Abstract
This paper reports a strategy that uses microfluidic networks to pattern self-assembled monolayers with gradient microislands for the attachment of individual cells. A microfluidic network is first used to pattern a monolayer into square regions that present maleimide groups and then used to flow a solution having a gradient of the cell adhesion peptide Arg-Gly-Asp over the substrate. In this way, the surface is patterned with microislands approximately 33x33 microns in size and each having a defined gradient of immobilized cell adhesion ligand. B16F10 cells were allowed to attach to the patterned islands and were found to display a non-uniform distribution of cytoskeletal structures in response to the gradient of adhesion ligand. This work is significant because it permits studies of the influence of a non-uniform microenvironment on the polarization, differentiation and signaling of adherent cells.
Keywords: surface chemical gradient, surface patterning, microfluidics, haptotaxis, cell polarity
Mammalian and bacterial cells live in gradients. Soluble and immobilized gradients of signaling proteins guide the trafficking of cells and direct their development and maintenance.1 New methods to determine the influence of gradients in solution on cellular function have been critical to understanding chemotaxis,2a differentiation,2b and morphogenesis.2c The development of strategies to prepare gradients of immobilized species, by contrast, has been more difficult and is still at an early stage.3 These strategies should combine surface chemistries to immobilize ligands in defined densities, orientations and conformations and accommodate the preparation of surfaces that are geometrically patterned—for example, to prepare shapes for the attachment and spreading of individual cells. In this paper, we report a method that combines gradients of soluble ligands in microfluidic networks (μFNs) with immobilization chemistries on self-assembled monolayers (SAMs) to prepare well-defined gradients of adhesion molecules for the attachment of cells.
Several methods have been reported for the generation of biologically relevant, substrate-bound gradients including the electrochemical desorption of alkanethiolates from monolayers,4a photochemical patterning,4b diffusive patterning,4c and the use of μFNs.5 These methods have enabled studies of cell migration, adhesion, and polarity, but are limited in the ability to define, at the molecular level, the activities and densities of ligands at the surface. Photochemical methods have been applied to create structurally well-defined surfaces, but are not compatible with all immobilization chemistries, including thiol addition to maleimide groups used in this work.6
In the current work we use μFNs to establish a soluble gradient of the peptide GRGDSC (the ‘RGD’ peptide) in contact with a monolayer that presents maleimide groups among tri(ethylene glycol) groups.7 In this way, the peptides are presented uniformly and at defined density. The RGD peptide is a ligand for cell-surface integrin receptors, which are used by most cells to attach to and sense the extracellular environment, and to establish a cytoskeleton.8 The glycol groups prevent non-specific interactions of proteins and cells with the surface.9 We demonstrate the preparation of approximately square features that present defined gradients to individual cells and show that the gradient of adhesion ligand leads to an non-uniform distribution of the cytoskeletal elements in adherent cells.
We used the μFN to combine a stream containing the RGD peptide with a stream containing the inactive GGRDGSC (the ‘RDG’) peptide at the same concentration.10 The two peptides mix by diffusion to establish the gradient, but maintain a uniform concentration of total peptide across the channel, ensuring that the immobilization reaction proceeds at a uniform rate and the immobilized gradient reflects the gradient of the RGD peptide in the μFN. To pattern a monolayer with an array of squares (measuring 33 μm) each having a gradient of RGD peptide, we first used a serpentine microfluidic channel to block the maleimide groups with the inactive peptide RDG (Figure 1B). We then removed the microfluidic stamp and applied a Y-channel stamp that was used to flow a gradient of RGD peptide across the reactive stripes on the substrate (Figure 1C). In this way, individual gradients were created which were bounded by both sides of the microfluidic channel and by the presence of the blocked regions of the substrate from the first patterning step. We then removed the microfluidic device and immersed the entire surface in a solution of nonadhesive peptide to block the remaining maleimide groups and to give an array of isolated gradients of adhesive peptide (Figure 1D).
Figure 1.

Scheme for preparing isolated gradient microislands. See the text for an explanation of each step.
This sequential patterning scheme requires that immobilization of the peptides at distinct regions of the monolayer proceeds in high yield. To verify the reactions, we used SAMDI mass spectrometry to analyze the composition of a surface patterned with μFNs.11 In this experiment, a 500 μm wide stripe of RGD was patterned on the surface. Subsequently, the entire surface was immersed in a solution of the RDG peptide. SAMDI analysis of each region showed that the first patterning reaction was complete, and no subsequent immobilization of RDG was observed in the patterned region (Figure 2).
Figure 2.
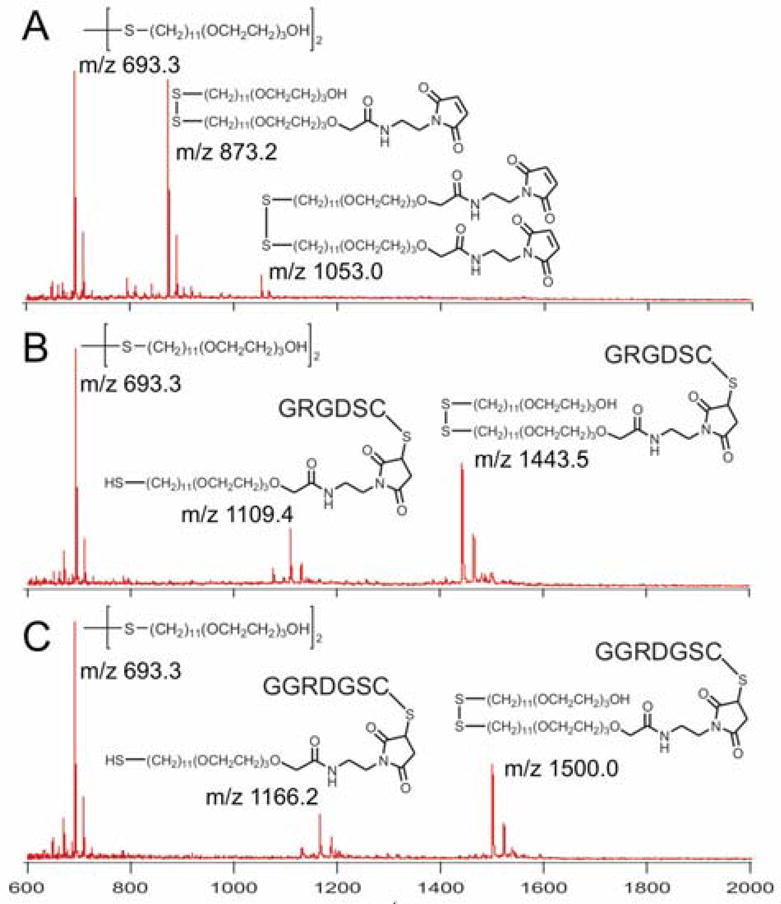
SAMDI-MS of the maleimide-terminated SAM (A) before and after reaction with the (B) RGD peptide and (C) RDG peptide.
We next report studies of cell adhesion to the micropatterned gradients. We note that our method generates a gradient of gradients—that is, the gradient of active peptide becomes more diffuse as the fluid moves through the channel, yielding a continuum of immobilized gradients. This property can be useful for experiments that survey the dependence of a cellular function on geometry of the gradient. Importantly, analytical solutions exist for defining the gradient of peptide as a function of initial peptide concentrations, channel geometry and flow rate5, and that account for the transverse diffusive broadening in laminar flows.12 The supporting information shows images of the gradients.
We placed the patterned substrates in a suspension of B16/F10 mouse melanoma cells. The cells attached efficiently, with individual cells spreading within the gradient microislands (Figures 3). Prior examples have used surfaces that were modified with gradients at length scales larger than that of the cell, and therefore focused on cell migration.4a Our use of islands that restrict individual cells allows a determination of the influence of a gradient of adhesion ligand on polarization of the cytoskeleton. Importantly, the microisland gradients preclude cell migration, which can also contribute to cell polarity.13 We fixed and immunostained the cells with an antibody against vinculin, which is found in the focal adhesion structures that integrate the cytoskeleton with the extracellular matrix.15 A visual inspection of the cells revealed a polarized FA distribution, with FAs preferentially located at regions of the substrate presenting a higher density of RGD.
Figure 3.
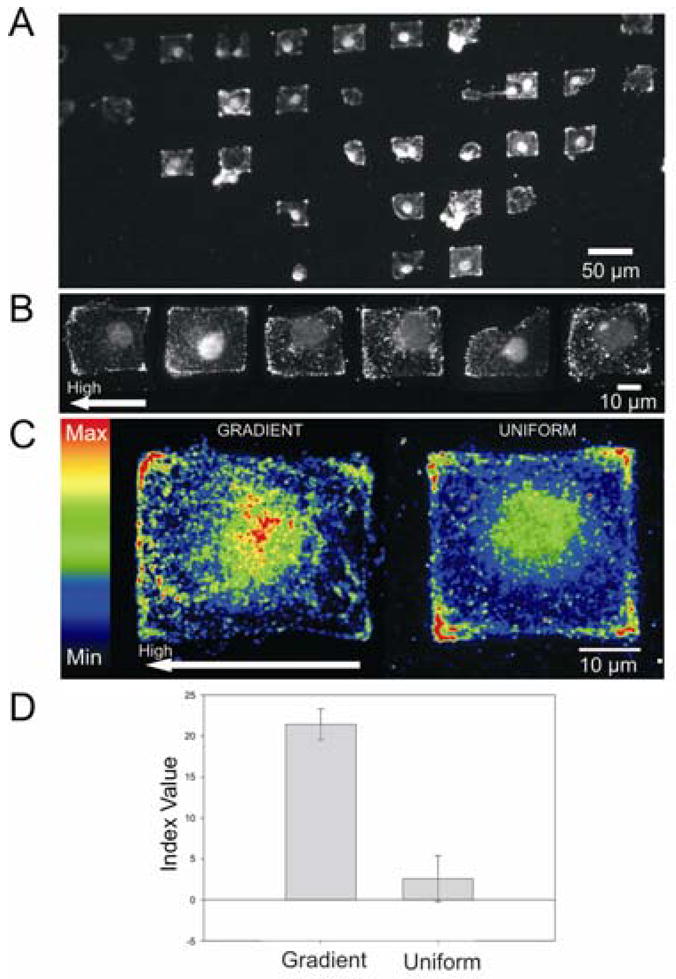
Representative views of B16/F10 melanoma cells stained for vinculin. (A) Fluorescent image of fixed and immunostained cells on an array of gradient microislands. (B) Representative images of single cells isolated on gradient islands. (C) Representative heat maps of cells on gradient (left) and uniform (right) microislands. High RGD concentration is located on the left side of the gradient heat map. The total area of the islands is ~1000 μm2 (gradient, n=22; uniform, n=11). (D) Average index values for four separate experiments. Error bars represent the standard error of measurement.
Vinculin heat maps from independent experiments were generated by aligning and stacking images of individual cells to obtain the integrated pixel intensity. We imaged cells that were on gradient islands having a maximum slope (at the mid-point of the channel) of approximately 1.0×10−12 to 7.6×10−12 nmol RGD/μm3. To quantitate the distribution of FAs on the high and low density edges of the cell, we integrated the pixel density (D, reflective of the density of FA in the cell) on each edge of the cell (using a ~5 μm stripe) and determined a polarity index as follows: I = (DHIGH − DLOW)/(DHIGH + DLOW). The average values of the index for four independent experiments gave a mean index of 21±2 (relative to a mean index of 2±2 for cells on islands of uniform density) and clearly showed that the gradient of adhesive ligand influenced the cyto-skeletal structure.
This communication demonstrates a flexible and straightforward method for generating non-uniform patterns of substrate bound ligands on the length scale of single cells. We have shown that the resulting monolayers are compatible with studies of adherent cell culture and that cells exhibit a non-uniform distribution of FAs in response to a gradient of peptide adhesion ligand. This technique offers significant benefits in preparing substrates having well-defined gradients and patterns of immobilized ligands. We believe these tools will be especially important in understanding the influence of non-uniform microenvironments on cell function, including polarization and migration.
Supplementary Material
Details for fabrication of μFNs, preparation of SAMs, cell culture, mass spectrometric analysis, fluorescent images of the gradient, and data analysis. This material is available free of charge via the internet at http://pubs.acs.org.
Acknowledgments
This work was supported by NSF-MRSEC and NIH. R.T.P. was supported by the NIH Chemistry & Biology Interface Pre-Doctoral Training Program (T32 GM008720).
References
- 1.(a) Van Haastert PJM, Devreotes PN. Nat Rev Mol Cell Bio. 2004;5:626–634. doi: 10.1038/nrm1435. [DOI] [PubMed] [Google Scholar]; (b) Gurdon JB, Bourillot PY. Nature. 2001;413:797–803. doi: 10.1038/35101500. [DOI] [PubMed] [Google Scholar]
- 2.(a) Mao H, Cremer PS, Manson MD. Proc Natl Acad Sci. 2003;100:5449–5454. doi: 10.1073/pnas.0931258100. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chung BG, Flanagan LA, Rhee SW, Schwartz PH, Lee AP, Monuki ES, Jeon NL. Lab Chip. 2005;5:401–406. doi: 10.1039/b417651k. [DOI] [PubMed] [Google Scholar]; (c) Lucchetta EM, Lee JH, Fu LA, Patel NH, Ismagilov RF. Nature. 2005;434:1134–1138. doi: 10.1038/nature03509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sniadecki NJ, Desai RA, Ruiz SA, Chen CS. Ann Biomed Eng. 2006;34:59–74. doi: 10.1007/s10439-005-9006-3. [DOI] [PubMed] [Google Scholar]
- 4.(a) Plummer ST, Wang Q, Bohn PW. Langmuir. 2003;19:7528–7536. [Google Scholar]; (b) Ryan D, Parviz BA, Linder V, Semetey V, Su J, Mrksich M, Whitesides GM. Langmuir. 2004;20:9080–9088. doi: 10.1021/la048443u. [DOI] [PubMed] [Google Scholar]; (c) Smith JT, Tomfohr JK, Wells MC, Beebe TP, Jr, Kepler TB, Reichert WM. Langmuir. 2004;20:8279–8286. doi: 10.1021/la0489763. [DOI] [PubMed] [Google Scholar]
- 5.(a) Jiang X, Xu Q, Dertinger SKW, Stroock AD, Fu T-M, Whitesides GM. Anal Chem. 2005;77:2338–2347. doi: 10.1021/ac048440m. [DOI] [PubMed] [Google Scholar]; (b) Fosser KA, Nuzzo RG. Anal Chem. 2003;75:5775–5782. doi: 10.1021/ac034634a. [DOI] [PubMed] [Google Scholar]; (c) Jeon NJ, Dertinger SKW, Chiu DT, Choi IS, Stroock AD, Whitesides GM. Langmuir. 2000;16:8311–8316. [Google Scholar]; (d) Dertinger SKW, Jiang X, LiZMurthy VN, Whitesides GM. Proc Natl Acad Sci. 2002;99:12542–12547. doi: 10.1073/pnas.192457199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dillmore WS, Yousaf MN, Mrksich M. Langmuir. 2004;20:7223–7231. doi: 10.1021/la049826v. [DOI] [PubMed] [Google Scholar]
- 7.Houseman BT, Gawalt ES, Mrksich M. Langmuir. 2003;19:1522–1531. [Google Scholar]
- 8.Geiger B, Bershadsky A, Pankov R, Yamada KM. Nat Rev Mol Cell Bio. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- 9.(a) Roberts C, Chen CS, Mrksich M, Martichonok V, Ingber DE, Whitesides GM. J Am Chem Soc. 1998;120:6548–6555. [Google Scholar]; (b) Houseman BT, Mrksich M. Biomaterials. 2001;22:943–955. doi: 10.1016/s0142-9612(00)00259-3. [DOI] [PubMed] [Google Scholar]
- 10.Giancotti FG. Curr Opin Cell Biol. 1997;9:691–700. doi: 10.1016/s0955-0674(97)80123-8. [DOI] [PubMed] [Google Scholar]
- 11.Su J, Mrksich M. Langmuir. 2003;19:4867–4870. [Google Scholar]
- 12.Ismagilov RF, Stroock AD, Kenis PJA, Whitesides G, Stone HA. Appl Phys Lett. 2000;76:2376–2378. [Google Scholar]
- 13.Huttenlocher A. Nat Cell Bio. 2005;7:336–337. doi: 10.1038/ncb0405-336. [DOI] [PubMed] [Google Scholar]
- 14.Ziegler WH, Liddington RC, Critchley DR. Trends Cell Biol. 2006;16:453–460. doi: 10.1016/j.tcb.2006.07.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Details for fabrication of μFNs, preparation of SAMs, cell culture, mass spectrometric analysis, fluorescent images of the gradient, and data analysis. This material is available free of charge via the internet at http://pubs.acs.org.


