rates from all causes, CHD, non-CHD CVD, and other causes of death among walkers and non-walkers with and without diabetes while accounting for loss to follow-up over the 10-year follow-up period. Follow-up time was calculated from date of clinic visit until death outcome, loss to follow-up from study, or December 31, 1996, whichever came first. Follow-up time ended in 1996 due to the relatively high completeness of the mortality data through that year (97.9%). Adjusted hazard ratios (HRs) and 95 percent confidence intervals (CIs) were calculated for each cause of death. Additionally, investigation of the proportional hazards assumption included visual inspection of the adjusted cumulative probability of death as a function of time graphed after stratification by diabetes and walking status as well as investigation of statistical significance of interaction with time.
Changes in BMI, exercise, and walking were investigated as explanatory variables in the 731 original participants who returned to the clinic between 1992 and 1996. Prevalence and weighted Kappa statistics were computed to examine consistency between visits. Shorter follow-up time precluded mortality analyses in this subset. Cox proportional hazard models were repeated after stratifying by known or unknown diabetes at baseline. All data manipulation and analyses were conducted using SAS® (Version 9.0, SAS Institute, Inc., Cary, North Carolina) [35].
RESULTS
Of the 1,664 older adults included in this study, 347 (20.9%) had type 2 diabetes, 32 percent by history and 68 percent newly diagnosed by OGTT. The mean age was 73.7 years among those with diabetes and 69.1 years among those with normal glucose tolerance (p-value < 0.0001). As shown in Table 1, slightly more than half were women and 40 percent were non-walkers, although 63 percent of non-walkers indicated that they exercised > 3 times a week. Few participants were current smokers (13%) or reported three or more alcoholic drinks per day (11%). However, hypertension (71%) and a history of CHD (32%) were common. Mean fasting plasma glucose levels for adults with and without diabetes were 125.5 and 93.5 mg/dl, respectively (p-value < 0.0001), and mean post challenge levels were 237.5 and 105.2 mg/dl, respectively (p-value < 0.0001). Table 1 also shows that adults with diabetes were significantly more likely than those with normal glucose tolerance to be male, older, former smokers, overweight or obese, hypertensive, consume ≥ three alcoholic drinks per day, have high triglycerides, low HDL cholesterol, and a history of coronary heart disease, and were less likely to report regular exercise. Walking was not significantly associated with diabetes status (p-value = 0.15).
Table 1.
Characteristics of Participants Stratified by Diabetes Status; Rancho Bernardo CA, 1984-87
| Variable | Total Population (N=1,664) |
Normal Glucose (N=1,317) |
Diabetes (N=347) |
p-value* |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Walking | ||||
| Non-walker | 666 (40.0) | 525 (39.8) | 141 (40.6) | 0.15 |
| > 1 mile walker | 585 (35.2) | 452 (34.3) | 133 (38.3) | |
| ≥ 1 mile walker | 413 (24.8) | 340 (25.8) | 73 (21.0) | |
| Gender | ||||
| Female | 923 (55.5) | 757 (57.5) | 166 (47.8) | 0.001 |
| Male | 741 (44.5) | 560 (42.5) | 181 (52.2) | |
| Age (years) | ||||
| 50-59 | 293 (17.6) | 266 (20.2) | 27 (7.8) | < 0.0001 |
| 60-69 | 439 (26.4) | 374 (28.4) | 65 (18.7) | |
| 70-79 | 655 (39.4) | 490 (37.2) | 165 (47.6) | |
| 80-89 | 277 (16.6) | 187 (14.2) | 90 (25.9) | |
| Smoking | ||||
| Never | 661 (39.8) | 522 (39.7) | 139 (40.1) | 0.03 |
| Past | 789 (47.5) | 611 (46.5) | 178 (51.3) | |
| Current | 211 (12.7) | 181 (13.8) | 30 (12.7) | |
| Body mass index | ||||
| Underweight (<18.5) | 30 (1.8) | 25 (1.9) | 5 (1.5) | < 0.0001 |
| Normal (18.5-24.9) | 872 (53.5) | 724 (56.1) | 148 (43.5) | |
| Overweight (25.0-29.9) | 596 (36.5) | 453 (35.1) | 143 (42.1) | |
| Obese (≥30.0) | 133 (8.2) | 89 (6.9) | 44 (12.9) | |
| Average daily alcohol intake | ||||
| None | 613 (36.9) | 465 (35.3) | 148 (42.7) | 0.001 |
| 1-30 grams (1-2 drinks) | 868 (52.1) | 717 (54.4) | 151 (43.5) | |
| 30+ grams (3 or more drinks) | 183 (11.0) | 135 (10.3) | 48 (13.8) | |
| Exercised ≥ 3 times past week | ||||
| Yes | 1,358 (81.7) | 1,088 (82.6) | 270 (78.0) | 0.05 |
| Hypertension† | ||||
| Yes | 1,180 (70.9) | 876 (66.5) | 304 (87.6) | < 0.0001 |
| Triglycerides | ||||
| ≥ 150 mg/dl (high)/ < 150 mg/dl | 353 (21.2) | 225 (17.1) | 128 (36.9) | < 0.0001 |
| HDL cholesterol | ||||
| Low‡ | 231 (13.9) | 144 (10.9) | 87 (25.1) | < 0.0001 |
| History coronary heart disease§ | ||||
| Yes | 534 (32.1) | 376 (28.5) | 158 (45.5) | < 0.0001 |
Unadjusted p-value based on a chi square test of association, comparing distribution among all categories.
Any antihypertensive in past two weeks or SBP≥130 mm Hg or DBP≥85 mm Hg.
High-density lipoprotein cholesterol characterized low when <40 mg/dl for men and <50 mg/dl women.
Table 2 compares characteristics after stratification by walking status. Walkers were significantly more likely than non-walkers to be male, report regular exercise, and have a history of CHD, but significantly less likely to be current smokers or have low HDL cholesterol. Although the mean age was similar by walking status (p-value > 0.10, means 69.8 for non-walkers, 70.7 for < 1 mile walkers, and 69.6 for ≥ 1 mile per day walkers), analyses with age as a categorical variable showed higher proportions of younger participants among non-walkers and higher proportions of older participants among walkers (p-value = 0.02). BMI, daily alcohol intake, hypertension, and triglycerides did not differ by walking status (p-value > 0.10).
Table 2.
Characteristics of Participants Stratified by Walking Status; Rancho Bernardo CA, 1984-87
| Variable | Non-Walker (N=666) |
< 1 Mile Walker (N=585) |
≥ 1 Mile Walker (N=413) |
p-value* |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Diabetes | ||||
| Normal glucose tolerance | 525 (78.8) | 452 (77.3) | 340 (82.3) | 0.15 |
| Type 2 diabetes | 141 (21.2) | 133 (22.7) | 73 (17.7) | |
| Gender | ||||
| Female | 418 (62.8) | 306 (52.3) | 199 (48.2) | < 0.0001 |
| Male | 248 (37.2) | 279 (47.7) | 214 (51.8) | |
| Age (years) | ||||
| 50-59 | 132 (19.8) | 95 (16.2) | 66 (16.0) | 0.02 |
| 60-69 | 176 (26.4) | 142 (24.3) | 121 (23.3) | |
| 70-79 | 245 (36.8) | 235 (40.2) | 175 (42.4) | |
| 80-89 | 113 (17.0) | 113 (19.3) | 51 (12.5) | |
| Smoking | ||||
| Never | 258 (38.9) | 223 (38.2) | 180 (43.6) | 0.001 |
| Past | 297 (44.7) | 288 (49.3) | 204 (49.4) | |
| Current | 109 (16.4) | 73 (12.5) | 29 (7.0) | |
| Body mass index | ||||
| Underweight (<18.5) | 10 (1.5) | 14 (2.5) | 6 (1.5) | 0.22 |
| Normal (18.5-24.9) | 334 (51.5) | 310 (54.2) | 228 (55.6) | |
| Overweight (25.0-29.9) | 242 (37.3) | 201 (35.1) | 153 (37.3) | |
| Obese (≥30.0) | 63 (9.7) | 47 (8.2) | 23 (5.6) | |
| Average daily alcohol intake | ||||
| None | 242 (36.3) | 230 (39.3) | 141 (34.1) | 0.08 |
| 1-30 grams (1-2 drinks) | 339 (50.9) | 293 (50.1) | 236 (57.1) | |
| 30+ grams (≥3 drinks) | 85 (12.8) | 62 (10.6) | 36 (8.7) | |
| Exercised ≥ 3 times past week | ||||
| Yes | 417 (62.7) | 532 (90.9) | 409 (99.0) | < 0.0001 |
| Hypertension† | ||||
| Yes | 479 (71.9) | 403 (68.9) | 298 (72.1) | 0.41 |
| Triglycerides | ||||
| ≥ 150 mg/dl (high)/ < 150 mg/dl | 153 (23.0) | 127 (21.7) | 73 (17.7) | 0.11 |
| HDL cholesterol | ||||
| Low‡ | 89 (13.4) | 96 (16.4) | 46 (11.1) | 0.05 |
| History coronary heart disease§ | ||||
| Yes | 192 (28.8) | 217 (37.1) | 125 (30.3) | 0.005 |
Unadjusted p-value based on a chi square test of association, comparing distribution among all categories.
Any antihypertensive in past two weeks or SBP≥130 mm Hg or DBP≥85 mm Hg.
High-density lipoprotein cholesterol characterized low when <40 mg/dl for men and <50 mg/dl women.
During the 10-year follow-up period there were 152 CHD deaths, 143 other CVD deaths, and 269 deaths from other causes totaling 564 deaths from all-causes. The lowest age-adjusted all-cause death rate was among those with diabetes who walked a mile or more each day (rate = 26.5 per 100; 95% CI = 13.6, 39.4). When analyzed by cause of mortality the age-adjusted rate for other CVD mortality was also lowest among adults with diabetes who walked a mile or more each day (rate = 2.3 per 100; 95% CI = 0, 6.1). However, there was no difference in age-adjusted rates of CHD or other mortality by walking status (data not shown). Among adults with normal glucose tolerance there was little difference between age-adjusted all-cause and cause-specific mortality by walking status (data not shown). In comparison to other subgroup age-adjusted all cause mortality rates, those with the lowest mortality rates included never smokers (rate = 28.6), normal body weight as measured by BMI (rate = 32.0), those who drank on average 1-2 drinks per day (rate = 32.3), those who exercised 3 or more times per week (rate = 32.7), and those without hypertension (rate = 30.5) (data not shown).
There were no significant differences in distributions of covariates between those with and without complete data. Regression diagnostics for investigation of the pair-wise correlations and the variance inflation suggested no discernible multicollinearity among the variables in each of the models presented in Table 3.
Table 3.
Adjusted Hazard Ratios for Death from All Causes, Coronary Heart Disease, Non-Coronary Heart Disease Cardiovascular Disease, and Other Causes; Rancho Bernardo CA, 1984-1996
| Variable | All-Cause* (N=538) |
CHD (N=143) |
Non-CHD CVD (N=138) |
Other Causes (N=257) |
||||
|---|---|---|---|---|---|---|---|---|
| HR† | 95% CI† | HR† | 95% CI† | HR† | 95% CI† | HR† | 95% CI† | |
| Diabetes | ||||||||
| Non-walker‡ | 1.00 | -- | 1.00 | -- | 1.00 | -- | 1.00 | -- |
| < 1 mile walker | 1.02 | 0.70, 1.43 | 1.07 | 0.55, 2.07 | 1.08 | 0.51, 2.30 | 0.94 | 0.56, 1.58 |
| ≥ 1 mile walker | 0.54 | 0.33, 0.88 | 1.05 | 0.48, 2.31 | 0.19 | 0.04, 0.86 | 0.48 | 0.23, 1.00 |
| Normal glucose tolerance | ||||||||
| Non-walker‡ | 1.00 | -- | 1.00 | -- | 1.00 | -- | 1.00 | -- |
| < 1 mile walker | 0.98 | 0.76, 1.25 | 1.14 | 0.67, 1.94 | 0.77 | 0.48, 1.21 | 1.05 | 0.74, 1.49 |
| ≥ 1 mile walker | 0.89 | 0.67, 1.18 | 1.29 | 0.73, 2.28 | 0.55 | 0.32, 0.96 | 0.94 | 0.63, 1.41 |
| Gender | ||||||||
| Male/ Female | 2.45 | 2.03, 2.96 | 2.87 | 1.96, 4.20 | 2.67 | 1.83, 3.89 | 2.18 | 1.66, 2.86 |
| Age (per year) | 1.13 | 1.11, 1.14 | 1.13 | 1.10, 1.16 | 1.17 | 1.13, 1.20 | 1.11 | 1.09, 1.13 |
| Smoking | ||||||||
| Past/Never | 1.21 | 1.00, 1.47 | 0.93 | 0.64, 1.34 | 1.07 | 0.73, 1.56 | 1.53 | 1.14, 2.04 |
| Current/ Never | 2.02 | 1.49, 2.73 | 1.39 | 0.72, 2.68 | 1.98 | 1.08, 3.63 | 2.43 | 1.60, 3.77 |
| Body mass index | ||||||||
| Underweight (<18.5)/Normal | 1.52 | 0.90, 2.59 | 1.10 | 0.27, 4.59 | 1.23 | 0.44, 3.42 | 1.85 | 0.93, 3.68 |
| Overweight (25.0-29.9)/Normal | 0.86 | 0.71, 1.04 | 1.18 | 0.82, 1.70 | 0.80 | 0.55, 1.17 | 0.74 | 0.56, 0.98 |
| Obese (≥30.0)/Normal | 1.02 | 0.72, 1.43 | 1.54 | 0.84, 2.82 | 0.53 | 0.21, 1.34 | 1.04 | 0.64, 1.67 |
| Average drinks per day | ||||||||
| 1-30 grams (1-2 drinks)/None | 0.79 | 0.65, 0.95 | 0.88 | 0.61, 1.27 | 0.65 | 0.45, 0.95 | 0.83 | 0.63, 1.09 |
| 30+ grams (3 or more drinks) /None | 0.88 | 0.65, 1.18 | 0.65 | 0.34, 1.25 | 0.86 | 0.49, 1.53 | 0.99 | 0.65, 1.49 |
| Exercised 3 or more times past week | ||||||||
| Yes/No | 0.69 | 0.55, 0.88 | 0.49 | 0.31, 0.77 | 0.86 | 0.53, 1.38 | 0.74 | 0.52, 1.04 |
| Hypertension§ | ||||||||
| Yes/No | 1.21 | 0.94, 1.57 | 1.51 | 0.84, 2.71 | 1.26 | 0.75, 2.12 | 1.09 | 0.77, 1.54 |
| Triglycerides# | ||||||||
| ≥ 150 mg/dl (high)/ < 150 mg/dl | 1.08 | 0.85, 1.36 | 1.25 | 0.82, 1.90 | 0.74 | 0.44, 1.26 | 1.16 | 0.83, 1.62 |
| High-density lipoprotein cholesterol | ||||||||
| Low/ Normal# | 1.13 | 0.86, 1.48 | 1.50 | 0.95, 2.38 | 1.05 | 0.56, 1.96 | 0.97 | 0.65, 1.46 |
| History coronary heart disease** | ||||||||
| Yes/No | 1.64 | 1.38, 1.95 | 2.67 | 1.89, 3.78 | 1.31 | 0.92, 1.86 | 1.43 | 1.11, 1.84 |
All-cause death is inclusive of CHD, CVD, and other.
Cox regression adjusted hazard ratio and 95% confidence interval for those with complete covariate data.
Reference category. Non-walkers are referenced within each of the diabetic and normal glucose tolerance groups.
Any antihypertensive in past two weeks or SBP≥130 mm Hg or DBP≥85 mm Hg.
High-density lipoprotein cholesterol characterized low when <40 mg/dl for men and <50 mg/dl women.
Covariate data were complete for 1,627 of the 1,664 participants (97.8%). Cox proportional hazard models adjusting for gender, age (continuous), smoking, BMI, average drinks per day, exercise, hypertension, triglycerides, high-density lipoprotein cholesterol, and history of coronary heart disease for all-cause and cause specific mortality are shown in Table 3. There was no evidence of a sex by walking/diabetes interaction (p-value = 0.77). After adjusting for other covariates, adults with diabetes who walked a mile or more each day were about half as likely to die in the next 10 years compared to adults with diabetes who did not walk this distance (HR = 0.54; 95% CI = 0.33, 0.88). Adults without diabetes who walked a mile or more each day were also at reduced risk for all-cause mortality, however this finding was not statistically significant (HR = 0.89; 95% CI = 0.67, 1.18) (Table 3). Other statistically significant independent predictors for all-cause mortality after adjustment for all other covariates included male sex (HR = 2.45; 95% CI = 2.03, 2.96), age in years (HR = 1.13; 95% CI = 1.11, 1.14), current smoking (HR = 2.02; 95% CI = 1.49, 2.73), and history of CHD (HR = 1.64; 95% CI = 1.38, 1.95); exercise ≥ 3 times per week was protective (HR = 0.69; 95% CI = 0.55, 0.88).
Only 32 percent of those with type 2 diabetes knew of their condition at the 1984-1987 clinic visit; the remainder were diagnosed by OGTT. The following reported results were consistent when stratified by known and unknown diabetes at baseline however, statistical significance was reduced due to smaller cell sizes. There was no statistically significant difference in reporting walking (p-value = 0.85) or exercising 3 or more times in the past week (p-value = 0.64) between those who did and did not know they had type 2 diabetes.
After adjusting for other covariates, walking at least one mile each day reduced the risk of other CVD death in those with diabetes (HR = 0.19; 95% CI = 0.04, 0.86) and those without (HR = 0.55; 95% CI = 0.32, 0.96) when compared to those who walked less (Table 3). In contrast, there were no statistically significant differences in risk of mortality attributed to CHD or other causes between walkers and non-walkers among adults with and without diabetes. Other risk factors for CHD, other CVD, and other causes of death included male sex, age, current smoking, and history of CHD. The only statistically significant protective factor for other CVD death was drinking 1-2 drinks per day, while the only protective factor for CHD death was exercising ≥ 3 times per week.
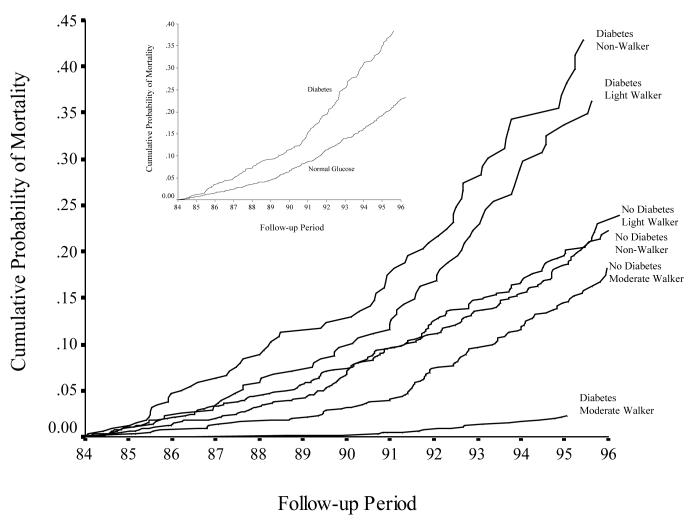
Plots of adjusted cumulative probability of death over the 10-year follow-up period are shown for adults with diabetes compared to adults without diabetes (Figure 1). Almost 40 percent of those with diabetes died compared to 23 percent of those without diabetes.
Figure 1.
Adjusted cumulative probability of all-cause mortality by diabetes status and all-cause mortality by walking and diabetes category during the period January 1, 1984, to December 31, 1996.
Figure 1 also shows the adjusted cumulative probability of death for adults for the six categories of walking and diabetes and appeared stable over the 10-year follow-up period. Those with and without diabetes who walked a mile or more each day had the lowest adjusted cumulative probability of death over the period. Proportional hazards assumption was confirmed by visual inspection of the curves as well as inclusion of a time interaction variable which was found to be insignificant (p-value=0.14).
Investigation of behavior change in the sub-sample of 731 participants who returned for a follow-up clinic visit in 1992-96 showed that 45 percent consistently reported being non-walkers, light walkers, and moderate walkers, and 71 percent consistently reported exercising three or more times per week. Of those whose walking habits changed, 30 percent increased to become light or moderate walkers from nonwalking, while 25 percent reduced their walking to become non- or light walkers from moderate walkers. Body mass index was stable (weighted kappa = 0.63).
DISCUSSION
The prevalence of type 2 diabetes has increased dramatically during the past two decades with further increases predicted and tremendous public health implications [1]. Regular exercise has been recommended to help reduce the cardiovascular complications and mortality among adults with diabetes [15-22]. Physical activity of moderate intensity and duration has been shown to reduce the risk of developing type 2 diabetes in observational studies and animal trials [36]. However, older adults with and without diabetes may have functional limitations and physical disabilities that do not allow for strenuous physical activity [37]. Therefore, this investigation focused on walking while controlling for many potential confounders including other exercise. Results of this study show that while walking more than one mile per day reduced all-cause and non-CHD CVD mortality in both adults with and without diabetes, the protective effect of walking was much stronger in those with diabetes compared to those without diabetes.
This decreased risk of all-cause and other CVD mortality among adults with diabetes walking more than one mile each day, independent of reporting regular exercise ≥ 3 times per week, is consistent with a recent report by Gregg [24]. In the present study, the magnitude of the effect of walking more than one mile per day on all-cause as well as non-CHD CVD mortality was noticeably different for adults with diabetes than for those with normal glucose tolerance. However, while visual inspection of cumulative probability of mortality among persons with diabetes indicated a relative benefit, the test of interaction suggested that these effect measures for walking do not statistically vary over diabetes status (p-value=0.14).
Results of the present study are in accord with other studies showing that exercise can reduce mortality linked to diabetes [7-10,16-22]. In a previous study of this cohort, sex differences were found in the contribution of diabetes to fatal heart disease, which was largely explained by the persistently more favorable survival rate of women without diabetes [38]. However, a metanalysis of cohort studies that corrected for common risk factors removed the evidence of a statistically significant sex difference [39]. These papers did not consider physical activity. In the present study, men walked slightly more than women, but there was no significant interaction between sex and the walking/diabetes variable.
Others have reported that those with diabetes have three- to five-fold increases in future cardiovascular events compared to those without diabetes [40] and nearly three quarters of all deaths in those with diabetes result from CHD [41]. In this study we found a greater cardio-protective benefit of walking among those with diabetes compared to those without diabetes. Diabetes is associated with obesity, higher blood pressure, and cholesterol, factors that improve with exercise [42-45]. Smoking, hypertension, obesity, sedentary lifestyle, hypercholesterolemia, and diabetes are known risk factors for CVD [46]. Thus, individuals with diabetes may experience a greater reduction in CVD mortality by improving in several of these risk factors. Those with diabetes who attempt to improve glycemic control through walking may also be more aggressive about dietary changes, which may in turn reduce other CVD risk factors [47-51]. People who walk more than a mile a day are more likely to do other regular exercise. In the present study there was a relatively low correlation between self-reported walking and exercise (correlation coefficient = 0.30) and no noticeable multicollinearity among the variables as measured by the variance inflation values, therefore exercising three or more times per week was included in the model to adjust for potential differences in exercise.
While moderate and intense physical exercise decrease with age, light exercise such as walking increases with age and is associated with other forms of exercise [52]. The transition from moderate or intense exercise to walking was not accounted for in the present analyses. However, investigation of habit changes in 731 who participated in a follow-up visit from 1992 to 1996 suggested only a modest change in this population that would likely reduce evidence of benefit if true.
It should be noted that this is a sample of ambulatory, older, Caucasian, middle-class adults living in southern California where the weather permits year-round walking, and may not be representative of the general US population. In addition, those who were ill may have chosen not to participate or may have lacked the capacity to do so. Finally, behavioral data were self-reported, which limits the ability to quantify behavioral trends such as walking and exercise. However, BMI was stable over approximately 10 years (weighted kappa = 0.63), suggesting no large shifts in body mass in the sub-group of the 1984-86 study population participating in a visit between 1992 and 1996. Lastly, although differences in characteristics such as gender, age, smoking, body mass index, alcohol consumption, exercise, hypertension, triglyceride levels, high-density lipoprotein cholesterol, and overall coronary heart disease were controlled for, it is possible that other factors not taken into account may explain the observed differences.
This study has a number of strengths. Use of a glucose-tolerance test-based diagnosis of type 2 diabetes allowed for more complete ascertainment of diabetes than relying on self-reported data alone. Restriction to those with diabetes and those without diabetes at baseline omitted an intermediate group where misclassification would be most likely, and should give more precise estimates of the effect moderate walking has on mortality. In addition, the prospective design of the present study allows for investigation of the risk of mortality among walkers and non-walkers with and without diabetes over a 10-year follow-up period. The sample size, the relatively homogeneous study population, and the use of Cox regression survival models allowed for fairly robust hazard ratio estimates and plots of adjusted cumulative probability of mortality over time while controlling for confounding and adjusting for minimal loss to follow up.
In summary, the present study demonstrates a two-fold reduction in adjusted risk of all-cause mortality and a five-fold reduction in adjusted risk of non-CHD CVD death associated with walking among persons with diabetes. This is likely a conservative estimate of the true benefit. Moderate activity appears to have a marked reduction in mortality in elderly adults with diabetes. These results are reassuring to those who are unable to achieve a higher intensity physical activity.
Acknowledgments
This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-31801 and the National Institute on Aging, grant # AG 07181. The authors would also like to thank Ricki Bettencourt, MS, for data management support and Cedric Garland, DrPH, Frank Garland, PhD, and Ed Gorham, PhD, for critical review of the presentation of data.
Abbreviations
- CI
confidence interval
- HR
hazard ratio
- CHD
coronary heart disease
- CVD
cardiovascular disease
- BMI
body mass index
- DBP
diastolic blood pressure
- SBP
systolic blood pressure
- OGTT
oral glucose tolerance test
References
- 1.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21(9):1414–1431. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 2.Hu FB, Manson JE. Walking: the best medicine for diabetes? Arch Intern Med. 2003;163(12):1397–1398. doi: 10.1001/archinte.163.12.1397. [DOI] [PubMed] [Google Scholar]
- 3.Egede LE, Zheng D. Modifiable cardiovascular risk factors in adults with diabetes: prevalence and missed opportunities for physician counseling. Arch Intern Med. 2002;162(4):427–433. doi: 10.1001/archinte.162.4.427. [DOI] [PubMed] [Google Scholar]
- 4.Lipman TH, Hayman LL, Fabian CV, et al. Risk factors for cardiovascular disease in children with type I diabetes. Nurs Res. 2000;49(3):160–166. doi: 10.1097/00006199-200005000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Hu FB, Stampfer MJ, Solomon CG, et al. The impact of diabetes mellitus on mortality from all causes and coronary heart disease in women: 20 years of follow-up. Arch Intern Med. 2001;161(14):1717–1723. doi: 10.1001/archinte.161.14.1717. [DOI] [PubMed] [Google Scholar]
- 6.Hu FB, Stampfer MJ, Solomon C, et al. Physical activity and risk for cardiovascular events in diabetic women. Ann Intern Med. 2001;134(2):96–105. doi: 10.7326/0003-4819-134-2-200101160-00009. [DOI] [PubMed] [Google Scholar]
- 7.Powell KE, Thompson PD, Caspersen CJ, Kendrick JS. Physical activity and the incidence of coronary heart disease. Annu Rev Public Health. 1987;8:253–287. doi: 10.1146/annurev.pu.08.050187.001345. [DOI] [PubMed] [Google Scholar]
- 8.Levenson JW, Skerrett PJ, Gaziano JM. Reducing the global burden of cardiovascular disease: the role of risk factors. Prev Cardiol. 2002;5(4):188–199. doi: 10.1111/j.1520-037x.2002.00564.x. [DOI] [PubMed] [Google Scholar]
- 9.Macera CA, Hootman JM, Sniezek JE. Major public health benefits of physical activity. Arthritis Rheum. 2003;49(1):122–128. doi: 10.1002/art.10907. [DOI] [PubMed] [Google Scholar]
- 10.Havranek EP. Primary prevention of CHD: nine ways to reduce risk. Am Fam Physician. 1999;59(6):1455–1463. 1466. [PubMed] [Google Scholar]
- 11.Wei M, Gibbons LW, Kampert JB, Nichaman MZ, Blair SN. Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med. 2000;132(8):605–611. doi: 10.7326/0003-4819-132-8-200004180-00002. [DOI] [PubMed] [Google Scholar]
- 12.Manson JE, Hu FB, Rich-Edwards JW, et al. A prospective study of walking as compared with vigorous exercise in the prevention of coronary heart disease in women. N Engl J Med. 1999;341(9):650–658. doi: 10.1056/NEJM199908263410904. [DOI] [PubMed] [Google Scholar]
- 13.Wannamethee SG, Shaper AG. Physical activity in the prevention of cardiovascular disease: an epidemiological perspective. Sports Med. 2001;31(2):101–114. doi: 10.2165/00007256-200131020-00003. [DOI] [PubMed] [Google Scholar]
- 14.Ryan DH, Diabetes Prevention Program Research, G. Diet and exercise in the prevention of diabetes. Int J Clin Pract. 2003;134:28–35. [PubMed] [Google Scholar]
- 15.Ratner R, Goldberg R, Haffner S, et al. Impact of intensive lifestyle and metformin therapy on cardiovascular disease risk factors in the diabetes prevention program. Diabetes Care. 2005;28(4):888–894. doi: 10.2337/diacare.28.4.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eriksson JG. Exercise and the treatment of type 2 diabetes mellitus. An update. Sports Med. 1999;27(6):381–391. doi: 10.2165/00007256-199927060-00003. [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes Association Diabetes mellitus and exercise. Diabetes Care. 2000;23(Suppl 1):S50–54. [PubMed] [Google Scholar]
- 18.Hamdy O, Goodyear LJ, Horton ES. Diet and exercise in type 2 diabetes mellitus. Endocrinol Metab Clin North Am. 2001;30(4):883–907. doi: 10.1016/s0889-8529(05)70220-6. [DOI] [PubMed] [Google Scholar]
- 19.Peirce NS. Diabetes and exercise. Br J Sports Med. 1999;33(3):161–172. doi: 10.1136/bjsm.33.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richter EA, Ruderman NB, Schneider SH. Diabetes and exercise. Am J Med. 1981;70(1):201–209. doi: 10.1016/0002-9343(81)90427-7. [DOI] [PubMed] [Google Scholar]
- 21.Zinman B. Diabetes and exercise. Postgrad Med. 1979;66(5):81–82. doi: 10.1080/00325481.1979.11715296. [DOI] [PubMed] [Google Scholar]
- 22.Holbrook TL, Barrett-Connor E, Wingard DL. The association of lifetime weight and weight control patterns with diabetes among men and women in an adult community. Int J Obes. 1989;13(5):723–729. [PubMed] [Google Scholar]
- 23.Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C. Physical activity/exercise and type 2 diabetes. Diabetes Care. 2004;27(10):2518–2539. doi: 10.2337/diacare.27.10.2518. [DOI] [PubMed] [Google Scholar]
- 24.Gregg EW, Gerzoff RB, Caspersen CJ, Williamson DF, Narayan KM. Relationship of walking to mortality among US adults with diabetes. Arch Intern Med. 2003;163(12):1440–1447. doi: 10.1001/archinte.163.12.1440. [DOI] [PubMed] [Google Scholar]
- 25.Criqui MH, Barrett-Connor E, Austin M. Differences between respondents and non-respondents in a population-based cardiovascular disease study. Am J Epidemiol. 1978;108(5):367–372. doi: 10.1093/oxfordjournals.aje.a112633. [DOI] [PubMed] [Google Scholar]
- 26.Wingard DL, Barrett-Connor E. Community-based study of prevalence of NIDDM in older adults. Diabetes Care. 1990;13(Suppl2):S3–S8. [Google Scholar]
- 27.Anonymous The Hypertension Detection and Follow-up Program: Hypertension Detection and Follow-up Program Cooperative Group. Prev Med. 1976;5(2):207–215. doi: 10.1016/0091-7435(76)90039-6. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization . Report of a WHO Consultation. Part 1: diagnosis and classification of diabetes mellitus. World Health Organization, Department of Noncommunicable Disease Surveillance; Geneva: 1999. Definition, diagnosis and classification of diabetes mellitus and its complications; pp. 1–59. [Google Scholar]
- 29.Wingard DL, Barrett-Connor EL, Scheidt-Nave C, McPhillips JB. Prevalence of cardiovascular and renal complications in older adults with normal or impaired glucose tolerance or NIDDM. A population-based study. Diabetes Care. 1993;16(7):1022–1025. doi: 10.2337/diacare.16.7.1022. [DOI] [PubMed] [Google Scholar]
- 30.The International Classification of Diseases, 9th Revision, Clinical Modification: ICD-9-CM. 2 ed. US Dept of Health and Human Services, Public Health Service; Washington DC: 1980. [Google Scholar]
- 31.Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 32.Rose G, McCartney P, Reid DD. Self-administration of a questionnaire on chest pain and intermittent claudication. Br J Prev Soc Med. 1977;31(1):42–48. doi: 10.1136/jech.31.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilcosky T, Harris R, Weissfeld L. The prevalence and correlates of Rose Questionnaire angina among women and men in the Lipid Research Clinics Program Prevalence Study population. Am J Epidemiol. 1987;125(3):400–409. doi: 10.1093/oxfordjournals.aje.a114546. [DOI] [PubMed] [Google Scholar]
- 34.Barrett-Connor E, Ferrara A. Isolated postchallenge hyperglycemia and the risk of fatal cardiovascular disease in older women and men. The Rancho Bernardo Study. Diabetes Care. 1998;21(8):1236–1239. doi: 10.2337/diacare.21.8.1236. [DOI] [PubMed] [Google Scholar]
- 35.SAS Institute Inc. SAS/STAT® software: changes and enhancements through release 6.11. SAS Institute Inc.; Cary, NC: 1996. p. 1104. [Google Scholar]
- 36.Molitch ME, Fujimoto W, Hamman RF, Knowler WC. The diabetes prevention program and its global implications. J Am Soc Nephrol. 2003;14(7 Suppl 2):S103–107. doi: 10.1097/01.asn.0000070140.62190.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gregg EW, Beckles GL, Williamson DF, et al. Diabetes and physical disability among older U.S. adults. Diabetes Care. 2000;23(9):1272–1277. doi: 10.2337/diacare.23.9.1272. [DOI] [PubMed] [Google Scholar]
- 38.Barrett-Connor EL, Cohn BA, Wingard DL, Edelstein SL. Why is diabetes mellitus a stronger risk factor for fatal ischemic heart disease in women than in men? The Rancho Bernardo Study.[erratum appears in JAMA 1991 Jun 26;265(24):3249] JAMA. 1991;265(5):627–631. [PubMed] [Google Scholar]
- 39.Kanaya AM, Grady D, Barrett-Connor E. Explaining the sex difference in coronary heart disease mortality among patients with type 2 diabetes mellitus: a meta-analysis. Arch Intern Med. 2002;162(15):1737–1745. doi: 10.1001/archinte.162.15.1737. [DOI] [PubMed] [Google Scholar]
- 40.Kannel WB, McGee DL. Diabetes and glucose tolerance as risk factors for cardiovascular disease: the Framingham study. Diabetes Care. 1979;2:120–126. doi: 10.2337/diacare.2.2.120. [DOI] [PubMed] [Google Scholar]
- 41.Gu K, Cowie CC, Harris MI. Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971-1993. Diabetes Care. 1998;21:1138–1145. doi: 10.2337/diacare.21.7.1138. [DOI] [PubMed] [Google Scholar]
- 42.Slentz CA, Duscha BD, Johnson JL, et al. Effects of the amount of exercise on body weight, body composition, and measures of central obesity: STRRIDE-a randomized controlled study. Arch Intern Med. 2004;164(1):31–39. doi: 10.1001/archinte.164.1.31. [DOI] [PubMed] [Google Scholar]
- 43.Roberts CK, Vaziri ND, Barnard RJ. Effect of diet and exercise intervention on blood pressure, insulin, oxidative stress, and nitric oxide availability. Circulation. 2002;106(20):2530–2532. doi: 10.1161/01.cir.0000040584.91836.0d. [DOI] [PubMed] [Google Scholar]
- 44.Hagberg JM, Goldring D, Ehsani AA, et al. Effect of exercise training on the blood pressure and hemodynamic features of hypertensive adolescents. Am J Cardiol. 1983;52(7):763–768. doi: 10.1016/0002-9149(83)90412-5. [DOI] [PubMed] [Google Scholar]
- 45.Couillard C, Despres JP, Lamarche B, et al. Effects of endurance exercise training on plasma HDL cholesterol levels depend on levels of triglycerides: evidence from men of the Health, Risk Factors, Exercise Training and Genetics (HERITAGE) Family Study. Arterioscler Thromb Vasc Biol. 2001;21(7):1226–1232. doi: 10.1161/hq0701.092137. [DOI] [PubMed] [Google Scholar]
- 46.McCunney RJ. A practical approach to occupational and environmental medicine. Lippincott Williams & Wilkins; Philadelphia, PA: 2003. p. 952. [Google Scholar]
- 47.Beaton SJ, Nag SS, Gunter MJ, Gleeson JM, Sajjan SS, Alexander CM. Adequacy of glycemic, lipid, and blood pressure management for patients with diabetes in a managed care setting. Diabetes Care. 2004;27(3):694–698. doi: 10.2337/diacare.27.3.694. [DOI] [PubMed] [Google Scholar]
- 48.Drexel H. Walking downhill lowers blood sugar, uphill lowers cholesterol levels. American Heart Association 2004 Scientific Session: Abstract 3826. 2004 [Google Scholar]
- 49.Salmeron J, Manson JE, Stampfer MJ, Colditz GA, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA. 1997:472–477. doi: 10.1001/jama.1997.03540300040031. [DOI] [PubMed] [Google Scholar]
- 50.Liu S, Willett WC, Stampfer MJ, et al. A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in US women. Am J Clin Nutr. 2000;71:1455–1461. doi: 10.1093/ajcn/71.6.1455. [DOI] [PubMed] [Google Scholar]
- 51.Angotti CM, Chan WT, Sample CJ, Levine MS. Combined dietary and exercise intervention for control of serum cholesterol in the workplace. Am J Health Promot. 2000;15(1):9–16. doi: 10.4278/0890-1171-15.1.9. [DOI] [PubMed] [Google Scholar]
- 52.McPhillips JB, Pellettera KM, Barrett-Connor EL, Wingard DL, Criqui MH. Exercise patterns in a population of older adults. Am J Prev Med. 1989;5(2):65–72. [PubMed] [Google Scholar]