Abstract
We investigated the role of heme oxygenase-1 (HO-1), a powerful anti-inflammatory and anti-oxidant enzyme, in modulating cigarette smoke (CS)-induced mucus secretion. In both rats and mice, 5-day CS exposure increased HO-1 expression and activity, mucus secretion, MUCIN 5AC (MUC5AC) gene and protein expression, and local inflammation, along with up-regulation of dual oxidase 1 gene expression and both the activity and phosphorylation of the epidermal growth factor receptor, which is involved in MUC5AC induction. Pharmacological induction of HO-1 prevented these actions and inhibition of HO-1 expression by a specific siRNA potentiated them. In French participants to the European Community Respiratory Health Survey II (n = 210, 30 to 53 years of age, 50% males) exposed to CS, a significant increase in the percentage of participants with chronic sputum was observed in those harboring at least one allele with a long (GT)n in the HO-1 promoter gene (>33 repeats), which is associated with a low level of HO-1 protein expression, compared with those with a short number of (GT)n repeats (21.7% versus 8.6%, P = 0.047). No such results were observed in those who had never smoked (n = 297). We conclude that HO-1 has a significant protective effect against airway mucus hypersecretion in animals and humans exposed to CS.
In the human respiratory tract, the airway epithelium provides a barrier against various environmental insults. The normal airway epithelium is coated with a mucus layer. Mucins (eg, MUC5AC) are major components of mucus,1 which assists in clearing inhaled foreign materials. In contrast with the physiological situation, airway mucus hypersecretion is now recognized as a key pathophysiological feature in different chronic inflammatory pulmonary diseases such as chronic obstructive pulmonary disease (COPD).2 Through airway obstruction and infection, mucus hypersecretion greatly contributes to the morbidity and mortality associated with COPD,3,4,5 a common disease that represents a major public health problem. Indeed, COPD is a global epidemic of major proportions that is predicted to become, by 2030, the fourth most common cause of death and the fifth most frequent cause of chronic disability.6 Cigarette smoking is the main etiological factor of COPD and is closely associated with mucus hypersecretion.7
The epidermal growth factor receptor (EGF-R) cascade plays a central role in mucin production by airway epithelial cells in response to cigarette smoke (CS) and a wide variety of inflammatory or oxidative stimuli.1,8 EGF-R activation involves two different pathways: ligand-dependent and -independent EGF-R tyrosine phosphorylation. Recent studies well characterized the molecular actors involved in these pathways.9,10,11,12,13,14 However, specific treatment for mucus hypersecretion in conditions such as COPD is lacking.
The microsomal enzyme heme oxygenase (HO) catalyzes the oxidation of heme to biliverdin (converted to bilirubin by biliverdin reductase) and carbon monoxide (CO) and is widely distributed in mammalian tissues.15 Two main isoforms, from different genes, have been identified: inducible heme oxygenase-1 (HO-1) (also known as heat shock protein 32), and constitutive HO-2. HO-1 expression is extremely sensitive to various agents that cause oxidative stress15 and exhibits, in turn, powerful anti-oxidant and anti-inflammatory properties. In airways, HO-1 is expressed in epithelium, smooth muscles, and macrophages,16 and is involved in protection against inflammation and oxidative stress in pathological conditions such as hyperoxia, asthma, or emphysema.16 Usually, the protective role of HO-1 is two faced: decreased consequences of aggression with HO-1 induction and potentiation of these phenomena as a result of a decreased HO-1 expression. This last point is particularly interesting from a clinical point of view because of the existence of a HO-1 gene promoter polymorphism leading to low levels of protein expression.17 This polymorphism has been associated with increased susceptibility to diseases such as pulmonary emphysema in smokers.18 In this sense, HO-1 can be seen either as a potential therapeutic target19 and/or a disease susceptibility marker. However, despite the protective properties of HO-1 against airway inflammation and oxidative aggression, whether HO-1 is part of a protective mechanism against mucus hypersecretion and mucin induction on exposure to CS has yet to be examined. Elucidation of such a mechanism would have important pathophysiological and therapeutic consequences.
To investigate the involvement of HO-1 as a protective mechanism against mucus hypersecretion induced by CS, we used a translational experimental approach. First, we examined the effect of HO-1 on airway mucus hypersecretion induced by exposure to CS in vivo in two animal species (rats and mice) and the involved molecular mechanisms. In initial experiments, we examined whether pharmacological induction of HO-1 expression and activity by hemin protected rats against airway mucus hypersecretion induced by CS exposure. We chose rats because the species reproduces many features of human exposure to tobacco smoke.20 Hemin is both an inductor and a substrate of HO-1, thus ensuring its high expression and activity. To complete these experiments, we examined whether down-regulating HO-1 potentiated mucus hypersecretion induced by CS by use of an in vivo siRNA strategy. We performed these experiments in mice,21,22 thus expanding on the data obtained in rats.
Finally, we analyzed the clinical implications of animal data by examining, in a sample of 507 participants from the European Community Respiratory Health Survey,23 whether the aforementioned HO-1 gene promoter polymorphism, leading to low levels of HO-1 protein expression, was associated with increased chronic sputum prevalence, a symptom of respiratory mucus hypersecretion.
Materials and Methods
Animal Studies
Animals
All of the animal experiments were approved by the local institutional animal care and use committee and the experimental protocol was in agreement with the recommendations related to animal studies of the French Law.
Experimental Protocol in Rats
Pathogen-free male Sprague-Dawley rats (150 to 200 g body wt; Janvier, France) were housed in individual cages and given free access to water and food. Two groups of animals were studied: the CS group was exposed to filtered mainstream smoke (2R4F research cigarettes; Tobacco Health Research, Lexington, KY) 10 cigarettes/hour, 2 hours/day (morning and evening) for 5 days by use of a smoking machine (Anitech, Paris, France). Animals were placed in a restraining box and CS was delivered cyclically (1 puff/minute, 15 seconds with the box closed and the remaining 45 seconds with the box open, to mimic as much as possible the behavior of a smoker). Preliminary experiments showed that HbCO was 10 to 12% at the end of the second exposure period (data not shown). The procedure was similar for control animals (group C), except that they were exposed to filtered room air instead of CS.
CS and C groups were randomly divided into three subgroups. One subgroup received 50 mg/kg body weight of hemin, an HO-1 inductor, administered intraperitoneally, on days 1 and 3. A second subgroup received 50 mg/kg body weight of hemin plus 35 mg/kg body weight of tin protoporphyrin IX (SnPP-IX),24 an inhibitor of HO activity, administered intraperitoneally on days 1 and 3 but 6 hours before hemin administration. The third subgroup received SnPP-IX or hemin vehicle (1 mmol/L NaOH in phosphate-buffered saline) at the same time points as subgroup 2. The solutions of SnPP-IX and hemin were protected from light because light can activate protoporphyrin to produce radicals, which are toxic to tissues.25 Each experimental subgroup included 30 to 35 animals.
In another set of experiments, animals received either 50 mg/kg/day of apocynin,26,27 an NADPH oxidase inhibitor, or its vehicle (phosphate-buffered saline) administered intraperitoneally during the 5-day exposure. Each experimental group included 10 to 12 animals. On day 5, the animals were sacrificed just after the last CS exposure. They were anesthetized with sodium pentobarbital (50 mg/kg body weight, delivered intraperitoneally), and a tracheal cannula was inserted via a tracheotomy into the lumen of the trachea. The abdominal cavity was opened immediately, and the animals were exsanguinated via the abdominal aorta. Bronchoalveolar lavage (BAL) was performed, and cells were counted. Samples of BAL supernatant were frozen for cytokine measurement. Samples of lung were removed rapidly for immediate measurement of HO activity. Then, samples of the trachea were removed rapidly and immediately frozen in liquid nitrogen and stored at −80°C until use. In a separate set of animals, the trachea was removed without lavage and divided in two pieces: one immerged in formaldehyde and the second cut into rings and placed in Krebs solution for measuring reactive oxygen species production.
Experimental Protocol in Mice
Pathogen-free male Balb/C mice (20 to 30 g body wt; Janvier) were exposed to CS or to room air (CS and C animals, respectively) for 2.5 days, following the same protocol of exposure as that for rats. We choose this duration of CS exposure because we observed in preliminary experiments that it corresponded to the maximal effect of the HO-1 siRNA in terms of HO-1 protein expression down-regulation.
Both CS and C animals were randomly divided into two groups: the first was injected with siRNA specifically targeted against HO-1 and the second with nonspecific siRNA, both delivered by a high-pressure technique.28 Briefly, an amount of 50 μg of siRNA diluted in 1 ml of ringer lactate was delivered by rapid injection (10 seconds) in the tail vein 4 hours after the first hour of exposure to CS or room air. HO-1-specific siRNA nucleotide sequences were as follows21,22: sense, 5′-AAGCCACACAGCACUAUGUAAdTdT-3′; and antisense, 5′-UUACAUAGUGCUGUGUGGCUUdTdT-3′. Nonspecific siRNA was provided by Qiagen (Courtaboeuf, France): sense, 5′-UUCUCCGAACGUGUCACGUdTdT-3′ and antisense: 5′-ACGUGACACGUUCGGAGAAdTdT-3′. Animals were sacrificed on day 3 after exposure to CS or room air. In a previous study we verified that this protocol of administration of HO-1 si-RNA specifically decreased HO-1 protein expression in different organs (joint, lungs, spleen, and liver) in mice.22 Sacrifice and lung sampling were as described in the experimental protocol for rats. Each experimental group included six to eight animals.
Measurement of HO Activity
HO activity was quantified by measuring bilirubin production by lung microsomes, as described previously.29
Immunohistochemical Evaluation of HO-1 Expression in Tracheal Rings
HO-1 immunohistochemical detection was performed as described previously30 using a polyclonal anti-HO-1 antibody (StressGen Biotechnologies Corp., Victoria, Canada).
Western Blot Assay of HO-1, HO-2, and Phosphorylated and Nonphosphorylated Expression of the of EGF-R
HO-1 and HO-2 protein expressions were detected in trachea homogenates by Western blot analysis, as described previously,31 using the same antibody as for immunohistochemistry (HO-1) and a polyclonal anti-HO-2 antibody (StressGen Biotechnologies Corp.). Phosphorylated (tyrosine 845) and nonphosphorylated expression of EGF-R was detected by Western blot using polyclonal antibodies from Cell Signaling Technology Inc. (Danvers, MA).
Immunoassay of MUC5AC Protein
MUC5AC protein in the BAL fluid of rats was measured with slight modifications of the method described previously32 using a MUC5AC monoclonal antibody (clone 45 M1, 1/500; NeoMarkers, Fremont, CA).The concentrations of mucins in BAL were determined using bovine submaxillary gland mucins (type I; Sigma-Aldrich, St. Louis, MO) as a standard. The results were reported as mg/ml of MUC5AC protein.
Evaluation of mRNA Expression
mRNA expression analysis was performed in trachea homogenates by quantitative real-time reverse transcriptase-polymerase chain reaction (RT-PCR) by use of the PCR ABI 7700 apparatus (Applied Biosystems, Foster City, CA) as described previously.31 Sequences of the primers used are provided in Table 1.
Table 1.
Primer Sequences
| Gene | Primer sequences |
|---|---|
| Rat MUC5AC | |
| Forward | 5′-CAAGAGGAGTGCCTCTGGTC-3′ |
| Reverse | 5′-GCTGCTGTAGTGCATGGAAA-3′ |
| Rat MUC5B | |
| Forward | 5′-CAGGTGTATGGCCCAGAACT-3′ |
| Reverse | 5′-CCAGGAAGTGTGTGTGGATG-3′ |
| Mouse MUC5AC | |
| Forward | 5′-GGACCTGGAAACTATAGCAACATT-3′ |
| Reverse | 5′-GCACACCAGGCCCACACT-3′ |
| Mouse HO-1 | |
| Forward | 5′-CACGCATATACCCGCTACCT-3′ |
| Reverse | 5′-CCAGAGTGTTCATTCGAGCA-3′ |
| Rat DUOX1 | |
| Forward | 5′-CAGGACAAGGAGGAGCTGAC-3′ |
| Reverse | 5′-GCGCTCATTCTAGGTGAAGG-3′ |
| Rat DUOX2 | |
| Forward | 5′-GTGCTCCGTACCATCCAACT-3′ |
| Reverse | 5′-GCTTGGTCACAGCCTTTCTC-3′ |
| Mouse DUOX1 | |
| Forward | 5′-CTTCTTTTGGCTTGCAGGAG-3′ |
| Reverse | 5′-GAGGCACTAAGGAGGCTGAC-3′ |
| Rat TGF-α | |
| Forward | 5′-GCAAGTTCTGCCTGTTCCTC-3′ |
| Reverse | 5′-GCACTGAACCAACCCACTTT-3′ |
| Mouse TGF-α | |
| Forward | 5′-GCCCAGATTCCCACACTCAGTA-3′ |
| Reverse | 5′-TCTGCATGCTCACAGCGAA-3′ |
| Rat β-Actin | |
| Forward | 5′-AGCCATGTACGTAGCCATCC-3′ |
| Reverse | 5′-CTCTCAGCTGTGGTGGTGAA-3′ |
| Mouse β-Actin | |
| Forward | 5′-GTGGGCCGCTCTAGGCACCA-3′ |
| Reverse | 5′-TGGCCTTAGGGTTCAGGGGG-3′ |
Measurement of Reactive Oxygen Species Release by Tracheal Rings
The release of reactive oxygen species by tracheal rings was assessed by luminol-dependent chemiluminescence, as described previously.11 Briefly, tracheal rings were placed in luminometer cuvettes containing 500 μmol/L of luminol in a final volume of 0.5 ml of Krebs solution. Chemiluminescence was measured every 15 seconds for 60 minutes at 37°C. The integrated response was determined with the use of a computer program supplied with the luminometer (AutoLumat LB 953; EG & G Instruments, Evry, France). Data are presented as the area under curve for 60 minutes, normalized to the wet weight of the ring.
Histology and PAS Staining
Trachea in paraffin blocks were sectioned in the coronal plane at 5 μm and affixed to slides. After paraffin removal and rehydration, slides were stained with hematoxylin and eosin for histology or Alcian blue/periodic acid-Schiff for detection of mucin glycoproteins and then were dehydrated in graded ethanol and mounted. In rats, a semiquantitative analysis of staining was performed by counting the number of positive epithelial cells. Results are expressed as the percentage of positive cells over the total number of epithelial cells. At least 10 fields (×20 magnification) were counted in each slide. A similar approach was applied to mice samples, by counting the number of positive epithelial cells in submucosal glands and expressing results as the percentage of positive cells over the total number of epithelial cells in the glands.
Drugs
Hemin chloride and SnPP-IX were purchased from Porphyrin Products (Logan, UK). Sodium thiopental (Nesdonal) was purchased from Specia-Rhone-Poulenc (Romainville, France). Reagents for Western blotting were from Bio-Rad Laboratories (Richmond, CA). All other chemicals were purchased from Sigma-Aldrich (St. Quentin-Fallavier, France).
Human Studies
We analyzed the relation between HO-1 gene promoter polymorphism and chronic sputum prevalence in smokers from a general population sample. Methods are described elsewhere.23 Data were collected in Grenoble, Montpellier, and Paris (France) as part of the multicenter longitudinal European Community Respiratory Health Survey.33 Of the 1650 participants (20 to 44 years of age) examined in 1992, 1066 were followed-up in 2000 (participation rate = 64.4%). We analyzed data for 297 participants who never smoked (never-smokers) and 210 smokers examined in 2000 for whom complete data, including forced expiratory volume in 1 second (FEV1) for both 1992 and 2000 as well as HO-1 genotype, were available.23 The participants were considered to have chronic sputum if they answered yes to both “Do you usually bring up with phlegm during the day, or at night, in the winter?” and “if yes, do you bring up with phlegm on most days for as much as three months each year?” Analysis of the length variability of (GT)n repeats in the HO-1 gene promoter was described previously.23 If the number of (GT)n repeats in the HO-1 gene promoter was higher than 33, the allele was defined as long (L). Participants with one or two L alleles (group L+) were compared to those with no L alleles (group L−).
L allele has already been associated with a low level of protein expression.17 We verified such association in the present study by measuring HO-1 levels in plasma (ELISA kit, Stressgen Biotechnologies Corp.) in subsets of patients representative for the different groups in which the relation between HO-1 genotype and chronic sputum phenotype was analyzed. HO-1 protein level in serum was quantified previously in different studies.34,35 The number of never-smoker patients in which HO-1 plasma levels were quantified was 16 L+ (3, 11, and 2 L/L, L/M, and L/S, respectively) and 24 L− (11, 10, and 3 M/S, M/M, and S/S, respectively) whereas the number of smokers in which this measurement was performed was 7 L+ (3 and 4 L/M and L/S, respectively) and 25 L− (13, 8, and 4 M/S, M/M, and S/S, respectively). Written informed consent was obtained from all participants before inclusion, and the protocol was approved the French Ethics Committee for Human Research.
Statistical Analysis
For animal studies values are given as mean ± SEM. Data obtained in animals were analyzed by one-way analysis of variance or nonparametric tests as appropriate. For human studies, values are given as either percentages for qualitative factors (center, sex, HO-1 alleles, HO-1 genotypes) or mean ± SD for quantitative factors (age, FEV1% predicted, FEV1%/FVC). A lack of underlying population stratification for the polymorphism (Hardy-Weinberg structure) was tested using a χ2 test. For descriptive purpose, nonsmokers and smokers were compared with the χ2 test for qualitative factors or Mann-Whitney test for quantitative factors. Differences in HO-1 serum levels were tested with Kruskal-Wallis and Dunn’s multiple comparison test. A separate analysis was performed to compare the risk of chronic sputum in group L+ versus group L−, in never-smokers and in smokers separately. Because of small numbers of participants with chronic sputum in nonsmokers, a Fisher test was used in this subgroup, whereas a χ2 was used in smokers. Significance for all statistics was accepted at P < 0.05.
Results
Animal Studies
CS Exposure in Rats Induced HO-1, MUC5AC, and Lung Inflammation
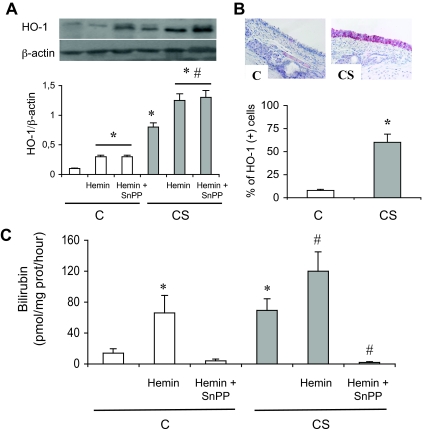
Rats exposed to CS for 5 days showed a fourfold increased HO-1 protein expression in trachea as compared to air-exposed animals (Figure 1A). HO-1 protein expression was observed in epithelial cells (Figure 1B). No change in HO-2 protein expression in trachea homogenates was observed (data not shown). Increased HO-1 protein expression was accompanied by a marked increase in HO activity, by fourfold, with cigarette-smoke exposure as compared to air exposure (P < 0.05, Figure 1C).
Figure 1.
HO-1 expression and HO activity in rats exposed or not to CS. A: Typical Western blot showing detection of HO-1 protein expression. The bottom part shows densitometric analysis of HO-1 expression expressed as a ratio of β-actin expression. C, Exposure to room air; CS, exposure to cigarette smoke; H, hemin; SnPP-IX, tin protoporphyrin IX. Values are mean ± SEM, n = 8 to 10 in each group. *P < 0.05 versus vehicle-treated C; #P < 0.05 versus vehicle-treated CS. B: Immunohistochemical analysis of HO-1 expression in the trachea of one rat exposed to room air (C) and in another exposed to cigarette smoke (CS). Staining was observed in the tracheal epithelium of the CS rat. No staining was observed with the isotype antibody (data not shown). The bottom part shows the percentage of HO-1 (+) cells over the whole number of epithelial cells. Values are mean ± SEM, n = 8 to 10 in each group. *P < 0.05 versus C. C: HO activity. C, Exposure to room air; CS, exposure to cigarette smoke; H, hemin; SnPP-IX, tin protoporphyrin IX. Abbreviations are the same as those in A. Values are mean ± SEM, n = 8 to 10 in each group. *P < 0.05 versus vehicle-treated C; #P < 0.05 versus vehicle-treated CS. Original magnifications, ×40.
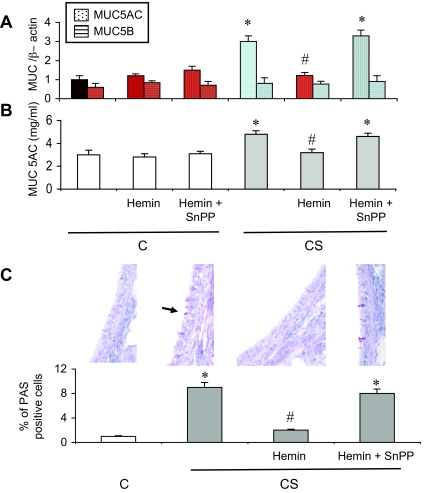
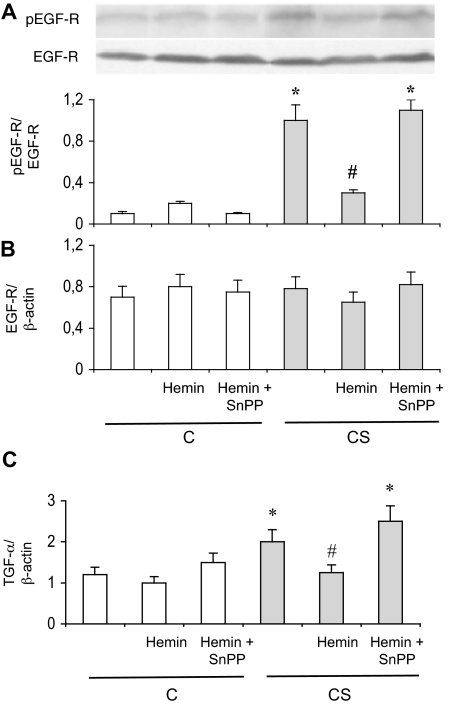
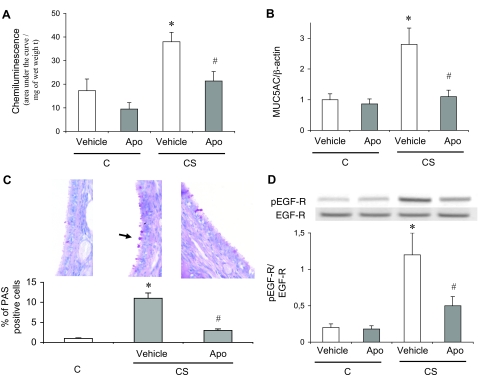
In parallel with HO-1 induction, exposure to CS increased MUC5AC mRNA expression by threefold in trachea, and MUC5AC protein concentration in BAL by twofold (P < 0.05 versus C rats, respectively; Figure 2, A and B), whereas no change in MUC5B mRNA expression was observed (Figure 2A). Mucus secretion paralleled induction of MUC5AC mRNA and protein, as revealed by a significant increase in the percentage of PAS-positive tracheal epithelial cells in CS animals (Figure 2C). MUC5AC induction and mucus hypersecretion were associated with an EGF-R activation, as seen by an increase in EGF-R phosphorylation on tyrosine 845 in trachea of CS rats as compared to C rats (Figure 3A). Interestingly, EGF-R protein and mRNA expression were unchanged in CS animals (Figure 3, A and B). Expression of the mRNA of transforming growth factor (TGF)-α, an EGF-R ligand involved in airways mucus production,1 was also increased in CS rats (Figure 3C). These modifications observed in CS animals were accompanied by an increased BAL cellularity. CS rats showed a 65% increase in the number of total inflammatory cells in BAL fluid as compared to C animals (from 6.98 ± 0.02 × 105 to 10.57 ± 0.06 × 105 cells/ml, P < 0.05; Table 2). The increased cellularity in CS animals concerned neutrophils and macrophages (Table 2).
Figure 2.
Mucin expression and mucus production in rats exposed or not to cigarette smoke. A: Real-time RT-PCR analysis of MUC5AC and MUC5B mRNA expression expressed as a ratio of β-actin mRNA level. Abbreviations are the same as those in Figure 1. Values are mean ± SEM, n = 12 to 15 in each group. *P < 0.05 versus vehicle-treated C; #P < 0.05 versus vehicle-treated CS. B: MUC5AC protein levels in BAL fluid. Abbreviations are the same as those in Figure 1. Values are mean ± SEM, n = 12 to 15 in each group. *P < 0.05 versus vehicle-treated C; #P < 0.05 versus vehicle-treated CS. C: PAS staining in trachea. PAS(+) cells are stained in red. The arrow shows a PAS(+) cell. The bottom part shows the percentage of PAS(+) cells over the whole number of epithelial cells. Abbreviations are the same as those in Figure 1. Values are mean ± SEM, n = 12 to 15 in each group. *P < 0.05 versus vehicle-treated C; #P < 0.05 versus vehicle-treated CS. Original magnifications, ×40.
Figure 3.
EGF pathway in rat epithelium. A: Typical Western blot showing detection of phosphorylated (pEGF-R, Tyr845) and nonphosphorylated EGF-R expression. The bottom part shows densitometric analysis of pEGF-R expression as a ratio of EGF-R expression. Abbreviations are the same as those in Figure 1. Values are mean ± SEM, n = 10 to 12 in each group. *P < 0.05 versus vehicle-treated C; #P < 0.05 versus vehicle-treated CS. B: Real-time RT-PCR analysis of EGF-R mRNA expression as a ratio of β-actin mRNA level. Abbreviations are the same as those in Figure 1. Values are mean ± SEM, n = 12 to 15 in each group. C: Real-time RT-PCR analysis of TGF-α mRNA expression as a ratio of β-actin mRNA level. Abbreviations are the same as those in Figure 1. Values are mean ± SEM, n = 12 to 15 in each group. *P < 0.05 versus vehicle-treated C; #P < 0.05 versus vehicle-treated CS.
Table 2.
Differential Cell Count in Bronchoalveolar Lavage (BAL) Fluid of Animals Exposed or Not to Cigarette Smoke
| Groups | Number of cells (105/ml)
|
||||
|---|---|---|---|---|---|
| Total of inflammatory cells | Neutrophils | Macrophages | Eosinophils | Lymphocytes | |
| C | 6.98 ± 0.02 | 0.45 ± 0.05 | 2.78 ± 0.2 | 0.13 ± 0.04 | 0.23 ± 0.04 |
| C+H | 4.85 ± 0.04 | 0.30 ± 0.04 | 2.39 ± 0.15 | 0.09 ± 0.03 | 0.09 ± 0.03 |
| C+H+SnPP | 8.12 ± 0.05 | 0.73 ± 0.09 | 3.491 ± 0.22 | 0.13 ± 0.02 | 0.26 ± 0.04 |
| CS | 10.57 ± 0.06* | 0.99 ± 0.09* | 4.744 ± 0.20* | 0.21 ± 0.04 | 0.31 ± 0.04 |
| CS+H | 5.57 ± 0.03† | 0.44 ± 0.08† | 2.655 ± 0.22† | 0.07 ± 0.03 | 0.17 ± 0.04 |
| CS+H+SnPP | 9.52 ± 0.06* | 0.94 ± 0.08* | 4.743 ± 0.10* | 0.18 ± 0.03 | 0.46 ± 0.04 |
C, animals exposed to room air; CS, animals exposed to cigarette smoke; H, hemin; SnPP, tin protoporphyrin IX. Values are mean ± SEM, n = 12 to 15 in each group.
P < 0.05 versus vehicle-treated C;
P < 0.05 versus vehicle-treated CS.
HO-1 Induction with Hemin Protects against Mucus Hypersecretion Induced by CS Exposure in Rats
Repeated administration of hemin, an HO-1 inducer and substrate, increased HO-1 protein expression and HO activity in C rats (P < 0.05; Figure 1, A and C), and further increased activity in CS rats (P < 0.05 versus vehicle treated CS animals). This effect was reversed by concomitant administration of the HO inhibitor SnPP (Figure 1C). Hemin did not change tracheal HO-2 protein expression (data not shown). Hemin administration in C rats did not significantly modify MUC5AC mRNA and protein expression, mucus production, EGF-R phosphorylation, TGF-α expression, and BAL cellularity (Figures 2 and 3, and Table 2). In CS rats, hemin administration significantly reduced MUC5AC induction and mucus secretion, EGF-R phosphorylation, TGF-α expression, and BAL cellularity (Figures 2 and 3, and Table 2). These changes were reversed by concomitant administration of SnPP (Figures 2 and 3, and Table 2).
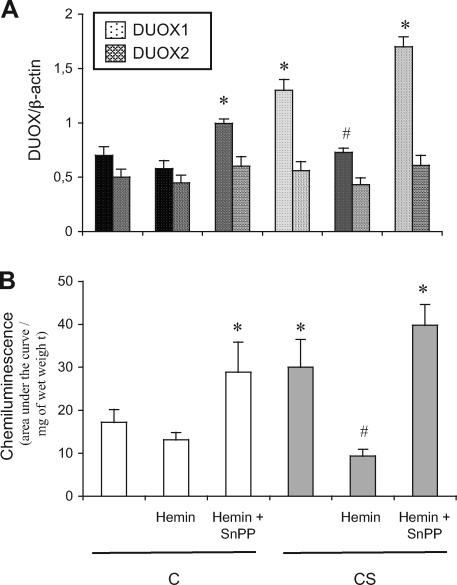
Down-Regulation of DUOX1/NADPH Oxidase Is Involved in the Protective Effect of HO-1 against Mucus Hypersecretion
Recent in vitro data showed that epithelial dual oxidase-1 (DUOX1)/NADPH oxidase is involved in ligand-dependent EGF-R activation.10 Therefore, we examined whether HO-1-attenuated MUC5AC induction by CS results from down-regulated DUOX1. We first examined DUOX1 expression and activity in the trachea of rats exposed to CS and then analyzed the effect of apocynin, an NADPH oxidase blocker, on MUC5AC induction. CS rats showed significantly increased DUOX1 mRNA expression (Figure 4A), and reactive oxygen species production (P < 0.05 versus C animals; Figure 4B). Furthermore, because expression of DUOX2 mRNA was not changed in CS as compared to C animals (Figure 4A) and no inflammatory cells were seen infiltrating the trachea (Figure 2C), the reactive oxygen species production might reflect the DUOX1 activity.
Figure 4.
DUOX1 pathway in rats exposed or not to cigarette smoke. A: Real-time RT-PCR analysis of DUOX1 and DUOX2 mRNA expression expressed as a ratio of β-actin mRNA level. Abbreviations are the same as those in Figure 1. Values are mean ± SEM, n = 12 to 15 in each group. *P < 0.05 versus vehicle-treated C; #P < 0.05 versus vehicle-treated CS. B: Reactive oxygen species production in tracheal rings evaluated by chemiluminescence. Values are expressed as the total chemiluminescence produced (cpm) throughout 60 minutes, normalized to the wet weight of the ring. Abbreviations are the same as those in Figure 1. Values are mean ± SEM, n = 10 to 12 in each group. *P < 0.05 versus vehicle-treated C; #P < 0.05 versus vehicle-treated CS.
Because DUOX activity is inhibited by apocynin,36 we used this molecule to examine the role of DUOX1 on mucus secretion induced by CS. Apocynin administration significantly reversed CS-induced reactive oxygen species production, MUC5AC induction, mucus secretion, and EGF-R phosphorylation in tracheal rings (Figure 5), which suggests a causative role of DUOX1 in these phenomena. Therefore, we next examined whether the protective effect of hemin involved attenuation of DUOX1 induction. Hemin administration to CS rats significantly attenuated DUOX1 mRNA induction and reactive oxygen species production (Figure 4), which were reversed by concomitant administration of SnPP. These results suggest that HO-1 up-regulation could prevent MUC5AC induction by attenuating a DUOX1/EGF-R pathway. Of note, in C rats, hemin and SnPP administration, which resulted in reduced HO activity as compared to vehicle-treated animals (Figure 1C), significantly increased DUOX1 expression and reactive oxygen species production (P < 0.05 versus vehicle-treated animals; Figure 4), with MUC5AC expression and EGF-R phosphorylation unchanged (Figures 2 and 3, respectively).
Figure 5.
Effects of apocynin treatment in rats exposed or not to cigarette smoke. A: Reactive oxygen species production in tracheal rings evaluated by chemiluminescence. Values are expressed as the total chemiluminescence produced (cpm) throughout 60 minutes, normalized to the wet weight of the ring. C, Animals exposed to room air; CS, animals exposed to cigarette smoke; Apo, apocynin. Values are mean ± SEM, n = 10 to 12 in each group. *P < 0.05 versus vehicle-treated C; #P < 0.05 versus vehicle-treated CS. B: Real-time RT-PCR analysis of MUC5AC expression expressed as a ratio of β-actin mRNA level. Abbreviations are the same as those in A. Values are mean ± SEM, n = 10 to 12 in each group. *P < 0.05 versus vehicle-treated C; #P < 0.05 versus vehicle-treated CS. C: PAS staining in trachea. PAS(+) cells are stained in red. The arrow shows a PAS(+) cell. The bottom part shows the percentage of PAS(+) cells over the whole number of epithelial cells. Abbreviations are the same as those in A. Values are mean ± SEM, n = 10 to 12 in each group. *P < 0.05 versus vehicle-treated C; #P < 0.05 versus vehicle-treated CS. D: Typical Western blot showing detection of phosphorylated (pEGF-R, Tyr845) and nonphosphorylated EGF-R expression. The bottom part shows densitometric analysis of pEGF-R expression expressed as a ratio of EGF-R expression. Abbreviations are the same as those in A. Values are mean ± SEM, n = 10 to 12 in each group. *P < 0.05 versus vehicle-treated C; #P < 0.05 versus vehicle-treated CS. Original magnifications, ×40.
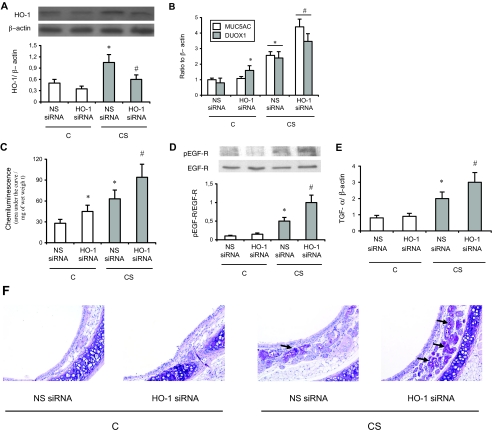
Down-Regulated HO-1 Expression in Mice Potentiates DUOX1 and MUC5AC Induction by CS Exposure
To complete the experiments with hemin, we examined whether down-regulating HO-1 expression potentiated DUOX1 expression, oxidants production, EGF-R phosphorylation, TGF-α expression, MUC5AC induction, and mucus secretion in mice exposed to CS. We used an in vivo siRNA strategy. Exposure of mice to CS induced HO-1 protein, DUOX1 mRNA expression, oxidants production, EGF-R phosphorylation, TGF-α expression, MUC5AC mRNA expression, and mucus secretion in trachea (Figure 6). Attenuating HO-1 expression with a specific siRNA further increased these phenomena (Figure 6). It has to be noted that, as described previously,37 mucus secretion was only observed in submucosal glands. PAS staining intensity was −/+ in C animals exposed to both nonspecific and HO-1-specific siRNA, and ++ and +++/++++ in CS animals exposed to nonspecific and HO-1-specific siRNA, respectively. Interestingly, although no change in MUC5AC level was observed in C animals, HO-1 siRNA administration in these animals increased DUOX1 expression and oxidants production. No such changes were observed with nonspecific siRNA. These results stress the critical role of HO-1 in the control of mucin expression on exposure to CS.
Figure 6.
Effect of HO-1 siRNA administration in mice. A: Western blot analysis of HO-1 protein expression in trachea. The bottom part shows densitometric analysis of HO-1 expression expressed as a ratio of β-actin levels. C, mice exposed to room air; CS, mice exposed to cigarette smoke; NS, nonspecific siRNA. Values are mean ± SEM, n = 8 in each group. *P < 0.05 versus C mice treated with nonspecific siRNA; #P < 0.05 versus CS mice treated with nonspecific siRNA. B: Real-time RT-PCR analysis of DUOX1 and MUC5AC expression expressed as a ratio of β-actin mRNA levels. Abbreviations are the same as those in A. Values are mean ± SEM, n = 8 in each group. *P < 0.05 versus C mice treated with nonspecific siRNA; #P < 0.05 versus CS mice treated with nonspecific siRNA. C: Reactive oxygen species production in tracheal rings evaluated by chemiluminescence. Values are expressed as the total chemiluminescence produced (cpm) throughout 60 minutes, normalized to the wet weight of the ring. Values are mean ± SEM, n = 6 to 8 in each group. *P < 0.05 versus C mice treated with nonspecific siRNA; #P < 0.05 versus CS mice treated with nonspecific siRNA. D: Typical Western blot showing detection of phosphorylated (pEGF-R, Tyr845) and nonphosphorylated EGF-R expression. The bottom part shows densitometric analysis of pEGF-R expression expressed as a ratio of EGF-R expression. Values are mean ± SEM, n = 6 to 8 in each group. *P < 0.05 versus C mice treated with nonspecific siRNA; #P < 0.05 versus CS mice treated with nonspecific siRNA. E: Real-time RT-PCR analysis of TGF-α expression expressed as a ratio of β-actin mRNA levels. Abbreviations are the same as those in A. Values are mean ± SEM, n = 8 in each group. *P < 0.05 versus C mice treated with nonspecific siRNA; #P < 0.05 versus CS mice treated with nonspecific siRNA. F: PAS staining in trachea. PAS(+) cells are stained in red (black arrows). Magnification is ×40. Abbreviations are the same as those in A. The bottom part shows the percentage of PAS(+) cells over the whole number of submucosal glands epithelial cells. Values are mean ± SEM, n = 6 to 8 in each group. *P < 0.05 versus C mice treated with nonspecific siRNA; #P < 0.05 versus CS mice treated with nonspecific siRNA. Original magnifications, ×40.
Human Studies
Increased (GT)n Repeats in the HO-1 Gene Promoter Is Associated with Chronic Sputum Prevalence in Smokers
To investigate the clinical implications of the animal studies, we examined human samples originating from smokers in the general population and focused on the relation between a HO-1 gene promoter polymorphism, which modulates HO-1 expression, and prevalence of chronic sputum, reflecting mucus hypersecretion. Group L+ participants harbor at least one allele with a long (GT)n in the HO-1 promoter gene (>33 repeats), which has been reported to be associated with a low level of HO-1 protein expression.17 The main characteristics of the sample are provided in Table 3. We observed no deviation from the Hardy-Weinberg structure (P = 0.53) and allele distributions were similar between nonsmokers and smokers (Table 3).
Table 3.
Characteristics of the Human Subjects
| Variables | Never-smokers (n = 297) | Smokers (n = 210) | P |
|---|---|---|---|
| Center | |||
| Grenoble | 38.0 | 33.8 | 0.04 |
| Montpellier | 19.5 | 13.3 | |
| Paris | 42.5 | 52.9 | |
| Sex (men) | 50.9 | 49.0 | 0.2 |
| Age (years) | 44.1 ± 7.2 | 43.8 ± 7.3 | 0.6 |
| FEV1% predicted | 105.9 ± 14.5 | 101.9 ± 14.8 | 0.002 |
| FEV1/FVC (%) | 83.6 ± 7.1 | 82.0 ± 7.9 | 0.04 |
| HO-1 allele (%) | |||
| Allele L | 7 | 6 | 0.3 |
| Allele M | 57 | 53 | |
| Allele S | 36 | 41 | |
| HO-1 genotype | |||
| Group L+ | 12.5 | 10.9 | 0.6 |
| Group L− | 87.5 | 89.1 |
Results in percentage for qualitative variables and mean ± SD for quantitative variables.
FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity.
Group L+: Subjects with one or two L alleles [number of (GT)n repeats in the HO-1 gene promoter higher than 33]. Group L−: Subjects with no L alleles.
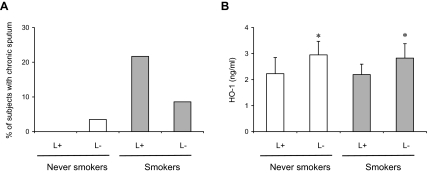
A higher proportion of smokers in group L+ than in group L− had chronic sputum (21.7% versus 8.6%, P = 0.047). In contrast, there was no difference in never-smokers (Figure 7A). As expected, HO-1 protein level in serum was significantly lower in L+ as compared to L− participants (both in never-smokers and smokers, P < 0.05 in each case respectively; Figure 7B). These results expand on our in vivo data in animals and strongly suggest that HO-1 plays a critical role in mucus hypersecretion induced by CS in humans.
Figure 7.
Consequences of HO-1 gene promoter polymorphism. A: Prevalence of chronic sputum by HO-1 genotype [group L+ (one or two L alleles) versus group L− (no L allele)] in 210 smokers and 297 never-smokers examined in 2000 in the European Community Respiratory Health Survey. B: HO-1 protein levels in plasma in a subset of L+ and L− patients (32 smokers and 40 never-smokers). Values are mean ± SD. *P < 0.05 versus respective L+ patients.
Discussion
Up-regulated HO-1 expression attenuated pathological phenomena in experimental models of pulmonary diseases such as ischemia-reperfusion injury,21 hyperoxia,38 influenza virus infection,39 ozone-induced lung injury,40 and asthma.41,42 In all of these models HO-1 was induced as a compensatory mechanism, and further pharmacological or transgenic induction had protective effects. The same concept can be applied to the consequences of CS exposure because HO-1 expression is induced in healthy smokers as compared to controls.43 However, smokers with severe COPD display a lower level of lung HO-1 expression as compared with smokers without COPD.44 Furthermore, the same (GT)n dinucleotide length polymorphism we analyzed in the present study has been linked to the development of emphysema in smokers18 and is associated with an accelerated rate of decline of FEV1 in heavy smokers (>1 pack/day).23 These observations imply that a low expression level of stress-defense mechanisms such as HO-1 could contribute to the pathophysiology of CS-induced diseases such as COPD.
The results of our study confirm and expand on these data, showing that HO-1 induction with hemin prevented MUC5AC mRNA and protein expression and mucus hypersecretion induced by CS exposure. We choose to up-regulate HO-1 with hemin and not with other strategies (ie, HO-1 gene delivery) because hemin is not only an inductor but also a substrate of the enzyme, thus ensuring a high HO activity. It should be noted however, that hemin might not be simply an inducer of HO-1, but also of HO-2.45 However, we think a role of HO-2 in our results unlikely because HO-2 expression was unmodified after CS and/or hemin exposure. Furthermore, we ensure that the effects of hemin were reversed by the HO inhibitor SnPP, which markedly reduced HO activity (Figure 1C), and opposite effects to those induced by hemin were observed by specific inhibition of HO-1 expression by an in vivo siRNA strategy. Moreover, we are confident about the clinical implications of animal results because we found a significant increase in chronic sputum in smokers with a long (GT)n in the HO-1 promoter gene, which is associated with a low level of HO-1 protein expression.17 We verified that this was the case in the present study and found low concentrations of HO-1 protein in the plasma of L+ as compared to L− never-smokers and smokers. Collectively, these results demonstrate, for the first time to the best of our knowledge, that HO-1 significantly protects against airway MUC5AC expression and mucus hypersecretion induced by cigarette-smoke exposure. HO-1 did not modify MUC5AC expression in nonsmoking animals and patients, suggesting that HO-1 acts only after the exposure to CS, which supports the stress-response characteristic of this system. These data agree with our recent results in patients from the same sample, showing the L+ genotype associated with accelerated decline in lung function among heavy smokers, with no such effect observed in nonsmokers and patients smoking less than one pack per day.23 Interestingly, the fact that the same factor (a relative HO-1 deficiency) is associated with both chronic sputum prevalence and accelerated decline of lung function in heavy smokers supports a cause-effect relation between the former and the latter factor.4,46,47
As stated previously, the EGF-R cascade plays a central role in mucin production by airway epithelial cells after exposure to CS and other stimuli.1 EGF-R activation involves two different pathways: ligand-dependent and -independent EGF-R tyrosine phosphorylation. The ligand-dependent pathway involves TACE (now ADAM 17) activation by DUOX1 with the consecutive cleavage of pro-TGF-α into soluble TGF-α, whereas the ligand-independent pathway involves EGF-R phosphorylation in response to oxidative stress. Some stimuli, such as CS, can involve both pathways. In the present study, CS exposure induced phosphorylation of EGF-R on tyrosine 845 in both rats and mice, as demonstrated previously with air pollution particles.48 HO-1 induction by hemin prevented both DUOX1 induction, and EGF-R activation induced by CS, and apocynin mimicked hemin effects. These data suggests that the preventive effect of HO-1 on EGF-R activation and MUC5AC expression could be secondary to the suppression of DUOX1 induction. Furthermore, suppression of TGF-α induction by hemin stress the implication of the ligand-dependent pathway as a mechanism involved in modulation of mucus production by HO-1. It must be noted that apocynin inhibits not only DUOX1,36 but also other NADPH oxidase gp91phox homologues. However, because DUOX1 is highly expressed in tracheal epithelial cells,12,36 the expression of DUOX2, which is also expressed in tracheal epithelial cells,49 was unchanged in CS rats, and no inflammatory cells were seen infiltrating the trachea (Figure 2C), we are confident in the specificity of apocynin as a DUOX1 inhibitor in the present experimental model. Furthermore, specific siRNA inhibition of HO-1 expression potentiated both DUOX1 expression and activity, EGF-R phosphorylation, TGF-α expression, MUC5AC induction, and mucus production by CS exposure in mice, stressing the role of HO-1 in modulating DUOX1 expression and ensuing signaling events. Modulation of DUOX1 expression by HO-1 could be secondary to the anti-inflammatory properties of this enzyme, demonstrated in the present study by the reduced BAL inflammatory cellularity in CS animals receiving hemin. Indeed, it has been shown in primary human airway epithelial cells that DUOX1 is up-regulated by inflammatory cytokines, such as IL-13.50 In addition, a decreased heme content secondary to the increased HO-1 activity could contribute to a decreased DUOX1 protein stability, as demonstrated previously in the case of other NADPH oxidase homologues.31 However, examination of these mechanism(s) deserves further investigations.
In addition to potentially blocking DUOX1-mediated EGF-R phosphorylation, HO-1 could suppress MUC5AC induction by interfering with the ligand-independent pathway. Indeed, suppression of HO-1 activity in both control rats and mice increased DUOX1 expression and oxidants production, but not EGF-R phosphorylation, TGF-α expression, MUC5AC expression, and mucus production. This result and data obtained with apocynin could mean that DUOX1-derived oxidants are necessary but not sufficient to phosphorylate the EGF-R in the context of CS exposure. Indeed, it has been previously shown that tobacco smoke induces mucin synthesis in airway epithelial cells and generation of reactive oxygen species, which can directly transactivate the EGF-R.8 Therefore, one can postulate that oxidants from DUOX1 and from CS can act in concert to phosphorylate the EGF-R and induce MUC5AC in CS animals. Considering the antioxidant properties of HO-1, mainly related to bilirubin production,51,52 interference with this last pathway could also explain the protective effect of HO-1 against EGF-R phosphorylation and MUC5AC induction by CS. Whatever the mechanisms involved, our results provide the first demonstration of modulation of EGF-R phosphorylation by HO-1. This finding has widespread implications, considering the involvement of EGF-R phosphorylation, including phosphorylation on tyrosine 845 found in the present study, in different inflammatory diseases and in cancer.53,54,55 For example, a low HO-1 expression can explain increased frequency of lung adenocarcinoma in smokers with the L+ HO-1 gene promoter genotype,56 because this cancer type presents an increased phosphorylation of the EGF-R, resulting in a loss of cell apoptosis.53
In conclusion, our study provides evidence that HO-1 counterbalances mucus hypersecretion induced by exposure to CS in rodents and humans. These results suggest that an induced HO pathway could be beneficial to attenuate mucus hypersecretion associated with exposure to CS and COPD. In this context, use of new or well-known HO-1 inductors already applied in humans (such as curcumin or probucol57,58) and/or DUOX1 inhibitors or blockers could become interesting new therapeutic approaches. These possibilities have important implications in terms of public health because mucus hypersecretion greatly contributes to the morbidity and mortality of COPD,1 which is actually the fifth cause of mortality and is predicted to be the fourth one in the next 30 years.6
Acknowledgments
We thank Delphine Goven for her very helpful technical assistance.
Footnotes
Address reprint requests to Jorge Boczkowski, INSERM U700, Faculté de Médecine Paris 7, Site X. Bichat, BP416, 75870 Paris Cedex 18, France. E-mail: jorge.boczkowski@inserm.fr.
Supported by INSERM and Université Paris 7, France (to A.A., A.G., N.A., R.B., J.E.B., B.L., M.A., and J.B.); INSERM and Université Paris 5 Paris-Descartes, France (to M.B. and F.R., as a Soutien à Equipe en Emergence”); Assistance Publique-Hopitaux de Paris, France (as a Contrat d’Interface to J.B.); Albaath University, Syria (to A.A.); the Association pour la Recherche sur les Nicotianées, France (to A.A. and A.G.); the Region Ile de France (to S.G. and J.D.); and the Ministry of Science and Higher Education, Poland (grant PBZ-KBN 107/P04/2004 to S.G. and J.D.).
References
- Burgel PR, Nadel JA. Roles of epidermal growth factor receptor activation in epithelial cell repair and mucin production in airway epithelium. Thorax. 2004;59:992–996. doi: 10.1136/thx.2003.018879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J. 2003;22:672–688. doi: 10.1183/09031936.03.00040703. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. Am Rev Respir Dis. 1987:225–244. doi: 10.1164/ajrccm/136.1.225. [DOI] [PubMed] [Google Scholar]
- Vestbo J, Prescott E, Lange P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study Group. Am J Respir Crit Care Med. 1996;153:1530–1535. doi: 10.1164/ajrccm.153.5.8630597. [DOI] [PubMed] [Google Scholar]
- Vestbo J. Chronic mucus hypersecretion, exacerbations and natural history of COPD. Exp Lung Res. 2005;31(Suppl 1):63–65. [PubMed] [Google Scholar]
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leikauf GD, Borchers MT, Prows DR, Simpson LG. Mucin apoprotein expression in COPD. Chest. 2002;121:166S–182S. doi: 10.1378/chest.121.5_suppl.166s. [DOI] [PubMed] [Google Scholar]
- Takeyama K, Jung B, Shim JJ, Burgel PR, Dao-Pick T, Ueki IF, Protin U, Kroschel P, Nadel JA. Activation of epidermal growth factor receptors is responsible for mucin synthesis induced by cigarette smoke. Am J Physiol. 2001;280:L165–L172. doi: 10.1152/ajplung.2001.280.1.L165. [DOI] [PubMed] [Google Scholar]
- Shao MX, Ueki IF, Nadel JA. Tumor necrosis factor alpha-converting enzyme mediates MUC5AC mucin expression in cultured human airway epithelial cells. Proc Natl Acad Sci USA. 2003;100:11618–11623. doi: 10.1073/pnas.1534804100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao MX, Nadel JA. Dual oxidase 1-dependent MUC5AC mucin expression in cultured human airway epithelial cells. Proc Natl Acad Sci USA. 2005;102:767–772. doi: 10.1073/pnas.0408932102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiszt M, Witta J, Baffi J, Lekstrom K, Leto TL. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J. 2003;17:1502–1504. doi: 10.1096/fj.02-1104fje. [DOI] [PubMed] [Google Scholar]
- Forteza R, Salathe M, Miot F, Conner GE. Regulated hydrogen peroxide production by Duox in human airway epithelial cells. Am J Respir Cell Mol Biol. 2005;32:462–469. doi: 10.1165/rcmb.2004-0302OC. [DOI] [PubMed] [Google Scholar]
- Goldkorn T, Balaban N, Matsukuma K, Chea V, Gould R, Last J, Chan C, Chavez C. EGF-receptor phosphorylation and signaling are targeted by H2O2 redox stress. Am J Respir Cell Mol Biol. 1998;19:786–798. doi: 10.1165/ajrcmb.19.5.3249. [DOI] [PubMed] [Google Scholar]
- Takeyama K, Dabbagh K, Jeong Shim J, Dao-Pick T, Ueki IF, Nadel JA. Oxidative stress causes mucin synthesis via transactivation of epidermal growth factor receptor: role of neutrophils. J Immunol. 2000;164:1546–1552. doi: 10.4049/jimmunol.164.3.1546. [DOI] [PubMed] [Google Scholar]
- Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24:449–455. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- Slebos DJ, Ryter SW, Choi AM. Heme oxygenase-1 and carbon monoxide in pulmonary medicine. Respir Res. 2003;4:7. doi: 10.1186/1465-9921-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exner M, Minar E, Wagner O, Schillinger M. The role of heme oxygenase-1 promoter polymorphisms in human disease. Free Radic Biol Med. 2004;37:1097–1104. doi: 10.1016/j.freeradbiomed.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Yamada N, Yamaya M, Okinaga S, Nakayama K, Sekizawa K, Shibahara S, Sasaki H. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to emphysema. Am J Hum Genet. 2000;66:187–195. doi: 10.1086/302729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker R, Perrella MA. Heme oxygenase-1: a novel drug target for atherosclerotic diseases? Circulation. 2006;114:2178–2189. doi: 10.1161/CIRCULATIONAHA.105.598698. [DOI] [PubMed] [Google Scholar]
- Nikula KJ, Green FH. Animal models of chronic bronchitis and their relevance to studies of particle-induced disease. Inhal Toxicol. 2000;12(Suppl 4):123–153. doi: 10.1080/089583700750019549. [DOI] [PubMed] [Google Scholar]
- Zhang X, Shan P, Jiang D, Noble PW, Abraham NG, Kappas A, Lee PJ. Small interfering RNA targeting heme oxygenase-1 enhances ischemia-reperfusion-induced lung apoptosis. J Biol Chem. 2004;279:10677–10684. doi: 10.1074/jbc.M312941200. [DOI] [PubMed] [Google Scholar]
- Benallaoua M, Francois M, Batteux F, Thelier N, Shyy JY, Fitting C, Tsagris L, Boczkowski J, Savouret JF, Corvol MT, Poiraudeau S, Rannou F. Pharmacologic induction of heme oxygenase 1 reduces acute inflammatory arthritis in mice. Arthritis Rheum. 2007;56:2585–2594. doi: 10.1002/art.22749. [DOI] [PubMed] [Google Scholar]
- Guenegou A, Leynaert B, Benessiano J, Pin I, Demoly P, Neukirch F, Boczkowski J, Aubier M. Association of lung function decline with the heme oxygenase-1 gene promoter microsatellite polymorphism in a general population sample. Results from the European Community Respiratory Health Survey (ECRHS), France. J Med Genet. 2006;43:e43. doi: 10.1136/jmg.2005.039743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landaw SA, Sassa S, Drummond GS, Kappas A. Proof that Sn-protoporphyrin inhibits the enzymatic catabolism of heme in vivo. Suppression of 14CO generation from radiolabeled endogenous and exogenous heme sources. J Exp Med. 1987;165:1195–1200. doi: 10.1084/jem.165.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundemar L, Ny L. Pitfalls using metalloporphyrins in carbon monoxide research. TiPS. 1997;18:193–195. doi: 10.1016/s0165-6147(97)01065-1. [DOI] [PubMed] [Google Scholar]
- Asaba K, Tojo A, Onozato ML, Goto A, Quinn MT, Fujita T, Wilcox CS. Effects of NADPH oxidase inhibitor in diabetic nephropathy. Kidney Int. 2005;67:1890–1898. doi: 10.1111/j.1523-1755.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- Hougee S, Hartog A, Sanders A, Graus YM, Hoijer MA, Garssen J, van den Berg WB, van Beuningen HM, Smit HF. Oral administration of the NADPH-oxidase inhibitor apocynin partially restores diminished cartilage proteoglycan synthesis and reduces inflammation in mice. Eur J Pharmacol. 2006;531:264–269. doi: 10.1016/j.ejphar.2005.11.061. [DOI] [PubMed] [Google Scholar]
- Lewis DL, Hagstrom JE, Loomis AG, Wolff JA, Herweijer H. Efficient delivery of siRNA for inhibition of gene expression in postnatal mice. Nat Genet. 2002;32:107–108. doi: 10.1038/ng944. [DOI] [PubMed] [Google Scholar]
- Taillé C, Foresti R, Lanone S, Zedda C, Green C, Aubier M, Motterlini R, Boczkowski J. Protective role of heme oxygenases against endotoxin-induced diaphragmatic dysfunction in rats. Am J Respir Crit Care Med. 2001;163:753–761. doi: 10.1164/ajrccm.163.3.2004202. [DOI] [PubMed] [Google Scholar]
- Samb A, Taille C, Almolki A, Megret J, Staddon JM, Aubier M, Boczkowski J. Heme oxygenase modulates oxidant-signaled airway smooth muscle contractility: role of bilirubin. Am J Physiol. 2002;283:L596–L603. doi: 10.1152/ajplung.00446.2001. [DOI] [PubMed] [Google Scholar]
- Taillé C, El-Benna J, Lanone S, Dang MC, Ogier-Denis E, Aubier M, Boczkowski J. Induction of heme oxygenase-1 inhibits NAD(P)H oxidase activity by down-regulating cytochrome b558 expression via the reduction of heme availability. J Biol Chem. 2004;279:28681–28688. doi: 10.1074/jbc.M310661200. [DOI] [PubMed] [Google Scholar]
- Burgel PR, Lazarus SC, Tam DC, Ueki IF, Atabai K, Birch M, Nadel JA. Human eosinophils induce mucin production in airway epithelial cells via epidermal growth factor receptor activation. J Immunol. 2001;167:5948–5954. doi: 10.4049/jimmunol.167.10.5948. [DOI] [PubMed] [Google Scholar]
- The European Community Respiratory Health Survey II. Eur Respir J. 2002;20:1071–1079. doi: 10.1183/09031936.02.00046802. [DOI] [PubMed] [Google Scholar]
- Kirino Y, Takeno M, Iwasaki M, Ueda A, Ohno S, Shirai A, Kanamori H, Tanaka K, Ishigatsubo Y. Increased serum HO-1 in hemophagocytic syndrome and adult-onset Still’s disease: use in the differential diagnosis of hyperferritinemia. Arthritis Res Ther. 2005;7:R616–R624. doi: 10.1186/ar1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Takeno M, Honma K, Yamauchi H, Saito Y, Sasaki T, Morikubo H, Nagashima Y, Takagi S, Yamanaka K, Kaneko T, Ishigatsubo Y. Heme oxygenase-1, a potential biomarker of chronic silicosis, attenuates silica-induced lung injury. Am J Respir Crit Care Med. 2006;174:906–914. doi: 10.1164/rccm.200508-1237OC. [DOI] [PubMed] [Google Scholar]
- Schwarzer C, Machen TE, Illek B, Fischer H. NADPH oxidase-dependent acid production in airway epithelial cells. J Biol Chem. 2004;279:36454–36461. doi: 10.1074/jbc.M404983200. [DOI] [PubMed] [Google Scholar]
- Pack RJ, Al-Ugaily LH, Morris G. The cells of the tracheobronchial epithelium of the mouse: a quantitative light and electron microscope study. J Anat. 1981;132:71–84. [PMC free article] [PubMed] [Google Scholar]
- Otterbein LE, Kolls JK, Mantell LL, Cook JL, Alam J, Choi AM. Exogenous administration of heme oxygenase-1 by gene transfer provides protection against hyperoxia-induced lung injury. J Clin Invest. 1999;103:1047–1054. doi: 10.1172/JCI5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashiba T, Suzuki M, Nagashima Y, Suzuki S, Inoue S, Tsuburai T, Matsuse T, Ishigatubo Y. Adenovirus-mediated transfer of heme oxygenase-1 cDNA attenuates severe lung injury induced by the influenza virus in mice. Gene Ther. 2001;8:1499–1507. doi: 10.1038/sj.gt.3301540. [DOI] [PubMed] [Google Scholar]
- Hisada T, Salmon M, Nasuhara Y, Chung K. Involvement of haemoxygeanse-1 in ozone-induced airway inflammation and hyperresponsiveness. Eur J Pharmacol. 2000;399:229–234. doi: 10.1016/s0014-2999(00)00369-1. [DOI] [PubMed] [Google Scholar]
- Jia YX, Sekizawa K, Okinaga S, Lie R, Sasaki H. Role of heme oxygenase in pulmonary response to antigen challenge in sensitized rats in vivo. Int Arch Allergy Immunol. 1999;120:141–145. doi: 10.1159/000024232. [DOI] [PubMed] [Google Scholar]
- Almolki A, Taille C, Martin GF, Jose PJ, Zedda C, Conti M, Megret J, Henin D, Aubier M, Boczkowski J. Heme oxygenase attenuates allergen-induced airway inflammation and hyperreactivity in guinea pigs. Am J Physiol. 2004;287:L26–L34. doi: 10.1152/ajplung.00237.2003. [DOI] [PubMed] [Google Scholar]
- Maestrelli P, El Messlemani AH, De Fina O, Nowicki Y, Saetta M, Mapp C, Fabbri LM. Increased expression of heme oxygenase (HO)-1 in alveolar spaces and HO-2 in alveolar walls of smokers. Am J Respir Crit Care Med. 2001;164:1508–1513. doi: 10.1164/ajrccm.164.8.2011083. [DOI] [PubMed] [Google Scholar]
- Maestrelli P, Paska C, Saetta M, Turato G, Nowicki Y, Monti S, Formichi B, Miniati M, Fabbri LM. Decreased haem oxygenase-1 and increased inducible nitric oxide synthase in the lung of severe COPD patients. Eur Respir J. 2003;21:971–976. doi: 10.1183/09031936.03.00098203. [DOI] [PubMed] [Google Scholar]
- Kaizu T, Tamaki T, Tanaka M, Uchida Y, Tsuchihashi S, Kawamura A, Kakita A. Preconditioning with tin-protoporphyrin IX attenuates ischemia/reperfusion injury in the rat kidney. Kidney Int. 2003;63:1393–1403. doi: 10.1046/j.1523-1755.2003.00882.x. [DOI] [PubMed] [Google Scholar]
- Vestbo J, Hogg JC. Convergence of the epidemiology and pathology of COPD. Thorax. 2006;61:86–88. doi: 10.1136/thx.2005.046227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innes AL, Woodruff PG, Ferrando RE, Donnelly S, Dolganov GM, Lazarus SC, Fahy JV. Epithelial mucin stores are increased in the large airways of smokers with airflow obstruction. Chest. 2006;130:1102–1108. doi: 10.1378/chest.130.4.1102. [DOI] [PubMed] [Google Scholar]
- Churg A, Xie C, Wang X, Vincent R, Wang RD. Air pollution particles activate NF-kappaB on contact with airway epithelial cell surfaces. Toxicol Appl Pharmacol. 2005;208:37–45. doi: 10.1016/j.taap.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Moskwa P, Lorentzen D, Excoffon KJ, Zabner J, McCray PB, Jr, Nauseef WM, Dupuy C, Banfi B. A novel host defense system of airways is defective in cystic fibrosis. Am J Respir Crit Care Med. 2007;175:174–183. doi: 10.1164/rccm.200607-1029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper RW, Xu C, Eiserich JP, Chen Y, Kao CY, Thai P, Setiadi H, Wu R. Differential regulation of dual NADPH oxidases/peroxidases, Duox1 and Duox2, by Th1 and Th2 cytokines in respiratory tract epithelium. FEBS Lett. 2005;579:4911–4917. doi: 10.1016/j.febslet.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- Lanone S, Bloc S, Foresti R, Almolki A, Taille C, Callebert J, Conti M, Goven D, Aubier M, Dureuil B, El-Benna J, Motterlini R, Boczkowski J. Bilirubin decreases nos2 expression via inhibition of NAD(P)H oxidase: implications for protection against endotoxic shock in rats. FASEB J. 2005;19:1890–1892. doi: 10.1096/fj.04-2368fje. [DOI] [PubMed] [Google Scholar]
- Franklin WA, Veve R, Hirsch FR, Helfrich BA, Bunn PA., Jr Epidermal growth factor receptor family in lung cancer and premalignancy. Semin Oncol. 2002;29:3–14. doi: 10.1053/sonc.2002.31520. [DOI] [PubMed] [Google Scholar]
- Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, Kris MG, Varmus H. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerner JL, Biscardi JS, Silva CM, Parsons SJ. Transactivating agonists of the EGF receptor require Tyr 845 phosphorylation for induction of DNA synthesis. Mol Carcinog. 2005;44:262–273. doi: 10.1002/mc.20138. [DOI] [PubMed] [Google Scholar]
- Kikuchi A, Yamaya M, Suzuki S, Yasuda H, Kubo H, Nakayama K, Handa M, Sasaki T, Shibahara S, Sekizawa K, Sasaki H. Association of susceptibility to the development of lung adenocarcinoma with the heme oxygenase-1 gene promoter polymorphism. Hum Genet. 2005;116:354–360. doi: 10.1007/s00439-004-1162-2. [DOI] [PubMed] [Google Scholar]
- Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R, Alam J, Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003;371:887–895. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu BJ, Kathir K, Witting PK, Beck K, Choy K, Li C, Croft KD, Mori TA, Tanous D, Adams MR, Lau AK, Stocker R. Antioxidants protect from atherosclerosis by a heme oxygenase-1 pathway that is independent of free radical scavenging. J Exp Med. 2006;203:1117–1127. doi: 10.1084/jem.20052321. [DOI] [PMC free article] [PubMed] [Google Scholar]