Abstract
Cell migration is an evolutionarily conserved mechanism that underlies the development and functioning of uni- and multicellular organisms and takes place in normal and pathogenic processes, including various events of embryogenesis, wound healing, immune response, cancer metastases, and angiogenesis. Despite the differences in the cell types that take part in different migratory events, it is believed that all of these migrations occur by similar molecular mechanisms, whose major components have been functionally conserved in evolution and whose perturbation leads to severe developmental defects. These mechanisms involve intricate cytoskeleton-based molecular machines that can sense the environment, respond to signals, and modulate the entire cell behavior. A big question that has concerned the researchers for decades relates to the coordination of cell migration in situ and its relation to the intracellular aspects of the cell migratory mechanisms. Traditionally, this question has been addressed by researchers that considered the intra- and extracellular mechanisms driving migration in separate sets of studies. As more data accumulate researchers are now able to integrate all of the available information and consider the intracellular mechanisms of cell migration in the context of the developing organisms that contain additional levels of complexity provided by extracellular regulation. This review provides a broad summary of the existing and emerging data in the cell and developmental biology fields regarding cell migration during development.
Keywords: Cell migration, embryonic development, actin, cytoskeleton, neural crest, embryogenesis, vasculogenesis, angiogenesis, primordial germ cells, neuritogenesis
INTRODUCTION
Cells of the developing embryo undergo a highly complex chain of events that define their correct shape and positioning during organogenesis and in the tissues and organs of the adult body. Among these events, a crucial role belongs to coordinated cell migration that involves movement of cells of different lineages over short and long distances throughout the body. Defects of migration at all stages of development lead to severe embryonic malformations and result in drastic overall consequences, ranging from early embryonic lethality to birth defects and accounting for multiple human syndromes, including neurological disorders, congenital heart diseases, and physical and mental retardation.
Migration is an evolutionarily conserved mechanism that underlies the development and functioning of uni- and multicellular organisms and takes place in normal and pathogenic processes, including various events of embryogenesis, wound healing, immune response, cancer metastases, and angiogenesis. Despite the differences in the cell types that take part in different migratory events, it is believed that all of these migrations occur by similar molecular mechanisms, whose major components have been functionally conserved in evolution for over a billion years, from protozoa to mammals.
Molecular mechanisms of migration have been extensively studied over the past decades (see Pollard and Borisy, 2003; Ridley et al., 2003; Vicente-Manzanares et al., 2005 and Cell Migration Gateway http://www.cellmigration.org for overviews). While many mysteries underlying the regulatory mechanisms of migration still remain to be uncovered, significant progress has been made in the mechanistic understanding of migration and the major molecular events that occur inside the cell during directional movement. Most of these studies have been conducted in culture, where cells move on hard, two-dimensional surfaces rather than in the three-dimensional environment of the tissues composing the embryo. Special aspects of cell migration on different matrices and surfaces of different stiffness that mimic the tissue environment in situ have recently become a focus of cell biology research, but a gap still exists between the mechanistic studies of cell migration in culture and embryonic migration in situ. Vast amounts of data have been accumulated in both fields, however developmental biologists traditionally devote more attention to the external signaling molecules that control the migration on the global tissue and organismal level, while cell biologists focus more on the intracellular molecules involved in migration of individual cells. The connection between the molecular mechanisms of cell movement in culture and in situ, as well as the general nature of the mechanisms of cell migration regardless of specific environment, are rarely emphasized in the current literature.
The goal of this review is to bridge these two fields and present a broad overview of the studies of the mechanisms of cell migration in culture and in embryogenesis, with emphasis on the similarities between the mechanisms that underlie the major embryonic migratory events. We focus on the intracellular mechanisms that control cell migration and are common for most of the migratory cell types and use mouse development as a model of the migration processes in situ.
BASIC PRINCIPLES OF CELL MIGRATION
Overall structure of the cell leading edge
To initiate migration in a developing organism, individual cells receive signals, which set in motion the complex and highly coordinated molecular machinery that drives these cells to move in the right direction with the appropriate speeds and to arrive at their destinations at exactly the right time. While on the organismal level migration is initiated and coordinated by global extracellular signaling, for each individual cell it is regulated from within, by forming transient structures that allow the cell to polarize, protrude, and retract in response to its environment.
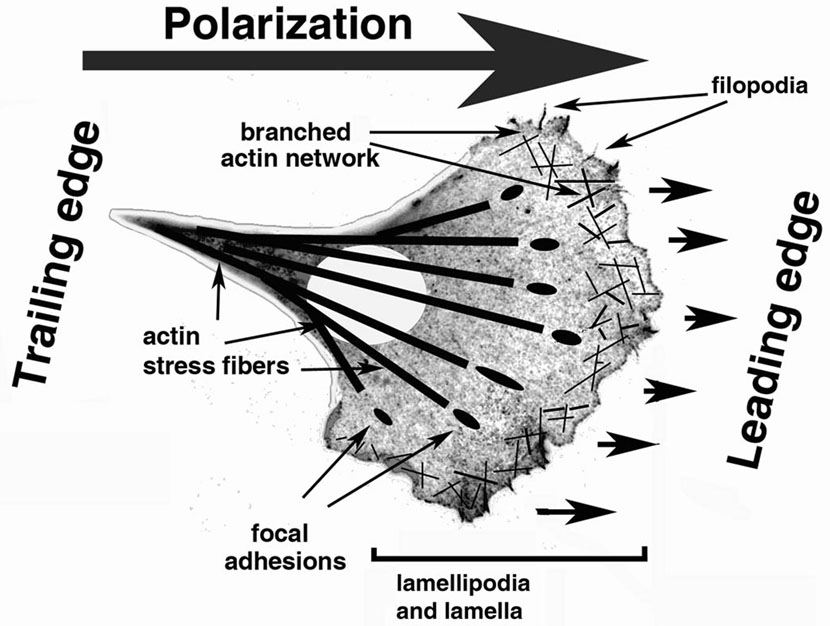
Migration is a cyclical process, in which the cell polarizes to form an active leading edge that extends dynamic protrusions toward the cell front, and a trailing edge that retracts as the cell moves forward (Fig. 1) (Lauffenburger and Horwitz, 1996; Pollard and Borisy, 2003; Ridley et al., 2003). The major structures that define the leading edge activity of a migrating cell are lamella, lamellipodia, and filopodia. Lamella is a broad, highly active zone that encompasses the area inward from the leading edge. Many of the mechanistic and regulatory events responsible for cell migration and leading edge activity take place in the lamella. Its outer zone, a thin area at the extreme cell edge, is defined as lamellipodia. In a polarized migrating cell, lamellipodia is highly active in defining the direction of the cell movement. Another type of leading edge structures, thin finger-like protrusions called filopodia, are believed to be responsible for exploring the environment during motility (Faix and Rottner, 2006; Gupton and Gertler, 2007). While it has been suggested that inhibition of lamellipodia (Gupton et al., 2005; Yang et al., 2007) or filopodia (Yang et al., 2007) does not inhibit cell motility, it results in defects in cell migration speed and directionality. While apparently subtle in vitro, such defects are likely to result in a critical handicap during in situ migration through tissues. Both lamellipodia and filopodia are always seen at the edge of the locomoting cells and their relative balance is believed to regulate specific aspects of migration of different cell types.
Figure 1. Cell migration.
A schematic representation of a generic cell of mesenchymal morphology migrating directionally along a two-dimensional substrate. Polarization, mediated by signaling molecules, defines the leading and the trailing edge of the cell and the direction of migration. Lamella, lamellipodia, and filopodia are responsible for the activity and directionality of the leading edge, whose protrusion is mediated by the branched actin network. A cell attaches to the substrate via dynamic focal adhesions. Actin filaments in the cytoplasm to the rear of the lamella form stress fibers that transmit the myosin-mediated tractional forces to propel the cell movement and regulate the trailing edge retraction via actomyosin contractility. See the explanation in the text for further details.
Four steps of migration: polarization, protrusion, adhesion, and retraction
1. Polarization
In order to move in a certain direction, a cell first needs to polarize in that direction, i.e., to define its front (that will move forward) and its back (that will remain in the rear and retract as the leading edge protrudes). The central regulators of establishing this polarity are Rho family small GTPase Cdc42 (Etienne-Manneville and Hall, 2001; Itoh et al., 2002), Par proteins, and atypical protein kinase (aPKC) (see (Ridley et al., 2003) for overview). Additional signals are provided by a gradient of phosphatidylinositol triphosphate (PIP3) (decreasing from front to rear), produced by the action of phosphoinositide-3 kinase (PI3K), and regulated by PTEN, a PIP3 phosphatase, which is located to the cell rear and controls the lower level of PIP3 at the trailing edge. Thus, polarization is a complex, highly coordinated process that encompasses the entire cell, from front to rear.
Signal gradients during cell polarization are closely followed by cytoplasm rearrangement and organelle repositioning. The central role in this rearrangement belongs to the actin filaments that provide the major driving force for the subsequent cell movement, but other organelles and cytoskeletal systems also participate in this process. The microtubule organizing center reorients to the front of the nucleus to serve as rails for vesicle transport toward the leading edge. This reorientation is closely coordinated with the reorientation of Golgi apparatus to the front of the nucleus. Both events occur rapidly after cell polarization (Etienne-Manneville and Hall, 2001; Gotlieb et al., 1981; Gundersen and Bulinski, 1988; Kupfer et al., 1983; Kupfer et al., 1982). In the cell rear, the increased PTEN activity is correlated with the activity of myosin II – an actin-dependent molecular motor that ensures the trailing edge contractility and retractive behavior.
Remarkably, cell fragments that lack nuclei and centrosomes are also capable of directional motility (Euteneuer and Schliwa, 1984; Verkhovsky et al., 1999), suggesting that organelle rearrangement is secondary to other polarization events and may not be required for cell motility. Enucleated centrosome-free cell fragments have been shown to possess a functional cytoskeletal array (Malikov et al., 2005; Rodionov and Borisy, 1997; 1998; Vorobjev et al., 2001), suggesting that the critical role in motility of cells as well as cytoplasmic fragments belongs to the cytoskeleton and regulatory molecules, regardless of the presence or absence of other major organelles.
It has been traditionally believed that cell polarization, especially when it occurs in response to external stimuli (e.g., chemotaxis), originates at the cell front and is initiated by protrusion of the leading edge. However, in spontaneously polarizing cells, the contraction of the rear end actually precedes the leading edge protrusion (Chen, 1979; Dunn and Zicha, 1995; Verkhovsky et al., 1999; Yam et al., 2007). Recent quantitative analysis of spontaneous polarization in fish keratinocytes (Yam et al., 2007) suggests that cell polarization signals are initiated near the nucleus and at the cell rear rather than at the leading edge. Since during development the migratory cell types are often not polarized at all before the initiation of the migration at the tissue and organismal level, it appears highly likely that polarization signals in situ may originate at different cell locations to define the leading and trailing edge, and that multiple mechanisms may exist to ensure that this polarization occurs promptly, uniformly, and unambiguously in all cells of the migrating layer.
2. Protrusion
Protrusion of the cell leading edge is the actual start of the cell migration cycle. The mechanical force for protrusion is provided by polymerization of actin filaments at the leading edge, either as long parallel bundles to form filopodia, or as a branched dendritic network during lamellipodia formation (Pollard and Borisy, 2003; Ponti et al., 2004; Ridley et al., 2003; Svitkina and Borisy, 1999; Vicente-Manzanares et al., 2005). In both cases, addition of actin subunits onto the fast-growing ‘barbed’ ends of the actin filaments physically pushes the membrane forward in the direction of migration. As the cell moves, the leading edge filaments are disassembled from the rear-facing, slow-growing ‘pointed’ ends, creating a polymerization-depolymerization-driven ‘machine’ often referred to as the ‘actin treadmill’. In such a ‘treadmill’ the force of actin polymerization at the front is coupled to the disassembly of the actin network at the rear of the lamellipodia, propelling the leading edge forward along the substrate (Machacek and Danuser, 2006; Pollard and Borisy, 2003; Ponti et al., 2004; Watanabe and Mitchison, 2002). Based on actin filament stiffness, it has been calculated that the ‘free’ length of the actin filaments pushing the leading edge has to be quite short (less than 150 nm) in order to create sufficient force for membrane protrusion (Mogilner and Oster, 1996), suggesting that the filament length in the treadmill must be very tightly regulated to achieve the most efficient forward movement.
Assembly of different types of actin structures at the leading edge is achieved by two different actin nucleating mechanisms (Pollard, 2007). The first mechanism is mediated by the Arp2/3 complex (Goley and Welch, 2006; Mullins and Pollard, 1999; Welch et al., 1997; Welch and Mullins, 2002) that interacts with the surface of the existing actin filaments to create sites of branching for new actin polymerization and ensure actin assembly into the dendritic network that drives the protrusion of lamellipodia. The second mechanism is mediated by formins (Goode and Eck, 2007; Pruyne et al., 2002; Zigmond, 2004; Zigmond et al., 1998) that drive actin assembly into parallel cross-linked bundles that serve as a structural basis for filopodia.
The predominant role in the signaling events that regulate cell protrusion during migration belongs to small GTPases of the Rho family (Etienne-Manneville and Hall, 2001). When bound to GTP, they are active and interact with their downstream targets, including protein kinases, lipid-modifying enzymes, and activators of the Arp2/3 complex. The major players in the cell protrusion machinery are Rac and Cdc42, whose downstream targets of the WASP/WAVE family activate the Arp2/3 complex that regulates the formation of branches on actin filaments at the leading edge. Additional factors that are important in the network formation and maintenance are regulators of actin polymerization, such as profilin that binds and targets actin monomers to the barbed ends, therefore preventing self-nucleation and ensuring actin incorporation into the proper network, and ADF/cofilin that severs the filaments at the branch points (debranching) and promotes depolymerization from the pointed ends. In filopodia, anti-capping proteins, such as Ena-VASP and their partners (Bailly et al., 1999; Krause et al., 2004; Lafuente et al., 2004) and bundling proteins, such as fascin (Adams, 2004a; Adams, 2004b), are especially important. A large number of other actin-binding proteins participate in the leading edge actin dynamics, facilitating different stages of actin assembly, disassembly, sequestering, and crosslinking. Identification of these proteins and their role in the leading edge dynamics constitutes an exciting field of research.
3. Adhesion
Once the protrusion of the leading edge has formed, it needs to attach to the surrounding matrix and stabilize, so that the moving cell can use it as a ‘push-off’ point to move forward. The central role in this attachment belongs to integrins, transmembrane proteins that indirectly bind the ends of the actin filaments on the inside and attach to the extracellular matrix on the outside, providing stable points of the cell interaction with the environment.
Integrins are heterodimeric proteins, consisting of different combinations of α and β chains that are responsible for binding to different matrices (Delon and Brown, 2007; Lock et al., 2008; Takada et al., 2007). As they bind to their extracellular ligands, they change conformation, promoting activation of intracellular signaling cascades that lead to changes in all the signaling molecules that participate in the leading edge activity. Integrin activation on the cytoplasmic side is mediated by talin binding and involves protein kinase C (PKC), small GTPases (Rap1) and PI3K signaling pathways. Such activation is followed by integrin clustering (achieved by binding to multivalent ligands and regulated by Rac) and recruitment of structural and signaling proteins to form nascent adhesions that eventually maturate to focal adhesion.
Focal adhesion formation and maturation is coupled to the assembly of prominent actin stress fibers and is believed to be mediated by actin interaction with the molecular motor myosin II (Galbraith et al., 2002; Geiger and Bershadsky, 2001; Giannone et al., 2007; Rottner et al., 1999; Schoenwaelder and Burridge, 1999). Myosin and its regulatory machinery play a major role in creating and maintaining tractional forces that enable the cell to attach and propel itself over the substrate (Even-Ram et al., 2007; Iwasaki and Wang, 2008). The strongest forces in a migrating cell are transmitted through the focal adhesions in the leading lamella and the retracting regions in the cell rear (Beningo et al., 2001).
As the cell moves forward, focal adhesions disassemble to release the cell for a new phase of the migration cycle. At the leading edge of the cells, focal adhesion disassembly accompanies the formation of new protrusions and assembly of new adhesions at the front (Webb et al., 2002). Some adhesions, however, mature and persist to form larger, more stable structures that provide firm attachment sites. While little is known about the mechanisms that determine the fate of each particular adhesion, it has been suggested that some focal adhesions are targeted for disintegration by the dynamic ends of the microtubules in a process sometimes referred to as the ‘kiss of death’ (Kaverina et al., 1999; Small and Kaverina, 2003). Activation of protein kinases FAK and Src, as well as signaling via Rac and ERK, have also been shown to contribute to the adhesion turnover at the leading edge.
4. Trailing edge retraction
As the cell moves forward, the trailing edge must retract to enable the cell to advance. An exception to this rule is the migration of the neuronal growth cones, which occurs by the mechanisms similar to those described above, but without the movement of the cell body that remains attached to the advancing neurite. This feature prompted some researchers to refer to growth cones as ‘lamellae on a leash’.
Persistent adhesions form at the front and stay as the cell moves forward to maintain the connection to the substratum. Their release at the cell’s trailing edge is accompanied by stretching, driven by myosin II contractility and regulated by FAK and Src signaling (Henson et al., 1999; Medeiros et al., 2006; Vallotton et al., 2004; Verkhovsky et al., 1999; Zhang et al., 2003). The stretching force created during the focal adhesion release is believed to be sufficient to open the stretch-activated Ca2+ channels that trigger activation of the calcium-dependent protease calpain that participates in focal adhesion disassembly by cleaving a number of focal adhesion proteins, including talin, vinculin, and FAK (Franco and Huttenlocher, 2005; Franco et al., 2004). The release of adhesions at the cell rear contributes to maintaining the polarity of the leading edge and promoting the leading edge activity, providing positive feedback for the continued cycle of migration.
Posttranslational regulation of cell migration
In addition to the activity of protein kinases and proteases that post-translationally regulate proteins involved in the cell migration cycle by phosphorylation or cleavage, emerging data suggest contribution of other post-translational modifications to various migration-dependent events. Of those, a special place belongs to post-translational modification of cytoskeletal proteins by amino acids. It has been long known that tubulin undergoes acetylation that correlates with microtubule stability and microtubule-based organelle transport (Bulinski, 2007; Reed et al., 2006), which is important for the delivery of membranous components for cell polarization and protrusion to the cell leading edge. Tubulin is also modified by post-translational phosphorylation and detyrosynation, as well as addition of tyrosine (Raybin and Flavin, 1977a; b), mono- and poly-glutamine (Audebert et al., 1993; Edde et al., 1990), and glycine (Plessmann and Weber, 1997) (see also (Luduena, 1998) for an overview of tubulin modifications). Some of these tubulin modifications have been associated with microtubule stability and found to serve as markers for cell polarization (Erck et al., 2005; Gundersen and Bulinski, 1986; Infante et al., 2000; Khawaja et al., 1988). Tubulin modifications by amino acids have also been implicated in modulation of microtubule functions in various types of motile cells (Ikegami et al., 2007; Raybin and Flavin, 1977a; b; Redeker et al., 2005).
Actin also undergoes a number of posttranslational modifications that have been implicated in normal and pathological actin-mediated processes (see (Sheterline et al., 1998) for an overview). N-Terminal aminopeptidation and acetylation is involved in the early maturation of the actin monomers and is shown to be essential for normal actin function (Hennessey et al., 1991; Redman and Rubenstein, 1984; Rubenstein and Martin, 1983). An emerging role in cell motility belongs to post-translational arginylation of actin and actin-associated proteins (Wong et al., 2007). Arginylation has been shown to modulate lamella formation in motile cells and determine directionality and speed of cell migration (Karakozova et al., 2006).
CELL MIGRATION IN A THREE-DIMENSIONAL ENVIRONMENT
While the basic principles of cell migration described above are generally applicable to all types of migratory events in different motile cells throughout the body, several aspects of cell migration in the developing organism are distinct from those in culture, which have served as the foundation for the model of the cell migration cycle described above. These differences have to do with the fact that during embryogenesis cells migrate through three-dimensional tissues and matrices that possess distinct physical and chemical properties, which in themselves could vary during migration. The dynamic properties of these environments introduce an additional level of complexity into the signals and molecular interactions that influence the migration of different cell types. In the section below, we give a general overview of the special aspects that distinguish cell migration in a three-dimensional environment of an organism compared to a two-dimensional culture.
Extracellular signaling: coordinated migration of layers and lineages
Cells are capable of chemical sensing, which enables them to migrate toward favorable directions and/or in response to extracellular chemical stimuli. While cells in culture often exhibit spontaneous, stimuli-independent polarization and migration, in the developing embryo cell migration is precisely coordinated by extracellular signals that define the timing, the direction, and the final destination for the migrating cells. Such signals control the complex events that occur during embryogenesis, including gastrulation, patterning, and tissue and organ formation. Perturbation of these signals and the underlying molecular mechanisms of their generation lead to drastic abnormalities of embryogenesis (Gilbert, 2000).
Extracellular signals define the initial steps of cell polarization, to ensure that large populations of cells in a certain layer or lineage initiate their migration synchronously on a tight temporal cue. However, while external signals indeed induce preferred directionality and provide the overall cues for the embryonic migratory events, their role in the migration cycle is restricted to the activation of the intracellular signals that drive the subsequent migration steps. Given their strictly extracellular role, such signals were therefore excluded from the current review.
Extracellular matrix and force sensing: paving the pathways of migration
Arguably the most important aspect that distinguishes between cell migration in culture and in the embryo is related to the fact that in situ cells migrate through a three-dimensional network of the extracellular matrix. While all cells basically employ similar mechanisms of migration, their pathways and the speeds of movement through the tissues of the developing embryo vary a great deal between cells of different lineages and types (Table 1). It is highly likely that this variability is achieved in large part by the properties of the surrounding matrix, which forms structures of different thickness, strength, and density, as well as biochemical composition, facilitating differential migration of cells intended for various destinations (Trelstad, 1984). Such differentially paved ‘roadways’ in the embryo participate in the complex embryonic mechanisms that regulate the formation of different tissues and organs (Gilbert, 2000).
Table 1.
Major types of embryonic migratory events
| Cell Types | Embryonic Events | Stages | Duration (hrs) | Origin | Destination | Maximum range | Distance* (µm) | Speed* (µm/hr) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Epiblast cells | Gastrulation | E6.5 | ~24hrs | Primitive streak | Mesoderm | Neighboring cell layers | 1 | <0.04 um/hr | (Gilbert, 2000; Nagy et al., 2003) |
| Primordial germ cells | General embryo patterning and organogenesis | E7.5–E12 | 96 | Base of the allantois | Genital ridge | Within the tail region | 1500 | 15 um/hr | (Gilbert, 2000; Molyneaux and Wylie, 2004; Molyneaux et al., 2001; Nagy et al., 2003) |
| Cranial neural crest | E8–E9 | 24 | Mid-diencephalon to somite 5 | Forebrain, craniofacial mesenchyme | Neck to front head | 1000 | 40 um/hr | (Hutson and Kirby, 2007; Nagy et al., 2003; Serbedzija et al., 1992; Trainor, 2005) | |
| Vagal neural crest | E9–E14.5 | 120 | Somites 1–5 | Neurons in the gut | Neck to end of gut | 10000 | 85 um/hr | (Anderson et al., 2006a; Durbec et al., 1996; Heanue and Pachnis, 2007; Young and Newgreen, 2001) | |
| 120 | Somites 6–7 | Neurons in the esophagus | Neck to stomach | 3000 | 25 um/hr | ||||
| Trunk neural crest | E8.5–E10.5 | 48 | Somite 5 to caudal tip of neural tube | Sensory and sympathetic neurons, adrenal gland, schwann cells, pigment cells | Back to front | 1500 | 30 um/hr | (Hutson and Kirby, 2007; Nagy et al., 2003) | |
| Sacral neural crest | E10–E10.5 | 12 | Caudal region to somite 24 | Hindgut | Back to front | 1000 | 85 um/hr | (Anderson et al., 2006a; Anderson et al., 2006b; Heanue and Pachnis, 2007; Kapur, 2000) | |
| E14.5–E15* | 12* | Hindgut | Spread within hindgut | Within an organ | 1000 | 85 um/hr* | |||
| Cardiac neural crest | Heart development | E8.5–E13.5 | 120 | Otic placode to the end of somite 3 | Cadiac outflow tract, septae, parts of ventricular myocardium via pharingeal arches | Neck to heart | 4000 | 35 um/hr | (Brown and Baldwin, 2006; Hutson and Kirby, 2007; Jiang et al., 2000) |
| Extraembryonic mesoderm, endothelial, smooth muscle | Vasculogenesis and angiogenesis | E7-postnatal | Extraembryonic tissues and primitive streak | Mature circulatory system | Circulatory system | NA | NA | (Gerthoffer, 2007; Gilbert, 2006; Mu et al., 2005; Patan, 2000) | |
| Nerve cells | Brain development | E14.5-adulthood | Brain and ganglia | Nerve endings in the brain and throughout the body | Up to adult body length | NA | NA | (da Silva and Dotti, 2002; Gilbert, 2006; Skaper, 2005) |
estimated
It has been found that cells migrating through three-dimensional matrices assume a different morphology compared to those migrating on hard two-dimensional surfaces, have different migration speeds, and may employ additional migratory mechanisms (Even-Ram and Yamada, 2005), such as proteolysis of the matrix at the leading edge, shown to be used by endothelial cells during angiogenesis (van Hinsbergh and Koolwijk, 2008). It has also been found that even on flat, matrix-coated surfaces, the amount of extracellular matrix determines the speed of migration by balancing the protrusion and adhesion steps of the cell migration cycle (Gupton and Waterman-Storer, 2006). When the concentration of the extracellular matrix is too low, cultured cells exhibit a decrease in the actin flow and form few highly dynamic adhesions that cannot sufficiently ‘stick’ to the substrate. When the concentration of the extracellular matrix is too high, cells form too many adhesions that are less dynamic and cannot retract enough to ensure movement. An optimal, medium, concentration of extracellular matrix ensures a normal migration cycle and results in the highest motility rates. It appears likely that similar mechanisms are employed in the developing organism, where varying amounts of extracellular matrix can ensure great variability in the migration speeds that are often observed for cells of the same lineage during the formation of different organs (Table 1).
An important property of different extracellular matrices in situ is defined by their mechanical rigidity, or stiffness. The relationship of cell adhesion and motility to the substrate stiffness has become the focus of major studies in recent years (Bershadsky et al., 2006; Geiger and Bershadsky, 2001; Georges and Janmey, 2005; Rehfeldt et al., 2007; Vogel and Sheetz, 2006). It has been found that force sensing and substrate stiffness define the formation and turnover of the adhesions to the extracellular matrix and determine the speed of migration, as well as cell shape and ability to differentiate (Engler et al., 2006). Loss of force sensing accompanies adhesive and migratory defects in vitro and in situ, including oncogenic transformation and cancer metastases, closely correlated with the ability of cells for substrate-independent growth (Kedrin et al., 2007; Yamaguchi and Condeelis, 2007).
A special type of force sensing is related to shear stress due to the application of external mechanical force onto the migrating or stationary cells. Such force can be applied via blood flow over the endothelial cells lining the blood vessels, or via muscle contraction onto the cells within the muscle tissue. Overall, cellular responses to mechanical signals are estimated to have major influence on their behavior in culture and in tissues.
Force is transduced through adhesion sites via the actin cytoskeleton and results in major signaling changes that define cell shape, polarization, and the entire complexity of the motility events (Dobereiner et al., 2005; Georges and Janmey, 2005). Stretch applied on a cell due to external mechanical force or attachment to the stiffer substrates affects actin near the adhesion site and is followed by recruitment of adhesion complex proteins and global cytoskeletal rearrangements (Galbraith et al., 2002; Riveline et al., 2001). Multiple actin-binding proteins at the cell adhesion sites directly respond to substrate rigidity (overviewed in Dobereiner et al., 2005), resulting in changes to all steps of the migration cycle and all of the structural and signaling molecules involved (Beningo et al., 2004; Bhadriraju et al., 2007; Gunzer et al., 2000; Knight et al., 2000).
Cell-cell adhesion
Cell-cell adhesions have an important role in tissue morphogenesis and are critical for the formation of interconnected cell layers, such as epithelial sheets (Gilbert, 2000). While such layers have only limited migratory capacity, they migrate jointly over short distances during gastrulation and are capable of short-distance migration with a free edge later in development and in adulthood during wound healing. Cell-cell adhesions are critical for maintaining the epithelial morphology and for enabling their proper in-tissue behavior.
Cell-cell adhesion molecules are also important for the migration of cells of the mesenchymal morphology. During migration through tissues and layers of the developing embryo, mesenchymal cells do not incorporate into sheets and do not form stable connections with each other, but they are capable of forming transient connections to the stationary cells of the neighboring layers and with each other as they come into immediate proximity. All of these connections participate in modulating the paths and speeds of migration via cell-cell adhesion molecules, such as cadherins and catenins that, like integrins, regulate intracellular signaling cascades by sensing the environment. Information about the regulation of mesenchymal cell migration by cell-cell adhesion molecules is so far limited to the general mechanisms and signaling pathways mediated by these molecules. For this reason, we do not review these mechanisms in detail, beyond mentioning the fact that these mechanisms are likely to participate in the in situ migration cycle.
CELL MIGRATION IN THE DEVELOPING EMBRYO
Major migratory cell types
Cell migration over short and long distances plays a critical role at all stages of development, from gastrulation to the formation of an adult organism (Gilbert, 2000). From the migratory point of view, all embryonic cells are divided into cells of the epithelial morphology that form interconnected sheets, and mesenchymal cells that originate from different lineages and migrate throughout the embryo as independent units to contribute to tissues and cell types throughout the body.
Interconnected epithelial sheets undergo short-range migration during gastrulation, however part of this migration is driven not by the movement of the sheet itself, but by the repositioning of the mesenchymal cells that define the embryonic mesodermal layer. Migration of epithelial sheets is an important part in the development of such organisms as fish and amphibia that do not dramatically increase in size during early development. Overall, the migration of epithelial sheets generally occurs at a short range at very slow speeds. While such migration is believed to be driven by different signaling events compared to the migration of the mesenchymal cells (Locascio and Nieto, 2001), it employs similar molecular mechanisms.
Cells of the mesenchymal morphology are responsible for most migratory events that take place during development (Table 1). In general terms, mesenchymal cells share the generic features of fibroblasts, which have been the classical model for studying cell migration in culture (Fig. 1 and Fig. 2), and it is believed that different embryonic mesenchymal cells during migration generally follow the mechanisms described in the first section of the review (Gerthoffer, 2007; Molyneaux and Wylie, 2004; Pak et al., 2008) (Fig. 2).
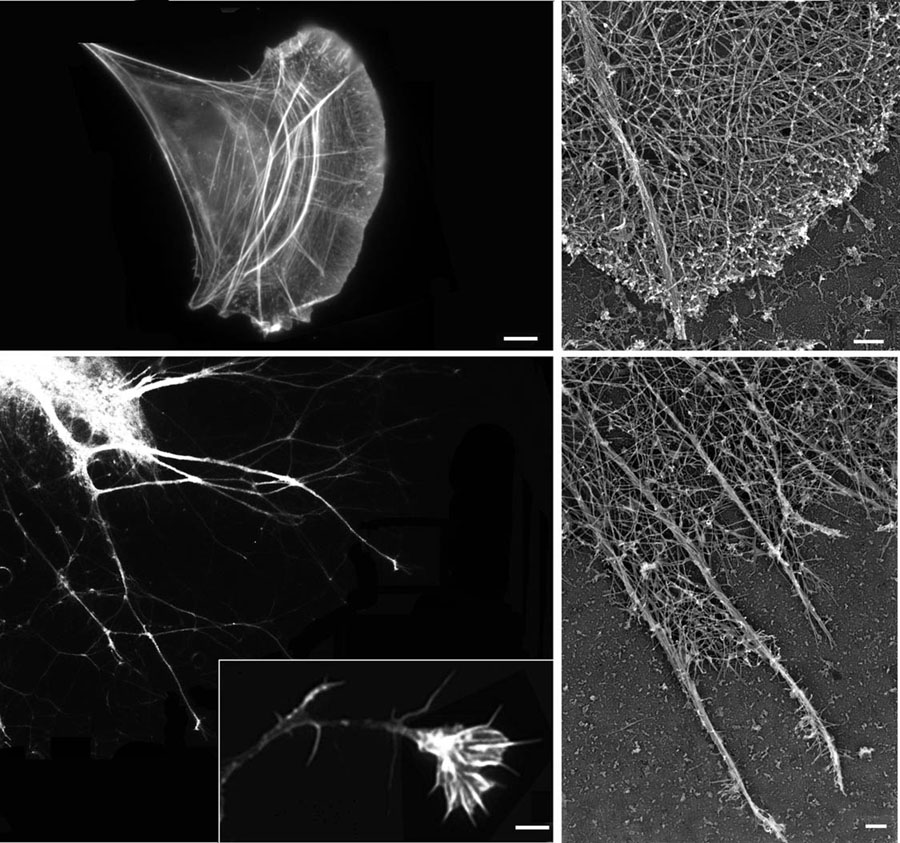
Figure 2. Migration of different cell types is driven by the same mechanisms.
Top left, immunofluorescence image of a migrating fibroblast stained with rhodamine-phalloidin to visualize actin filaments. Top right, closeup electron microscopy image of a platinum replica of the leading edge cytoskeletal network of a migrating fibroblast, similar to that shown on the left. Bottom left, immunofluorescence image of neurite outgrowth and closeup image of a neuronal growth cone (boxed) illustrates the migration events during neuritogenesis and the laying down of the nervous system. Bottom right, close-up electron microscopy image of a platinum replica of the leading edge cytoskeletal network in a neuronal growth cone, similar to that shown on the left. Despite morphological differences, variations in the amount of filopodia and the relative density of the actin cytoskeleton, the major structural features of the leading edge, are similar in both cell types. Images in the top panels, bottom right panel, and the close-up of the neuronal growth cone in the bottom left panel courtesy of Dr. T. M. Svitkina (University of Pennsylvania). Bars, 2 µm for immunofluorescence images and 200 nm for the electron micrographs.
A special type of migration takes place during development and patterning of the nervous system. While during pre-differentiation stages neuronal precursors of the neural crest lineage follow the same migration mechanisms as any other mesenchymal cells, after differentiation the neuronal cell bodies eventually find stationary positions within the developing nervous system and only their neurites continue to migrate through tissues to reach their destinations and form connections with their effectors as part of the mature nervous system. This process can occur over considerable distances and with relatively high speeds, and constitutes a global and important part of development. In humans, brain development alone involves the laying down of approximately 1011 neurons, each forming approximately 1,000 connections with other neurons (Williams and Herrup, 1988) and often traveling over distances of more than one meter, five orders of magnitude larger than the size of the neuron cell body. This migration, while highly specialized and morphologically distinct, is believed to occur by the same mechanisms as those described above for other cell types (Dent et al., 2007; Mongiu et al., 2007; Pak et al., 2008, Fig. 1 and 2). From the mechanistic perspective, the growth cone of each neurite is considered a separate leading edge and generally follows the same mechanisms as whole cells migrating through tissues (Fig. 2).
Embryonic migratory events
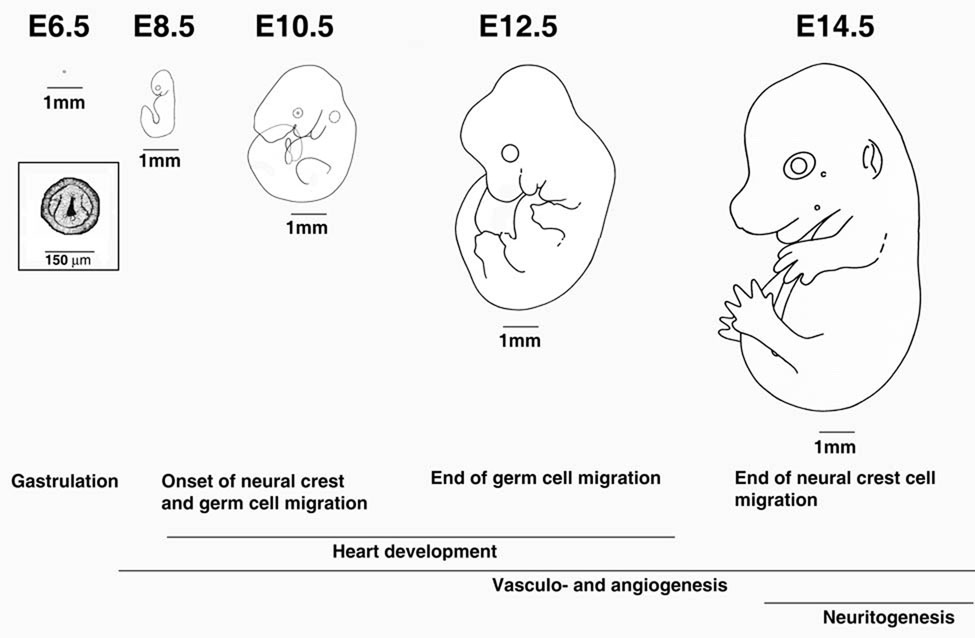
Table 1 shows an overview of the major migratory events that take place during different stages of development in a mouse. Most of these migrations take part after gastrulation and can be loosely divided into global redistribution of undifferentiated cells throughout the embryo (days 7–15 in mouse development) and local rearrangements of differentiating cells into the mature vascular and nervous system (see Fig. 3 and Fig. 4 for mouse developmental stages and migration pathways). Since the embryo continues to grow as migration takes place, both global and local migration can occur over large distances and at relatively high speeds.
Figure 3. Timing of the major migratory events in mouse embryogenesis in relation to the embryo growth.
Letters and numbers on the top denote days postcoitum. 1mm scale bar of the same size is shown next to each embryo to enable the direct size comparison. Boxed image showed an enlargement of the gastrula shown above. The major migratory events corresponding to those shown in Table 1 are written for the appropriate stages underneath. Images and sizes are adapted from Kaufman (1992) and have been used as the basis for the calculation of migration distances shown in Table 1.
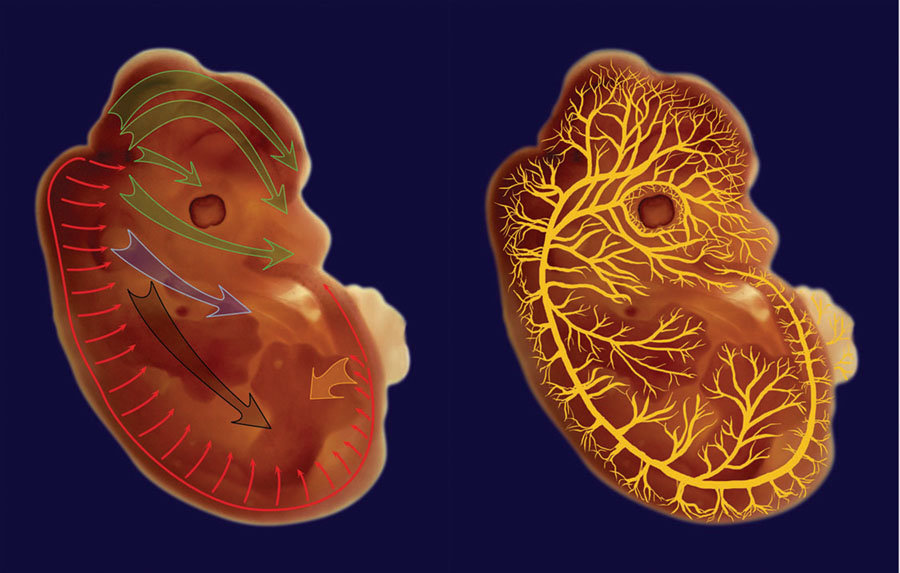
Figure 4. Pathways of neural crest migration, neuritogenesis, and angiogenesis during mouse development.
Neural crest cells (left) generally migrate from the back area of the embryo, originating from different somite regions at different embryonic stages as listed in Table 1. Large green arrows in the head area denote cranial neural crest, blue arrow – cardiac neural crest, black – vagal neural crest, orange arrow – sacral neural crest, and thin red arrows – trunk neural crest. Neurites and blood vessels sprout from the structures laid out during earlier stages of development and reach to all the areas of the mature organism along the pathways illustrated on the right hand image. Illustrations were prepared by O. Karengina based on an image of a mouse embryo at E13.5.
Early embryonic migration is dominated by cells of the neural crest lineage (see Bronner-Fraser et al., 1991; Fraser, 1991; Le Douarin, 1983; Saint-Jeannet, 2006; Tucker, 2004, for overview) that are classified into several types based on their origin and destination in the embryo (Fig. 3, Fig. 4, and Table 1). Cranial neural crest cells originate from the head and neck region and give rise to cartilage, bone, cranial neurons, glia, connective tissue of the face, thymus, thyroid, teeth, bones of the middle ear, and jaw (Hutson and Kirby, 2007; Nagy et al., 2003; Serbedzija et al., 1992; Trainor, 2005). Cardiac neural crest cells originate from a similar head and neck region and form cardiac outflow tract, as well as heart septae and parts of the venricular myocardium (Brown and Baldwin, 2006; Hutson and Kirby, 2007; Jiang et al., 2000). Vagal and sacral neural crest cells migrate from two opposite sides to populate the gastrointestinal system and differentiate into the neurons that innervate the digestive tract (Anderson et al., 2006a; Durbec et al., 1996; Young and Newgreen, 2001). Trunk neural crest cells migrate from the back of the embryo to form sensory and sympathetic neurons, adrenal gland, Schwann cells, and pigment cells of the skin (Hutson and Kirby, 2007; Nagy et al., 2003).
In addition to the neural crest cells, primordial germ cells also migrate within the mid-rear area of the embryo into the genital ridges. This migration takes place over a considerable period of time (5 embryonic days for a mouse embryo, which constitutes approximately ¼ of the entire development), and is believed to occur in stages of rapid migration followed by relatively stationary periods (Molyneaux and Wylie, 2004; Molyneaux et al., 2001). The actual speed of this migration is difficult to estimate, because as the cells migrate, the embryo itself continues to grow and increases several times in size during the migration process (Fig. 3). Therefore it is possible that primordial germ cells actually migrate very little, and instead ‘ride’ the expanding tissues to reach their destination (Freeman, 2003).
Some sources refer to the positioning of the blood cell precursors in the fetal liver and bone marrow as a type of migration in embryogenesis. However, while individual blood cells (e.g., macrophages) are capable of active migration, most of the embryonic repositioning of the blood cell precursors occurs by passive circulation through the blood stream. For this reason, we are not considering this type of movement in the current review.
Duration of each type of migration and maximum distance between origin and destination at the end of each migratory event can serve as a basis for an estimation of the overall migration speed of each cell type during different embryonic events (Table 1). The estimated speeds shown in Table 1 are based on the assumption that cells of each lineage migrate uniformly and continuously during the appropriate stages; however, for some lineages and cell types (e.g., germ cells) it may not be the case. Based on such estimation, all types of migrating cells can be classified into groups that differ from each other by their migration speeds. Sacral and most of the vagal neural crest cells that populate the digestive tract are the fastest (migration speeds >80 µm/hr), while cranial, cardiac, and trunk cells migrate at less than half the speed (30–40 µm/hr), suggesting that distinct mechanisms within the body regulate the speed of migration of these highly similar cell types.
Different mechanisms of the regulation of cell migration speeds, acting jointly or separately, could be envisioned. One mechanism involves the creation of differential extracellular matrix ‘pavement’ for different migration pathways, making paths of different stiffness, composition, and density that could tightly control the migration speed. To regulate short ‘bursts’ of migration followed by relatively stationary periods, signals may be used that would switch the migration of certain cell types on and off in coordination with other developmental events.
Live observations of in situ migration of labeled neural crest tissue explants in chick embryos suggested that over the periods of observation, cells were able to migrate faster than the estimates shown in Table 1 for mouse cells, on the order of 170 µm/hr (Kulesa et al., 2000), arguing in favor of the ‘short burst’ hypothesis. However, live imaging of neural crest cell migration in mouse ranged between cell populations migrating at 30–40 and 84 µm/hr, which is extremely close to our calculations presented in Table 1 (Druckenbrod and Epstein, 2005; Druckenbrod and Epstein, 2007), arguing in favor of uniform, continuous migration. Overall, it is likely that both mechanisms may contribute to the regulation of cell migration speeds in situ.
During later stages of development, global migratory events of neuritogenesis and angiogenesis encompass the developing embryo (Fig. 4) and drive tissue rearrangements that continue after birth, and, in the case of neuritogenesis, into adulthood. Due to the extensive duration of these processes, it is difficult to estimate their distances and speeds. However, since the molecular mechanisms that drive these migratory events are similar for different cell types (Gerthoffer, 2007; Pak et al., 2008), the order of magnitude of these migration speeds is expected to be similar to those observed at the earlier embryonic stages. It seems likely that angiogenesis and neuritogenesis do not occur continuously with constant speeds, but are modulated in response to the type and availability of matrices and external signals from the neighboring cells and tissues.
MIGRATION-DEPENDENT DEVELOPMENTAL DEFECTS
Key cell migration components in mouse embryogenesis
Since all cell types during embryonic migration employ similar molecular mechanisms, perturbation of the key players in these mechanisms is expected to lead to drastic developmental defects. Over the years, numerous data on mouse knockouts of cell migration components have been accumulated (Table 2). Analysis of these data and comparison of defects that develop in response to each gene knockout provide evidence of the unique mechanisms whose perturbation results in early lethality, and those mechanisms that are either cell type specific or can be compensated by the function of different genes.
Table 2.
Mouse knockouts of cell migration components or other proteins with direct effects on cell migration
| Protein | Function | Type | Phenotype | Reference |
|---|---|---|---|---|
| α skeletal muscle actin | Actin Cytoskeleton | Total | Postnatal death at P1-9, marked loss of body weight; upregulation of other actin isoforms | (Crawford et al., 2002) |
| α smooth muscle actin | Actin Cytoskeleton | Total | Viable; impaired vascular contractility and blood pressure homeostasis; upregulation of other actin isoforms | (Schildmeyer et al., 2000) |
| α cardiac actin | Actin Cytoskeleton | Total | Perinathal lethality; cardiac hypertrophy and heart muscle abnormalities; upregulation of other actin isoforms | (Kumar et al., 1997) |
| β non-muscle actin | Actin Cytoskeleton | Total | Death after E9.5 | (Shawlot et al., 1998) |
| γ non-muscle actin | Actin Cytoskeleton | Skeletal muscle-specific | Muscle weakness, necrosis and degeneration | (Sonnemann et al., 2006) |
| Tropomyosin | Actin Cytoskeleton | Total | Death before morula stage | (Hook et al., 2004) |
| Mena | Actin Cytoskeleton | Total | Viable, with misrouted axons and defects in the nervous system | (Lanier et al., 1999) |
| Mena, VASP, Evl triple null | Actin Cytoskeleton | Total | Defects in brain development, neuritogenesis, and neural tube closure | (Kwiatkowski et al., 2007) |
| Filamin-B | Actin Cytoskeleton | Total | Skeletal malformations and impaired microvascular development | (Zhou et al., 2007) |
| Gelsolin (or ADF) | Actin Cytoskeleton | Total | Defects in fibroblast and platelet motility and lamellar responses | (Witke et al., 1995) |
| Nonmuscle myosin II-B | Actin Cytoskeleton | Total | Embryonic and perinatal lethality with severe heart defects | (Tullio et al., 1997) |
| Myosin heavy chain II-A | Actin Cytoskeleton | Total | Failure in embryonic patterning, embryonic lethality by E7.5 | (Conti et al., 2004) |
| Cardiac alpha myosin, heavy chain | Actin Cytoskeleton | Total | Embryonic lethality between E11 and 12 with gross heart defects | (Jones, 1996) |
| Profilin I | Actin Cytoskeleton | Total | Very early embryonic lethality - no viable knockout blastocysts found. Heterozygotes show reduced embryonic survival | (Witke et al., 2001) |
| Profilin II | Actin Cytoskeleton | Total | Neurons have altered kinetics of synapse recycling. Mice are anxious, do not react to stress, and lack maternal behavior | Witke Group EMBL 201 Research Reports http://www.embl-heidelberg.de/emblGroup/researchReport/rr01_73.pdf |
| VASP | Actin Cytoskeleton | Total | Mice viable, with significant reduction in cAMP- and cGMP-mediated inhibition of platelet aggregation; hyperplasia of megakaryocytes in bone marrow and spleen; abnormalities in cortical neuron positioning | (Aszódi, 1999; Goh et al., 2002; Hauser et al., 1999) |
| N-cofilin | Actin Cytoskeleton | Total | NC cells fail to polarize and form F-actin bundles | (Gurniak et al., 2005) |
| Paxillin | Focal adhesions | Total | Embryonic lethality due to abnormal development of mesodermally derived structures; phenotype similar to fibronectin null | (Hagel et al., 2002) |
| Talin | Focal adhesions | Total | Embryonic lethality at E8.5–9.5 with disorganization of embryos at gastrulation. | (Monkley, 2000) |
| Tensin | Focal adhesions | Total | Viable and healthy at birth: weakness, renal failure and cysts in proximal kidney tubules later in life. Analysis of cysts show disruptions in cell matrix junctions and weakening of focal adhesion | (Lo et al., 1997) |
| Tensin | Focal adhesions | Total | Delayed regeneration of wounded skeletal muscle correlated with delayed expression of myosin, paxillin and dystrophin | (Ishii and Lo, 2001) |
| Fak | Focal adhesions | Total | Mesodermal defects post-gastrulation. Death at E8.5 with impaired blood vessel development | (Furuta, 1995; Ilic et al., 2003) |
| Vinculin | Focal adhesions | Total | Death by E10, with embryos displaying 30–40% reduction in size, lack of midline fusion of the rostral neural tube and developmental defects in heart, somites and limbs | (Xu et al., 1998) |
| Zyxin | Focal adhesions | Total | Viable, fertile, no obvious abnormalities | (Hoffman et al., 2003) |
| α4 integrin | Integrins | Total | Embryonic lethality, defects in placenta and vascular system, cardiac hemmorhages | (Yang et al., 1995) |
| αv integrin | Integrins | Total | Partial embryonic lethality, intracerebral and intestinal hemorrhages and cleft palates | (Bader et al., 1998) |
| α5 integrin | Integrins | Total | Embryonic lethal and E10–11 with migration-type defects in posterior trunk and yolk sac mesodermal structures | (Yang et al., 1993) |
| α7 integrin | Integrins | Total | Partial embryonic lethality, cerebral vascular defects, placental defects | (Flintoff-Dye et al., 2005; Mayer et al., 1997; Rooney et al., 2006; Welser et al., 2007) |
| β1 integrin | Integrins | Endothelium | Failure of normal vascular patterning, severe fetal growth retardation, and embryonic death at E9.5–10 | (Lei et al., 2008; Tanjore, 2008) |
| Total | Death shortly after implantation | (Fassler and Meyer, 1995) | ||
| In vitro studies of cells from knockout mice | Reduced neural crest cell motility | (Breau et al., 2006) | ||
| β3 integrin | Integrins | Total | Defects in platelet aggregation and clot retraction, hemmorhages, postnatal anemia, and reduced survival | (Hodivala-Dilke, 1999) |
| β8 integrin | Integrins | Total | Embryonic or perinatal lethality with profound defects in vascular development, insufficient vascularization of the placenta and yolk sac | (Zhu et al., 2002) |
| VCAM-1 | Integrins | Total | Embrynic lethality at E10–12, abnormal placental development | (Gurtner et al., 1995) |
| Syndecan-4 | Extracellular matrix | Total | Blood vessel defects in the placenta; defects in angiogenesis and wound repair | (Echtermeyer, 2001; Ishiguro, 2000; Kojima, 2002) |
| N-syndecan | Extracellular matrix | Total | Impaired neural migration in the developing brain | (Hienola et al., 2006) |
| Perlecan | Extracellular matrix | Total | Defects in heart integrity, invasion of brain tissue into the overlaying ectoderm, severe defect in cartilage, cleft palates and death shortly after birth from respiratory failure. | (Costell et al., 1999) |
| Tenascin C | Extracellular matrix | Total | NC cells fail to disperse laterally | (Tucker, 2001) |
| Laminin γ1 | Extracellular matrix | Total | Death at E5.5, lack of basement membranes | (Smyth et al., 1999) |
| Laminin β2 | Extracellular matrix | Total | Postnatal death between P15–30, neuromuscular junctions and glomerular defects | (Noakes et al., 1995a; Noakes et al., 1995b; Patton et al., 1997) |
| Laminin α2 | Extracellular matrix | Total | Death by 5 weeks postnatal, severe muscular dystrophy and peripheral neurophathy | (Miyagoe et al., 1997) |
| Laminin α2 (dy/dy) | Extracellular matrix | Spontaneous | Adult lethality, severe muscular dystrophy and peripheral nerve dysmyelination | (Patton et al., 1999; Patton et al., 1997) |
| Laminin α3 | Extracellular matrix | Total | Death at P2–3, epithelial adhesion defect | (Ryan et al., 1999) |
| Laminin α4 | Extracellular matrix | Total | Transient microvascular defect with hemorrhages and misalignment of neuromuscular junctions | (Patton et al., 2001; Thyboll et al., 2002) |
| Laminin α5 | Extracellular matrix | Total | Death at E14–E17, with placental vessel, neural tube, limb, and kidney defects | (Miner et al., 1998; Miner and Li, 2000) |
| Fibronectin | Extracellular matrix | Total | Death before E14.5, shortened anterior-posterior axes, deformed neural tubes, and defects in mesodermally derived tissues. | (George et al., 1993) |
| Collagen XVIII | Extracellular matrix | Total | Eye abnormalities modeling Knoblock syndrome | (Fukai et al., 2002) |
| Extracellular matrix | Total | Basement membrane defects | (Utriainen et al., 2004) | |
| Collagen XV | Extracellular matrix | Total | Skeletal myopathy and cardiovascular defects | (Eklund et al., 2001) |
| betaglycan | Extracellular matrix | Total | Embryonic lethality of heart and liver defects | (Stenvers et al., 2003) |
| Connexin 43 | Cell-cell adhesion | In vitro studies of cells from knockout mice | Increased cell protrusive activity, loss of polarized cell movement, rearrangement of actin stress fibers | (Xu et al., 2006) |
| Total | Die at birth from severe pulmonary outflow tract obstruction | (Reaume et al., 1995) | ||
| PECAM-1 | Cell-cell adhesion | In vitro studies of cells froom knockout mice | Reduced migration, inability to undergo capillary morphogenesis in matrigel | (Kondo et al., 2007) |
| Total | Mice viable, defects in retinal vasculature | (Dimaio et al., 2008) | ||
| β-catenin | Cell-cell adhesion | Conditional EC-specific knockout | Death between E11.5 and E13.5; impaired vascular patterning in the head, vitelline and umbilical vessels, and placenta | (Cattelino et al., 2003) |
| N-cadherin | Cell-cell adhesion | Conditional NC specific | Defects in remodeling of cardiac outflow tract | (Luo et al., 2006) |
| Total | Death by E10 | (Radice et al., 1997) | ||
| Conditional EC specific | Death at midgestation, severe vascular defects, decrease of VE-cadherin | (Luo and Radice, 2005) | ||
| E-cadherin | Cell-cell adhesion | Total | Early lethality, fail to form trophectoderm. | (Larue et al., 1994) |
| VE-cadherin | Cell-cell adhesion | Total | Death at E9.5, endothelial cells are assembled in vascular plexi, but their subsequent remodeling and maturation are impaired. | (Carmeliet et al., 1999) |
| L1-CAM | Cell-cell adhesion | Total | Malformations of the nervous system, decreased nerve sensitivity, weak and uncoordinated hindlegs. | (Dahme et al., 1997) |
| Ate1 | Arginyltransferase | Total | Embryonic lethality at E12–17 with defects in cardiovascular development and angiogenesis | (Kwon et al., 2002) |
| Calpain IV (Capn4) | Protease | Total | Embryonic lethality at mid-gestation with defects in the cardiovascular system, hemorrhaging, and accumulation of erythroid progenitors | (Arthur et al., 2000) |
| C3G | Guanine nucleotide exchange factor 1 | Total | Death before E7.5; Death at E11.5 due to hemorrhages and vascular integrity defects | (Ohba, 2001; Voss et al., 2003) |
| Rap1b | Small GTPase | Total | Decreased level of Matrigel and neonatal retinal neovascularization, impaired endothelial cell motility in culture | (Chrzanowska-Wodnicka et al., 2008) |
| ROCK-II | Small GTPase | Total | 90% embryonic lethal 13.5 days postcoitum due to placental abnormalities | (Thumkeo et al., 2003) |
| Csk | C-srk tyrosine kinase | Total | Neural tube defects, death between E9 and E10; developmental arrest in the 10–12 somite stage | (Imamoto and Soriano, 1993; Nada et al., 1993) |
| Ephrin B1 | Transmembrane signaling | Total | Neural crest cell misguidance (cranial and cardiac, but not trunk) | (Davy et al., 2004) |
| Angiomotin | Transmembrane signaling | Total | Death between E11–E11.5, severe vascular insufficiency in intersomitic regions, dilated vessels in the brain | (Aase et al., 2007) |
Table 2 summarizes the current data on mouse knockouts of the intra- and extracellular components that are directly relevant to the cell migration mechanisms outlined above. These data are grouped by functions and separated into sections based on their role at different migration stages. Actin cytoskeleton proteins play an important role in all of the mechanical steps of the migration cycle, while focal adhesion proteins, integrins, and components of the extracellular matrix predominantly participate in adhesion and retraction. In addition, knockouts of some of the regulatory molecules directly involved in the regulation of the mechanistic components of cell migration have been described and are listed separately in the table. Knockouts of other regulatory molecules, such as PI3K and PTEN, have also been characterized (Di Cristofano et al., 1998; Jou et al., 2002; Katso et al., 2001; Stambolic et al., 2000; Suzuki et al., 2003), but are too general to be implicated directly in migration and have therefore been removed from our list.
A separate body of literature concerns tissue signaling molecules and growth factors, as well as transcription factors that regulate cell migration and coordinate global developmental migratory events (see Gilbert, 2000 for an overview, and Alva and Iruela-Arispe, 2004; Chi and Epstein, 2002; Gruber and Epstein, 2004; High and Epstein, 2008; Hofmann and Iruela-Arispe, 2007 for some recent reviews). Since these knockouts do not directly affect the intracellular events of the cell migration cycle, they were not included in the tables or discussion of the current review.
As seen in Table 2, certain knockouts lead to early embryonic lethality (e.g., β actin or tropomyosin), suggesting that these proteins play a universal role in multiple embryonic events. Other knockouts result in organ-specific phenotypes (cardiac defects, placenta, neuritogenesis, or vascular patterning), suggesting that these genes either play a tissue-specific role or their function can be compensated for by similar molecules leading to normal, or semi-normal development of the tissues, in which migration plays a less critical role. Analysis of these phenotypes provides insights into the underlying molecular reasons for birth defects, and may allow us to predict major functional roles for proteins, whose functions have not been well established. An example of such a protein is arginyltransferase, Ate1, whose knockout in mice results in embryonic lethality with specific defects in heart development and angiogenesis that suggest that this gene and the corresponding posttranslational modification plays a critical role in cell migration in situ (Kwon et al., 2002). In agreement with that, Ate1 has been recently shown to regulate actin cytoskeleton, lamella formation, and lamellipodial protrusion (Karakozova et al., 2006), however the exact role of Ate1 in embryogenesis and cell motility remains to be uncovered.
Cell migration and human disease
The data presented above demonstrate the critical importance of cell migration to embryonic development. Not surprisingly, perturbations of migration can lead to severe disorders with direct relevance to human survival and health. Cancer, the second leading cause of death in the developed countries worldwide, is characterized by impaired attachment of cells to the matrix and perturbation of the cell migratory mechanisms (see (Yamaguchi et al., 2005 for an overview).
On the developmental level, a number of human diseases and congenital disorders have been associated with defects in cell migration. Heart septation defects (ventricular and atrial septal defects, and persistent truncus arteriosus, abbreviated as VSD, ASD, and PTA, respectively), associated with defects of migration of the cardiac neural crest cells (Table 1), are among the most highly occurring human congenital heart defects. DiGeorge syndrome, characterized by heart defects, craniofacial abnormalities and severe retardation, has been linked to chromosome deletions that result in migratory defects of the neural crest cells (Epstein, 2001; Epstein and Parmacek, 2005; Gitler et al., 2002; Hutson and Kirby, 2007; Stoller and Epstein, 2005). Hirschsprung's disease, characterized by impaired bowel movement, is related to defects in migration of the vagal and sacral neural crest lineages that populate the gastrointestinal system and subsequently differentiate into the neurons responsible for gut innervation (Amiel et al., 2008; Heanue and Pachnis, 2007; Tucker, 2004). Waardenburg's syndrome, characterized by abnormal pigmentation, results from the impaired migration of neural crest cells that give rise to the melanocytes of the skin (Tachibana et al., 2003; Tucker, 2004). Other human syndromes, including Alagille, Carpenter, CHARGE, Ivemark, and Leopard/Noonan syndromes, result in abnormalities that suggest impairments of the neural crest migration, with known or unknown molecular reasons (Hutson and Kirby, 2003). Increasing molecular understanding of the embryonic migratory mechanisms provides means for the development of new therapies for these diseases in humans.
CONCLUSIONS
Cell migration is a highly conserved mechanism that is critical for normal embryonic development and the functioning of an adult organism. Despite the differences between cell types and lineages that undergo migration, all cells migrate by similar mechanisms, whose perturbation leads to severe developmental defects. These mechanisms involve intricate molecular machines that can sense the environment, respond to signals, and modulate the entire cell behavior. Molecular machines driving migration have a cytoskeleton-based ‘mechanical drive’ and the regulatory molecules that provide direction and determine the migration speed.
A big question that has concerned the researchers for decades relates to the coordination of cell migration in situ and its relation to the intracellular aspects of the cell migratory mechanisms. Indeed, all cells migrate by similar mechanisms, yet in a developing organism they migrate along highly specified paths with different speeds that can vary by orders of magnitude for different cell types (Table 1). Traditionally, this question has been addressed by researchers that considered the intra- and extracellular mechanisms driving migration in separate sets of studies. As more data accumulate, researchers are now able to integrate all of the available information and consider the intracellular mechanisms of cell migration in the context of the developing organisms that contain additional levels of complexity provided by extracellular regulation. This synthesis of the existing and emerging data would eventually enable us to understand the complexity and the mystery of cell migration during development.
Note on references
Since the current review contains a very broad overview of several extensive fields, we have tried, where possible, to cite recent reviews on the appropriate subjects rather than original papers. We apologize to those authors whose very important articles were not included in our reference list due to space constraints.
Note on nomenclature
In the two fields overviewed here, different conventions have been in place to define in vitro and in vivo studies. In cell biology, in vitro is usually related to the biochemical tests done in a cell-free system or with purified proteins, while in vivo defines studies of living cells in culture. In developmental biology, in vitro usually refers to cells and tissue explants in culture, and in vivo is used to describe studies in a living embryo. To side-step these conventions, we used ‘in culture’ when referring to cultured cells and ‘in situ’ when referring to cells in an embryo.
Acknowledgements
We thank Drs. Farida Korobova and Tatyana Svitkina for providing the images for figure 2, Olga Karengina for preparing the illustrations for figure 4, and Dr. Sougata Saha for critical reading of the manuscript. Work in our lab is currently supported by NIH grant 5R01HL084419-01A1 and awards from WW Smith Charitable Trust and Philip Morris Research Management Group to AK.
References
- Aase K, Ernkvist M, Ebarasi L, Jakobsson L, Majumdar A, Yi C, Birot O, Ming Y, Kvanta A, Edholm D, Aspenstrom P, Kissil J, Claesson-Welsh L, Shimono A, Holmgren L. Angiomotin regulates endothelial cell migration during embryonic angiogenesis. Genes Dev. 2007;21(16):2055–2068. doi: 10.1101/gad.432007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JC. Fascin protrusions in cell interactions. Trends Cardiovasc Med. 2004a;14(6):221–226. doi: 10.1016/j.tcm.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Adams JC. Roles of fascin in cell adhesion and motility. Curr Opin Cell Biol. 2004b;16(5):590–596. doi: 10.1016/j.ceb.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Alva JA, Iruela-Arispe ML. Notch signaling in vascular morphogenesis. Curr Opin Hematol. 2004;11(4):278–283. doi: 10.1097/01.moh.0000130309.44976.ad. [DOI] [PubMed] [Google Scholar]
- Amiel J, Sproat-Emison E, Garcia-Barcelo M, Lantieri F, Burzynski G, Borrego S, Pelet A, Arnold S, Miao X, Griseri P, Brooks AS, Antinolo G, de Pontual L, Clement-Ziza M, Munnich A, Kashuk C, West K, Wong KK, Lyonnet S, Chakravarti A, Tam PK, Ceccherini I, Hofstra RM, Fernandez R. Hirschsprung disease, associated syndromes and genetics: a review. J Med Genet. 2008;45(1):1–14. doi: 10.1136/jmg.2007.053959. [DOI] [PubMed] [Google Scholar]
- Anderson RB, Newgreen DF, Young HM. Neural crest and the development of the enteric nervous system. Adv Exp Med Biol. 2006a;589:181–196. doi: 10.1007/978-0-387-46954-6_11. [DOI] [PubMed] [Google Scholar]
- Anderson RB, Stewart AL, Young HM. Phenotypes of neural-crest-derived cells in vagal and sacral pathways. Cell Tissue Res. 2006b;323(1):11–25. doi: 10.1007/s00441-005-0047-6. [DOI] [PubMed] [Google Scholar]
- Arthur JS, Elce JS, Hegadorn C, Williams K, Greer PA. Disruption of the murine calpain small subunit gene, Capn4: calpain is essential for embryonic development but not for cell growth and division. Mol Cell Biol. 2000;20(12):4474–4481. doi: 10.1128/mcb.20.12.4474-4481.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aszódi A, Pfeifer A, Ahmad M, Glauner M, Zhou XH, Ny L, Andersson KE, Kehrel B, Offermanns S, Fässler R. The vasodilator-stimulated phosphoprotein (VASP) is involved in cGMP- and cAMP-mediated inhibition of agonist-induced platelet aggregation, but is dispensable for smooth muscle function. EMBO Journal. 1999;18(1):37–48. doi: 10.1093/emboj/18.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audebert S, Desbruyeres E, Gruszczynski C, Koulakoff A, Gros F, Denoulet P, Edde B. Reversible polyglutamylation of alpha- and beta-tubulin and microtubule dynamics in mouse brain neurons. Mol Biol Cell. 1993;4(6):615–626. doi: 10.1091/mbc.4.6.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader BL, Rayburn H, Crowley D, Hynes RO. Extensive Vasculogenesis, Angiogenesis, and Organogenesis Precede Lethality in Mice Lacking All [alpha]v Integrins. Cell. 1998;95(4):507–519. doi: 10.1016/s0092-8674(00)81618-9. [DOI] [PubMed] [Google Scholar]
- Bailly M, Macaluso F, Cammer M, Chan A, Segall JE, Condeelis JS. Relationship between Arp2/3 complex and the barbed ends of actin filaments at the leading edge of carcinoma cells after epidermal growth factor stimulation. J Cell Biol. 1999;145(2):331–345. doi: 10.1083/jcb.145.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beningo KA, Dembo M, Kaverina I, Small JV, Wang YL. Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J Cell Biol. 2001;153(4):881–888. doi: 10.1083/jcb.153.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beningo KA, Dembo M, Wang YL. Responses of fibroblasts to anchorage of dorsal extracellular matrix receptors. Proc Natl Acad Sci U S A. 2004;101(52):18024–18029. doi: 10.1073/pnas.0405747102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershadsky AD, Ballestrem C, Carramusa L, Zilberman Y, Gilquin B, Khochbin S, Alexandrova AY, Verkhovsky AB, Shemesh T, Kozlov MM. Assembly and mechanosensory function of focal adhesions: experiments and models. Eur J Cell Biol. 2006;85(3–4):165–173. doi: 10.1016/j.ejcb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Bhadriraju K, Yang M, Alom Ruiz S, Pirone D, Tan J, Chen CS. Activation of ROCK by RhoA is regulated by cell adhesion, shape, and cytoskeletal tension. Exp Cell Res. 2007;313(16):3616–3623. doi: 10.1016/j.yexcr.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breau MA, Pietri T, Eder O, Blanche M, Brakebusch C, Fassler R, Thiery JP, Dufour S. Lack of beta1 integrins in enteric neural crest cells leads to a Hirschsprung-like. Development. 2006;133(9):1725–1734. doi: 10.1242/dev.02346. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M, Stern CD, Fraser S. Analysis of neural crest cell lineage and migration. J Craniofac Genet Dev Biol. 1991;11(4):214–222. [PubMed] [Google Scholar]
- Brown CB, Baldwin HS. Neural crest contribution to the cardiovascular system. Adv Exp Med Biol. 2006;589:134–154. doi: 10.1007/978-0-387-46954-6_8. [DOI] [PubMed] [Google Scholar]
- Bulinski JC. Microtubule modification: acetylation speeds anterograde traffic flow. Curr Biol. 2007;17(1):R18–R20. doi: 10.1016/j.cub.2006.11.036. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oostuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, de Ruiter MC, Gittenberger-de Groot A, Poelmann R, Lupu F, Herbert JM, Collen D, Dejana E. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98(2):147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Cattelino A, Liebner S, Gallini R, Zanetti A, Balconi G, Corsi A, Bianco P, Wolburg H, Moore R, Oreda B, Kemler R, Dejana E. The conditional inactivation of the beta-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility. J Cell Biol. 2003;162(6):1111–1122. doi: 10.1083/jcb.200212157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WT. Induction of spreading during fibroblast movement. J Cell Biol. 1979;81(3):684–691. doi: 10.1083/jcb.81.3.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi N, Epstein JA. Getting your Pax straight: Pax proteins in development and disease. Trends Genet. 2002;18(1):41–47. doi: 10.1016/s0168-9525(01)02594-x. [DOI] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Kraus AE, Gale D, White GC, 2nd, Vansluys J. Defective angiogenesis, endothelial migration, proliferation, and MAPK signaling in Rap1b-deficient mice. Blood. 2008;111(5):2647–2656. doi: 10.1182/blood-2007-08-109710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti MA, Even-Ram S, Liu C, Yamada KM, Adelstein RS. Defects in Cell Adhesion and the Visceral Endoderm following Ablation of Nonmuscle Myosin Heavy Chain II-A in Mice. J Biol Chem. 2004;279(40):41263–41266. doi: 10.1074/jbc.C400352200. [DOI] [PubMed] [Google Scholar]
- Costell M, Gustafsson E, Aszodi A, Morgelin M, Bloch W, Hunziker E, Addicks K, Timpl R, Fassler R. Perlecan maintains the integrity of cartilage and some basement membranes. J Cell Biol. 1999;147(5):1109–1122. doi: 10.1083/jcb.147.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford K, Flick R, Close L, Shelly D, Paul R, Bove K, Kumar A, Lessard J. Mice lacking skeletal muscle actin show reduced muscle strength and growth deficits and die during the neonatal period. Mol Cell Biol. 2002;22(16):5887–5896. doi: 10.1128/MCB.22.16.5887-5896.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva JS, Dotti CG. Breaking the neuronal sphere: regulation of the actin cytoskeleton in neuritogenesis. Nat Rev Neurosci. 2002;3(9):694–704. doi: 10.1038/nrn918. [DOI] [PubMed] [Google Scholar]
- Dahme M, Bartsch U, Martini R, Anliker B, Schachner M, Mantei N. Disruption of the mouse L1 gene leads to malformations of the nervous system. Nat Genet. 1997;17(3):346–349. doi: 10.1038/ng1197-346. [DOI] [PubMed] [Google Scholar]
- Davy A, Aubin J, Soriano P. Ephrin-B1 forward and reverse signaling are required during mouse development. Genes Dev. 2004;18(5):572–583. doi: 10.1101/gad.1171704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delon I, Brown NH. Integrins and the actin cytoskeleton. Curr Opin Cell Biol. 2007;19(1):43–50. doi: 10.1016/j.ceb.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Dent EW, Kwiatkowski AV, Mebane LM, Philippar U, Barzik M, Rubinson DA, Gupton S, Van Veen JE, Furman C, Zhang J, Alberts AS, Mori S, Gertler FB. Filopodia are required for cortical neurite initiation. Nat Cell Biol. 2007;9(12):1347–1359. doi: 10.1038/ncb1654. [DOI] [PubMed] [Google Scholar]
- Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19(4):348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- Dimaio TA, Wang S, Huang Q, Scheef EA, Sorenson CM, Sheibani N. Attenuation of retinal vascular development and neovascularization in PECAM-1-deficient mice. Dev Biol. 2008;315(1):72–88. doi: 10.1016/j.ydbio.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobereiner HG, Dubin-Thaler BJ, Giannone G, Sheetz MP. Force sensing and generation in cell phases: analyses of complex functions. J Appl Physiol. 2005;98(4):1542–1546. doi: 10.1152/japplphysiol.01181.2004. [DOI] [PubMed] [Google Scholar]
- Druckenbrod NR, Epstein ML. The pattern of neural crest advance in the cecum and colon. Dev Biol. 2005;287(1):125–133. doi: 10.1016/j.ydbio.2005.08.040. [DOI] [PubMed] [Google Scholar]
- Druckenbrod NR, Epstein ML. Behavior of enteric neural crest-derived cells varies with respect to the migratory wavefront. Dev Dyn. 2007;236(1):84–92. doi: 10.1002/dvdy.20974. [DOI] [PubMed] [Google Scholar]
- Dunn GA, Zicha D. Dynamics of fibroblast spreading. J Cell Sci. 1995;108(Pt 3):1239–1249. doi: 10.1242/jcs.108.3.1239. [DOI] [PubMed] [Google Scholar]
- Durbec PL, Larsson-Blomberg LB, Schuchardt A, Costantini F, Pachnis V. Common origin and developmental dependence on c-ret of subsets of enteric and sympathetic neuroblasts. Development. 1996;122(1):349–358. doi: 10.1242/dev.122.1.349. [DOI] [PubMed] [Google Scholar]
- Echtermeyer F, Streit M, Wilcox-Adelman S, Saoncella S, Denhez F, Detmar M, Goetinck P. Delayed wound repair and impaired angiogenesis in mice lacking syndecan-4. J Clin Invest. 2001;107(2):R9–R14. doi: 10.1172/JCI10559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edde B, Rossier J, Le Caer JP, Desbruyeres E, Gros F, Denoulet P. Posttranslational glutamylation of alpha-tubulin. Science. 1990;247(4938):83–85. doi: 10.1126/science.1967194. [DOI] [PubMed] [Google Scholar]
- Eklund L, Piuhola J, Komulainen J, Sormunen R, Ongvarrasopone C, Fassler R, Muona A, Ilves M, Ruskoaho H, Takala TE, Pihlajaniemi T. Lack of type XV collagen causes a skeletal myopathy and cardiovascular defects in mice. Proc Natl Acad Sci U S A. 2001;98(3):1194–1199. doi: 10.1073/pnas.031444798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Epstein JA. Developing models of DiGeorge syndrome. Trends Genet. 2001;17(10):S13–S17. doi: 10.1016/s0168-9525(01)02450-7. [DOI] [PubMed] [Google Scholar]
- Epstein JA, Parmacek MS. Recent advances in cardiac development with therapeutic implications for adult cardiovascular disease. Circulation. 2005;112(4):592–597. doi: 10.1161/CIRCULATIONAHA.104.479857. [DOI] [PubMed] [Google Scholar]
- Erck C, Peris L, Andrieux A, Meissirel C, Gruber AD, Vernet M, Schweitzer A, Saoudi Y, Pointu H, Bosc C, Salin PA, Job D, Wehland J. A vital role of tubulin-tyrosine-ligase for neuronal organization. Proc Natl Acad Sci U S A. 2005;102(22):7853–7858. doi: 10.1073/pnas.0409626102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 2001;106(4):489–498. doi: 10.1016/s0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- Euteneuer U, Schliwa M. Persistent, directional motility of cells and cytoplasmic fragments in the absence of microtubules. Nature. 1984;310(5972):58–61. doi: 10.1038/310058a0. [DOI] [PubMed] [Google Scholar]
- Even-Ram S, Doyle AD, Conti MA, Matsumoto K, Adelstein RS, Yamada KM. Myosin IIA regulates cell motility and actomyosin-microtubule crosstalk. Nat Cell Biol. 2007;9(3):299–309. doi: 10.1038/ncb1540. [DOI] [PubMed] [Google Scholar]
- Even-Ram S, Yamada KM. Cell migration in 3D matrix. Curr Opin Cell Biol. 2005;17(5):524–532. doi: 10.1016/j.ceb.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Faix J, Rottner K. The making of filopodia. Curr Opin Cell Biol. 2006;18(1):18–25. doi: 10.1016/j.ceb.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Fassler R, Meyer M. Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 1995;9(15):1896–1908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- Flintoff-Dye NL, Welser J, Rooney J, Scowen P, Tamowski S, Hatton W, Burkin DJ. Role for the alpha7beta1 integrin in vascular development and integrity. Dev Dyn. 2005;234(1):11–21. doi: 10.1002/dvdy.20462. [DOI] [PubMed] [Google Scholar]
- Franco SJ, Huttenlocher A. Regulating cell migration: calpains make the cut. J Cell Sci. 2005;118(Pt 17):3829–3838. doi: 10.1242/jcs.02562. [DOI] [PubMed] [Google Scholar]
- Franco SJ, Rodgers MA, Perrin BJ, Han J, Bennin DA, Critchley DR, Huttenlocher A. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat Cell Biol. 2004;6(10):977–983. doi: 10.1038/ncb1175. [DOI] [PubMed] [Google Scholar]
- Fraser SE. Pattern formation in the vertebrate nervous system. Curr Opin Genet Dev. 1991;1(2):217–220. doi: 10.1016/s0959-437x(05)80073-5. [DOI] [PubMed] [Google Scholar]
- Freeman B. The active migration of germ cells in the embryos of mice and men is a myth. Reproduction. 2003;125(5):635–643. doi: 10.1530/rep.0.1250635. [DOI] [PubMed] [Google Scholar]
- Fukai N, Eklund L, Marneros AG, Oh SP, Keene DR, Tamarkin L, Niemela M, Ilves M, Li E, Pihlajaniemi T, Olsen BR. Lack of collagen XVIII/endostatin results in eye abnormalities. Embo J. 2002;21(7):1535–1544. doi: 10.1093/emboj/21.7.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Ilic D, Kanazawa S, Takeda N, Yamamoto T, Aizawa S. Mesodermal defect in late phase of gastrulation by a targeted mutation of focal adhesion kinase, FAK. Oncogene. 1995;11(10):1989–1995. [PubMed] [Google Scholar]
- Galbraith CG, Yamada KM, Sheetz MP. The relationship between force and focal complex development. J Cell Biol. 2002;159(4):695–705. doi: 10.1083/jcb.200204153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A. Assembly and mechanosensory function of focal contacts. Curr Opin Cell Biol. 2001;13(5):584–592. doi: 10.1016/s0955-0674(00)00255-6. [DOI] [PubMed] [Google Scholar]
- George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119(4):1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- Georges PC, Janmey PA. Cell type-specific response to growth on soft materials. J Appl Physiol. 2005;98(4):1547–1553. doi: 10.1152/japplphysiol.01121.2004. [DOI] [PubMed] [Google Scholar]
- Gerthoffer WT. Mechanisms of Vascular Smooth Muscle Cell Migration. Circ Res. 2007;100(5):607–621. doi: 10.1161/01.RES.0000258492.96097.47. [DOI] [PubMed] [Google Scholar]
- Giannone G, Dubin-Thaler BJ, Rossier O, Cai Y, Chaga O, Jiang G, Beaver W, Dobereiner HG, Freund Y, Borisy G, Sheetz MP. Lamellipodial actin mechanically links myosin activity with adhesion-site formation. Cell. 2007;128(3):561–575. doi: 10.1016/j.cell.2006.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SF. Developmental Biology. Sunderland, MA: Sinauder Associates, Inc.; 2000. [Google Scholar]
- Gilbert SF. Developmental Biology. Eighth Edition. Sunderland, MA: Sinauder Associates, Inc.; 2006. [Google Scholar]
- Gitler AD, Brown CB, Kochilas L, Li J, Epstein JA. Neural crest migration and mouse models of congenital heart disease. Cold Spring Harb Symp Quant Biol. 2002;67:57–62. doi: 10.1101/sqb.2002.67.57. [DOI] [PubMed] [Google Scholar]
- Goh KL, Cai L, Cepko CL, Gertler FB. Ena/VASP Proteins Regulate Cortical Neuronal Positioning. Current Biology. 2002;12(7):565–569. doi: 10.1016/s0960-9822(02)00725-x. [DOI] [PubMed] [Google Scholar]
- Goley ED, Welch MD. The ARP2/3 complex: an actin nucleator comes of age. Nat Rev Mol Cell Biol. 2006;7(10):713–726. doi: 10.1038/nrm2026. [DOI] [PubMed] [Google Scholar]
- Goode BL, Eck MJ. Mechanism and function of formins in the control of actin assembly. Annu Rev Biochem. 2007;76:593–627. doi: 10.1146/annurev.biochem.75.103004.142647. [DOI] [PubMed] [Google Scholar]
- Gotlieb AI, May LM, Subrahmanyan L, Kalnins VI. Distribution of microtubule organizing centers in migrating sheets of endothelial cells. J Cell Biol. 1981;91(2 Pt 1):589–594. doi: 10.1083/jcb.91.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber PJ, Epstein JA. Development gone awry: congenital heart disease. Circ Res. 2004;94(3):273–283. doi: 10.1161/01.RES.0000116144.43797.3B. [DOI] [PubMed] [Google Scholar]
- Gundersen GG, Bulinski JC. Microtubule arrays in differentiated cells contain elevated levels of a post-translationally modified form of tubulin. Eur J Cell Biol. 1986;42(2):288–294. [PubMed] [Google Scholar]
- Gundersen GG, Bulinski JC. Selective stabilization of microtubules oriented toward the direction of cell migration. Proc Natl Acad Sci U S A. 1988;85(16):5946–5950. doi: 10.1073/pnas.85.16.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunzer M, Schafer A, Borgmann S, Grabbe S, Zanker KS, Brocker EB, Kampgen E, Friedl P. Antigen presentation in extracellular matrix: interactions of T cells with dendritic cells are dynamic, short lived, and sequential. Immunity. 2000;13(3):323–332. doi: 10.1016/s1074-7613(00)00032-7. [DOI] [PubMed] [Google Scholar]
- Gupton SL, Anderson KL, Kole TP, Fischer RS, Ponti A, Hitchcock-DeGregori SE, Danuser G, Fowler VM, Wirtz D, Hanein D, Waterman-Storer CM. Cell migration without a lamellipodium: translation of actin dynamics into cell movement mediated by tropomyosin. J Cell Biol. 2005;168(4):619–631. doi: 10.1083/jcb.200406063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupton SL, Gertler FB. Filopodia: the fingers that do the walking. Sci STKE. 2007;2007(400):re5. doi: 10.1126/stke.4002007re5. [DOI] [PubMed] [Google Scholar]
- Gupton SL, Waterman-Storer CM. Spatiotemporal feedback between actomyosin and focal-adhesion systems optimizes rapid cell migration. Cell. 2006;125(7):1361–1374. doi: 10.1016/j.cell.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Gurniak CB, Perlas E, Witke W. The actin depolymerizing factor n-cofilin is essential for neural tube morphogenesis and neural crest cell migration. Dev Biol. 2005;278(1):231–241. doi: 10.1016/j.ydbio.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Gurtner GC, Davis V, Li H, McCoy MJ, Sharpe A, Cybulsky MI. Targeted disruption of the murine VCAM1 gene: essential role of VCAM-1 in chorioallantoic fusion and placentation. Genes Dev. 1995;9(1):1–14. doi: 10.1101/gad.9.1.1. [DOI] [PubMed] [Google Scholar]
- Hagel M, George EL, Kim A, Tamimi R, Opitz SL, Turner CE, Imamoto A, Thomas SM. The Adaptor Protein Paxillin Is Essential for Normal Development in the Mouse and Is a Critical Transducer of Fibronectin Signaling. Mol Cell Biol. 2002;22(3):901–915. doi: 10.1128/MCB.22.3.901-915.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser W, Knobeloch K-P, Eigenthaler M, Gambaryan S, Krenn V, Geiger J, Glazova M, Rohde E, Horak I, Walter U, Zimmer M. Megakaryocyte hyperplasia and enhanced agonist-induced platelet activation in vasodilator-stimulated phosphoprotein knockout mice. Proceedings of the National Academy of Sciences. 1999;96(14):8120–8125. doi: 10.1073/pnas.96.14.8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heanue TA, Pachnis V. Enteric nervous system development and Hirschsprung's disease: advances in genetic and stem cell studies. Nat Rev Neurosci. 2007;8(6):466–479. doi: 10.1038/nrn2137. [DOI] [PubMed] [Google Scholar]
- Hennessey ES, Drummond DR, Sparrow JC. Post-translational processing of the amino terminus affects actin function. Eur J Biochem. 1991;197(2):345–352. doi: 10.1111/j.1432-1033.1991.tb15917.x. [DOI] [PubMed] [Google Scholar]
- Henson JH, Svitkina TM, Burns AR, Hughes HE, MacPartland KJ, Nazarian R, Borisy GG. Two components of actin-based retrograde flow in sea urchin coelomocytes. Mol Biol Cell. 1999;10(12):4075–4090. doi: 10.1091/mbc.10.12.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hienola A, Tumova S, Kulesskiy E, Rauvala H. N-syndecan deficiency impairs neural migration in brain. J Cell Biol. 2006;174(4):569–580. doi: 10.1083/jcb.200602043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High FA, Epstein JA. The multifaceted role of Notch in cardiac development and disease. Nat Rev Genet. 2008;9(1):49–61. doi: 10.1038/nrg2279. [DOI] [PubMed] [Google Scholar]
- Hodivala-Dilke K, McHugh KP, Tsakiris DA, Rayburn H, Crowley D, Ullman-Culleré M, Ross FP, Coller BS, Teitelbaum S, Hynes RO. β3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. The Journal of Clinical Investigation. 1999;103(2):229–238. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman LM, Nix DA, Benson B, Boot-Hanford R, Gustafsson E, Jamora C, Menzies AS, Goh KL, Jensen CC, Gertler FB, Fuchs E, Fassler R, Beckerle MC. Targeted Disruption of the Murine zyxin Gene. Mol Cell Biol. 2003;23(1):70–79. doi: 10.1128/MCB.23.1.70-79.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann JJ, Iruela-Arispe ML. Notch signaling in blood vessels: who is talking to whom about what? Circ Res. 2007;100(11):1556–1568. doi: 10.1161/01.RES.0000266408.42939.e4. [DOI] [PubMed] [Google Scholar]
- Hook J, Lemckert F, Qin H, Schevzov G, Gunning P. Gamma tropomyosin gene products are required for embryonic development. Mol Cell Biol. 2004;24(6):2318–2323. doi: 10.1128/MCB.24.6.2318-2323.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson MR, Kirby ML. Neural crest and cardiovascular development: a 20-year perspective. Birth Defects Res C Embryo Today. 2003;69(1):2–13. doi: 10.1002/bdrc.10002. [DOI] [PubMed] [Google Scholar]
- Hutson MR, Kirby ML. Model systems for the study of heart development and disease. Cardiac neural crest and conotruncal malformations. Semin Cell Dev Biol. 2007;18(1):101–110. doi: 10.1016/j.semcdb.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami K, Heier RL, Taruishi M, Takagi H, Mukai M, Shimma S, Taira S, Hatanaka K, Morone N, Yao I, Campbell PK, Yuasa S, Janke C, Macgregor GR, Setou M. Loss of alpha-tubulin polyglutamylation in ROSA22 mice is associated with abnormal targeting of KIF1A and modulated synaptic function. Proc Natl Acad Sci U S A. 2007;104(9):3213–3218. doi: 10.1073/pnas.0611547104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic D, Kovacic B, McDonagh S, Jin F, Baumbusch C, Gardner DG, Damsky CH. Focal adhesion kinase is required for blood vessel morphogenesis. Circ Res. 2003;92(3):300–307. doi: 10.1161/01.res.0000055016.36679.23. [DOI] [PubMed] [Google Scholar]
- Imamoto A, Soriano P. Disruption of the csk gene, encoding a negative regulator of Src family tyrosine kinases, leads to neural tube defects and embryonic lethality in mice. Cell. 1993;73(6):1117–1124. doi: 10.1016/0092-8674(93)90641-3. [DOI] [PubMed] [Google Scholar]
- Infante AS, Stein MS, Zhai Y, Borisy GG, Gundersen GG. Detyrosinated (Glu) microtubules are stabilized by an ATP-sensitive plus-end cap. J Cell Sci. 2000;113(Pt 22):3907–3919. doi: 10.1242/jcs.113.22.3907. [DOI] [PubMed] [Google Scholar]
- Ishiguro K, Kadomatsu Kenji, Kojima Tetsuhito, Muramatsu Hisako, Nakamura Eishin, Ito Masafumi, Nagasaka Tetsuro, Kobayashi Hiroshi, Kusugami Kazuo, Saito Hidehiko, Muramatsu Takashi. Syndecan-4 deficiency impairs the fetal vessels in the placental labyrinth. Developmental Dynamics. 2000;219(4):539–544. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1081>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Ishii A, Lo SH. A role of tensin in skeletal-muscle regeneration. Biochem J. 2001;356(3):737–745. doi: 10.1042/0264-6021:3560737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh RE, Kurokawa K, Ohba Y, Yoshizaki H, Mochizuki N, Matsuda M. Activation of rac and cdc42 video imaged by fluorescent resonance energy transfer-based single-molecule probes in the membrane of living cells. Mol Cell Biol. 2002;22(18):6582–6591. doi: 10.1128/MCB.22.18.6582-6591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki T, Wang YL. Cytoplasmic force gradient in migrating adhesive cells. Biophys J. 2008;94(5):L35–L37. doi: 10.1529/biophysj.107.124479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development. 2000;127(8):1607–1616. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- Jones W, Grupp IL, Doetschman T, Grupp G, Osinska H, Hewett TE, Boivin G, Gulick J, Ng WA, Robbins J. Ablation of the murine alpha myosin heavy chain gene leads to dosage effects and functional deficits in the heart. J Clin Invest. 1996;98(8):1906–1917. doi: 10.1172/JCI118992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou ST, Carpino N, Takahashi Y, Piekorz R, Chao JR, Wang D, Ihle JN. Essential, nonredundant role for the phosphoinositide 3-kinase p110delta in signaling by the B-cell receptor complex. Mol Cell Biol. 2002;22(24):8580–8591. doi: 10.1128/MCB.22.24.8580-8591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur RP. Colonization of the murine hindgut by sacral crest-derived neural precursors: experimental support for an evolutionarily conserved model. Dev Biol. 2000;227(1):146–155. doi: 10.1006/dbio.2000.9886. [DOI] [PubMed] [Google Scholar]
- Karakozova M, Kozak M, Wong CC, Bailey AO, Yates JR, 3rd, Mogilner A, Zebroski H, Kashina A. Arginylation of beta-actin regulates actin cytoskeleton and cell motility. Science. 2006;313(5784):192–196. doi: 10.1126/science.1129344. [DOI] [PubMed] [Google Scholar]
- Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield MD. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2001;17:615–675. doi: 10.1146/annurev.cellbio.17.1.615. [DOI] [PubMed] [Google Scholar]
- Kaufman MH. The Atlas of Mouse Development. San Diego, CA: Academic Press; 1992. [Google Scholar]
- Kaverina I, Krylyshkina O, Small JV. Microtubule targeting of substrate contacts promotes their relaxation and dissociation. J Cell Biol. 1999;146(5):1033–1044. doi: 10.1083/jcb.146.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedrin D, van Rheenen J, Hernandez L, Condeelis J, Segall JE. Cell motility and cytoskeletal regulation in invasion and metastasis. J Mammary Gland Biol Neoplasia. 2007;12(2–3):143–152. doi: 10.1007/s10911-007-9046-4. [DOI] [PubMed] [Google Scholar]
- Khawaja S, Gundersen GG, Bulinski JC. Enhanced stability of microtubules enriched in detyrosinated tubulin is not a direct function of detyrosination level. J Cell Biol. 1988;106(1):141–149. doi: 10.1083/jcb.106.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight B, Laukaitis C, Akhtar N, Hotchin NA, Edlund M, Horwitz AR. Visualizing muscle cell migration in situ. Curr Biol. 2000;10(10):576–585. doi: 10.1016/s0960-9822(00)00486-3. [DOI] [PubMed] [Google Scholar]
- Kojima T. Targeted gene disruption of natural anticoagulant proteins in mice. Int J Hematol. 2002;76 Suppl:36–39. doi: 10.1007/BF03165083. [DOI] [PubMed] [Google Scholar]
- Kondo S, Scheef EA, Sheibani N, Sorenson CM. PECAM-1 isoform-specific regulation of kidney endothelial cell migration and capillary morphogenesis. Am J Physiol Cell Physiol. 2007;292(6):C2070–C2083. doi: 10.1152/ajpcell.00489.2006. [DOI] [PubMed] [Google Scholar]
- Krause M, Leslie JD, Stewart M, Lafuente EM, Valderrama F, Jagannathan R, Strasser GA, Rubinson DA, Liu H, Way M, Yaffe MB, Boussiotis VA, Gertler FB. Lamellipodin, an Ena/VASP ligand, is implicated in the regulation of lamellipodial dynamics. Dev Cell. 2004;7(4):571–583. doi: 10.1016/j.devcel.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Kulesa P, Bronner-Fraser M, Fraser S. In ovo time-lapse analysis after dorsal neural tube ablation shows rerouting of chick hindbrain neural crest. Development. 2000;127(13):2843–2852. doi: 10.1242/dev.127.13.2843. [DOI] [PubMed] [Google Scholar]
- Kumar A, Crawford K, Close L, Madison M, Lorenz J, Doetschman T, Pawlowski S, Duffy J, Neumann J, Robbins J, Boivin GP, O'Toole BA, Lessard JL. Rescue of cardiac alpha-actin-deficient mice by enteric smooth muscle gamma-actin. Proc Natl Acad Sci U S A. 1997;94(9):4406–4411. doi: 10.1073/pnas.94.9.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A, Dennert G, Singer SJ. Polarization of the Golgi apparatus and the microtubule-organizing center within cloned natural killer cells bound to their targets. Proc Natl Acad Sci U S A. 1983;80(23):7224–7228. doi: 10.1073/pnas.80.23.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A, Louvard D, Singer SJ. Polarization of the Golgi apparatus and the microtubule-organizing center in cultured fibroblasts at the edge of an experimental wound. Proc Natl Acad Sci U S A. 1982;79(8):2603–2607. doi: 10.1073/pnas.79.8.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski AV, Rubinson DA, Dent EW, Edward van Veen J, Leslie JD, Zhang J, Mebane LM, Philippar U, Pinheiro EM, Burds AA, Bronson RT, Mori S, Fassler R, Gertler FB. Ena/VASP Is Required for Neuritogenesis in the Developing Cortex. Neuron. 2007;56(3):441–455. doi: 10.1016/j.neuron.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Kwon YT, Kashina AS, Davydov IV, Hu RG, An JY, Seo JW, Du F, Varshavsky A. An essential role of N-terminal arginylation in cardiovascular development. Science. 2002;297(5578):96–99. doi: 10.1126/science.1069531. [DOI] [PubMed] [Google Scholar]
- Lafuente EM, van Puijenbroek AA, Krause M, Carman CV, Freeman GJ, Berezovskaya A, Constantine E, Springer TA, Gertler FB, Boussiotis VA. RIAM, an Ena/VASP and Profilin ligand, interacts with Rap1-GTP and mediates Rap1-induced adhesion. Dev Cell. 2004;7(4):585–595. doi: 10.1016/j.devcel.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Lanier LM, Gates MA, Witke W, Menzies AS, Wehman AM, Macklis JD, Kwiatkowski D, Soriano P, Gertler FB. Mena Is Required for Neurulation and Commissure Formation. Neuron. 1999;22(2):313–325. doi: 10.1016/s0896-6273(00)81092-2. [DOI] [PubMed] [Google Scholar]
- Larue L, Ohsugi M, Hirchenhain J, Kemler R. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc Natl Acad Sci U S A. 1994;91(17):8263–8267. doi: 10.1073/pnas.91.17.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84(3):359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM. The Neural Crest (Developmental and Cell Biology Series) Cambridge University Press; 1983. [Google Scholar]
- Lei L, Liu D, Huang Y, Jovin I, Shai S-Y, Kyriakides T, Ross RS, Giordano FJ. Endothelial Expression of 1 Integrin Is Required for Embryonic Vascular Patterning and Postnatal Vascular Remodeling. Mol Cell Biol. 2008;28(2):794–802. doi: 10.1128/MCB.00443-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo SH, Yu Q-C, Degenstein L, Chen LB, Fuchs E. Progressive Kidney Degeneration in Mice Lacking Tensin. J Cell Biol. 1997;136(6):1349–1361. doi: 10.1083/jcb.136.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locascio A, Nieto MA. Cell movements during vertebrate development: integrated tissue behaviour versus individual cell migration. Curr Opin Genet Dev. 2001;11(4):464–469. doi: 10.1016/s0959-437x(00)00218-5. [DOI] [PubMed] [Google Scholar]
- Lock JG, Wehrle-Haller B, Stromblad S. Cell-matrix adhesion complexes: master control machinery of cell migration. Semin Cancer Biol. 2008;18(1):65–76. doi: 10.1016/j.semcancer.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Luduena RF. Multiple forms of tubulin: different gene products and covalent modifications. Int Rev Cytol. 1998;178:207–275. doi: 10.1016/s0074-7696(08)62138-5. [DOI] [PubMed] [Google Scholar]
- Luo Y, High FA, Epstein JA, Radice GL. N-cadherin is required for neural crest remodeling of the cardiac outflow tract. Dev Biol. 2006;299(2):517–528. doi: 10.1016/j.ydbio.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Radice GL. N-cadherin acts upstream of VE-cadherin in controlling vascular morphogenesis. J Cell Biol. 2005;169(1):29–34. doi: 10.1083/jcb.200411127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machacek M, Danuser G. Morphodynamic profiling of protrusion phenotypes. Biophys J. 2006;90(4):1439–1452. doi: 10.1529/biophysj.105.070383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malikov V, Cytrynbaum EN, Kashina A, Mogilner A, Rodionov V. Centering of a radial microtubule array by translocation along microtubules spontaneously nucleated in the cytoplasm. Nat Cell Biol. 2005;7(12):1213–1218. doi: 10.1038/ncb1332. [DOI] [PubMed] [Google Scholar]
- Mayer U, Saher G, Fassler R, Bornemann A, Echtermeyer F, von der Mark H, Miosge N, Poschl E, von der Mark K. Absence of integrin alpha 7 causes a novel form of muscular dystrophy. Nat Genet. 1997;17(3):318–323. doi: 10.1038/ng1197-318. [DOI] [PubMed] [Google Scholar]
- Medeiros NA, Burnette DT, Forscher P. Myosin II functions in actin-bundle turnover in neuronal growth cones. Nat Cell Biol. 2006;8(3):215–226. doi: 10.1038/ncb1367. [DOI] [PubMed] [Google Scholar]
- Miner JH, Cunningham J, Sanes JR. Roles for laminin in embryogenesis: exencephaly, syndactyly, and placentopathy in mice lacking the laminin alpha5 chain. J Cell Biol. 1998;143(6):1713–1723. doi: 10.1083/jcb.143.6.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH, Li C. Defective glomerulogenesis in the absence of laminin alpha5 demonstrates a developmental role for the kidney glomerular basement membrane. Dev Biol. 2000;217(2):278–289. doi: 10.1006/dbio.1999.9546. [DOI] [PubMed] [Google Scholar]
- Miyagoe Y, Hanaoka K, Nonaka I, Hayasaka M, Nabeshima Y, Arahata K, Takeda S. Laminin alpha2 chain-null mutant mice by targeted disruption of the Lama2 gene: a new model of merosin (laminin 2)-deficient congenital muscular dystrophy. FEBS Lett. 1997;415(1):33–39. doi: 10.1016/s0014-5793(97)01007-7. [DOI] [PubMed] [Google Scholar]
- Mogilner A, Oster G. Cell motility driven by actin polymerization. Biophys J. 1996;71(6):3030–3045. doi: 10.1016/S0006-3495(96)79496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneaux K, Wylie C. Primordial germ cell migration. Int J Dev Biol. 2004;48(5–6):537–544. doi: 10.1387/ijdb.041833km. [DOI] [PubMed] [Google Scholar]
- Molyneaux KA, Stallock J, Schaible K, Wylie C. Time-lapse analysis of living mouse germ cell migration. Dev Biol. 2001;240(2):488–498. doi: 10.1006/dbio.2001.0436. [DOI] [PubMed] [Google Scholar]
- Mongiu AK, Weitzke EL, Chaga OY, Borisy GG. Kinetic-structural analysis of neuronal growth cone veil motility. J Cell Sci. 2007;120(Pt 6):1113–1125. doi: 10.1242/jcs.03384. [DOI] [PubMed] [Google Scholar]
- Monkley SJ, Zhou Xiao-Hong, Kinston Sarah J, Giblett Susan M, Hemmings Lance, Priddle Helen, Brown Jane E, Pritchard Catrin A, Critchley David R, Fässler Reinhard. Disruption of the <I>talin</I> gene arrests mouse development at the gastrulation stage. Developmental Dynamics. 2000;219(4):560–574. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1079>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Mu H, Ohashi R, Lin P, Yao Q, Chen C. Cellular and molecular mechanisms of coronary vessel development. Vascular Medicine. 2005;10(1):37–44. doi: 10.1191/1358863x05vm584ra. [DOI] [PubMed] [Google Scholar]
- Mullins RD, Pollard TD. Structure and function of the Arp2/3 complex. Curr Opin Struct Biol. 1999;9(2):244–249. doi: 10.1016/s0959-440x(99)80034-7. [DOI] [PubMed] [Google Scholar]
- Nada S, Yagi T, Takeda H, Tokunaga T, Nakagawa H, Ikawa Y, Okada M, Aizawa S. Constitutive activation of Src family kinases in mouse embryos that lack Csk. Cell. 1993;73(6):1125–1135. doi: 10.1016/0092-8674(93)90642-4. [DOI] [PubMed] [Google Scholar]
- Nagy A, Gertsenstein M, Vintersten K, Behringer RR. Manipulating the Mouse Embryo: A Laboratory Manual. Third Edition. Cold Sprong Harbor, NY: Cold Sprong Harbor Laboratory Press; 2003. [Google Scholar]
- Noakes PG, Gautam M, Mudd J, Sanes JR, Merlie JP. Aberrant differentiation of neuromuscular junctions in mice lacking s-laminin/laminin beta 2. Nature. 1995a;374(6519):258–262. doi: 10.1038/374258a0. [DOI] [PubMed] [Google Scholar]
- Noakes PG, Miner JH, Gautam M, Cunningham JM, Sanes JR, Merlie JP. The renal glomerulus of mice lacking s-laminin/laminin beta 2: nephrosis despite molecular compensation by laminin beta 1. Nat Genet. 1995b;10(4):400–406. doi: 10.1038/ng0895-400. [DOI] [PubMed] [Google Scholar]
- Ohba Y, Ikuta K, Ogura A, Matsuda J, Mochizuki N, Nagashima K, Kurokawa K, Mayer BJ, Maki K, Miyazaki J, Matsuda M. Requirement for C3G-dependent Rap1 activation for cell adhesion and embryogenesis. EMBO Journal. 2001;20:3333–3341. doi: 10.1093/emboj/20.13.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak CW, Flynn KC, Bamburg JR. Actin-binding proteins take the reins in growth cones. 2008;9(2):136–147. doi: 10.1038/nrn2236. [DOI] [PubMed] [Google Scholar]
- Patan S. Vasculogenesis and angiogenesis as mechanisms of vascular network formation, growth and remodeling. J Neurooncol. 2000;50(1–2):1–15. doi: 10.1023/a:1006493130855. [DOI] [PubMed] [Google Scholar]
- Patton BL, Connoll AM, Martin PT, Cunningham JM, Mehta S, Pestronk A, Miner JH, Sanes JR. Distribution of ten laminin chains in dystrophic and regenerating muscles. Neuromuscul Disord. 1999;9(6–7):423–433. doi: 10.1016/s0960-8966(99)00033-4. [DOI] [PubMed] [Google Scholar]
- Patton BL, Cunningham JM, Thyboll J, Kortesmaa J, Westerblad H, Edstrom L, Tryggvason K, Sanes JR. Properly formed but improperly localized synaptic specializations in the absence of laminin alpha4. Nat Neurosci. 2001;4(6):597–604. doi: 10.1038/88414. [DOI] [PubMed] [Google Scholar]
- Patton BL, Miner JH, Chiu AY, Sanes JR. Distribution and function of laminins in the neuromuscular system of developing, adult, and mutant mice. J Cell Biol. 1997;139(6):1507–1521. doi: 10.1083/jcb.139.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plessmann U, Weber K. Mammalian sperm tubulin: an exceptionally large number of variants based on several posttranslational modifications. J Protein Chem. 1997;16(5):385–390. doi: 10.1023/a:1026332621215. [DOI] [PubMed] [Google Scholar]
- Pollard TD. Regulation of actin filament assembly by Arp2/3 complex and formins. Annu Rev Biophys Biomol Struct. 2007;36:451–477. doi: 10.1146/annurev.biophys.35.040405.101936. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112(4):453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- Ponti A, Machacek M, Gupton SL, Waterman-Storer CM, Danuser G. Two Distinct Actin Networks Drive the Protrusion of Migrating Cells. Science. 2004;305(5691):1782–1786. doi: 10.1126/science.1100533. [DOI] [PubMed] [Google Scholar]
- Pruyne D, Evangelista M, Yang C, Bi E, Zigmond S, Bretscher A, Boone C. Role of formins in actin assembly: nucleation and barbed-end association. Science. 2002;297(5581):612–615. doi: 10.1126/science.1072309. [DOI] [PubMed] [Google Scholar]
- Radice GL, Rayburn H, Matsunami H, Knudsen KA, Takeichi M, Hynes RO. Developmental defects in mouse embryos lacking N-cadherin. Dev Biol. 1997;181(1):64–78. doi: 10.1006/dbio.1996.8443. [DOI] [PubMed] [Google Scholar]
- Raybin D, Flavin M. Enzyme which specifically adds tyrosine to the alpha chain of tubulin. Biochemistry. 1977a;16(10):2189–2194. doi: 10.1021/bi00629a023. [DOI] [PubMed] [Google Scholar]
- Raybin D, Flavin M. Modification of tubulin by tyrosylation in cells and extracts and its effect on assembly in vitro. J Cell Biol. 1977b;73(2):492–504. doi: 10.1083/jcb.73.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267(5205):1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- Redeker V, Levilliers N, Vinolo E, Rossier J, Jaillard D, Burnette D, Gaertig J, Bre MH. Mutations of tubulin glycylation sites reveal cross-talk between the C termini of alpha- and beta-tubulin and affect the ciliary matrix in Tetrahymena. J Biol Chem. 2005;280(1):596–606. doi: 10.1074/jbc.M408324200. [DOI] [PubMed] [Google Scholar]
- Redman KL, Rubenstein PA. Actin amino-terminal acetylation and processing in a rabbit reticulocyte lysate. Methods Enzymol. 1984;106:179–192. doi: 10.1016/0076-6879(84)06018-3. [DOI] [PubMed] [Google Scholar]
- Reed NA, Cai D, Blasius TL, Jih GT, Meyhofer E, Gaertig J, Verhey KJ. Microtubule acetylation promotes kinesin-1 binding and transport. Curr Biol. 2006;16(21):2166–2172. doi: 10.1016/j.cub.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Rehfeldt F, Engler AJ, Eckhardt A, Ahmed F, Discher DE. Cell responses to the mechanochemical microenvironment--implications for regenerative medicine and drug delivery. Adv Drug Deliv Rev. 2007;59(13):1329–1339. doi: 10.1016/j.addr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell Migration: Integrating Signals from Front to Back. Science. 2003;302(5651):1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, Kam Z, Geiger B, Bershadsky AD. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol. 2001;153(6):1175–1186. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov VI, Borisy GG. Self-centring activity of cytoplasm. Nature. 1997;386(6621):170–173. doi: 10.1038/386170a0. [DOI] [PubMed] [Google Scholar]
- Rodionov VI, Borisy GG. Self-centering in cytoplasmic fragments of melanophores. Mol Biol Cell. 1998;9(7):1613–1615. doi: 10.1091/mbc.9.7.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney JE, Welser JV, Dechert MA, Flintoff-Dye NL, Kaufman SJ, Burkin DJ. Severe muscular dystrophy in mice that lack dystrophin and alpha7 integrin. J Cell Sci. 2006;119(Pt 11):2185–2195. doi: 10.1242/jcs.02952. [DOI] [PubMed] [Google Scholar]
- Rottner K, Hall A, Small JV. Interplay between Rac and Rho in the control of substrate contact dynamics. Curr Biol. 1999;9(12):640–648. doi: 10.1016/s0960-9822(99)80286-3. [DOI] [PubMed] [Google Scholar]
- Rubenstein PA, Martin DJ. NH2-terminal processing of actin in mouse L-cells in vivo. J Biol Chem. 1983;258(6):3961–3966. [PubMed] [Google Scholar]
- Ryan MC, Lee K, Miyashita Y, Carter WG. Targeted disruption of the LAMA3 gene in mice reveals abnormalities in survival and late stage differentiation of epithelial cells. J Cell Biol. 1999;145(6):1309–1323. doi: 10.1083/jcb.145.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Jeannet J-P, editor. Neural Crest Induction and Differentiation. New Yory, NY: Springer Science + Business Media, LLC; 2006. [Google Scholar]
- Schildmeyer LA, Braun R, Taffet G, Debiasi M, Burns AE, Bradley A, Schwartz RJ. Impaired vascular contractility and blood pressure homeostasis in the smooth muscle alpha-actin null mouse. Faseb J. 2000;14(14):2213–2220. doi: 10.1096/fj.99-0927com. [DOI] [PubMed] [Google Scholar]
- Schoenwaelder SM, Burridge K. Bidirectional signaling between the cytoskeleton and integrins. Curr Opin Cell Biol. 1999;11(2):274–286. doi: 10.1016/s0955-0674(99)80037-4. [DOI] [PubMed] [Google Scholar]
- Serbedzija GN, Bronner-Fraser M, Fraser SE. Vital dye analysis of cranial neural crest cell migration in the mouse embryo. Development. 1992;116(2):297–307. doi: 10.1242/dev.116.2.297. [DOI] [PubMed] [Google Scholar]
- Shawlot W, Deng JM, Fohn LE, Behringer RR. Restricted beta-galactosidase expression of a hygromycin-lacZ gene targeted to the beta-actin locus and embryonic lethality of beta-actin mutant mice. Transgenic Res. 1998;7(2):95–103. doi: 10.1023/a:1008816308171. [DOI] [PubMed] [Google Scholar]
- Sheterline P, Clayton J, C SJ. Actin. Fourth Edition. New York, NY: Oxford University Press; 1998. [Google Scholar]
- Skaper SD. Neuronal growth-promoting and inhibitory cues in neuroprotection and neuroregeneration. Ann N Y Acad Sci. 2005;1053:376–385. doi: 10.1196/annals.1344.032. [DOI] [PubMed] [Google Scholar]
- Small JV, Kaverina I. Microtubules meet substrate adhesions to arrange cell polarity. Curr Opin Cell Biol. 2003;15(1):40–47. doi: 10.1016/s0955-0674(02)00008-x. [DOI] [PubMed] [Google Scholar]
- Smyth N, Vatansever HS, Murray P, Meyer M, Frie C, Paulsson M, Edgar D. Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J Cell Biol. 1999;144(1):151–160. doi: 10.1083/jcb.144.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnemann KJ, Fitzsimons DP, Patel JR, Liu Y, Schneider MF, Moss RL, Ervasti JM. Cytoplasmic gamma-actin is not required for skeletal muscle development but its absence leads to a progressive myopathy. Dev Cell. 2006;11(3):387–397. doi: 10.1016/j.devcel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Stambolic V, Tsao MS, Macpherson D, Suzuki A, Chapman WB, Mak TW. High incidence of breast and endometrial neoplasia resembling human Cowden syndrome in pten+/− mice. Cancer Res. 2000;60(13):3605–3611. [PubMed] [Google Scholar]
- Stenvers KL, Tursky ML, Harder KW, Kountouri N, Amatayakul-Chantler S, Grail D, Small C, Weinberg RA, Sizeland AM, Zhu HJ. Heart and liver defects and reduced transforming growth factor beta2 sensitivity in transforming growth factor beta type III receptor-deficient embryos. Mol Cell Biol. 2003;23(12):4371–4385. doi: 10.1128/MCB.23.12.4371-4385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoller JZ, Epstein JA. Cardiac neural crest. Seminars in Cell & Developmental Biology. 2005;16(6):704–715. doi: 10.1016/j.semcdb.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Itami S, Ohishi M, Hamada K, Inoue T, Komazawa N, Senoo H, Sasaki T, Takeda J, Manabe M, Mak TW, Nakano T. Keratinocyte-specific Pten deficiency results in epidermal hyperplasia, accelerated hair follicle morphogenesis and tumor formation. Cancer Res. 2003;63(3):674–681. [PubMed] [Google Scholar]
- Svitkina TM, Borisy GG. Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. J Cell Biol. 1999;145(5):1009–1026. doi: 10.1083/jcb.145.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M, Kobayashi Y, Matsushima Y. Mouse models for four types of Waardenburg syndrome. Pigment Cell Res. 2003;16(5):448–454. doi: 10.1034/j.1600-0749.2003.00066.x. [DOI] [PubMed] [Google Scholar]
- Takada Y, Ye X, Simon S. The integrins. Genome Biol. 2007;8(5):215. doi: 10.1186/gb-2007-8-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanjore H, Zeisberg Elisabeth M, Gerami-Naini Behzad, Kalluri Raghu. beta1 integrin expression on endothelial cells is required for angiogenesis but not for vasculogenesis. Developmental Dynamics. 2008;237(1):75–82. doi: 10.1002/dvdy.21385. [DOI] [PubMed] [Google Scholar]
- Thumkeo D, Keel J, Ishizaki T, Hirose M, Nonomura K, Oshima H, Oshima M, Taketo MM, Narumiya S. Targeted disruption of the mouse rho-associated kinase 2 gene results in intrauterine growth retardation and fetal death. Mol Cell Biol. 2003;23(14):5043–5055. doi: 10.1128/MCB.23.14.5043-5055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyboll J, Kortesmaa J, Cao R, Soininen R, Wang L, Iivanainen A, Sorokin L, Risling M, Cao Y, Tryggvason K. Deletion of the laminin alpha4 chain leads to impaired microvessel maturation. Mol Cell Biol. 2002;22(4):1194–1202. doi: 10.1128/MCB.22.4.1194-1202.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor PA. Specification of neural crest cell formation and migration in mouse embryos. Semin Cell Dev Biol. 2005;16(6):683–693. doi: 10.1016/j.semcdb.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Trelstad RL, editor. Role of Extracellular Matrix in Development (Society for Developmental Biology Symposium//(Proceedings)); John Wiley & Sons; 1984. [Google Scholar]
- Tucker RP. Abnormal neural crest cell migration after the in vivo knockdown of tenascin-C expression with morpholino antisense oligonucleotides. Dev Dyn. 2001;222(1):115–119. doi: 10.1002/dvdy.1171. [DOI] [PubMed] [Google Scholar]
- Tucker RP. Neural crest cells: a model for invasive behavior. Int J Biochem Cell Biol. 2004;36(2):173–177. doi: 10.1016/s1357-2725(03)00243-7. [DOI] [PubMed] [Google Scholar]
- Tullio AN, Accili D, Ferrans VJ, Yu ZX, Takeda K, Grinberg A, Westphal H, Preston YA, Adelstein RS. Nonmuscle myosin II-B is required for normal development of the mouse heart. Proc Natl Acad Sci U S A. 1997;94(23):12407–12412. doi: 10.1073/pnas.94.23.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utriainen A, Sormunen R, Kettunen M, Carvalhaes LS, Sajanti E, Eklund L, Kauppinen R, Kitten GT, Pihlajaniemi T. Structurally altered basement membranes and hydrocephalus in a type XVIII collagen deficient mouse line. Hum Mol Genet. 2004;13(18):2089–2099. doi: 10.1093/hmg/ddh213. [DOI] [PubMed] [Google Scholar]
- Vallotton P, Gupton SL, Waterman-Storer CM, Danuser G. Simultaneous mapping of filamentous actin flow and turnover in migrating cells by quantitative fluorescent speckle microscopy. Proc Natl Acad Sci U S A. 2004;101(26):9660–9665. doi: 10.1073/pnas.0300552101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hinsbergh VW, Koolwijk P. Endothelial sprouting and angiogenesis: matrix metalloproteinases in the lead. Cardiovasc Res. 2008 doi: 10.1093/cvr/cvm102. [DOI] [PubMed] [Google Scholar]
- Verkhovsky AB, Svitkina TM, Borisy GG. Self-polarization and directional motility of cytoplasm. Curr Biol. 1999;9(1):11–20. doi: 10.1016/s0960-9822(99)80042-6. [DOI] [PubMed] [Google Scholar]
- Vicente-Manzanares M, Webb DJ, Horwitz AR. Cell migration at a glance. J Cell Sci. 2005;118(Pt 21):4917–4919. doi: 10.1242/jcs.02662. [DOI] [PubMed] [Google Scholar]
- Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7(4):265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- Vorobjev I, Malikov V, Rodionov V. Self-organization of a radial microtubule array by dynein-dependent nucleation of microtubules. Proc Natl Acad Sci U S A. 2001;98(18):10160–10165. doi: 10.1073/pnas.181354198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss AK, Gruss P, Thomas T. The guanine nucleotide exchange factor C3G is necessary for the formation of focal adhesions and vascular maturation. Development. 2003;130(2):355–367. doi: 10.1242/dev.00217. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Mitchison TJ. Single-molecule speckle analysis of actin filament turnover in lamellipodia. Science. 2002;295(5557):1083–1086. doi: 10.1126/science.1067470. [DOI] [PubMed] [Google Scholar]
- Webb DJ, Parsons JT, Horwitz AF. Adhesion assembly, disassembly and turnover in migrating cells -- over and over and over again. Nat Cell Biol. 2002;4(4):E97–E100. doi: 10.1038/ncb0402-e97. [DOI] [PubMed] [Google Scholar]
- Welch MD, DePace AH, Verma S, Iwamatsu A, Mitchison TJ. The human Arp2/3 complex is composed of evolutionarily conserved subunits and is localized to cellular regions of dynamic actin filament assembly. J Cell Biol. 1997;138(2):375–384. doi: 10.1083/jcb.138.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch MD, Mullins RD. Cellular control of actin nucleation. Annu Rev Cell Dev Biol. 2002;18:247–288. doi: 10.1146/annurev.cellbio.18.040202.112133. [DOI] [PubMed] [Google Scholar]
- Welser JV, Lange ND, Flintoff-Dye N, Burkin HR, Burkin DJ. Placental Defects in [alpha]7 Integrin Null Mice. Placenta. 2007;28(11–12):1219–1228. doi: 10.1016/j.placenta.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RW, Herrup K. The control of neuron number. Annu Rev Neurosci. 1988;11:423–453. doi: 10.1146/annurev.ne.11.030188.002231. [DOI] [PubMed] [Google Scholar]
- Witke W, Sharpe AH, Hartwig JH, Azuma T, Stossel TP, Kwiatkowski DJ. Hemostatic, inflammatory, and fibroblast responses are blunted in mice lacking gelsolin. Cell. 1995;81(1):41–51. doi: 10.1016/0092-8674(95)90369-0. [DOI] [PubMed] [Google Scholar]
- Witke W, Sutherland JD, Sharpe A, Arai M, Kwiatkowski DJ. Profilin I is essential for cell survival and cell division in early mouse development. Proceedings of the National Academy of Sciences. 2001;98(7):3832–3836. doi: 10.1073/pnas.051515498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CC, Xu T, Rai R, Bailey AO, Yates JR, 3rd, Wolf YI, Zebroski H, Kashina A. Global analysis of posttranslational protein arginylation. PLoS Biol. 2007;5(10):e258. doi: 10.1371/journal.pbio.0050258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Baribault H, Adamson ED. Vinculin knockout results in heart and brain defects during embryonic development. Development. 1998;125(2):327–337. doi: 10.1242/dev.125.2.327. [DOI] [PubMed] [Google Scholar]
- Xu X, Francis R, Wei CJ, Linask KL, Lo CW. Connexin 43-mediated modulation of polarized cell movement and the directional migration of cardiac neural crest cells. Development. 2006;133(18):3629–3639. doi: 10.1242/dev.02543. [DOI] [PubMed] [Google Scholar]
- Yam PT, Wilson CA, Ji L, Hebert B, Barnhart EL, Dye NA, Wiseman PW, Danuser G, Theriot JA. Actin myosin network reorganization breaks symmetry at the cell rear to spontaneously initiate polarized cell motility. J Cell Biol. 2007;178(7):1207–1221. doi: 10.1083/jcb.200706012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Condeelis J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim Biophys Acta. 2007;1773(5):642–652. doi: 10.1016/j.bbamcr.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Wyckoff J, Condeelis J. Cell migration in tumors. Curr Opin Cell Biol. 2005;17(5):559–564. doi: 10.1016/j.ceb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Yang C, Czech L, Gerboth S, Kojima S, Scita G, Svitkina T. Novel roles of forming mDia2 in lamellipodia and filopodia formation in motile cells. PLoS Biol. 2007;5(11):e317. doi: 10.1371/journal.pbio.0050317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Rayburn H, Hynes R. Embryonic mesodermal defects in alpha 5 integrin-deficient mice. Development. 1993;119(4):1093–1105. doi: 10.1242/dev.119.4.1093. [DOI] [PubMed] [Google Scholar]
- Yang J, Rayburn H, Hynes R. Cell adhesion events mediated by alpha 4 integrins are essential in placental and cardiac development. Development. 1995;121(2):549–560. doi: 10.1242/dev.121.2.549. [DOI] [PubMed] [Google Scholar]
- Young HM, Newgreen D. Enteric neural crest-derived cells: origin, identification, migration, and differentiation. Anat Rec. 2001;262(1):1–15. doi: 10.1002/1097-0185(20010101)262:1<1::AID-AR1006>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Zhang XF, Schaefer AW, Burnette DT, Schoonderwoert VT, Forscher P. Rho-dependent contractile responses in the neuronal growth cone are independent of classical peripheral retrograde actin flow. Neuron. 2003;40(5):931–944. doi: 10.1016/s0896-6273(03)00754-2. [DOI] [PubMed] [Google Scholar]
- Zhou X, Tian F, Sandzen J, Cao R, Flaberg E, Szekely L, Cao Y, Ohlsson C, Bergo MO, Boren J, Akyurek LM. Filamin B deficiency in mice results in skeletal malformations and impaired microvascular development. Proceedings of the National Academy of Sciences. 2007;104(10):3919–3924. doi: 10.1073/pnas.0608360104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Motejlek K, Wang D, Zang K, Schmidt A, Reichardt LF. {beta}8 integrins are required for vascular morphogenesis in mouse embryos. Development. 2002;129(12):2891–2903. doi: 10.1242/dev.129.12.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond SH. Formin-induced nucleation of actin filaments. Curr Opin Cell Biol. 2004;16(1):99–105. doi: 10.1016/j.ceb.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Zigmond SH, Joyce M, Yang C, Brown K, Huang M, Pring M. Mechanism of Cdc42-induced actin polymerization in neutrophil extracts. J Cell Biol. 1998;142(4):1001–1012. doi: 10.1083/jcb.142.4.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]