Abstract
The Ras family G-proteins RalA and RalB make critical non-overlapping contributions to the generation of a tumorigenic regulatory network, supporting bypass of the normal restraints on both cell proliferation and survival. The Sec6/8 complex, or exocyst, has emerged as a principal direct effector complex for Ral GTPases. Here, we show that RalA and RalB support mitotic progression through mobilization of the exocyst for two spatially and kinetically distinct steps of cytokinesis. RalA is required to tether the exocyst to the cytokinetic furrow in early cytokinesis. RalB is then required for recruitment of the exocyst to the midbody of this bridge to drive abscission and completion of cytokinesis. The collaborative action of RalA and RalB is specified by discrete subcellular compartmentalization and unique pairs of RalGEF proteins that provide inputs from both Ras-family protein-dependent and protein-independent regulatory cues. This suggests that Ral GTPases integrate diverse upstream signals to choreograph multiple roles for the exocyst in mitotic progression.
Keywords: cytokinesis, exocyst, Ral GTPases, RalGEFs
Introduction
The Ral GTPases belong to the Ras branch of the superfamily of monomeric GTPases (Valencia et al, 1991). Interest in Ral function increased following the discovery that Ral proteins acted as proximal effectors of oncogenic Ras, that their activity is essential to support tumorigenesis of human epithelial cells of diverse origin and can be even sufficient in some genetic backgrounds to drive oncogenic transformation (Hamad et al, 2002; Chien and White, 2003; Rangarajan et al, 2004; Sablina et al, 2007).
The two Ral genes in mammals, RalA and RalB, encode highly homologous proteins (81% identity, 89% similarity), which make distinct and sometimes collaborative contributions to diverse cellular functions. RalA drives exocyst assembly and polarized exocytosis in epithelial cells (Moskalenko et al, 2002; Shipitsin and Feig, 2004), whereas RalB drives exocyst assembly during polarized cell migration (Rosse et al, 2006). RalA and B cooperate to support oncogenesis, as RalA is required for anchorage-independent proliferation and tumorigenesis, and RalB for metastasis formation and cell survival (Chien and White, 2003; Chien et al, 2006; Lim et al, 2006).
In contrast to the diversity of their biological functions (Feig, 2003; Camonis and White, 2005), Ral GTPases exert an effect through a limited number of well-documented Ral-GTP functional effectors, RalBP1/RLIP76 and the exocyst complex, being the best documented. RalBP1/RLIP76 participates in the regulation of a subset of endocytic pathways (Nakashima et al, 1999; Jullien-Flores et al, 2000; Rosse et al, 2003). The exocyst complex, in addition to its structural role in vesicle–plasma membrane tethering, functions as a signalling complex through direct interactions with effector kinases (Moskalenko et al, 2002; Feig, 2003; Camonis and White, 2005; Balakireva et al, 2006; Chien et al, 2006).
The exocyst complex is required for appropriate completion of cytokinesis (Wang et al, 2002; Dobbelaere and Barral, 2004; Gromley et al, 2005; VerPlank and Li, 2005). The correct course of cytokinesis, the ultimate step of cell division, is essential for high-fidelity chromosomal segregation. In metazoans, cytokinesis can be divided into two steps. Actino-myosin ring formation and cleavage furrow ingression generate a cytoplasmic bridge, whose abscission requires mobilization of secretory vesicles and exocytosis (Echard et al, 2004; Eggert et al, 2004; Schweitzer and D'Souza-Schorey, 2004; Skop et al, 2004; Albertson et al, 2005; Glotzer, 2005; Gromley et al, 2005; Schweitzer et al, 2005). In metazoans, the exocyst complex is required for late cytokinesis and is recruited to the midbody for the resolution of the intracellular bridge (Gromley et al, 2005).
Here, we have examined the role of Ral GTPases, their effectors and their activators, the RalGEFs, during cytokinesis. Although RalA has been shown to be required for the completion of cytokinesis (Chen et al, 2006), we found that RalA and RalB make collaborative exocyst-dependent contributions to cytokinesis that are distinct in space and time. The non-overlapping roles of RalA and RalB are specified by unique pairs of RalGEF proteins that provide inputs from both Ras-family protein-dependent and protein-independent regulatory cues, suggesting that each Ral GTPase functions as a signalling integration node from several transduction pathways. In this model, the concomitant correct functioning of these pathways would be required to permit progression of cytokinesis beyond RalA- and RalB-controlled specific steps.
Results
RalA and RalB make distinct contributions to cytokinesis
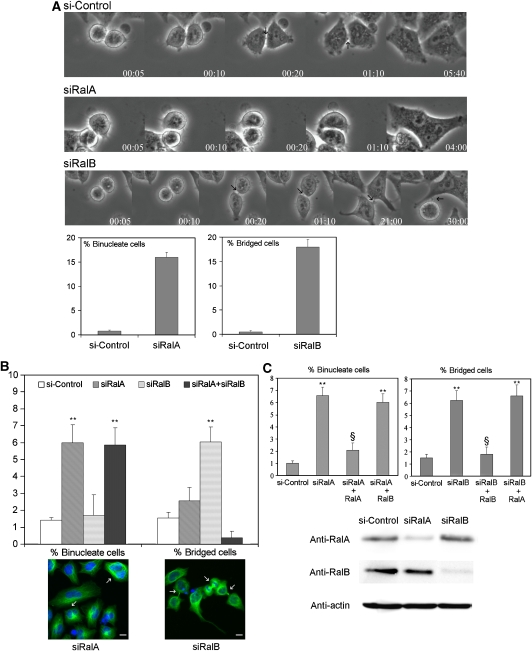
Mitotic failure as a consequence of cytokinesis defects can manifest as the fusion of nascent ‘daughter cells' leading to binucleate cells or in prolonged daughter cell attachments as a consequence of delayed or failed abscission (Echard et al, 2004; Fielding et al, 2005; Gromley et al, 2005). To examine the explicit contributions of RalA and RalB to cytokinesis, we monitored mitotic progression upon siRNA-mediated depletion. Real-time imaging of control cells demonstrated complete cleavage furrow ingression 5 min post-telophase initiation and the appearance of intracellular bridges around 10–20 min post-telophase initiation. Cytokinesis appeared complete by approximately 5 h after telophase start (Figure 1A, upper panel, and Supplementary Movie S1). By contrast, depletion of RalA resulted in cytokinesis regression, whereas depletion of RalB resulted in abscission failure. RalA-depleted cells (Figure 1A, middle panel, and Supplementary Movie S2) often did not spread properly, and in 16% of cells entering mitosis, furrows regressed, resulting in binucleate cells (Figure 1A). In siRalB-treated cells (Figure 1A, lower panel, and Supplementary Movie S3), the intracellular bridge appeared correctly (00:20, arrow), but approximately 20% of the cells failed to complete abscission.
Figure 1.
Depletion of RalA induces accumulation of binucleate cells, whereas depletion of RalB induces accumulation of cells with an intracellular bridge. (A) Selected phase-contrast time-lapse videomicroscopy frames of representative cytokinesis steps from HeLa cells transfected with control siRNA (upper panel) or siRNA targeting RalA (central panel) or RalB (lower panel). In control cells, the intracellular bridge appeared 20 min after the beginning of the process (arrows). In RalA-depleted cells, the intracellular bridge did not appear and cells fused back giving binucleate cells. In RalB-depleted cells, the intracellular bridge formed timely and stayed for an extended period without abscission. In some cases, one of the daughter cells re-entered mitosis still bound to its sister cell (Figure 1A, arrow in last image of third panel). For every video, 50 cells entering mitosis were tracked. In RalA-depleted populations, we counted the frequency of binucleate cells (lower left graph), and in RalB-depleted population, the frequency of cells failing to resolve their intracellular bridge at the end of the video (lower right graph). (B) HeLa cells were depleted of RalA or RalB or both by siRNA (10 nM). Cells were fixed after 72 h and stained (lower panels) with a nuclear marker (DAPI) (blue) and an anti-tubulin FITC mAb to mark the intracellular bridge (green). Binucleate cells (arrows in the left picture) or cells with an intracellular bridge (arrows in the right picture) were counted. In the graph, the percentages of binucleate or bridged cells are shown, with an indicated statistical significance (**P<0.01). (C) HeLa cells were depleted of RalA or RalB by a second independent siRNA, and transfected with plasmids expressing Myc–RalA or Myc–RalB, or an empty vector. Myc–RalA was made insensitive to siRalA by making silent mutations in three nucleotides within the siRNA target sequence. Myc–RalB was not mutagenized, as the siRalB target sequence is within the 3′-UTR and as pRK5–Myc–RalB carries only RalB coding region. Binucleate cells or cells with an intracellular bridge were counted as in (B). In the graphs, the percentage of binucleate or bridged cells are shown, and statistical significance of the difference with control siRNA (**P<0.01) is indicated, as is the case for rescue compared with siRal (§P<0.01). In all experiments, at least 1000 cells were counted, and results are from at least three independent experiments. Efficiency and specificity of depletion of RalA and RalB by the siRNAs were tested by western blots (lower panel).
The RalA- and RalB-dependent cytokinesis phenotypes were quantified in fixed samples (Figure 1B). Depleting RalA significantly increased the percentage of binucleate cells and not intracellular bridges, whereas depleting RalB had no effect on the percentage of binucleate cells but strongly induced accumulation of bridged cells. When RalA and RalB were depleted together, the RalA phenotype dominated (Figure 1B). These results were reproduced in a different cell line, the telomerase-immortalized human bronchial epithelial cell line (Chien et al, 2006) (Supplementary Figure S4A).
We confirmed the specificity of the RalA and RalB phenotypes with a second independent set of specific siRNAs and with rescue experiments (Figure 1C). siRalA-treated cells were transfected with plasmids expressing Myc–RalA made resistant to the siRalA by three conservative point mutations, Myc–RalB or control plasmid. siRalA-treated cells transfected with empty plasmid displayed the expected RalA-dependent phenotype. Expressing Myc–RalA suppressed the phenotype, whereas expressing Myc–RalB did not (Figure 1C). The same approach was applied to siRalB-treated cells using an siRalB targeted to the 3′-UTR. siRalB-transfected cells displayed the ‘bridge' phenotype (Figure 1C), which was suppressed by expression of Myc–RalB and not of Myc–RalA. These data demonstrate that the binucleate phenotype is due to the specific depletion of RalA and that the bridge phenotype is due to the specific depletion of RalB. Thus, RalA participates in the progress of cytokinesis beyond a point where fusion is no longer possible, whereas RalB contributes to the termination of cytokinesis.
To examine the consequences of Ral gain of function, HeLa cells overexpressing dominant active mutants of RalA (RalADA) or RalB (RalBDA) was followed by time-lapse videomicroscopy and cells entering mitosis were tracked. Expressions of RalADA and RalBDA caused cytokinesis failure: 15% of transfected cells that entered mitosis resulted in binucleate cells (data not shown). Statistical analysis of the accumulation of binucleate cells after 48 h of transfection but in fixed samples confirmed that RalADA- or RalBDA-expressing cells significantly accumulated binucleate cells (Supplementary Figure S4B and C). The Ral binding domain (RalBD) of RalBP1 interacts with the effector loop of both activated RalA and RalB and blocks Ral downstream signalling by sequestering active Ral proteins away from their endogenous effectors (Wolthuis et al, 1998; Moskalenko et al, 2002). RalBD expression induced accumulation of binucleate cells (Supplementary Figure S4B), suggesting that Ral GTPases participate in cytokinesis through the activation of one or several effectors.
Ral variants selectively uncoupled from downstream effectors were employed to examine the G-protein/effector relationships required for Ral-GTP to engage cytokinesis machinery. D49N uncouples Ral from RLIP76/RalBP1 and D49E from both Exo84 and Sec5 as described previously (Jullien-Flores et al, 2000; Moskalenko et al, 2002, 2003). To generate variants selectively uncoupled from Exo84 versus Sec5, we examined the consequences of point mutations defined previously as making selective contributions to Ral-GTP/Exo84 and Ral-GTP/Sec5 complex formation in vitro (Jin et al, 2005). As shown in Supplementary Figure S4D, A48W and E38R dramatically and specifically decrease affinity of RalB G23V for Exo84 and Sec5, respectively. Similar results were obtained for RalA G23V (not shown). We also employed a deletion of the NH2-terminal 11 amino acids (Δ11) of Ral, which are required for association with active Phospholipase D (PLD) (Jiang et al, 1995). RalADA and RalBDA uncoupled from RLIP76/RalBP1 or PLD still induced a binucleate phenotype. In contrast, impairing interaction with any exocyst component abolished the capacity of RalADA and RalBDA to impair cytokinesis (Supplementary Figure S4B and D).
RalA and RalB use common and distinct effectors to progress cytokinesis
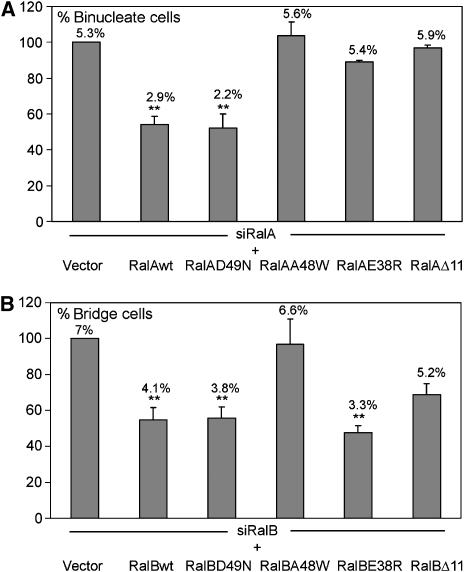
The above-mentioned data show that constitutively activated Ral impacts cytokinesis through its effector, the exocyst complex. Previous observations from our group and others strongly suggest that overexpression of constitutively active Ral GTPases obscures selectivity of biological control observed with the endogenous proteins. To circumvent this problem in the context of complementation experiments, we employed effector-selective Ral variants that are otherwise wild type rather than dominant active. Figure 2 shows that RalA uncoupled from RLIP76 (D49N) can rescue cytokinesis upon depletion of native RalA, whereas variants uncoupled from PLD, Exo84 or Sec5 (A48W and E38R, respectively) cannot. In contrast, only RalB variants uncoupled from Exo84 and PLD fail to support abscission. All alleles were expressed at similar levels (Supplementary Figure S4E). The RalB variant uncoupled from both Sec5 and Exo84 (D49E) failed to support abscission (data not shown). These results show that RalA and RalB share Exo84 and PLD1 as effectors for cytokinesis, and Sec5 is a specific effector of RalA. They do not imply that no other partner of Ral has an important function in cytokinesis.
Figure 2.
RalA and RalB use common and different effectors. HeLa cells were transfected with siRNA for RalA or RalB, and plasmids expressing Ral proteins resistant to these siRNA (see legend of Figure 1C). These variants carry a mutation in the effector loop as indicated in the figure. Δ11 refers to a deletion of the first 11 amino acids of Ral. Binucleate (A) and bridged (B) cells were counted as described in Figure 1C. Percentage of binucleate and bridged cells are indicated on top of each bar. In ordinate are indicated the percentages of rescue by each allele calculated versus the siRalA or siRalB phenotypes. Statistical significance of differences is indicated.
RalA is localized to the cleavage furrow, whereas RalB is at the midbody
Overexpressed RalA has been reported to localize at the cleavage furrow region (Chen et al, 2006). We have examined the localization of Ral proteins in early and late cytokinesis in synchronized cells by immunofluorescence. Mitotic-kinesin like protein-1 (MKLP1) was used as a marker of the midbody ring in early and late stages of cytokinesis (Gromley et al, 2005). The early midbody ring appears during acto-myosin ring constriction, which occurs 8 h post-release from G1-M cell-cycle blockade. The late midbody ring encircles the midbody per se, which appears as a dense structure in phase-contrast microscopy in elongated intracellular bridges 10 h post-release.
We found that, in early cytokinesis, RalA was concentrated at the cleavage furrow region surrounding MKLP1 (Figure 3). Globally, RalA localized mostly to the plasma membrane delimited by the cortical cytoskeleton revealed by β-spectrin (Supplementary Figure S5A). This accumulation was not a ‘passive' effect from membrane apposition, as RalA was not enriched at the double membrane of two just mitotic adjacent cells (arrowheads in Supplementary Figure S5A). The staining of RalA was specific as verified by loss of signal in RalA-depleted cells (Supplementary Figure S5B). This localization is in contrast to that reported previously employing overexpression of green fluorescent protein (GFP)–RalA fusions (Chen et al, 2006). We did not find any discrete localization of endogenous RalA during the late stage of cytokinesis (Figure 3).
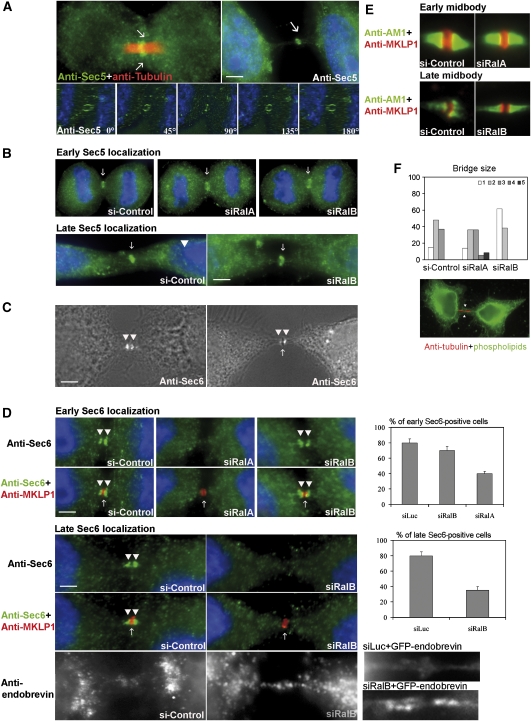
Figure 3.
RalA localizes at the cleavage furrow and RalB at the midbody. Synchronized HeLa cells were stained for endogenous RalA with anti-RalA mAb (green) or for overexpressed Flag-fused RalB with anti-Flag mAb (green) and co-stained with anti-MKLP1. Early and late cytokineses are defined by timing of cell synchronization, 8 and 10 h after the release of the thymidine block, respectively. During these steps, MKLP1 localized to the early and late midbody ring. RalA localized at the cleavage furrow membrane at early cytokinesis but could not be detected in discrete regions at late cytokinesis. RalB was localized at the midbody at late cytokinesis but did not display detectable discrete localization earlier.
Given the absence of appropriate antibodies to detect endogenous RalB, we had to turn to Myc- and Flag-fusions to localize RalB. Results are shown for Flag–RalB. During early cytokinesis, we could not detect any discrete localization of RalB. Later in cytokinesis, Flag–RalB was enriched at the midbody (Figure 3). This is not a Flag-dependent effect, as Myc–RalB displayed the same localization as Flag–RalB, nor a tag-fusion effect, as both Myc–RalA and Flag–RalA were enriched at the cleavage furrow (data not shown), displaying the same localization as endogenous RalA at an early stage, and were never detected at the midbody. We could not analyse the localization of RalA and tagged-RalB in the same cells due to different fixation protocols.
Exocyst components are already mobilized at early cytokinesis
Sec5 together with other components of the exocyst complex is localized at the midbody ring in late cytokinesis (Gromley et al, 2005), which is what we observed here (Figure 4A and B). As Sec5 is an effector of RalA in cytokinesis, we asked whether Sec5 was specifically localized in early cytokinesis. We detected a discrete localization of Sec5 encircling the microtubule bundles as soon as furrow ingression terminated, suggesting that the exocyst complex is already engaged at this early step (Figure 4A). Three-dimensional reconstruction of deconvolution microscope images revealed that Sec5 was present in a ring-like structure, which is presumably the early midbody ring, which starts to organize at this step (Figure 4A, lower panel). This signal was lost in Sec5-depleted cells (Supplementary Figure S5B).
Figure 4.
RalA and RalB regulate Sec6 localizations. (A) Synchronized HeLa cells were fixed at early (upper left panel) and late (upper right panel) cytokinesis as shown in Figure 3 and stained with anti-Sec5 Ab (green) and co-stained with anti-tubulin mAb (upper row). Sec5 (arrows) can be observed in late as well as early cytokinesis on the midbody ring. Cells stained with anti-Sec5 Ab (green) were imaged with a deconvolution microscope. Images show a 180° 3D reconstruction: the staining of Sec5 is in a ring-like structure (arrow). (B) Synchronized HeLa cells transfected with siRalA, siRalB or control siRNA were stained with anti-Sec5 mAb (green). Early (8 h after release of the thimidine block) and late (10 h after release of the thimidine block) localisations of Sec5 did not appear sensitive to a depletion of RalA or B. (C) Synchronized HeLa cells were stained with anti-Sec6 Ab at early (left panel) and late (right panel) cytokineses as shown in Figure 3. Sec6 (arrow heads) accumulated on two discrete regions in early cytokinesis and on both sides of the midbody in late cytokinesis on elongated bridges. On these phase-contrast microscopic pictures, a dense midbody can be seen (arrow) at late times (right panel) and not at early times (left panel). (D) Synchronized HeLa cells were transfected with an siRalB or control siRNA, and stained for Sec6 (green) and MKLP1 (red). The early localization of Sec6 on both sides of the early midbody ring marked by the anti-MKLP1 disappeared in RalA- but not in RalB-depleted cells. The late localization of Sec6 was lost in RalB-depleted cells. Graphs on the right give a quantification of the rate of Sec6-positive cells in the various situations. Data are from at least three independent experiments where at least 40 cells have been counted. Elongated bridges of RalB-depleted cells stained for endobrevin, which marks exocytotic vesicle, displayed an accumulation compared with the control (low row) as was the case in cells where the exocyst was disrupted (Gromley et al, 2005). Elongated bridges of the RalB-depleted cells overexpressing GFP-endobrevin (Martineau et al, 2008) accumulated GFP (n=40). The physiological late intracellular bridges of the siLuc-treated cells overexpressing GFP-endobrevin did not show any accumulation of the GFP (n=10). (E, F) The absence of RalA and RalB does not impact formation of the spindle per se but modifies bridges. The organization of the central spindle and of the intracellular bridge was controlled by co-staining synchronized cells with an anti-AuroraB mAb (green) and an anti-MKLP1 Ab (red). In controls, RalA-depleted cells and RalB-depleted cells, AuroraB concentrated in the region of the bundled microtubules on both sides of the midbody ring stained by MKLP1, showing that cytokinetic spindle was correctly formed (E). Cells were stained with FITC-coupled phospholipids, which stains plasma membranes. This allowed to measure the diameter of the intracellular channel (as in the given example) in cells transfected with siRalA, siRalB and a control siRNA. We defined five categories (0–1, 1–2, 2–3, 3–4 and 4–5 μm) and distributed the number of diameters per category. The distribution is shown on the graph, which reveals that the intracellular diameters are rather wider in RalA-depleted cells and are rather thinner in RalB-depleted cells, as opposed to the distribution in control cells.
In PC12 cells, Sec5 and Sec6 are present in the same heavy plasma membrane fraction (Moskalenko et al, 2003). We examined whether they localize similarly at the early midbody ring. Whereas Sec5 was localized at the early midbody ring, Sec6 could only be detected at the Golgi apparatus during its post-mitotic reassembly (data not shown). However, following furrow ingression termination, Sec6 displayed a discrete pattern of accumulation (arrowheads in Figure 4C, left panel), which appeared to be on both sides of the early midbody ring and the midbody as indicated by MKLP1 staining (Figure 4D, upper panel) and phase-contrast images, respectively (arrowheads in Figure 4C, right panel; arrow to the midbody). Specificity of Sec6 mAb labelling was validated by Sec6 depletion (Supplementary Figure S5B).
Ral GTPases control Sec6 but not Sec5 localization during cytokinesis
As RalA participates in early cytokinesis events and is localized to the cleavage furrow (Figure 3), we examined if the early Sec5 and Sec6 recruitment were dependent on RalA. As shown in Figure 4B, recruitment of Sec5 to the early midbody ring was not perturbed by siRNA-mediated depletion of RalA. In contrast, the early recruitment of Sec6 was blocked by RalA depletion; perturbing RalB had no effect (Figure 4D).
RalB appears to make an obligate contribution to abscission. RNAi-mediated depletion of RalB induced an accumulation of elongated bridges (Figure 2) with normal Sec5 localization (Figure 4B); however, Sec6 was absent (Figure 4D). We observed an increase of endobrevin/VAMP8 staining (Figure 4D, lower panels). Elongated bridges of the RalB-depleted but not control cells overexpressing GFP-endobrevin accumulated GFP flanking the midbody, but we were unable to discriminate single vesicles (Figure 4D, lower panels).
The loss of Sec6 recruitment to cytokinesis machinery in RalA- and RalB-depleted cells was not due to a general defect of central spindle organization, as bridge architecture was globally unaffected (Figure 4E). However, when the intracellular diameter channels were measured in the various populations, we found that the average sizes of the diameter were different according to the presence of Ral. When RalA was depleted, the intracellular diameters were on average wider, and when RalB was depleted, they were thinner, as compared with cells with full complements of RalA and RalB (Figure 4F). We did not find any defect in microtubule morphology (data not shown).
RalA and Sec5 are both required for recruitment of exocyst components at early stages of cytokinesis
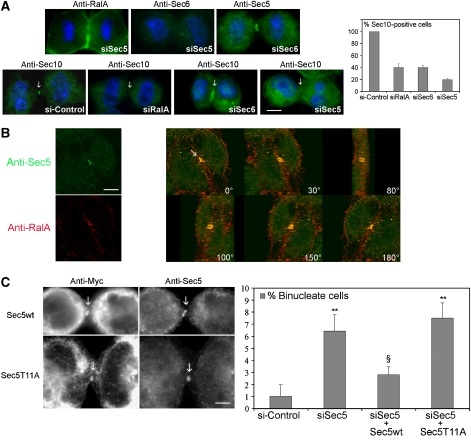
RalB drives late cytokinesis events as has been described for the exocyst (Gromley et al, 2005), but our observations indicate that Sec5 and RalA are already mobilized at early steps, and they colocalize at the early midbody ring during furrow ingression (Figure 5B and Supplementary Movie S6). Depletion of Sec5 induced cytokinesis defects (Gromley et al, 2005) and accumulation of binucleate cells similar to the depletion of RalA (Supplementary Figure S7). To further investigate the role of RalA in early cytokinesis, we depleted cells of RalA, Sec5 or Sec6 and tracked the consequences on the localization of exocyst components in early cytokinesis. Sec6 depletion led to the same cytokinesis defects as those caused by Sec5 or RalA depletion (Supplementary Figure S7) but did not perturb Sec5 or RalA localization (Figure 5A and data not shown, respectively). In contrast, Sec5 depletion blocked recruitment of Sec6, whereas RalA was unaffected (Figure 5A). Sec10 is a component of the exocyst present on vesicles and assembles with the core Sec6/8 exocyst complex in a Ral-dependent manner (Moskalenko et al, 2002; Rosse et al, 2006). During cytokinesis, Sec10 localized similarly to Sec5 at the midbody ring, and this localization was lost when RalA, Sec5 or Sec6 were depleted (Figure 5A, lower row, and graph on the right). Thus, both Sec5 and RalA constitute a spatial landmark for further early localization or stabilization of exocyst holocomplexes. These results also show that in addition to its role in abscission, Sec5 has a previous role in early cytokinesis.
Figure 5.
RalA and Sec5 cooperate at the early midbody ring. (A) Synchronized RalA-, Sec6- or Sec5-depleted cells or control cells were investigated for the early localization (8 h after release of the thimidine block) of the indicated proteins by staining with anti-RalA mAb, anti-Sec6 mAb, anti-Sec5 Ab or anti-Sec10 Ab. RalA was not delocalized in Sec5-depleted cells, Sec5 disruption delocalizes Sec6, whereas Sec6 disruption has not effect on Sec5 localization. Cell depleted in RalA or Sec5 or Sec6 showed a defect in Sec10 localization. This defect is quantified in the adjacent graph (data from at least three experiments, with ⩾50 cytokinetic cells counted). (B) HeLa cells were co-stained with anti-Sec5 Ab (green) and anti-RalA mAb (red). Pictures were taken on a confocal microscope and images of the 180° 3D reconstruction showing views under different angles of the early midbody ring where the two proteins colocalized are shown (arrow). The separate channel for anti-RalA and anti-Sec5 are shown on the left for one frame. (C) Synchronized Sec5 wild-type or Sec5T11A-overexpressing cells were stained with anti-Myc and anti-Sec5 Ab. The Sec5T11A mutant is unable to bind to Ral GTPases, but it localized as wild-type Sec5 at the early midbody ring (arrows). HeLa cells depleted of Sec5 by siRNA were analyzed for the appearance of a binucleate cell phenotype. These cells were transfected to express an siRNA-resistant wild-type Sec5 or Sec5T11A allele, and the binucleate phenotype was quantified, with an indicated statistical significance (**P<0.01). The wild-type Sec5 rescued the siSec5 binucleate phentoype (§P<0.01) but the Sec5T11A allele did not.
Sec5 and RalA interact functionally and colocalize at the midbody ring
A RalA mutant (E38R) unable to interact with Sec5 is unable to support cytokinesis (Figure 2), and RalA and Sec5 colocalize at the early midbody ring (Figure 5B). To confirm that a physical interaction between RalA and Sec5 is required to mediate RalA-dependent progression of cytokinesis, we employed a Sec5 variant (T11A) defective for RalA binding (Fukai et al, 2003). As shown in Figure 5C, both wild-type and mutant Sec5 correctly localized to the midbody ring during cytokinesis, confirming that the localization of Sec5 is independent of RalA. RNAi-mediated depletion of endogenous Sec5 resulted in cytokinesis failures similar to those observed upon depletion of RalA (Supplementary Figure S7). These phenotypes were rescued upon expression of siRNA-resistant wild-type Sec5 but not Sec5T11A (Figure 5C, right panel). These observations indicate that a physical interaction between RalA and Sec5 is required for progression of cytokinesis.
Specific Ras-dependent and Ras-independent RalGEFs are involved at different steps of cytokinesis
There are six RalGEFs encoded by the human genome, which can mediate Ral-GTP loading. Four of them (RalGDS, RGL, RGL2 and RGL3) are themselves activated by the Ras GTPases and two of them (RalGPS1 and 2) activate Ral in a Ras-independent manner (Rebhun et al, 2000; Ceriani et al, 2007).
We individually depleted all RalGEFs by siRNAs, as validated by qRT–PCR (Supplementary Figure S8). Remarkably, we found that depletion of RalGDS or RalGPS2 caused the accumulation of binucleate cells similarly to the depletion of RalA (Figure 6A). In contrast, the depletion of RGL or RalGPS2 caused the accumulation of cells connected by an intracellular bridge, similarly to the depletion of RalB (Figure 6A). These phenotypes were confirmed with an independent siRNA for each of these genes (Supplementary Figure S8). We never observed both phenotypes upon depletion of any RalGEF. The depletion of RGL2 or RGL3 had no significant effect despite effective depletion (Figure 6A and Supplementary Figure S8). These results suggest that some RalGEFs, during cytokinesis, display specificity towards RalA or RalB.
Figure 6.
The many Ral activators (RalGEFs) have distinct targets and functions during cytokinesis. (A) HeLa cells were depleted of RalGEFs (RalGDS, RGL, RGL2 and RGL3, RalGPS1 and 2) by specific siRNA (10 nM). After 72 h, cells were fixed and counted as described in Figure 1 for the percentage of binucleate cells and bridged cells. The graph displays the percentage of binucleate or bridge cells, and statistical significance is indicated (**P<0.01). These results were reproduced with a second independent siRNA for each gene (Supplementary Figure S8). In all experiments, at least 1000 cells were counted, and results are from at least three independent experiments. (B) Selected phase-contrast time-lapse videomicroscopy frames of representative cytokinesis steps from HeLa cells transfected with control siRNA or siRNA targeting RalGDS, RalGPS2, RGL or RalGPS1. RalGDS- or RalGPS2-depleted cells did not form proper intracellular bridges; RGL- or RalGPS1-depleted cells stayed for an extended period with the intracellular bridges. Even one RGL-depleted cell still attached to its sister cell can be observed entering mitosis.
We used time-lapse videomicroscopy to evaluate the stage of cytokinesis impacted by these RalGEFs. In cells treated with siRNA-targeting RalGDS or RalGPS2, furrow ingression took place but cells failed to spread and form intracellular bridges, and daughter cells ultimately fused and formed binucleate cells. Cells treated with siRNA-targeting RGL or RalGPS1 were defective in abscission and maintained intracellular bridges for extended periods (Figure 6B). The depletion of RalGDS or RalGPS2 impacted cytokinesis dynamics very similarly to what was observed upon depletion of RalA, and the depletion of RGL or RalGPS1 impacted cytokinesis dynamics very similarly to the depletion of RalB: these data suggest that RalGDS and RalGPS2 might have RalA as substrate during cytokinesis, and RGL and RalGPS1 would have RalB. Notably, one member of each pair of RalGEF is a Ras-responsive RalGEF, whereas the other member is not and contains a PH domain that may associate with discrete membrane microdomains and transduce signals whose nature remains elusive.
RalGEFs localize differently
RalGEFs are able to induce the activation of RalA and RalB (Albright et al, 1993), but their in vivo specificity for RalA or RalB under physiological conditions is unknown. In interphase cells, RalA and RalB have been reported to have overlapping but not identical localizations: RalA was found more at the plasma membrane and RalB was found more on endomembranes (Shipitsin and Feig, 2004). We also found distinct and exclusive localizations of RalA and B during cytokinesis.
We questioned whether RalGEFs might also display specific intracellular localizations, which might be the basis of a preferential activation of RalA or B. Owing to the lack of antibodies to localize endogenous proteins, we used tagged versions of the RalGEFs. In HeLa cells, Flag–RalGDS and Myc–RalGPS2 localized to the plasma membrane (Supplementary Figure S8), which is enriched in RalA, and Flag–RGL and Flag–RalGPS1 displayed a punctuate localization through the cytoplasm (Supplementary Figure S8), similar to RalB (Shipitsin and Feig, 2004). We examined the localization of these RalGEFs during cytokinesis in synchronized cells. We found that Flag–RalGDS localized to the early midbody ring (Figure 7), where RalA and Sec5 colocalized (Figure 5), and persisted there till maturation of the midbody (Figure 7). Myc–RalGPS2 was found, also like RalA, on the ingressed cleavage furrow membrane, and was lost later in cytokinesis (Figure 7). Upon depletion of RalGDS or RalGPS2, Sec6 failed to accumulate at the midbody ring (Figure 8, upper panels) as was observed upon RalA depletion. Similar to RalB, Flag–RGL and Flag–RalGPS1 did not display any discrete localization at the early stages of cytokinesis but were enriched on the midbody during late-stage cytokinesis (Figure 7). When RGL or RalGPS1 were depleted, Sec6 was still localized normally (Figure 8).
Figure 7.
Different RalGEFs localize in different cellular compartments. Synchronized HeLa cells were transfected with plasmids expressing Flag-fusions of RalGDS, RGL, RalGPS1 or Myc-fusion of RalGPS2. Cells were fixed at different times as shown in Figure 3. RalGEFs were detected by indirect immunofluorescence using anti-Flag mAb (green), co-stained with anti-MKLP1 Ab (red) and DAPI (blue).
Figure 8.
Impact of RalGEFs on Sec6 localization. Synchronized HeLa cells were transfected with siRNA targeting the indicated genes, fixed and stained for MKLP1 (red) and Sec6 (green). Nuclei were visualized by DAPI staining (blue). Graphs on the right represent the percentage of cells in cytokinesis where Sec6 was correctly localized. Depleting RalGDS and RalGPS2 impacted Sec6 localization (upper graph), whereas depleting RGL and RalGPS1 did not.
Discussion
Ral GTPases RalA and RalB are effectors of the Ras GTPases, and Ral in turn signals through a small set of effectors, the best documented being the exocyst complex (Feig, 2003; Camonis and White, 2005). The latter is involved in exocytosis and is required for the abscission of the bridge joining the sister cells emerging from mitosis (Echard et al, 2004; Gromley et al, 2005). Here, we show that the multistep process of cytokinesis integrates the contribution of both Ral GTPase family members to distinct spatial and temporal mobilization of exocyst components and that this integration is specified by distinct RalGEFs.
We find that the exocyst is not only required for abscission, but it is also already engaged upon the formation of the cleavage furrow. Second, we find that RalA and RalB drive distinct tasks: RalA supports the stabilization and elongation of the intracellular bridge, whereas RalB supports abscission. RalA cooperates with Sec5 to allow tethering of other components of the exocyst to the early midbody ring; upon completion of bridge formation, RalB takes over to mediate exocyst tethering and exocytosis for abscission. The specificity of engagement of the RalA-exocyst and RalB-exocyst modules appears to be driven by different Ral activators; RalGDS and RalGPS2 for RalA, RGL and RalGPS1 for RalB. The diversity of regulatory inputs made available through these GEF family members suggests that RalA and RalB may integrate multiple signals to allow transition through distinct phases of cytokinesis and function as signal integration nodes.
The exocyst complex is required for abscission, which involves endobrevin/VAMP8 secretory vesicle tethering to the midbody (Gromley et al, 2005). We found that Sec5 is already recruited at the early midbody ring during actomyosin-ring constriction where it colocalizes with RalA. At this step, Sec6 is at the Golgi apparatus during its post-mitotic reassembly (data not shown) but soon displays a discrete pattern on both sides of the early midbody ring, a region of active exocytic and endocytic processes (Albertson et al, 2005). This exocyst localization might correspond to the symmetric flow of exocytosis that has been documented recently as taking place during cytokineiss (Goss and Toomre, 2008), which might be under the control of RalA. Although Sec5 or Sec6 have a late role in cytokinesis (Gromley et al, 2005), we have also observed earlier failures upon Sec5 and Sec6 depletions, which led to binucleate cells. This suggests that at least some exocyst components have timely distinct functions during cytokinesis. During cytokinesis, Sec5 localization appears to orchestrate recruitment of the other subcomplexes. As opposed to its ‘late' function, this ‘early' function of Sec5 is not dependent on centriolin. Upon centriolin depletion by siRNA, we observed a ‘bridge phenotype' reflecting the defect in abscission but not in furrow formation (Supplementary Figure S5D), and Sec5 was properly recruited to the early midbody ring, although it was lost in the midbody ring of matured intracellular bridges as published (Supplementary Figure S5D; Gromley et al, 2005). Sec5 localization at the midbody ring was Ral independent. Similarly, centriolin localization was not modified by depletion of RalA or B (Supplementary Figure S5C). However, the Sec5–RalA interaction was required for cytokinesis to proceed, demonstrating that RalA does not control Sec5 localization but does control its function. Hence, RalA and Sec5 cooperate to mediate and control exocyst assembly as soon as the early midbody ring is formed.
In RalA-perturbed cells, the cleavage furrow and the central spindle organization progresses normally, but the intracellular diameter of daughter cells after cleavage furrow ingression is wider, suggesting that stability and elongation, rather than formation of the intracellular bridge, was affected. Control cells show that the maturation of the intracellular bridge and cell spreading are concomitant, and both events were impaired in RalA-perturbed cells. A role of RalA could be to tether vesicles carrying a Rab11/FIP3-4/exocyst subcomplex to Sec5-carrying receptor membranes. RalA would assemble the exocyst complex by interacting with Exo84 and Sec5 (Moskalenko et al, 2003), allowing tethering of these vesicles before fusion. Our data with RalA are consistent with the binucleate phenotype observed upon depletion of Rab11, FIP3 and ARF6 together with Rab11 (Wilson et al, 2005; Yu et al, 2007). We also found that both RalA and RalB may need to interact with PLD1 to support cytokinesis. This interaction might be an integrating node for signals coming from other GTPases, like ARF6. ARF6 and Ral are able to coactivate PLD1 (Xu et al, 2003), and on the other hand, ARF6 is involved in cytokinesis, where it supports correct localization of exocyst subunits (Fielding et al, 2005). Bridge stability requires signals carried by the trafficking machinery (Echard et al, 2004): complementary or alternatively to its function as a ‘membrane supplier', the exocyst complex could also be in charge of targeting signalling molecules during cytokinesis, as observed for cell survival and innate immunity (Balakireva et al, 2006; Chien et al, 2006).
Similarly, the participation of RalB in abscission further highlights functional coupling of Ral GTPases and the exocyst, and RalB appears as a required conductor of the exocyst-dependent membrane dynamics that drive abscission. However, the process is complex, with RalB potentially engaging an Exo84-dependent but Sec5-independent activity. The fact that, in RalB-depleted cells, endobrevin-carrying vesicles display a pattern similar to the one observed when exocyst function was impaired (Gromley et al, 2005) suggests that RalB and the exocyst complex are involved in exocytosis of the same pool of vesicles. However, this requires more investigation, as depleting RGL1 and RalB, although leading to the same failure in abscission and accumulation of endobrevin-positive vesicles near the midbody, did not have the same impact on Sec6: in the former depletion, Sec6 was correctly localized, in the latter it was not. This suggests that RalB even under its GDP bound form might have a function which may be related to PLD activation (Xu et al, 2003).
A role of RalA in abscission and its localization at the midbody has been reported (Chen et al, 2006), which does not fit with our observations. We do not know the source of this discrepancy. The localization of RalA at the midbody might be due to the use of a GFP-fused RalA, and GFP fusions in fixed cells can get mislocalized (Schmitz and Bereiter-Hahn, 2001). Our data are supported by tracking endogenous RalA as well as Myc-tagged and Flag-tagged RalA. As for the role in abscission, our data are supported by the use of several different siRNA as well as by siRNA-complementation experiments.
Ral activation of effector complexes requires GTP loading through the action of RalGEFS. We found that depletion of RalGDS or RalGPS2 phenocopied depletion of RalA, whereas depletion of RGL and RalGPS1 phenocopied depletion of RalB. This suggests pathways where RalA activation requires both Ras-dependent (RalGDS) and Ras-independent (RalGPS2) GEFs, and RalB activation requires a distinct Ras-dependent (RGL) and Ras-independent (RalGPS1) pair. RalGTPases could therefore integrate information from two pathways, which must be active to mobilize the exocyst for both progression and termination of cytokinesis. The required concomitant actions of two GEFs on one GTPase for functional activation is reminiscent of the participation of RhoA in cytokinesis, which also requires two GEFs to function properly: one, Ect2, localizes RhoA in the vicinity of the other, GEF-H1, in charge of the activating GDP/GTP exchange per se (Birkenfeld et al, 2007). The fact that the RalGEFs are responsible for timely engagement of RalA or RalB during cytokinesis might explain the observation that activated mutants of RalA and RalB display nearly the same phenotype: in these mutants, RalGEFs are no longer required and thus activated RalA and RalB would be similarly activated, without the time separation provided by their specific GEFs.
How the specificity for RalA and RalB is achieved by RalGEFs is unknown. Although it might be an intrinsic in vivo biochemical property of each RalGEF, it is possibly (and more likely) the consequence of differential localizations, and indeed we show that RalGEFs can have specific localizations. Taking into account the recently described capacity of Ras to impact cytokinesis during tumorigenesis (Thullberg et al, 2007), an unified theory would be that a RalGPS, perhaps through its interaction with specific membrane domains, localizes its partnering Ral in the proximity of its activating Ras-dependent RalGEF.
Materials and methods
Antibodies, plasmids and siRNA oligonucleotides
RalA, RalB, Myc–RalB, RalBDA (RalBG23V), RalBDA/D49N, RalBDA/D49E, RalBDA/A48E and RalBΔN11 (deleted of their 11 first codons) (Jullien-Flores et al, 1995, 2000; Bauer et al, 1999; Moskalenko et al, 2002), and RalADA (RalAQ72L), RalADA/D49N, RalADA/D49E and ΔN11RalA (Lalli and Hall, 2005) were expressed using pRK5. RalA G23V/E38R, RalA G23V/A48W, RalB G23V/E38R and RalB G23V/A48W were expressed using pCMV10–3FLAG as their controls RalA G23V and RalB G23V. Wild-type RalA and RalB, and RalA and RalB carrying ΔN11, D49N, D49E, A48W and E38R in an otherwise wild-type background were expressed from the pRK5–Myc or 3XFlag–CMV10 plasmids. Myc–Sec5wt and Myc–Sec5T11A were expressed in pCMV.
siRal_II resistant RalA was obtained by PCR mutagenesis using oligonucleotides GAAGATAAAAGGCaaGtaAGTGTAGAAGAGGCAAA AAACAGAGC and GCCTCTTCTACACTtaCttGCCTTTTATCTTCTAA ATCTG. PCR product was cloned in pRK5–Myc and sequenced.
Synthetic siRNAs targeting Ral GTPases and RalGEFs were designed by standard methods (sequences in Supplementary Methods S9). Exocyst subunits were depleted by published siRNA (Chien and White, 2003; Chien et al, 2006; Lim et al, 2006).
Antibodies anti-RalA and anti-Aurora B kinase (AM1) were obtained from Beckton Dickinson; anti-tubulin FITC-coniugated mAb from Sigma; anti-Myc mAb from Roche; anti-Sec5 and anti-Sec10 Abs as previously described (Gromley et al, 2005). Anti-Sec6 was obtained from Stressgen Bioreagents; anti-MKLP1 from Santa-Cruz; anti-PRC1 was a kind gift of W Jiang (Burnham Institute, La Jolla, CA, USA). Anti αII-spectrin was a gift of G Nicolas and MC Lecomte (Inserm, U665, Paris); anti-endobrevin was a gift of T Galli (IJM, Paris, France).
Cell culture
HeLa cells were cultivated in Dulbecco's modified Eagle medium (DMEM) and 10% fetal calf serum at 37 °C under 5% CO2 in humidified chamber on Falcon plastic dishes. Cells (60–120 × 103/ml) were plated on treated slides in 24-wells (Falcon) synchronized by double thymidine block (Bootsma et al, 1964), maintained in 2.5 mM thymidine (Sigma) for 18 h, washed three times in DMEM and incubated in DMEM containing 10% fetal calf serum and 24 μM 2′-deoxycytidine hydrochloride (Sigma) for 8 h. Cells were then blocked again in 2.5 mM thymidine for 16 h. The second block was released as described above. Cells entered G2/M ∼6.5 h later.
Transfection and biochemistry
HeLa cells (12 × 104/ml) were plated in 24-wells (Falcon) and synchronized as described above. They were transfected with plasmids (0.4 μg) between the first and the second thymidine block with Lipofectamine PLUS reagents (Invitrogen) in Optimem for 3 h according to the manufacturer's instructions. Where indicated, cells were co-transfected with a plasmid encoding for the GFP, used as a tracer.
siRNA transfection for immunolocalization studies was performed using the Hiperfect forward-protocol (Qiagen) on the first day of synchronization. A total of 60 × 103 cells per ml were plated on treated slides in 24-well microplate (Falcon) in growth medium containing the indicated concentrations of siRNA and 3 μl of Hiperfect. For phenotyping, 20 × 103 cells per ml were plated on black 96-well microplate with clear bottom (Perkin Elmer) in growth medium containing 5 nM of pooled siRNAs and 1 μl of Hiperfect for 72 h. In rescue experiments, cells were transfected with siRNA in 96-well plates as described above and the next day they were transfected with plasmids using JETPEI reagents (Polyplus Transfection). mRNA levels were measured by quantitative RT–PCR using TaqMan Universal PCR or SYBR Green PCR Master Mix (Applied Biosytems) and 7500 Real-Time PCR System (see Taqman probes and primers used with SYBR Green in Supplementary Methods S9).
Immunofluorescence and time-lapse videomicroscopy
HeLa cells were fixed and stained, and images were acquired and analysed using classical immunofluorscence methods. Specific treatments according to antibodies and aims are described in Supplementary Methods S9.
For time-lapse videomicroscopy, HeLa cells were synchronized and transfected on 35-mm glass dishes (Iwaki) and maintained at 37 °C in an open chamber (Life imaging) equilibrated in 5% CO2. When GFP was used as a tracer, cells were transfected with a ratio of 5:1 between the plasmid expressing the studied gene and the plasmid expressing GFP. Time-lapse sequences were recorded at 5-min intervals on a Leica DMIRBE microscope using a × 20 objective controlled by the Metamorph software. This microscope was equipped with a cooled CCD camera (Micro Max 5 MHz; Ropper Scientific). Video analysis was performed by the Metamorph software.
Supplementary Material
Supplementary Movies S1
Supplementary Movies S2
Supplementary Movies S3
Supplementary Figure S4
Supplementary Figure S5
Supplementary Movies S6
Supplementary Figure S7
Supplementary Figure S8
Supplementary Methods S9
Supplementary Information Legends
Acknowledgments
We are very grateful to A Hall, L Quilliam, M Ceriani, H Bos and T Galli for RalA, RalGPS1, RalGPS2, RGL2/Rlf, RGL3 and GFP-VAMP8 constructs, A Echard, S Doxsey and C Counter for enlightening discussions, N Brandon for technical help and C Camonis and P Gary for expert film editing. This work was supported by grant no. 3131 from Association de Recherche sur la Cancer (ARC)(JC), by grant ANR05BLAN033802 from Agence Nationale de la Recherche (ANR) (JC), by Association Christelle Bouillot (JC), by CA129451 from the National Cancer Institute (MAW), by I-1414 from the Robert Welch foundation (MAW). IC was supported by a Long-Term Fellowship from EMBO, and RS by a PhD fellowship from Institut National du Cancer (INCa).
References
- Albertson R, Riggs B, Sullivan W (2005) Membrane traffic: a driving force in cytokinesis. Trends Cell Biol 15: 92–101 [DOI] [PubMed] [Google Scholar]
- Albright CF, Giddings BW, Liu J, Vito M, Weinberg RA (1993) Characterization of a guanine nucleotide dissociation stimulator for a ras-related GTPase. EMBO J 12: 339–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakireva M, Rosse C, Langevin J, Chien YC, Gho M, Gonzy-Treboul G, Voegeling-Lemaire S, Aresta S, Lepesant JA, Bellaiche Y, White M, Camonis J (2006) The Ral/Exocyst effector complex counters c-Jun N-terminal kinase-dependent apoptosis in drosophila melanogaster. Mol Cell Biol 26: 8953–8963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer B, Mirey G, Vetter IR, Garcia-Ranea JA, Valencia A, Wittinghofer A, Camonis JH, Cool RH (1999) Effector recognition by the small GTP-binding proteins Ras and Ral. J Biol Chem 274: 17763–17770 [DOI] [PubMed] [Google Scholar]
- Birkenfeld J, Nalbant P, Bohl BP, Pertz O, Hahn KM, Bokoch GM (2007) GEF-H1 modulates localized RhoA activation during cytokinesis under the control of mitotic kinases. Dev Cell 12: 699–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootsma D, Budke L, Vos O (1964) Studies on synchronous division of tissue culture cells initiated by excess thymidine. Exp Cell Res 33: 301–309 [DOI] [PubMed] [Google Scholar]
- Camonis JH, White MA (2005) Ral GTPases: corrupting the exocyst in cancer cells. Trends Cell Biol 15: 327–332 [DOI] [PubMed] [Google Scholar]
- Ceriani M, Scandiuzzi C, Amigoni L, Tisi R, Berruti G, Martegani E (2007) Functional analysis of RalGPS2, a murine guanine nucleotide exchange factor for RalA GTPase. Exp Cell Res 313: 2293–2307 [DOI] [PubMed] [Google Scholar]
- Chen XW, Inoue M, Hsu SC, Saltiel AR (2006) RalA-exocyst-dependent recycling endosome trafficking is required for the completion of cytokinesis. J Biol Chem 281: 38609–38616 [DOI] [PubMed] [Google Scholar]
- Chien Y, Kim S, Bumeister R, Loo YM, Kwon SW, Johnson CL, Balakireva MG, Romeo Y, Kopelovich L, Gale M Jr, Yeaman C, Camonis JH, Zhao Y, White MA (2006) RalB GTPase-mediated activation of the IkappaB family kinase TBK1 couples innate immune signaling to tumor cell survival. Cell 127: 157–170 [DOI] [PubMed] [Google Scholar]
- Chien Y, White MA (2003) RAL GTPases are linchpin modulators of human tumour-cell proliferation and survival. EMBO Rep 4: 800–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbelaere J, Barral Y (2004) Spatial coordination of cytokinetic events by compartmentalization of the cell cortex. Science 305: 393–396 [DOI] [PubMed] [Google Scholar]
- Echard A, Hickson GR, Foley E, O'Farrell PH (2004) Terminal cytokinesis events uncovered after an RNAi screen. Curr Biol 14: 1685–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert US, Kiger AA, Richter C, Perlman ZE, Perrimon N, Mitchison TJ, Field CM (2004) Parallel chemical genetic and genome-wide RNAi screens identify cytokinesis inhibitors and targets. PLoS Biol 2: e379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig LA (2003) Ral-GTPases: approaching their 15 minutes of fame. Trends Cell Biol 13: 419–425 [DOI] [PubMed] [Google Scholar]
- Fielding AB, Schonteich E, Matheson J, Wilson G, Yu X, Hickson GR, Srivastava S, Baldwin SA, Prekeris R, Gould GW (2005) Rab11-FIP3 and FIP4 interact with Arf6 and the exocyst to control membrane traffic in cytokinesis. EMBO J 24: 3389–3399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukai S, Matern HT, Jagath JR, Scheller RH, Brunger AT (2003) Structural basis of the interaction between RalA and Sec5, a subunit of the sec6/8 complex. EMBO J 22: 3267–3278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer M (2005) The molecular requirements for cytokinesis. Science 307: 1735–1739 [DOI] [PubMed] [Google Scholar]
- Goss JW, Toomre DK (2008) Both daughter cells traffic and exocytose membrane at the cleavage furrow during mammalian cytokinesis. J Cell Biol 181: 1047–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromley A, Yeaman C, Rosa J, Redick S, Chen CT, Mirabelle S, Guha M, Sillibourne J, Doxsey SJ (2005) Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell 123: 75–87 [DOI] [PubMed] [Google Scholar]
- Hamad NM, Elconin JH, Karnoub AE, Bai W, Rich JN, Abraham RT, Der CJ, Counter CM (2002) Distinct requirements for Ras oncogenesis in human versus mouse cells. Genes Dev 16: 2045–2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Luo JQ, Urano T, Frankel P, Lu Z, Foster DA, Feig LA (1995) Involvement of Ral GTPase in v-Src-induced phospholipase D activation. Nature 378: 409–412 [DOI] [PubMed] [Google Scholar]
- Jin R, Junutula JR, Matern HT, Ervin KE, Scheller RH, Brunger AT (2005) Exo84 and Sec5 are competitive regulatory Sec6/8 effectors to the RalA GTPase. EMBO J 24: 2064–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jullien-Flores V, Dorseuil O, Romero F, Letourneur F, Saragosti S, Berger R, Tavitian A, Gacon G, Camonis J (1995) Bridging the Ral GTPase to Rho pathways: RLIP76, a Ral effector with CDC42/Rac GTPase activating protein (GAP) activity. J Biol Chem 270: 22473–22477 [DOI] [PubMed] [Google Scholar]
- Jullien-Flores V, Mahe Y, Mirey G, Leprince C, Meunier-Bisceuil B, Sorkin A, Camonis JH (2000) RLIP76, an effector of the GTPase Ral, interacts with the AP2 complex: involvement of the Ral pathway in receptor endocytosis. J Cell Sci 113: 2837–2844 [DOI] [PubMed] [Google Scholar]
- Lalli G, Hall A (2005) Ral GTPases regulate neurite branching through GAP-43 and the exocyst complex. J Cell Biol 171: 857–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KH, O'Hayer K, Adam SJ, Kendall SD, Campbell PM, Der CJ, Counter CM (2006) Divergent roles for RalA and RalB in malignant growth of human pancreatic carcinoma cells. Curr Biol 16: 2385–2394 [DOI] [PubMed] [Google Scholar]
- Martineau M, Galli T, Baux G, Mothet JP (2008) Confocal imaging and tracking of the exocytotic routes for D-serine-mediated gliotransmission. Glia 56: 1271–1284 [DOI] [PubMed] [Google Scholar]
- Moskalenko S, Henry DO, Rosse C, Mirey G, Camonis JH, White MA (2002) The exocyst is a Ral effector complex. Nat Cell Biol 4: 66–72 [DOI] [PubMed] [Google Scholar]
- Moskalenko S, Tong C, Rosse C, Mirey G, Formstecher E, Daviet L, Camonis J, White MA (2003) Ral GTPases regulate exocyst assembly through dual subunit interactions. J Biol Chem 278: 51743–51748 [DOI] [PubMed] [Google Scholar]
- Nakashima S, Morinaka K, Koyama S, Ikeda M, Kishida M, Okawa K, Iwamatsu A, Kishida S, Kikuchi A (1999) Small G protein Ral and its downstream molecules regulate endocytosis of EGF and insulin receptors. Embo J 18: 3629–3642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangarajan A, Hong SJ, Gifford A, Weinberg RA (2004) Species- and cell type-specific requirements for cellular transformation. Cancer Cell 6: 171–183 [DOI] [PubMed] [Google Scholar]
- Rebhun JF, Chen H, Quilliam LA (2000) Identification and characterization of a new family of guanine nucleotide exchange factors for the Ras-related GTPase Ral. J Biol Chem 275: 13406–13410 [DOI] [PubMed] [Google Scholar]
- Rosse C, Hatzoglou A, Parrini MC, White MA, Chavrier P, Camonis J (2006) RalB mobilizes the exocyst to drive cell migration. Mol Cell Biol 26: 727–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosse C, L'Hoste S, Offner N, Picard A, Camonis J (2003) RLIP, an effector of the Ral GTPases, is a platform for Cdk1 to phosphorylate epsin during the switch off of endocytosis in mitosis. J Biol Chem 278: 30597–30604 [DOI] [PubMed] [Google Scholar]
- Sablina AA, Chen W, Arroyo JD, Corral L, Hector M, Bulmer SE, DeCaprio JA, Hahn WC (2007) The tumor suppressor PP2A Abeta regulates the RalA GTPase. Cell 129: 969–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz HD, Bereiter-Hahn J (2001) GFP associates with microfilaments in fixed cells. Histochem Cell Biol 116: 89–94 [DOI] [PubMed] [Google Scholar]
- Schweitzer JK, Burke EE, Goodson HV, D'Souza-Schorey C (2005) Endocytosis resumes during late mitosis and is required for cytokinesis. J Biol Chem 280: 41628–41635 [DOI] [PubMed] [Google Scholar]
- Schweitzer JK, D'Souza-Schorey C (2004) Finishing the job: cytoskeletal and membrane events bring cytokinesis to an end. Exp Cell Res 295: 1–8 [DOI] [PubMed] [Google Scholar]
- Shipitsin M, Feig LA (2004) RalA but not RalB enhances polarized delivery of membrane proteins to the basolaterla surface of epithelial cells. Mol Cell Biol 24: 5746–5756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skop AR, Liu H, Yates J III, Meyer BJ, Heald R (2004) Dissection of the mammalian midbody proteome reveals conserved cytokinesis mechanisms. Science 305: 61–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thullberg M, Gad A, Le Guyader S, Stromblad S (2007) Oncogenic H-Ras V12 promotes anchorage-independent cytokinesis in human fibroblasts. Proc Natl Acad Sci USA 104: 20338–20343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia A, Chardin P, Wittinghofer A, Sander C (1991) The ras protein family: evolutionary tree and role of conserved aminoacids. Biochemistry 30: 4637–4648 [DOI] [PubMed] [Google Scholar]
- VerPlank L, Li R (2005) Cell cycle-regulated trafficking of Chs2 controls actomyosin ring stability during cytokinesis. Mol Biol Cell 16: 2529–2543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Tang X, Liu J, Trautmann S, Balasundaram D, McCollum D, Balasubramanian MK (2002) The multiprotein exocyst complex is essential for cell separation in Schizosaccharomyces pombe. Mol Biol Cell 13: 515–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson GM, Fielding AB, Simon GC, Yu X, Andrews PD, Hames RS, Frey AM, Peden AA, Gould GW, Prekeris R (2005) The FIP3-Rab11 protein complex regulates recycling endosome targeting to the cleavage furrow during late cytokinesis. Mol Biol Cell 16: 849–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolthuis RM, Franke B, van Triest M, Bauer B, Cool RH, Camonis JH, Akkerman JW, Bos JL (1998) Activation of the small GTPase Ral in platelets. Mol Cell Biol 18: 2486–2491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Frankel P, Jackson D, Rotunda T, Boshans RL, D'Souza-Schorey C, Foster DA (2003) Elevated phospholipase D activity in H-Ras- but not K-Ras-transformed cells by the synergistic action of RalA and ARF6. Mol Cell Biol 23: 645–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Prekeris R, Gould GW (2007) Role of endosomal Rab GTPases in cytokinesis. Eur J Cell Biol 86: 25–35 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Movies S1
Supplementary Movies S2
Supplementary Movies S3
Supplementary Figure S4
Supplementary Figure S5
Supplementary Movies S6
Supplementary Figure S7
Supplementary Figure S8
Supplementary Methods S9
Supplementary Information Legends