Abstract
The specific recognition and binding of biological molecules by antibodies is fundamentally important. Natural antibodies are multivalent, having at least two identical ligand-binding sites; this permits them to bind tightly at cell surfaces, which present multiple copies of their target ligands. Antibodies that bind to soluble monovalent ligands, such as most small molecules, do not share this multivalent advantage. Nor do engineered fragments of antibodies, such as single-chain Fv proteins or Fab fragments, which generally possess only a single ligand-binding site. Engineered monovalent antibody/ligand pairs that retain the binding specificity of the antibody, but do not dissociate, are promising components of new delivery systems. These are based on a combination of genetic manipulation of the protein and chemical synthesis of appropriate ligands, examples of which are reviewed here.
Keywords: antibody, receptor, protein engineering, bifunctional chelating agent, macrocycle, DOTA, affinity label, mutagenesis
1. Introduction
Most of the articles in this special issue deal with probe molecules that have selective affinity for cancer cells. In some cases the target cells effectively trap the probes, usually by internalization. In other cases multivalent binding acts to hold the probe molecules at the target for long periods of time. Here we review an alternative approach that aims to enhance targeted radiotherapy by using probe molecules developed for reduced uptake in normal tissue, and examine an important aspect of that approach: irreversible capture of the probe molecule at the target.
The motivation for irreversible capture originated in the concept of pretargeted imaging and therapy, in the first step of which a receptor that could capture a man-made molecule is bound to target cells. After clearance of unbound receptor from the circulation, the patient exhibits that receptor only on target cells. The next step involves injection of a small synthetic probe molecule that has specific affinity for the receptor. This leads to the capture of probe molecules by pretargeted receptors; uncaptured probe molecules clear quickly through the kidneys, leaving almost no background.
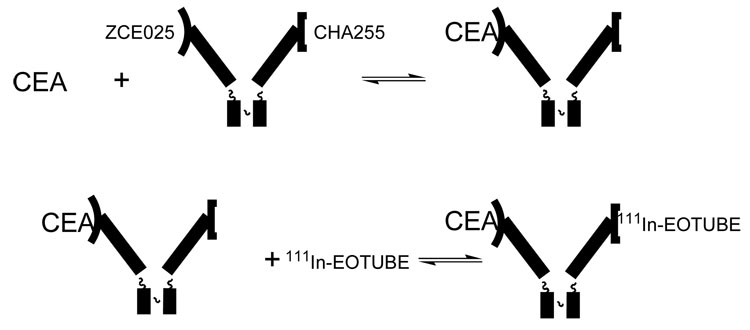
In our work, the first pretargeted receptor was a monoclonal antibody, CHA255, that specifically bound indium-111 chelates based on S-2-benzyl-EDTA [i]. In order to produce a cancer-targeting molecule, a Fab′ fragment of antibody CHA255 was cross-linked to a Fab′ fragment of the anti-CEA antibody ZCE025 (Figure 1). This bifunctional antibody was studied in a small number of human patients, showing excellent images of metastatic colon cancer in the liver [ii]. This was particularly important, because the metabolism of antibodies conventionally labeled with metal chelates generally leads to high levels of signal in the liver, which precludes detection of tumors there. However, the use of this bifunctional antibody was technically difficult, because the monovalent complexes formed by the bifunctional antibody (ZCE025 with CEA, CHA255 with the indium chelate) were not sufficiently stable.
Figure 1.
Bifunctional antibody for imaging human tumors; prepared by cross-linking Fab′ fragments of ZCE025, which binds carcinoembryonic antigen (CEA), and CHA255, which binds benzyl-EDTA(indium) chelates such as 111In-EOTUBE [ii]. In practice, the bifunctional antibody is injected and allowed to localize in the target as ZCE025 binds to CEA, and then the 111In chelate is injected and captured by the pretargeted CHA255. Uncaptured 111In chelate is efficiently eliminated via the kidneys, leaving little background signal in normal tissue.
How to extend the bound lifetimes of both entities? For binding to the carcinoembryonic antigen on cancer cells it is straightforward to extend the bound lifetime by using a multivalent antibody in place of a monovalent fragment, because many copies of carcinoembryonic antigen are expressed on the surface of a single cancer cell. As is well known, one of the most important aspects of antibody function is that two binding sites are much better than one when the target is a molecule expressed in great numbers on a cell surface.
A means of extending the bound lifetime of a small synthetic probe molecule is less obvious, because making the small molecule multivalent for its antibody also makes it larger. This can alter the required clearance properties, degrading the performance of the probe. For a long time we tried to design a triggerable mechanism that would permanently capture the probe when bound to the antibody, without success. When the crystal structure of the antibody-chelate complex became available [iii], a way forward occurred to us. We would use site-directed mutagenesis to prepare the CHA255 binding site for a specific chemical reaction, and we would use synthetic chemistry to prepare an indium chelate that would attach permanently to the mutated residue, but not react otherwise under physiological conditions [iv]. The elements of this plan have their roots in biochemistry and molecular biology.
2. Affinity Labeling
Site-specific derivatization of proteins (affinity labeling) began with the attachment of haloacetyl reagents to enzyme active sites, resulting in irreversible inhibition [e.g., v,vi], and the diazonium coupling of a hapten to an antibody’s binding site [vii]. The derivatizing reagents were prepared by synthesizing a substrate or ligand for the macromolecular target, and incorporating a reactive group. The ligand’s strong affinity for a particular site concentrates the reactive group there, where its reaction with a nearby amino acid side chain is promoted by mutual proximity, or effective local concentration. Affinity labeling has been widely employed in biomedical research, from protein structural studies to the development of new pharmaceuticals [e.g., viii]. Notably, even the antibiotic penicillin may be regarded as an affinity label [ix]. Several examples of drugs that act as affinity labels by binding to a site on a biological molecule and then forming a covalent bond are described in Section 3 below.
Producing targeting molecules in cultured cells allows an enhanced form of affinity labeling because control of the genes for the targeting molecule allows the production of mutant proteins with new properties. This is convenient for the development of macromolecules that permanently capture small probe molecules for targeted imaging and therapy [iv, x, xi, xii, xiii, xiv]. Because the macromolecular target site is genetically modified to make a reactive partner for the affinity label, it permits rapid, permanent attachment by modestly reactive synthetic ligands that bind near the modified site. Systems that exhibit such irreversible binding are referred to as having infinite affinity. The reason is that the on-rate is finite while the off-rate is zero; under these circumstances the apparent value of the equilibrium binding constant becomes .
2.1 Effective Local Concentration
To put this on a more quantitative footing, we turn to the simple example illustrated in Figure 2.
Figure 2.
Imagine that the reaction between molecules A and B can also occur with A and B connected by a linker. Compare the bimolecular reaction on the left to the unimolecular reaction on the right. The bimolecular reaction has a second-order rate constant k2 M−1s−1, while the unimolecular reaction has a first-order rate constant k1 s−1. The effective local concentration of A in the presence of B in the unimolecular reaction may be defined = k1/k2 (units: moles/liter, M). Several examples are described in reference [xv]
In organic chemistry this might be referred to as neighboring group assistance, and the linker might be a hydrocarbon chain. This phenomenon is also related to the chelate effect in inorganic coordination chemistry, where again the linker might be a hydrocarbon chain. In medicinal chemistry and biochemistry, irreversible inhibitors of enzyme action work on this principle; here the linker is usually more complex and the inhibitor usually binds reversibly to its target site and then makes a covalent bond with a nearby residue. The probe capture strategy we devised works similarly [iv].
2.2 Nucleophiles and Electrophiles
Because proteins and other biological molecules commonly possess an array of (electron-rich) nucleophilic sites such as amines and thiols but rarely contain electrophilic groups, the placement of an electrophile on the synthetic ligand to react with a nucleophile on the macromolecule seems to be a universal practice [e.g., xvi,xvii,xviii,xix,xx]. A potential weakness of this strategy is that the electrophilic reagent may react with nucleophiles other than the target because there are so many. This is manifested by the occasional development of allergic reactions to drugs such as penicillin [xxi]. Examples of this and other electrophiles are given below.
3. Pharmaceutical Examples of Affinity Labeling
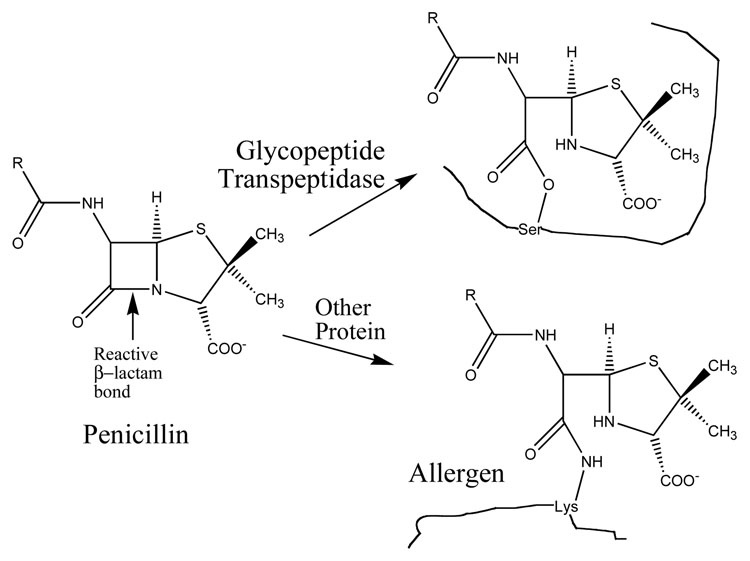
3.1 Penicillin (Figure 3)
Figure 3.
Penicillin and its reactions with target and non-target proteins [ix,xxi,xxii]. The arrow indicates the scissile bond of penicillin; the adjacent carbon atom acts as an electrophile, while the protein provides the nucleophile. The target glycopeptide transpeptidase has a nucleophilic oxygen on the side chain of a serine residue in the active site; alternatively, amino groups on the lysine side chains of other proteins may serve as nucleophiles to form stable amide linkages, resulting in allergens.
A prominent example of a small drug molecule that acts by covalent attachment to its target, the antibiotic penicillin binds to and reacts with an active site nucleophile of bacterial cell wall synthesizing enzymes to form an inactive acyl complex [ix,xxii]. The acyl-enzyme is subject to hydrolysis under physiological conditions, with a half-life of several minutes; a system like this would be of only marginal use for attachment of a probe molecule to a target. It is important to be aware that in common parlance, formation of a covalent bond between inhibitor and enzyme is often referred to as “irreversible” even when it obviously is not.
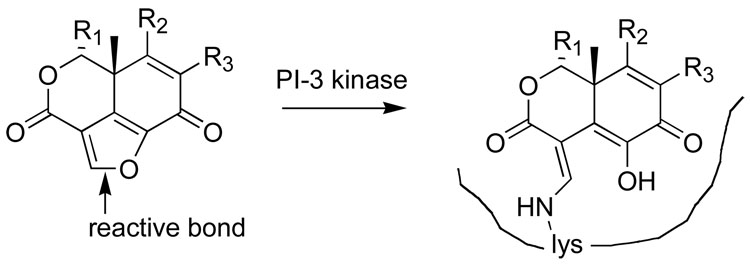
3.2 Wortmannin (Figure 4)
Figure 4.
The reaction of wortmannin with its target, phosphoinositide 3- kinase, showing the proposed product [xxv].
Wortmannin, a fungal metabolite, is a potent and specific inhibitor of phosphoinositide (PI) 3 kinase [xxiii,xxiv]. It contains a furan ring that is subject to nucleophilic attack by amines, and it has been proposed that a particular lysine in PI-3 kinase opens the furan ring to form the covalent link shown in Figure 4.
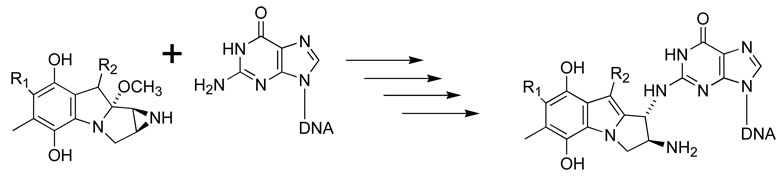
3.3 The Mitomycins (Figure 5)
Figure 5.
The reaction of mitomycin A hydroquinone with a guanine residue in the minor groove of DNA, by a complex mechanism involving the three-membered aziridine ring [xxvi,xxvii].
Mitomycins A and C are two of the most familiar of a highly elaborated family of DNA-alkylating drugs [xxvi,xxvii]. Their chemical mechanism of action is complex, ultimately resulting in the opening of the strained 3-membered ring.
The examples above suggest that natural products can possess specific chemical reactivity with a high order of subtlety, based on complex structures whose reactivity may be modified by binding to their targets. The molecular groups on proteins or DNA that become conjugated to the drugs may be rather surprising, particularly the alcohol oxygen of a serine side chain and the 2-amino group of guanine, both of which are weak nucleophiles under normal circumstances. Evidently the binding of the drug near the target residue has a strong influence on the course, or even the occurrence, of the reaction.
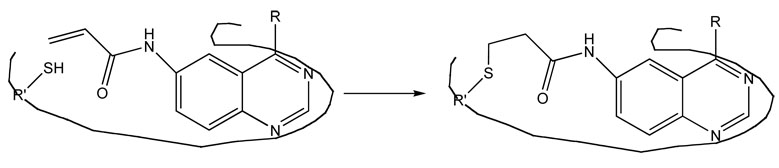
3.4 PD168393 (Figure 6)
Figure 6.
Permanent attachment of PD168393 to the naturally occurring Cys- 773 of the human epidermal growth factor receptor [viii].
These examples, which originate from natural products found in fungi and other organisms, provide a framework for the design of synthetic molecules that will bind permanently to a chosen site on a protein or some other biological molecule. An instructive example is the candidate drug PD168393, a synthetic, irreversible enzyme inhibitor that blocks the function of the epidermal growth factor receptor [viii]. The active site of the epidermal growth factor receptor is rich in cysteine residues. Here a Michael addition of a cysteine side chain in the protein to the double bond of an acryloyl group in the synthetic drug molecule leads to a robust thioether bond that is not likely to degrade significantly over time (Figure 6).
4. Engineered receptors
For the capture of probe molecules, it is attractive to use an antibody’s binding site and engineer the antibody to display an appropriately located cysteine thiol side chain because of its distinctive nucleophilicity [xiv].
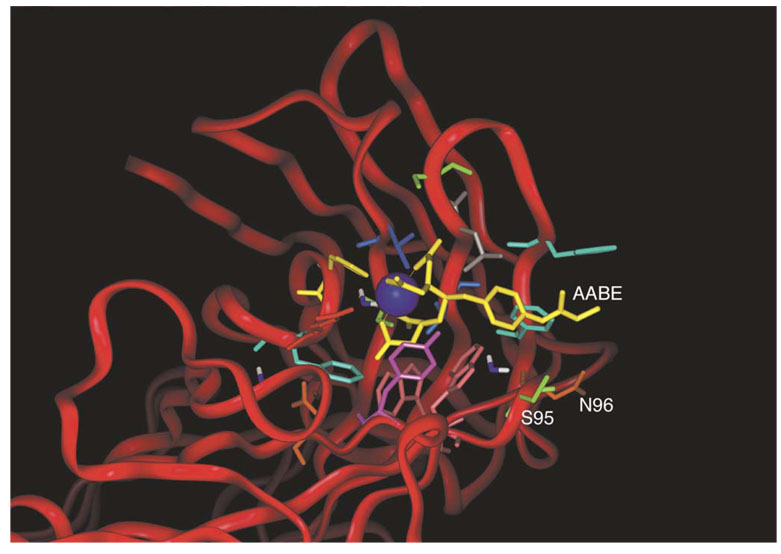
4.1 Antibody CHA255 (Figure 7)
Figure 7.
Selection of amino-acid residues for mutation [iv]. The crystal structure of the CHA255-ligand complex from reference [iii] was modified to simulate an acryloyl substituent at the para-position of the aromatic ring of the ligand. Residues 95 and 96 of the antibody light chain (Kabat numbering 93 and 94) showed side chains that were not in van der Waals contact with the ligand, yet were only a few Ångstroms away from the para-substituent. Reproduced from A.J. Chmura, M.S. Orton, C.F. Meares, Antibodies with infinite affinity, Proc. Natl. Acad. Sci. USA 98 (2001) 8480-8484.
Choosing the location of this cysteine poses an interesting challenge. As shown in Figure 7, we initially studied the crystal structure of the CHA255 antibody-chelate complex [iii] to look for amino-acid residues whose side chains were close to the para position of the aromatic ring of the chelate [iv].
A set of candidate ligands for irreversible binding was synthesized and tested for side reactions in a mouse model as described in [x]. Briefly, it was observed that chelates carrying an acryloyl group were cleanly excreted from mice over the course of 24 hr, while chelates carrying more reactive electrophiles such as chloro- or bromoacetyl groups were substantially retained. These experimental results set the stage for a reaction dominated by the effective local concentration of a chelate associated in the binding site of the antibody.
The two amino-acid residues highlighted in Figure 7 initially appeared equally likely to react with the acryloyl-bearing ligand, but we found that only the Ser95Cys mutant did so [iv]. The failure of the Asn96Cys mutant to react with the ligand might have been due to alternative folding of the mutant, or other causes related to the expression of mutant proteins in insect cells. Because the reaction between the ligand and the Ser95Cys mutant of CHA255 was rapid and efficient, we did not investigate the Asn96Cys further.
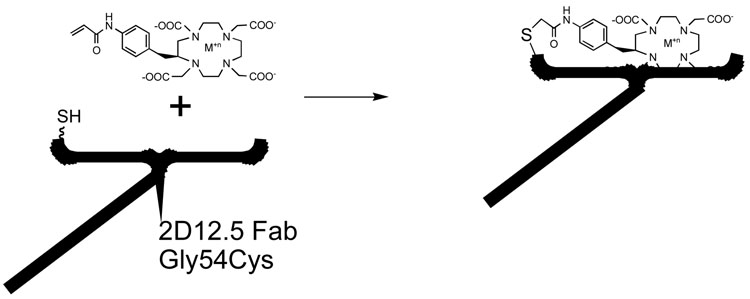
4.2 Antibody 2D12.5 (Figure 8)
Figure 8.
Permanent capture of Acryloyl-bearing DOTA chelates by the Gly54Cys mutant of antibody 2D12.5. A variety of metals may be used, including not only all the rare earths but also indium and copper [xi].
We later used a similar strategy to select residues for mutation of the DOTA-binding antibody 2D12.5 [xii]. Here again the crystal structure of the complex [xxviii] was very useful in choosing mutation sites, because the region of the 2D12.5 antibody that lies close to the para position of the ligand is very different from that of antibody CHA255. The sites we chose to mutate were on the heavy chain of antibody 2D12.5, as opposed to the light chain of CHA255, and were at sequence positions 54, 55, and 56, all of which were glycine residues located on a loop of complementarity determining region 2 [xii]. We used the same chemical strategy of site-directed mutation to cysteine, and found that the Gly54Cys mutant of antibody 2D12.5 reacted very well with acryloyl-substituted aminobenzyl-DOTA chelates containing any of a number of different metal ions [xi,xii].
5. Metal selectivity of anti-chelate antibodies
One of the surprising aspects of antibodies that bind metal chelates is that the details of the molecular interactions involved are characteristic of each system. When antibody CHA255 binds a benzyl-EDTA(indium) chelate, a histidine side chain coordinates directly to the indium ion. This antibody-chelate system is remarkably metal-selective, with significant affinity only for the indium chelate and to a lesser degree for the iron chelate, which binds in a different way [iii]. In contrast, antibody 2D12.5 binds DOTA complexes with all the rare earths with similar affinity [xxix]. The crystal structures of 2D12.5 with DOTA chelates of yttrium and gadolinium show that there are no direct interactions between the protein and the metal [xxviii]. As illustrated in Figure 8, DOTA chelates of metals whose properties are far from the rare earths still bind to 2D12.5 well enough for permanent attachment to occur [xi]. This is true even for copper-DOTA, whose structure is quite different from the rare-earth DOTA chelates.
6. Other examples of irreversible capture
Shortly after the development of antibodies with infinite affinity, Belshaw reported an application based on similar acryloyl + cysteine attachment [xix], and more recently used this engineered cyclophilin A– cyclosporin A receptor–ligand system to fluorescently label fusion proteins in live cells [xxx]. Sames reported the use of an epoxide affinity label to alkylate a specific histidine in native carbonic anhydrase II [xx].
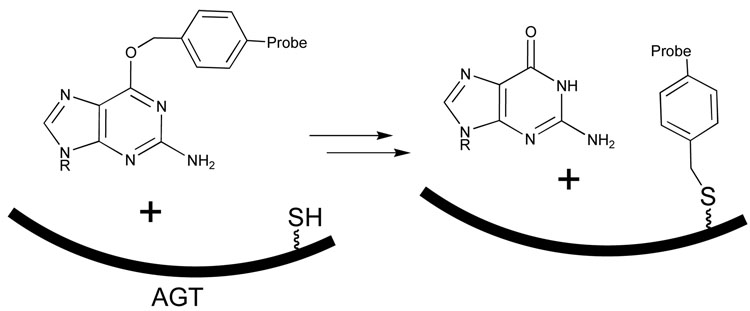
Another potentially interesting approach is the use of the enzyme O6- alkylguanine alkyltransferase (AGT), which can form a thioether link to a synthetic molecule [xxxi]. If the synthetic molecule is chosen for this type of application, a guanosine derivative carrying a fluorescent or other imaging probe can be used to label AGT [xxxii]. The AGT enzyme might be developed further to play a role similar to the antibodies with infinite affinity described above.
7. Outlook
There now are well-established complementary reaction partners that possess the properties necessary for in vivo applications of irreversible probe capture. The next stage, development and validation of fusion proteins and protocols for their use in targeted radiotherapy, is nearing maturity. New approaches to inserting this chemistry into a variety of biological situations are being explored. One obvious topic of interest is the biotin-avidin interaction, which is extraordinarily strong and specific and is widely used for biochemical assays in vitro. Many of the irreversible systems described above have properties of unique specificity, and chemical and genetic accessibility, that should easily lead to improvements on current avidin-biotin technology. In particular, synthetic probe molecules and matching antibodies can be prepared that do not compete with naturally occurring molecules such as biotin or guanine for binding to an engineered protein, and the choices are limitless. The use of libraries of such molecular pairs could make possible multiplex analyses and imaging/therapeutic technologies that will yield more benefit with much less background interference than are available currently.
Figure 9.
Transfer of an alkyl group from an O-benzyl-guanine derivative to form a stable bond to cysteine in the active site of the enzyme O6- alkylguanine alkyltransferase (AGT), with the potential to capture a probe molecule at the site [xxxi,xxxii].
Acknowledgment
I thank the members of the Meares laboratory, from the beginning to the present, for their contributions to this research program; also our medical collaborators over the years, in particular David Goodwin, for sharing their insights and ideas. This research has been supported by NIH research grants CA016861 and CA098207 from the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- i.Reardan DT, Meares CF, Goodwin DA, McTigue M, David GS, Stone MR, Leung JP, Bartholomew RM, Frincke JM. Antibodies Against Metal Chelates. Nature. 1985;316:265–268. doi: 10.1038/316265a0. [DOI] [PubMed] [Google Scholar]
- ii.Stickney DR, Anderson LD, Slater JB, Ahlem CN, Kirk GA, Schweighardt SA, Frincke JM. Bifunctional Antibody: A Binary Radiopharmaceutical Delivery System for Imaging Colorectal Carcinoma. Cancer Research. 1991;51:6650–6655. [PubMed] [Google Scholar]
- iii.Love RA, Villafranca JE, Aust RM, Nakamura KK, Jue RA, Major JG, Radhakrishnan R, Butler WF. How the anti-(metal chelate) antibody CHA255 is specific for the metal ion of its antigen: X-ray structures for two Fab'/hapten complexes with different metals in the chelate. Biochemistry. 1993;32:10950–10959. doi: 10.1021/bi00092a004. [DOI] [PubMed] [Google Scholar]
- iv.Chmura AJ, Orton MS, Meares CF. Antibodies with infinite affinity. Proc. Natl. Acad. Sci. USA. 2001;98:8480–8484. doi: 10.1073/pnas.151260298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- v.Baker BR, Lee WW, Tong E, Ross LO. Potential Anticancer Agents. LXVI. Non-classical Antimetabolites. III. 4-(Iodoacetamido)-Salicylic Acid, an Exo Alkylating Irreversible Inhibitor of Glutamic Dehydrogenase. J. Am. Chem. Soc. 1961;83:3713–3714. [Google Scholar]
- vi.Schoellmann G, Shaw E. Direct evidence for the presence of histidine in the active center of chymotrypsin. Biochemistry. 1963;2:252–255. doi: 10.1021/bi00902a008. [DOI] [PubMed] [Google Scholar]
- vii.Wofsy L, Metzger H, Singer SJ. Affinity labeling-A general method for labeling the active sites of antibody and enzyme molecules. Biochemistry. 1962;1:1031–1039. doi: 10.1021/bi00912a013. [DOI] [PubMed] [Google Scholar]
- viii.Fry DW, Bridges AJ, Denny WA, Doherty A, Greis KD, Hicks JL, Hook KE, Keller PR, Leopold WR, Loo JA, McNamara DJ, Nelson JM, Sherwood V, Smaill JB, Trumpp-Kallmeyer S, Dobrusin EM. Specific, irreversible inactivation of the epidermal growth factor receptor and erbB2, by a new class of tyrosine kinase inhibitor. Proc. Natl. Acad. Sci. USA. 1998;95:12022–12027. doi: 10.1073/pnas.95.20.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ix.Yocum RR, Waxman DJ, Rasmussen JR, Strominger JL. Mechanism of penicillin action: penicillin and substrate bind covalently to the same active site serine in two bacterial D-alanine carboxypeptidases. Proc. Natl. Acad. Sci. USA. 1979;76:2730–2734. doi: 10.1073/pnas.76.6.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- x.Chmura AJ, Schmidt BD, Corson DT, Traviglia ST, Meares CF. Electrophilic chelating agents for binding of metal chelates to engineered antibodies. J. Controlled Release. 2002;78:249–258. doi: 10.1016/s0168-3659(01)00485-0. [DOI] [PubMed] [Google Scholar]
- xi.Corneillie TM, Whetstone PA, Lee KC, Wong JP, Meares CF. Converting weak binders into infinite binders. Bioconjugate Chem. 2004;15:1389–1391. doi: 10.1021/bc049825e. [DOI] [PubMed] [Google Scholar]
- xii.Corneillie TM, Lee KC, Whetstone PA, Wong JP, Meares CF. Irreversible Engineering of the Multielement-Binding Antibody 2D12.5 and Its Complementary Ligands. Bioconjugate Chem. 2004;15:1392–1402. doi: 10.1021/bc049824m. [DOI] [PubMed] [Google Scholar]
- xiii.Corneillie TM, Whetstone PA, Meares CF. Irreversibly binding anti-metal chelate antibodies: Artificial receptors for pretargeting. J. Inorg. Biochem. 2006;100:882–890. doi: 10.1016/j.jinorgbio.2006.01.004. [DOI] [PubMed] [Google Scholar]
- xiv.Butlin NG, Meares CF. Antibodies with infinite affinity: origins and applications. Accts. Chem. Res. 2006;39:780–787. doi: 10.1021/ar020275e. [DOI] [PubMed] [Google Scholar]
- xv.Fersht A. Enzyme Structure and Mechanism. 2nd Ed. New York: Freeman; 1985. pp. 56–63. [Google Scholar]
- xvi.Pollack SJ, Nakayama GR, Schultz PG. Introduction of nucleophiles and spectroscopic probes into antibody combining sites. Science (Washington, DC) 1988;242:1038–1040. doi: 10.1126/science.3194752. [DOI] [PubMed] [Google Scholar]
- xvii.Salerno A, Lawrence DS. Covalent modification with concomitant inactivation of the cAMP-dependent protein kinase by affinity labels containing only L-amino acids. J. Biol. Chem. 1993;268:13043–13049. [PubMed] [Google Scholar]
- xviii.Yan XW, Corbin JD, Francis SH, Lawrence DS. Precision targeting of protein kinases - An affinity label that inactivates the cGMP- but not the cAMP-dependent protein kinase. J. Biol. Chem. 1996;271:1845–1848. doi: 10.1074/jbc.271.4.1845. [DOI] [PubMed] [Google Scholar]
- xix.Levitsky K, Ciolli CJ, Belshaw PJ. Selective inhibition of engineered receptors via proximity-accelerated alkylation. Org Lett. 2003;5:693–696. doi: 10.1021/ol027448k. [DOI] [PubMed] [Google Scholar]
- xx.Chen G, Heim A, Riether D, Yee D, Milgrom Y, Gawinowicz MA, Sames D. Reactivity of functional groups on the protein surface: development of epoxide probes for protein labeling. J. Am. Chem. Soc. 2003;125:8130–8133. doi: 10.1021/ja034287m. [DOI] [PubMed] [Google Scholar]
- xxi.Blanca M, Cornejo-Garcia JA, Torres MJ, Mayorga C. Specificities of B cell reactions to drugs. The penicillin model. Toxicology. 2005;209:181–184. doi: 10.1016/j.tox.2004.12.018. [DOI] [PubMed] [Google Scholar]
- xxii.Nicholas RA, Strominger JL. Site-directed Mutants of a Soluble Form of Penicillin-binding Protein 5 from Escherichia coli and Their Catalytic Properties. J. Biol. Chem. 1988;263:2034–2040. [PubMed] [Google Scholar]
- xxiii.Wymann MP, Bulgarelli-Leva G, Zvelebil MJ, Pirola L, Vanhaesebroeck B, Waterfield MD, Panayotou G. Wortmannin Inactivates Phosphoinositide 3-Kinase by Covalent Modification of Lys-802, a Residue Involved in the Phosphate Transfer Reaction. Mol. Cellular Biol. 1996;16:1722–1733. doi: 10.1128/mcb.16.4.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xxiv.Wipf P, Halter RJ. Chemistry and biology of wortmannin. Org. Biomol. Chem. 2005;3:2053–2061. doi: 10.1039/b504418a. [DOI] [PubMed] [Google Scholar]
- xxv.Norman BH, Paschal J, Vlahos CJ. Synthetic Studies On The Furan Ring Of Wortmannin. Bioorg. Med. Chem. Lett. 1995;5:1183–1186. [Google Scholar]
- xxvi.Kunz KR, Iyengar BS, Dorr RT, Alberts DS, Remers WA. Structure-Activity Relationships for Mitomycin C and Mitomycin A Analogues. J. Med. Chem. 1991;34:2281–2286. doi: 10.1021/jm00111a051. [DOI] [PubMed] [Google Scholar]
- xxvii.Boamah EK, White DE, Talbott KE, Arva NC, Berman D, Tomasz M, Bargonetti J. Mitomycin–DNA Adducts Induce p53-Dependent and p53-Independent Cell Death Pathways. ACS Chem. Biol. 2007;2:399–407. doi: 10.1021/cb700060t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xxviii.Corneillie TM, Fisher AJ, Meares CF. Crystal Structures of Two Complexes of the Rare-Earth-DOTA-Binding Antibody 2D12.5: Ligand Generality from a Chiral System. J. Am. Chem. Soc. 2003;125:15039–15048. doi: 10.1021/ja037236y. [DOI] [PubMed] [Google Scholar]
- xxix.Corneillie TM, Whetstone PA, Fisher AJ, Meares CF. A rare earth- DOTA-binding antibody: Probe properties and binding affinity across the lanthanide series. J. Am. Chem. Soc. 2003;125:3436–3437. doi: 10.1021/ja029363k. [DOI] [PubMed] [Google Scholar]
- xxx.Krusemark CJ, Belshaw PJ. Covalent labelling of fusion proteins in live cells via an engineered receptor-ligand pair. Organic & Biomolecular Chem. 2007;5:2201–2204. doi: 10.1039/b705185a. [DOI] [PubMed] [Google Scholar]
- xxxi.Noll DM, Clarke ND. Covalent capture of a human O(6)-alkylguanine alkyltransferase-DNA complex using N(1),O(6)-ethanoxanthosine, a mechanism-based crosslinker. Nucleic Acids Res. 2001;29:4025–4034. doi: 10.1093/nar/29.19.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xxxii.Heinis C, Schmitt S, Kindermann M, Godin G, Johnsson K. Evolving the Substrate Specificity of O6-Alkylguanine-DNA Alkyltransferase through Loop Insertion for Applications in Molecular Imaging. ACS Chem. Biol. 2006;1:575–584. doi: 10.1021/cb6003146. [DOI] [PubMed] [Google Scholar]