Abstract
Gram-negative bacilli with acquired metallo-β-lactamase (MBL) production have been increasingly reported in some countries, necessitating their detection. The aim of this study was to evaluate the performance of the Hodge test and those of the imipenem (IPM)-EDTA, ceftazidime (CAZ)-mercaptopropionic acid (MPA), and CAZ-sodium mercaptoacetic acid (SMA) double-disk synergy tests (DDSTs). The efficiencies of testing CAZ-resistant and IPM-nonsusceptible isolates were also compared. Strains used for the evaluation were known IMP-1 and VIM-2 MBL-producing isolates and consecutive and CAZ-nonsusceptible isolates of pseudomonads and acinetobacters. The performance of the Hodge test was improved by addition of zinc sulfate (140 μg/disk) to an IPM disk. In DDSTs, EDTA (ca. 1,900 μg) disks were better at detecting MBL-producing strains among pseudomonads, while MPA (3 μl) and SMA (3 mg) disks performed better for acinetobacters. EDTA (ca. 750 μg)-plus-SMA (ca. 2 mg) disks performed better than EDTA, MPA, or SMA disks with both organisms. CAZ-SMA DDSTs failed to detect 22 of 80 (28%) MBL-producing acinetobacters. In conclusion, use of an IPM disk and an EDTA (750 μg)-plus-SMA (2 mg) disk improves performance, and testing IPM-nonsusceptible isolates rather than CAZ-resistant isolates could reduce screening work. Further evaluation of the test is required for the detection of other types of MBL-producing gram-negative bacilli.
Carbapenems are often used as antibiotics of last resort for treating infections due to multidrug-resistant gram-negative bacilli, because they are stable even in response to extended-spectrum and AmpC β-lactamases. However, gram-negative bacilli producing the acquired metallo-β-lactamases (MBLs) IMP and VIM have been increasingly reported in Asia and Europe (4, 6, 8, 9, 19), and more recently, they have been detected in Canada (5) and the United States (M. A. Tolman, K. Rolston, R. N. Jones, and T. R. Walsh, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. C1-1851, 2002). Another type, SPM-1, has been reported in South America (18).
The MBLs efficiently hydrolyze all β-lactams, except for aztreonam, in vitro (2). Therefore, detection of MBL-producing gram-negative bacilli is crucial for the optimal treatment of patients and to control the spread of resistance (14). However, NCCLS documents (11) do not yet contain a method for detection of MBL-producing isolates.
Lee et al. have reported that the Hodge test can be used to screen carbapenemase-producing gram-negative bacilli and that the imipenem (IPM)-EDTA double-disk synergy test (DDST) can distinguish MBL-producing from MBL-nonproducing gram-negative bacilli (7). Arakawa et al. (1) have reported a DDST using a ceftazidime (CAZ) and a 2-mercaptopropionic acid (MPA) disk. Recently, a commercial disk (Eiken Co., Tokyo, Japan) containing 3 mg of sodium mercaptoacetic acid (SMA) became available, obviating handling of the disagreeable reagent MPA.
Our DDST (7) and that of Arakawa et al. (1) were based on studies of VIM-2 and IMP-1 MBL-producing isolates, respectively. The two tests were also different in terms of the β-lactams and chelating agents used: IPM versus CAZ and EDTA versus MPA, respectively. We tested IPM-nonsusceptible isolates for detection, while Arakawa et al. (1) tested CAZ-resistant isolates. With the appearance of IMP-1 MBL, in addition to VIM-2 MBL, which was already prevalent in Korea, reevaluation of our method became necessary. Application of the Hodge test to a large number of isolates revealed occasional false-negative results, indicating the need to modify the test (K. Lee, Y. S. Lim, D. Yong, J. H. Yum, and Y. Chong, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. D-529, 2002).
The aims of this study were to modify the Hodge test so as to improve its performance, to determine the effects of different kinds of β-lactams and chelating agents on the DDSTs for detection of pseudomonad and acinetobacter isolates producing either the VIM or the IMP type of MBL, and finally to compare the efficiencies of testing CAZ-resistant and IPM-nonsusceptible isolates.
MATERIALS AND METHODS
Evaluation of the Hodge test.
The Hodge test was performed as described previously (7) by using known MBL-producing isolates and both consecutive and CAZ-nonsusceptible isolates of Pseudomonas aeruginosa and acinetobacters. Briefly, the indicator organism, Escherichia coli ATCC 25922, at a turbidity of 0.5 McFarland standard, was used to swab inoculate the surface of a Mueller-Hinton agar plate (Becton Dickinson, Cockeysville, Md.), and the test strain was heavily streaked from the center to the plate periphery. After the plate was allowed to stand for 15 min at room temperature, a 10-μg IPM disk (Becton Dickinson) was placed at the center, and the plate was incubated overnight. The presence of a distorted inhibition zone was interpreted as a positive result for carbapenem hydrolysis screening. To determine the effect of zinc ions on the test, a 50 mM zinc sulfate solution (ZnSO4 · 7H2O; Junsei Chemical, Tokyo, Japan) was added in amounts of 5 to 20 μl (ca. 70 to 280 μg) to an IPM disk or in amounts of 0.5 to 50 μl/ml (ca. 7 to 700 μg) to the Mueller-Hinton agar.
Comparison of the performances of different DDSTs.
Strains used for the evaluation of DDSTs were stock cultures of known MBL-producing strains of pseudomonads and acinetobacters isolated from 1995 to 2001 at a tertiary-care hospital in Korea. Transconjugants of P. aeruginosa and one isolate each of MBL-producing Serratia marcescens and Achromobacter xylosoxidans subsp. denitrificans were also used.
The chelating agents used for DDSTs were EDTA (a 0.5 M solution used for molecular biology), MPA (Sigma Chemical Co., St. Louis, Mo.), and SMA (Sigma Chemical Co.). To evaluate the performance of the SMA disk, a commercial product containing 3 mg of SMA (Eiken Co.) was used. A mixture of 4 volumes of 0.5 M EDTA plus 6 volumes of a 300-mg/ml SMA solution (EDTA + SMA) was prepared.
Test strains were adjusted to the McFarland 0.5 standard and used to inoculate Mueller-Hinton agar plates (7). Depending on the test, a 10-μg IPM disk or a 30-μg CAZ disk (Becton Dickinson) was placed on the plate, and a blank filter paper disk or an SMA disk was placed at a distance of 10 mm (edge to edge). To the blank disk, either 10 μl of a 0.5 M EDTA solution (ca. 1,900 μg of disodium salt, dihydrate), 2 or 3 μl of MPA (1, 7), or 10 μl of an EDTA + SMA solution (ca. 750 μg of EDTA and ca. 2 mg of SMA per disk) was added. After overnight incubation, the presence of even a small synergistic inhibition zone was interpreted as positive, but the manufacturer's recommended interpretation criterion for the CAZ-SMA test was not followed. Production of MBL by the DDST-positive isolates was confirmed by PCR amplification of blaIMP-1 and blaVIM-2 alleles as described previously (3, 13).
Efficiency of screening and susceptibility of isolates.
Consecutive isolates and CAZ-nonsusceptible isolates of P. aeruginosa and acinetobacters recovered in 2001 at the same hospital were tested by the Hodge test and DDSTs to detect the presence of MBL-producing strains among IPM-susceptible isolates. Routine NCCLS disk diffusion test (11) data for the year 2000 at the hospital were used to determine the efficiency of testing CAZ-resistant versus IPM-nonsusceptible isolates for the screening of MBL-producing isolates.
RESULTS
Evaluation of the Hodge test.
Among the 39 MBL-producing P. aeruginosa and acinetobacter isolates tested by the original Hodge method, 26 gave positive results, 10 gave equivocal results, and 3 gave negative results (data not shown). All of the initially equivocal or false-negative results became positive when the IPM disks were supplemented with 10 μl of 50 mM zinc sulfate (140 μg/disk). Addition of more than 280 μg of zinc sulfate resulted in light growth of the indicator organism in an inhibition zone, which made interpretation more difficult. Alternatively, addition of zinc sulfate to Mueller-Hinton agar to a final concentration of 70 μg/ml also improved test performance.
Comparison of the performances of different DDSTs.
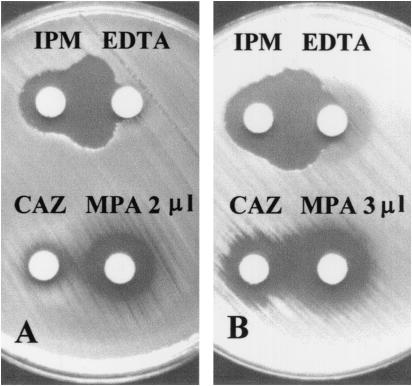
In the first phase of the study, the performances of the IPM-EDTA and CAZ-MPA DDSTs were compared by using known VIM-2-producing strains of 52 pseudomonads and 6 transconjugants (data not shown). All of these strains were synergy positive by both the IPM-EDTA and CAZ-MPA (3 μl of MPA/disk) tests. However, use of a disk with 2 μl of MPA failed to detect three MBL-producing isolates (Fig. 1 and data not shown).
FIG. 1.
Performances of DDSTs using disks of IPM or CAZ and disks of EDTA (1,900 μg) or MPA. Panels A and B show results for two different P. aeruginosa isolates. An IPM disk and an EDTA disk produced large synergistic inhibition zones for both isolates (A, top, and B, top). A CAZ disk and a 2-μl MPA disk failed to produce a synergistic zone (A, bottom), but a CAZ disk and a 3-μl MPA disk produced a small synergistic zone (B, bottom).
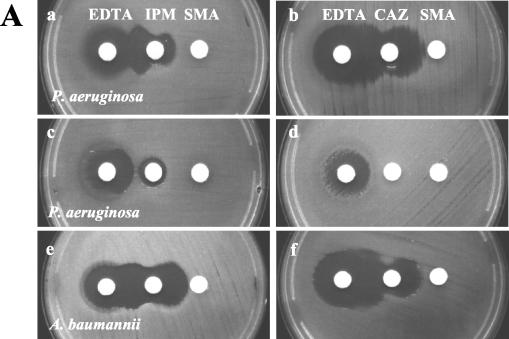
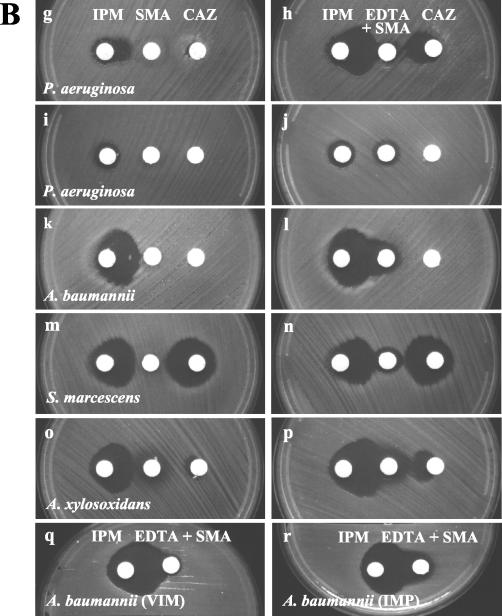
The second phase of the study became necessary because the commercial SMA disks became available after the completion of the first phase of the study. IPM-EDTA, IPM-SMA, and CAZ-SMA DDSTs were compared by using 19 and 80 isolates of known MBL-producing pseudomonads and acinetobacters, respectively. Typical DDST patterns are shown in Fig. 2. Among the isolates, all pseudomonads had blaVIM-2 alleles, while 42 and 38 acinetobacters had blaVIM-2 and blaIMP-1 alleles, respectively (Table 1). All of the 19 pseudomonads showed large synergistic inhibition zones by the IPM-EDTA test, but 2 isolates were negative by both the IPM-SMA and CAZ-SMA tests. All of the 80 MBL-producing acinetobacters (mostly Acinetobacter baumannii) were positive by the IPM-SMA DDSTs, but by the IPM-EDTA test, 5 (6%) were negative and synergistic zones were often smaller. The CAZ-SMA test failed to detect 22 (28%) isolates of MBL-producing acinetobacters.
FIG. 2.
Performances of DDSTs with different β-lactams and chelating agents. (A) Synergistic inhibition zones tended to be larger with EDTA (1,900 μg) disks for P. aeruginosa (a and b), while they tended to be larger with SMA (3 mg) disks for Acinetobacter spp. (e and f). EDTA disks occasionally produced small, suspect synergistic zones with MBL-nonproducing isolates (c and d), but SMA (3 mg) disks alone did not inhibit growth (a to f). (B) The synergistic inhibition zones produced by an IPM disk and an EDTA (750 μg) + SMA (2 mg) disk were large for all MBL-producing isolates (h, l, n, and p), but with an IPM disk and an SMA (3 mg) disk, a small zone was produced for an isolate of P. aeruginosa (g). The MBL-nonproducing isolate which showed an equivocal zone (c) was not inhibited by an SMA (3 mg) or an EDTA + SMA disk (i and j, respectively). CAZ disks failed to show synergistic zones for an isolate of A. baumannii (k and l). An isolate with VIM β-lactamase showed an arrowhead-shaped synergistic zone (q), and an isolate with an IMP enzyme showed an oval synergistic zone (r).
TABLE 1.
Comparison of performances of IPM-EDTA, IPM-SMA, and CAZ-SMA DDSTs using known MBL-producing isolates
| MBL-producing isolatesa (no. tested) and IPM-EDTA (1,900 μg) DDST result | No. of isolates with the indicated result by IPM-EDTA (1,900 μg) DDSTb | No. of isolates with the following result by:
|
|||||
|---|---|---|---|---|---|---|---|
| IPM-SMA (3 mg) DDSTb
|
CAZ-SMA (3 mg) DDSTb
|
||||||
| Clearly positive | Weakly positive | Negative | Clearly positive | Weakly positive | Negative | ||
| Pseudomonas spp. (19) | |||||||
| Clearly positive | 19 | 17 | 0 | 2 | 15 | 2 | 2 |
| Total | 19 | 17 | 0 | 2 | 15 | 2 | 2 |
| Acinetobacter spp. (80) | |||||||
| Clearly positive | 59 | 59 | 0 | 0 | 38 | 4 | 17 |
| Weakly positive | 16 | 16 | 0 | 0 | 10 | 2 | 4 |
| Negative | 5 | 5 | 0 | 0 | 4 | 0 | 1 |
| Total | 80 | 80 | 0 | 0 | 52 | 6 | 22 |
Fifteen isolates of P. aeruginosa and 4 Pseudomonas putida isolates had blaVIM-2 alleles. Among the acinetobacters, 42 and 38 isolates had blaVIM-2 and blaIMP-1 alleles, respectively.
Sensitivities of DDSTs for pseudomonads and acinetobacters were as follows: 100 and 93.8% for the IPM-EDTA DDST, 89.4 and 100% for the IPM-SMA DDST, and 89.4 and 72.5% for the CAZ-SMA DDST, respectively. The differences in sensitivity between the tests were statistically significant (P < 0.01 by the chi-square test), except for IPM-SMA versus CAZ-SMA for pseudomonads.
Disks containing 750 μg of EDTA and 2 mg of SMA were chosen for further study, based on a preliminary study (data not shown) which showed improved detection and elimination of a nonspecific inhibition zone for both P. aeruginosa and acinetobacters (Fig. 2B). In a comparison using known blaVIM-2-positive P. aeruginosa isolates, all of the 50 isolates showed positive results by both IPM-EDTA and IPM-EDTA + SMA DDSTs, but the synergistic zone was smaller in 41 (82%) of the isolates by the latter method (Table 2). All of the 15 blaVIM-2- and 16 blaIMP-1-positive acinetobacters showed larger synergistic zones when the EDTA + SMA disks were used. Interestingly, the shape of the synergistic inhibition zone was arrowhead-like in blaVIM-2-positive isolates, while it was oval in blaIMP-1-positive isolates (Fig. 2Bq and r, respectively).
TABLE 2.
Comparison of performances of IPM-EDTA and IPM-EDTA + SMA DDSTs using known MBL-producing isolates
| Isolates and MBL type | No. of isolates positive by IPM-EDTA (1,900 μg) DDST | No. of isolates for which IPM-EDTA (750 μg) + SMA (2 mg) DDST synergistic inhibition zone was:
|
||
|---|---|---|---|---|
| Larger | Smaller | Much smaller | ||
| P. aeruginosa, VIM-2 | 50 | 9 | 40 | 1 |
| Acinetobacter spp. | ||||
| VIM-2 | 15 | 15 | 0 | 0 |
| IMP-1 | 16 | 16 | 0 | 0 |
| Total | 81 | 40 | 40 | 1 |
Efficiency of screening in relation to susceptibility of isolates.
The possible presence of MBL-producing strains among CAZ-resistant but IPM-susceptible isolates was tested using 199 consecutive isolates and 241 CAZ-nonsusceptible isolates of P. aeruginosa and acinetobacters by the Hodge test and the IPM-EDTA and CAZ-MPA DDSTs (Table 3). All of the 49 MBL-producing isolates detected were either resistant or intermediate to IPM, and one was intermediate to CAZ (data not shown). In 2000 at our hospital, rates of CAZ-resistant and IPM-nonsusceptible isolates were 19.5 and 26.4%, respectively, for P. aeruginosa and 68.8 and 2.3%, respectively, for acinetobacters (data not shown). Therefore, MBL production screening could be reduced by 59.6% if IPM-nonsusceptible rather than CAZ-resistant isolates were tested.
TABLE 3.
Determination of presence of MBL producers among CAZ-resistant but IPM-susceptible isolates of P. aeruginosa and Acinetobacter spp.a
| Isolates (no. tested) | blaVIM-2 or blaIMP-1 status (no. of isolates) | No. of isolates positive byc:
|
No. of isolates with the following CAZ/IPM result:
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Hodge testb | IPM-EDTA (1,900 μg) DDSTb | CAZ-MPA (3 μl) DDSTb | R/R | I or R/I or R | I or R/I or S | S/R | S/S | ||
| Consecutive | |||||||||
| P. aeruginosa (100) | Positive (5) | 4 (1) | 5 | 5 | 3 | 2 | 0 | 0 | 0 |
| Negative (95) | 1 | 1 | 1 | 4 | 3 | 13 | 10 | 65 | |
| Acinetobacter spp. (99) | Positive (2) | 1 (1) | 1 (1) | 1 (1) | 1 | 1 | 0 | 0 | 0 |
| Negative (97) | 0 | 0 | 0 | 15 | 0 | 56 | 2 | 24 | |
| CAZ-nonsusceptible | |||||||||
| P. aeruginosa (168) | Positive (38) | 33 (5) | 36 (2) | 32 (6) | 36 | 2 | 0 | NA | NA |
| Negative (130) | 4 | 10 | 8 | 102 | 18 | 10 | NA | NA | |
| Acinetobacter spp. (73) | Positive (4) | 3 (1) | 3 (1) | 3 (1) | 4 | 0 | 0 | NA | NA |
| Negative (69) | 5 | 3 | 0 | 46 | 17 | 6 | NA | NA | |
| Total (440) | Positive (49) | 41 (8) | 45 (4) | 41 (8) | 44 | 5 | 0 | 0 | 0 |
| Negative (391) | 10 | 14 | 9 | 167 | 38 | 85 | 12 | 89 | |
Abbreviations: R, resistant; I, intermediate; S, susceptible; NA, not applicable.
Numbers of isolates that were positive by a repeat Hodge test with Zn2+-added IPM disks, and numbers of isolates that were DDST positive by repeated tests, are given in parentheses. All of the false-positive Hodge test and DDST results were equivocal.
Sensitivities and specificities, excluding repeated tests, were as follows: for the Hodge test, 83.7 and 97.4%, respectively; for the IPM-EDTA DDST, 91.8 and 96.4%, respectively; and for the CAZ-MPA DDST, 83.7 and 97.7%, respectively. The differences in sensitivity and specificity between the two DDSTs were statistically significant (P < 0.01 by the chi-square test).
DISCUSSION
Simple and accurate tests are needed to detect MBL-producing isolates, given the increasing prevalence of MBL-producing gram-negative bacilli in many countries (9, 14). Yigit et al. (20) used an inhibition assay, which was similar in principle to the Hodge test, to test the hydrolysis of carbapenem by an isolate of KPC-1-producing Klebsiella pneumoniae. The Hodge test is a simple method for screening MBL-producing isolates, but occasional isolates show false-negative results. The test can be improved by using an IPM disk to which 10 μl of 50 mM zinc sulfate (140 μg/disk) has been added (Table 3) or by using Mueller-Hinton agar to which zinc sulfate has been added to a final concentration of 70 μg/ml.
Inhibition of enzyme activity by EDTA is an important characteristic used to distinguish MBLs from other β-lactamases (12). IPM-EDTA and CAZ-MPA DDSTs (1, 7) were the only simple disk diffusion screening methods for MBL production at the time of initiation of the present study. Therefore, these two tests were compared by using a collection of VIM-2-producing pseudomonads, which were the only MBL-producing organisms in Korea at that time. The use of 2 to 3 μl of MPA per disk was recommended for the test (1), but occasionally, MBL-producing isolates failed to show positive results with 2-μl MPA disks in our study (Fig. 1). The IPM-EDTA and CAZ-MPA tests showed identical results when 3-μl MPA disks were used, but the synergistic inhibition zones for P. aeruginosa were larger by the IPM-EDTA test. It was a disadvantage that both the MPA and EDTA disks alone frequently produced undesirably large inhibition zones (Fig. 1 and 2A).
In another comparison using blaIMP-1- or blaVIM-2-positive isolates (Table 1), only the IPM-EDTA DDST detected all MBL-producing pseudomonads, while the IPM-SMA test detected all MBL-producing acinetobacters. It was noteworthy that the CAZ-SMA test failed to detect 28% of the MBL-producing acinetobacters.
Yong et al. reported that the IPM-EDTA disks produced smaller inhibition zones for isolates of acinetobacters than for isolates of pseudomonads (21). A mixture of EDTA plus 1,10-phenanthroline was used to detect MBL-producing P. aeruginosa by a microdilution method (10). In our present study, a disk containing 750 μg of EDTA plus 2 mg of SMA was chosen, because it produced a larger synergistic zone with MBL-producing acinetobacters than the EDTA disk and because it alone did not produce the undesirably large inhibition zones (Fig. 2B).
Both the IPM-EDTA and IPM-EDTA + SMA DDSTs detected all of the blaVIM-2- and blaIMP-1-positive isolates (Table 2). The latter test showed larger synergistic zones for acinetobacters but smaller zones for most P. aeruginosa isolates. One isolate each of blaVIM-2-positive S. marcescens and A. xylosoxidans subsp. denitrificans also showed large synergistic zones with the EDTA + SMA disks (Fig. 2Bm to p). Therefore, we decided that the EDTA + SMA disk is better than the EDTA, MPA, or SMA disk.
The first phase of our study did not determine the presence of MBL-producing strains among IPM-susceptible isolates, since the MBL-producing isolates of pseudomonads used were detected by testing IPM-nonsusceptible isolates. Therefore, in the second phase of the study, consecutive isolates and a collection of CAZ-nonsusceptible isolates were subjected to screening. However, MBL-producing isolates were not detected among the IPM-susceptible isolates (Table 3). Some MBL-producing gram-negative bacilli were considered difficult to detect, because they were inhibited by low concentrations of IPM (1). This was the reason that Arakawa et al. (1) and the SMA disk producer recommended testing CAZ-resistant isolates for MBL production. MBL-producing strains may also have another CAZ resistance mechanism. With such strains, DDSTs using an IPM disk can show positive results, but a CAZ disk cannot, just as a cefepime disk but not a CAZ disk can detect extended-spectrum β-lactamase production in AmpC β-lactamase-producing strains (15). Even in Japan, Sugino et al. (16) subjected carbapenem-nonsusceptible isolates to MBL screening. The rate of resistance of acinetobacters to CAZ has been much higher than that to IPM in Korea. Therefore, if IPM-nonsusceptible isolates rather than CAZ-resistant isolates are tested, screening work can be reduced.
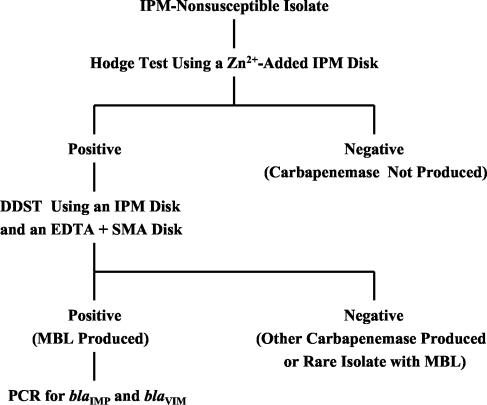
Figure 3 is our proposed scheme for the detection of IMP-1- and VIM-2-producing pseudomonads and acinetobacters. This scheme should be suitable in countries where these organisms are endemic but may also be used in laboratories with small numbers of suspected MBL-producing isolates. The Hodge test is performed using an IPM disk with zinc sulfate added. Use of both the Hodge test and an IPM-EDTA + SMA DDST should improve detection. Screening-positive isolates can be further investigated by PCR to detect blaIMP, blaVIM, or other genes. The dried EDTA + SMA disks were stable for at least 12 weeks at room temperature. We occasionally observed total or partial loss of MBL-producing cells during room temperature storage of the isolates, indicating the importance of testing IPM susceptibility and MBL production at the same time. Loss of blaIMP-1 from A. baumannii isolates stored at room temperature has also been reported by other investigators (17).
FIG. 3.
Proposed scheme for detection of MBL-producing gram-negative bacilli. IPM-nonsusceptible isolates are tested by the Hodge test using an IPM disk to which 140 μg of zinc sulfate has been added. Hodge test-positive isolates are then tested to confirm MBL production by a DDST using an IPM disk and a disk containing 750 μg of EDTA plus 2 mg of SMA. Synergy-positive isolates are then further tested by PCR to detect the blaIMP or blaVIM allele.
In conclusion, the performance of the Hodge test is reliable when IPM disks to which zinc sulfate has been added are used. The IMP-EDTA, CAZ-MPA, and CAZ-SMA DDSTs have both strengths and weaknesses for the detection of MBL-producing pseudomonads and acinetobacters. Use of an IPM disk and an EDTA (750 μg) + SMA (2 mg) disk improves performance, and testing IPM-nonsusceptible isolates rather than CAZ-resistant isolates may reduce screening work. Further evaluation of the tests is required for the detection of other types of MBL-producing gram-negative bacilli.
Acknowledgments
This work was supported in part by the Brain Korea 21 Project for Medical Sciences, Yonsei University, in 2002.
We are grateful to M. Inoue, Kitasato University School of Medicine, Sagamihara, Japan, for providing SMA disks and to Younghee Suh and Chasoon Lee for technical assistance.
REFERENCES
- 1.Arakawa, Y., N. Shibata, K. Shibayama, H. Kurokawa, T. Yagi, H. Fujiwara, and M. Goto. 2000. Convenient test for screening metallo-β-lactamase-producing gram-negative bacteria by using thiol compounds. J. Clin. Microbiol. 38:40-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bush, K., J. A. Jacoby, and A. A. Medeiros. 1995. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob. Agents Chemother. 39:1211-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chong, Y., K. Lee, R. Okamoto, and M. Inoue. 1999. Characteristics of extended-spectrum β-lactam hydrolyzing activity of Klebsiella pneumoniae and Escherichia coli strains isolated from clinical specimens. Korean J. Infect. Dis. 29:477-485. [Google Scholar]
- 4.Chu, Y.-W., M. Afzal-Shah, E. T. S. Houang, M.-F. I. Palepou, D. J. Lyon, N. Woodford, and D. M. Livermore. 2001. IMP-4, a novel metallo-β-lactamase from nosocomial Acinetobacter spp. collected in Hong Kong between 1994 and 1998. Antimicrob. Agents Chemother. 45:710-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gibb, A. P., C. Tribuddharat, R. A. Moore, T. J. Louie, W. Krulicki, D. M. Livermore, M.-F. I. Palepou, and N. Woodford. 2002. Nosocomial outbreak of carbapenem-resistant Pseudomonas aeruginosa with a new blaIMP allele, blaIMP-7. Antimicrob. Agents Chemother. 46:255-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iyobe, S., H. Kusadokoro, J. Ozaki, N. Matsumura, S. Minami, S. Haruta, T. Sawai, and K. O'Hara. 2000. Amino acid substitution in a variant of IMP-1 metallo-β-lactamase. Antimicrob. Agents Chemother. 44:2023-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee, K., Y. Chong, H. B. Shin, Y. A. Kim, D. Yong, and J. H. Yum. 2001. Modified Hodge test and EDTA-disk synergy tests to screen metallo-β-lactamase-producing strains of Pseudomonas and Acinetobacter species. Clin. Microbiol. Infect. 7:88-91. [DOI] [PubMed] [Google Scholar]
- 8.Lee, K., J. B. Lim, J. H. Yum, D. Yong, Y. Chong, J. M. Kim, and D. M. Livermore. 2002. blaVIM-2 cassette-containing novel integrons in metallo-β-lactamase-producing Pseudomonas aeruginosa and Pseudomonas putida isolates disseminated in a Korean hospital. Antimicrob. Agents Chemother. 46:1053-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Livermore, D. M., and N. Woodford. 2000. Carbapenemases: a problem in waiting? Curr. Opin. Microbiol. 3:489-495. [DOI] [PubMed] [Google Scholar]
- 10.Migliavacca, R., J.-D. Docquier, C. Mugnaioli, G. Amicosante, R. Daturi, K. Lee, G. M. Rossolini, and L. Pagani. 2002. Simple microdilution test for detection of metallo-β-lactamase production in Pseudomonas aeruginosa. J. Clin. Microbiol. 40:4388-4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards. 2001. Performance standards for antimicrobial susceptibility testing; 11th informational supplement. M100-S11. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 12.Payne, D. J., R. Cramp, J. H. Bateson, J. Neale, and D. Knowles. 1994. Rapid identification of metallo- and serine β-lactamases. Antimicrob. Agents Chemother. 38:991-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poirel, R., T. Naas, D. Nicolas, L. Collect, S. Bellais, J.-D. Cavallo, and P. Nordmann. 2000. Characterization of VIM-2, a carbapenem-hydrolyzing metallo-β-lactamase, and its plasmid- and integron-borne gene from a Pseudomonas aeruginosa clinical isolate in France. Antimicrob. Agents Chemother. 44:891-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richet, H. M., J. Mohammed, L. C. McDonald, and W. R. Jarvis. 2001. Building communication networks: International Network for the Study and Prevention of Emerging Antimicrobial Resistance. Emerg. Infect. Dis. 7:319-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steward C. D., J. K. Rasheed, S. K. Hubert, J. W. Biddle, P. M. Raney, G. J. Anderson, P. P. Williams, K. L. Brittain, A. Oliver, J. E. McGowan, Jr., and F. C. Tenover. 2001. Characterization of clinical isolates of Klebsiella pneumoniae from 19 laboratories using the National Committee for Clinical Laboratory Standards extended-spectrum β-lactamase detection method. J. Clin. Microbiol. 39:2864-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugino, Y., Y. Iinuma, T. Nada, Y. Tawada, H. Amano, T. Nakamura, Y. Hasegawa, K. Shimokata, N. Shibata, and Y. Arakawa. 2001. Antimicrobial activities and mechanism of carbapenem resistance in clinical isolates of carbapenem-resistant Pseudomonas aeruginosa and Acinetobacter spp. J. Jpn. Assoc. Infect. Dis. 75:662-670. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi, A., S. Yomoda, I. Kobayashi, T. Okubo, M. Tsunoda, and S. Iyobe. 2000. Detection of carbapenemase-producing Acinetobacter baumannii in a hospital. J. Clin. Microbiol. 38:526-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tolman, M. A., A. M. Simm, T. A. Murphy, A. C. Gales, D. J. Biedenbach, R. N. Jones, and T. R. Walsh. 2002. Molecular characterization of SPM-1, a novel metallo-β-lactamase isolated in Latin America: report from the SENTRY antimicrobial surveillance programme. J. Antimicrob. Chemother. 50:673-679. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe, M., S. Iyobe, M. Inoue, and S. Mitsuhashi. 1991. Transferable imipenem resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 35:147-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yigit, H., A. M. Queenan, G. Y. Anderson, A. Domenech-Sanchez, J. W. Biddle, C. D. Steward, S. Alberti, K. Bush, and F. C. Tenover. 2001. Novel carbapenem-hydrolyzing β-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 45:1151-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yong, D., K. Lee, J. H. Yum, H. B. Shin, G. M. Rossolini, and Y. Chong. 2002. Imipenem-EDTA disk method for differentiation of metallo-β-lactamase-producing clinical isolates of Pseudomonas spp. and Acinetobacter spp. J. Clin. Microbiol. 40:3798-3801. [DOI] [PMC free article] [PubMed] [Google Scholar]