Abstract
The molecular requirements for the translocation of secretory proteins across, and the integration of membrane proteins into, the plasma membrane of Escherichia coli were compared. This was achieved in a novel cell-free system from E. coli which, by extensive subfractionation, was simultaneously rendered deficient in SecA/SecB and the signal recognition particle (SRP) components, Ffh (P48), 4.5S RNA, and FtsY. The integration of two membrane proteins into inside-out plasma membrane vesicles of E. coli required all three SRP components and could not be driven by SecA, SecB, and ΔμH+. In contrast, these were the only components required for the translocation of secretory proteins into membrane vesicles, a process in which the SRP components were completely inactive. Our results, while confirming previous in vivo studies, provide the first in vitro evidence for the dependence of the integration of polytopic inner membrane proteins on SRP in E. coli. Furthermore, they suggest that SRP and SecA/SecB have different substrate specificities resulting in two separate targeting mechanisms for membrane and secretory proteins in E. coli. Both targeting pathways intersect at the translocation pore because they are equally affected by a blocked translocation channel.
INTRODUCTION
The export of secretory, i.e., periplasmic and outer membrane proteins across the inner membrane of the Gram-negative bacterium Escherichia coli involves a distinct set of Sec proteins. SecA and SecB function in the posttranslational chaperoning and membrane targeting of the signal sequence-containing precursors of these secretory proteins (Duong et al., 1997). SecA, which possesses ATPase activity, is also the motor for the subsequent translocation across the bacterial plasma membrane. A minimal translocation pore consists of SecY/SecE/SecG whereas SecD/SecF and YajC improve the efficiency of translocation. In addition to ATP, ΔμH+ represents a second source of energy for the bacterial protein export.
In addition, E. coli contains a 4.5S RNA, a protein termed either Ffh or P48, and the FtsY protein, which are the structural homologues to the 7S RNA, the 54-kDa subunit of the mammalian SRP (signal recognition particle), and SRα (α-subunit of the SRP receptor), respectively (Poritz et al., 1988; Struck et al., 1988; Bernstein et al., 1989; Römisch et al., 1989). SRP and its receptor function in targeting precursor and membrane proteins to the membrane of the endoplasmic reticulum (recently reviewed by Rapoport et al., 1996). In view of the well characterized Sec apparatus, the physiological function of the bacterial SRP/SR homologues, all of which are essential for viability, has remained enigmatic for some time. Pulse-chase kinetics performed with conditional mutants of 4.5S RNA, Ffh (P48), and FtsY originally suggested an involvement of these components in the export of a subset of secretory proteins (Poritz et al., 1990; Phillips and Silhavy, 1992; Luirink et al., 1994). The effects observed, however, were only minor. They were interpreted as Ffh and FtsY being SecB-like chaperones. On the other hand, SRP/SR-like functions were inferred from the cross-linking of Ffh/4.5S RNA to signal sequences of eukaryotic (Luirink et al., 1992) and various bacterial nascent proteins (Valent et al., 1995, 1997). Similarly, replacing subunits of the eukaryotic SRP by the bacterial homologues led to partially active chimeric SRPs in assay systems developed for the mammalian SRP (Ribes et al., 1990; Bernstein et al., 1993).
Consistent with the observation that polytopic inner membrane proteins of E. coli integrate without the help of SecA (Werner et al., 1992; Yamato, 1992; MacFarlane and Müller, 1995), we demonstrated a decrease in the assembly of lactose permease when E. coli cells were partially deprived of 4.5S RNA and Ffh (MacFarlane and Müller, 1995). For the first time, these studies pointed toward an involvement of the bacterial SRP in the biogenesis of polytopic membrane proteins that are usually synthesized without cleavable signal sequences. The results were later confirmed by others (de Gier et al., 1996, 1998; Seluanov and Bibi, 1997) but also modified in a manner that only a subset of membrane proteins would be substrates of the bacterial SRP/SR (Ulbrandt et al., 1997).
We have now developed an SRP-dependent in vitro system from E. coli cells allowing the analysis of authentic activities of the bacterial SRP/SR. Using this in vitro system, we demonstrate the integration of two different E. coli membrane proteins by purified Ffh (P48), 4.5S RNA, and FtsY. Furthermore, these studies clearly discriminate between the SRP/SR-dependent integration of polytopic membrane proteins and the SecA/SecB requiring translocation of a secretory protein. Finally, the sensitivity of the in vitro system identifies mannitol permease (MtlA) as a substrate for Ffh/FtsY, although previously it did not score as an SRP target in an in vivo genetic screen (Ulbrandt et al., 1997).
MATERIALS AND METHODS
Strains, Plasmids, and Media
For conditional expression of FtsY, the E. coli strain N4156, harboring the plasmid pAra14-FtsY′, was used (Luirink et al., 1994). Subcloning the mtlA gene under the control of the SP6 phage promoter was performed by ligating the StuI–SalI fragment of pCD7.5 (Werner et al., 1992) into vector pSELECT (Promega, Madison, WI) cut with SmaI and SalI yielding plasmid pMtlII-9. Plasmid pDMB contains the ompA gene under the T7 phage promoter (Behrmann et al., 1998). Plasmid pJM8CS7, carrying the secY gene under the T7 phage promotor, was constructed by subcloning the KpnI–PstI fragment of pNO1573 (Akiyama and Ito, 1985) into pBluescript (Stratagene, La Jolla, CA), resulting in pJMsecY7. By site-directed mutagenesis CC was inserted in front of the ATG start codon to create a NcoI site. The resulting plasmid, pJMsecY73, was digested with NcoI/BamHI yielding a 1.6-kilobase (kb) fragment that was ligated into pET-3d (Novagen, Madison, WI). This plasmid was subsequently termed pJM8CS7 and used for in vitro expression of SecY. To obtain FtsY-depleted inside-out vesicles, E. coli N4156 pAra14-FtsY′ was grown overnight on media previously described by Müller and Blobel (1984), supplemented with 0.4% fructose and 0.2% l-arabinose and subcultered on the same medium containing only 0.4% fructose. The same strategy was used for the preparation of the FtsY-depleted subfraction of cytosolic translation factors (CTF). In contrast to the original protocol (Hoffschulte et al., 1994), the medium described by Müller and Blobel (1984) was used.
In Vitro Reactions
The components of the in vitro system, salt-washed ribosomes, initiation factors, and the CTF (formerly denoted UDF), were prepared as described previously (Hoffschulte et al., 1994). For obtaining RNA-free CTF, 500 μl of CTF were incubated with 600 μl DEAE-Sepharose CL-6B (Amersham Pharmacia Biotech, Arlington Heights, IL) that had been preequilibrated first with buffer A (50 mM triethanolamine acetate [TeaOAc] pH 7.5, 50 mM KCH3COO [KOAc], 5 mM Mg(CH3COO)2 [MgOAc]) containing 2 M KOAc and subsequently with buffer A. After several wash steps with buffer A, proteins were eluted with buffer A containing 600 mM KOAc, buffer-exchanged against buffer A, and concentrated twofold (with respect to the starting material) by ultrafiltration using Centricon 10 microconcentrators (Amicon, Danvers, MA). In vitro protein synthesis was performed in 25-μl aliquots according to Hoffschulte et al. (1994) with the following modifications: initiation factor 1 and 3 were omitted; the system was run in a coupled transcription/translation mode adding 5 U per reaction of SP6- and T7 RNA polymerase, respectively, together with 8 U of placental RNAse inhibitor. Incubations were for 30 min at 37°C. Inside-out inner membrane vesicles (INV) and translocation factors were added from the beginning. Gradient-purified inner membrane vesicles were obtained as described previously (Hoffschulte et al., 1994) and extracted with 6 M urea as detailed elsewhere (Helde et al., 1997).
Subfractionation of Translation Products
After chilling on ice, 50-μl samples were applied on a two-step sucrose gradient in an airfuge tube (Beckman, Fullerton, CA) consisting of 50 μl 0.77 M and 50 μl 1.44 M sucrose in 40 mM TeaOAc, pH 7.5, 70 mM KOAc, and 8 mM Mg(OAc)2, respectively. After a 30-min centrifugation at 30 psi in a A100/18 rotor at 4°C, the following fractions were sequentially withdrawn from the top: 80 μl representing soluble material and 70 μl to give the membrane fraction. The pelleted material was directly dissolved in 20 μl of SDS-PAGE loading buffer. Proteins contained in the supernatant fractions were precipitated by the addition of 1 vol 10% trichloroacetic acid.
Purification of SecA, SecB, F1-ATPase, Ffh (P48), and FtsY
The purification of SecA (Helde et al., 1997), SecB (Hoffschulte et al., 1994), and F1-ATPase (Müller et al., 1987) followed previously reported protocols. His-tagged Ffh (P48) was expressed from pDS12–48His6 in E. coli strain MC4100 containing the lac repressor-encoding pDMI,1 (Lentzen et al., 1994) and purified in a manner similar to that described by Lentzen and associates. LB-plates containing 0.4% (wt/vol) glucose, 100 μg/ml ampicillin, and 25 μg/ml kanamycin were inoculated from a glycerol stock and incubated overnight at 37°C. Several single colonies were used to inoculate 1-l batch cultures prepared with the above-mentioned medium and grown to an optical density of 0.3 at 578 nm when 1 mM isopropyl-β-d-thiogalactopyranoside was added. After an additional 2.5 h of growth, cells were harvested, washed with buffer B (50 mM TeaOAc, pH 8.0, 300 mM NaCl, 5 mM imidazol) and quickly frozen. After resuspension in buffer B at a 1:1 ratio, they were broken by three passages through a French Pressure Cell at 8000 psi, and an S-20 was prepared, which was applied in three batches to a 10-ml column of Ni2+-NTA Agarose (Qiagen, Chatsworth, CA) equilibrated with buffer B. The matrix was washed first with buffer B and subsequently with buffer B containing 40 mM imidazol until no more material absorbing at 280 nm was eluted and Ffh (P48) was desorbed within 3 ml of buffer B containing 150 mM imidazol. All buffers had been supplemented with 0.5 mg/ml Pefabloc. The eluate was fourfold concentrated by ultrafiltration and exchanged against buffer A. FtsY was purified according to Luirink et al. (1994) except that the first MonoQ column was replaced by Q-Sepharose. Polyclonal antibodies directed against SDS-PAGE–purified Ffh-His (P48) and FtsY were raised in rabbits.
Preparation and Analysis of 4.5S RNA and tRNA
For in vitro synthesis of 4.5S RNA, pT7/T3α19, carrying the 4.5S RNA coding sequence (Wood et al., 1992), was linearized with BamHI and transcribed for 2 h at 37° in the presence of 1 mM CTP, 1 mM GTP, 1 mM UTP, 1 mM ATP, 0.1 mg/ml BSA, 0.4 U/μl RNasin, 10 μg/ml pyrophosphatase, 0.25 U/μl T7-RNA Polymerase, 80 mM HEPES-NaOH, pH 7.5, 24 mM MgCl2, 2 mM spermidine, and 40 mM DTT. The reaction mixture was subsequentely treated for 30 min at 37° with RNase-free DNase (0.5 U/μl), extracted once with acid phenol, and precipitated with ethanol and 3 M NaOAc, pH 5.2. The RNA pellet was dissolved in H20 and adjusted to a concentration of 20 μg/ml.
Commercially available E. coli tRNA (Roche Diagnostics, Nutley, NJ) was purified by preparative native PAGE on 8% polyacrylamide gels prepared in TAE buffer. The 4S (tRNA) RNA bands were excised and the RNA was extracted from the gel pieces by treatment with 0.1 M NH4OAc, 2 mM MgOAc, 0.2 mM EGTA by end-over-end rotation at 4°C for several hours. The extracted RNAs were adsorbed to DEAE-Sepharose CL-6B as described above, eluted with 2 M NaOAc, pH 7.2, and precipitated by adding 2 vol of ethanol.
Northern blot analysis of glyoxal/DMSO-denaturated samples was performed as described (Sambrook et al., 1989). The oligodeoxynucleotide 5′-TGC TTC CTT CCG GAC CTG ACC TGG T-3′, which is complementary to the central portion of the conserved domain IV of the 4.5S RNA (Poritz et al., 1990), was end labeled with Digoxygenin-conjugated nucleotides (Roche Diagnostics) and used as hybridization probe for the detection of 4.5S RNA by alkaline phosphatase-coupled anti-Digoxygenin-Fabs and Lumigen-mediated chemiluminescence (Roche Diagnostics).
Preparation of OmpA::tRNA as a Translocation Intermediate
Ribosome-associated, nascent chains of pOmpA were synthesized with the β5-oligodeoxynucleotides as described earlier (Behrmann et al., 1998) in a 20-fold scaled-up reaction. Urea was added to 500 μl of reaction mixture to a final concentration of 8 M, and the mixture was incubated for 30 min at 4°C. After 1:1 dilution with translocation buffer (Behrmann et al., 1998), it was buffer exchanged against translocation buffer and concentrated twofold (with respect to the starting material) by ultrafiltration using Centricon 3 microconcentrators (Amicon). The OmpA::tRNA-containing mixture (250 μl) was incubated with 7.5 μl INV (10 eq; 1 eq is the amount used per 25 μl cell-free synthesis reaction) in the presence of 2.5 mM ATP, 8 mM creatine phosphate, 40 μg/ml creatine phosphokinase, 2 mM DTT for 15 min at 37°C. To remove the OmpA::tRNA portion not associated with the membranes, the entire mixture was separated by centrifugation through a 30% (wt/vol) sucrose cushion (in 40 mM TeaOAc, pH 7.5, 1 mM DTT) for 10 min at 30 psi in a Beckmann Airfuge. The supernatant was discarded and the pOmpA::tRNA-carrying INV were resuspended in 15 μl membrane buffer. For in vitro assays with OmpA and MtlA, 3 μl of these INV were used per 25 μl reaction mixture. As a control, the ribosome-associated nascent chains were treated with 1.5 mM puromycin for 15 min at 37°C to release the ribosomes and the tRNA before the urea treatment. They were subsequently isolated and incubated with INV as described above. These mock-INV were then employed for in vitro assays.
Sample Analysis and Quantification
All samples were analyzed on 13 or 15% SDS-polyacrylamide gels. Radiolabeled proteins were visualized by phosphorimaging using a Molecular Dynamics (Sunnyvale, CA) PhosphorImager and quantified using Imagequant software from Molecular Dynamics.
RESULTS
In Vitro Synthesis in the Absence of SRP/SR
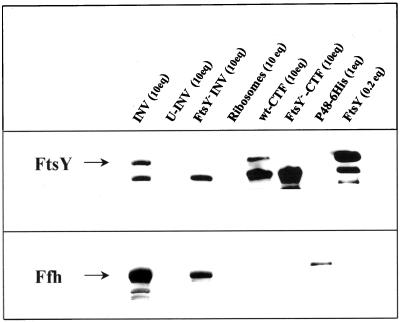
The primary goal of this work was to directly show the involvement of the E. coli SRP in the assembly of polytopic membrane proteins by reproducing the integration process in vitro with purified components. We have previously reported (Hoffschulte et al., 1994) the preparation of a distinct, SecA/B-deficient cell-free translation system from E. coli to follow translocation of secretory proteins into inside-out inner membrane vesicles (INV). This so-called reconstituted system (RCS) translates mRNA, which is transcribed from various promoters, by means of salt-extracted ribosomes, largely purified translation initiation factors, and a subfraction of cytosolic proteins enriched in the additional translation factors required (denoted CTF, cytosolic translation factors). The various components of this in vitro system were first analyzed by immunoblotting for the presence of Ffh (P48) and FtsY (Figure 1). Both, FtsY and Ffh (P48) exist in a membrane-associated and a soluble form (Luirink et al., 1994). In agreement with this, both proteins could be detected in INV but were removed by treatment of the INV with 6 M urea (U-INV) similar to what has been reported for SecA (Cunningham et al., 1989). The CTF, as the major fraction of soluble proteins of this in vitro system, contained no Ffh (P48) and, as reported previously (Hoffschulte et al., 1994), only traces of SecA and SecB. As such, it was strictly dependent on exogenously added SecA and SecB for translocation of a secretory protein into U-INV (see Figure 3B). The CTF, however, contained significant amounts of FtsY (Figure 1). If the CTF was therefore prepared from the conditional FtsY mutant N4156 pAra14-FtsY′ (Figure 1), a cell-free translation system was obtained, which at the same time lacks soluble SecA, SecB, Ffh (P48), and FtsY.
Figure 1.
Localization of the components of the E. coli SRP/SRP receptor in the cell-free integration system used. Immunoblot of the fractions indicated using rabbit antisera directed against Ffh (P48) and FtsY and a horseradish peroxidase-based enhanced chemiluminescence (ECL) detection system (Amersham). The same blot was subsequently probed with both antisera. One equivalent (eq) of a particular fraction is the amount used per 25 μl cell-free synthesis reaction. For P48–6His and FtsY, 1 eq corresponds to ∼0.2 and 0.5 μg protein, respectively. The molecular nature of the lower band detected by the FtsY antiserum even in FtsY-free samples is unknown. INV, Inside-out inner membrane vesicles; U-INV, urea-treated INV; FtsY−-INV, INV prepared from a conditional FtsY-mutant strain; CTF, cytosolic translation factors, prepared either from wild-type E. coli cells (wt-CTF) or from the conditional ftsY-mutant (FtsY−-CTF).
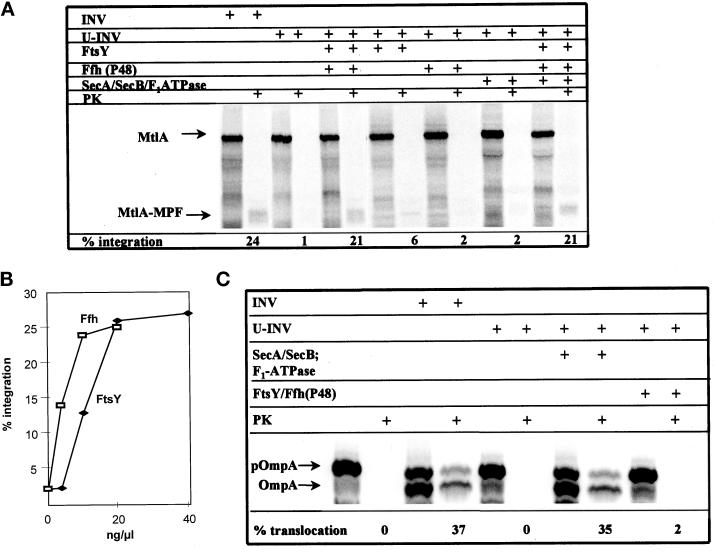
Figure 3.
The integration of a membrane protein into, and the translocation of a secretory protein across, the cytoplasmic membrane of E. coli have different molecular requirements. MtlA (A) and OmpA (C) were synthesized in vitro in the presence of the reagents indicated (INV, E. coli membrane vesicles; U-INV, urea-extracted INV; FtsY [20 ng/μl], Ffh [8 ng/μl], SecA [80 ng/μl], SecB [80 ng/μl] and F1-ATPase [40 ng/μl] indicate the purified proteins and the final concentrations used). For further details see Figure 2 legend. (B) The integration of MtlA into U-INV was analyzed in the presence of increasing amounts of Ffh (P48) while keeping the FtsY concentration constant and vice versa. Integration was quantified as detailed in Figure 2.
FtsY-depleted INV Are Exclusively Impaired in the Integration of Polytopic Membrane Proteins
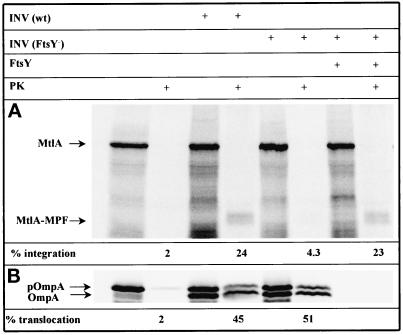
In a first set of experiments, a secretory and a membrane protein were synthesized by this FtsY- and Ffh (P48)-deprived in vitro system, and their translocation and integration were studied using INV prepared from the ftsY mutant cells. As a polytopic membrane protein we chose mannitol permease (MtlA). Due to a large cytoplasmic domain at its COOH terminus, the 60-kDa MtlA, when integrated into INV, is cleaved by proteinase K to leave a 30-kDa membrane-embedded, NH2-terminal fragment (Werner et al., 1992). The appearance of this membrane-protected fragment, called MPF, is thus an indication that the integration of MtlA into INV has occurred. As a secretory protein we used the outer membrane protein OmpA whose precursor (pOmpA) is partially converted to the signal sequence-free form (OmpA) by SecA-containing INV concomitantly with the translocation into the lumen of INV. The latter is indicated by the acquirement of resistance against proteinase K. As shown in Figure 2A, no partial resistance of MtlA toward proteinase K was visible when the protein was synthesized in the absence of INV. The presence of wild-type INV resulted in conversion of 24% of the input MtlA into the 30-kDa fragment (MtlA-MPF) resistant to proteinase K. The integration efficiency decreased to 4% when FtsY-depleted INV were used in this assay. This integration defect could be restored solely by addition of purified FtsY, demonstrating that FtsY is indispensable for the integration of MtlA. In contrast to the results obtained with MtlA, the translocation of the secretory protein pOmpA was not impaired by using FtsY-depleted INV (Figure 2B). For both wild-type and FtsY-depleted INV, basically the same translocation efficiency was observed. However, the translocation of pOmpA was diminished as well when FtsY-depleted INV were isolated from cells grown under FtsY-limiting conditions for an extended period of time (our unpublished results). This defect in translocation was most likely caused by a lack of the integral membrane protein SecY (see below). In summary, the complete removal merely of FtsY from the in vitro system resulted in a selective impairment of the integration of a polytopic membrane protein.
Figure 2.
Depletion of FtsY affects integration of membrane proteins but does not impair translocation of secretory proteins. MtlA and OmpA were synthesized in vitro by coupled transcription/translation of plasmids pMtlII-9, encoding mtlA under the SP6 promoter (A), and pDMB, coding for ompA under the T7 promoter (B), in a reconstituted cell-free translation system from E. coli (for further details see MATERIALS AND METHODS). Cell-free translation products labeled with [35S]methionine were either directly precipitated with trichloroacetic acid or only after incubation with 0.5 mg/ml proteinase K for 20 min at 25°C (PK). Thereafter, proteins were separated by SDS-PAGE (15% acrylamide) and visualized by phosphorimaging. Indicated are the positions of full-length MtlA, the fragment of MtlA resistant toward proteinase K (MtlA-MPF), the precursor (pOmpA), and the signal sequence-free form of OmpA. In vitro syntheses were performed in the presence of the reagents indicated at the top of the figure (INV (wt), wild-type E. coli inner-membrane vesicles; INV (FtsY−), membrane vesicles prepared from the conditional FtsY mutant E. coli N4156 pAra14-FtsY′; FtsY, purified FtsY [20 ng/μl final concentration]). The percentage of integration was calculated after quantitation of the radioactivity of individual protein bands using a Phosphorimager and calculating the ratio between MtlA-MPF and MtlA. The values thus obtained were corrected for the loss of Met residues occurring during cleavage by proteinase K. The percentage of translocation equals the ratio of radioactivity present in the proteinase K-resistant bands of pOmpA and OmpA and that recovered from the corresponding bands before proteolytic digestion (the INV used here usually give rise to some proteinase K-resistant pOmpA representing a fraction of uncleaved yet translocated precursor).
Both FtsY and Ffh (P48) Are Essential for the Integration of Polytopic Membrane Proteins
For demonstrating the involvement of Ffh (P48) in the integration of polytopic membrane proteins, urea-treated INV (U-INV) were employed. The use of U-INV in combination with the FtsY-depleted CTF yielded an in vitro system, which by immunoblotting was completely deficient in FtsY and Ffh (P48) (c.f. Figure 1). Under these conditions, the integration of MtlA into U-INV was almost completely abolished (Figure 3A). However, it could totally be restored by the addition of both FtsY and Ffh (P48). This restoration was dosage dependent, and the addition of FtsY and Ffh (P48) showed saturable behavior in this reaction (Figure 3B). Adding either Ffh (P48) or FtsY alone did not significantly improve the background level of integration of MtlA into U-INV, demonstrating that both components are essential for the integration of MtlA. Furthermore, supplementation of the RCS with SecA and SecB and restoration of the ΔμH+ with F1-ATPase had no effect on the integration process (Figure 3A): in the presence of these three components alone, no integration could be observed, and their addition in combination with Ffh (P48) and FtsY did not enhance the integration beyond the level achieved with FtsY and Ffh (P48) alone. This is completely consistent with our previous findings indicating that membrane integration of MtlA is independent of SecA, SecB, and F1-ATPase (Werner et al., 1992). On the other hand, the translocation of pOmpA into U-INV was strictly dependent on the addition of SecA, SecB, and F1-ATPase (Figure 3C). FtsY and Ffh (P48), however, had no stimulatory effect on the translocation of pOmpA into U-INV. In summary, the data obtained with this in vitro system reveal a clear-cut distinction between the molecular mechanisms of translocation of pOmpA into INV and the integration process of MtlA. The former requires SecA, SecB, and restoration of ΔμH+; the latter depends on at least FtsY and Ffh (P48). No overlapping specificities were observed between the translocation and the integration pathways of pOmpA and MtlA under these conditions.
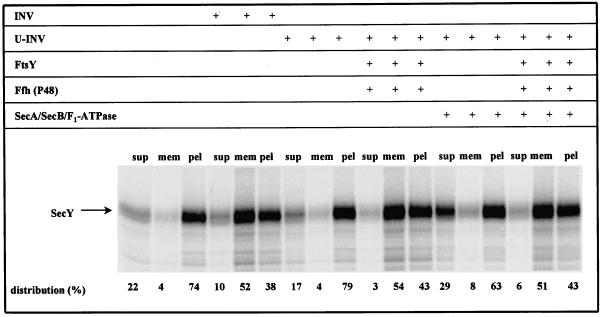
To further verify the requirement of Ffh (P48) and FtsY for the integration of polytopic membrane proteins, a second substrate was analyzed. During the preparation of INV from FtsY-depleted cells, we noticed a high sensitivity of SecY toward reduced FtsY concentrations, consistent with data published previously (Seluanov and Bibi, 1997). This suggested that the integration of SecY requires FtsY and, most likely, also Ffh (P48). In contrast to MtlA, the integration of SecY into INV could not be followed via protease protection assay because no discrete protease-resistant fragments of membrane-embedded SecY were obtained. Instead, the SecY translation products were subfractionated on a two-step sucrose gradient into soluble, membrane-associated and pelletable proteins. It had been shown previously for both SecY and LacY that cosedimentation of the in vitro synthesized membrane proteins with INV indeed reflects a functional integration into the membrane (Ahrem et al., 1989; Swidersky et al., 1992). In the absence of INV, only 4% of the SecY synthesized was recovered from the membrane fraction (Figure 4). This changed drastically by cotranslationally adding INV; under these conditions, 52% of the in vitro synthesized SecY cosedimented with the INV. That this cosedimentation with membranes requires functional interaction between SecY and the membranes was demonstrated by using U-INV. In the presence of these FtsY- and Ffh (P48)-free INV, membrane association dropped to the level of an INV-free assay. However, by providing FtsY and Ffh (P48), more than 50% of the SecY was again recovered from the U-INV. SecA, SecB, and F1-ATPase did not support association of SecY with U-INV, nor did their addition further increase the membrane association of SecY obtained in the presence of only FtsY and Ffh (P48). These data are equivalent to the ones observed with MtlA and confirm that in E. coli the SRP/SR pathway is specifically utilized by polytopic membrane proteins with no involvement of SecA, SecB, and the ΔμH+.
Figure 4.
SRP is also required for the membrane integration of SecY. SecY was synthesized using plasmid pJM8CS7, encoding secY under the T7 promotor, in the presence of the components indicated (for experimental details see legends of Figures 2 and 3). Membrane integration of newly synthesized SecY was analyzed by subfractionation of the translation products on a two-step sucrose gradient (see MATERIALS AND METHODS for details). Radioactivity present in the individual SecY bands was quantitated and the sum of the three subfractions was set at 100%.
The Effect of 4.5S RNA on the Integration of Membrane Proteins
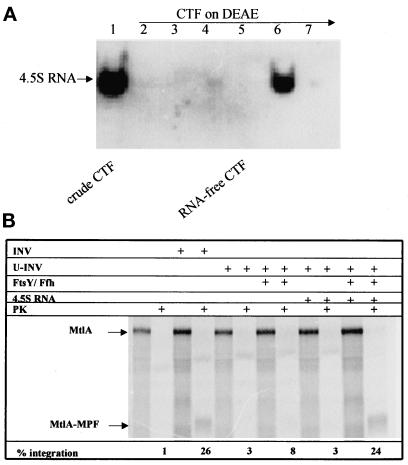
The 4.5S RNA together with Ffh (P48) constitutes the bacterial SRP. With the in vitro system thus far described, we had not been able to detect any stimulation of the integration process by the addition of 4.5S RNA (our unpublished results), most likely due to the significant amount of 4.5S RNA present in the CTF (Figure 5A). To further verify that this in vitro system reflects an authentic SRP-dependent system, the CTF was therefore treated with DEAE-Sepharose CL-6B as described in MATERIALS AND METHODS to quantitatively remove small RNAs (Figure 5A). This treatment of the CTF, however, rendered the in vitro system dependent on exogenously added tRNA. Because commercially available tRNA contained significant amounts of 4.5S RNA, pure tRNA had to be prepared by preparative polyacrylamide gel electrophoresis. The removal of 4.5 RNA from the CTF did not impair the synthesis of MtlA and its integration into INV (Figure 5B). However, in contrast to the data described in the previous section, the addition of FtsY and Ffh (P48) to U-INV was no longer sufficient to mediate efficient integration of MtlA in this 4.5S RNA-free system. Efficient integration of MtlA into U-INV now required the addition of 4.5S RNA together with Ffh (P48) and FtsY, demonstrating that all three components are essential for the integration of polytopic membrane proteins.
Figure 5.
Integration of MtLA into U-INV is dependent on 4.5S RNA. (A) Northern blot analysis using a 4.5S RNA probe (see MATERIALS AND METHODS for details). Lane 1, 25 μl of CTF (cytosolic translation factors); lane 2, flow-through (FT) of CTF applied to DEAE-Sepharose CL-6B; lane 3, 50 mM KOAc-eluate; lanes 4 and 5, 0.6 M KOAc-eluate before and after concentration by ultrafiltration, respectively; the latter was used as RNA-free CTF; lanes 6 and 7, 1 M and 2 M KOAc–eluates, respectively. (B) The RNA-free CTF after DEAE-treatment (lane 5) was used for in vitro synthesis of MtlA in the presence of 4.5S RNA-free tRNA (80 ng/μl final concentration) and the reagents indicated (INV, E. coli membrane vesicles; U-INV, urea-extracted INV; FtsY [20 ng/μl] and Ffh [8 ng/μl] indicate the purified proteins and the final concentrations used. 4.5S RNA, purified 4.5S RNA [1 ng/μl final concentration]; PK, Proteinase K [0.5 mg/ml final concentration]).
Secretory and Integral Membrane Proteins Use the Same Translocation Pore
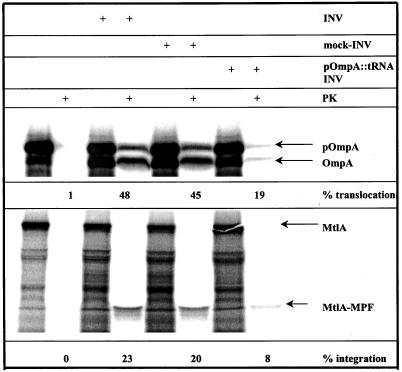
Substantial evidence exists that the SecYEG complex provides the translocation pore for secretory proteins (reviewed by Duong et al., 1997). In addition, an involvement of SecY in the integration of MtlA has been demonstrated (Werner et al., 1992). This was confirmed recently by a cross-linking approach demonstrating an interaction between the membrane protein FtsQ and components of the Sec translocon (Valent et al., 1998). In the same study, however, cross-links between FtsQ and SecA were identified, an observation that is not consistent with the SecA-independent integration of the polytopic membrane proteins MtlA and SecY described above. We therefore designed an in vitro competition assay to examine by functional means a SecY involvement in the integration of membrane proteins. Matlack et al. (1997) have recently described the use of tRNA-tethered polypeptides as translocation intermediates for studying Kar2p-dependent protein transport through the Sec61p channel in yeast. This technique was applied to generate a pOmpA derivative carrying a tRNA at its COOH terminus (pOmpA::tRNA). The fusion was synthesized in vitro by transcription/translation of the ompA gene in the presence of an oligonucleotide to generate 125-amino acid ribosome-associated nascent chains (Behrmann et al., 1998), which were subsequently released from the ribosomes with 8 M urea, leaving the peptidyl-tRNA bond intact. Upon translocation into INV, the bulky tRNA moiety should cause stalling of the translocating polypeptide, resulting in a blocked SecYEG translocation pore. Such INV were then isolated and assayed for translocation of pOmpA as a control and integration of MtlA (Figure 6). By using untreated INV, 48% of OmpA synthesized was proteinase K resistant, and almost the same translocation efficiency was observed with mock-treated INV, i.e., when the ribosome-associated nascent chains were treated with puromycin to release the ribosome and the tRNA before urea treatment and incubation with the membranes. This translocation efficiency, however, dropped to 19% when INV preincubated with pOmpA::tRNA were used. The decreased translocation efficiency indicates that pOmpA::tRNA, in fact, blocks the translocation pore of INV (a tight association of the pOmpA::tRNA intermediate with the SecYEG complex was further verified by an in vitro cross-linking approach [Beck and Müller, manuscript in preparation]). We consequently repeated this assay with MtlA as a substrate. Like for pOmpA, there was no significant difference in the integration efficiency between untreated and mock-treated INV. However, with INV preincubated with pOmpA::tRNA the integration efficiency decreased drastically to 8%, demonstrating that both integration of MtlA and translocation of pOmpA are dependent on the same translocation pore.
Figure 6.
Secretory and integral membrane proteins use the same translocon. pOmpA nascent chains were synthesized in the presence of an oligonucleotide causing a translational stop after 125 amino acids (Behrmann et al., 1998). They were subsequently released from the ribosomes by treatment with 8 M urea (Matlack et al., 1997), resulting in a pOmpA derivative carrying a tRNA at its COOH terminus. The tRNA-tethered OmpA (pOmpA::tRNA) was incubated with INV. After centrifugation through a sucrose cushion, these INV carrying the pOmpA::tRNA translocation intermediates (pOmpA::tRNA-INV) were resuspended and assayed for the translocation of OmpA and the integration of MtlA. As a control (mock-INV) the pOmpA nascent chains were incubated with puromycin to remove the ribosome and the tRNA before urea treatment and incubation with the membranes.
DISCUSSION
To understand the mode of action of the bacterial SRP the development of an in vitro system was mandatory. The major problem was removal of both the soluble and membrane-associated SRP/SR together with SecA/B from the transcription/translation system and the membranes. Only in the absence of SecA/B was an individual substrate specificity of SRP/SR likely to be recognized and vice versa. In an in vitro system simultaneously deficient in SRP/SR, SecA, and SecB, membrane integration of mannitol permease and SecY was strictly dependent on the presence of the bacterial SRP/SR, whereas SecA, SecB, and ΔμH+, all of which were found to be required for the efficient translocation of pOmpA under identical experimental conditions, were without any influence on the integration of the two membrane proteins. These results suggest that the SecA/SecB- and SRP-mediated pathways have different substrate specificities.
The results obtained with the in vitro system confirm by biochemical means the SRP-dependent integration of membrane proteins that had previously been suggested by studies with conditional mutants of the 4.5S RNA-, Ffh (P48)-, and FtsY-encoding genes (MacFarlane and Müller, 1995; de Gier et al., 1996, 1998; Seluanov and Bibi, 1997). The identification in vitro of MtlA as a substrate for SRP is in contradiction, however, to results of a genetic screen designed to detect potential substrates of the E. coli SRP (Ulbrandt et al., 1997). In that study, which classified E. coli inner-membrane proteins according to their strong, moderate, and missing susceptibility toward a lack of Ffh (P48), MtlA did not score as a substrate of SRP. Possibly, if MtlA is a membrane protein requiring less SRP than others for its assembly, it would likely be missed by an in vivo approach biased by residual levels of SRP in the depletion strain.
An integration of polytopic membrane proteins that depends exclusively on SRP is also in line with earlier reports that SecA was not required for this process. Independence of SecA was described for mannitol and lactose permease, both in vitro (Werner et al., 1992) and in vivo (Werner et al., 1992; Yamato, 1992; MacFarlane and Müller, 1995). In contrast, MalF, originally also reported to be a SecA-independent protein (McGovern and Beckwith, 1991), was found more recently to require SecA for proper membrane assembly (Traxler and Murphy, 1996). MalF is different from many multispanning membrane proteins in that it contains an ∼180-amino acid long periplasmic loop. This is reminiscent of the membrane-anchored leader peptidase harboring a large periplasmic tail, which is the only domain of the protein that renders it dependent on SecA (Wolfe et al., 1985): no SecA is needed for integration when the protein is forced to insert in an inverted orientation (von Heijne, 1989). Membrane integration of the inverted leader peptidase, however, still needs SRP for integration (de Gier et al., 1996, 1998) as does the wild-type protein. Similarly, a cross-linking approach recently revealed an interaction between SecA and the membrane protein FtsQ (Valent et al., 1998). Again, FtsQ possesses an extended periplasmic domain, which would explain an involvement of SecA in its assembly. Neither MtlA nor SecY was found here to require SecA for membrane integration in vitro, indicating that polytopic membrane proteins without substantial periplasmic moieties require exclusively SRP for their assembly.
Original studies with conditional ffh (Phillips and Silhavy, 1992) and ftsY (Luirink et al., 1994) mutants had suggested that deprivation of SRP can interfere also with the translocation of some, particularly SecB-independent, signal sequence-bearing proteins. However, since SecY is a strictly SRP-dependent protein, any SRP/SR depletion of cells will ultimately lead to reduced levels of SecY and thereby negatively affect also the translocation of proteins otherwise targeted by SecA/B. Similarly, by use of an in vitro system composed of wheat germ ribosomes and mammalian microsomes, 4.5S RNA, Ffh (P48), and FtsY were found recently to be sufficient to yield cotranslational targeting of a eukaryotic secretory protein (Powers and Walter, 1997). However, nascent chains assembled on wheat germ ribosomes do not necessarily exhibit the authentic properties found in a homologous bacterial environment (Valent et al., 1997; Behrmann et al., 1998). The idea that the bacterial SRP represents a specialized targeting mechanism for membrane proteins is further supported by the finding that only the more hydrophobic signal sequences of E. coli proteins, such as those present in membrane proteins, efficiently cross-link to Ffh (P48) in vitro (Valent et al., 1997). Moreover, in chloroplasts, which have evolved from bacterial ancestors, a homologue of the 54-kDa subunit of the mammalian SRP is specifically involved in the integration of a protein into thylakoidal membranes (Li et al., 1995). Collectively, several lines of evidence now support the view that in E. coli, SRP and SecA/SecB possess independent targeting specificities for polytopic membrane and secreted proteins, respectively, with no detectable overlaps.
Our results clearly demonstrate that Ffh (P48) and FtsY are essential for the integration of polytopic membrane proteins. The E. coli 4.5S RNA constitutes a third component of the bacterial SRP/SR system that is required for this process. It has been shown that 4.5S RNA and Ffh (P48) form a particle (Poritz et al., 1990; Ribes et al., 1990; Lentzen et al., 1994) to which FtsY binds in vitro (Miller et al., 1994). We have examined the localization of the 4.5S RNA within the various subfractions of our in vitro translation system and identified the CTF as a major source of the 4.5S RNA. Removal of 4.5S RNA did not significantly affect translation of MtlA. This observation argues against a general role of the 4.5S RNA in the translation process. Such a function was concluded from the findings that a deficiency of 4.5S RNA is suppressed by mutations mapping to translation factors (Brown, 1987, 1989) and that protein-synthesizing, total E. coli extracts prepared from 4.5S RNA-deficient strains showed less translational activity toward a natural mRNA which, however, could not be overcome by the addition of pure 4.5S RNA (Bourgaize and Fournier, 1987). Recently, specific binding of 4.5S RNA to elongation factor EF-G was reported (Nakamura et al., 1999). Although we cannot rule out the occurrence of residual amounts of this RNA in our subfractionated system, the important result is that the addition of pure 4.5S RNA did not affect translation but allowed integration of MtlA. Our results therefore suggest that the major function of 4.5S RNA is the formation of the bacterial SRP together with P48 (Ffh) involved in the integration of membrane proteins. An interesting speculation would be that the observed suppressor mutations developing under limitation of 4.5S RNA actually help the cell to prevent the accumulation of nonintegrated membrane proteins by slowing down the rate of elongation of their mRNAs. That such a situation would be detrimental is indicated solely by the development of a heat shock response when 4.5S RNA becomes limiting (Bourgaize et al., 1990; Poritz et al., 1990).
Considering that two independent targeting processes are active in E. coli the question arises whether both use the same translocation pore. In eukaryotic cells, the Sec61 complex mediates both the translocation of proteins into the lumen of the endoplasmic reticulum as well as the integration of proteins into the ER membrane (Rapoport et al., 1996). There might be, however, some modulation in the exact composition of the translocation pore, depending on whether cotranslational or posttranslational protein transport is mediated (Rapoport et al., 1996). In E. coli, the SecYEG complex provides the translocation pore for secretory proteins. The involvement of SecY in the integration of membrane proteins has been deduced from impaired MtlA activity in a conditional secY mutant (Werner et al., 1992), by obtaining cross-links between two membrane proteins and SecY (Valent et al., 1998) and by the observation of synthetic lethality in a conditional secY mutant under reduced Ffh (P48) concentrations (Newitt and Bernstein, 1998). Our experimental approach using a blocked translocation pore now extends these initial observations and provides direct evidence that the translocation of OmpA, as well as the integration of MtlA, depends on the same translocation pore.
ACKNOWLEDGMENTS
We gratefully acknowledge Dr. B. Dobberstein and Dr. Joen Luirink for providing plasmids pDS12–48His6 and pET9-FtsY; Dr. P. Werner for plasmid pMtlII-9; and M. Behrmann for plasmid pDMB. This work was supported by the Sonderforschungsbereiche 184 and 388 and the Fonds der chemischen Industrie.
REFERENCES
- Ahrem B, Hoffschulte HK, Müller M. In vitro membrane assembly of a polytopic, transmembrane protein results in an enzymatically active conformation. J Cell Biol. 1989;108:1637–1646. doi: 10.1083/jcb.108.5.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama Y, Ito K. The SecY membrane component of the bacterial protein export machinery: analysis by new electrophoretic methods for integral membrane proteins. EMBO J. 1985;4:3351–3356. doi: 10.1002/j.1460-2075.1985.tb04088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrmann M, Koch H-G, Hengelage T, Wieseler B, Hoffschulte HK, Müller M. Requirements for the translocation of elongation-arrested, ribosome-associated OmpA across the plasma membrane of Escherichia coli. J Biol Chem. 1998;273:13898–13904. doi: 10.1074/jbc.273.22.13898. [DOI] [PubMed] [Google Scholar]
- Bernstein HD, Poritz MA, Strub K, Hoben PJ, Brenner S, Walter P. Model for signal sequence recognition from amino acid sequence of 54K subunit of signal recognition particle. Nature. 1989;340:482–486. doi: 10.1038/340482a0. [DOI] [PubMed] [Google Scholar]
- Bernstein HD, Zopf D, Freymann DM, Walter P. Functional substitution of the signal recognition particle 54-kDa subunit by its Escherichia coli homolog. Proc Natl Acad Sci USA. 1993;90:5229–5233. doi: 10.1073/pnas.90.11.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgaize DB, Fournier MJ. Initiation of translation is impaired in E. coli cells deficient in 4.5S RNA. Nature. 1987;325:281–284. doi: 10.1038/325281a0. [DOI] [PubMed] [Google Scholar]
- Bourgaize DB, Phillips TA, Van Bogelen RA, Jones PG, Neidhardt FC, Fournier MJ. Loss of 4.5S RNA induces the heat shock response and lambda prophage in Escherichia coli. J Bacteriol. 1990;172:1151–1154. doi: 10.1128/jb.172.2.1151-1154.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. Mutations in the gene for EF-G reduce the requirement for 4.5S RNA in the growth of E. coli. Cell. 1987;49:825–833. doi: 10.1016/0092-8674(87)90620-9. [DOI] [PubMed] [Google Scholar]
- Brown S. Time of action of 4.5S RNA in Escherichia coli translation. J Mol Biol. 1989;209:79–90. doi: 10.1016/0022-2836(89)90171-x. [DOI] [PubMed] [Google Scholar]
- Cunningham K, Lill R, Crooke E, Rice M, Moore K, Wickner W, Oliver D. SecA protein, a peripheral protein of the Escherichia coli plasma membrane, is essential for the functional binding and translocation of proOmpA. EMBO J. 1989;8:955–959. doi: 10.1002/j.1460-2075.1989.tb03457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gier JWL, Mansournia P, Valent QA, Phillips GJ, Luirink J, von Heijne G. Assembly of a cytoplasmic membrane protein in Escherichia coli is dependent on the signal recognition particle. FEBS Lett. 1996;399:307–309. doi: 10.1016/s0014-5793(96)01354-3. [DOI] [PubMed] [Google Scholar]
- de Gier JWL, Scotti PA, Sääf A, Valent QA, Kuhn A, Luirink J, von Heijne G. Differential use of the signal recognition particle translocase targeting pathway for inner membrane protein assembly in Escherichia coli. Proc Natl Acad Sci USA. 1998;95:14646–14651. doi: 10.1073/pnas.95.25.14646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong F, Eichler J, Price A, Leonard MR, Wickner W. Biogenesis of the Gram-negative bacterial envelope. Cell. 1997;91:567–573. doi: 10.1016/s0092-8674(00)80444-4. [DOI] [PubMed] [Google Scholar]
- Helde R, Wieseler B, Wachter E, Neubüser A, Hoffschulte HK, Hengelage T, Schimz KL, Stuart RA, Müller M. Comparative characterization of SecA from the α-subclass purple bacterium Rhodobacter capsulatus and Escherichia coli reveals differences in membrane and precursor specificity. J Bacteriol. 1997;179:4003–4012. doi: 10.1128/jb.179.12.4003-4012.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffschulte HK, Drees B, Müller M. Identification of a soluble SecA/SecB-complex by means of a subfractionated cell-free export system. J Biol Chem. 1994;269:12833–12839. [PubMed] [Google Scholar]
- Lentzen G, Dobberstein B, Wintermeyer W. Formation of SRP-like particle induces a conformational change in E. coli 4.5S RNA. FEBS Lett. 1994;348:233–238. doi: 10.1016/0014-5793(94)00599-0. [DOI] [PubMed] [Google Scholar]
- Li X, Henry R, Yuan J, Cline K, Hoffman NE. A chloroplast homologue of the signal recognition particle subunit SRP54 is involved in the posttranslational integration of a protein into thylakoid membranes. Proc Natl Acad Sci USA. 1995;92:3789–3793. doi: 10.1073/pnas.92.9.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luirink J, High S, Wood H, Giner A, Tollervey D, Dobberstein B. Signal-sequence recognition by an Escherichia coli ribonucleoprotein complex. Nature. 1992;359:741–743. doi: 10.1038/359741a0. [DOI] [PubMed] [Google Scholar]
- Luirink J, ten Hagen-Jongman CM, van der Weijden CC, Oudega B, High S, Dobberstein B, Kusters R. An alternative protein targeting pathway in Escherichia coli: studies on the role of FtsY. EMBO J. 1994;13:2289–2296. doi: 10.1002/j.1460-2075.1994.tb06511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane J, Müller M. The functional integration of a polytopic membrane protein of Escherichia coli is dependent on the bacterial signal recognition particle. Eur J Biochem. 1995;233:766–771. doi: 10.1111/j.1432-1033.1995.766_3.x. [DOI] [PubMed] [Google Scholar]
- Matlack KES, Plath K, Misselwitz B, Rapoport TA. Protein transport by purified yeast Sec complex and Kar2p without membranes. Science. 1997;277:938–941. doi: 10.1126/science.277.5328.938. [DOI] [PubMed] [Google Scholar]
- McGovern K, Beckwith J. Membrane insertion of the Escherichia coli MalF protein in cells with impaired secretion machinery. J Biol Chem. 1991;266:20870–20876. [PubMed] [Google Scholar]
- Miller JD, Bernstein HD, Walter P. Interaction of E. coli Ffh/4.5S ribonucleoprotein and FtsY mimics that of mammalian signal recognition particle and its receptor. Nature. 1994;367:657–659. doi: 10.1038/367657a0. [DOI] [PubMed] [Google Scholar]
- Müller M, Blobel G. In vitro translocation of bacterial proteins across the plasma membrane of Escherichia coli. Proc Natl Acad Sci USA. 1984;81:7421–7425. doi: 10.1073/pnas.81.23.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M, Fisher RP, Rienhöfer-Schweer A, Hoffschulte HK. DCCD inhibits protein translocation into plasma membrane vesicles from Escherichia coli at two different steps. EMBO J. 1987;6:3855–3861. doi: 10.1002/j.1460-2075.1987.tb02723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Fujii Y, Shibata T, Yamane K. Depletion of Escherichia coli 4.5S RNA leads to an increase in the amount of protein elongation factor EF-G associated with ribosomes. Eur J Biochem. 1999;259:543–550. doi: 10.1046/j.1432-1327.1999.00077.x. [DOI] [PubMed] [Google Scholar]
- Newitt JA, Bernstein HD. A mutation in the Escherichia coli secY gene that produces distinct effects on inner membrane protein insertion and protein export. J Biol Chem. 1998;20:12451–12456. doi: 10.1074/jbc.273.20.12451. [DOI] [PubMed] [Google Scholar]
- Phillips GJ, Silhavy TJ. The E. coli ffh gene is necessary for viability and efficient protein export. Nature. 1992;359:744–746. doi: 10.1038/359744a0. [DOI] [PubMed] [Google Scholar]
- Poritz MA, Bernstein HD, Strub K, Zopf D, Wilhelm H, Walter P. An E. coli ribonucleoprotein containing 4.5S RNA resembles mammalian signal recognition particle. Science. 1990;250:1111–1117. doi: 10.1126/science.1701272. [DOI] [PubMed] [Google Scholar]
- Poritz MA, Strub K, Walter P. Human SRP RNA and E. coli 4.5S RNA contain a highly homologous structural domain. Cell. 1988;55:4–6. doi: 10.1016/0092-8674(88)90003-7. [DOI] [PubMed] [Google Scholar]
- Powers T, Walter P. Cotranslational protein targeting catalyzed by the Escherichia coli signal recognition particle and its receptor. EMBO J. 1997;16:4880–4886. doi: 10.1093/emboj/16.16.4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport TA, Jungnickel B, Kutay U. Protein transport across the eukaryotic endoplasmic reticulum and bacterial inner membranes. Annu Rev Biochem. 1996;65:271–303. doi: 10.1146/annurev.bi.65.070196.001415. [DOI] [PubMed] [Google Scholar]
- Ribes V, Römisch K, Giner A, Dobberstein B, Tollervey D. E. coli 4.5S RNA is part of a ribonucleoprotein particle that has properties related to signal recognition particle. Cell. 1990;63:591–600. doi: 10.1016/0092-8674(90)90454-m. [DOI] [PubMed] [Google Scholar]
- Römisch K, Webb J, Herz J, Prehn S, Frank R, Vingron M, Dobberstein B. Homology of 54K protein of signal recognition particle, docking protein and two E. coli proteins with putative GTP-binding domains. Nature. 1989;340:478–482. doi: 10.1038/340478a0. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning –A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Seluanov A, Bibi E. FtsY, the prokaryotic signal recognition particle receptor homologue, is essential for biogenesis of membrane proteins. J Biol Chem. 1997;272:2053–2055. doi: 10.1074/jbc.272.4.2053. [DOI] [PubMed] [Google Scholar]
- Struck JCR, Toschka HY, Specht T, Erdmann VA. Common structural features between eukaryotic 7SL RNAs, eubacterial 4.5S RNA and scRNA and archaebacterial 7S RNA. Nucleic Acids Res. 1988;16:7740. doi: 10.1093/nar/16.15.7740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swidersky UE, Rienhöfer-Schweer A, Werner PK, Ernst F, Benson SA, Hoffschulte HK, Müller M. Biochemical analysis of the biogenesis and function of the Escherichia coli export factor SecY. Eur J Biochem. 1992;207:803–811. doi: 10.1111/j.1432-1033.1992.tb17111.x. [DOI] [PubMed] [Google Scholar]
- Traxler B, Murphy C. Insertion of the polytopic membrane protein MalF is dependent on the bacterial secretion machinery. J Biol Chem. 1996;271:12394–12400. doi: 10.1074/jbc.271.21.12394. [DOI] [PubMed] [Google Scholar]
- Ulbrandt ND, Newitt JA, Bernstein HD. The E. coli signal recognition particle is required for the insertion of a subset of inner membrane proteins. Cell. 1997;88:187–196. doi: 10.1016/s0092-8674(00)81839-5. [DOI] [PubMed] [Google Scholar]
- Valent QA, de Gier JWL, von Heijne G, Kendall DA, ten Hagen-Jongman C, Oudega B, Luirink J. Nascent membrane and presecretory proteins synthesized in Escherichia coli associate with signal recognition particle and trigger factor. Mol Microbiol. 1997;25:53–64. doi: 10.1046/j.1365-2958.1997.4431808.x. [DOI] [PubMed] [Google Scholar]
- Valent QA, Kendall DA, High S, Kusters R, Oudega B, Luirink J. Early events in preprotein recognition in E. coli: interaction of SRP and trigger factor with nascent polypeptides. EMBO J. 1995;14:5494–5505. doi: 10.1002/j.1460-2075.1995.tb00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valent QA, Scotti PA, High S, de Gier JWL, von Heijne G, Lentzen G, Wintermeyer W, Oudega B, Luirink J. The Escherichia coli SRP and SecB targeting pathways converge at the translocon. EMBO J. 1998;17:2504–2512. doi: 10.1093/emboj/17.9.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Control of topology and mode of assembly of a polytopic membrane protein by positively charged residues. Nature. 1989;341:456–458. doi: 10.1038/341456a0. [DOI] [PubMed] [Google Scholar]
- Wolfe PB, Rice M, Wickner W. Effects of two sec genes on protein assembly into the plasma membrane of Escherichia coli. J Biol Chem. 1985;260:1836–1841. [PubMed] [Google Scholar]
- Wood H, Luirink J, Tollervey D. Evolutionary conserved nucleotides within the E. coli 4.5S RNA are required for association with P48 in vitro and for optimal function in vivo. Nucleic Acid Res. 1992;20:5919–5925. doi: 10.1093/nar/20.22.5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner PK, Saier MH, Jr, Müller M. Membrane insertion of the mannitol permease of Escherichia coli occurs under conditions of impaired SecA function. J Biol Chem. 1992;267:24523–24532. [PubMed] [Google Scholar]
- Yamato I. Membrane assembly of lactose permease of Escherichia coli. J Biochem. 1992;111:444–450. doi: 10.1093/oxfordjournals.jbchem.a123777. [DOI] [PubMed] [Google Scholar]