Abstract
We recently established that the elastin-binding protein, which is identical to the spliced variant of β-galactosidase, forms a cell surface-targeted complex with two proteins considered “classic lysosomal enzymes”: protective protein/cathepsin A and neuraminidase-1 (Neu1). We also found that cell surface-residing Neu1 can desialylate neighboring microfibrillar glycoproteins and facilitate the deposition of insoluble elastin, which contributes to the maintenance of cellular quiescence. Here we provide evidence that cell surface-residing Neu1 contributes to a novel mechanism that limits cellular proliferation by desialylating cell membrane-residing sialoglycoproteins that directly propagate mitogenic signals. We demonstrated that treatment of cultured human aortic smooth muscle cells (SMCs) with either a sialidase inhibitor or an antibody that blocks Neu1 activity induced significant up-regulation in SMC proliferation in response to fetal bovine serum. Conversely, treatment with Clostridium perfringens neuraminidase (which is highly homologous to Neu1) decreased SMC proliferation, even in cultures that did not deposit elastin. Further, we found that pretreatment of aortic SMCs with exogenous neuraminidase abolished their mitogenic responses to recombinant platelet-derived growth factor (PDGF)-BB and insulin-like growth factor (IGF)-2 and that sialidosis fibroblasts (which are exclusively deficient in Neu1) were more responsive to PDGF-BB and IGF-2 compared with normal fibroblasts. Furthermore, we provide direct evidence that neuraminidase caused the desialylation of both PDGF and IGF-1 receptors and diminished the intracellular signals induced by the mitogenic ligands PDGF-BB and IGF-2.
The sialidases (neuraminidases; NEUs) are widely distributed in nature. They have been identified in numerous viral, bacterial, fungal, protozoan, avian, and mammalian species.1,2 In mammalian cells, four genetically distinct neuraminidases (sialidases), which differ in their tissue distribution, subcellular localization, and substrate specificity, have been characterized. They have been localized to lysosomes; neuraminidase 1 (Neu1)3,4,5,6,7 to cytosol, neuraminidase 2 (Neu2)8,9 to the plasma membrane, neuraminidase 3 (Neu3, also known as ganglioside sialidase)10,11,12 to mitochondria and lysosomes, neuraminidase 4 (Neu4).13,14,15 But only Neu1, which is expressed in all mammalian tissues and is active mostly toward sialylated glycoproteins, has been detected in a lysosome-targeted multiprotein complex with β-galactosidase (β-Gal) and protective protein/cathepsin A (PPCA).16,17,18,19 Both lysosomal Neu1 and PPCA have also been immunolocalized to the cell surface of normal fibroblasts, activated lymphocytes, and neutrophils, but this has been attributed to mistargeting or to alternative transport involving their subsequent exocytosis and endocytosis.20,21,22,23
We have previously established that such unusual localization of these “classic lysosomal enzymes” can be attributed to the fact that these two proteins can also form a molecular complex with S-gal, a 67-kDa, enzymatically inactive, spliced variant of β-galactosidase. We found that S-gal serves as the elastin-binding protein, the major subunit of the elastin receptor, which is targeted to the cell surface instead of to the lysosomes.24,25,26 We demonstrated that proteins copurified with the 67-kDa S-Gal/elastin-binding protein from the cell membrane fraction of human fibroblasts by elastin affinity columns were immunoreactive with antibodies raised to human Neu1 and PPCA. Moreover, anti-Neu1 and anti-PPCA antibodies showed an identical pattern of immunolocalization on the surface of elastin-producing cells, as did an antibody recognizing the elastin-binding protein.27 We further demonstrated that the sialidase activity of cell-surface-residing Neu1 causes the removal of terminal sialic acids from carbohydrate chains of matrix-residing microfibrillar glycoproteins, which is a prerequisite step for the subsequent binding of newly secreted tropoelastin and the proper assembly of elastic fibers containing crosslinked (insoluble) elastin. Because endogenous Neu1 is necessary for the effective formation of insoluble elastin,27 which, in turn, can sequester such mitogenic growth factors as platelet-derived growth factor (PDGF) and fibroblast growth factor,28 we propose that Neu1 may contribute in this “indirect” way to inhibition of the proliferative phenotype of arterial SMCs and other elastin-producing cells.
Because cell-surface-residing Neu1 is capable of removing terminal ketosidically linked sialic acids from pericellular matrix glycoproteins, we further speculated that this sialidase would also catalyze the desialylation of other adjacent glycoproteins anchored to the plasma membrane. Because the cell surface receptors interacting with two mitogenic growth factors, PDGF and insulin-like growth factor (IGF)-2, are sialylated glycoproteins,29,30,31,32,33 we decided to investigate whether cell-surface-residing Neu1 would target these two moieties and thereby modulate the proliferative response of cultured aortic SMCs to the mitogenic ligands PDGF-BB and IGF-2.
Both growth factors are potent stimulators of SMC proliferation and have been implicated in the progression of atherosclerosis.34,35,36,37,38,39 It has been established that the mature cell-surface-residing PDGF receptor activates intracellular pro-mitogenic signaling in arterial SMCs via the PI3K/Akt, PLCγ, and Ras-Raf1-MEKs-ERK1/2 pathways.34,35,36,37 The proliferative effect of IGF-2 is mediated after its interaction with the dimeric IGF-1 receptor,32,38,39,40,41,42,43,44,45 triggering tyrosine kinase-dependent IGF-1 receptor autophosphorylation and subsequent activation of downstream PI3K-Akt, PLCγ, and Ras-Raf1-MEKs-ERK1/2 signaling pathways.46,47
Results of the present study provide evidence for the existence of a novel mechanism in which the enzymatic activity of cell-surface-residing Neu1 contributes to the down-regulation of net cellular proliferation by desialylating cell surface receptors interacting with PDGF-BB and IGF-2.
Materials and Methods
Materials
Chemicals and reagents were obtained as follows. Minimal essential α media, medium 199, PBS, fetal bovine serum (FBS), and other tissue culture reagents were obtained from GIBCO (Burlington, Ontario). Neuraminidase from Clostridium perfringens Type V (Nase), neuraminidase inhibitor 2,3-dehydro-2-deoxy-n-acetylneuraminic-acid (ddNANA), inhibitor of lysyl oxidase inhibitor, β-aminopropionitrile fumerate (βAPN), human recombinant IGF-2, human recombinant PDGF-BB, and all reagent grade chemicals were purchased from Sigma (St. Louis, MO). Human recombinant interleukin 1-β (IL-1β) came from Pepro Tech (Rocky Hill, NJ). Polyclonal anti Ki-67 antibody was obtained from Chemicon (Temecula, CA), and polyclonal anti-tropoelastin antibody from EPC Elastin Products (Owensville, MO). Antibodies used for western blotting (polyclonal anti-PDGF receptor β subunit, polyclonal anti-IGF-1 receptor β subunit, and monoclonal anti-phosphotyrosine [PY-20]) were provided by Santa Cruz Biotechnology (Santa Cruz, CA). Polyclonal anti-phospho-Akt (Ser473), anti-Akt, anti-phospho-Erk1/2, and anti-Erk1/2 were purchased from Cell Signaling Technology (Beverly, MA), and monoclonal anti-β-actin from ABCam (Cambridge, MA). Polyclonal antibody specific to human Neu 1 (the IgG fraction was initially purified from rabbit antiserum by ammonium sulfate fractionation and shown not to cross-react with other human sialidases)22 and the IgG fraction similarly purified from the respective preimmune rabbit serum were generous gifts from Dr. Alexey V. Pshezhetsky of the Department of Medical Genetics, Sainte-Justine Hospital, University of Montreal (Québec, Canada). The digoxigenin glycan differentiation kit (used to identify sialylated glycoproteins) was purchased from Roche (Mannheim, Germany). The fluorescein-labeled F(ab′)2 fragments of goat anti-rabbit secondary immunoglobulin, nuclear stains, propidium iodide, and 4,6-diamidino-2-phenylindole were purchased from Sigma (St Louis, MO). The Cytomation LSAB2 System (horseradish peroxidase liquid diaminobenzidine kit and hematoxylin counterstain) was supplied by DakoCytomation (Carpinteria, CA). Species and type-specific horseradish peroxidase-conjugated secondary antibodies used in western immunoblotting were supplied by Boehringer Mannheim (Indianapolis, IN). The enhanced chemiluminescence western blotting detection kit, [3H]-leucine, and [3H]-valine were obtained from Amersham Life Science (Oakville, Ontario). Bromodeoxyuridine (BrdU) cell proliferation labeling reagent and anti-BrdU monoclonal antibody were obtained from Amersham Biosciences (Buckinghamshire, UK). The ApopTag Plus Peroxidase In Situ Apoptosis Detection Kit was obtained from Chemicon International (Temecula, CA). The DNeasy Tissue kit for DNA isolation was obtained from Qiagen (Mississauga, ON).
Cell Cultures
With parental consent and approval of the Ethics Committee at the Hospital for Sick Children in Toronto, endothelial cells and smooth muscle cells (AoSMCs) were propagated from small aortic fragments obtained during the autopsies of three patients who died as a result of road accidents. Smooth muscle cells isolated from the coronary arteries (CaSMCs) and the small intestines of three 2-month-old pigs were used for comparison. Comparisons were also made using fibroblasts (4028, 4029, and 4079) derived from skin biopsies of three children (2 months, 8 months, and 4 years old) diagnosed with congenital sialidosis caused by primary lesions in the Neu1 gene.5,7,27 These cells demonstrated 0.2, 0.4, and 0.8% of residual Neu1 activity (measured against a specific artificial substrate) as compared with fibroblasts (4212, 3858, and 4992) obtained from skin biopsies of three normal children of matching ages. We have previously reported that fibroblasts derived from these sialidosis patients do not deposit elastic fibers.27 All cells were originally isolated by collagenase digestion of the initial tissues and then passaged two to five times by trypsinization and maintained in α-minimum essential medium supplemented with 20 mmol/L HEPES, 1% antibiotics/antimycotics, and 10% FBS.
The cultured cells were then used in numerous experiments testing whether experimental inhibition of endogenous sialidase activity would modulate cell proliferation in response to 10% FBS (containing numerous growth factors) or proliferative response to the recombinant proliferative growth factors PDGF-BB and IGF-2 in serum-free conditions. To eliminate the enzymatic activity of cell-surface-residing Neu1, we used the endogenous sialidase inhibitor ddNANA27,48,49,50 and a blocking antibody to human Neu1 (purified IgG) that did not cross-react with other human sialidases.21 For comparison, the appropriate preimmune IgG was also tested.
To enhance the putative cellular effects caused by desialylation of cell surface sialo-conjugates, C. perfringens Type V Nase, which displays similar substrate specificity and extensive homology to Neu1 (but not to the other three human sialidases), was also used.51,52,53,54 This particular bacterial neuraminidase was chosen because it (like human Neu1) preferentially removes terminal sialic acids from monovalent sialylated glycoproteins but not from sialylated gangliosides, as other bacterial sialidases do. We anticipated that the biological effects observed after treatment of cultured SMCs with the C. perfringens neuraminidase would differ from the effects recorded after treatment with Vibrio cholerae neuraminidase,55,56,57 which preferentially hydrolyzes both −2,3-and −2,6-linked sialic acid residues from higher-order gangliosides.58,59,60 V. cholerae neuraminidase also has an additional carbohydrate-binding domain,61,62 which would potentially enhance the association of certain growth factors with their cell surface receptors and probably stimulate cellular proliferation independently of its enzymatic action.
Assessments of Elastin Production
AoSMCs were initially plated (100,000 cells/dish) in minimal essential α media with 10% FBS to achieve the immediate confluency and then maintained for the next 6 days in the presence and absence of 500 μmol/L ddNANA, 2 μg/ml of anti-Neu1, or 50 mU/ml of Nase. The parallel confluent cultures of AoSMCs were also incubated with 100 μmol/L βAPN, as indicated in the figure legends. In a separate set of experiments, elastin deposition was also monitored in subconfluent cultures of AoSMCs. The 6-day-old cultures were then fixed in 100% cold methanol. The deposition of elastin was detected with polyclonal antibody to tropoelastin. The immunoreactions were visualized with fluorescein isothiocyanate-conjugated goat anti-rabbit secondary antibody. Nuclei were counterstained red with propidium iodide, as previously described.63,64 The slides were mounted and were examined at original magnification ×600 with a fluorescence microscope (Nikon Eclipse E1000).
The production of a new (metabolically labeled) insoluble elastin was also assessed in parallel cultures that were incubated with [3H]-valine, as described previously.56 Deposition of insoluble elastin was indicated by the levels of radioactive valine present in residues remaining after boiling the cell layers of the same cultures in 0.1N NaOH for 45 minutes. This procedure removes all cellular and extracellular components except the cross-linked elastin. The results were expressed as cpm and normalized per DNA content determined in the soluble fractions of the NaOH extracts.
Assessments of Cellular Proliferation
The influence of all reagents modulating sialidase activity on net cellular proliferation was initially assessed: (1) in 6-day-old confluent cultures of human AoSMCs that deposited elastic fibers, (2) in 6-day-old confluent cultures of human AoSMCs in which deposition of these ECM components was eliminated by treatment with an inhibitor of the elastin cross-linker βAPN,65,66,67 (3) in 6-day-old subconfluent cultures of human AoSMCs and porcine CaSMCs, in which these cells could not deposit elastic fibers, and (4) in confluent cultures of porcine intestinal SMCs that never deposit elastin. Finally, we tested, using a 3-day time course, whether immediate treatment of the subconfluent cultures of AoSMCs (that do not deposit elastin) with exogenous neuraminidase would modulate their proliferative response to recombinant PDGF-BB and IGF-2. For comparison, we also tested whether preincubation with C. perfringens Nase would modulate the proliferative response of cultured human AoSMCs to recombinant human IL-1β. The IL-1β was chosen because its receptor is not sialylated glycoprotein68 and it can interact with the cell membrane GM4 sialylated glycolipid, Neu5Aca2-3Galβ1-ceramide.69 Thus, the detection of any diminution of the proliferative effects of IL-1β in cultures pretreated with C. perfringens Nase would indicate some collateral action of this enzyme (eg, through desialylation of cell membrane gangliosides) that might indirectly modify cellular proliferation. Then, we compared the magnitude of the proliferative effects of the same doses of PDGF-BB and IGF-2 in 3-day-old subconfluent cultures of normal human skin fibroblasts and Neu1-deficient fibroblasts derived from patients with congenital sialidosis.
The following parallel methods were used to assess cellular proliferation rates in cultures that were maintained in the presence or absence of different reagents, as described in the figure legends.
Cell Counting and Total DNA Assay
Cultures were trypsinized and resuspended in 1 ml medium with 10% FBS to inactivate trypsin. We counted the number of cells present in three 10 μl aliquots of each culture in a hemocytometer. Results from four parallel cultures in each experimental group were averaged. Three aliquots from each culture were also used for an assay of total DNA, using the DNeasy Tissue Kit from QIAGEN according to the manufacturer’s instructions.
[3H]-Thymidine Incorporation
One μCi of [3H]-thymidine/ml of media was added to cultures of all experimental groups at the same time as different reagents were applied (day 1 and 3). The 6-day-old cultures were then washed twice with cold 5% trichloroacetic acid at 4°C and incubated with 0.5 ml of 0.3N NaOH for 30 minutes. Aliquots (200 μl) from each well were added to 5 ml of liquid scintillation cocktail and counted with a Win Spectral 1414 Liquid Scintillation Counter, as previously described.55,56
Immunohistochemical Detection of Proliferating Cells Expressing Ki-67 and BrdU and Detection of Apoptotic Cells
One set of cultures treated with different reagents was also used for the ultimate detection of Ki-67 proliferative antigen.70,71 In the parallel set of cultures, BrdU71,73 was added at the same time as different reagents were applied (days 1 and 3). The 6-day-old cultures of both groups were fixed in cold 100% methanol. The DakoCytomation LSAB2 System was then used, according to the manufacturer’s instructions, to detect BrdU-positive and Ki-67 positive cells. The cultures were then counterstained with hematoxylin.
Apoptotic cells were detected by TdT-mediated dUTP nick-end labeling,74 using an ApopTag Plus Peroxidase In Situ Apoptosis Detection Kit, following the manufacturer’s instructions. All cultures were examined with a Light Microscope (Leica DC500, Leica Microsystems, Wetzlar, Gemany) using Openlab software (Improvision Inc, Lexington, MA). For each culture, the number of positively and negatively stained cells was counted under original magnification ×200 in 30 separate fields. The percentage of positively staining cells was determined within each field and averaged over the 30 fields examined.
Western Blot Analysis
AoSMCs were initially plated at a density of 500,000 cells/culture and grown in medium with 10% FBS for 24 hours. The subconfluent cultures were then serum starved for 24 hours. Cultures kept in serum-free medium were then preincubated for 30 minutes in the presence or absence of 50 mU of exogenous Nase before they were exposed for 10 minutes to 10 ng/ml of human recombinant PDGF-BB or to 100 ng/ml of human recombinant IGF-2 or to 25 ng/ml of human recombinant IL-1β. At the end of this incubation period, cultures were washed in PBS and then lysed in radioimmunoprecipitation assay buffer containing a proteinase inhibitor cocktail and 1 mmol/L of the endogenous phosphatase inhibitor sodium orthovanadate. The aliquots of each cell lysate (containing equal protein concentration) were then re-suspended in sample buffer (0.5 mol/L Tris-HCl, pH 6.8; 10% SDS; 10% glycerol; 4% 2-β-mercaptoethanol; and 0.05% bromophenol blue), resolved by 10% SDS-polyacrylamide gel electrophoresis (PAGE), transferred to the nitrocellulose membranes, and immunoblotted with polyclonal antibodies directed against the PDGF receptor β subunit and IGF-1 receptor β subunit, and the IL-1β receptor. The PDGF-and IGF-1 receptor blots were then stripped and reprobed with antibodies recognizing phosphotyrosines (PY-20 and PY-99). The parallel blots were also probed with antibodies against phospho-Akt and phospho-Erk1/2 and reprobed with anti-Akt and anti-Erk1/2 antibodies. Loading of equivalent amounts of protein was additionally confirmed by stripping the membranes and reprobing with monoclonal antibody to β-actin.
Glycoprotein Analysis
To provide direct evidence that the IGF-1 and PDGF receptors present on the surface of AoSMCs are sialylated glycoproteins and that exposure of these cells to exogenous sialidase would cause their desialylation, the following procedures were performed. The confluent cultures of AoSMCs (initially plated at 105 cells/plate) were transferred for 18 hours to serum-free minimal essential media D. The media were then changed and the cultures were maintained for 10 minutes in the presence or absence of 50 mU/ml of exogenous Nase. At the end of this incubation period, cultures were washed in PBS and then lysed in radioimmunoprecipitation assay buffer containing a proteinase inhibitor cocktail. The aliquots of each cell lysate (containing equal 400 μg protein concentrations) were then incubated for 1 hour at 4°C with an antibody recognizing the β subunit of the PDGF receptor or with an antibody to the β subunit of the IGF-1 receptor. The immunocomplexes were then precipitated with 4% protein A-beaded agarose overnight. The resulting final pellets containing the immunoprecipitation products were centrifuged and washed four times with PBS and then resuspended in sample buffer with 2-β-mercaptoethanol, resolved by 10% SDS-PAGE, and transferred onto a nitrocellulose membrane. To visualize the sialylated glycoprotein bands, the membranes were then probed with the Maackia amurensis agglutinin lectin (recognizing α2-3-linked sialic acids) and with Sambucus nigra agglutinin lectin (recognizing α2-6-linked sialic acids) from the Roche digoxigenin glycan differentiation kit, in accordance with the manufacturer’s protocol.
Statistical Analysis of Data
All experimental groups described above consisted of quadruplicate cultures of cells derived from each of three different donors. Means and standard deviations obtained from two separate experiments were calculated for each experimental group, and statistical analyses were performed by analysis of variance, followed by a Tukey posttest to establish which groups were different.
Results
Inhibition of Endogenous Neu1 Activity and Treatment with Exogenous Nase (Homologous to Neu1) Inversely Affect the Deposition of Elastin and the Proliferation Rates of Cultured Aortic SMCs
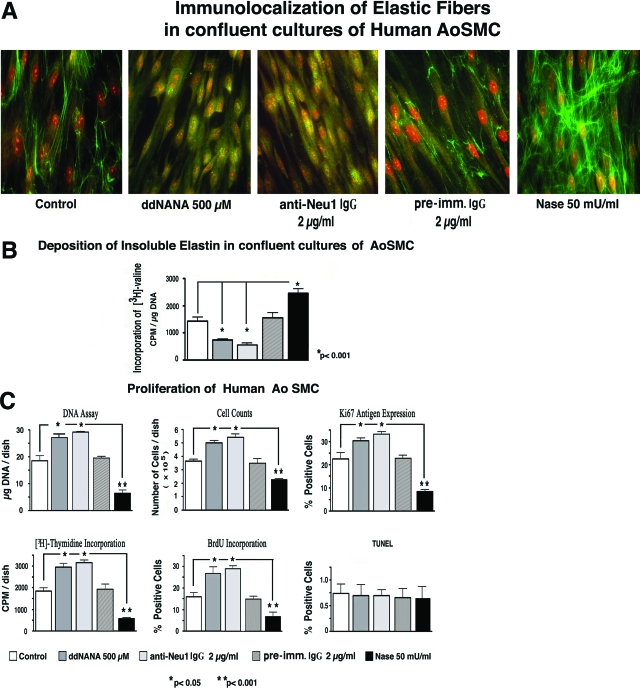
We previously established that Neu1 is a component of the elastin receptor and that its enzymatic activity is required for normal assembly of elastic fibers.27 Because the major component of elastic fibers, insoluble elastin, is capable of sequestering certain growth factors, including PDGF and FGF,28 we proposed that Neu1 might indirectly contribute to the complicated mechanisms controlling cellular proliferation via its pro-elastogenic effect. Indeed, the results of our first series of experiments demonstrated that treating confluent cultures of human AoSMCs (kept in medium with 10% FBS) with 500 μmol/L of the sialidase inhibitor ddNANA or with 2 μg/ml of blocking antibody to human Neu1 for 6 days caused a significant decrease in their deposition of immunodetectable elastic fibers. Conversely, cultures treated with 50 mU/ml of exogenous Nase, which shares extensive sequence homology and substrate specificity with human Neu1 but not with other known human sialidases, demonstrated a significant increase in elastic fiber deposition (Figure 1A). Metabolic labeling of parallel SMC cultures with [3H]-valine, followed by a quantitative assay of radioactive insoluble elastin, confirmed results obtained by immunocytochemistry (Figure 1B). Because the quantitative values obtained from the biochemical assay of metabolically labeled insoluble elastin have been normalized per DNA content in each culture, we realized that cultures treated with ddNANA or anti-Neu1 contained more DNA, whereas exogenous Nase-treated cultures contained less DNA, than their untreated counterparts. Results from assays quantifying cellular proliferation rates (DNA content, cell counts, Ki-67 antigen expression, and [3H]-thymidine and BrdU incorporation) in 6-day-old cultures of elastin-producing SMCs further confirmed that experimental down-regulation of sialidase activity in confluent cultures of SMCs not only inhibited the net deposition of elastin in these cultures but simultaneously induced a significant up-regulation in proliferation rates. Conversely, cultures treated with exogenous Nase displayed significantly lower cell proliferation rates than their untreated counterparts (Figure 1C). Statistical evaluation of results obtained from two separate experiments using five parallel proliferation assays indicated that inhibition of endogenous sialidase activity with ddNANA or with blocking anti-Neu1 antibody was associated with an average 41.4 ± 6.6% and 48.6 ± 4.6% increase in the SMC proliferation rate, respectively. The proliferation rate in cultures treated with nonimmune IgG did not differ from untreated controls (data not shown). The cultures treated with exogenous Nase demonstrated an average 63.8 ± 12.3% decrease in their proliferation rates, compared with values obtained from untreated control cultures. It is notable that cultures exposed to all of the tested reagents modulating net sialidase activity did not demonstrate any significant change in their apoptosis rates, as assessed by TdT-mediated dUTP nick-end labeling assay (Figure 1C).
Figure 1.
(A) Representative photomicrographs depicting 6-day-old confluent cultures of human AoSMCs immunostained with specific anti-tropoelastin antibody. Cultures maintained in medium with 10% FBS and treated with ddNANA or with blocking anti-Neu1 antibody did not deposit elastic fibers. On the other hand, treatment of parallel cultures with exogenous Nase significantly increased elastic fiber deposition. (B) Results of quantitative assessment of insoluble elastin in confluent cultures metabolically labeled with [3H]-valine demonstrate the similar inverse effects of blocking endogenous Neu1 activity and of exogenous cPNase treatment on elastic fiber deposition in human AoSMC cultures. (C) Results of parallel assays (DNA assay, cell counts, immunohistochemical detection of Ki-67 antigen, BrdU and [3H]-thymidine incorporation) assessing net cellular proliferation rates in 6-day-old confluent cultures of human AoSMCs maintained in medium with 10% FBS. The results demonstrate that treatment with exogenous Nase resulted in a significant decrease in cellular proliferation rate. Conversely, treatment with the sialidase inhibitor ddNANA or with blocking anti-Neu1 antibody (but not with preimmune IgG) significantly up-regulated cellular proliferation. No significant difference in apoptosis levels determined by TdT-mediated dUTP nick-end labeling assay was observed between control cultures and those treated with ddNANA, anti-Neu1 antibody, or Nase.
Experimental Modulation of Neu1 Activity Also Affects the Cellular Proliferation Rate in Cultures of Aortic SMCs that Do Not Deposit Elastic Fibers
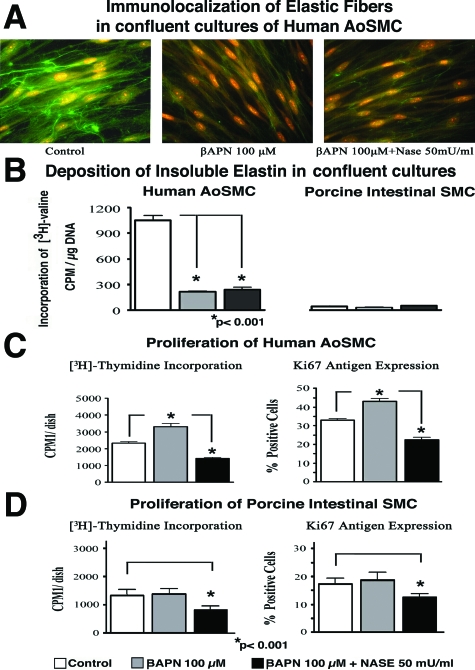
Further experiments attempted to determine whether cell-surface-residing Neu1 would also contribute to a direct modulation of cellular proliferation in a parallel elastin-independent mechanism. To test this possibility, we exposed cultures of AoSMCs to the lysyl oxidase inhibitor βAPN60,61 to prevent the deposition of cross-linked elastin. We then tested whether inhibition of endogenous sialidase (through treatments with ddNANA and with blocking anti-Neu1 IgG) or up-regulation of the net sialidase activity (through treatment with exogenous Nase) would also modulate the proliferative response of βAPN-treated cells maintained in growth-factor-rich (10% FBS) media. Both immunohistochemistry and the results of quantitative biochemical assays demonstrated that pretreatment of confluent cultures of AoSMCs with βAPN prevented the effective deposition of immunodetectable elastic fibers and metabolically labeled insoluble elastin. This was also observed in cultures of Nase-treated AoSMCs (Figure 2A and B, left panel). Results of a [3H]-thymidine incorporation assay and an immunohistochemical assessment of Ki-67 expression indicated that whereas the inhibition of elastin deposition by βAPN was associated with an average 29.2 ± 2.6% increase in the cellular proliferation rate of AoSMCs, simultaneous treatment with exogenous Nase and βAPN induced an average 32.6 ± 0.4% decrease in the proliferation rate of aortic SMCs (*P < 0.001; Figure 2C).
Figure 2.
Representative photomicrographs of 6-day-old human AoSMC cultures immunostained with specific anti-tropoelastin antibody (A) and the results of quantitative assessments of insoluble elastin in parallel cultures metabolically labeled with [3H]-valine (B) demonstrated that βAPN treatment, applied alone or in conjunction with exogenous Nase, caused a significant decrease in elastin deposition in confluent cultures of human AoSMCs maintained in 10% FBS media. This assay also confirmed that porcine intestinal SMCs did not deposit any insoluble elastin in the presence or absence of βAPN and Nase treatment. (C) Results of assays measuring [3H]-thymidine incorporation and Ki-67 antigen expression demonstrate that 6-day-old confluent cultures of human AoSMCs maintained in medium with 10% FBS significantly increased their cellular proliferation rates when deposition of elastin was inhibited by βAPN. In contrast, the parallel cultures that were simultaneously treated with βAPN and exogenous Nase demonstrated a significant decrease in cellular proliferation rate in comparison to control values. (D) In addition, porcine intestinal SMCs that did not change their basal proliferation rate after βAPN treatment demonstrated a significant decrease in proliferation rate after the simultaneous addition of exogenous Nase.
Next we found that βAPN treatment of confluent cultures of intestinal SMCs that do not deposit elastin (Figure 2B, right panel) did not increase their proliferation rate over those of untreated control cultures (Figure 2D). Intestinal SMCs simultaneously treated with βAPN and Nase, however, demonstrated a significant (33 ± 6.6%) decrease in proliferation rate (*P < 0.001). The magnitude of the Nase-induced inhibitory effect was similar to that observed in cultures of AoSMCs in which elastin deposition had been inhibited with βAPN (Figure 2D).
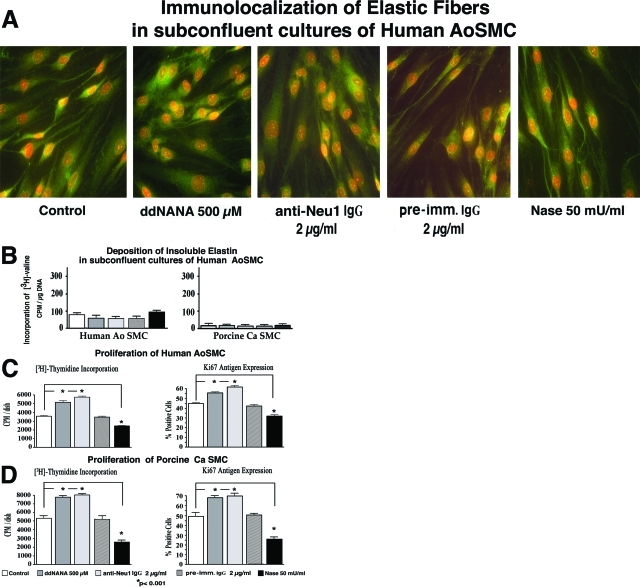
Additional experiments tested the proliferation of AoSMCs in subconfluent cultures that do not deposit elastic fibers, even when maintained in medium with 10% FBS (Figure 3A and B). Results from these experiments indicated that inhibition of endogenous sialidase (through treatments with ddNANA or with blocking anti-Neu1 antibody) led to up-regulation of cellular proliferation (by 32 ± 2.6% and 40 ± 3.8% respectively). The proliferation rate in cultures treated with nonimmune IgG did not differ from untreated controls (data not shown). Conversely, increasing net sialidase activity (through treatment with exogenous Nase) down-regulated the proliferation of AoSMCs by 32 ± 4.1% (Figure 3C). Similar results were seen in CaSMCs (Figure 3D).
Figure 3.
Representative photomicrographs of 6-day-old subconfluent cultures of human AoSMCs immunostained with specific anti-tropoelastin antibody (A) and results of quantitative assays of metabolically labeled insoluble elastin in cultures of human AoSMCs and porcine CaSMCs (B) demonstrate that arterial cells (either untreated or treated with reagents inhibiting endogenous Neu1) did not deposit elastin when maintained in subconfluent conditions. (C) Results of proliferation assays measuring [3H]-thymidine incorporation and Ki-67 antigen expression indicate that inhibiting endogenous Neu1 activity with ddNANA or with blocking anti-Neu1 antibody led to a significant increase in the proliferation rate in subconfluent cultures of AoSMCs maintained in medium with 10% FBS. The proliferation rate of subconfluent cultures treated with preimmune IgG did not differ from untreated controls. In contrast, the addition of exogenous Nase caused a significant down-regulation of the cellular proliferation rate in comparison to untreated control cultures. (D) Porcine CaSMCs exhibited a similar increase in proliferation after inhibiting endogenous Neu1 activity but displayed a decrease in proliferation rate after the addition of exogenous Nase.
Jointly, these results strongly suggest the existence of a parallel, elastin-independent mechanism in which fluctuations of sialidase activity modulate the cellular response to growth-factor-rich FBS.
Pretreatment with Exogenous Nase Abolished the Proliferative Response of Subconfluent Cultures of Aortic SMCs to Recombinant PDGF-BB and IGF-2
The results reported above led to speculation that cell-surface-residing Neu1 may desialylate certain adjacent growth factor receptors and thereby render them unresponsive to their mitogenic ligands. Before we began to explore whether desialylation of the cell surface PDGF and IGF-1 receptors would alter cellular responsiveness to their respective mitogenic ligands, PDGF-BB and IGF-2, we first confirmed that neither PDGF-BB nor IGF-2 stimulates elastogenesis in subconfluent cultures of aortic SMCs maintained in media with 2% FBS (data not shown).
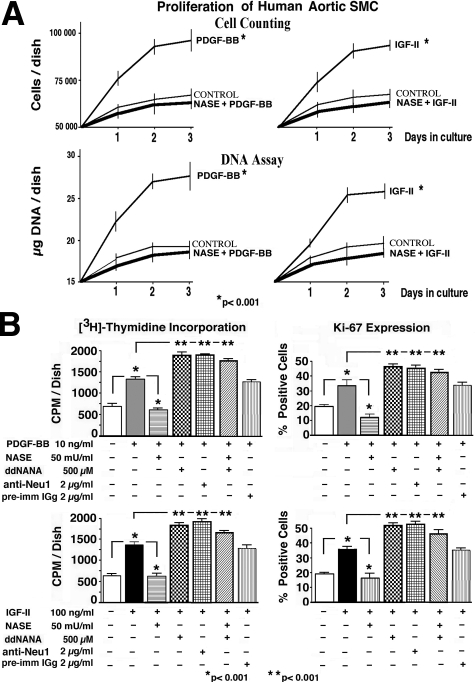
Then we demonstrated (by cell counting and DNA assay) that the proliferative effects of PDGF-BB and IGF-2 can be detected as early as 24 hours after the addition of these growth factors to subconfluent cultures of AoSMCs maintained in serum-free medium. In contrast, parallel cultures that were pretreated with exogenous Nase and simultaneously maintained in the presence of tested growth factors did not demonstrate any increase over their basal proliferation rate (Figure 4A). Subsequent quantitative assays ([3H]-thymidine incorporation and detection of Ki-67) measured the net effects of PDGF-BB and IGF-2 on proliferation rate of subconfluent cultures of AoSMCs maintained for 3 days in serum-free conditions. The obtained results indicate that PDGF-BB and IGF-2 significantly stimulated the proliferation of the cultured SMCs maintained in serum free medium. In contrast, cultures pretreated and simultaneously treated with C. perfringens Nase did not demonstrate any mitogenic response to either of the tested growth factors. Cultures, in which activity of endogenous neuraminidase has been inhibited by pretreatment with ddNANA or with blocking anti-Neu1 antibody, demonstrated more potent responses to the same doses of the tested growth factors. It is also meaningful that pretreatment with preimmune IgG did not induce any increase in the magnitude of mitogenic responsiveness to the same doses of tested growth factors. Interestingly, cultures treated with ddNANA also demonstrated a heightened proliferatve response to both growth factors, even after the addition of exogenous Nase (Figure 4B).
Figure 4.
(A) Results of cell counting and DNA assays performed in time course fashion indicate that subconfluent cultures of AoSMCs maintained in serum-free medium rapidly (within the first 24 hours) up-regulate their proliferation rate in response to either 10 ng/ml of PDGF-BB or 100 ng/ml of IGF-2. In contrast, parallel cultures that were pretreated with 50 mU/ml of exogenous Nase and subsequently treated with the same concentrations of tested growth factors did not increase their proliferation rate as compared with untreated control cultures. Cells were initially plated at 5 × 106 cells/30 mm dish and maintained in serum-free medium. The exogenous Nase was added 2 hours after plating, when the majority of cells had attached to the dish. The growth factors were added 30 minutes later. (B) Results of [3H]-thymidine incorporation and Ki-67 antigen expression assays measuring the proliferation rate of 3-day-old subconfluent cultures of human AoSMCs maintained in serum-free medium. The data indicate that PDGF-BB and IGF-2 significantly stimulated the proliferation of the cultured SMCs maintained in serum free medium. In contrast, cultures pretreated and simultaneously treated with exogenous C. perfringens Nase did not demonstrate any mitogenic response to either of the tested growth factors. Cultures preincubated with ddNANA or with blocking anti-Neu1 antibody, which inhibits endogenous sialidase, demonstrated more potent responses to the same doses of the tested growth factors. Interestingly, cultures treated with ddNANA (a known inhibitor of Neu1) also demonstrated a heightened proliferative response to both growth factors, even after the addition of exogenous Nase.
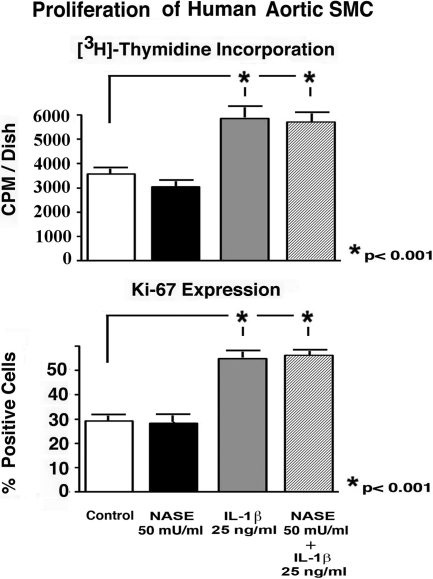
The results of additional experiments demonstrated that preincubation with C. perfringens Nase did not diminish the increase (an average 60%) in mitogenic response induced by 25 ng/ml of IL-1β (Figure 5).
Figure 5.
Results of [3H]-thymidine incorporation assay and Ki-67 detection demonstrate that preincubation with exogenous Nase did not significantly diminish the proliferative response of human aortic SMCs to recombinant IL-1β. SMCs cultures were plated at initial density (1 × 105/cells dish) and then maintained for 3 days in serum-free medium, either in the presence and absence of 25 ng/ml of IL-1β (SMCs). The parallel cultures were also treated with 50 mU of C. perfringens Nase, which was administered one hour before the addition of the growth factors.
Fibroblasts Derived from Three Patients with Genetic Sialidosis Bearing Exclusive Deficiency in Neu1 Demonstrate Higher Basal Proliferation Rates and Greater Responsiveness to PDGF-BB and IGF-2 than Normal Fibroblasts
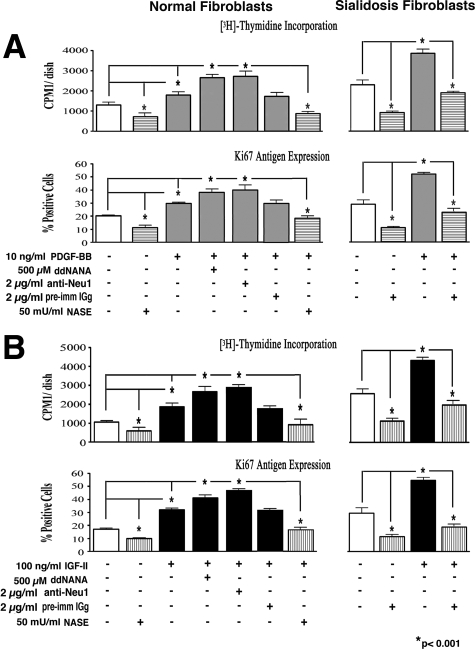
To further support our notion that the enzymatic activity of cell-surface-residing Neu1 is responsible for modulating the mitogenic response to PDGF-BB and IGF-2 but the enzymatic activity of other human sialidases (Neu2, Neu3, or Neu4) is not, we also compared the proliferation rates of dermal fibroblasts derived from three normal individuals with dermal fibroblasts from three patients with sialidosis, which are exclusively deficient in Neu1. Results of proliferation assays ([3H]-thymidine incorporation and immunodetection of Ki-67 expression) indicated that subconfluent cultures of sialidosis fibroblasts (passage 4) exhibited higher proliferation rates than their normal counterparts (also passage 4) when maintained in media with 2% FBS. Moreover, sialidosis fibroblasts displayed a proportionally higher mitogenic response to identical doses of both PDGF-BB and IGF-2 than normal fibroblasts (Figure 6). Results of statistical analyses comparing the proliferation rates of fibroblasts from three normal and three sialidosis patients demonstrated that, whereas treatment with 10 ng/ml of PDGF-BB caused an average 47.0 ± 5.1% increase in the proliferation rate of normal fibroblasts, the same dose of this growth factor caused an average 74.1 ± 3.2% increase in proliferation rate in the sialidosis fibroblasts, compared with untreated cultures. Likewise treatment with 100 ng/ml of IGF-2 resulted in an average 54.1 ± 4.6% increase in the proliferation of normal fibroblasts and a 68.9 ± 4.5% increase in sialidosis fibroblasts, as compared with the respective control cultures. Significantly, inhibiting endogenous sialidase activity in cultures of normal fibroblasts by pretreating them with 500 μmol/L of ddNANA or with 2 μg/ml of blocking anti-Neu1 IgG caused a further increase in their responsiveness to the same doses of tested growth factors, with magnitudes 98.1 ± 9.4% and 109.9 ± 10.0% respectively. It is also meaningful that pretreatment with preimmune IgG did not induce any increase in the magnitude of mitogenic responsiveness to the same doses of tested growth factors. The proliferation rate of normal fibroblasts treated with preimmune IgG did not differ from untreated controls (data not shown). We also observed that pretreatment of normal and sialidosis fibroblast cultures with exogenous Nase abolished their proliferative responsiveness to PDGF-BB or IGF-2 (Figure 6, A and B, respectively).
Figure 6.
Results of [3H]-thymidine incorporation and Ki-67 antigen expression assays comparing the proliferation rates of 3-day-old subconfluent cultures of human skin fibroblasts derived from normal individuals and from sialidosis patients. Results indicate that sialidosis fibroblasts demonstrated a significantly higher basic proliferation rate than normal fibroblasts. They also demonstrate that exposure to similar doses of PDGF-BB and IGF-2 induced a proportionally greater increase in the cellular proliferation rate in cultures of sialidosis fibroblasts than in cultures of normal fibroblasts. The addition of exogenous Nase significantly decreased the basal proliferation rates of both normal and sialidosis fibroblasts and eliminated the proliferative effect of PDGF-BB and IGF-2 in both experimental groups. Importantly, inhibition of endogenous sialidase activity in cultures of normal fibroblasts by pretreatment with 500 μmol/L of ddNANA or with 2 μg/ml of blocking anti-Neu1 IgG caused a further increase in their responsiveness to the same doses of the tested growth factors. All cultures were plated at the same initial density (1 × 105/cells dish) and maintained for 3 days in medium with 2% FBS in the presence and absence of 10 ng/ml of PDGF-BB (A) or in the presence and absence of 100 ng/ml of IGF-2 (B).
Preincubation of AoSMCs with Exogenous Nase Causes Desialylation of the β Subunits of the PDGF and IGF-1 Receptors
Because PDGF-BB and IGF-2 use respective cell-surface residing PDGF and IGF-1 receptors, which are sialylated glycoproteins, we tested whether Neu1 may desialylate and thereby modulate the intracellular mitogenic signaling propagated by these receptors.
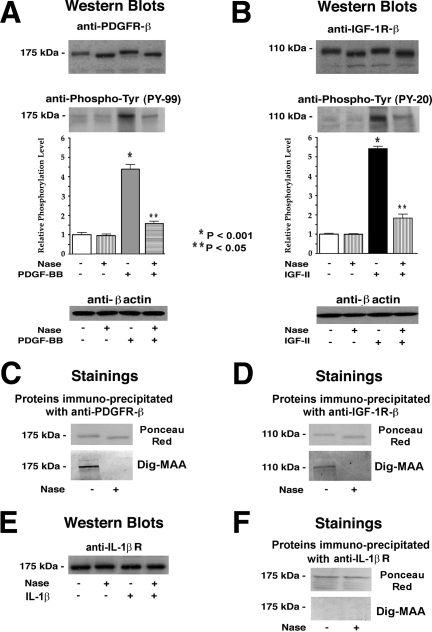
Indeed, immunoblotting with antibodies recognizing the β subunits of the PDGF and IGF-1 receptors demonstrated that cell extracts of AoSMCs preincubated for 30 minutes with exogenous Nase contain immunodetectable proteins whose molecular masses are slightly lower (by ∼5 kDa) than those of their counterparts derived from untreated cells (Figure 7A and B). Consecutive reblotting with antibodies to phosphotyrosine indicated that the β subunits of the PDGF and IGF-1 receptors isolated from control AoSMCs were not phosphorylated, and that a 10 minute exposure of AoSMCs to PDGF-BB or IGF-2 led to a significant increase in tyrosine phosphorylation of these proteins. Significantly, the β subunit of the PDGF receptor detected in lysates of cells preincubated with Nase and subsequently treated with PDGF-BB demonstrated a lower level of phosphorylation (Figure 7A). Likewise pretreating the AoSMCs with Nase completely eliminated any IGF-2-induced increase in the phosphorylation of the β subunit of the IGF-1 receptor (Figure 7B).
Figure 7.
Representative micrographs and results of densitometric evaluations of multiple Western blots presenting the results of quadruplicate experiments indicate that pretreatment of AoSMCs derived from three individuals with exogenous Nase caused desialylation of the β subunits of their PDGF and IGF-1 receptors. (A and B) Western blots with antibodies recognizing the β subunit of the PDGF receptor and the β subunit of the IGF-1 receptor demonstrate that 30 minutes of pretreatment of AoSMCs with Nase caused a 5-kDa downshift in the molecular weight of both the PDGF and IGF-1 receptors β subunits. Cultures of AoSMCs derived from three individuals were preincubated for 30 minutes in the presence or absence of 50 mU/ml of Nase and then exposed to PDGF (10 ng/ml) or IGF-2 (100 ng/ml) for 10 minutes. Cells were then lysed in 2× sample buffer and lysate aliquots were chromatographed on SDS-PAGE and then immunoblotted with antibodies directed to the PDGF receptor β subunit or the IGF-1 receptor β subunit. The blots were then stripped and reprobed with antibodies recognizing phosphotyrosines. The intensity of these β subunits’ phosphorylation was additionally quantified by densitometry. Loadings of equivalent amounts of protein were additionally confirmed by the immunodetection of β-actin. The following panels (C and D) present direct evidence that the IGF-1 and PDGF receptors expressed on the surface of AoSMCs are sialylated glycoproteins and that exposure of these cells to exogenous sialidase caused their desialylation. The lysates of AoSMCs preincubated for 30 minutes in the presence or absence of 50 mU/ml of Nase were immunoprecipitated with an antibody recognizing the β subunit of the PDGF receptor (anti-PDGFR-β) or with an antibody to the β subunit of the IGF-1 receptor (anti-IGF-1R-β). The resulting immunoprecipitation products were resolved by 10% SDS-PAGE and transferred onto nitrocellulose membranes. Transient staining with Ponceau red demonstrated that the β subunits of the PDGF and IGF-1 receptors immunoprecipitated from cultures preincubated with Nase had a molecular mass that was slightly lower (by ∼5 kDa) than their counterparts derived from untreated cells. Consecutive staining with Dig-MAA indicated that both of the receptors immunoprecipitated from control cultures contained α2-3-linked sialic acid residues and that the proteins of lower molecular weight, immunoprecipitated from Nase-treated cells, did not contain any α2-3-linked sialic acid residues, as detected by Dig-MAA staining. Similar blots were obtained from two separate experiments performed with cultures of AoSMCs derived from three human subjects. In contrast, Western blotting demonstrated that pretreatment of AoSMCs with Nase did not affect the molecular size of immunodetectable IL-1β receptors isolated from SMC lysates (E). Transient staining with Ponceau red demonstrated that the IL-1β receptors immunoprecipitated from cultures preincubated with Nase had the same molecular mass as those derived from untreated cells. Negative staining of anti-IL-1β receptor antibody immunoprecipitation products with Dig-MAA further confirmed that the IL-1β receptor does not contain α2-3-linked sialic acid residues (F).
Using digoxigenin-labeled M. amurensis agglutinin lectin (Dig-MAA) to recognize the presence of α2–3-linked sialic acids, we then confirmed that the β subunits of the PDGF and IGF-1 receptors immunoprecipitated from the control AoSMCs were sialylated glycoproteins. In contrast, the β subunits of the PDGF and IGF-1 receptors that had been immunoprecipitated from cells preincubated with exogenous Nase failed to react with Dig-MAA, even though visualization with Ponceau red stain indicated that they were present as proteins of slightly lower (∼5 kDa) molecular mass (Figure 7, C and D). It is noteworthy that pretreatment of cultured AoSMCs with 50 mU/ml of Nase did not cause any reduction in the molecular size of their immunodetected IL-1β receptors. Moreover, IL-1β receptors immunoprecipitated from both control and Nase-treated cells did not react with Dig-MAA (Figure 7, E and F).
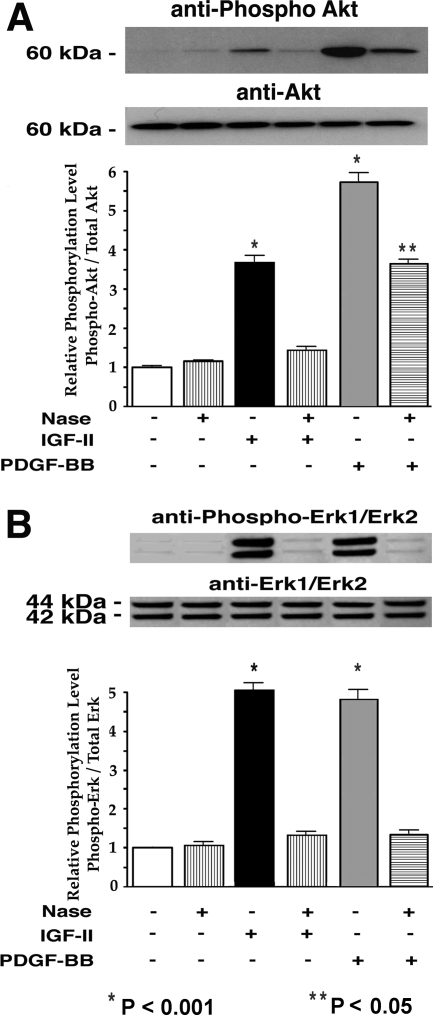
Furthermore, results of the Western blot analysis indicated that pretreatment of AoSMCs with exogenous Nase, which desialylated the PDGF and the IGF-1 receptors, also diminished PDGF-BB-or IGF-2-induced increases in Akt and Erk1/2 phosphorylation (Figure 8, A and B, respectively).
Figure 8.
Representative micrographs and results of densitometric evaluations of multiple Western blots with anti-phospho-Akt (A) and anti-phospho-Erk1/Erk2 (B) antibodies demonstrate that pretreatment of AoSMCs with Nase, which caused desialylation of the β subunits of their PDGF and IGF-1 receptors, also resulted in the inhibition of downstream Akt and Erk1/Erk2 phosphorylation normally induced by their respective ligands, IGF-2 and PDGF-BB. Quadruplicate cultures of AoSMCs derived from three different individuals were preincubated for 30 minutes in the presence or absence of 50 mU/ml of exogenous Nase and then exposed for 10 minutes to IGF-2 (100 ng/ml) or PDGF (10 ng/ml). Cells were then lysed in 2× sample buffer. The lysate aliquots were subjected to SDS-PAGE, transferred to nitrocellulose, and subsequently immunoblotted with the indicated antibodies. In each experiment the parallel Western blots were first probed with antibodies against phospho-Akt or phospho-Erk1/2 and, respectively reprobed with anti-Akt or anti-Erk1/2 antibodies. Loadings of equivalent amounts of protein were additionally confirmed by the immunodetection of β-actin. The quantitative densitometric evaluations of phospho-Akt and Phospho-Erk1/Erk2 immunoblots were normalized against their immunodetectable non-phosphorylated forms. The final results are presented in arbitrary units and reflect the proportional change from the untreated control.
Discussion
The heightened proliferation of arterial SMCs has long been defined as a significant mechanism contributing to the progression of occlusive arterial diseases.32,33,34,35,36,37,38,39,40,41,42,43 Proteolytic degradation of existing elastic fibers28,75,76,77 and the impaired formation of new elastic fibers have been recognized among the many factors associated with elevated vascular SMC migration and proliferation. Increased proliferation of arterial SMCs has been reported in transgenic mice lacking elastin.78,79 Studies from our laboratory have particularly focused on the role of elastin in modulating cellular proliferation in humans. We demonstrated a direct relationship between impaired elastogenesis and heightened proliferation of human aortic SMCs derived from patients with supravalvular aortic stenosis and Williams-Beuren syndrome,80 both of which are characterized by haploinsufficiency of the elastin gene and by development of arterial occlusions.81,82 We also reported the association between impaired elastogenesis and heightened cellular proliferation in a mechanism leading to the development of hypertrophic cardiopathy and arterial occlusions in Hurler’s disease and Costello syndrome.63,64 We further found that insoluble elastin is capable of sequestering certain mitogenic growth factors, including PDGF28 and that heightened proliferation of cultured arterial SMCs and fibroblasts from patients with supravalvular aortic stenosis and Williams-Beuren syndrome could be reversed by the addition of exogenous insoluble elastin.80 Because we have recently established that the activity of the cell-surface-residing neuraminidase Neu1 is required for normal assembly of elastic fibers,27 we suggested that Neu1 may indirectly contribute to the down-regulation of cellular proliferation, via its pro-elastogenic effect. Whereas the results of our initial experiments endorsed this notion, we also found that the addition of exogenous Nase to cultures that did not deposit elastin (namely, confluent cultures of AoSMCs treated with βAPN, subconfluent cultures of AoSMCs, and cultures of intestinal SMCs) also caused a significant down-regulation in their proliferation rates. Conversely, inhibition of endogenous Neu1 (through treatment with ddNANA or with blocking anti-Neu1 antibody) in subconfluent cultures of AoSMCs coincided with up-regulation in their proliferation rates. Because these cultures were maintained in the presence of mitogenic factor-rich (10% FBS) media, we hypothesized that Neu1 might also modulate the proliferative response of arterial SMCs via a parallel elastin-independent mechanism.
We speculated that such a putative Neu1-dependent mechanism must either induce desialylation and consecutive functional inactivation of certain serum-derived mitogenic factors or desialylation of certain plasma membrane-residing receptors engaged in the transduction of mitogenic signals.
PDGF-BB and IGF-2 are potent stimulants of cellular proliferation34,35,36,37,38,39 that have been implicated in the development of hyperproliferative vascular diseases in humans34,40,41 and have been extensively studied in several animal models.35,36,39 We therefore explored the possibility that cell-surface-residing Neu1 would down-regulate the cellular response to these mitogenic growth factors.
As anticipated, treating cultured human AoSMCs with recombinant PDGF-BB or IGF-2 caused a significant increase in their proliferation rates. Importantly, in subconfluent cultures of AoSMCs maintained in serum-free media, pretreatment with C. perfringens Nase, which like mammalian Neu1 exclusively removes sialic acid residues from monovalent sialylated glycoproteins,51 completely abolished the response of the AoSMCs to recombinant PDGF-BB or IGF-2, but not diminish the mitogenic effect of IL-1β, which receptors are not sialylated glycoprotein’s.68,69 Thus, the exogenous C. perfringens neuraminidase altered cellular responsiveness to PDGF-BB or IGF-2, probably mimicking the overexpression of cell surface Neu1.
We did not attempt to test this notion further with AoSMCs displaying heightened endogenous Neu1 activity, because transfection of cells with the Neu1 gene does not lead to a consequent increase in the activity of this enzyme.4,5,6,22 To obtain heightened Neu1 activity, cells have to be transfected with the double construct encoding Neu1 and PPCA, which is needed for the proteolytic trimming of the Neu1 precursor and its consecutive processing to the active form.27 Because increased proteolytic activity of cell-surface-delivered PPCA in transfected cells could potentially inactivate other mitogenic polypeptides (eg, endothelin-1 or angiotensin II),83,84 results obtained from such an experimental model might not be conclusive. However, we demonstrated that treatment of cultured human AoSMCs with blocking antibody raised to human Neu 122 or with the competitive sialidase inhibitor ddNANA, which inhibits the activity of Neu1,27 induced a significant up-regulation in their proliferation in response to fetal bovine serum.
Further confirmation of the exclusive involvement of Neu1 in altering the mitogenic effects of PDGF-BB and IGF-2 clearly emerged from studies involving a natural experimental model of cells, derived from three sialidosis patients, that are specifically deficient in Neu1 but not in other cellular sialidases.4,5 We found that fibroblasts derived from all three sialidosis patients, cultured in medium with 2% FBS, demonstrated a significantly higher basic proliferating rate than normal fibroblasts and a stronger mitogenic response to the same doses of PDGF-BB and IGF-2. We therefore postulate that the selective genetic deficiency in Neu1 created a permanent condition that resulted in a greater number of cell surface receptors remaining sialylated and thus more responsive to their respective mitogenic growth factors. This notion was additionally endorsed by the fact that inhibiting the action of endogenous Neu1 in cultures of normal human fibroblasts (through treatments with ddNANA and anti-Neu1) up-regulated their mitogenic responsiveness to the same doses of PDGF-BB and IGF-2 to the levels observed in sialidosis fibroblasts.
An important result of our research is that we have established that the lack of a proliferative response to PDGF or IGF-2 in cultures preincubated with exogenous Nase coincided with a shift in the molecular size of the β subunits of their PDGF-BB and IGF-1 receptors and with a lack of staining with the sialic acid recognizing reagent, Dig-MAA. This clearly indicated that exposure of cells to neuraminidase caused desialylation of these cell-surface-residing receptors. A similar neuraminidase-induced decrease in the molecular mass of both receptors has been previously described.85,86 Despite the fact that we did not test whether desialylation of the PDGF and IGF-1 receptors would change the binding kinetics of their mitogenic ligands, our consecutive immunoblotting with antibodies to phosphotyrosine consistently indicated that treatment of cultured cells with exogenous Nase eliminated IGF-2-induced increases in the phosphorylation of the IGF-1 receptor β subunit. This is consistent with the results of previous studies, which demonstrated that the IGF-1 receptor, like the insulin and EGF receptors, requires substantial posttranslational processing, including sialylation, before effective ligand binding is possible.87 Our results also demonstrate that exogenous Nase desialylates the β subunit of the human PDGF receptor and attenuates PDGF-BB-induced increases in receptor tyrosine phosphorylation. Our results, obtained from studies with human cells, differ from those of some other investigators, who have used mouse-derived BALB/c3T3 fibroblasts to demonstrate that autocrine ligand-dependent tyrosine phosphorylation of the PDGF receptor may not require the addition of N-or 0-linked oligosaccharides, including sialic acids.30,88,89 These data obtained from mouse cells have not been verified in studies of human cells. However, more recent studies from the same group have acknowledged that several subpopulations of PDGF receptors exist, which may differ with respect to subcellular distribution, durability, and the extent of ligand-induced phosphorylation.31 Moreover, the autocrine ligand-dependent tyrosine phosphorylation of nonglycosylated PDGF receptor molecules detected in subcellular fractions of mouse cells could not be linked with the subsequent induction of physiologically significant signaling. In contrast, our results, obtained from a human cell experimental model, directly demonstrated that the desialylation of the PDGF and IGF-1 receptors coincides with significant inhibition of PDGF-BB-and IGF-2-induced downstream signaling and subsequent reductions in cellular proliferation rates.
In addition, our results demonstrating that treatment with exogenous C. perfringens neuraminidase, (which like the Neu1 desialylates sialoglycoproteins) down-regulated mitogenic response of cultured SMCs to PDGF-BB, differ from results of other investigators who demonstrated that pretreated with V. cholerae neuraminidase stimulated the growth of embryonic fibroblasts86 and enhance the proliferative response of SMCs to serum and PDGF.56,57 We anticipate that this difference is because of the fact that V. cholerae neuraminidase desialylates glycolipids, and after binding to the cell surface gangliosides it may additionally enhance the association of certain growth factors with their cell surface receptors and probably stimulate cellular proliferation independently of its enzymatic action.61,62
Our major claim that it is the activity of cell-surface-residing Neu1 that contributes to the down-regulation of arterial SMC proliferation and not Neu3, the other sialidase associated with the cell membrane, is consistent with the results of previous studies examining the role of Neu1 in rapidly proliferating cancer cells. These studies demonstrated that overexpression of Neu1 led to suppression of metastasis and was associated with reversion of the malignant phenotype in B16 melanoma cells through mechanisms that suppress cell growth and promote apoptosis.90,91,92 Importantly, other studies have indicated that, in contrast to Neu1, plasma membrane-associated Neu3, which is a key enzyme responsible for the desialylation of gangliosides, is essential for cancer cell survival. It has been established that Neu3 is markedly up-regulated in human cancers and that it facilitates EGF-receptor-generated signals leading to the stimulation of a Ras signaling cascade and to the inhibition of cell apoptosis.93,94,95 In addition, treatment with Cholera toxin neuraminidase, which desialylates gangliosides, has been shown to stimulate the growth of embryonic fibroblasts55 and enhance the proliferative response of SMCs to serum and PDGF.56,57
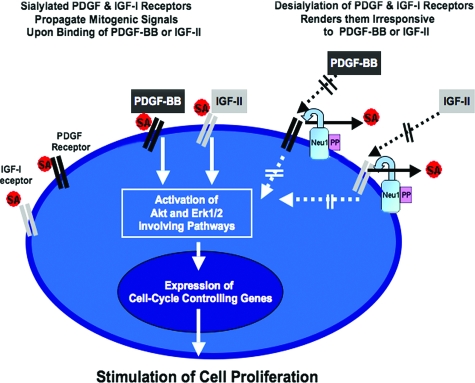
In summary, the present study provides the first experimental evidence that Neu1 plays an important role in the down-regulation of arterial SMC proliferation. We postulate that the enzymatic activity of this particular sialidase residing on the cell surface (as a subunit of the elastin receptor) could structurally alter and functionally inactivate adjacent sialoconjugates, including the PDGF and IGF-1 receptors, and thereby decrease net cellular responsiveness to PDGF-BB and IGF-2 (Figure 9). The findings presented not only introduce another level of complexity by which the cellular proliferation of human arterial SMCs may be regulated but also suggest that the application of exogenous C. perfringens Nase, which displays high structural homology and has similar substrate specificity to human Neu1, may be conceivable as an adjuvant therapeutic agent for inhibiting the development of hyperproliferative neointima after surgical angioplasty, stenting, or vascular grafting.
Figure 9.
A diagram depicting the proposed mechanisms by which Neu1 modulates the proliferation of human arterial SMCs. The enzymatic activity of cell-surface-residing Neu1 causes desialylation of the adjacent cell surface PDGF-and IGF-1 receptors, decreasing cellular responsiveness to their respective mitogenic ligands, PDGF-BB and IGF-2.
Footnotes
Address reprint requests to Aleksander Hinek, M.D., Ph.D., D.Sc., Heart Centre, The Hospital for Sick Children, 555 University Avenue, Toronto, ON, M5G 1X8, CANADA. M5G 1X8. E-mail: alek.hinek@sickkids.ca.
Supported by the Canadian Institute of Health Research through grant PG 13920 and by the Heart and Stroke Foundation of Ontario through grants NA 5435 and NA 4381 and a Career Investigator Award (CI 4198) to A.H.
References
- Achyuthan KE, Achyuthan AM. Comparative enzymology, biochemistry and pathophysiology of human exo-alpha-sialidases (neuraminidases). Comp Biochem Physiol B Biochem Mol Biol. 2001;129(1):29–64. doi: 10.1016/s1096-4959(01)00372-4. [DOI] [PubMed] [Google Scholar]
- Monti E, Preti A, Venerando B, Borsani G. Recent development in mammalian sialidase molecular biology. Neurochem Res. 2002;(7–8):649–663. doi: 10.1023/a:1020276000901. [DOI] [PubMed] [Google Scholar]
- Pshezhetsky AV, Richard C, Michaud L, Igdoura S, Wang S, Elsliger MA, Qu J, Leclerc D, Gravel R, Dallaire L, Potier M. Cloning, expression and chromosomal mapping of human lysosomal sialidase and characterization of mutations in sialidosis. Nat Genet. 1997;15:316–320. doi: 10.1038/ng0397-316. [DOI] [PubMed] [Google Scholar]
- Igdoura SA, Gafuik C, Mertineit C, Saberi F, Pshezhetsky AV, Potier M, Trasler JM, Gravel RA. Cloning of the cDNA and gene encoding mouse lysosomal sialidase and correction of sialidase deficiency in human sialidosis and mouse SM/J fibroblasts. Hum Mol Genet. 1998;7(1):115–121. doi: 10.1093/hmg/7.1.115. [DOI] [PubMed] [Google Scholar]
- Seyrantepe V, Poupetova H, Froissart R, Zabot MT, Maire I, Pshezhetsky AV. Molecular pathology of NEU1 gene in sialidosis. Hum Mutat. 2003;22(5):343–352. doi: 10.1002/humu.10268. [DOI] [PubMed] [Google Scholar]
- Pattison S, Pankarican M, Rupar CA, Graham FL, Igdoura SA. Five novel mutations in the lysosomal sialidase gene (NEU1) in type II Sialidosis patients and assessment of their impact on enzyme activity and intracellular targeting using adenovirus-mediated expression. Hum Mutat. 2004;23:32–39. doi: 10.1002/humu.10278. [DOI] [PubMed] [Google Scholar]
- Bonten EJ, Arts WF, Beck M, Covanis A, Donati MA, Parini R, Zammarchi E, d'Azzo A. Novel mutations in lysosomal neuraminidase identify domains and determine clinical severity in sialidosis. Hum Molec Genet. 2000;9:2715–2725. doi: 10.1093/hmg/9.18.2715. [DOI] [PubMed] [Google Scholar]
- Monti E, Preti A, Nesti C, Ballabio A, Borsani G. Expression of a novel human sialidase encoded by the NEU2 gene. Glycobiology. 1999;9:1313–1321. doi: 10.1093/glycob/9.12.1313. [DOI] [PubMed] [Google Scholar]
- Chavas LM, Tringali C, Fusi P, Venerando B, Tettamanti G, Kato R, Monti E, Wakatsuki S. Crystal structure of the human cytosolic sialidase Neu2. Evidence for the dynamic nature of substrate recognition. J Biol Chem. 2005;280(1):469–475. doi: 10.1074/jbc.M411506200. [DOI] [PubMed] [Google Scholar]
- Miyagi T, Wada T, Iwamatsu A, Hata K, Yoshikawa Y, Tokuyama S, Sawada M. Molecular cloning and characterization of a plasma membrane-associated sialidase specific for gangliosides. J Biol Chem. 1999;274(8):5004–5011. doi: 10.1074/jbc.274.8.5004. [DOI] [PubMed] [Google Scholar]
- Zanchetti G, Colombi P, Manzoni M, Anastasia L, Caimi L, Borsani G, Venerando B, Tettamanti G, Preti A, Monti E, Bresciani R. Sialidase NEU3 is a peripheral membrane protein localized on the cell surface and in endosomal structures. Biochem J. 2007;408(2):211–219. doi: 10.1042/BJ20070503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K, Hata K, Wada T, Moriya S, Miyagi T. Epidermal growth factor-induced mobilization of a ganglioside-specific sialidase (NEU3) to membrane ruffles. Biochem Biophys Res Commun. 2006;346(2):484–490. doi: 10.1016/j.bbrc.2006.05.136. [DOI] [PubMed] [Google Scholar]
- Seyrantepe V, Landry K, Trudel S, Hassan JA, Morales CR, Pshezhetsky AV. Neu4, a novel human lysosomal lumen sialidase, confers normal phenotype to sialidosis and galactosialidosis cells. J Biol Chem. 2004;279(35):37021–37029. doi: 10.1074/jbc.M404531200. [DOI] [PubMed] [Google Scholar]
- Monti E, Bassi MT, Bresciani R, Civini S, Croci GL, Papini N, Riboni M, Zanchetti G, Ballabio A, Preti A, Tettamanti G, Venerando B, Borsani G. Molecular cloning and characterization of NEU4, the fourth member of the human sialidase gene family. Genomics. 2004;83(3):445–453. doi: 10.1016/j.ygeno.2003.08.019. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Hata K, Koseki K, Shiozaki K, Akita H, Wada T, Moriya S, Miyagi T. Evidence for mitochondrial localization of a novel human sialidase (NEU4). Biochem J. 2005;390:85–93. doi: 10.1042/BJ20050017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Spoel A, Bonten E, D'Azzo A. Transport of human lysosomal neuraminidase to mature lysosomes requires protective protein/cathepsin A. EMBO J. 1998;17:1588–1597. doi: 10.1093/emboj/17.6.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonten EJ, d'Azzo Lysosomal neuraminidase – catalytic activation in insect cells is controlled by the protective protein/cathepsin A. J Biol Chem. 2000;275:17657–37663. doi: 10.1074/jbc.M007380200. [DOI] [PubMed] [Google Scholar]
- Lukong KE, Elsliger MA, Chang Y, Richard C, Thomas G, Carey W, Tylki-Szymanska A, Czartoryska B, Buchholz T, Criado GR, Palmeri S, Pshezhetsky AV. Characterization of the sialidase molecular defects in sialidosis patients suggests the structural organization of the lysosomal multienzyme complex. Hum Mol Genet. 2000;9(7):1075–1085. doi: 10.1093/hmg/9.7.1075. [DOI] [PubMed] [Google Scholar]
- Pshezhetsky AV, Ashmarina M. Lysosomal multienzyme complex: biochemistry, genetics, and molecular pathophysiology. Prog Nucleic Acid Res Mol Biol. 2001;69:81–114. doi: 10.1016/s0079-6603(01)69045-7. [DOI] [PubMed] [Google Scholar]
- Cross AS, Wright DG. Mobilization of sialidase from intracellular stores to the surface of human neutrophils and its role in stimulated adhesion responses of these cells. J Clin Investig. 1991;88:2067–2076. doi: 10.1172/JCI115536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukong KE, Seyrantepe V, Landry K, Trudel S, Ahmad A, Gal WA, Lefrancois S, Morales CR, Pshezhetsky AV. Intracellular distribution of lysosomal sialidase is controlled by the internalization signal in its cytoplasmic tail. J Biol Chem. 2001;276:46172–46181. doi: 10.1074/jbc.M104547200. [DOI] [PubMed] [Google Scholar]
- Vinogradova MV, Michaud L, Mezentsev AV, Lukong KE, El-Alfy M, Morales CR, Potier M, Pshezhetsky AV. Molecular mechanisms of lysosomal sialidase deficiency in galactosialidosis involves its rapid degradation. Biochem J. 1998;330:641–650. doi: 10.1042/bj3300641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang F, Seyrantepe V, Landry K, Ahmad R, Ahmad A, Stamatos NM, Pshezhetsky AV. Monocyte differentiation up-regulates the expression of the lysosomal sialidase. Neu1, and triggers its targeting to the plasma membrane via major histocompatibility complex class II-positive compartments. J Biol Chem. 2006;281(37):27526–27538. doi: 10.1074/jbc.M605633200. [DOI] [PubMed] [Google Scholar]
- Hinek A, Rabinovitch M, Keeley F, Okamura OY, Callahan JW. The 67 kD elastin/laminin-binding protein is related to an alternatively spliced β-galactosidase. J Clin Investig. 1993;91:1198–1205. doi: 10.1172/JCI116280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinek A. Nature and multiple functions of the 67 kD elastin/laminin binding protein. Cell Adhes Commun. 1994;2:185–193. doi: 10.3109/15419069409004436. [DOI] [PubMed] [Google Scholar]
- Privitera S, Prody CA, Callahan JW, Hinek A. The 67-kDa enzymatically inactive alternatively spliced variant of β-galactosidase is identical to the elastin/laminin-binding protein. J Biol Chem. 1998;273:6319–6326. doi: 10.1074/jbc.273.11.6319. [DOI] [PubMed] [Google Scholar]
- Hinek A, Pshezhetsky AV, von Itzstein M, Starcher B. Lysosomal sialidase (neuraminidase-1) is targeted to the cell surface in a multiprotein complex that facilitates elastic fiber assembly. J Biol Chem. 2006;281:3698–3710. doi: 10.1074/jbc.M508736200. [DOI] [PubMed] [Google Scholar]
- Hinek A. Impaired elastogenesis and development of the non-atherosclerotic occlusive arterial diseases in children. Ann Diagnost Pediatr Pathol. 2003;7(1–2):7–14. [Google Scholar]
- Waldbillig RJ, Fletcher RT, Somers RL, Chader GJ. IGF-I receptors in the bovine neural retina: structure, kinase activity and comparison with retinal insulin receptors. Exp Eye Res. 1988;47:587–607. doi: 10.1016/0014-4835(88)90097-8. [DOI] [PubMed] [Google Scholar]
- Keating MT, Harryman CC, Williams LT. Platelet-derived growth factor receptor inducibility is acquired immediately after translation and does not require glycosylation. J Biol Chem. 1989;264:9129–9132. [PubMed] [Google Scholar]
- Coats SR, Pledger WJ, Awazu M, Daniel TO. Detergent solubility defines an alternative itinerary for a subpopulation of PDGF beta receptors. J Cell Physiol. 1996;168:412–423. doi: 10.1002/(SICI)1097-4652(199608)168:2<412::AID-JCP20>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Masnikosa R, Baricević I, Jones DR, Nedić O. Characterization of insulin-like growth factor receptors and insulin receptors in the human placenta using lectin affinity methods. Growth Horm IGF Res. 2006;16(3):174–184. doi: 10.1016/j.ghir.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Siddals KW, Marshman E, Westwood M, Gibson JM. Abrogation of insulin-like growth factor-I (IGF-I) and insulin action by mevalonic acid depletion: synergy between protein prenylation and receptor glycosylation pathways. J Biol Chem. 2004;279:38353–38359. doi: 10.1074/jbc.M404838200. [DOI] [PubMed] [Google Scholar]
- Raines EW. PDGF and cardiovascular disease. Cytokine Growth Factor Rev. 2004;15:237–254. doi: 10.1016/j.cytogfr.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Tallquist M, Kazlauskas A. PDGF signaling in cells and mice. Cytokine Growth Factor Rev. 2004;15:205–213. doi: 10.1016/j.cytogfr.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Betsholtz C. Insight into the physiological functions of PDGF through genetic studies in mice. Cytokine Growth Factor Rev. 2004;15:215–228. doi: 10.1016/j.cytogfr.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Myllarniemi M, Calderon L, Lemstrom K, Buchdunger E, Hayry P. Inhibition of platelet-derived growth factor receptor tyrosine kinase inhibits vascular smooth muscle cell migration and proliferation. FASEB J. 1997;11:1119–1126. doi: 10.1096/fasebj.11.13.9367346. [DOI] [PubMed] [Google Scholar]
- LeRoith D, Werner H, Beitner-Johnson D, Roberts CT. Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr Rev. 1995;16:143–163. doi: 10.1210/edrv-16-2-143. [DOI] [PubMed] [Google Scholar]
- Zaina S, Pettersson L, Ahren B, Branen L, Hassan AB, Lindholm M, Mattsson R, Thyberg J, Nilsson J. Insulin-like growth factor II plays a central role in atherosclerosis mouse model. J Biol Chem. 2002;277:4504–4511. doi: 10.1074/jbc.M108061200. [DOI] [PubMed] [Google Scholar]
- Zaina S, Nilsson J. Insulin-like growth factor II and its receptors in atherosclerosis and in conditions predisposing to atherosclerosis. Curr Op Lipidol. 2003;14:483–489. doi: 10.1097/00041433-200310000-00009. [DOI] [PubMed] [Google Scholar]
- Bayes-Genis A, Conover CA, Schwartz RS. The insulin-like growth factor axis: a review of atherosclerosis and restenosis. Circ Res. 2000;86(2):125–130. doi: 10.1161/01.res.86.2.125. [DOI] [PubMed] [Google Scholar]
- Eckardt K, May C, Koenen M, Eckel J. IGF-1 receptor signalling determines the mitogenic potency of insulin analogues in human smooth muscle cells and fibroblasts. Diabetol. 2007;50(12):2534–2543. doi: 10.1007/s00125-007-0815-9. [DOI] [PubMed] [Google Scholar]
- Adams TE, Epa VC, Garrett TPJ, Ward CW. Structure and function of the type 1 insulin-like growth factor receptor. Cell Mol Life Sci. 2000;57:1050–1093. doi: 10.1007/PL00000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CE, Rotwein P. Growth, differentiation, and survival: multiple physiological functions for insulin-like growth factors. Physiol Rev. 1996;76:1005–1026. doi: 10.1152/physrev.1996.76.4.1005. [DOI] [PubMed] [Google Scholar]
- Tomizawa M, Saisho H. Insulin-like growth factor (IGF)-II regulates CCAAT/enhancer binding protein alpha expression via phosphatidyl-inositol 3 kinase in human hepatoblastoma cell lines. J Cell Biochem. 2007;102(1):161–170. doi: 10.1002/jcb.21293. [DOI] [PubMed] [Google Scholar]
- Duan C. The chemotactic and mitogenic responses of vascular smooth muscle cells to insulin-like growth factor-I require the activation of ERK1/2. Molec Cell Endocrinol. 2003;206:75–83. doi: 10.1016/s0303-7207(03)00212-0. [DOI] [PubMed] [Google Scholar]
- Feng Y, Zhu Z, Xiao X, Choudhry V, Barrett JC, Dimitriov DS. Novel human monoclonal antibodies to insulin-like growth factor (IGF)-II that potently inhibit the IGF receptor type I signal transduction function. Molec Canc Ther. 2006;5:114–120. doi: 10.1158/1535-7163.MCT-05-0252. [DOI] [PubMed] [Google Scholar]
- Holzer CT, von Itzstein M, Jin B, Pegg MS, Stewart WS, Wu W-Y. Inhibition of sialidases from viral, bacterial and mammalian sources by analogues of 2-deoxy-2,3-didehydro-N-acetylneuraminic acid modified at the C-4 position. Glycoconj J. 1993;10:40–45. doi: 10.1007/BF00731185. [DOI] [PubMed] [Google Scholar]
- von Itzstein M, Wu W-Y, Jin B. The synthesis of 2,3-didehydro-2,4-dideoxy-4-guanidinyl-N-acetylneuraminic acid: a potent influenza virus sialidase inhibitor. Carbohydr Res. 1994;259(2):301–305. doi: 10.1016/0008-6215(94)84065-2. [DOI] [PubMed] [Google Scholar]
- Florio P, Thomson RJ, Alafaci A, Abo S, von Itzstein M. Synthesis of delta4-beta-d-glucopyranosiduronic acids as mimetics of 2,3-unsaturated sialic acids for sialidase inhibition. Bioorg Med Chem Lett. 1999;9:2065–2068. doi: 10.1016/s0960-894x(99)00331-5. [DOI] [PubMed] [Google Scholar]
- Newstead SL, Potter JA, Wilson JC, Xu G, Chien CH, Watts AG, Withers SG, Taylors GL. The structure of Clostridium perfringens Nanl sialidase and its catalytic intermediates. J Biol Chem. 2008;283(14):9080–9088. doi: 10.1074/jbc.M710247200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thobhani S, Ember B, Siriwardena A, Boons GJ. Multivalency and the mode of action of bacterial sialidases. J Am Chem Soc. 2003;125(24):7154–7155. doi: 10.1021/ja029759w. [DOI] [PubMed] [Google Scholar]
- Boraston AB, Ficko-Blean E, Healey M. Carbohydrate recognition by a large sialidase toxin from Clostridium perfringens. Biochemistry. 2007;46(40):11352–11360. doi: 10.1021/bi701317g. [DOI] [PubMed] [Google Scholar]
- Magesh S, Suzuki T, Miyagi T, Ishida H, Kiso M. Homology modeling of human sialidase enzymes NEU1, NEU3 and NEU4 based on the crystal structure of NEU2: hints for the design of selective NEU3 inhibitors. J Mol Graph Model. 2006;25(2):196–207. doi: 10.1016/j.jmgm.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Vaheri A, Ruoslahti E, Nordling S. Neuraminidase stimulates division and sugar uptake in density-inhibited cell cultures. Nat New Biol. 1972;238(85):211–212. doi: 10.1038/newbio238211a0. [DOI] [PubMed] [Google Scholar]
- Nilsson J, Ksiazek T, Thyberg J. Effects of neuraminidase on DNA synthesis, proliferation and endocytosis of cultivated arterial smooth muscle cells. Exp Cell Res. 1982;142(2):333–339. doi: 10.1016/0014-4827(82)90375-5. [DOI] [PubMed] [Google Scholar]
- Nilsson J, Ksiazek T, Thyberg J, Wasteson A. Cell surface components and growth regulation in cultivated arterial smooth muscle cells. J Cell Sci. 1983;64:107–121. doi: 10.1242/jcs.64.1.107. [DOI] [PubMed] [Google Scholar]
- Moustafa I, Connaris H, Taylor M, Zaitsev V, Wilson JC, Kiefel MJ, von Itzstein M, Taylor G. Sialic Acid Recognition by Vibrio cholerae Neuraminidase. J Bol Chem. 2004;279:40819–40826. doi: 10.1074/jbc.M404965200. [DOI] [PubMed] [Google Scholar]
- Sedlacek HH, Stärk J, Seiler F, Ziegler W, Wiegandt H. Cholera toxin induced redistribution of sialoglycolipid receptor at the lymphocyte membrane. Febs Lett. 1976;61(2):272–276. doi: 10.1016/0014-5793(76)81055-1. [DOI] [PubMed] [Google Scholar]
- Galen JE, Ketley JM, Fasano A, Richardson SH, Wasserman SS, Kaper JB. Role of Vibrio cholerae neuraminidase in the function of cholera toxin. Infect Immun. 1992;60:406–415. doi: 10.1128/iai.60.2.406-415.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crennell S, Garman E, Laver G, Vimr E, Taylor G. Crystal structure of Vibrio cholerae neuraminidase reveals dual lectin-like domains in addition to the catalytic domain. Structure (Lond) 1994;2:535–544. doi: 10.1016/s0969-2126(00)00053-8. [DOI] [PubMed] [Google Scholar]
- Gaskell A, Crennell S, Taylor GT. The three domains of a bacterial sialidase: a beta-propeller, an immunoglobulin module and a galactose-binding jelly roll. Structure. 1995;3(11):1197–1205. doi: 10.1016/s0969-2126(01)00255-6. [DOI] [PubMed] [Google Scholar]
- Hinek A, Wilson SE. Impaired elastogenesis in Hurler disease: dermatan sulfate accumulation linked to deficiency in the elastin-binding protein and elastic fiber assembly. Am J Pathol. 2000;156:925–938. doi: 10.1016/S0002-9440(10)64961-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinek A, Smith AC, Cutiongco EM, Callahan JW, Gripp KW, Weksberg R. Decreased elastin deposition and high proliferation of fibroblasts from Costello syndrome are related to functional deficiency in the 67-kD elastin-binding protein. Am J Hum Genet. 2000a;66:859–872. doi: 10.1086/302829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LE, Faris B, Martin BM, Jones HV, Rich CB, Foster JA, Franzblau C. The effect of beta-aminopropionitrile on elastin gene expression in smooth muscle cell cultures. Biochem Biophys Res Commun. 1991;179(2):939–944. doi: 10.1016/0006-291x(91)91909-v. [DOI] [PubMed] [Google Scholar]
- Wilmarth KR, Froines JR. In vitro and in vivo inhibition of lysyl oxidase by aminopropionitriles. J Toxicol Environ Health. 1992;37(3):411–423. doi: 10.1080/15287399209531680. [DOI] [PubMed] [Google Scholar]
- Long JL, Tranquillo RT. Elastic fiber production in cardiovascular tissue-equivalents. Matrix Biol. 2003;22(4):339–350. doi: 10.1016/s0945-053x(03)00052-0. [DOI] [PubMed] [Google Scholar]
- Simon PL, Kumarn V, Lillquist JS, Bhatnagar P, Einstein R, Lee J, Porter T, Green D, Sathell G, Young PR. Mapping of neutralizing epitopes and the receptor binding site of human interleukin 1β. J Biol Chem. 1993;268:9771–9779. [PubMed] [Google Scholar]
- Cebo C, Dambrouck T, Maes E, Laden C, Strecker G, Michalski J-C, Zanetta J-P. Recombinant human interleukins IL-1a, IL-1b, IL-4, IL-6, and IL-7 show different and specific calcium-independent carbohydrate-binding properties. J Biol Chem. 2001;276:5685–5691. doi: 10.1074/jbc.M008662200. [DOI] [PubMed] [Google Scholar]
- Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- Okabe M, Kimura I, Kimura M. Competence effect of PDGF on Ki-67 antigen and DNA contents, and its inhibition by trichostatin-A and a butylydene phthalide BP-421 in primary smooth muscle cells of rat aorta by flow cytometry. Biol Pharm Bull. 1995;18(12):1665–1670. doi: 10.1248/bpb.18.1665. [DOI] [PubMed] [Google Scholar]
- Zeymer U, Fishbein MC, Forrester JS, Cercek B. Proliferating cell nuclear antigen immunohistochemistry in rat aorta after balloon denudation. Comparison with thymidine and bromodeoxyuridine labeling. Am J Pathol. 1992;141(3):685–690. [PMC free article] [PubMed] [Google Scholar]
- Magaud JP, Sargent I, Mason DY. Detection of human white cell proliferative responses by immunoenzymatic measurement of bromodeoxyuridine uptake. J Immunol Methods. 1988;106:95–100. doi: 10.1016/0022-1759(88)90276-1. [DOI] [PubMed] [Google Scholar]
- Yang G, Sun X, Wang R. Hydrogen sulfide-induced apoptosis of human aorta smooth muscle cells via the activation of mitogen-activated protein kinases and caspase-3. FASEB J. 2004;18:1782–1784. doi: 10.1096/fj.04-2279fje. [DOI] [PubMed] [Google Scholar]
- Hinek A, Mecham RP, Keeley F, Rabinovitch M. Impaired elastin fiber assembly is related to reduced 67-kD elastin-binding protein in fetal lamb ductus arteriosus and in cultured aortic smooth muscle cells treated with chondroitin sulfate. J Clin Investig. 1991;88:2083–2094. doi: 10.1172/JCI115538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinek A, Boyle J, Rabinovitch M. Vascular smooth muscle cells detachment from elastin and migration through elastic laminae is promoted by chondroitin sulfate-induced shedding of the 67-kD cell surface elastin binding protein. Exp Cell Res. 1992;203:344–353. doi: 10.1016/0014-4827(92)90008-v. [DOI] [PubMed] [Google Scholar]
- Mochizuki S, Brassart A, Hinek A. Signaling pathways transduced through the elastin receptor facilitate proliferation of arterial smooth muscle cells. J Biol Chem. 2002;277:44854–44863. doi: 10.1074/jbc.M205630200. [DOI] [PubMed] [Google Scholar]
- Li DY, Faury G, Taylor DG, Davis EC, Boyle WA, Mecham RP, Stenzel P, Boak B, Keating MT. Novel arterial pathology in mice and humans hemizygous for elastin. J Clin Invest. 1998b;102:1783–1787. doi: 10.1172/JCI4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DY, Toland AE, Boak BB, Atkinson DL, Ensing GJ, Morris CA, Keating MT. Elastin point mutations cause an obstructive vascular disease, supravalvular aortic stenosis. Hum Mol Gen. 1997;6:1021–1028. doi: 10.1093/hmg/6.7.1021. [DOI] [PubMed] [Google Scholar]
- Urban Z, Riazi S, Siedl TL, Katahira J, Smoot LB, Chitayat D, Boyd CD, Hinek A. Connection between elastin haploinsufficiency and increased cell proliferation in patients with supravalvular aortic stenosis and Williams-Beuren syndrome. Am J Hum Genet. 2002;71:30–44. doi: 10.1086/341035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JCP, Berratt-Boyes BG, Lowe JB. Supravalvular aortic stenosis. Circulation. 1961;24:1311–1318. doi: 10.1161/01.cir.24.6.1311. [DOI] [PubMed] [Google Scholar]
- Beuren AJ, Apitz J, Harmjanz D. Supravalvular aortic stenosis in association with mental retardation and a certain facial seemance. Circulation. 1962;26:1235–1240. doi: 10.1161/01.cir.26.6.1235. [DOI] [PubMed] [Google Scholar]
- Bonten EJ, Galjart NJ, Willemsen R, Usmany M, Vlak JM, d'Azzo A. Lysosomal protective protein/cathepsin A. Role of the “linker” domain in catalytic activation. J Biol Chem. 1995;270:26441–26445. doi: 10.1074/jbc.270.44.26441. [DOI] [PubMed] [Google Scholar]
- Seyrantepe V, Hinek A, Peng J, Fedjaev M, Ernest S, Kadota Y, Canuel M, Itoh K, Morales CR, Lavoie J, Tremblay J, Pshezhetsky AV. Enzymatic activity of lysosomal carboxypeptidase (Cathepsin) A is required for proper elastic fiber formation and inactivation of endothelin-1. Circulation. 2008;117(15):1973–1981. doi: 10.1161/CIRCULATIONAHA.107.733212. [DOI] [PubMed] [Google Scholar]
- Heidenreich K, Brandenburg D. Oligosaccharide heterogeneity of insulin receptors. Comparison of N-linked glycosylation of insulin receptors in adipocytes and brain. Endicrinol. 1986;118(5):1835–1842. doi: 10.1210/endo-118-5-1835. [DOI] [PubMed] [Google Scholar]
- Neilsen D, Gyllberg H, Ostlund P, Bergman T, Bedecs K. Increased levels of insulin and insulin-like growth factor-1 hybrid receptors and decreased glycosylation of the insulin receptor alpha-and beta-subunits in scrapie-infected neuroblastoma N2a cells. Biochem J. 2004;380(2):571–579. doi: 10.1042/BJ20040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald RG, Czech MP. Biosynthesis and processing of the type II insulin-like growth factor receptor in H-35 hepatoma cells. J Biol Chem. 1985;260:11357–11365. [PubMed] [Google Scholar]
- Daniel TO, Milfay DF, Escobedo J, Williams LT. Biosynthetic and glycosylation studies of cell surface platelet-derived growth factor receptors. J Biol Chem. 1987;262(20):9778–9784. [PubMed] [Google Scholar]
- Hosang M. Characterization of a platelet-derived growth factor receptor on Swiss 3T3 cells by affinity crosslinking. J Recept Res. 1988;8(1–4):455–466. doi: 10.3109/10799898809049004. [DOI] [PubMed] [Google Scholar]
- Miyagi T, Sato K, Hata K, Taniguchi S. Metastatic potential of transformed rat 3Y1 cell lines is inversely correlated with lysosomal-type sialidase activity. FEBS Lett. 1994;349:255–259. doi: 10.1016/0014-5793(94)00682-2. [DOI] [PubMed] [Google Scholar]
- Tokuyama S, Moriya S, Yasui A, Miyazaki J, Taniguchi S, Miyagi T. Suppression of pulmonary metastasis in mouse B16 melanoma cells. Int J Cancer. 1997;73:410–415. doi: 10.1002/(sici)1097-0215(19971104)73:3<410::aid-ijc16>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Kato T, Wang Y, Yamaguchi K, Milner CM, Shineha R, Satomi S, Miyagi T. Overexpression of lysosomal-type sialidase leads to suppression of metastasis associated with reversion of malignant phenotype in murine B16 melanoma cells. Int J Cancer. 2001;92(6):797–804. doi: 10.1002/ijc.1268. [DOI] [PubMed] [Google Scholar]
- Kakugawa Y, Wada T, Yamaguchi K, Yamanami H, Ouchi K, Sato I, Miyagi T. Up-regulation of plasma membrane-associated ganglioside sialidase (Neu3) in human colon cancer and its involvement in apoptosis suppression. Proc Natl Acad Sci. 2002;99(16):10718–10723. doi: 10.1073/pnas.152597199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S, Saito S, Wada T, Yamaguchi K, Satoh M, Arai Y, Miyagi T. Plasma membrane-associated sialidase is up-regulated in renal cell carcinoma and promotes interleukin-6-induced apoptosis suppression and cell motility. J Biol Chem. 2006;281(12):7756–7764. doi: 10.1074/jbc.M509668200. [DOI] [PubMed] [Google Scholar]
- Wada T, Hata K, Yamaguchi K, Shiozaki K, Koseki K, Moriya S, Miyagi TA. Crucial role of plasma membrane-associated sialidase in the survival of human cancer cells. Oncogene. 2007;26(17):2483–2490. doi: 10.1038/sj.onc.1210341. [DOI] [PubMed] [Google Scholar]