Abstract
Human metapneumovirus (hMPV), a newly discovered paramyxovirus, has been associated with acute respiratory tract infections (ARIs) ranging from upper ARIs to severe bronchiolitis and pneumonia. Important questions remain on the contribution of hMPV to ARIs and its impact on public health. During the 2001-2002 season, we conducted a collaborative study with four provincial public health laboratories to study the prevalence of this new virus in the Canadian population. A total of 445 specimens were collected from patients of all age groups with ARIs and were tested for the presence of hMPV by reverse transcription-PCR. Of these, 66 (14.8%) tested positive for hMPV. Positive specimens were found in all age groups and in all four provinces studied. Virus activity peaked in February and March. The age range of the patients with hMPV infection was 2 months to 93 years (median age, 25 years), with similar numbers of females (35%) and males (41%). Thirty-three percent (n = 22) of hMPV-infected patients were hospitalized; of these, 27% (n = 6) had rhinitis and pneumonia, 23% (n = 5) had bronchiolitis, and 9% (n = 2) had bronchitis. The hospitalization rates were significantly higher among patients <5 years of age (P = 0.0005) and those >50 years of age (P = 0.0044) than among those 6 to 50 years of age. Phylogenetic analysis of the F gene showed that two hMPV genetic clusters were cocirculating in the 2001-2002 season, and comparison with earlier studies suggests a temporal evolutionary pattern of hMPV isolates. These results provide further evidence of the importance of hMPV in ARIs, particularly in young children and elderly individuals.
Viral respiratory diseases are a major health problem and represent the leading cause of death from infectious diseases in Canada (5). They affect people of all ages and exert a great economic impact on the health care system. The viruses most frequently associated with respiratory tract infections include rhinoviruses, coronaviruses, influenza viruses, parainfluenza viruses, respiratory syncytial viruses (RSVs), and adenoviruses. However, the etiological agents for a large number of respiratory infections remain unknown. In 2001, a new paramyxovirus, human metapneumovirus (hMPV), that was tentatively assigned to the Metapneumovirus genus of the Pneumovirinae subfamily was identified by Van den Hoogen et al. (16) and has been associated with respiratory illnesses ranging from upper respiratory tract disease to severe bronchiolitis and pneumonia (2, 12, 15, 16). Earlier recognition of hMPV was delayed because it has been difficult to detect in cell culture due to its slow growth and mild cytopathic effect and awaited the development of molecular techniques such as reverse transcription (RT)-PCR. hMPV has been isolated from patients with respiratory diseases in several countries, suggesting that hMPV may be circulating worldwide (6, 10-12, 15). The relative importance of hMPV in viral respiratory tract illnesses is still not known, but serological studies have demonstrated that by the age of 5 years, virtually all children in The Netherlands have been in contact with the virus and that it has been present in the population for at least 50 years (16). It was also reported that hMPV can be associated with severe lower respiratory tract infections in young children, elderly individuals, and immunocompromised patients (2). In the United Kingdom, 2.2% of patients with influenza-like illnesses were found to be infected with hMPV (15). In this study, we looked at hMPV isolates from Canadian patients with respiratory tract infections during the 2001-2002 influenza season in order to assess the impact of hMPV infections on acute respiratory tract infections (ARIs) and to describe the presenting signs and symptoms of this illness. Furthermore, to evaluate the genetic diversity of the different Canadian hMPV isolates, we performed phylogenetic analysis based on F gene sequences.
MATERIALS AND METHODS
Primer sequences.
The primers used for amplification and sequencing were based on the published hMPV F gene sequence (GenBank accession no. AF371367) (16). Primers MPVF1f (5′-CTTTGGACTTAATGACAGATG-3) and MPVF1r (5′-GAGAAGAGCTGGGTAGAAG-3′) were used to amplify a 450-bp fragment of the F gene (12).
RT-PCR and sequence analysis.
Viral RNA was extracted from 100 μl of original samples or tissue culture fluid by using the RNeasy Mini kit (QIAGEN). Viral RNA was amplified in a one-step RT-PCR (QIAGEN) according to the recommendations of the manufacturer. Briefly, 5 μl of RNA was added to the RT-PCR mixture, which contained 2 μl of QIAGEN OneStep RT-PCR enzyme mixture, 10 μl of 5× QIAGEN OneStep RT-PCR buffer, 400 μM deoxynucleoside triphosphates, 0.6 μM each primer, and 10 μl of Q-solution in a final volume of 50 μl. The thermocycler conditions used were 50°C for 30 min for RT; 95°C for 15 min for activation of the HotStart DNA polymerase; and then 35 cycles of 94°C for 1 min, 56°C for 1 min, and 72°C for 1 min, followed by an extension of 10 min at 72°C. The PCR products were purified with the QIAquick PCR purification kit (QIAGEN) and sequenced on an ABI 377 sequencer with a fluorescent dye terminator kit (Applied Biosystems). The DNA sequences were assembled and analyzed with the SEQMAN, EDITSEQ, and MEGALIGN programs in Lasergene software (DNASTAR, Madison, Wis.). Phylogenetic trees were generated by the neighbor-joining method with the MEGA program (7).
Nucleotide sequence accession number.
The hMPV sequences described in this paper have been deposited in GenBank under accession numbers AY286135 to AY286189. The nucleotide sequence accession numbers for hMPV Netherlands isolate 00-1 (NLD00-1) is AF371367, and those of previous Canadian isolates CAN97-82 to CAN00-16 (see Fig. 3) are AY145287 to AY145301, respectively.
FIG.3.
Phylogenetic analysis of hMPV isolates. The sequences of nucleotides 688 to 1032 of the F gene were determined. The corresponding gene sequences of previously reported Canadian and Dutch hMPV isolates were also included. Phylogenetic analysis was performed by the neighbor-joining method of the MEGA program. The previous Canadian isolates are indicated by CAN followed by the year of collection and the isolate number (e.g., CAN97-83). The isolates presented in this study are indicated by the isolate number and the year (e.g., 172-02). The Dutch isolate is indicated by NDL00-1.
RESULTS
Specimens.
A total of 445 specimens collected from patients with ARIs from October 2001 to April 2002 were tested for the presence of hMPV by RT-PCR. The specimens originated from four provincial public health laboratories across Canada: Nova Scotia (75 specimens), Manitoba (191 specimens), Saskatchewan (143 specimens), and British Columbia (36 specimens). All but three specimens were negative for influenza viruses A and B; parainfluenza virus 1, 2, and 3; adenovirus; and RSVs by direct or indirect fluorescence assays and virus isolation. Of the remaining three specimens, two were positive for influenza virus A and one was positive for RSV. Specimens were collected from individuals in all age groups: 125 (28%) from patients 0 to 5 years old, 20 (4%) from patients 6 to 10 years old, 43 (10%) from patients 11 to 20 years old, 112 (25%) from patients 20 to 50 years old, and 140 (31%) from patients >50 years old. The age of the patients was unknown for 5 (1%) specimens. The specimen types analyzed were 173 throat swabs, 97 nasopharyngeal swabs, 73 nasal aspirates, 42 nasal swabs, 11 lung tissue specimens, 13 tracheal secretions, 7 bronchoaveolar lavage specimens, 7 pharyngeal swabs, 4 throat wash specimens, 3 nasal secretions, 9 miscellaneous specimens, and 6 unknown respiratory specimens.
hMPV specimens.
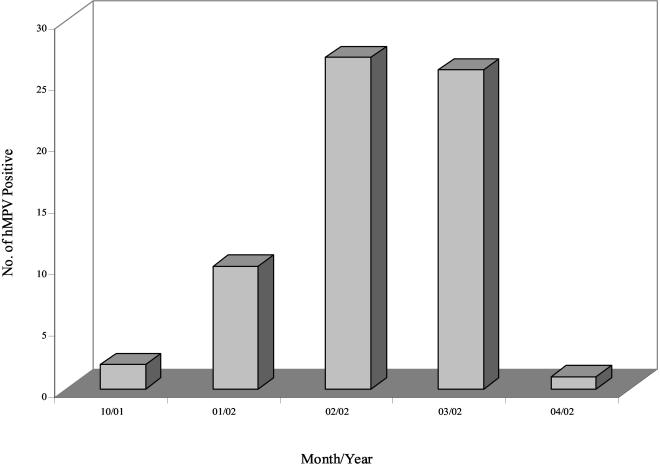
Of the 445 specimens tested, 66 (14.8%) were positive for hMPV by RT-PCR. Positive specimens were identified from all four provinces, with the highest number identified in Saskatchewan (n = 28; 42%) (Table 1). The positive specimens comprised 21 throat swabs, 16 nasopharyngeal swabs, 11 nasal aspirates, 9 nasal swabs, 6 tracheal secretions, 2 bronchoalveolar lavage specimens, and 1 nasal secretion. hMPV activity peaked in February (n = 27; 41%) and March (n = 26; 39%) and subsided in late spring (Fig. 1). The patients infected with hMPV were ages 2 months to 93 years (median age, 25 years), and overall, no significant difference in infection rates was observed between age groups. The sex distribution was 41% (n = 27) male and 35% (n = 23) female; the sex was not reported for 24% (n = 16) of the patients (Table 1). A total of 4.5% of the hMPV-infected patients were coinfected with influenza virus A (n = 2; 3%) or RSV (n = 1; 1.5%).
TABLE 1.
Characteristics of specimens positive for hMPV isolates by province, gender, and patient statusa
| Characteristic | % of specimens |
|---|---|
| Province | |
| British Columbia | 18 |
| Saskatchewan | 42 |
| Manitoba | 24 |
| Nova Scotia | 15 |
| Gender | |
| Male | 41 |
| Female | 35 |
| Not reported | 24 |
| Patient status | |
| Outpatients | 67 |
| Hospitalized | 33 |
A total of 66 specimens were positive.
FIG. 1.
Number of hMPV-positive specimens per month in the 2001-2002 season.
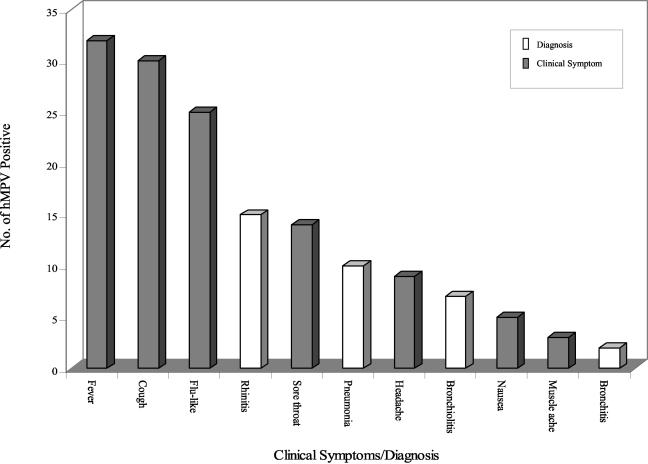
Information on clinical symptoms was available for 56 of the 66 hMPV-positive patients. The main clinical presentations reported were fever (n = 32; 57%), cough (n = 30; 54%), and influenza-like illnesses (n = 25; 45%) (Fig. 2). Other clinical symptoms also included sore throat (n = 14; 25%), headache (n = 9; 16%), nausea (n = 5; 9%), and muscle ache (n = 3; 5%) (Fig. 2). Twenty-seven percent (n = 15) of patients were observed to have rhinitis, 18% (n = 10) had pneumonia, 13% (n = 7) had bronchiolitis, and 4% (n = 2) had bronchitis (Fig. 2). Five percent (n = 3) of patients had both bronchiolitis and pneumonia. Twenty-two (33%) hMPV-infected patients were hospitalized; of these, 6 (27%) had rhinitis, 6 (27%) had pneumonia, 5 (23%) had bronchiolitis, and 2 (9%) had bronchitis (Table 2). The incidence of lower respiratory tract infection was lower in outpatients: 4 (12%) had pneumonia, 2 (6%) had bronchiolitis, and none (0%) had bronchitis (Table 2). Although the infection rates were similar in all age groups, there was a significant increase in hospitalization rates among those <5 years of age and >50 years of age compared to the rate among those ages 6 to 50 years (11 of 19 versus 2 of 25 [P = 0.0005] and 9 of 20 versus 2 of 25 [P = 0.0044]) (Table 3). The majority of ARIs (9 [90%] cases of pneumonia, 6 [86%] cases of bronchiolitis, 2 [100%] cases of bronchitis) were also reported among individuals in these two age groups.
FIG. 2.
Clinical symptoms and diagnoses reported in hMPV-positive patients.
TABLE 2.
Comparison of clinical diagnoses reported for hospitalized patients and outpatientsa
| Diagnosis | No. (%) of hMPV-infected patients
|
||
|---|---|---|---|
| Hospitalized (n = 22) | Outpatient (n = 34) | Total (n = 56) | |
| Rhinitis | 6 (27) | 9 (26) | 15 (27) |
| Pneumonia | 6 (27) | 4 (12) | 10 (18) |
| Bronchiolitis | 5 (23) | 2 (6) | 7 (13) |
| Bronchitis | 2 (9) | 0 (0) | 2 (4) |
Information on the diagnosis was reported for 56 of the 66 hMPV-positive patients.
TABLE 3.
hMPV isolates by age group and patient status
| Age group (yr) | No. of hMPV-positive patients
|
||
|---|---|---|---|
| Total | Hospitalized | Outpatients | |
| <5 | 19 | 11 | 8 |
| 6-10 | 2 | 0 | 2 |
| 11-20 | 9 | 0 | 9 |
| 21-50 | 16 | 2 | 14 |
| >50 | 20 | 9 | 11 |
Phylogenetic analysis.
The sequences from nucleotides 688 to 1032 of the F gene were determined. Sequence analysis showed that the F genes were relatively well conserved, with the rates of nucleic acid identity between specimens ranging from 83.8 to 100%. Phylogenetic comparisons of the F gene sequences of the hMPV isolates recovered in 2001-2002 with published Canadian and Dutch hMPV F gene sequences were performed (Fig. 3). Consistent with recent reports (1, 12, 16), the phylogenetic tree of the hMPV isolates recovered in 2001-2002 showed the existence of two major groups or clusters, with the majority of the isolates clustering in cluster 1 (Fig. 3). Similar hMPV strains were isolated from adults and children in all four provinces and in different epidemics. Cluster 1, which contained all the specimens recovered in 2002, was subdivided into two subclusters (subclusters 1a and 1b), and the two specimens recovered in 2001 were found in cluster 2. Previously reported Canadian isolates and Dutch isolates from 1997, 1999, and 2000 were found in both clusters, whereas isolates from 1998 were found only in cluster 2 (1, 12, 16).
DISCUSSION
The prevalence of hMPV in the human population and the frequency of morbidity associated with hMPV infections are not well characterized. This study demonstrates that hMPV was widespread across several Canadian provinces during the 2001-2002 season. The relatively high incidence of hMPV infections reported here provides further evidence of the contribution of hMPV to ARIs and of the significant burden that it may present to health care systems. hMPV infection occurs mainly in the winter to early spring, when influenza viruses and RSVs are also prevalent, and account for a substantial number of hospitalizations. The clinical symptoms associated with hMPV infection are also comparable to those associated with influenza virus and RSV infections, making it impossible to differentiate between these viral infections on the basis of seasonality and clinical manifestations. Previous estimates of the contribution of RSV and other etiological agents to ARIs on the basis of these parameters may have been biased because the involvement of hMPV had been overlooked (9). Although the rate of infection appears to be similar in patients from all age groups, the risk of severe hMPV disease and hospitalization rates were increased in patients <5 years old or >50 years old. Individuals in these age groups represented 91% of all individuals hospitalized because of infection with this virus. The concentration of individuals in these susceptible groups in day care centers or long-term-care facilities may be contributing to the transmission of hMPV. These results suggest that hMPV plays a role as a common respiratory pathogen among infants, young children, and elderly individuals, as is observed with RSV. A similar study conducted in England and Wales reported an incidence of hMPV infection in patients with ARIs of 2.2% during the winter of 2000-2001, which was lower than the incidence of 14.8% found in this study, suggesting that hMPV may follow different epidemiological patterns in different settings (15). However, the difference observed between the two studies could also be due to differences in study design, more particularly, to the different number of specimens tested from individuals <5 and >50 years of age, who seem to be more at risk for hMPV infection. A previous report showed that respiratory virus coinfections occur at a rate of 1 to 3% in various sample sets (8, 17). The coinfections with hMPV and other respiratory viruses observed in this study suggest that hMPV could be implicated in similar coinfections.
Phylogenetic analysis based on the F genes confirmed previous results and showed the presence of two distinct genetic clusters (1, 12, 16). The increased number of isolates tested provided further evidence of additional genetic diversity and the presence of subclusters, suggesting that hMPV might be more heterogeneous than was reported previously (16). Similar to what is observed for RSV (3, 4, 6, 13, 14), multiple hMPV lineages were circulating during the same epidemics. Furthermore, comparison of the isolates from the 2001-2002 season with those from previous epidemics suggests a shift in the predominant genotypes over time, suggesting a temporal evolution of hMPV. The close clustering of hMPV isolates recovered from different Canadian provinces as well as The Netherlands also suggests that the evolutionary pattern of hMPV correlates with temporal rather than geographic variation. These results suggest that the F gene of hMPV is constantly evolving, which might be due to immune pressure. No correlation between the hMPV genetic clusters and either the clinical symptoms observed or the severity of illness could be established.
In summary, our data suggest that hMPV may be responsible for a significant number of ARIs, especially in young children and elderly individuals. More comprehensive studies including data on prevalence, risk factors, and the use of health care services are needed to determine the importance of hMPV in ARIs and its impact on health care services.
REFERENCES
- 1.Bastien, N., S. Normand, T. Taylor, D. Ward, T. C. T. Peret, G. Boivin, L. J. Anderson, and Y. Li. 2003. Sequence analysis of the N, P, M and F genes of Canadian human metapneumovirus strains. Virus Res. 93:51-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boivin, G., Y. Abed, G. Pelletier, L. Ruel, D. Moisan, S. Cote, T. C. Peret, D. D. Erdman, and L. J. Anderson. 2002. Virological features and clinical manifestations associated with human metapneumovirus: a new paramyxovirus responsible for acute respiratory-tract infections in all age groups. J. Infect. Dis. 186:1330-1334. [DOI] [PubMed] [Google Scholar]
- 3.Choi, E. H., and H. J. Lee. 2000. Genetic diversity and molecular epidemiology of the G protein of subgroups A and B of respiratory syncytial viruses isolated over 9 consecutive epidemics in Korea. J. Infect. Dis. 181:1547-1556. [DOI] [PubMed] [Google Scholar]
- 4.Coggins, W. B., E. J. Lefkowitz, and W. M. Sullender. 1998. Genetic variability among group A and group B respiratory syncytial viruses in a children's hospital. J. Clin. Microbiol. 36:3552-3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Editiorial Board of Respiratory Disease in Canada. 2001. Respiratory disease in Canada 2001. Health Canada. [Online.] http://www.hc-sc.gc.ca/pphb-dgspsp/.
- 6.Freymuth, F., A. Vabret, L. Legrand, N. Eterradossi, F. Lafay-Delaire, J. Brouard, and B. Guillois. 2003. Presence of the new human metapneumovirus in French children with bronchiolitis. Pediatr. Infect. Dis. J. 22:92-94. [DOI] [PubMed] [Google Scholar]
- 7.Kumar, S., K. Tamura, I. B. Jakobsen, and M. Nei. 2001. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17:1244-1245. [DOI] [PubMed] [Google Scholar]
- 8.Lina, B., M. Valette, S. Foray, J. Luciani, J. Stagnara, D. M. See, and M. Aymard. 1996. Surveillance of community-acquired viral infections due to respiratory viruses in Rhone-Alpes (France) during winter 1994 to 1995. J. Clin. Microbiol. 34:3007-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller-Pebody, B., W. J. Edmunds, M. C. Zambon, N. J. Gay, and N. S. Crowcroft. 2002. Contribution of RSV to bronchiolitis and pneumonia-associated hospitalizations in English children, April 1995-March 1998. Epidemiol. Infect. 129:99-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nissen, M. D., D. J. Siebert, I. M. Mackay, T. P. Sloots, and S. J. Withers. 2002. Evidence of human metapneumovirus in Australian children. Med. J. Aust. 176:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pelletier, G., P. Dery, Y. Abed, and G. Boivin. 2002. Respiratory tract reinfections by the new human metapneumovirus in an immunocompromised child. Emerg. Infect. Dis. 8:976-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peret, T. C., G. Boivin, Y. Li, M. Couillard, C. Humphrey, A. D. Osterhaus, D. D. Erdman, and L. J. Anderson. 2002. Characterization of human metapneumoviruses isolated from patients in North America. J. Infect. Dis. 185:1660-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peret, T. C., C. B. Hall, K. C. Schnabel, J. A. Golub, and L. J. Anderson. 1998. Circulation patterns of genetically distinct group A and B strains of human respiratory syncytial virus in a community. J. Gen. Virol. 79:2221-2229. [DOI] [PubMed] [Google Scholar]
- 14.Seki, K., H. Tsutsumi, M. Ohsaki, H. Kamasaki, and S. Chiba. 2001. Genetic variability of respiratory syncytial virus subgroup a strain in 15 successive epidemics in one city. J. Med. Virol. 64:374-380. [DOI] [PubMed] [Google Scholar]
- 15.Stockton, J., I. Stephenson, D. Fleming, and M. Zambon. 2002. Human metapneumovirus as a cause of community-acquired respiratory illness. Emerg. Infect. Dis. 8:897-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van den Hoogen, B. G., J. C. de Jong, J. Groen, T. Kuiken, R. de Groot, R. A. Fouchier, and A. D. Osterhaus. 2001. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 7:719-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zambon, M. C., J. D. Stockton, J. P. Clewley, and D. M. Fleming. 2001. Contribution of influenza and respiratory syncytial virus to community cases of influenza-like illness: an observational study. Lancet 358:1410-1416. [DOI] [PubMed] [Google Scholar]