Abstract
Alterations in CD151 have been associated with primary glomerular disease in both humans and mice, implicating CD151 as a key component of the glomerular filtration barrier. CD151 belongs to the tetraspanin family and associates with cell-matrix adhesion complexes such as α3β1-integrin. Here we show that Cd151-deficient mice develop severe kidney disease on an FVB background but are healthy on a B6 background, providing a new and unique tool for the identification of genes that modulate the onset of proteinuria. To better understand the function of CD151 in the kidney, we studied its expression pattern and characterized early ultrastructural defects in Cd151-null kidneys. CD151 is expressed in podocytes of the mouse kidney and co-localizes with α3-integrin at the base of podocyte foot processes, at the site of anchorage to the glomerular basement membrane (GBM). Interestingly, the first ultrastructural lesions seen at the onset of proteinuria in Cd151-null kidneys were severe alterations of the GBM, reminiscent of Alport syndrome and consisting of massive thickening and splitting of the GBM. These lesions are associated with increased expression of GBM components. Podocyte abnormalities, effacement of foot processes, and podocyte loss appear to occur consequently to the GBM damage. In conclusion, CD151 appears to be involved in the establishment, maturation, and/or maintenance of the GBM structure in addition to its role in integrin-mediated adhesion strengthening.
Primary glomerular diseases are characterized by proteinuria and are due to intrinsic alterations of the glomerular filtration barrier, which is composed of a tight association between the podocytes, the glomerular basement membrane (GBM), and the fenestrated endothelium. The podocytes are highly organized octopus-like epithelial cells. They present with a cell body floating in the urinary space, sending out primary processes that divide into secondary processes (foot processes) interdigitating around each capillary loop and tightly attached to each other by a specific cell-cell junction called the slit diaphragm. In most glomerular diseases proteinuria is associated with effacement of podocyte foot processes. For many years, there has been some controversy about whether effacement of podocyte foot processes is a cause or a consequence of proteinuria. The podocyte cytoskeleton and the slit diaphragm often tend to be considered as the central players in the filtration barrier, because mutations in several proteins associated with the slit diaphragm and cytoskeleton have been associated with glomerular diseases in humans in recent years.1 Clearly, in these cases the effacement of podocyte foot processes is believed to be the causal event for proteinuria. However, GBM integrity and proper podocyte anchorage are also required for a functional kidney filter. Indeed, alterations in any of the α3, α4, and α5 type IV collagen chains cause Alport syndrome, which is characterized by glomerular disease and sensorineural deafness,2,3 while mutations in the laminin β2 gene cause Pierson syndrome,4 comprising severe glomerular disease, ocular abnormalities, and psychomotor defects. In these two pathologies, the glomerular disease is associated early on with ultrastructural defects of the GBM. As shown by Jarad and colleagues5 in the mouse model of Pierson’s syndrome, proteinuria and GBM abnormalities precede effacement of podocyte foot processes. Interestingly, alterations in the α3β1 laminin binding integrin, the major integrin involved in attachment of the podocyte foot processes to the GBM, cause a severe glomerular disease in mice6,7 characterized by proteinuria associated with both GBM abnormalities and effacement of podocyte foot processes.
Recent work in humans has shown that the tetraspanin CD151 is essential for the function of the kidney, as mutations in CD151 have been identified in three patients presenting with hereditary nephrotic syndrome leading to end stage renal failure, pretibial bullous skin lesions, sensorineural deafness, and thalassemia.8 CD151 is part of the tetraspanin family of proteins that are ubiquitously expressed, membrane-embedded proteins that share a similar structure, and form dynamic complexes with each other and with integrins. While tetraspanins clearly play a role in cell migration, maintenance of cell-cell contacts, and membrane remodeling, their exact function remains poorly understood at the molecular level. CD151 is expressed in a wide range of cell types throughout the body9,10 and strongly associates with laminin-binding integrins such as α3β1 integrin.11,12,13,14 CD151 does not seem to regulate static integrin mediated cell adhesion15,16 but instead seems to mediate the strengthening of integrin-based cell-extracellular matrix adhesion.17,18 In the glomerular filter, CD151 could therefore have a collaborative role with α3β1 integrin in strengthening the hold of podocytes onto the GBM to counteract the mechanical forces due to the flow of primary urine and constant stretch. Recently, Sachs et al reported that Cd151 deletion in mice on a mixed FVB/N x129 background resulted in severe glomerular disease.7 In the kidneys of 3-month-old Cd151-null mice, they observed partial loss of podocyte foot processes and slit diaphragms, together with thickening and protrusion of the GBM, and decreased expression of podocyte proteins. At this age however, the disease is already advanced, with massive proteinuria and lesions of glomerulosclerosis, and it is thus impossible to identify the primary glomerular changes. At this point, it was therefore not clear whether CD151 was involved in foot processes adhesion to the GBM, development/maintenance of the slit diaphragm, and/or regulation of the cytoskeleton, or all of the above.
Here, we investigated this question and characterized the primary and causative defect in Cd151-null diseased glomeruli, providing new insights into CD151’s function in the glomerular filtration barrier. In the present study, we show that our Cd151 knock-out mice, which are grossly normal and healthy on the C57Bl/6 (B6) background,19,20,21 develop a severe glomerular disease after backcross onto a FVB/N (FVB) background. To better understand the role of CD151 in the kidney filter, we analyzed the pattern of expression of CD151 in the mouse kidney and characterized the ultrastructural changes associated with the onset of proteinuria in young Cd151-null mice. Our study shows that CD151 plays an important role not only in the GBM-podocyte interaction but also in the GBM integrity. It also provides new evidence for a primary role of the GBM network and the podocyte-GBM adhesion in the maintenance of a selective kidney filter.
Materials and Methods
Animals, Backcrossing, and Genotyping
The use of animals for this study was approved by the University of Newcastle (Australia) Animal Care and Ethics Committee. Cd151-null mice on a B6 strain19 were backcrossed onto the FVB background for 10 generations. Genotyping was performed as previously described.19 Male and female 10th generation heterozygous mice were then crossed to obtain null mice. Both male and female Cd151+/+ and Cd151−/− progeny have been included in the study and no gender difference was observed.
Proteinuria Analysis
Urine samples were diluted in SDS sample buffer (375 mmol/L Tris–HCL, 2% (w/v) SDS, and 10% (w/v) sucrose, pH (6.8), containing 2% (v/v) β-mercaptoethanol, and boiled at 100°C for 5 minutes. One microliter of each sample was separated on a 10% denaturing polyacrylamide gel and stained using Coomassie brilliant blue G250.
Histological and Ultrastructural Analysis
For histology, kidneys were halved and fixed overnight in 4% paraformaldehyde or 10% neutral buffered formalin. Kidneys were then washed in PBS, dehydrated, and embedded in paraffin. Sections (4 μm) were stained with H&E or PAS.
For scanning and transmission electron microscopy (SEM and TEM), kidney cortex was cut into small pieces, fixed in glutaraldehyde (2.5% in PBS) for a minimum of 12 hours at 4°C, postfixed in 1% osmium tetroxide for 1 hour at room temperature, and dehydrated. For SEM, the blocks were then critical-point dried, cracked, fixed onto metal stubs, coated with gold and imaged using a Philips XL30 SEM. For TEM, post fixed tissue was embedded in Spurr resin. Sections (70 nm) were cut on an Ultracut S ultramicrotome (Reichert-Jung, Austria) with a diamond knife (Diatome Ltd, Bienne, Switzerland), placed on formvar coated copper grids, and stained in saturated uranyl acetate and lead citrate. Micrographs were taken on a JEM-1200EXII transmission electron microscope (Tokyo, Japan) operating at 80 kV. To estimate the extent of GBM splitting, and effacement of foot processes over time, TEM pictures of several capillary loops from two mice of each genotype were assessed as follows: Normal and split regions of GBM were measured and the number of podocyte foot processes was counted. The percentage of split GBM and number of foot processes per micrometer of GBM were calculated.
Antibodies
For immunohistochemistry the following antibodies and dilutions were used: rabbit anti-WT1 (Wilms’ Tumor) antibody (sc-192, Santa Cruz Biotechnology, Santa Cruz, CA) at 1:100; rat anti-laminin γ1 (MAB1914, Chemicon, Temecula, CA) at 1:1500; rat anti-nidogen (MAB1883, Chemicon) at 1:200; rat anti-CD31 (550274, BD Biosciences, Franklin Lakes, NJ) at 1:200; rabbit anti-α3 integrin22 (a kind gift from Dr. Fiona Watt, Wellcome Trust Centre for Stem Cell Research, Cambridge, UK) at 1:1000; rabbit anti-podocin23 (P35, a kind gift from Dr. Corinne Antignac, INSERM U574, Hôpital Necker-Enfants Malades, Université René Descartes, Paris, France) at 1:2000; rabbit anti-laminin α524 (a kind gift from Dr. Jeffrey Miner, Washington University, St. Louis, Missouri) at 1:500; rabbit anti-laminin β225 (a kind gift from Dr. Takako Sasaki, Shriners Hospital for Children, Portland, Oregon) at 1:500; rat anti- α1, α2, α3, α4, and α5 chains of collagen type IV22,26,27,28 (H11, H22, H31, RH42, and H53 respectively; a kind gift from Dr. Yoshikadu Sado, Shigei Medical Research Institute, Okayama, Japan) at 1:100. Fluorescent secondary antibodies (Molecular Probes) were donkey anti-rat AlexaFluor 555, Invitrogen, Carlsbad, CA at 1:300; and donkey anti-rabbit AlexaFluor 488 and 555 at 1:300. The anti-CD151 antibody (LAI-2) was generated by immunizing rabbits with a peptide corresponding to mouse C- and N-terminal tails fused at cysteine (ie, N-GEFNEKKATCLYRSLKLEHY-C) and affinity purified.
Immunofluorescence Labeling
Kidneys were snap frozen in liquid nitrogen using Tissue-Tek optimal cutting temperature compound and stored at −80°C and routine immunolabeling was performed as previously described.23 Briefly, 3-μm-thick frozen sections were fixed in acetone, air dried, and rehydrated in PBS. Sections were then blocked with 5% donkey serum for 30 minutes, and incubated with primary antibodies for 1 hour. Slides were washed in PBS, and incubated with secondary antibodies for 45 minutes at room temperature. Slides were washed in PBS, and either mounted in Vectashield, or stained with DAPI before mounting. All dilutions were made in 1% bovine serum albumin and 0.1% Tween in PBS. For double labeling where two rabbit primary antibodies were used, we followed established methods.29 In this case, the two labeling procedures were done sequentially. The anti-CD151 rabbit antibody was applied first and revealed by an AlexaFluor 488 secondary antibody (green) (Molecular Probes). CD151 labeling was then fixed with 4% paraformaldehyde, blocked for 1 hour with purified rabbit IgG followed by 1 hour with donkey anti-rabbit Fab fragment (Jackson ImmunoResearch Laboratories, West Grove, PA). Then, labeling with the second rabbit antibody (podocin or integrin-α3) was performed and revealed with an AlexaFluor 555 (red) secondary antibody (Molecular Probes). Appropriate controls were included in the double rabbit staining.
Determination of Podocyte Number and Podocyte Loss
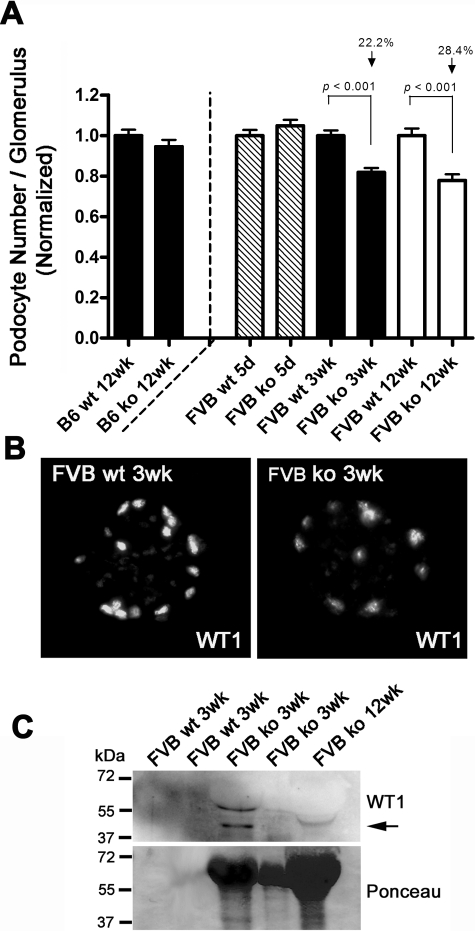
Kidney sections from 5-day-old, 3-week-old, and 12-week-old FVB wild-type and Cd151−/− mice, and 12-week-old B6 mice were stained for the podocyte marker WT1 and counterstained with DAPI. Podocytes from a minimum of 15 glomeruli were counted, from at least three individual mice. Only glomeruli measuring between 70 to 100 μm in diameter were scored in adult mice to ensure sectioning through the center of each glomerulus counted. In 5-day old mice, only fully developed glomeruli were counted. Slides were coded and counted blind by two separate people. Glomerular cross-sectional areas were calculated and showed a slight but significant increase for Cd151−/− versus Cd151+/+ mice (data not shown). Because glomerular cross-sectional areas were significantly different, numbers of podocytes per cross-sectional area could not be directly compared. Therefore, values of podocyte number per cross-sectional area were corrected for average podocyte size, glomerular area, and glomerular volume using published methodology.30 Data are presented as a percentage of wild-type values for each mouse strain. For Western blot analysis, 5 μl urine samples were run out by SDS-polyacrylamide gel electrophoresis and transferred onto nitrocellulose membrane. Membranes were stained with Ponceau stain, probed with anti-WT1 antibody at 1:100, and with goat anti-rabbit horseradish peroxidase secondary antibody.
Data Analysis
Experiments were replicated at least three times before statistical assessment by non-parametric t-test (Mann-Whitney U). Samples with a P value of <0.05 were considered significant (*).
Results
FVB, but Not B6 Cd151-Null Mice, Develop Severe Glomerular Disease
After backcross of the B6 Cd151+/− heterozygous mice for 10 generations onto the FVB background, they were mated, and the phenotype of FVB Cd151-null mice was analyzed. Five FVB Cd151−/− mice died unexpectedly at around 5 months of age. Then, two 5-month old mice looking unwell and edematous were euthanized and sent to pathology; the reports pointed out that these mice were suffering from end stage renal failure. From this point onward, the Animal Care and Ethics committee allowed us to keep FVB Cd151-null mice only up to 4 months of age. We found that these FVB Cd151-null mice exhibited a glomerular disease comparable to what has been described by Sachs et al. Briefly, FVB Cd151-null mice presented with proteinuria by 5 days of age, which became more severe by 2 and 6 weeks of age (Figure 1). At 4 weeks of age, the kidneys from Cd151-null mice looked quite normal by light microscopy and H&E staining, with most glomeruli indistinguishable from wild-type mice (not shown). However, in some glomeruli at this age, the H&E staining showed focal thickening of the extracellular matrix (Figure 2B, arrow), which appeared to reflect important thickening of the GBM, as shown by PAS staining (Figure 2J). In fact, most glomeruli in the 3- to 4-week-old FVB Cd151-null mice showed similar extensive thickening of the GBM, whereas they did not present with sclerotic areas (assessed by trichrome staining, data not shown) at this age. The GBM thickening became more dramatic with age and by the age of 12 weeks, all of the FVB Cd151-null mice showed significant glomerular damage, the severity of which varied between littermates at this age (Figure 2, D–F). Indeed, most of the 12 week-old FVB Cd151-null mice analyzed (4 out of 5 mice) presented with mild to moderate glomerular lesions (Figure 2D) made of abnormal thickening of the GBM (Figure 2L) associated with obstruction of the tubules with proteinaceous casts. One of the mice examined at 12 weeks of age already had severe and advanced glomerular lesions associated with massive tubular dilations and proteinaceous casts (Figure 2E). Similar severe renal changes were observed in the 4 to 5 month-old Cd151−/− FVB mice that presented with edema, had enlarged and granular kidneys, and developed progressive kidney failure, eventually leading to death (data not shown).
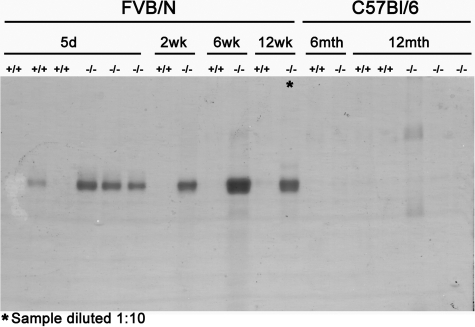
Figure 1.
Massive Proteinuria in Cd151-Null Mice on the FVB but Not B6 Background. Urine from Cd151+/+ and Cd151−/− mice at different ages was analyzed by SDS- polyacrylamide gel electrophoresis. One microliter of urine from each mouse was loaded on the same gel. Proteinuria was observed as early as 5 days of age in the FVB Cd151-null mouse and increased substantially with age to become massive at 12 weeks (note that the urine sample of the 12 week-old FVB Cd151-null mouse has been diluted 1:10). B6 Cd151-null mice were analyzed up to 12 months of age and did not exhibit any signs of proteinuria.
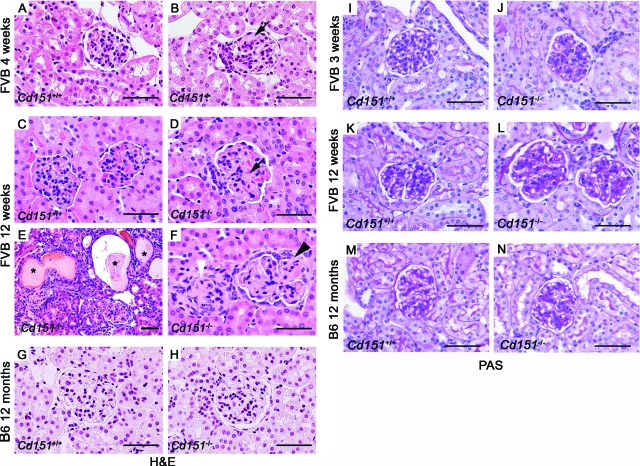
Figure 2.
Histological Analysis of Cd151−/− Kidneys. Cd151+/+ and Cd151−/− Kidneys from both FVB (A–F; I–L) and B6 strains (G, H, M, N) were analyzed by H&E (A–H) and PAS staining (I–N). At 4 weeks of age, FVB Cd151-null mice presented with mild glomerular damage as seen by H&E staining (black arrow head in B) compared to wild-type (A). At this age, the glomerular damage consisted exclusively of GBM thickening as seen on the PAS stains (I, J). By 12 week of age, all FVB Cd151-null mice showed significantly increased kidney damage (D–F) compared to wild-type control (C), ranging from moderate glomerular damage (D, black arrow) to more severe glomerular damage associated with tubular lesions (E). The GBM of Cd151−/− mice at this age was extensively thickened (L). In addition, a few 12-week-old FVB Cd151-null mice presented with massive glomerulosclerosis and collapse of the glomerular tuft (E, F, white arrowhead) associated with massive tubular dilations and protein casts (E*). Some sclerotic glomeruli showed partial (F, black arrowhead) or complete (F, white arrowhead) adhesion to the Bowman’s capsule. The 12 month-old B6 Cd151-null kidneys appeared totally normal (H, N) as compared with wild-type controls (G, M). Scale bars = 50 μm.
We had previously reported that Cd151−/− mice on the B6 background were grossly normal.19 Nevertheless, given the strikingly different phenotype for the same deletion in the FVB strain, we wondered whether the B6 Cd151-null mice might develop a milder disease with a later onset. However the kidneys of B6 Cd151−/− mice up to 12 months of age were histologically normal as compared with age-matched controls (Figure 2, G, H, M, N) and mice of this age did not show any sign of proteinuria (Figure 1).
Expression Pattern of CD151 in the Mouse Kidney
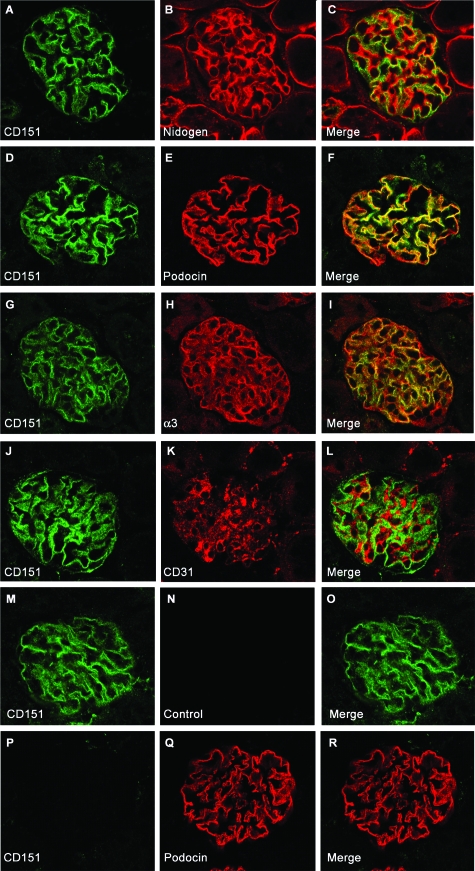
A rabbit antibody generated against mouse CD151 showed that Cd151 is mainly expressed by the glomeruli in mouse kidney, in contrast to what has been shown before in human kidney.11 The specificity of our antibody was shown by the absence of staining in Cd151-null kidneys (Figure 3, P–R). To better define the subcellular localization of CD151 we performed double immunofluorescence labeling and confocal analysis on wild-type mouse kidneys with anti-CD151 antibodies and antibodies directed against nidogen (Figure 3A–C), α3-integrin (Figure 3G–I), podocin (Figure 3, D–F), and CD31 (Figure 3, J–L). The anti-nidogen antibody gives a strong labeling of the GBM and the dual staining showed tight association of the green and the red staining without superposition, indicating that CD151 is localized adjacent to the GBM, at the basal side of podocytes (Figure 3, A–C). The anti-α3-integrin antibody stains the laminin receptor α3β1 integrin in the glomerulus, which is exclusively expressed at the basal side of podocytes at the sites of anchorage to the GBM. Dual labeling showed overlapping of the α3-integrin and CD151 staining (Figure 3, G–I), suggesting that CD151 probably associates with the α3β1 integrin heterodimer in the podocytes, similarly to other cell types in the body. Interestingly, CD151 co-labeling with the slit diaphragm-associated protein podocin showed substantial overlapping (Figure 3, D–F). This could be due to the close proximity of integrin adhesion complexes and slit diaphragms in the podocyte foot processes, but could also reflect some partial association of CD151 with the slit diaphragm complex. Finally, because CD151 has previously been shown to be expressed in endothelial cells, we performed some co-staining experiments with an antibody against the endothelial cell marker CD31. These experiments did not show any co-localization with CD151 (Figure 3, J–L), in accordance with the nidogen co-staining that showed CD151 to be localized only on the external side of the GBM. CD151 is therefore not expressed by the glomerular fenestrated endothelium. In conclusion, in the mouse kidney, CD151 is expressed mainly in the glomerulus, localizes exclusively in podocytes, along the GBM in association with the integrin adhesion complexes.
Figure 3.
Localization of CD151 in the Mouse Kidney. Dual immunofluorescence labeling and confocal analysis using CD151 antibody (green) and antibodies (red) directed against nidogen (A–C), podocin (D–F), α3 integrin (G–I), and CD31 (J–L). The CD151 antibody labeled mainly glomeruli in the kidney (A–O). The specificity of the CD151 antibody was shown by absence of CD151 labeling on Cd151−/− section (P–R). The green CD151 labeling follows the GBM (red, C) and colocalizes substantially with podocin (F) and α3 integrin (I) at the base of podocytes. CD151 does not colocalize with CD31 in the glomerular endothelium (L). M–O: For labeling where two rabbit antibodies were used, an appropriate control (where the second primary antibody was omitted) was included in the experiments (M–O). The absence of labeling by the second secondary antibody (red) in this control proves sufficient blocking of the first primary (CD151) and secondary antibody (green). Pictures were taken using a confocal microscope. Original magnification ×400.
Early Ultrastructural Changes in the Glomeruli of Cd151-Null Kidneys
To further elucidate the sequence of events occurring in the diseased kidneys, and therefore better understand CD151’s function in the filter, we performed ultrastructural analysis of kidneys in young Cd151-null animals (5 days and 3 weeks of age), when the mice are proteinuric, but do not yet present with glomerulosclerosis. By looking at the early stages of the disease, we wished to specify which defect (podocyte or GBM) comes first and to determine whether the podocyte foot processes and slit diaphragms develop normally in the Cd151−/− mice.
GBM maturation, which involves fusion between the endothelial- and podocyte-secreted basement membranes, is not yet complete at birth, and in the 5-day-old wild-type FVB mice, there was still evidence of a few double layered GBM segments (Figure 4A, box). The GBM was strikingly different in Cd151-null mice: many GBM segments looked highly disorganized in the 5-day-old mice (Figure 4, B and C*) and more GBM areas were affected at 3 weeks (Figure 4, F–H*). The very irregular GBM thickening and splitting seen in Cd151-null mice was reminiscent of what can be seen in Alport syndrome in mice or humans. Surprisingly, podocyte foot processes were mostly preserved (Figure 4C, D, and H), even along the hugely thickened GBM segments where they could be irregular in shape (Figure 4B, C, F, and G). Effacement of foot processes was observed only focally (Figure 4, B and G). Between adjacent foot processes, the slit diaphragms looked ultrastructurally normal (Figure 4C, D, and H, arrows) suggesting that there was no developmental defect in foot process and slit diaphragm formation. To estimate the extent of the ultrastructural lesions over the time course of the disease, we performed a semiquantitative analysis on the TEM pictures (Figure 4, I and J). At 5 days of age, when proteinuria was already present, the GBM thickening and splitting affected most of the GBM, whereas the overall number of foot processes per micrometer of GBM was not significantly affected (Figure 4J), confirming that proteinuria precedes effacement of foot processes in FVB Cd151-null mice. At 3 weeks of age, there was a slight but significant effacement of foot processes as shown by the significant decrease in number of foot processes per micrometer of GBM (Figure 4J).
Figure 4.
TEM study of FVB Cd151−/− Kidneys. Five-day-old (A–D) and 3-week-old (E–H) FVB kidneys were analyzed by TEM. The GBM is abnormally and irregularly thickened in the 5-day- and 3-week-old FVB Cd151-null animals (*in B, C, F, and G). Normal differentiation and architecture of the glomerular filtration barrier in FVB wild-type animals is shown at 5 days (A) and 3 weeks (E) of age. GBM maturation is not yet complete in the 5-day-old wild-type FVB mice (box in A, white arrowhead). The striking feature in Cd151-null kidneys is the massive thickening and splitting of the GBM observed as early as 5 days in some segments of glomeruli (B, C*). The GBM disorganization becomes more important and involves more capillary loops with progression of the disease as shown here at 3 weeks of age (F, G*). Foot processes and slit diaphragms seem to develop normally as observed in the 5-day-old (C, D, arrows) and 3-week-old (H, arrow) Cd151−/− animals (C, D arrows). The foot processes were effaced in a few areas (arrowhead in B and G) but they were surprisingly well maintained in other areas, given the extent of GBM damage (C, D, and H). A semiquantitative analysis of the extent of GBM splitting (I) and effacement of foot processes (J) over time was performed. The results are expressed as percentage of split GBM and number of podocyte foot processes (FP) per micrometer of GBM, respectively. The representative results from two mice in each group at 5 days and 3 weeks are shown. Scale bars = 2 μm (A, B, E, F); 500 nm C, D, G, H.
In conclusion, the podocyte cytoskeleton and slit diaphragm did not seem to be significantly affected by the absence of CD151. Seven-month-old adult B6 Cd151+/+ and Cd151−/− kidneys (n = 2 in each group, data not shown) were also examined and did not show any ultrastuctural defect. To address the question of whether young B6 mutant animals might present with a mild defect in GBM ultrastructure that might later be repaired, TEM was also performed on 5-day-old B6 Cd151+/+ and Cd151−/− kidneys and did not show any obvious sign of GBM alterations (n = 2 in each group, data not shown).
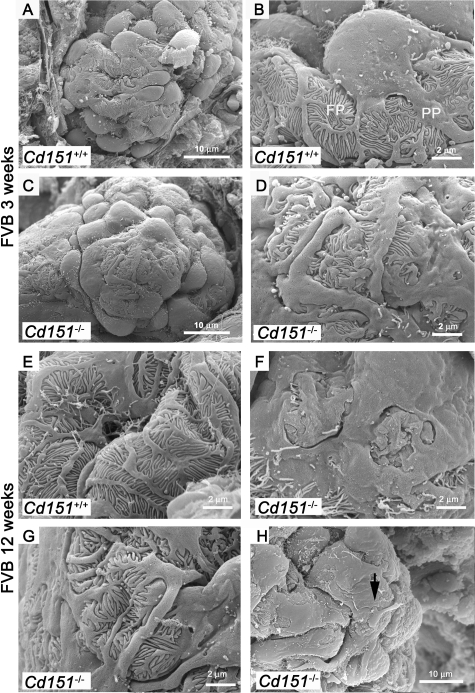
As a complement to the TEM in the ultrastructural study of the FVB diseased glomeruli and to better assess the podocyte changes, we performed SEM. The SEM experiments revealed that the podocytes were damaged in Cd151-null mice, from the age of 3 weeks (Figure 5). In fact, all of the glomeruli observed by SEM showed some extent of podocyte damage. In wild-type animals, the outline of the cell bodies was well defined and smooth and the foot processes tightly and regularly interdigitated (Figure 5A, B, and E). In the Cd151-null mice, most podocytes looked abnormal: the cell bodies were flattened and the processes were disorganized but still present at 3 weeks (Figure 5C and D), in accordance with the TEM data. The podocyte alterations progressed with age and some glomeruli at 12 weeks of age showed complete disappearance of foot processes by SEM (Figure 5F). Interestingly, in one 12-week old glomerulus, we noticed a damaged podocyte sending a large process attaching indiscriminately onto the cell body of its neighbor, suggesting podocyte detachment (Figure 5H, arrow). Overall, podocytes appeared more damaged in Cd151-null mice by SEM analysis than what was seen by TEM.
Figure 5.
SEM Analysis of Podocyte Structure in FVB Cd151-Null Mice. Three-and 12-week old FVB Cd151+/+ and Cd151−/− kidneys were analyzed by SEM to determine the extent of glomerular and more specifically podocyte damage. In wild-type animals (A, B, and E) the podocyte structure is well defined with the cell body dividing into primary processes (PP) that divide into foot processes (FP). At 3 weeks of age, the podocytes look abnormal in Cd151−/− kidneys (C, D) compared to wild-type controls (A, B). Indeed, the podocyte cell bodies look flattened at 3 weeks of age (C). Primary processes and foot processes are still present in most areas as seen by TEM but appear disorganized (C, D, and G). Podocyte alterations progress with age and foot process effacement has become more pronounced by 12 weeks (F). However, even at 12 weeks of age, there are still a number of places where disorganized foot processes are still visible (G). Also note the unhealthy podocyte sending a process onto the cell body of its neighbor (arrow in H). The representative results from 3 mice in each group at 3 weeks of age and two mice in each group at 12 weeks of age are shown. Three to 6 glomeruli were analyzed for each mouse. Original magnification: ×6000 (A, C); ×20,000 (B, D–G); ×12,000 (H).
Progressive Podocyte Loss in Cd151-Null Kidneys
To determine whether podocytes eventually become detached from the GBM, as suggested by the electron microscopy study, we evaluated the number of podocytes per glomerulus by immunofluorescence, using the transcription factor WT1 as a podocyte marker. The podocyte number was equal after birth in Cd151-null kidneys as compared with wild-type, but showed a progressive decrease in the Cd151-null kidneys of the FVB background from 3 to 12 weeks of age (Figure 6A). Podocyte loss was seen as early as 3 weeks after birth when the glomeruli presented only with GBM thickening but did not present with sclerotic areas. In accordance with this result, WT1 Western blot revealed the presence of podocytes in the urine of 3 week-old and 12 week-old FVB Cd151-null mice (Figure 6C). As expected, no podocyte loss was observed in the 12-week-old B6 Cd151-null mice, as compared with age matched wild-type controls.
Figure 6.
Progressive Podocyte Loss in FVB Cd151−/− Kidneys. A: Podocyte number per glomerulus was estimated by counting nuclei positive for both WT1 and DAPI stainings. Results were normalized to the average podocyte number in the wild-type control for each age. At 5 days of age podocyte number in Cd151−/− kidneys was equal to control, showing that the podocytes differentiate normally. At 3 weeks and 12 weeks of age FVB Cd151-deficient mice present with a significant decrease in podocyte number per glomerulus (22.2% and 28.4%, respectively), compared to wild-type controls (P < 0.001). In B6 Cd151-null animals there is no loss in podocyte number compared to wild-type controls. B: Representative pictures of the WT1 immunolabeling used to assess the number of podocytes per glomerulus in both wild-type (wt) and Cd151 knock-out (ko) kidney sections. C: Representative Western blot of urine from Cd151+/+ and Cd151−/− mice probed with WT1 antibody (top panel), showing presence of podocytes in urine of some Cd151−/− mice at 3 weeks and 12 weeks of age (black arrow). Ponceau staining of the same membrane shows the extent of proteinuria for each mouse (bottom panel).
Defect in GBM Assembly and Maturation in Cd151−/− Mice
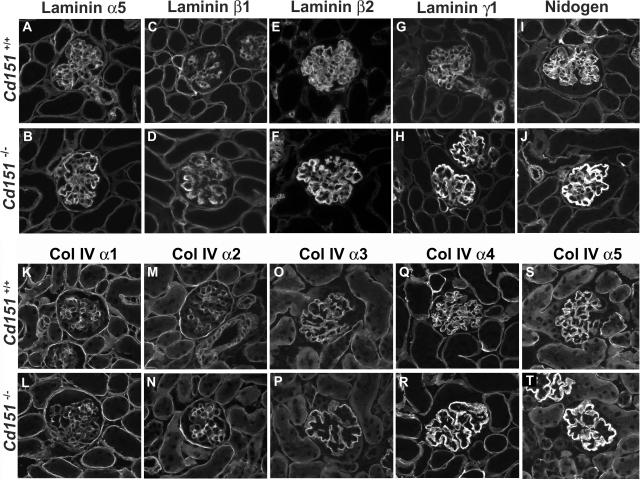
GBM maturation normally occurs within the first 2 weeks after birth, and involves a switch in the laminin and collagen IV networks: the laminin 511 (α5β1γ1) and type IV collagen α1α1α2 networks are replaced by laminin 521 (α5β2γ1) and collagen α3α4α5 during glomerular maturation. Because the morphological and TEM study showed thickening and splitting of the GBM, we investigated further this GBM defect and whether it could reflect alterations in GBM assembly and maturation. To evaluate expression of GBM components, we performed immunofluorescence labeling with antibodies toward laminin chains, nidogen, and collagen IV chains in 3-week-old kidneys (Figure 7); this is an age at which GBM maturation is normally complete. In wild-type kidneys, the GBM laminin network was composed of the α5, β2, and γ1 laminin chains (ie, laminin 521) (Figure 7 A, E, G) while the β1 chain of laminin had switched off in the GBM and was restricted to the mesangial matrix (Figure 7C). In the Cd151 knock-out kidneys, however, the laminin β1 was clearly expressed in the GBM (Figure 7D), together with the α5, β2, and γ1 laminin. All four chains of laminin also showed increased intensity of staining specifically in the GBM and a thicker pattern of expression, suggesting that they were components of the split and thickened GBM. Interestingly, the immunolabeling of nidogen/entactin (Figure 7, I–J), a GBM molecule involved in bridging the laminin and collagen IV networks, was also significantly increased. In both wild-type and Cd151−/− 3-week-old kidneys, the switch from the α1α1α2 collagen network to the α3α4α5 network had normally occurred (Figure 7, K–T). However there was a strong increase in expression of the α4 and α5 chains of collagen type IV in the thickened GBM of Cd151−/− kidneys, whereas the α3 chain did not seem particularly affected. It is also important to note that there was no increase in mesangial labeling of the collagen and laminin chains, in accordance with the histology, showing no mesangiosclerosis at this age.
Figure 7.
Expression of Glomerular Basement Membrane Components in Cd151-Null Kidneys. Immunofluorescence analysis of GBM laminin chains (A–H), nidogen (I, J), and collagen type IV α1–5 chains (K–T) in 3-week old Cd151+/+ and Cd151−/− kidneys. In wild-type kidneys at this age, the laminin network is composed of the α5, β2, and γ1 laminin chains (ie, laminin 521) (A, E, G) while the β1 chain of laminin has switched off in the GBM and is restricted to the mesangial matrix (C). In the Cd151 knock-out kidneys however there is persistence of the laminin β1 chain in the GBM (D) together with expression of α5, β2, and γ1 laminin chains. All four chains of laminin also show increased expression specifically in the GBM, as does nidogen/entactin (I–J). In both 3-week-old wild-type and Cd151−/− kidneys, the switch from the α1α1α2 collagen network to the α3α4α5 network has normally occurred (K–T). However there is a strong increase in expression of the α4 and α5 chains of collagen type IV in the thickened GBM of Cd151−/− kidneys (R, T). The representative results from two mice in each group are shown. Original magnification: ×400.
Discussion
In summary, in the present study we confirmed that CD151 plays a major role in the maintenance and function of the kidney filter and most importantly, we showed that CD151 appears to be directly involved in GBM assembly and maturation. In addition, we showed that the glomerular disease developed by Cd151-null mice is totally dependent on the genetic background, as only FVB Cd151-null mice develop a severe glomerular disease whereas B6 are healthy. The kidney phenotype developed by FVB Cd151-null mice was expected, as CD151 mutations have been found in patients with kidney failure,8 and Cd151-deficient mice (FVBx129) generated by Sachs and colleagues developed a similar disease.7 However, a detailed phenotypical analysis of the disease in young FVB Cd151-null animals was required to point out the initial event occurring in the Cd151-null kidneys. Indeed, this study allowed us to provide new insights into CD151’s function as we identified the thickening and splitting of the GBM as the early event associated with proteinuria in Cd151−/− kidneys, leading ultimately to podocyte loss and glomerulosclerosis. In addition, double immunofluorescence labeling allowed us to show that CD151 is expressed mainly in the glomerulus in mouse kidneys and localizes to the base of podocytes, along the GBM. Our results in the mouse kidney are different from what has been shown before for CD151 localization in the human kidney.11 A study by Sterk et al showed expression of CD151 in the tubules in addition to the glomeruli whereas our antibody labeled only the podocyte. This difference in labeling with different CD151 antibodies could be due to a slight difference of expression pattern between human and mouse CD151, or could reflect a limitation in the sensitivity of our antibody as CD151 might be expressed to a lesser extent in tubules.
The detailed phenotypic analysis allowed us to better understand the mechanism of the disease and therefore the function of CD151 in the kidney filter. FVB Cd151-deficient mice develop proteinuria shortly after birth, at a time when their kidneys look normal by light microscopy. At this young age however, they have already developed focal but severe thickening and splitting of the GBM, whereas podocyte foot processes are mostly conserved, as seen by electron microscopy in the 5-day-old mice. The GBM changes strongly resemble those observed in Alport syndrome in humans and mice. Alport syndrome is a nephropathy characterized by irregular thickening and splitting of the GBM and is due to mutations in the genes encoding either of α3, α4, and α5 type IV collagen chains, which are major components of the mature GBM. Given the similarities between Alport syndrome and the nephropathy caused by CD151 deletion, it would be interesting to screen Alport-like patients, who are not linked to any of the known ColIV loci, for mutations in CD151.
As the disease progresses with age, ultrastructural GBM lesions get worse and extend. Loss of podocytes is apparent at 3 weeks of age and glomerulosclerosis follows, becoming well visible by 12 weeks of age. Therefore, in FVB Cd151-null kidneys, the first defect observed shortly after birth in albuminuric mice, is the massive disorganization of the GBM, whereas podocyte foot processes and slit diaphragms seem quite well maintained. There are two hypotheses concerning the mechanism by which the GBM thickening might occur: first, some breakdown or loosening in the podocyte anchorage to the GBM may occur at birth due to the sudden increase in the mechanical forces inflicted on the podocytes during the establishment of normal kidney function. This could be the signal for the podocyte to synthesize excessive amounts of GBM, in response to the loosening of the adhesions. The second and most likely hypothesis is that CD151 could be involved in the regulation of GBM production, maturation and assembly in collaboration with integrins. Indeed, the early GBM disorganization observed in the Cd151-deficient mice suggests that CD151 is directly implicated in the assembly and/or maintenance of the basement membrane integrity, rather than just playing a role in strengthening the α3β1-mediated podocyte adhesion to the GBM. At birth the glomeruli have to cope with increased blood pressure and filtration rate, although GBM maturation is not yet completed. It is therefore tempting to speculate that a defect in GBM assembly on top of this mechanical stress inflicted to the glomerular filtration barrier might cause a breakdown of the GBM as observed in Cd151−/− mice, leading to proteinuria. In addition, immunolabeling experiments revealed altered expression of GBM components: laminin chains, nidogen, and α4 and α5 chains of type IV collagen were over-expressed in the diseased thickened GBM. Moreover, co-expression of laminin β1 and β2 chains in the GBM of Cd151−/− kidneys clearly suggests a defect in GBM maturation. A recently published study also reported persistence of the laminin β1 chain in the GBM of integrin linked kinase and β1 integrin mutant animals,31 as observed in FVB Cd151−/− mice. Altogether these data suggest that CD151 might act with α3β1 integrin and integrin linked kinase downstream pathway to regulate GBM assembly and maturation. We propose that the defective maturation and assembly of the laminin network could be the initial event causing the GBM alterations. Indeed, it has been shown before that effective formation of basement membranes is dependent on proper assembly of the laminin network.32
Interestingly, α3β1 integrin is also known to be responsible for the structural organization of the GBM and the skin basement membrane, based on the phenotype of α3-integrin knock-out mice and the study of β1 integrin mutant mice mentioned above.6,24,33 Therefore, because CD151 has been shown to be implicated in integrin trafficking34 and is well known to modulate integrin function, one could argue that the phenotype observed in Cd151-null mice is due to a defect in integrin targeting. It is important to note however that immunolabeling experiments showed that expression and localization of α3-integrin in Cd151-null kidneys was not altered at early stages (data not shown), indicating that CD151 has a specific function in GBM integrity beyond its role in integrin trafficking. In contrast, the glomerular disease developed by mice deficient for integrin linked kinase is associated with mislocalization of α3 integrin and therefore altered integrin-mediated matrix assembly.35 Whereas segmental flattening of podocyte foot processes was observed where the GBM was severely affected in Cd151-null mice, overall the foot processes’ architecture did not seem much affected, suggesting that the podocyte changes occur consequently to the ultrastructural GBM changes. Therefore, CD151 seems to be specifically involved in the establishment and/or maintenance of the GBM structure rather than the integrity of the slit diaphragm, whereas integrin linked kinase together with α3β1 integrin have been shown to be more widely implicated in GBM-cytoskeleton-slit diaphragm maintenance and cross talk.
Another important finding of our study is the fact that Cd151 knock-out mice develop a severe kidney disease on FVB background but no disease at all on B6 background. This strongly suggests the presence of modifier genes influencing the onset of the disease in FVB versus B6 mice. Genetic modifiers are known to be involved in the progression of numerous diseases and are well documented in mice, as knock-out phenotypes are often more severe on one given background versus another. In accordance with this finding, B6 mice appear to be relatively resistant to proteinuria, in comparison to other mouse strains such as 129/Sv. For example, B6 mice have been shown to be less susceptible than 129/Sv mice to proteinuria induced by protein overload or deoxycortisone acetate.36,37 The B6 strain has also shown an increased resistance to intrinsic GBM damage due to collagen IV alterations in Alport mice, as the rate of progression to end stage renal disease of Col4a3−/− mice is significantly slower on a B6 than on a 129/Sv background.38 Surprisingly, mice deficient for the slit-diaphragm-associated protein podocin developed a more severe glomerular disease on B6 than 129/Sv background.39 This suggests that the genes modifying the progression of glomerular disease associated with podocyte slit diaphragm defects are different from those implicated in the onset of proteinuria due to GBM alterations. Because the effect of the genetic modifiers in Cd151-null mice results in presence or complete absence of disease, these mice represent a powerful and unique tool to identify these genes modulating the onset of glomerular diseases consequent to alterations in the podocyte-GBM contacts. As a first step toward the mapping of these genes, we performed an outcross to generate Cd151−/− mice on the F1 hybrid (B6xFVB) background. These mice have now been monitored for 6 months and do not show any sign of proteinuria (data not shown) suggesting that the alleles involved in the resistance to glomerular disease in B6 mice are dominant. A backcross of this F1 hybrid generation to parental FVB Cd151+/− mice will allow us to perform linkage analysis and identify the loci segregating with the glomerular disease in the FVB strain, but this is beyond the scope of the present study. Mapping and identifying these genetic modifiers in mice and their homologous genes in humans will help to better understand the pathogenesis of glomerular diseases and disease variations from patient to patient, and ultimately will help to improve disease classification and design new treatments.
Acknowledgments
We thank Corinne Antignac, Jeffrey Miner, Takako Sasaki, Yoshikazu Sado, and Fiona Watt for their gifts of antibodies. We are very grateful to David Phelan for excellent assistance with the electron microscopes. We also thank Amanda Harman and Géraldine Mollet for excellent advice with preparation of the TEM samples; and Anne Prins and Cathy Gillepsie for assistance with preparation of the histology and TEM samples, respectively.
Footnotes
Address reprint requests to: Dr. Séverine Roselli, School of Biomedical Sciences, University of Newcastle, Callaghan, NSW 2308, Australia. E-mail: severine.roselli@newcastle.edu.au.
Supported by grant 351140 to L.K.A. from the National Health & Medical Research Council (NHMRC) of Australia. S.R. is a fellow of the Cancer Institute of New South Wales, Australia.
L.K.A. is NHMRC Principal Research Fellow and Gladys Brawn Professional Fellow at the University of Newcastle. We thank Hunter Medical Research Institute for infrastructure support.
References
- Johnstone DB, Holzman LB. Clinical impact of research on the podocyte slit diaphragm. Nat Clin Pract Nephrol. 2006;2:271–282. doi: 10.1038/ncpneph0180. [DOI] [PubMed] [Google Scholar]
- Hudson BG, Reeders ST, Tryggvason K. Type IV collagen: structure, gene organization, and role in human diseases. Molecular basis of Goodpasture and Alport syndromes and diffuse leiomyomatosis. J Biol Chem. 1993;268:26033–26036. [PubMed] [Google Scholar]
- Antignac C. Molecular genetics of basement membranes: the paradigm of Alport syndrome. Kidney Int Suppl. 1995;49:S29–S33. [PubMed] [Google Scholar]
- Zenker M, Aigner T, Wendler O, Tralau T, Muntefering H, Fenski R, Pitz S, Schumacher V, Royer-Pokora B, Wuhl E, Cochat P, Bouvier R, Kraus C, Mark K, Madlon H, Dotsch J, Rascher W, Maruniak-Chudek I, Lennert T, Neumann LM, Reis A. Human laminin beta2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum Mol Genet. 2004;13:2625–2632. doi: 10.1093/hmg/ddh284. [DOI] [PubMed] [Google Scholar]
- Jarad G, Cunningham J, Shaw AS, Miner JH. Proteinuria precedes podocyte abnormalities inLamb2−/ − mice, implicating the glomerular basement membrane as an albumin barrier. J Clin Invest. 2006;116:2272–2279. doi: 10.1172/JCI28414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, Jaenisch R. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development. 1996;122:3537–3547. doi: 10.1242/dev.122.11.3537. [DOI] [PubMed] [Google Scholar]
- Sachs N, Kreft M, van den Bergh Weerman MA, Beynon AJ, Peters TA, Weening JJ, Sonnenberg A. Kidney failure in mice lacking the tetraspanin CD151. J Cell Biol. 2006;175:33–39. doi: 10.1083/jcb.200603073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamatic Crew V, Burton N, Kagan A, Green CA, Levene C, Flinter F, Brady RL, Daniels G, Anstee DJ. CD151, the first member of the tetraspanin (TM4) superfamily detected on erythrocytes, is essential for the correct assembly of human basement membranes in kidney and skin. Blood. 2004;104:2217–2223. doi: 10.1182/blood-2004-04-1512. [DOI] [PubMed] [Google Scholar]
- Sincock PM, Mayrhofer G, Ashman LK. Localization of the transmembrane 4 superfamily (TM4SF) member PETA-3 (CD151) in normal human tissues: comparison with CD9. CD63, and alpha5beta1 integrin. J Histochem Cytochem. 1997;45:515–525. doi: 10.1177/002215549704500404. [DOI] [PubMed] [Google Scholar]
- Sincock PM, Fitter S, Parton RG, Berndt MC, Gamble JR, Ashman LK. PETA-3/CD151, a member of the transmembrane 4 superfamily, is localised to the plasma membrane and endocytic system of endothelial cells, associates with multiple integrins and modulates cell function. J Cell Sci. 1999;112 (Pt 6):833–844. doi: 10.1242/jcs.112.6.833. [DOI] [PubMed] [Google Scholar]
- Sterk LM, Geuijen CA, van den Berg JG, Claessen N, Weening JJ, Sonnenberg A. Association of the tetraspanin CD151 with the laminin-binding integrins alpha3beta1, alpha6beta1, alpha6beta4, and alpha7beta1 in cells in culture and in vivo. J Cell Sci. 2002;115:1161–1173. doi: 10.1242/jcs.115.6.1161. [DOI] [PubMed] [Google Scholar]
- Yauch RL, Berditchevski F, Harler MB, Reichner J, Hemler ME. Highly stoichiometric, stable, and specific association of integrin alpha3beta1 with CD151 provides a major link to phosphatidylinositol 4-kinase, and may regulate cell migration. Mol Biol Cell. 1998;9:2751–2765. doi: 10.1091/mbc.9.10.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serru V, Le Naour F, Billard M, Azorsa DO, Lanza F, Boucheix C, Rubinstein E. Selective tetraspan-integrin complexes (CD81/alpha4beta1, CD151/alpha3beta1, CD151/alpha6beta1) under conditions disrupting tetraspan interactions. Biochem J. 1999;340 (Pt 1):103–111. [PMC free article] [PubMed] [Google Scholar]
- Yauch RL, Hemler ME. Specific interactions among transmembrane 4 superfamily (TM4SF) proteins and phosphoinositide 4-kinase. Biochem J. 2000;351 Pt 3:629–637. [PMC free article] [PubMed] [Google Scholar]
- Berditchevski F, Zutter MM, Hemler ME. Characterization of novel complexes on the cell surface between integrins and proteins with 4 transmembrane domains (TM4 proteins). Mol Biol Cell. 1996;7:193–207. doi: 10.1091/mbc.7.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berditchevski F, Odintsova E. Characterization of integrin-tetraspanin adhesion complexes: role of tetraspanins in integrin signaling. J Cell Biol. 1999;146:477–492. doi: 10.1083/jcb.146.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammerding J, Kazarov AR, Huang H, Lee RT, Hemler ME. Tetraspanin CD151 regulates alpha6beta1 integrin adhesion strengthening. Proc Natl Acad Sci USA. 2003;100:7616–7621. doi: 10.1073/pnas.1337546100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiuchi R, Sanzen N, Nada S, Sumida Y, Wada Y, Okada M, Takagi J, Hasegawa H, Sekiguchi K. Potentiation of the ligand-binding activity of integrin alpha3beta1 via association with tetraspanin CD151. Proc Natl Acad Sci USA. 2005;102:1939–1944. doi: 10.1073/pnas.0409493102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MD, Geary SM, Fitter S, Moseley GW, Lau LM, Sheng KC, Apostolopoulos V, Stanley EG, Jackson DE, Ashman LK. Characterization of mice lacking the tetraspanin superfamily member CD151. Mol Cell Biol. 2004;24:5978–5988. doi: 10.1128/MCB.24.13.5978-5988.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau LM, Wee JL, Wright MD, Moseley GW, Hogarth PM, Ashman LK, Jackson DE. The tetraspanin superfamily member CD151 regulates outside-in integrin alphaIIbbeta3 signaling and platelet function. Blood. 2004;104:2368–2375. doi: 10.1182/blood-2003-12-4430. [DOI] [PubMed] [Google Scholar]
- Cowin AJ, Adams D, Geary SM, Wright MD, Jones JC, Ashman LK. Wound healing is defective in mice lacking tetraspanin CD151. J Invest Dermatol. 2006;126:680–689. doi: 10.1038/sj.jid.5700142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berditchevski F, Odintsova E, Sawada S, Gilbert E. Expression of the palmitoylation-deficient CD151 weakens the association of alpha 3 beta 1 integrin with the tetraspanin-enriched microdomains and affects integrin-dependent signaling. J Biol Chem. 2002;277:36991–37000. doi: 10.1074/jbc.M205265200. [DOI] [PubMed] [Google Scholar]
- Roselli S, Gribouval O, Boute N, Sich M, Benessy F, Attie T, Gubler MC, Antignac C. Podocin localizes in the kidney to the slit diaphragm area. Am J Pathol. 2002;160:131–139. doi: 10.1016/S0002-9440(10)64357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH, Patton BL, Lentz SI, Gilbert DJ, Snider WD, Jenkins NA, Copeland NG, Sanes JR. The laminin alpha chains: expression, developmental transitions, and chromosomal locations of alpha1–5, identification of heterotrimeric laminins 8–11, and cloning of a novel alpha3 isoform. J Cell Biol. 1997;137:685–701. doi: 10.1083/jcb.137.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Mann K, Miner JH, Miosge N, Timpl R. Domain IV of mouse laminin beta1 and beta2 chains. Eur J Biochem. 2002;269:431–442. doi: 10.1046/j.0014-2956.2001.02663.x. [DOI] [PubMed] [Google Scholar]
- Sado Y, Kagawa M, Kishiro Y, Sugihara K, Naito I, Seyer JM, Sugimoto M, Oohashi T, Ninomiya Y. Establishment by the rat lymph node method of epitope-defined monoclonal antibodies recognizing the six different alpha chains of human type IV collagen. Histochem Cell Biol. 1995;104:267–275. doi: 10.1007/BF01464322. [DOI] [PubMed] [Google Scholar]
- Kagawa M, Kishiro Y, Naito I, Nemoto T, Nakanishi H, Ninomiya Y, Sado Y. Epitope-defined monoclonal antibodies against type-IV collagen for diagnosis of Alport’s syndrome. Nephrol Dial Transplant. 1997;12:1238–1241. doi: 10.1093/ndt/12.6.1238. [DOI] [PubMed] [Google Scholar]
- Heidet L, Borza DB, Jouin M, Sich M, Mattei MG, Sado Y, Hudson BG, Hastie N, Antignac C, Gubler MC. A human-mouse chimera of the alpha3alpha4alpha5(IV) collagen protomer rescues the renal phenotype in Col4a3−/− Alport mice. Am J Pathol. 2003;163:1633–1644. doi: 10.1016/s0002-9440(10)63520-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis Carl SA, Gillete-Ferguson I, Ferguson DG. An indirect immunofluorescence procedure for staining the same cryosection with two mouse monoclonal primary antibodies. J Histochem Cytochem. 1993;41:1273–1278. doi: 10.1177/41.8.7687266. [DOI] [PubMed] [Google Scholar]
- Macconi D, Bonomelli M, Benigni A, Plati T, Sangalli F, Longaretti L, Conti S, Kawachi H, Hill P, Remuzzi G, Remuzzi A. Pathophysiologic implications of reduced podocyte number in a rat model of progressive glomerular injury. Am J Pathol. 2006;168:42–54. doi: 10.2353/ajpath.2006.050398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanasaki K, Kanda Y, Palmsten K, Tanjore H, Lee SB, Lebleu VS, Gattone VH, Jr, Kalluri R. Integrin beta1-mediated matrix assembly and signaling are critical for the normal development and function of the kidney glomerulus. Dev Biol. 2008;313:584–593. doi: 10.1016/j.ydbio.2007.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Harrison D, Carbonetto S, Fassler R, Smyth N, Edgar D, Yurchenco PD. Matrix assembly, regulation, and survival functions of laminin and its receptors in embryonic stem cell differentiation. J Cell Biol. 2002;157:1279–1290. doi: 10.1083/jcb.200203073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPersio CM, Hodivala-Dilke KM, Jaenisch R, Kreidberg JA, Hynes RO. alpha3beta1 integrin is required for normal development of the epidermal basement membrane. J Cell Biol. 1997;137:729–742. doi: 10.1083/jcb.137.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, He B, Liu WM, Zhou D, Cox JV, Zhang XA. Tetraspanin CD151 promotes cell migration by regulating integrin trafficking. J Biol Chem. 2007;282:31631–31642. doi: 10.1074/jbc.M701165200. [DOI] [PubMed] [Google Scholar]
- El-Aouni C, Herbach N, Blattner SM, Henger A, Rastaldi MP, Jarad G, Miner JH, Moeller MJ, St-Arnaud R, Dedhar S, Holzman LB, Wanke R, Kretzler M. Podocyte-specific deletion of integrin-linked kinase results in severe glomerular basement membrane alterations and progressive glomerulosclerosis. J Am Soc Nephrol. 2006;17:1334–1344. doi: 10.1681/ASN.2005090921. [DOI] [PubMed] [Google Scholar]
- Ishola DA, Jr, van der Giezen DM, Hahnel B, Goldschmeding R, Kriz W, Koomans HA, Joles JA. In mice, proteinuria and renal inflammatory responses to albumin overload are strain-dependent. Nephrol Dial Transplant. 2006;21:591–597. doi: 10.1093/ndt/gfi303. [DOI] [PubMed] [Google Scholar]
- Hartner A, Cordasic N, Klanke B, Veelken R, Hilgers KF. Strain differences in the development of hypertension and glomerular lesions induced by deoxycorticosterone acetate salt in mice. Nephrol Dial Transplant. 2003;18:1999–2004. doi: 10.1093/ndt/gfg299. [DOI] [PubMed] [Google Scholar]
- Andrews KL, Mudd JL, Li C, Miner JH. Quantitative trait loci influence renal disease progression in a mouse model of Alport syndrome. Am J Pathol. 2002;160:721–730. doi: 10.1016/S0002-9440(10)64892-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli S, Heidet L, Sich M, Henger A, Kretzler M, Gubler MC, Antignac C. Early glomerular filtration defect and severe renal disease in podocin-deficient mice. Mol Cell Biol. 2004;24:550–560. doi: 10.1128/MCB.24.2.550-560.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]