Abstract
Tumor progression is regulated through paracrine interactions between tumor cells and stromal cells in the microenvironment, including endothelial cells and myofibroblasts. Nitric oxide (NO) is a key molecule in the regulation of tumor-microenvironment interactions, although its precise role is incompletely defined. By using complementary in vitro and in vivo approaches, we studied the effect of endothelial NO synthase (eNOS)-derived NO on liver tumor growth and metastasis in relation to adjacent stromal myofibroblasts and matrix because liver tumors maintain a rich, vascular stromal network enriched with phenotypically heterogeneous myofibroblasts. Mice with an eNOS deficiency developed liver tumors more frequently in response to carcinogens compared with control animals. In a surgical model of pancreatic cancer liver metastasis, eNOS overexpression in the tumor microenvironment attenuated both the number and size of tumor implants. NO promoted anoikis of tumor cells in vitro and limited their invasive capacity. Because tumor cell anoikis and invasion are both regulated by myofibroblast-derived matrix, we explored the effect of NO on tumor cell protease expression. Both microarray and Western blot analysis revealed eNOS-dependent down-regulation of the matrix protease cathepsin B within tumor cells, and silencing of cathepsin B attenuated tumor cell invasive capacity in a similar manner to that observed with eNOS overexpression. Thus, a NO gradient within the tumor microenvironment influences tumor progression through orchestrated molecular interactions between tumor cells and stroma.
Epithelial tumors constitute a constellation of not only neoplastic cells, but also a tumor-associated stroma comprised of endothelial cells, myofibroblasts, as well as extracellular components including cytokines and matrix.1 Indeed, it is becoming increasingly recognized that tumor stroma importantly influences tumor growth.1 The tumor microenvironment in liver is unique from other organ beds in that the hepatic vasculature is enriched in resident pericytes, termed hepatic stellate cells,2,3,4 which are activated into myofibroblasts in response to cytokine stimulation, such as that which occurs in tumors.5
Nitric oxide (NO) is a multifunctional signaling molecule with potentially high bioavailability in the tumor microenvironment, owing to its potential for generation from endothelial NO synthase (eNOS) within endothelial cells (ECs), a prevalent cell type within tumors in vivo.6 Although eNOS has been shown to promote angiogenesis and vascular permeability through autocrine actions on endothelial cells, its effects on tumor cell interactions with stromal myofibroblasts has not been explored, especially in the complex liver microenvironment where NO manifests diverse effects on various liver cell populations.3,7,8,9,10,11
In this study, we use a series of complementary tumor models to better determine the effects of eNOS-derived NO on tumor cell interaction with stromal myofibroblasts and how these interactions influence tumor growth. We demonstrate that NO limits metastatic implantation and tumor development in liver in complementary models in vivo. This is achieved by reducing tumor cell resistance to anoikis and invasive capacity, functions that are mediated by interaction of tumor cells with myofibroblast-derived stromal matrix. Furthermore, we identify down-regulation of the matrix protease, cathepsin B, as an integral step that mediates this process. Thus, NO limits tumor progression in liver by down-regulating matrix-degrading proteases that are responsible for tumor cell implantation and tumor progression, thereby identifying a new role for NO in tumor cell interactions with adjacent stromal cells.
Materials and Methods
Microscopic X-Ray Computed Tomography (Micro-CT) Analysis of Tumor Vasculature of Albumin-SV40 T-Antigen Transgenic Rats
All animal procedures were performed in accordance with the Institutional Animal Care and Use Committee guidelines of Mayo Clinic. Nine-month-old female albumin-SV40 T antigen transgenic rats, which develop spontaneous liver tumors,12,13 were used for this study. Approximately 10 minutes before laparotomy, rats were given 0.1 μg/g heparin sulfate. After laparotomy, the aorta was exposed, cannulated retrograde with PE 50 tubing, and ligated above the celiac trunk. Saline, containing 10 IU/ml heparin was infused through the aortic cannula at a rate of 4 ml/minute. When the hepatic vasculature was cleared of blood, the infusion was continued with 10% buffered formalin for 6 to 10 minutes. The formalin was flushed from the vasculature with 0.9% saline for 10 to 15 minutes. Silicone polymer containing lead chromate (Microfil; Flow Tech, Inc., Carver, MA) was infused at a constant pressure between 120 to 130 mmHg. At the completion of the infusion procedure and after the silicone had polymerized, liver was removed and placed in 10% buffered formalin. Liver tissue was encased in paraffin and scanned at 20-μm voxel resolution using a bench-top scanner.14 Reconstructed images were studied using Analyze 7.0 software (Biomedical Imaging Resources; Mayo Clinic, Rochester, MN). After the scans, the tissue was removed from the paraffin encasements, dehydrated, and embedded in paraffin for complementary histology analysis (see below).
Generation of Mouse Liver Metastasis by Portal Vein Injection of Tumor Cells
Mouse liver metastasis was induced by portal vein implantation of tumor cells as we had done previously.15 In brief, under aseptic technique, SCID mice (CB-17/1cr-Prkdcscid/Crl; Charles River, Wilmington, MA) were anesthetized with pentobarbital (50 μg/g) and a small midline laparotomy was performed. Tumor cells were suspended in 1× phosphate-buffered saline (PBS) at a density of 1 × 107 cells/ml and 100 μl of tumor cells were injected into mouse portal vein. Hemostasis was attained by direct pressure and the abdominal incision was closed. Mice were sacrificed 1 week or 2 weeks after implantation. Microscopic micrometastases were analyzed by staining liver sections with hematoxylin and eosin (H&E), LacZ staining for L3.6-LacZ-derived metastasis, and eNOS immunofluorescence for L3.6-eNOS-derived metastasis. Gross metastases were isolated and total mass was calculated by weight. The same protocol was used to generate mouse liver metastasis using L3.6 shRNA Plk0.1-cathepsin B-Puro cells and L3.6 empty vector Plk0.1-Puro cells (Sigma, St. Louis, MO), and control cells.
Diethylamine (DEA)-Induced Liver Carcinogenesis
eNOS−/− and littermate control mice eNOS+/+ mice were injected at the age of 15 days with 10 mg/kg of DEA (N-diethylnitrosamine) or saline solution, intraperitoneally, to induce liver tumors using a protocol previously described.16 Animals were sacrificed after 14 months or earlier if they appeared unhealthy because of extensive tumor burden. After sacrifice, incidence of tumorigenesis was assessed by counting the number of tumor-bearing animals per group, and evidenced tumor nodules were measured by a caliber followed by histochemical analysis.
Immunofluorescent Staining and Confocal Microscopy
Mouse liver samples were snap-frozen in OCT embedding compound and 7-μm frozen sections were obtained. After sections were fixed with 4% paraformaldehyde or acetone for 10 minutes, immunostaining was performed with primary antibodies recognizing CD31/PECAM-1 (BD Pharmingen, Franklin Lakes, NJ), eNOS (Transduction Laboratories, Lexington, KY), α-smooth muscle actin (SMA) (Novocastra Laboratories Ltd., Newcastle upon Tyne, UK), desmin (Calbiochem, La Jolla, CA), and NG2 (Chemicon International, Temecula, CA). For immunofluorescent double staining, anti-mouse IgG and anti-rabbit IgG conjugated with different Alexa fluorochromes were used as secondary antibodies (Molecular Probes, Eugene, OR). Nuclear dye Toto-3 was used as 1:1000 for counterstaining. For triple-immunofluorescent staining, sections were first incubated with Alex Fluor 488-labeled anti-SMA (Microscale protein labeling kit, Molecular Probes). After washing and blocking with mouse IgG blocking reagent (M.O.M. kit; Vector Laboratories, Burlingame, CA), fluorescent double staining described above, was resumed. Confocal microscopy was performed by a LSM 5 Pascal (Carl Zeiss, Thornwood, NY) in which appropriate laser and filter combinations were selected according to excitation and emission spectrum features of the Alexa fluorochromes.
Cell Culture and Generation of Stably Transduced Cell Lines
L3.6 pancreatic cancer cells were grown in minimal essential medium supplemented with essential vitamins, l-glutamine, sodium pyruvate, and streptomycin/penicillin. LacZ and eNOS cDNA were subcloned into the retroviral vector pMMP to generate pMMP-LacZ and pMMP-eNOS using standard molecular approaches.17 All constructs were sequenced for confirmation. To generate retroviruses, 5 × 106 293T/17 cells in 100-mm dishes were co-transfected with three plasmids, 1.5 μg of pMD.MLV gag.pol, 0.5 μg of pMD.G, and 2 μg of relevant retroviral vector using Effectene transfection reagent (Qiagen, Valencia, CA). Medium containing retroviruses was collected 48 hours after transfection. To transduce L3.6 cells, 2 ml of viral stock and 8 ml of fresh Dulbecco’s modified Eagle’s medium were mixed and added to 10-mm dishes of 1 × 106 cells supplemented with 8 μg/ml of polybrene. Transduction efficiency using this approach was uniformly greater than 90%. Cells were used for experiments 18 to 24 hours after viral transduction. To generate cells stably transduced with shRNA, L3.6 cells were transfected with an expression vector encoding cathepsin B shRNA Plk0.1-cathepsin B-Puro, and transfected cells were enriched by using puromycin (5 μg/ml) selection for ∼2 weeks. Cells transfected with empty vector Plk0.1-Puro (Sigma) were used as control cells. Knockdown of cathepsin B protein in puromycin selected cells was confirmed by Western blot analysis using anti-cathepsin B (AHP591; Serotech, Oxford, UK).
Cell Suspension Cultures and Anoikis Assay
The susceptibility of cells to anoikis as result of substratum detachment was assessed by using standard techniques.18 Tumor cell suspension cultures were performed by plating cells in Petri dishes that had been previously coated with polyhydroxyethylmethacrylate (poly-HEMA). Poly-HEMA-coated dishes were prepared by two applications of a 10-mg/ml solution of poly-HEMA in 95% ethanol onto Petri dishes and washed three times with 1× PBS before use. L3.6-LacZ and L3.6-eNOS cells were detached from tissue culture plates by trypsin/ethylenediaminetetraacetic acid, seeded onto 100-mm poly-HEMA-coated Petri dishes (1 × 106 cells/dish), and cultured in medium containing reduced concentration of serum (0.1% fetal bovine serum) with gentle shaking (100 rpm/minute). Cell suspensions were harvested and assessed for apoptosis by morphological analysis for detection of nuclear 4,6-diamidino-2-phenylindole (DAPI) (5 μg/ml) as we have previously done.19 All cells (500 to 1000) in multiple random fields were counted from three separate cell preparations, with characteristic DAPI-stained nuclei being defined as positive for apoptosis. Cell suspensions were also analyzed by Western blot analysis for cleaved caspase-3 (data not shown).
Western Blot Analysis and Antibodies
Cultured cells or mouse tissues were lysed in RIPA buffer (1× PBS, 1% Nonidet P-40, 0.5% sodium deoxychoate, 0.1% sodium dodecyl sulfate) supplemented with Protease inhibitors (Roche, Indianapolis, IN). After cleared of cell debris, samples were quantified and samples containing equal amounts of protein were loaded for sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Proteins immobilized onto nitrocellulose membranes were detected with various antibodies recognizing eNOS (BD Transduction Laboratory), cathepsin B (AHP591, Serotech), proliferating cell nuclear antigen (BD Transduction Laboratory), CD31 (BD Pharmingen), and β-actin (A5441, Sigma).
Superarray Analysis
Total RNA was isolated from L3.6-LacZ or L3.6-eNOS cells using the RNeasy kit according to the manufacturer’s instruction (Qiagen).20 Three μg of RNA was used for the probe preparation using the GEArray AmpoLabeling-LPR kit and biotin-16-2′-deoxy-uridine-5′-triphosphate. GEArray Q Series (SuperArray Bioscience Corp., Frederick, MD) human metastasis gene array (HS-036) membrane was used for hybridization with the synthesized probe and detected by a chemiluminescent detection kit according to the manufacturer’s protocol. Changes in expression were assessed by the software provided by the manufacturer.
In Vitro Tumor Cell Invasion Assay
The capacity of tumor cell invasion and pass through matrix was measured in vitro by a modified Boyden chamber invasion assay. In brief, 10 μl of rat collagen I stock solution (60 to 70 μg/ml) or Matrigel (1:1 dilution in Dulbecco’s modified Eagle’s medium) was added to the upper chamber and incubated at 37°C for 30 minutes so that the bottom of upper wells was covered by a thin layer of Matrigel or collagen I gel. Tumor cells suspended in serum-free medium were seeded to upper wells (20,000 cells/well) and lower chambers were filled with 26 μl of serum-free medium with or without chemoattractant epidermal growth factor (EGF) (25 ng/ml). After overnight incubation at 37°C the polycarbonated filter was removed and stained with HEMA-3, cells passed through the matrix were quantified from random microscopic fields as we have previously done.3 Each experiment was repeated at least three times with six replicates of each group per experiment.
Statistical Analysis
The data in bar graphs represent the mean ± SD of at least three independent experiments. Blots, autoradiographs, and micrographs represent typical experiments reproduced at least three times with similar results. Statistical analyses were performed by two-tailed Student’s t-test or two-way analysis of variance if two factors were involved (Graphpad Prism 4; Graphpad Software, San Diego, CA). Kaplan Meier survival curves were generated and analyzed by using Graphpad Prism 4. A value of P < 0.05 was considered statistically significant. The χ2 test was used to compare observed tumor frequency to expected tumor frequency. The Mann-Whitney test was used for nonparametric analysis of data that did not follow normal distribution.
Results
Microenvironment of liver tumors in SV40 T-antigen transgenic rats.
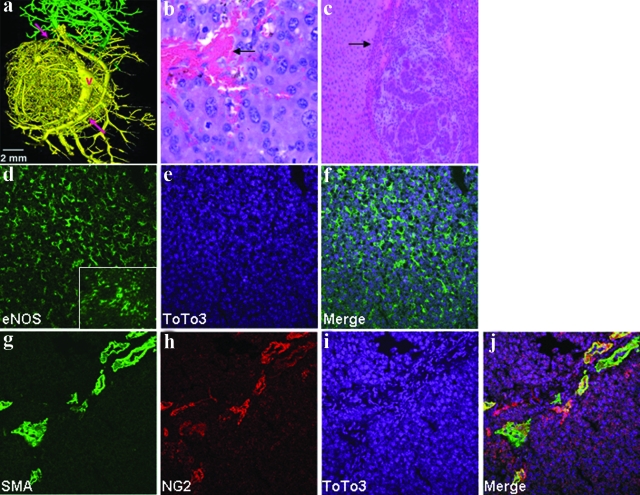
As an initial step to evaluate the microenvironment of liver cancers, we pursued micro-CT analysis, which allows a detailed examination of the vascular compartment.14 This analysis was performed in the SV40 T-antigen transgenic rat, a well-characterized model of primary hepatic neoplasia.13 A rat model was chosen for this analysis because micro-CT analysis was not technically feasible in the mouse models at this time. As seen in Figure 1a, micro-CT analysis evidenced marked derangements in the tumor vasculature in these rats, characterized by disorganized and tortuous vessels with atypical branching patterns (tumor vessels in yellow compared to normal liver sinusoidal vessels in green). At the level of H&E (Figure 1b), tumors that formed in SV40 rats were highly vascularized; often vascular lakes were found in the mass of the large tumor, reflecting the high permeability of the tumor vessels. Tumor microenvironment in liver is distinct from other sites owing to a rich pericyte network within the liver that evolves into a myofibroblast phenotype in the context of hepatic neoplasia.21 These cells respond to NO signals from ECs, to regulate hepatic vascular structure and function. Tumor myofibroblasts and stromal compartment were examined in the SV40 rat model by H&E, as shown in Figure 1c, that shows a rim of stromal network surrounding the tumor. Immunohistochemically, tumor ECs were positive for eNOS (Figure 1, d–f), with no apparent reduction compared to surrounding normal liver sinusoidal ECs (Figure 1d, boxed inset). Interestingly, tumor myofibroblasts showed phenotypic heterogeneity based on partial but not complete co-localization of NG2 and SMA (Figure 1, g–j). This was examined in greater detail in subsequent tumor models in mice.
Figure 1.
Albumin-SV40 Tag rats evidence highly vascular primary liver tumors in phenotypically and heterogeneous myofibroblasts. a: Micro-CT analysis of tumor vasculature demonstrates marked derangement of tumor blood vessels including tortuous vessels with atypical branching patterns (yellow). Arrows point to parallel arrangement of arterial and portal venous vascular supply and V indicates vein draining the tumor. Normal liver vascular tree is shown in green. b: Light microscopy of H&E-stained sections of primary liver tumors in albumin-SV40 Tag rats shows prominent tumor blood vessels among neoplastic cells that display polymorphic hyperchromatic nuclei with prominent nucleoli. c: Lower magnification view shows normal hepatocytes on the left with multiple neoplastic foci surrounded by a rim of stromal network (arrow). d–f: Fluorescent confocal microscopy of frozen sections from the primary tumor shows positive staining of eNOS, which reflects the high vascularity of the tumor. eNOS staining of the surrounding normal hepatic tissue is shown in the bottom right corner. Double-immunofluorescent staining (g–j) with myofibroblast markers (SMA and NG2) reveals co-localization of the markers in some cells but with some cells displaying only one of the two markers. Scale bar = 2 mm. Original magnifications: 40× (b); 10× (c).
Influence of eNOS on tumor growth in liver.
Because eNOS-derived NO is a key signaling molecule within the liver microvasculature that importantly regulates liver pericytes,3,21 we next explored the effects of NO on liver tumor growth, progression, and implantation in liver. First, eNOS−/− mice or littermate control eNOS+/+ mice, were exposed to the carcinogen DEA in the perinatal period, and tumor size was examined after 14 months. In this model, light microscopy of sections stained by H&E revealed tumor foci showing cellular neoplastic changes in the form of finely granular eosinophilic cytoplasm with polymorphic hyperchromatic nuclei (Figure 2a, liver section from eNOS−/− mouse is shown). The desmoplastic reaction in this model was discernible but not so prominent (Figure 2b). Staining for eNOS revealed no detection in the eNOS−/− tumor model (as expected) compared to the prominent tumor EC staining in the eNOS+/+ model (Figure 2c, inset). Immunofluorescent staining of tumor-associated myofibroblasts with NG2, SMA, and desmin again showed heterogeneity of these pericyte/myofibroblast markers (Figure 2, d–i). However, less staining intensity and co-localization is evident in this model compared to the SV40 rat model, perhaps because of different stages of tumor differentiation. The frequency of DEA-induced tumorigenesis was increased in eNOS−/− mice (15 of 17) as compared to eNOS+/+ mice (7 of 14) and was associated with a threefold greater cumulative tumor burden in eNOS−/− mice (Figure 2j).
Figure 2.
Increased tumor incidence in eNOS−/− mice as compared to eNOS+/+ mice. eNOS+/+ and eNOS−/− mice were injected at the age of 15 days with DEA carcinogen (10 mg/kg) or saline as control. Animals were sacrificed after 14 months. DEA-induced tumors were assessed by counting and measuring tumor nodules in mouse liver followed by H&E staining. a: Light microscopy of H&E-stained section of liver tumor in eNOS−/− mice showing part of the normal liver tissue (top) and neoplastic tissue (bottom) with hyperchromatic nuclei and cellular and nuclear polymorphism. b: In lower magnification image an arrow points to desmoplastic reaction at the interface between normal liver tissue (left) and the tumor foci (top right). c: Immunofluorescent staining of eNOS in eNOS−/− mice showed negative staining compared to the positive staining in the eNOS+/+ mice shown in the bottom right corner. d–i: Fluorescent confocal images after staining frozen sections of the eNOS−/− mice with myofibroblast markers such as NG2, SMA, and desmin showed positivity for the three markers. Although some co-localization was noticed between some markers many of the cells displayed only one of the markers. j: eNOS deficiency resulted in a higher tumor incidence in response to DEA challenge (50% in eNOS+/+ mice and 89% in eNOS−/− mice, P < 0.01; n = 18 eNOS−/− mice and n = 15 eNOS+/+ mice). Original magnifications: 20× (a); 10× (b).
To explore the aforementioned observations in more mechanistic detail, we pursued further studies in the surgical model of liver metastasis to liver. L3.6 pancreatic cancer cells were transduced with LacZ control or bovine eNOS using replication-defective retroviruses. The retroviral transduction system resulted in nearly 100% transfection efficiency as we have previously described.22 As seen in the left panel of Figure 3a, L3.6-LacZ-transduced cells stained positive for LacZ (top right), whereas L3.6-eNOS cells evidenced green fluorescence when analyzed for eNOS expression with a fluorescently labeled secondary antibody (bottom right). eNOS overexpression by Western blot was also prominently detected in L3.6-eNOS cells (Figure 3b). When L3.6-LacZ cells and L3.6-eNOS cells attached and grew on plates, they proliferated at a similar rate in vitro indicating that eNOS did not affect basal tumor cell proliferation thereby allowing us to subsequently explore the effects of tumor microenvironment on tumor growth in vivo using these cells (Figure 3c).
Figure 3.
Characterization of human pancreatic cancer cells with retroviral transduction. a: L3.6 cells were transduced with retroviruses encoding LacZ or eNOS and transduction efficiency was assessed by LacZ staining (blue, top) and eNOS immunofluorescence (green, bottom). Nearly 100% of cells were transduced to express respective transgenes. High-power images are shown in insets at the bottom demonstrating perinuclear distribution of eNOS within L3.6 cells. Nuclei were counterstained with Hoechst 33342 (blue). Nontransduced controls are shown as isolated. b: Expression of eNOS protein was confirmed by Western blot analysis using anti-eNOS antibody. β-Actin levels are shown for protein loading control. c: L3.6-eNOS and L3.6-LacZ exhibited comparable proliferation rates as analyzed by MTS assay when cultured in serum-containing media. d–i: Fluorescent confocal microscopy evidenced a close relationship of tumor blood vessel endothelial cells and pericytes. Frozen sections of liver metastasis were doubled-stained with anti-CD31 or eNOS antibodies (endothelial cell markers) and anti-NG2 (pericyte marker). j–o: Heterogeneity of pericytes is demonstrated by fluorescent staining of pericyte/fibroblast markers such as NG2, SMA, and desmin. Double-immunofluorescent staining (j–o) or triple-immunofluorescent staining (p–s) was performed on frozen sections and fluorescent confocal images were captured. Pericytes were differentiated as expressing single markers (red, green, or blue), double markers (yellow), or all three markers (white in s). Although some co-localization is detected, many of the cells display prominent phenotypic heterogeneity. t: Light microscopy of H&E-stained section of liver metastasis showing the tumor tissue (center) marked by increased basophilia because of hyperchromatic nuclei and part of normal liver tissue (top right). It also displays a prominent desmoplastic reaction (arrow) in the interface between normal liver tissue and the tumor. Original magnification, 10× (t).
After characterizing the in vitro transduction and proliferation characteristics of the L3.6-LacZ and L3.6-eNOS cells, we next used these cells in a model of liver metastasis. L3.6-LacZ or L3.6-eNOS cells (1 × 106) were injected into the portal vein of SCID mice, and tumors were analyzed histopathologically and for quantitative tumor burden. First, tumor myofibroblasts were examined immunohistochemically in this model to compare and contrast the observations of this metastatic model to that of the aforementioned primary liver tumor models, especially because pancreatic cancer metastasis to liver is a highly desmoplastic tumor enriched with myofibroblasts.23 As seen in Figure 3, d–i, tumor myofibroblasts, which stain positively for the pericyte myofibroblast marker NG2, reside in close apposition to tumor ECs that stain positively for CD31 and eNOS, especially within a stromal network that surrounds tumor nodules (TOTO-3 staining of tumor cell nuclei was removed from images to allow better delineation of stromal cell network).24 Interestingly, co-localization studies revealed significant heterogeneity of various pericyte/myofibroblast markers within the tumor microenvironment (Figure 3, j–o). For example, although pools of NG2-, SMA-, and desmin-positive cells did co-localize, there were quite distinct pools of cells that did not co-localize. This is particularly evident in the triple stain analysis of all three markers (Figure 3, p–s), indicating that pericyte pools within the tumor microenvironment may reside at different stages in the process of myofibroblast differentiation or may derive from alternative phenotypic lineages. Furthermore, the desmoplastic reaction generated by myofibroblasts, was quite prominent in the pancreatic metastatic model (Figure 3t), especially as compared to the other two models (Figures 1c and 2b). This may reflect the different role of myofibroblasts in the distinct tumor microenvironments of primary and metastatic liver tumors, or alternatively may reflect the influence of pancreatic versus hepatocellular origin of tumor cells.
As seen in Figure 4A, mice injected with L3.6-eNOS tumor cells evidenced smaller liver tumor mass as compared to mice injected with L3.6-LacZ cells. Similar results were obtained when data were analyzed as liver weight:body weight ratio (data not shown). This corresponded with a statistically significant improvement in survival in mice injected with L3.6-eNOS cells (Figure 4B). However, a marker of vascular density, CD31/PECAM-1, did not evidence significant changes between tumors from L3.6-LacZ cells or L3.6-eNOS cells (Figure 4C), suggesting that the inhibitory effects of eNOS on tumor cell growth may be occurring before initiation of the angiogenic switch.
Figure 4.
Tumor cells overexpressing eNOS form smaller metastatic lesions. L3.6-LacZ and L3.6-eNOS cells were implanted into SCID mouse liver via portal vein injection. Micrometastases formed in 1 week and gross metastases formed in 2 weeks. A: Two weeks after implantation metastases were isolated from each mouse liver and total tumor weight was measured. Representative photographs of liver tumors are depicted (top). L3.6-eNOS cells formed smaller liver metastasis in mice compared to L3.6-LacZ cells (bottom) (n = 15 animals per group, *P < 0.01). B: Kaplan-Meier survival plot of SCID mice implanted with L3.6-LacZ and L3.6-eNOS cells. eNOS expression in tumor cells extended median survival of mice (n = 13 mice per group, P < 0.05). C: Western blot analyses confirmed that liver metastases were derived from L3.6-eNOS. However, eNOS expression in tumor cells did not alter CD31 expression level. β-Actin levels were also shown for equal protein loading control. D: One week after implantation of L3.6-eNOS or L3.6 LacZ cells in portal vein of SCID mice, mouse liver frozen sections were stained for LacZ or eNOS and micrometastases were quantified by microscopy. L3.6-LacZ-derived metastases are blue (top left, LacZ staining) and L3.6-eNOS-derived tumors are green (top right, eNOS immunofluorescence). Micrometastatic density was significantly lower in tumors derived from L3.6-eNOS cells as compared to L3.6-LacZ cells (n = 6; *P < 0.05 by t-test).
To examine this possibility further, we performed additional analyses in animals that were sacrificed at 1 week after transplantation of tumor cells, a time point at which macrometastatic lesions were not detected and which corresponded with events before the angiogenic switch. Micrometastatic lesions were easily discernible on histological analysis at this time point (Figure 4D). Tumors from mice injected with L3.6-eNOS cells were positive for eNOS expression in tumor metastatic foci (right micrograph), whereas tumors injected with L3.6-LacZ cells were positive for LacZ staining (left micrograph) (Figure 4D, top). Also as seen in Figure 4D (bottom), micrometastatic density was significantly reduced in L3.6-eNOS cell-derived tumors as compared to L3.6-LacZ tumors. These studies suggested that the effects of eNOS on tumor cells may occur at the level of implantation of the tumor and also point out an important interplay between tumor cell growth, NO, and tumor myofibroblasts, which was subsequently elucidated in further in vitro studies.
NO promotes anoikis of tumor cells. Next, we pursued more mechanistically how eNOS-derived NO may regulate tumor cell implantation especially because stromal myofibroblasts deposit matrix constituents that importantly regulate tumor cell growth.25,26 Because a major step for successful tumor cell implantation from the vasculature into the organ parenchyma relates to the ability of tumor cells to gain resistance to anoikis,18 that is the ability to grow independent of myofibroblast-derived matrix, we next examined resistance to anoikis of L3.6-LacZ cells and L3.6-eNOS cells. Cells were maintained in suspension, and cell death was analyzed morphologically by DAPI staining. As seen in Figure 5A, L3.6-eNOS cells evidenced increased anoikis as compared to L3.6-LacZ cells (Figure 5A, apoptotic cells showing positive DAPI stain are marked by arrows). These data were also confirmed using Western blot analysis for levels of cleaved caspase 3 from cell lysates (data not shown). This effect was specific for apoptosis in response to matrix-independent suspension, because L3.6-eNOS cells and L3.6-LacZ cells, did not evidence differences in proliferation or apoptosis in response to serum deprivation while plated in culture by Western blot analysis for proliferating cell nuclear antigen and cleaved caspase 3 (data not shown).
Figure 5.
The resistance to anoikis and in vitro invasion capability are reduced in L3.6-eNOS cells. Cell suspension cultures were performed on HEMA-coated Petri dishes in medium containing reduced concentration of serum and with gentle shaking. A: Cell suspensions were harvested and assessed for apoptosis by morphological analysis of nuclear DAPI staining (5 μg/ml), based on counting cells (500 to 1000) in random fields (n = 3). Cell suspension cultures from L3.6 eNOS cells show more apoptotic nuclei than L3.6 LacZ cells (apoptotic nuclei are shown by the arrows, top). The bar chart (bottom) quantifies cumulative data from three independent experiments (*P < 0.01). B: Tumor cell ability to invade and cross a thin layer of Matrigel in the presence and absence of EGF (25 ng/ml) was measured by using a modified Boyden chamber cell invasion assay. The number of cells that invaded across the Matrigel in the presence of EGF was highly significantly reduced in L3.6-eNOS cells as compared to L3.6-LacZ cells (*P < 0.001) (three independent experiments with six replicates of each group per experiment).
To determine whether the effects of eNOS on L3.6 tumor cell anoikis corroborated with reduction in invasion, another key step in the process of implantation that is influenced by myofibroblast matrix, invasion of L3.6-eNOS cells was compared to L3.6-LacZ cells across Matrigel. L3.6-eNOS cells evidenced reduced cell invasion, both basally and in response to EGF (Figure 5B). Similar results were obtained using collagen I as well although the EGF response was not as robust with collagen I (data not shown). Thus, eNOS overexpression in L3.6 cells reduces metastatic foci and density in vivo in correlation with promoting anoikis and reducing cell invasion in vitro, both steps that are integrally related to myofibroblast-derived matrix. Thus, these studies support a model whereby NO gradients within the tumor microenvironment regulate the ability of tumor cells to implant and invade into the matrix of the metastatic target organ.
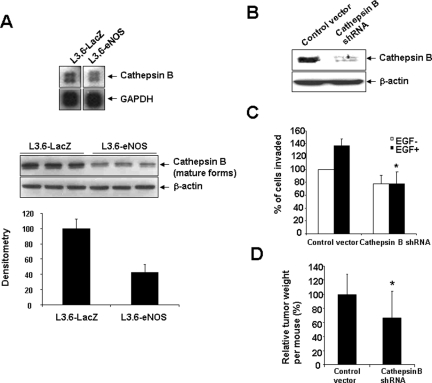
Next, we explored mechanisms by which myofibroblast-derived matrix might contribute to diminished invasion and increased susceptibility to anoikis in L3.6-eNOS cells as compared to L3.6-LacZ cells by using a focused microarray approach. Total RNA was extracted from cells and various genes important in the process of metastasis were examined using a spotted microarray. Although several genes varied in their expression between L3.6-eNOS cells compared to L3.6-LacZ cells (data not shown); in particular, the matrix protease, cathepsin B was down-regulated in L3.6-eNOS cells (Figure 6A, top). Furthermore, Western blot analysis confirmed this observation, evidencing a reduction in cathepsin B levels from lysates of L3.6-eNOS cells compared to L3.6-LacZ cells (Figure 6A, bottom, shows triplicate samples from independent cell lysates).
Figure 6.
Cathepsin B is down-regulated in L3.6-eNOS cells, limiting tumor cell invasion capacity. A: Total RNA was extracted from L3.6-LacZ or L3.6-eNOS cells, labeled, and hybridized to a focused array membrane. Cathepsin B mRNA level was down-regulated in L3.6-eNOS cells compared to L3.6-LacZ cells. GAPDH was used as control (top). Western blot analysis confirmed down-regulation of cathepsin B in L3.6-eNOS cells (bottom, triplicate samples are shown from separate cells). Result of densitometrical analysis of the blot is also shown. B: Cathepsin B was efficiently knocked-down by transfection with cathepsin B shRNA vector. C: L3.6 cell invasion capacity was reduced by cathepsin B shRNA transfection as assessed by the modified Boyden chamber cell invasion assay. In the presence or absence of EGF, the number of cells that invaded and crossed collagen I gel was significantly reduced in cathepsin B shRNA-transfected cells as compared to control cells (three independent experiments with six replicates of each group per experiment, *P < 0.05 by two-way analysis of variance). D: Cathepsin B shRNA-transfected L3.6 cells or control vector-transfected cells were implanted into the portal vein of SCID mice then sacrificed after 2 weeks. Gross liver metastasis were isolated and weighed and tumor mass of cathepsin B shRNA-transfected L3.6 cells was normalized to the control group. Cathepsin B shRNA-transfected L3.6 cells generated significantly less macrometastasis than control cells (n = 7; *P = 0.04, Mann-Whitney test).
NO-dependent loss of cathepsin B reduces pancreatic cancer cell-invasive capacity. Because cathepsin B is a matrix-cleaving enzyme implicated in cell-invasive capacity,27 we postulated that deficiency in this protease may contribute to the reduced invasive capacity in vitro and reduced tumor growth in vivo observed in eNOS-overexpressing cells. To test this concept, we used a shRNA approach to directly examine the effect of cathepsin B depletion on tumor cell-invasive capacity. L3.6 cells were stably transfected with control vector or cathepsin B shRNA and invasive capacity through collagen I gels was examined in response to EGF (25 ng/ml). Cathepsin B shRNA-transfected cells evidenced significant depletion of cathepsin B protein levels (Figure 6B), and also demonstrated impaired invasion both basally and in response to EGF stimulation as compared to cells transfected with control vector (Figure 6C).
Finally, to recapitulate these in vitro studies in the in vivo model, we implanted cathepsin B shRNA or control vector-transfected L3.6 cells into the portal vein of SCID mice. Mice were sacrificed 2 weeks later and gross liver metastases were isolated and weighed. Cathepsin B shRNA-transfected L3.6 cells generated significantly less metastatic tumor burden compared to the control L3.6 cells (n = 7, *P < 0.05 by Mann-Whitney test) (Figure 6D).
Discussion
Tumor cells are closely interpositioned with NO-generating ECs as well as with myofibroblasts and the matrix that they deposit.1 Indeed, tumor cells are highly dependent on extracellular matrix because disruption of cell-matrix contacts triggers anoikis, ultimately leading to caspase-dependent cell death. Furthermore, matrix also importantly regulates the ability of tumor cells to implant and invade into target tissues. Thus, resistance to anoikis and invasion are key steps in the metastatic process that are influenced by tumor cell interaction with myofibroblast-deposited matrix. The present study reveals a novel role of eNOS-derived NO in the process of regulating tumor cell anoikis and invasion in vitro, which culminates in diminished tumor progression in vivo. Mechanistically, this was achieved by NO-dependent decrease in tumor cell protein levels of cathepsin B, a multifunctional protease that is capable of degrading several components of tumor stroma, and activating zymogens of matrix metalloproteinases.27 Indeed, depletion in cellular levels of this key matrix protease within tumor cells, resulted in decreased tumor cell invasion through matrix. Thus, these studies highlight important NO-dependent paracrine interplay between tumor cells and stromal cells that act in concert to regulate tumor progression through a coordinated balance of matrix proteolysis (Figure 7).
Figure 7.
Summary mechanism. NO within the tumor microenvironment regulates tumor cell interactions with myofibroblasts and the matrix that they generate. This includes NO reduction in tumor cell resistance to anoikis and cathepsin B-dependent impairment of tumor cell invasion capacity. This cross-talk between tumor cells, NO, and stromal cells limits liver tumor growth.
Although EC-lined vascular networks that support tumor growth have traditionally been targeted for therapeutic anti-angiogenesis, pericytes have also recently been recognized as a compelling target for cancer therapy, especially owing to the unique and large pericyte population within liver.21,28 On activation, pericytes transform into myofibroblasts, which are responsible for the matrix deposition that importantly influences tumor cell anoikis and invasion.29,30 The present studies reveal that tumor myofibroblasts evidence significant phenotypic heterogeneity based on divergence of staining of different pericyte/myofibroblast protein markers. Furthermore, the density of myofibroblast infiltration and level of heterogeneity varied between the different tumor models. Although these observations may reflect cells residing at varying stages of transdifferentiation from pericyte to tumor-associated myofibroblast, an alternative interpretation may be that tumor myofibroblasts are derived from phenotypically divergent pools of liver cells (ie, portal fibroblasts versus pericytes), or through epithelial-mesenchymal transformation,31,32 a process by which epithelial cells lose cell-cell contacts and acquire mesenchymal properties and develop an invasive phenotype.33,34,35 Indeed, epithelial cancer cells or even normal epithelial cells adjacent to a tumor, might share a common origin with stromal fibroblasts and/or undergo further genetic rearrangements under a distinct selection pressure that facilitates transition into mesenchymal-like cells.34 These complementary mechanisms may contribute to myofibroblast heterogeneity. Regardless of their origin however, pericyte transformation into a tumor-associated myofibroblast creates important matrix-based paracrine signaling interactions with tumor cells themselves.
Owing to the close apposition of tumor cells, ECs, and myofibroblasts within the tumor microenvironment, we postulated that NO derived from ECs by virtue of eNOS is likely to initiate important paracrine signaling that would regulate tumor growth and progression. Of course eNOS contributes to angiogenic EC signaling,8,22 however, previous work from us and others has also demonstrated that NO limits hepatic stellate cell migration, motility, and other features of activation that lead pericytes toward a myofibroblast phenotype.3,7 These observations led us to hypothesize that NO may have beneficial effects on tumor progression by attenuating the tumor-progressing effects of myofibroblasts and the matrix derived from these cells. Interestingly, the present studies uncover an additional mechanism by which NO regulates tumor progression by disrupting tumor cell interactions with myofibroblast-derived matrix that determine anoikis and invasion. Our results are supported by the congruent effects of NO in two independent models of liver cancer as well as the mechanistic link to cathepsin B, thus highlighting the proteolytic balance in the tumor microenvironment that influences tumor growth. However, the complexity and diversity of NO signaling pathways that are governed by NOS isoform, cell type-specificity, and other factors within the tumor microenvironment, certainly accommodate the divergent actions of NO that are observed in the process of tumor progression in different models and organ systems.36,37,38,39 Indeed, administration of the NO donor compound, NCX-1000 did not significantly reduce tumor size in our SV40 rat model of hepatocellular carcinoma (data not shown), suggesting a specific role for eNOS-derived NO within the local tumor microenvironment, in the process of reducing metastatic tumor burden associated with tumor cell implantation into the liver vasculature. These observations are reminiscent of those from Wang and colleagues40 whereby the arrest of intravascular B16F1 melanoma cells in the liver induced a rapid local release of NO that causes apoptosis of the melanoma cells and inhibits their subsequent development into hepatic metastases.
In summary, these studies reveal a highly coordinated and integrated relationship between tumor cells and their host microenvironment whereby local NO gradients regulate tumor cell anoikis, implantation, and invasion through balanced interactions of stromal cells, matrix, and the tumor cell protease, cathepsin B. Thus, this study extends the concept that tumor stromal cells are key modifiers of cancer progression.
Footnotes
Address reprint requests to Vijay Shah or Ningling Kang Decker, GI Research Unit, Gu 10-21, Mayo Clinic Rochester, 200 First St. SW, Rochester, MN 55905. Soha S. Abdel-Moneim, Department of Tropical Medicine and Gastroenterology and Hepatology, Assuit University, Assuit, EGYPT. E-mail: shah.vijay@mayo.edu and decker.ningling@mayo.edu.
Supported by the National Institutes of Health (grants R01 DK059615 to V.H.S., R01 HL86990 to V.H.S., and K01 CA118722-01A1 to N.K.D.), the Mayo Clinic (pancreatic cancer SPORE seed grant to V.H.S.), and the American Liver Foundation/American Association for the Study of Liver Diseases (2006 Sheila Sherlock grant to N.K.D.).
References
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- Yang C, Zeisberg M, Mosterman B, Sudhakar A, Yerramalla U, Holthaus K, Lieming X, Eng F, Afdhal N, Kalluri R. Liver fibrosis: insights into migration of hepatic stellate cells in response to extracellular matrix and growth factors. Gastroenterology. 2003;124:147–159. doi: 10.1053/gast.2003.50012. [DOI] [PubMed] [Google Scholar]
- Lee J, Decker N, Chatterjee S, Yao J, Friedman S, Shah V. Mechanisms of nitric oxide interplay with Rho GTPase family members in modulation of actin membrane dynamics in pericytes and fibroblasts. Am J Pathol. 2005;166:1861–1870. doi: 10.1016/S0002-9440(10)62495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parola M, Marra F, Pinzani M. Myofibroblast-like cells and liver fibrogenesis: Emerging concepts in a rapidly moving scenario. Mol Aspects Med. 2008;29(1–2):58–66. doi: 10.1016/j.mam.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Chan EP, Wells RG. Today’s hepatic stellate cells: not your father’s Sternzellen. Hepatology. 2007;45:1326–1327. doi: 10.1002/hep.21725. [DOI] [PubMed] [Google Scholar]
- Farzaneh-Far R, Moore K. Nitric oxide and the liver. Liver. 2001;21:161–174. doi: 10.1034/j.1600-0676.2001.021003161.x. [DOI] [PubMed] [Google Scholar]
- Failli P, DeFranco R, Caligiuri A, Gentilini A, Romanelli R, Marra F, Batignani G, Guerra C, Laffi G, Gentilini P, Pinzani M. Nitrovasodilators inhibit platelet-derived growth factor-induced proliferation and migration of activated human hepatic stellate cells. Gastroenterology. 2000;119:479–492. doi: 10.1053/gast.2000.9354. [DOI] [PubMed] [Google Scholar]
- Gratton J, Lin M, Yu J, Weiss E, Jiang Z, Fairchild T, Iwakiri Y, Groszmann R, Claffey K, Cheng Y, Sessa W. Selective inhibition of tumor microvascular permeability by cavtratin blocks tumor progression in mice. Cancer Cell. 2003;4:31–39. doi: 10.1016/s1535-6108(03)00168-5. [DOI] [PubMed] [Google Scholar]
- Ying L, Hofseth LJ. An emerging role for endothelial nitric oxide synthase in chronic inflammation and cancer. Cancer Res. 2007;67:1407–1410. doi: 10.1158/0008-5472.CAN-06-2149. [DOI] [PubMed] [Google Scholar]
- Chinje EC, Stratford IJ. Role of nitric oxide in growth of solid tumours: a balancing act. Essays Biochem. 1997;32:61–72. [PubMed] [Google Scholar]
- Landar A, Darley-Usmar VM. Nitric oxide and cell signaling: modulation of redox tone and protein modification. Amino Acids. 2003;25:313–321. doi: 10.1007/s00726-003-0019-7. [DOI] [PubMed] [Google Scholar]
- Hully J, Su Y, Lohse J, Griep A, Sattler C, Haas M, Dragan Y, Peterson J, Neveu M, Pitot H. Transgenic hepatocarcinogenesis in the rat. Am J Pathol. 1994;145:384–397. [PMC free article] [PubMed] [Google Scholar]
- Czarny MJ, Babcock K, Baus RM, Manoharan H, Pitot HC. Hepatocellular carcinomas of the albumin SV40 T-antigen transgenic rat display fetal-like re-expression of lgf2 and deregulation of H19. Mol Carcinog. 2007;46:747–757. doi: 10.1002/mc.20286. [DOI] [PubMed] [Google Scholar]
- Jorgensen S, Demirkaya O, Ritman E. Three-dimensional imaging of vasculature and parenchyma in intact rodent organs with x-ray micro-CT. Am J Physiol. 1998;275:H1103–H1114. doi: 10.1152/ajpheart.1998.275.3.H1103. [DOI] [PubMed] [Google Scholar]
- Wendt MK, Johanesen PA, Kang-Decker N, Binion DG, Shah V, Dwinell MB. Silencing of epithelial CXCL12 expression by DNA hypermethylation promotes colonic carcinoma metastasis. Oncogene. 2006;25:4986–4997. doi: 10.1038/sj.onc.1209505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MR, Factor VM, Fantozzi A, Helin K, Huh CG, Thorgeirsson SS. Reduced hepatic tumor incidence in cyclin G1-deficient mice. Hepatology. 2003;37:862–870. doi: 10.1053/jhep.2003.50137. [DOI] [PubMed] [Google Scholar]
- Zeng H, Sanyal S, Mukhopadhyay D. Tyrosine residues 951 and 1059 of vascular endothelial growth factor receptor-2 (KDR) are essential for vascular permeability factor/vascular endothelial growth factor-induced endothelium migration and proliferation, respectively. J Biol Chem. 2001;276:32714–32719. doi: 10.1074/jbc.M103130200. [DOI] [PubMed] [Google Scholar]
- Toruner M, Fernandez-Zapico M, Sha JJ, Pham L, Urrutia R, Egan LJ. Antianoikis effect of nuclear factor-kappaB through up-regulated expression of osteoprotegerin, BCL-2, and IAP-1. J Biol Chem. 2006;281:8686–8696. doi: 10.1074/jbc.M512178200. [DOI] [PubMed] [Google Scholar]
- Langer D, Semela D, Decker N, Hendrickson H, Bronk S, Katusic Z, Gores G, Shah V. Nitric oxide promotes caspase independent hepatic stellate cell apoptosis through generation of reactive oxygen species. Hepatology. 2008;47(6):1983–1993. doi: 10.1002/hep.22285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Fernandez Zapico M, Cao S, Yao J, Fiorucci S, Hebbel R, Urrutia R, Shah V. Disruption of an SP2/KLF6 repression complex by SHP is required for farnesoid X receptor-induced endothelial cell migration. J Biol Chem. 2006;281:39105–39113. doi: 10.1074/jbc.M607720200. [DOI] [PubMed] [Google Scholar]
- Lee JS, Semela D, Iredale J, Shah VH. Sinusoidal remodeling and angiogenesis: a new function for the liver-specific pericyte? Hepatology. 2007;45:817–825. doi: 10.1002/hep.21564. [DOI] [PubMed] [Google Scholar]
- Kang-Decker NCS, Chatterjee S, Yao J, Egan J, Semela D, Mukhopadhyay D, Shah V. Nitric oxide promotes endothelial cell survival signaling through S-nitrosylation and activation of dynamin. J Cell Sci. 2007;120:492–501. doi: 10.1242/jcs.03361. [DOI] [PubMed] [Google Scholar]
- Ghaneh P, Costello E, Neoptolemos JP. Biology and management of pancreatic cancer. Gut. 2007;56:1134–1152. doi: 10.1136/gut.2006.103333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G. PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol. 2005;7:870–879. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ET. Stromal effects in breast cancer. N Engl J Med. 2007;357:2537–2538. doi: 10.1056/NEJMp0707576. [DOI] [PubMed] [Google Scholar]
- Gaça MD, Zhou X, Issa R, Kiriella K, Iredale JP, Benyon RC. Basement membrane-like matrix inhibits proliferation and collagen synthesis by activated rat hepatic stellate cells: evidence for matrix-dependent deactivation of stellate cells. Matrix Biol. 2003;22:229–239. doi: 10.1016/s0945-053x(03)00017-9. [DOI] [PubMed] [Google Scholar]
- Canbay A, Guicciardi ME, Higuchi H, Feldstein A, Bronk SF, Rydzewski R, Taniai M, Gores GJ. Cathepsin B inactivation attenuates hepatic injury and fibrosis during cholestasis. J Clin Invest. 2003;112:152–159. doi: 10.1172/JCI17740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaso E, Salado C, Egilegor E, Gutierrez V, Santisteban A, Sancho-Bru P, Friedman S, Vidal-Vanaclocha F. Proangiogenic role of tumor-activated hepatic stellate cells in experimental melanoma metastasis. Hepatology. 2003;37:674–685. doi: 10.1053/jhep.2003.50068. [DOI] [PubMed] [Google Scholar]
- Theret N, Musso O, Turlin B, Lotrian D, Bioulac-Sage P, Campion JP, Boudjema K, Clement B. Increased extracellular matrix remodeling is associated with tumor progression in human hepatocellular carcinomas. Hepatology. 2001;34:82–88. doi: 10.1053/jhep.2001.25758. [DOI] [PubMed] [Google Scholar]
- Sato M, Suzuki S, Senoo H. Hepatic stellate cells: unique characteristics in cell biology and phenotype. Cell Struct Funct. 2003;28:105–112. doi: 10.1247/csf.28.105. [DOI] [PubMed] [Google Scholar]
- Wells RG, Kruglov E, Dranoff JA. Autocrine release of TGF-beta by portal fibroblasts regulates cell growth. FEBS Lett. 2004;559:107–110. doi: 10.1016/S0014-5793(04)00037-7. [DOI] [PubMed] [Google Scholar]
- Magness ST, Bataller R, Yang L, Brenner DA. A dual reporter gene transgenic mouse demonstrates heterogeneity in hepatic fibrogenic cell populations. Hepatology. 2004;40:1151–1159. doi: 10.1002/hep.20427. [DOI] [PubMed] [Google Scholar]
- Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel) 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen OW, Nielsen HL, Gudjonsson T, Villadsen R, Rank F, Niebuhr E, Bissell MJ, Ronnov-Jessen L. Epithelial to mesenchymal transition in human breast cancer can provide a nonmalignant stroma. Am J Pathol. 2003;162:391–402. doi: 10.1016/S0002-9440(10)63834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lala PK, Chakraborty C. Role of nitric oxide in carcinogenesis and tumour progression. Lancet Oncol. 2001;2:149–156. doi: 10.1016/S1470-2045(00)00256-4. [DOI] [PubMed] [Google Scholar]
- Sessa WC. eNOS at a glance. J Cell Sci. 2004;117:2427–2429. doi: 10.1242/jcs.01165. [DOI] [PubMed] [Google Scholar]
- Qiu H, Orr FW, Jensen D, Wang HH, McIntosh AR, Hasinoff BB, Nance DM, Pylypas S, Qi K, Song C, Muschel RJ, Al-Mehdi AB. Arrest of B16 melanoma cells in the mouse pulmonary microcirculation induces endothelial nitric oxide synthase-dependent nitric oxide release that is cytotoxic to the tumor cells. Am J Pathol. 2003;162:403–412. doi: 10.1016/S0002-9440(10)63835-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KH, Ancrile BB, Kashatus DF, Counter CM. Tumour maintenance is mediated by eNOS. Nature. 2008;452:646–649. doi: 10.1038/nature06778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HH, McIntosh AR, Hasinoff BB, Rector ES, Ahmed N, Nance DM, Orr FW. B16 melanoma cell arrest in the mouse liver induces nitric oxide release and sinusoidal cytotoxicity: a natural hepatic defense against metastasis. Cancer Res. 2000;60:5862–5869. [PubMed] [Google Scholar]