Abstract
Mammalian neuronal DEG/ENaC channels known as ASICs (acid-sensing ion channels) mediate sensory perception and memory formation. ASICS are closed at rest and are gated by protons. Members of the DEG/ENaC family expressed in epithelial tissues are called ENaCs and mediate Na+ transport across epithelia. ENaCs exhibit constitutive activity and strict Na+ selectivity. We report here the analysis of the first DEG/ENaC in Caenorhabditis elegans with functional features of ENaCs that is involved in sensory perception. ACD-1 (acid-sensitive channel, degenerin-like) is constitutively open and impermeable to Ca2+, yet it is required with neuronal DEG/ENaC channel DEG-1 for acid avoidance and chemotaxis to the amino acid lysine. Surprisingly, we document that ACD-1 is required in glia rather than neurons to orchestrate sensory perception. We also report that ACD-1 is inhibited by extracellular and intracellular acidification and, based on the analysis of an acid-hypersensitive ACD-1 mutant, we propose a mechanism of action of ACD-1 in sensory responses based on its sensitivity to protons. Our findings suggest that channels with ACD-1 features may be expressed in mammalian glia and have important functions in controlling neuronal function.
Keywords: C. elegans, DEG/ENaC channels, glia, sensory perception
Introduction
DEG/ENaC channel subunits (named after the Caenorhabditis elegans degenerins, the first member cloned causes degeneration, and the mammalian epithelial Na+ channels) are two transmembrane domain proteins that trimerize (Jasti et al, 2007) to form Na+ (Canessa et al, 1993, 1994) and Na+/Ca2+-selective (Bianchi et al, 2004; Xiong et al, 2004) ion channels. Neuronally expressed DEG/ENaCs are primarily ASICs (acid-sensing ion channels) and are gated by extracellular protons (Krishtal, 2003). Members of the family that are expressed in epithelial tissues (called ENaCs) are inhibited by intracellular (Garty et al, 1987) but not extracellular acidification (Awayda et al, 2000) and mediate vectorial Na+ transport required for fluid clearance in the lung (Hummler et al, 1996; Barker et al, 1998) and Na+ reabsorption in intestine and kidney essential for Na+ homoeostasis (Garty and Palmer, 1997).
Several members of the DEG/ENaC family across species have been implicated in sensory processes, including mechanosensation (Chalfie and Sulston, 1981; Price et al, 2000, 2001), pain sensation (Chen et al, 2002; Sluka et al, 2003), thermo-sensation (Askwith et al, 2001), pheromone perception (Lin et al, 2005) and proprioception (Shreffler et al, 1995; Tavernarakis et al, 1997). ASICs have been implicated in pain sensation. For example, ASIC3 has been proposed to contribute to angina pectoris and to moderate- to high-intensity pain sensation (Chen et al, 2002; Naves and McCleskey, 2005). The role of DEG/ENaC channels in mediating sour taste is controversial. ENaC and ASIC proteins are found in taste buds (Kretz et al, 1999; Ugawa et al, 2003), but the ASIC2 knockout has no effects on sour taste sensitivity (Richter et al, 2004). Prior to this study, no DEG/ENaC channels have been linked to the detection of amino acids. Finally, some members of the DEG/ENaC family including C. elegans MEC-4 and mouse ASIC1a can induce neurodegeneration under conditions in which they become hyperactivated (Driscoll and Chalfie, 1991; Xiong et al, 2004). MEC-4 is hyperactivated by dominant gain-of-function mutations in the pore (MEC-4(A713), the d position) (Driscoll and Chalfie, 1991), ASIC1a is hyperactivated by prolonged acidosis of ischaemic brain (Xiong et al, 2004). Despite the difference in the mechanism of hyperactivation, we and others have proposed that the neurotoxic capacity of both these channels is linked to their permeability to Ca2+ ions (Bianchi et al, 2004; Xiong et al, 2004).
DEG/ENaC channels have also been found in glia. One group documented mRNA and protein for ENaC and ASIC family members in retinal Muller cells (Golestaneh et al, 2000; Brockway et al, 2005) and found that electroretinograms in the presence of the DEG/ENaC blocker amiloride are altered, suggesting that these channels play a role in retinal function (Brockway et al, 2005). Another group documented DEG/ENaC expression in astrocytes and gliomas, and proposed a role for these channels in glioma malignancy (Berdiev et al, 2003).
We present here the cloning and functional characterization of a novel C. elegans DEG/ENaC channel with greatest sequence similarity to mammalian hINaC (human intestinal Na+ channel) and BLINaC (brain, liver and intestinal Na+ channel) that we named ACD-1 (acid sensitive channel, degenerin-like). We document that ACD-1 is expressed in C. elegans amphid glia where it is needed for behavioural function rather than glia, or associated neuron, viability. We show that when acd-1 is knocked out in conjunction with DEG/ENaC deg-1-null mutation, deg-1 sensory defects in acidic solution avoidance and reduced attraction to lysine acetate are markedly exacerbated. We characterize properties of the ACD-1 channel to show that it is sensitive to both extracellular and intracellular acidification. Lastly, we engineer a mutation in ACD-1 that potentiates the channel sensitivity to extracellular protons and show that this mutant exacerbates acid avoidance behaviour in vivo. We propose a mechanism for the glial channel ACD-1 in controlling acid avoidance and lysine chemotaxis behaviours based on its acid sensitivity.
Results
C24G7.2 gene encodes a novel DEG/ENaC channel subunit
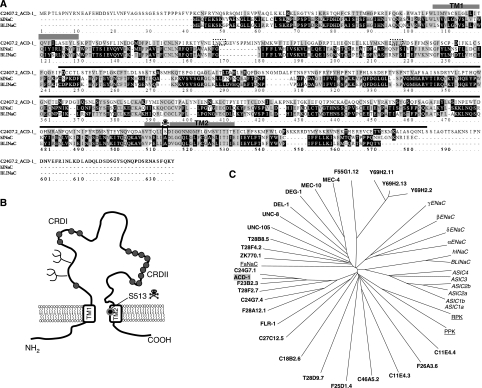
The C. elegans genome contains 28 predicted genes that encode proteins with similarity to DEG/ENaC subunits (reviewed in Bianchi and Driscoll, 2004). Of these, only three have been characterized using electrophysiological tools in heterologous expression systems and in vivo (Garcia-Anoveros et al, 1998; Goodman et al, 2002; Jospin et al, 2004; O'Hagan et al, 2005). The three functionally characterized C. elegans DEG/ENaCs are expressed in mechanosensory neurons (MEC-4 and MEC-10) (Goodman et al, 2002; O'Hagan et al, 2005) and muscles (UNC-105) (Garcia-Anoveros et al, 1998; Jospin et al, 2004), share the highest similarity with mammalian ASIC and are involved in mediating touch sensation and muscle contraction. C. elegans degenerins homologous to other types of mammalian DEG/ENaCs have not been functionally studied to date. Of the mammalian DEG/ENaCs, hINaC (human) and BLINaC (mouse and rat) are the least understood. Messenger RNA for hINaC and BLINaC has been found in intestine, liver and brain, but roles of these DEG/ENaCs in the physiology of these tissues remain unexplored (Sakai et al, 1999; Schaefer et al, 2000). We thus sought to identify C. elegans DEG/ENaC subunits with similarity with hINaC and BLINaC. We focused on gene C24G7.2 that shares the highest similarity with human hINaC (29% identity and 45% similarity) and mouse and rat BLINaC (28% identity and 44% similarity) (Figure 1A). We isolated a knockout mutant of C24G7.2 by screening a library of EMS-mutagenized animals using gene-specific primers. We identified a 0.9 kb deletion that removed part of the seventh, the eighth and part of the ninth exons, resulting in a frameshift that prematurely terminates the protein at amino acid 245 and is thus likely a null mutant (Figure 1A). For reasons documented by experiments below, we renamed C24G7.2 as ACD-1. It may be noteworthy that although ACD-1 shares greatest sequence similarity with mammalian hINaC and BLINaC, at the position corresponding to the channel-activating ‘d' substitutions in C. elegans MEC-4 and DEG-1, ACD-1 encodes a Ser instead of an Ala (Figure 1A) (a Ser substitution at this site is not expected to be channel hyperactivating; Driscoll and Chalfie, 1991). Other features of the ACD-1 protein include a cysteine-rich region preceding the second transmembrane domain (conserved in DEG/ENaC subunits across species; Benos and Stanton, 1999), and two potential glycosylation sites (Figure 1B). The phylogenetic tree in Figure 1C shows evolutionary relationships between ACD-1 and other DEG/ENaCs.
Figure 1.
The C. elegans DEG/ENaC channel ACD-1 (acid-sensitive channel degenerin-like) shares similarity with mammalian BLINaC and hINaC. (A) We designated C24G7.2 as ACD-1 because of its sensitivity to acidic solutions. C24G7.2 protein sequence and alignments with hINaC (29% identity and 45% similarity) and mouse and rat BLINaC (28% identity and 44% similarity). The transmembrane domains TM1 and TM2 and the region of the protein deleted in acd-1(bz90) knockout are indicated by the grey boxes and black line, respectively. The transmembrane domains were identified by threading ACD-1 sequence onto the sequence of chicken ASIC1a (Jasti et al, 2007), using PyMOL (www.PyMOL.org). The box and the skull designate position S513 in ACD-1 corresponding to the amino acid that when mutated to Val or Thr in MEC-4 induces hyperactivation of the channel (A713V or T, the (d) mutation). The dotted boxes indicate potential glycosylation sites. Alignment by ClustalW (available at bioweb.pasteur.fr). (B) A schematic diagram representing ACD-1 predicted topology. The potential glycosylation sites are indicated by the branches. The circles depict cysteine residues; a region just preceding TM2 is rich in cysteines. This feature is conserved in DEG/ENaC subunits across species (Benos and Stanton, 1999). (C) Dendrogram of C. elegans (bold), Drosophila (RPK and PPK, underlined)), Helix apersa (FaNaC, underlined) and mammalian (italic) DEG/ENaC channel subunits. ACD-1 subunit has a grey background.
ACD-1 subunits form Na+-selective channels
We next sought to functionally characterize ACD-1 by electrophysiological analysis in Xenopus oocytes. We found that ACD-1 produced large voltage-independent currents (Figure 2A) that reversed at ∼+10 mV and that were sensitive to amiloride (Figure 2B). When we looked at the permeability to NaCl, LiCl and KCl, we found that the ACD-1 is permeable to Na+ and to a lower degree to Li+ and K+ (PNa≫PLi>PK) (Figure 2C). As ACD-1 is only marginally permeable to K+, we argue that the relatively low reversal potential of the current reflects the phenomenon of Na+ overload previously described in oocytes expressing constitutively active DEG/ENaC channels (Garcia-Anoveros et al, 1998; Goodman et al, 2002; Bianchi et al, 2004). In support of this, we found that smaller currents reversed at a potential closer to ENaC (not shown). We conclude that ACD-1 subunits form Na+-selective homomeric channels in Xenopus oocytes.
Figure 2.
Properties of wild-type and mutated ACD-1 channel. (A) Typical Na+ currents in an oocyte injected with acd-1 cRNA, perfused with a physiological NaCl solution. Voltage steps were from −160 to +100 mV from a holding potential of −30 mV. (B) Current–voltage relationships for oocytes injected with acd-1 cRNA when the oocytes are perfused with a physiological NaCl solution (open circles, n=15) and with a physiological saline plus 500 μM of DEG/ENaC channel blocker amiloride (filled circles, n=14). (C) Selectivity of ACD-1 channels to monovalent cations, established by determining the ratio of the inward current at −160 mV when perfusing the oocytes with NaCl, LiCl and KCl (n=8). (D) Typical Na+ currents in an oocyte injected with acd-1(S513V) cDNA, perfused with a physiological NaCl solution. Voltage steps were from −160 to +100 mV from a holding potential of −30 mV. The (S513V) mutation in ACD-1 is analogous to A713V in MEC-4(d) (Driscoll and Chalfie, 1991). (E) We perfused the same oocyte shown in (D) with a solution in which we substituted NaCl with CaCl2. Note that perfusing the oocytes with the CaCl2 solution does not activate the oocyte endogenous Ca2+-activated Cl− current that we have used in the past as a measure of MEC-4(d) permeability to Ca2+ (Bianchi et al, 2004). Similar results were obtained for wild-type ACD-1 channels. (F) S513V mutation does not hyperactivate ACD-1. Average current amplitude at −160 mV from oocytes injected with wild-type and S513V mutant ACD-1, n=5. The averages are not significantly different by t-test (P=0.12). (G) Amiloride dose–response curves for ACD-1 and ACD-1(S513V) (Ki=99 and 17 μM, respectively, n=5 for both). Data are expressed as mean±s.e. (H) Lack of voltage dependence of amiloride blockade in ACD-1 channels (n=5). (I) The (S513V) mutation in ACD-1 introduces voltage dependence of amiloride blockade in ACD-1 channels, suggesting that amino acid 513 is located within the membrane electric field (n=5). Data points were fitted with a Woodhull model (=0.14). Data are expressed as mean±s.e.
ACD-1 is not further activated by hyperactivating mutations and is impermeable to Ca2+
C. elegans DEG/ENaC channel MEC-4 is hyperactivated by Ala to Val or Thr substitutions at positions 713 in the channel pore (the (d) mutation) (Driscoll and Chalfie, 1991; Goodman et al, 2002). MEC-4(d) channels have higher open probability than MEC-4(+) channel, resulting in larger whole-cell currents (Brown et al, 2007). Other members of the family including C. elegans UNC-105 and mammalian MDEG, hINaC and BLINaC are hyperactivated by analogous substitutions (Garcia-Anoveros et al, 1998; Sakai et al, 1999; Schaefer et al, 2000). We wondered whether ACD-1 channels, which display large whole-cell currents in the wild-type form, could be further activated by mutating Ser513 (corresponding to Ala713 in MEC-4) to Val. We found that currents produced by injection of ACD-1(S513V) into Xenopus oocytes were similar in amplitude to currents generated by wild-type ACD-1 (Figure 2D and F).
We recently reported that MEC-4(d) is Ca2+ permeable and may contribute to necrosis initiation by elevating intracellular Ca2+ concentration (Bianchi et al, 2004). We thus asked whether ACD-1(d) channels were Ca2+ permeable. We perfused oocytes expressing ACD-1(d) with a solution in which we substituted NaCl with CaCl2 and found that the oocyte endogenous Ca2+-activated Cl− current, which we have used as a sign of Ca2+ permeability through MEC-4(d) (Bianchi et al, 2004), was not activated (Figure 2E). Similar results were obtained in oocytes expressing wild-type ACD-1 (not shown). These results indicate that ACD-1 and ACD-1(d) channels are not permeable to Ca2+. However, we found that S513V substitution changed amiloride block by reducing the concentration of amiloride needed to inhibit 50% of the current (Ki) from 99 to 17 μM (measured at −160 mV; Figure 2G).
Amiloride inhibits DEG/ENaC channels by interacting with sites within the pore, in the membrane electric field (Palmer, 1985; Garcia-Anoveros et al, 1998; Goodman et al, 2002). Thus, amiloride block in DEG/ENaCs is usually influenced by membrane voltage. We wondered whether this was the case for ACD-1 as well. We found that the Ki for amiloride is unaffected by the membrane potential in wild-type ACD-1, suggesting that the blocker interacts with an extracellular binding site (Figure 2H). However, the Ki becomes voltage dependent in ACD-1(S513V), suggesting that S513V mutation introduces or uncovers an amiloride-binding site within the channel pore (Figure 2I). These results indicate that S513 is in the pore, similar to analogous residue A432 in chicken ASIC1a (Jasti et al, 2007). Taken together, these data suggest ACD-1 pore structure is similar to other DEG/ENaCs.
DEG/ENaC channel ACD-1 is expressed in C. elegans glia
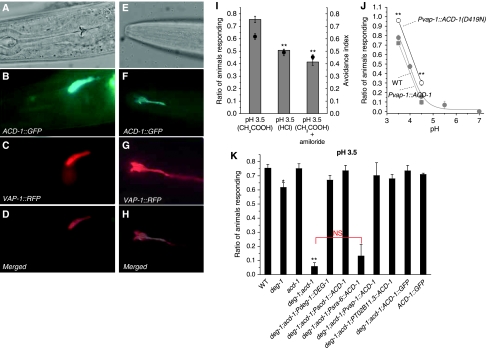
To gain insight into ACD-1 physiological function, we sought to determine ACD-1 expression pattern by analysing transgenic animals harbouring a translational ACD-1∷GFP construct. We found that transgenic animals strongly expressed ACD-1∷GFP in the amphid sheath cells, a pair of glial-like cells located in the head of the worm that can be visualized by the expression of sheath cell-specific marker VAP-1∷RFP (Figure 3A–H; Perens and Shaham, 2005). Amphid sheath cell processes run down to the tip of the nematode nose and wrap around the sensory neuron dendrites forming a channel through which sensory neurons, cilia (the sensory endings of sensory neurons) pass before reaching the external environment (Shaham, 2006).
Figure 3.
ACD-1∷GFP is expressed in the glial amphid sheath cells where it is needed for acid avoidance behaviour. (A, B) DIC and fluorescent (GFP channel) micrographs of a transgenic animal expressing Ex[ACD-1∷GFP;VAP-1∷RFP] showing the expression of ACD-1∷GFP in two large cells in the head of the worm (the other cell is on another focal plane) whose cell bodies are on either side of the pharyngeal bulb. vap-1 encodes for a venom-allergen-like-protein expressed in amphid sheath cells (Perens and Shaham, 2005). (C) The same animal was photographed using the rhodamine filter. This revealed overlapping of green (ACD-1∷GFP) and red (VAP-1∷RFP) signals (see (D), merged), establishing that ACD-1∷GFP is expressed in amphid sheath cells. (E–H) DIC and fluorescent micrographs taken with GFP and rhodamine filters of the tip of the nose of the same animal shown in (A–D), showing the expression of ACD-1∷GFP in amphid sheath cell processes (only one is shown here, the other one is on another focal plane). Front is left. (I, J) C. elegans exhibit an amiloride-sensitive aversive behaviour towards acidic solutions. (I) Average avoidance index to solutions in which pH was adjusted to 3.5 using acetic acid (CH3COOH) or HCl, measured using our drop test assay (see Materials and methods). For experiments in which we added amiloride, we preincubated animals in 1 mM amiloride for 30 min and then assayed adding amiloride to the pH 3.5 solution. Number of experiments performed was 8, 5 and 3, respectively. In each experiment, at least 10 animals were tested. (J) Quantification of ratio of animals responding (grey circles) at different pHs for wild-type C. elegans, deg-1;acd-1 mutants expressing wild-type ACD-1, and deg-1;acd-1 mutants expressing ACD-1(D419N). ACD-1 expression was driven in the glial amphid sheath cells by the vap-1 promoter (Perens and Shaham, 2005). We fitted the wild-type C. elegans pH/avoidance curve by sigmoidal equation, which gave pH0.5 values of 3.8. Data are expressed as mean±s.e. n of trials was 6–9 at pH 3.5 and 4.5 and 4–11 at the other pHs; at least 10 animals in each trial were tested. At least two transgenic lines were generated per construct and no statistical difference was found between data obtained from these lines. (K) ACD-1 functions with DEG-1 to mediate acid sensation. Ratio of animals responding to M13 solution buffered to pH 3.5 with acetic acid for wild-type, deg-1, acd-1, deg-1;acd-1 double mutants and deg-1;acd-1 double mutants expressing exogenous ACD-1 cDNA under the control, of acd-1 promoter, sra-6 promoter (Troemel et al, 1999) and glial-specific vap-1 and T02B11.3 promoters (Perens and Shaham, 2005) and (Supplementary Figure 2). ACD-1 expression in sheath cells but not ASH neurons rescues the acid insensitivity of deg-1;acd-1 mutants. ACD-1 construct used for the determining ACD-1 expression pattern, which includes acd-1 promoter and genomic sequence (B, F), also rescued acid avoidance defect of deg-1;acd-1 mutants but did not enhance acid avoidance when expressed in wild-type C. elegans. The figure also shows that a construct containing the deg-1 promoter that we have used for our expression pattern studies (Figure 4A) and deg-1 cDNA sequence (Pdeg-1∷DEG-1) rescues the acid avoidance deficit of deg-1;acd-1 mutant. Number of experiments was from 4 to 8 with at least 10 animals tested each. Data are expressed as mean±s.e. **, *P<0.01 and 0.05 by comparison with wild-type (WT) animals by t-test, respectively. NS stands for nonsignificant difference between the averages indicated by the red bracket by t-test.
ACD-1 channel functions with neuronal DEG/ENaC channel DEG-1 in acid avoidance behaviour
As C. elegans amphid sheath cells encircle the dendritic endings of sensory neurons, we considered the possibility that the glial channel ACD-1 might be involved in sensory perception. Because mammalian ASICs are gated by protons, we hypothesized that ACD-1 may have a function in acid sensation. C. elegans exhibit a strong aversive response to low pH environments on agar pH gradient plates (Sambongi et al, 2000). To investigate the contribution of ACD-1 channels to acid avoidance, we assayed acute responses in individual animals using ‘drop tests', in which a drop of acidic solution (either HCl or acetic acid) is delivered to a single animal (Hilliard et al, 2004). We observed that wild-type C. elegans showed pronounced escape responses to solutions of pH 3.5 in the drop test assay (Figure 3I). This response was partially dependent on the type of acid used for equal pH, as acetic acid, which induces intracellular acidification (Thomas, 1984; Zampighi et al, 1988), elicited a stronger avoidance behaviour than HCl, suggesting that intracellular acidification may have a key function in eliciting the response (Speake and Elliott, 1998). Interestingly, we also observed that the response was partially amiloride-sensitive (Figure 3I) and that the robustness of the escape response was dependent on the proton concentration (pH0.5 ∼4; Figure 3J). We next assayed acd-1 knockout animals (acd-1(bz90)) but found that they responded to pH 3.5 (Figure 3K).
Sambongi et al (2000) reported that combined laser ablation of ADF, ASE, ASK and ASH chemosensory neurons as well as of ADF/ASE, ASK/ASH, ADF/ASK and ASE/ASH pairs of sensory neurons diminishes C. elegans sensitivity to acidic solutions. We hypothesized that acd-1 knockout may exacerbate sensory defects caused by reduced sensory neuron function. The DEG/ENaC channel DEG-1 was previously reported to be expressed in ASH chemosensory neurons (Hall et al, 1997) and thus we first looked at the contribution DEG-1 to acid avoidance behaviour. We found deg-1-null mutant animals (deg-1(u38u421)) escaped pH 3.5 with reduced frequency compared with wild type, although the effect of the deg-1-null mutation was modest (Figure 3K). Strikingly, however, when we combined the deg-1-null mutation with acd-1 deletion, we found that more than 90% of the deg-1;acd-1 double mutants failed to respond to acidic solutions (Figure 3K). Thus, we conclude that ACD-1 and DEG-1 channels function in concert to mediate acid avoidance behaviour.
ACD-1 channel functions cell-autonomously in the glial cells to orchestrate acid avoidance behaviour
We found that ACD-1 is expressed in the amphid sheath cells. Oddly, neurons shown to contribute to acid sensation, including ASH (Sambongi et al, 2000), did not express ACD-1∷GFP. However, there are caveats to GFP expression experiments (reviewed in Rhoads et al, 2005) that leave open questions about whether ACD-1 might in fact act in the glial cells. To better test where ACD-1 channels function to control acid sensation, we expressed ACD-1∷GFP in ASH neurons and amphid sheath cells of deg-1;acd-1 mutants using cell-specific promoters. We found that although the expression of ACD-1 driven in ASH neurons by the sra-6 promoter (Troemel et al, 1995) did not rescue the acid avoidance defective phenotype of the deg-1;acd-1 double mutant, ACD-1 expression driven in amphid glia by two distinct and independent promoters (the promoters of the vap-1 and T02B11.3 genes Perens and Shaham, 2005) and Supplementary Figure 2) did (Figure 3K). Taken together, these data indicate that ACD-1 acts in glia to orchestrate acid avoidance behaviour in C. elegans.
DEG-1 is expressed in ASK sensory neurons
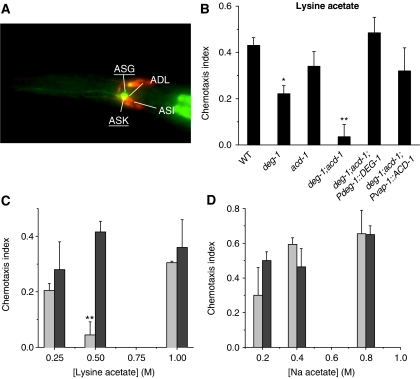
We expected that precise evaluation of the DEG-1 expression pattern would help clarify the molecular and cellular mechanisms of DEG-1-mediated acid sensing. We thus analysed transgenic animals harbouring a translational DEG-1∷GFP construct. We found that DEG-1∷GFP was expressed in ASK and ASG chemosensory neurons (Figure 4A). We did not detect expression of DEG-1∷GFP in ASH neurons (Hall et al, 1997) (although as our GFP construct did not include all deg-1 sequences, we cannot rule out that it might be). Interestingly though, we found that the exogenous expression of DEG-1 in deg-1;acd-1 double mutants under the control of the promoter that we used in our expression pattern studies, rescued acid avoidance behaviour (Figure 3K), suggesting that perhaps DEG-1 acts in ASK to contribute to acid avoidance (Sambongi et al, 2000). Importantly, ASK neurons mediate both acid avoidance and chemotaxis to lysine (Bargmann and Horvitz, 1991; Sambongi et al, 2000)—thus, our expression studies suggested one neuronal type that we could further probe for function in deg-1, acd-1 and deg-1;acd-1 mutants.
Figure 4.
DEG/ENaC channels ACD-1 and DEG-1 are also needed for lysine chemotaxis. (A) DEG-1 is expressed in ASK and ASG chemosensory neurons. Merged photographs of a transgenic C. elegans expressing DEG-1∷GFP stained with lipophylic dye DiO. Overlapping of green and red fluorescence identifies ASK sensory neurons as one of the neurons that expresses DEG-1∷GFP. Another nearby neuron that expresses DEG-1∷GFP was tentatively identified as ASG. DiO staining (red) is also evident in ADF and ASI sensory neurons. Dorsal view, front to the left. (B) Chemotaxis index towards lysine acetate was determined for wild-type, deg-1, acd-1 mutants and deg-1;acd-1 double mutant, deg-1;acd-1 double mutants expressing exogenous ACD-1 in amphid glia and deg-1;acd-1 double mutants expressing DEG-1 in sensory neurons. ACD-1 expression was under the control of glial-specific vap-1 promoter (Perens and Shaham, 2005), whereas DEG-1 expression was under its native promoter (see Materials and methods). The compromised chemotaxis of deg-1;acd-1 to lysine is rescued by the exogenous expression of ACD-1 in the glial amphid sheath cells and DEG-1 in sensory neurons. Number of trials was 5–14, with at least 30 animals assayed in each trial. (C) The chemotaxis index of wild-type (dark grey) and deg-1;acd-1 double mutant animals (light grey) was determined at different concentrations of lysine acetate (0.25, 0.5 and 1 M). n=4–11. (D) We determined the chemotaxis index to Na acetate for wild-type (dark grey) and deg-1;acd-1 double mutant animals (light grey) using different concentrations of Na acetate (0.2, 0.4 and 0.8 M). n=4–11. Data are expressed as mean±s.e. **, *P<0.01 and 0.05 by comparison with wild-type (WT) animals by t-test, respectively.
Because deg-1 is not thought to be expressed in the amphid sheath cells, our results also suggest that DEG-1 and ACD-1 are not part of the same channel complex; rather they function in different cell types to mediate acid sensation. Further support for this is that the addition of DEG-1 cRNA to oocytes expressing ACD-1 does not change ACD-1 currents in any measurable way (not shown).
ACD-1 and DEG-1 are needed for lysine chemotaxis
We assayed chemotaxis to lysine acetate on gradient plates (Bargmann and Horvitz, 1991). We found that, similar to acid avoidance, deg-1-null mutants are less attracted than wild-type animals to lysine and that acd-1 knockout greatly exacerbates deg-1-null phenotype (Figure 4B). We wondered whether the severe defect of deg-1;acd-1 could be explained by a reduced sensitivity to lysine acetate across a range of concentrations. We found that deg-1;acd-1 double mutant animals respond similarly to wild type to 0.25 and 1 M lysine acetate but they are severely impaired in detecting 0.5 M lysine acetate (Figure 4C). These results suggest that channels or receptors other than DEG-1 and ACD-1 may contribute to the mediation of lysine chemotaxis at concentrations lower and higher than 0.5 M. As lysine chemotaxis and acid avoidance are assayed using solutions in which acetic acid is present, we wondered whether reduced sensitivity to acetic acid could only explain the behavioural deficits that we observed in deg-1;acd-1 mutant animals. To address this question, we determined chemotaxis to a range of Na acetate concentrations. We found that deg-1;acd-1 chemotax to Na acetate, similar to wild-type animals at all the concentrations tested (Figure 4D).
Importantly, in deg-1;acd-1 mutant animals, glia and sensory neurons are viable and structurally normal, and deg-1;acd-1 mutants respond normally to all other attractants and noxious stimuli tested (Supplementary Figure 1). Thus, these results identify the first gene in C. elegans that specifically affects chemotaxis to lysine and acid avoidance and highlight glial channel ACD-1 as a modulator of these sensory functions.
ACD-1 channels are inhibited by protons
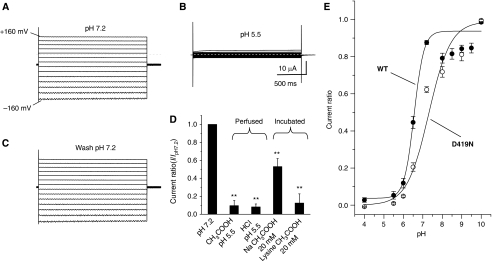
Given our demonstration that C. elegans DEG/ENaC channels DEG-1 and ACD-1 are needed for responding to acid, we investigated the possibility that protons directly affect DEG-1 and ACD-1 channels, similar to the mammalian ASICs (Krishtal, 2003). When we perfused oocytes expressing ACD-1 channels with a solution that was adjusted to pH 5.5 with acetic acid or HCl, we observed a strong suppression of ACD-1 currents (Figure 5A and B). The effect was completely reversible (Figure 5C). To establish whether ACD-1 channels are sensitive to intracellular acidification (Garty et al, 1987; Awayda et al, 2000), we assayed current amplitude after incubation of the oocytes expressing ACD-1 in a physiological saline at neutral pH containing 20 mM Na acetate known to cause intracellular acidification (the solution was washed away prior to electrophysiological recordings; Thomas, 1984; Zampighi et al, 1988). We found that ACD-1 currents in these oocytes were suppressed as compared with control oocytes (Figure 5D). This result suggests that intracellular acidification can inhibit the channel as well. In principle, lysine acetate should produce a similar effect because acetic acid acidifies the intracellular environment by entering the cell as uncharged HAcetate (Speake and Elliott, 1998) and this chemical species is present in both Na acetate and lysine acetate solutions. Indeed, when we assayed oocytes that were incubated in 20 mM lysine acetate, we found that ACD-1 currents were suppressed (Figure 5D). We did not observe any effect of lysine acetate at neutral pH (2–200 mM) acutely perfused onto the oocytes expressing ACD-1. By testing different extracellular proton concentrations, we observed that 6.4 is the pH that gives half-maximal inhibition and that the channel appears to have a single extracellular binding site for H+ (Figure 5E; fit with Hill's equation: Ki=6.4, n=0.9).
Figure 5.
DEDG/ENaC channel ACD-1 exhibits a strong sensitivity to acidic solutions. (A) Example of currents elicited by voltage steps from −160 to +100 mV from a holding potential of −30 mV in a Xenopus oocyte injected with acd-1 cRNA and exposed to a physiological NaCl saline solution at pH 7.2. (B) The same oocyte was exposed to a solution at pH 5.5, which resulted in the inhibition of ACD-1 currents. (C) When we returned to physiological pH 7.2, ACD-1 currents returned to control levels. (D) ACD-1 currents are equally suppressed by extracellular solutions buffered with acetic acid and HCl and are reduced by incubation with 20 mM extracellular Na acetate and lysine acetate, suggesting that both extracellular and intracellular acidification cause channel inhibition (Thomas, 1984; Zampighi et al, 1988). For incubation with 20 mM Na acetate and 20 mM lysine acetate, we incubated oocytes in the NaCl solution in which we added Na acetate or lysine acetate for 1 h at room temperature. Control oocytes were incubated under the same conditions in NaCl physiological saline. Oocytes were then transferred to control NaCl solution for 15 min prior to recordings. We could not recover currents to control levels after 3 h of wash in Na acetate- and lysine acetate-treated oocytes. This is not unusual and it has been observed by others using this technique for intracellular acidification (Thomas, 1984; Zampighi et al, 1988). n was 8 for each bar. (E) Sensitivity of wild-type ACD-1 and ACD-1(D419N) channels to extracellular pH. Currents were recorded at -160 mV, normalized to maximal currents and best fitted with the Hill's equation (Ki=pH 6.4, n=0.9 for wild-type ACD-1; Ki for ACD-1(D419N) was 7.3). n is 4–27. Data are expressed as mean±s.e. **Indicates P<0.01 by comparison with untreated oocytes by t-test.
What are the properties of DEG-1 channel? When we expressed deg-1 cDNA in Xenopus oocytes, we did not detect any current. Introducing the hyperactivating (d) mutation, or addition of postulated accessory subunits did not change the outcome of our experiments (Rajaram et al, 1998; Chelur et al, 2002; Goodman et al, 2002), thus we conclude that DEG-1 does not function in Xenopus oocytes probably because this system lacks nematode proteins/factors needed for DEG-1 function.
Analysis of an ACD-1 mutant uncovers a link between the channel pH sensitivity and C. elegans behaviour
If glial ACD-1 pH sensitivity has a function in acid avoidance behaviour in C. elegans, then the whole animal behaviour should be affected by changes in ACD-1 pH sensitivity. To test this hypothesis, we engineered mutation D419N in the postulated extracellular proton-binding region of ACD-1 (Jasti et al, 2007) (Supplementary Figure 3). D419 corresponds to D346 in chicken ASIC1a that when mutated to N renders the channel less sensitive to protons (Jasti et al, 2007). We expressed ACD-1(D419N) in Xenopus oocytes and assayed pH sensitivity. We found that ACD-1(D419N) generates currents with amplitude and properties similar to wild-type ACD-1 (not shown), but surprisingly, these currents are more rather than less sensitive to acidic pH, being inhibited with a Ki of pH 7.3 instead of 6.4 (Figure 5E, open circles). These data suggest that ACD-1 proton-binding site may be different from ASIC1a. We postulate that the increased acid sensitivity of ACD-1(D419N) may be due to rearrangements of the charges involved in proton binding resulting in higher net negative charge exposed in the extracellular domain of the mutant channel. Regardless of the structure/function implications of this finding, this mutant afforded us the possibility to establish whether a link exists between ACD-1 acid sensitivity and animal behaviour. We thus tested the effect of a more acid-sensitive ACD-1 on animal behaviour by expressing ACD-1(D419N) in the glial amphid sheath cells of deg-1;acd-1 mutant. We found that a higher percentage of deg-1;acd-1 animals that express ACD-1(D419N) respond to acidic solutions than wild-type animals (N2) or deg-1;acd-1 mutant animals that express wild-type ACD-1 (Figure 3J, open circles and filled squares). These results support that there is a link between ACD-1 acid sensitivity and C. elegans acid avoidance behaviour, suggesting that ACD-1 acid sensitivity plays a role in controlling acid avoidance.
Discussion
We have reported here the characterization of novel C. elegans DEG/ENaC channel ACD-1, which shares similarity with mammalian hINaC and BLINaC. We have shown that ACD-1 is a Na+-selective, constitutively open ion channel that cannot be further activated by hyperactivating (d) mutations and that is not permeable to Ca2+ ions. Our in vivo analysis of ACD-1 reveals that ACD-1 is expressed in C. elegans glia and that acd-1 knockout exacerbates sensory defects caused by deg-1 null, another DEG/ENaC ion channel that we show is expressed in ASK chemosensory neurons (among other neurons). We propose a mechanism of regulation of sensory activity by ACD-1 channels dependent on its sensitivity to intracellular and extracellular acidification.
A role for DEG/ENaC channels in chemosensation
Members of the DEG/ENaC channel family have been suggested as potential transduction channels for acid chemosensation in other systems. In particular, the mammalian ASIC channels, which are proton-activated cation channels, have been suggested as possible sensors in sour taste (Ugawa et al, 2003). However, studies of knockout mice lacking one such candidate taste receptor, ASIC2, failed to observe sensory defects in vivo (Richter et al, 2004). Although additional ASIC channel knockouts remain to be tested in this way, evidence is still lacking to definitively implicate this channel family in sour taste transduction. It is interesting that in C. elegans, a proton-inactivated DEG/ENaC channel with very different physiological properties from mammalian ASICs has a function in what can be viewed as the nematode counterpart of the sour taste sense. The physiological properties of ACD-1 are perhaps surprising given its role in regulating sensory perception. DEG/ENaC channels previously implicated in sensory transduction, such as the mechanosensory channel MEC-4, appear to share certain common characteristics: they are closed in the resting state, are gated by a sensory stimulus and in some cases are permeable to Ca2+ as well as Na+ (Bianchi et al, 2004; Xiong et al, 2004; O'Hagan et al, 2005). Channels similar to the canonical ENaC channel were thought to function only in epithelial cells and serve a primarily homoeostatic function (Canessa et al, 1994). Our findings here suggest that the functional distinctions between these DEG/ENaC subtypes may not be so clear cut, as a member of the latter class appears to have an important (perhaps non-canonical) function in sensory perception. Interestingly, Kretz et al (1999) reported the presence of mRNA for the α-, β- and γ-subunits of ENaCs as well as amiloride-binding sites in rat tongue, raising the possibility that a similar mechanism for sour (and perhaps salt) sensation, mediated by ENaCs, exists in mammals.
Glial cells and chemosensation in C. elegans
In the adult C. elegans hermaphrodite, there are 56 glial cells. The study of glia in C. elegans is still in its infancy, but elegant work to date has shown that glia are important for neuronal development and assembly (Shaham, 2006; Colon-Ramos et al, 2007; Yoshimura et al, 2008). For example, laser ablation of the amphid sheath cells leads to morphological abnormalities at the dendritic tips of several sensory neurons (T Bacaj and S Shaham, personal communication) (Shaham, 2006). We found that knockout of the glial sheath cell-expressed DEG/ENaC channel acd-1 does not have an effect on viability or morphology of associated neurons; rather, deficiency specifically affects acid and lysine sensitivity.
How might glia control sensory function in nematodes through the activity of an ion channel? One possibility is that the glial ACD-1 may help DEG-1-expressing neurons ASK and ASH, and other acid-sensing sensory neurons (i.e. ASE and ADL; Sambongi et al, 2000), to respond to sensory cues and that ACD-1 regulation of the function of these neurons may depend on the pH of the environment. In support of this hypothesis are our data that show acid insensitivity of the deg-1;acd-1 mutants (in which the activity of most acid-sensing neurons is possibly lost) being more severe than acid insensitivity of deg-1 null, and our data that link behaviour to the pH sensitivity of ACD-1. Another question is how to reconcile the fact that acd-1 knockout (loss of function) combined with deg-1 null causes behavioural loss of sensitivity to acidic solutions, when ACD-1 channels are actually inhibited by acidic pH. We speculate that transient changes in ACD-1 activity such as those caused by exposure to acidic environments mediate sensory response, whereas permanent loss of the channel causes acid insensitivity through a mechanism similar to sensory adaptation.
How might ACD-1 help sensory neurons respond to sensory cues? One possibility is that ACD-1 inhibition causes amphid sheath cell membrane hyperpolarization that in turn affects sensory neuron function through reduced release of neuromodulators or other factors by the sheath cells. This model implies that amphid sheath cells in C. elegans would actually contain and use neuromodulators. Although this has not been shown so far, amphid sheath cells do contain vesicles suggestive of secretion (Perkins et al, 1986). Another possibility is that proton block of ACD-1 increases the extracellular K+ concentration near sensory neuron dendrites through reduced activity of the Na+/K+ pump that relies on intracellular Na+ concentration for function and that this localized change in K+ environment changes sensory perception. Although our experiments do not distinguish between these possibilities, they clearly suggest a role of a glial-expressed channel in acid and lysine sensation. Future experiments in which the activity of sensory neurons and glia is probed by calcium imaging or electrophysiology will clarify the physiological consequences of acd-1 channel knockout on glia and neuronal function. Could mammalian hINaC and BLINaC be expressed in glia and function similarly? Mouse and rat BLINaC messenger RNA is found in the forebrain, cerebellum and brainstem (Sakai et al, 1999)—this channel may also be expressed in glia where it could have a function in controlling neuronal function.
Intracellular acidification and sour taste
Richter et al (2003) have shown that pH dose–response curves for Ca2+ transients elicited by sour stimuli in the mouse tongue differ depending on whether the acid stimulus used is HCl or citric acid, with the weaker citric acid being more potent. They argued that this is because weaker acids can more easily permeate the plasma membrane as undissociated species, thus causing more efficient acidification of the cytosol (Speake and Elliott, 1998). Thus, they conclude that taste receptor cells are responsive to both intracellular and extracellular acidification and suggest that intracellular acidification may have a key function in sour taste signal, as hypothesized earlier by Lyall et al (2001).
We found that the ACD-1 channel expressed in Xenopus oocytes was inhibited by both extracellular and intracellular acidification. When we tested the effect of strong and weak acid on behaviour, we found that acetic acid elicits a stronger response, suggesting that if stronger inhibition of the channel can be achieved by the action of both extracellular and intracellular protons, then the effect on behaviour is more robust. It is interesting though that the dose–response curves for pH effect on ACD-1 currents and animal behaviour do not overlap; this result may indicate that a nematode protein or factor tunes ACD-1 sensitivity to more acidic pH in vivo as shown for other DEG/ENaCs (reviewed in Bianchi and Driscoll, 2002). Although we have not identified the receptor for lysine, nor could we test whether DEG-1 is sensitive to this amino acid, we suggest that chemotaxis to lysine may be affected by ACD-1 inhibition by intracellular acidification. Thus, our results support the hypothesis of Richter et al (2003) that intracellular acidification may have a function in sour (and possibly umami) taste transduction.
Insights into the role of glia in sensory perception and pain
Our work is in line with this newly discovered role of glia in neuronal function. Indeed, recent studies have shown that glia are critical for the development of the nervous system and have a key function in neuronal disorders, including multiple sclerosis, amyotrophic lateral sclerosis, spinocerebellar ataxia, Parkinson's disease, Huntington's disease, ischaemia, neuropathic pain and epilepsy (reviewed in Iadecola and Nedergaard, 2007; Lobsiger and Cleveland, 2007; Rossi et al, 2007; Scholz and Woolf, 2007). Indirectly, glia regulate neuronal function by controlling the concentration of ions, neurotransmitters and modulators in the extracellular space (Kuffler, 1967; Cohen et al, 1968). Glial cells also modulate neuronal excitability directly, by releasing transmitters or co-agonists onto neurons (Volterra and Meldolesi, 2005; Vesce et al, 2007).
Our study proposes considerations on the mechanism of pain sensation associated with inflammation and tissue damage as well. Both these conditions produce a significant local increase of the proton concentration that causes the excitation of nociceptors that in turn evoke pain (Meyer et al, 2005). Our results suggest that the glial ion channels may participate in the amplification of the pain signal and thus may be considered as targets for the treatment of inflammation and neuropathic pain.
Materials and methods
Molecular biology
The ACD-1∷GFP vector was created by introducing an MscI fragment including the acd-1 promoter (2.55 kb prior to the start codon) and genomic sequence (minus stop codon) in frame with GFP into vector pPD95.77 (constructed by Scott Clark, NYU). To drive the expression of ACD-1∷GFP in ASH neurons, we used the sra-6 promoter (Troemel et al, 1995) and acd-1 cDNA sequence, respectively. To construct Pacd-1∷ACD-1, we substituted acd-1 genomic sequence in ACD-1∷GFP vector with acd-1 cDNA. To drive the expression of ACD-1 in amphid sheath cells, we swapped the sra-6 promoter with vap-1 promoter (2 kb upstream of the start codon) and the promoter of the T02B11.3 gene (2 kb upstream of the start codon) to generate Pvap-1∷ACD-1 and PT02B11.3∷ACD-1. DEG-1∷GFP was constructed by the amplification of the promoter (2.5 kb) and most genomic sequence (4.8 kb) of deg-1 and subcloning into pPD95.75 vector in frame with GFP. This DNA construct is expected to be translated into a DEG-1 protein missing the second transmembrane domain and C terminus. The DEG-1 rescue construct (Pdeg-1∷DEG-1) was generated by swapping the deg-1 genomic sequence in DEG-1∷GFP with deg-1 cDNA sequence after the BglII restriction site.
deg-1 cDNA was amplified from total C. elegans RNA using the RT–PCR Titan kit from Clontech. Restriction sites BamHI and HindIII were added to sense and antisense primers to allow subcloning into PGEM-HE. The 5′ end of acd-1 cDNA (from the start to 464 bp, corresponding to SwaI restriction site) was amplified similarly using the Clontech Titan kit, whereas the 3′ end (from 464 bp to the stop codon) was obtained from EST clone yk1553g01, kindly provided by Yuji Kohara. D419N mutation was introduced into ACD-1 using QuikChange from Stratagene. Prior to subcloning into pGEM-HE, pPD95.75 or pPD95.77, RT–PCR products were cloned into TOPO pCR2.1 vector for amplification and sequencing.
C. elegans strains and growth
Nematode strains were maintained at 20°C on standard nematode growth medium seeded with Escherichia coli strain OP50 (Brenner, 1974). For GFP expression and ACD-1∷GFP rescue studies, plasmids were injected into lin-15(n765) and deg-1(u38u421);acd-1(bz90) strains, respectively. Mutant strain deg-1(u38u421);acd-1(bz90) was obtained by standard crossing. Mutations were followed and confirmed by PCR. deg-1(u38u421) allele likely encodes for a non-functional, as it suppresses neurodegeneration caused by neurotoxic hypermorphic deg-1(u38) mutant (Chalfie and Wolinsky, 1990; Hart et al, 1999). acd-1(bz90) deletion mutant strain was outcrossed three times.
Dye-filling experiments
Animals were picked to a plate containing 1,1′-dioctodecyl-3,3,3′,3′-tetramethylindodicarbocyanine, 4-chlorobenzinesulphonate (DiO; Molecular Probes) diluted in M9 buffer (in mM: 85 NaCl, 42.3 Na2HPO4 and 22.2 KH2PO4) (0.01 mg/ml) and allowed to stain for 3 h at room temperature. Worms were then transferred to an agar plate and allowed to crawl on the bacterial lawn for 15 min to destain. Photographs were taken with a Leica microscope equipped with digital camera using rhodamine and GFP filters.
Behavioural assays
A drop of M13 solution (30 mM Tris, 100 mM NaCl, 10 mM KCl) at a specific pH was delivered near the tail of the forward-moving animal on an unseeded plate. When the solution was acidic, the animal reversed; otherwise it continued to move forward. Each animal was tested with five drops. We waited for at least 1 min between each drop. The response to each drop delivered was recorded as either a positive or negative response and the animal avoidance index was calculated (AI=positive/total drops). We also expressed the data as ratio of animals responding (a ‘responder' responded to at least three out of five trails). For lysine acetate and Na acetate assays, we followed procedures described in Bargmann and Horvitz (1991). Chemotaxis index (CI) was determined as follows: (number of animals at attractant−number of animals at control)/(total number of animals).
Oocyte expression and electrophysiology
Capped cRNAs were synthesized using T7 mMESSAGE mMACHINE kit (Ambion), purified (Qiagen RNAeasy columns) and run on denaturating agarose gels to check for size and integrity. cRNA quantification was then performed spectroscopically. Stage V–VI oocytes were manually defolliculated after selecting them among multistaged oocytes dissected by 2 h collagenase treatment (2 mg/ml in Ca2+-free OR2 solution) from Xenopus laevis ovaries (NASCO). Oocytes were incubated in OR2 media (82.5 mM NaCl, 2.5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 1 mM Na2HPO4, 0.5 g/l polyvinyl pyrrolidone and 5 mM HEPES, pH 7.2), supplemented with penicillin and streptomycin (0.1 mg/ml) and 2 mM Na pyruvate. Oocytes were injected with 52 nl of cRNA mix for a final amount of 5 ng/oocyte. Oocytes were incubated in OR2 at 20°C for 2–4 days before recording.
Currents were measured using a two-electrode voltage clamp amplifier (GeneClamp 500B; Axon Instruments) at room temperature. Electrodes (0.3–1 M) were filled with 3 M KCl and oocytes were perfused with a NaCl solution containing (in mM): 100 NaCl, 2 KCl, 1 CaCl2, 2 MgCl2, 10 HEPES, pH 7.2 or with a CaCl2 solution containing 73 CaCl2, 2 KCl, 10 HEPES, pH 7.2. When we tested solutions at pH lower than 6, we used 10 mM 2-(N-morpholino)ethanesulphonic acid instead of 10 mM HEPES to buffer the solutions. We used the pCLAMP suite of programs (Axon Instruments) for data acquisition and analysis. Currents were filtered at 200 Hz and sampled at 1 kHz.
Supplementary Material
Supplementary Figures 1
Supplementary Figures 2
Supplementary Figures 3
Supplementary Information
Acknowledgments
We thank the C. elegans Genetics Center and Theresa Stiernagle for providing nematode strains and Yuji Kohara for providing the yk1553g01 EST clone. We thank Ann Hart for the deg-1(u38u421) strain. We are grateful to Steven Roper for critical reading of the paper. This study is dedicated to the memory of Silvio Gavioli. This study was supported by grants from the National Institutes of Health (NS049511 to LB, NS034435 and NS37955 to MD, and DA016445 and DA018341 to WRS). SS is a Klingenstein fellow in the neurosciences and a Monique Weill-Caulier scholar.
References
- Askwith CC, Benson CJ, Welsh MJ, Snyder PM (2001) DEG/ENaC ion channels involved in sensory transduction are modulated by cold temperature. Proc Natl Acad Sci USA 98: 6459–6463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awayda MS, Boudreaux MJ, Reger RL, Hamm LL (2000) Regulation of the epithelial Na(+) channel by extracellular acidification. Am J Physiol Cell Physiol 279: C1896–C1905 [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Horvitz HR (1991) Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron 7: 729–742 [DOI] [PubMed] [Google Scholar]
- Barker PM, Nguyen MS, Gatzy JT, Grubb B, Norman H, Hummler E, Rossier B, Boucher RC, Koller B (1998) Role of gammaENaC subunit in lung liquid clearance and electrolyte balance in newborn mice. Insights into perinatal adaptation and pseudohypoaldosteronism. J Clin Invest 102: 1634–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benos DJ, Stanton BA (1999) Functional domains within the degenerin/epithelial sodium channel (Deg/ENaC) superfamily of ion channels. J Physiol 520 (Part 3): 631–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdiev BK, Xia J, McLean LA, Markert JM, Gillespie GY, Mapstone TB, Naren AP, Jovov B, Bubien JK, Ji HL, Fuller CM, Kirk KL, Benos DJ (2003) Acid-sensing ion channels in malignant gliomas. J Biol Chem 278: 15023–15034 [DOI] [PubMed] [Google Scholar]
- Bianchi L, Driscoll M (2002) Protons at the gate: DEG/ENaC ion channels help us feel and remember. Neuron 34: 337–340 [DOI] [PubMed] [Google Scholar]
- Bianchi L, Driscoll M (2004) The molecular basis of touch sensation as modeled in Caenorhabditis elegans. Transduction Channels in Sensory Cells, Frings S, Bradley J (eds), pp 1–30. Weinheim, Germany: Wiley Publisher [Google Scholar]
- Bianchi L, Gerstbrein B, Frokjaer-Jensen C, Royal DC, Mukherjee G, Royal MA, Xue J, Schafer WR, Driscoll M (2004) The neurotoxic MEC-4(d) DEG/ENaC sodium channel conducts calcium: implications for necrosis initiation. Nat Neurosci 7: 1337–1344 [DOI] [PubMed] [Google Scholar]
- Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77: 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockway LM, Benos DJ, Keyser KT, Kraft TW (2005) Blockade of amiloride-sensitive sodium channels alters multiple components of the mammalian electroretinogram. Vis Neurosci 22: 143–151 [DOI] [PubMed] [Google Scholar]
- Brown AL, Fernandez-Illescas SM, Liao Z, Goodman MB (2007) Gain-of-function mutations in the MEC-4 DEG/ENaC sensory mechanotransduction channel alter gating and drug blockade. J Gen Physiol 129: 161–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canessa CM, Horsiberger JD, Rossier BC (1993) Functional cloning of the epithelial sodium channel relation with genes involved in neurodegeneration. Nature 349: 588–593 [Google Scholar]
- Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC (1994) Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 367: 412–413 [DOI] [PubMed] [Google Scholar]
- Chalfie M, Sulston J (1981) Developmental genetics of the mechanosensory neurons of Caenorhabditis elegans. Dev Biol 82: 358–370 [DOI] [PubMed] [Google Scholar]
- Chalfie M, Wolinsky E (1990) The identification and suppression of inherited neurodegeneration in Caenorhabditis elegans. Nature 345: 410–416 [DOI] [PubMed] [Google Scholar]
- Chelur DS, Ernstrom GG, Goodman MB, Yao CA, Chen L, O'Hagan R, Chalfie M (2002) The mechanosensory protein MEC-6 is a subunit of the C. elegans touch-cell degenerin channel. Nature 420: 669–673 [DOI] [PubMed] [Google Scholar]
- Chen CC, Zimmer A, Sun WH, Hall J, Brownstein MJ (2002) A role for ASIC3 in the modulation of high-intensity pain stimuli. Proc Natl Acad Sci USA 99: 8992–8997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MW, Gerschenfeld HM, Kuffler SW (1968) Ionic environment of neurones and glial cells in the brain of an amphibian. J Physiol 197: 363–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colon-Ramos DA, Margeta MA, Shen K (2007) Glia promote local synaptogenesis through UNC-6 (netrin) signaling in C. elegans. Science 318: 103–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll M, Chalfie M (1991) The mec-4 gene is a member of a family of Caenorhabditis elegans genes that can mutate to induce neuronal degeneration. Nature 349: 588–593 [DOI] [PubMed] [Google Scholar]
- Garcia-Anoveros J, Garcia JA, Liu JD, Corey DP (1998) The nematode degenerin UNC-105 forms ion channels that are activated by degeneration- or hypercontraction-causing mutations. Neuron 20: 1231–1241 [DOI] [PubMed] [Google Scholar]
- Garty H, Asher C, Yeger O (1987) Direct inhibition of epithelial Na+ channels by a pH-dependent interaction with calcium, and by other divalent ions. J Membr Biol 95: 151–162 [DOI] [PubMed] [Google Scholar]
- Garty H, Palmer LG (1997) Epithelial sodium channels: function, structure, and regulation. Physiol Rev 77: 359–396 [DOI] [PubMed] [Google Scholar]
- Golestaneh N, Nicolas C, Picaud S, Ferrari P, Mirshahi M (2000) The epithelial sodium channel (ENaC) in rodent retina, ontogeny and molecular identity. Curr Eye Res 21: 703–709 [PubMed] [Google Scholar]
- Goodman MB, Ernstrom GG, Chelur DS, O'Hagan R, Yao CA, Chalfie M (2002) MEC-2 regulates C. elegans DEG/ENaC channels needed for mechanosensation. Nature 415: 1039–1042 [DOI] [PubMed] [Google Scholar]
- Hall DH, Gu G, García-Añoveros J, Gong L, Chalfie M, Driscoll M (1997) Neuropathology of degenerative cell death in C. elegans. J Neurosci 17: 1033–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AC, Kass J, Shapiro JE, Kaplan JM (1999) Distinct signaling pathways mediate touch and osmosensory responses in a polymodal sensory neuron. J Neurosci 19: 1952–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard MA, Bergamasco C, Arbucci S, Plasterk RH, Bazzicalupo P (2004) Worms taste bitter: ASH neurons, QUI-1, GPA-3 and ODR-3 mediate quinine avoidance in Caenorhabditis elegans. EMBO J 23: 1101–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummler E, Barker P, Gatzy J, Beermann F, Verdumo C, Schmidt A, Boucher R, Rossier BC (1996) Early death due to defective neonatal lung liquid clearance in alpha-ENaC-deficient mice. Nat Genet 12: 325–328 [DOI] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M (2007) Glial regulation of the cerebral microvasculature. Nat Neurosci 10: 1369–1376 [DOI] [PubMed] [Google Scholar]
- Jasti J, Furukawa H, Gonzales EB, Gouaux E (2007) Structure of acid-sensing ion channel 1 at 1.9 Å resolution and low pH. Nature 449: 316–323 [DOI] [PubMed] [Google Scholar]
- Jospin M, Mariol MC, Segalat L, Allard B (2004) Patch clamp study of the UNC-105 degenerin and its interaction with the LET-2 collagen in Caenorhabditis elegans muscle. J Physiol 557: 379–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretz O, Barbry P, Bock R, Lindemann B (1999) Differential expression of RNA and protein of the three pore-forming subunits of the amiloride-sensitive epithelial sodium channel in taste buds of the rat. J Histochem Cytochem 47: 51–64 [DOI] [PubMed] [Google Scholar]
- Krishtal O (2003) The ASICs: signaling molecules? Modulators? Trends Neurosci 26: 477–483 [DOI] [PubMed] [Google Scholar]
- Kuffler SW (1967) Neuroglial cells: physiological properties and a potassium mediated effect of neuronal activity on the glial membrane potential. Proc R Soc Lond B Biol Sci 168: 1–21 [DOI] [PubMed] [Google Scholar]
- Lin H, Mann KJ, Starostina E, Kinser RD, Pikielny CW (2005) A Drosophila DEG/ENaC channel subunit is required for male response to female pheromones. Proc Natl Acad Sci USA 102: 12831–12836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobsiger CS, Cleveland DW (2007) Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disease. Nat Neurosci 10: 1355–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall V, Alam RI, Phan DQ, Ereso GL, Phan TH, Malik SA, Montrose MH, Chu S, Heck GL, Feldman GM, DeSimone JA (2001) Decrease in rat taste receptor cell intracellular pH is the proximate stimulus in sour taste transduction. Am J Physiol Cell Physiol 281: C1005–C1013 [DOI] [PubMed] [Google Scholar]
- Meyer R, Ringkamp M, Campbell J, Raja S (2005) Peripheral mechanisms of cutaneous nociception. In Wall and Melzack's Textbook of Pain, McMahon SB, Koltzenburg M (eds). Edinburgh, Scotland: Churchill Livingstone [Google Scholar]
- Naves LA, McCleskey EW (2005) An acid-sensing ion channel that detects ischemic pain. Braz J Med Biol Res 38: 1561–1569 [DOI] [PubMed] [Google Scholar]
- O'Hagan R, Chalfie M, Goodman MB (2005) The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat Neurosci 8: 43–50 [DOI] [PubMed] [Google Scholar]
- Palmer LG (1985) Interactions of amiloride and other blocking cations with the apical Na channel in the toad urinary bladder. J Membr Biol 87: 191–199 [DOI] [PubMed] [Google Scholar]
- Perens EA, Shaham S (2005) C. elegans daf-6 encodes a patched-related protein required for lumen formation. Dev Cell 8: 893–906 [DOI] [PubMed] [Google Scholar]
- Perkins LA, Hedgecock EM, Thomson JN, Culotti JG (1986) Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev Biol 117: 456–487 [DOI] [PubMed] [Google Scholar]
- Price MP, Lewin GR, McIlwrath SL, Cheng C, Xie J, Heppenstall PA, Stucky CL, Mannsfeldt AG, Brennan TJ, Drummond HA, Qiao J, Benson CJ, Tarr DE, Hrstka RF, Yang B, Williamson RA, Welsh MJ (2000) The mammalian sodium channel BNC1 is required for normal touch sensation. Nature 407: 1007–1011 [DOI] [PubMed] [Google Scholar]
- Price MP, McIlwrath SL, Xie J, Cheng C, Qiao J, Tarr DE, Sluka KA, Brennan TJ, Lewin GR, Welsh MJ (2001) The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron 32: 1071–1083 [DOI] [PubMed] [Google Scholar]
- Rajaram S, Sedensky MM, Morgan PG (1998) Unc-1: a stomatin homologue controls sensitivity to volatile anesthetics in Caenorhabditis elegans. Proc Natl Acad Sci USA 95: 8761–8766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads RE, Dinkova TD, Korneeva NL (2005) Mechanism and regulation of translation in C. elegans. WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.7.1, http://www.wormbook.org [DOI] [PMC free article] [PubMed]
- Richter TA, Caicedo A, Roper SD (2003) Sour taste stimuli evoke Ca2+ and pH responses in mouse taste cells. J Physiol 547: 475–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter TA, Dvoryanchikov GA, Roper SD, Chaudhari N (2004) Acid-sensing ion channel-2 is not necessary for sour taste in mice. J Neurosci 24: 4088–4091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Brady JD, Mohr C (2007) Astrocyte metabolism and signaling during brain ischemia. Nat Neurosci 10: 1377–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H, Lingueglia E, Champigny G, Mattei MG, Lazdunski M (1999) Cloning and functional expression of a novel degenerin-like Na+ channel gene in mammals. J Physiol 519 (Part 2): 323–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambongi Y, Takeda K, Wakabayashi T, Ueda I, Wada Y, Futai M (2000) Caenorhabditis elegans senses protons through amphid chemosensory neurons: proton signals elicit avoidance behavior. NeuroReport 11: 2229–2232 [DOI] [PubMed] [Google Scholar]
- Schaefer L, Sakai H, Mattei M, Lazdunski M, Lingueglia E (2000) Molecular cloning, functional expression and chromosomal localization of an amiloride-sensitive Na(+) channel from human small intestine. FEBS Lett 471: 205–210 [DOI] [PubMed] [Google Scholar]
- Scholz J, Woolf CJ (2007) The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci 10: 1361–1368 [DOI] [PubMed] [Google Scholar]
- Shaham S (2006) Glia–neuron interactions in the nervous system of Caenorhabditis elegans. Curr Opin Neurobiol 16: 522–528 [DOI] [PubMed] [Google Scholar]
- Shreffler W, Magardino T, Shekdar K, Wolinsky E (1995) The unc-8 and sup-40 genes regulate ion channel function in Caenorhabditis elegans motorneurons. Genetics 139: 1261–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ (2003) Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain 106: 229–239 [DOI] [PubMed] [Google Scholar]
- Speake T, Elliott AC (1998) Modulation of calcium signals by intracellular pH in isolated rat pancreatic acinar cells. J Physiol 506 (Part 2): 415–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavernarakis N, Shreffler W, Wang S, Driscoll M (1997) unc-8, a DEG/ENaC family member, encodes a subunit of a candidate mechanically gated channel that modulates C. elegans locomotion. Neuron 18: 107–119 [DOI] [PubMed] [Google Scholar]
- Thomas RC (1984) Experimental displacement of intracellular pH and the mechanism of its subsequent recovery. J Physiol 354: 3P–22P [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troemel ER, Chou JH, Dwyer ND, Colbert HA, Bargmann CI (1995) Divergent seven transmembrane receptors are candidate chemosensory receptors in C. elegans. Cell 83: 207–218 [DOI] [PubMed] [Google Scholar]
- Troemel ER, Sagasti A, Bargmann CI (1999) Lateral signaling mediated by axon contact and calcium entry regulates asymmetric odorant receptor expression in C. elegans. Cell 99: 387–398 [DOI] [PubMed] [Google Scholar]
- Ugawa S, Yamamoto T, Ueda T, Ishida Y, Inagaki A, Nishigaki M, Shimada S (2003) Amiloride-insensitive currents of the acid-sensing ion channel-2a (ASIC2a)/ASIC2b heteromeric sour-taste receptor channel. J Neurosci 23: 3616–3622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesce S, Rossi D, Brambilla L, Volterra A (2007) Glutamate release from astrocytes in physiological conditions and in neurodegenerative disorders characterized by neuroinflammation. Int Rev Neurobiol 82: 57–71 [DOI] [PubMed] [Google Scholar]
- Volterra A, Meldolesi J (2005) Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci 6: 626–640 [DOI] [PubMed] [Google Scholar]
- Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, MacDonald JF, Wemmie JA, Price MP, Welsh MJ, Simon RP (2004) Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell 118: 687–698 [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Murray JI, Lu Y, Waterston RH, Shaham S (2008) mls-2 and vab-3 control glia development, hlh-17/Olig expression and glia-dependent neurite extension in C. elegans. Development 135: 2263–2275 [DOI] [PubMed] [Google Scholar]
- Zampighi G, Kreman M, Ramon F, Moreno AL, Simon SA (1988) Structural characteristics of gap junctions. I. Channel number in coupled and uncoupled conditions. J Cell Biol 106: 1667–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1
Supplementary Figures 2
Supplementary Figures 3
Supplementary Information