Abstract
The compound 4-hydroxynonenal (4-HNE) is the major aldehyde formed during lipid peroxidation of ω-6-polyunsaturated fatty acids and has been suggested to regulate inflammatory responses because it inhibits tumor necrosis factor (TNF) mRNA production in the human monocytic cell line THP-1. Here we demonstrate that 4-HNE inhibits TNF and interleukin-1β production in human monocytes in response to lipopolysaccharide. The main action of 4-HNE occurred at the pretranscriptional level; there was no effect on TNF mRNA production or stability when 4-HNE was added after stimulation. The mechanism of action of 4-HNE appears to be downstream of lipopolysaccharide-receptor binding. In the human monocytic MonoMac 6 cell line, 4-HNE caused selective inhibition of the activity of the mitogen-activated protein kinases p38 and ERK1/ERK2, but not JNK. However, in monocytes, the activities of all three kinases were inhibited, suggesting that the effects of 4-HNE were exerted at points upstream of ERK1/ERK2 and JNK as the levels of the phosphorylated kinases were reduced. In contrast, p38 phosphorylation was not inhibited, suggesting that 4-HNE affects kinase activity. 4-HNE also inhibited nuclear factor-κB activation in monocytes. In view of the roles of p38, ERK1/ERK2, JNK, and nuclear factor-κB in inflammation, the data suggest that 4-HNE, at nontoxic concentrations, has anti-inflammatory properties, most likely through an effect on these signaling molecules, and could lead to the development of novel treatments for inflammatory diseases.
Lipid peroxidation has been implicated in the pathogenesis of many diseases including atherosclerosis. A key feature of lipid peroxidation is the breakdown of polyunsaturated fatty acids to yield a broad array of smaller molecules including aldehydes. Among these, 4-hydroxynonenal (4-HNE) is known to be the major aldehyde formed during lipid peroxidation of ω-6-polyunsaturated fatty acids such as linoleic acid and arachidonic acid.1,2
Previous reports have shown that a 15-lipoxygenase-derived metabolite of arachidonic acid, 15-hydroperoxyeicosatetraenoic acid (15-HPETE), inhibits lipopolysaccharide (LPS)-induced tumor necrosis factor (TNF) production in monocytes at both the pretranscriptional level and by accelerating TNF mRNA degradation.3 Since it has been shown that 15-HPETE breaks down to 4-HNE through enzymatic and nonenzymatic pathways4,5,6 and previous studies showed the inhibition of TNF production by 4-HNE,7 it is possible that 15-HPETE may exert some of its effects through the more stable breakdown product, 4-HNE.
At very high concentrations >100 μmol/L, 4-HNE is acutely toxic to mammalian cells and leads to cell death within 1 hour or less. However it is unlikely to reach such concentrations in cells and organs, although such concentrations could be built up locally in peroxidizing membranes for a short time.8 It has been suggested that 4-HNE may accumulate in membranes at concentrations of 5 to 20 μmol/L in response to oxidative insults.1,2 The effects of such concentrations include the stimulation of neutrophil chemotaxis, modulation of adenylate cyclase activity, and stimulation of phospholipase C.1 Besides its effects on neutrophils, 4-HNE has displayed various other pro-inflammatory effects. At 0.1 to 10 μmol/L it up-regulated TGF-β mRNA in J774 murine macrophages and human U937 promonocytes differentiated with phorbol 12-myristate 13-acetate.9 The aldehyde (25 to 50 μmol/L) also displayed pro-inflammatory activity in inducing COX-2 mRNA in RAW264.7 murine macrophages by activating the p38 MAPK kinase pathway10,11 and chemotactic activity toward monocytes/macrophages.12 More recently however, 4-HNE was found to inhibit nuclear factor (NF)-κΒ activation in a dose-dependent manner by inhibiting phosphorylation of the inhibitory protein, IκΒα, in the monocytic cell line THP-1.7 4-HNE has also been found to inhibit the metabolism of arachidonic acid to form cyclooxygenase and lipoxygenase metabolites,13 as well as inhibiting the production of adhesion molecules ICAM-1, VCAM-1, and E-selectin.14 These results suggest that 4-HNE plays a role in regulating the inflammatory reaction.
The signs and symptoms of a diverse range of such seemingly unrelated diseases as rheumatoid arthritis, multiple sclerosis, cancer, septic shock, and hematopoietic disorders such as aplastic anemia and myelodysplastic syndrome can be attributed to the overproduction of TNF. The overproduction of TNF can be acute and intense, as in the case of sepsis, or chronic and low grade, as in the case of chronic disorders such as rheumatoid arthritis. In the case of bacterial sepsis, endotoxins cause intense production of TNF from monocytes and macrophages resulting in the clinical manifestations of septic shock.15 The damaging effects of such high and intense TNF levels are attributable to its capacity as a master initiator of the cytokine cascade leading to the end-organ failure that often proves to be fatal.
The major source of secreted TNF is cells of the monocyte/macrophage lineage and one of the most potent stimuli for inducing TNF release is bacterial LPS. LPS forms complexes with the plasma protein LBP (LPS binding protein) and is transferred to the CD14 receptors a membrane protein found specifically on monocytes/macrophages. CD14 then transfers LPS to a complex of TLR4 (Toll-like receptor) and MD-2 (myeloid differentiation factor) and thereby initiates the LPS response.16,17 The main pathways involved in the activation of monocytes/macrophages by LPS include the p38, ERK, and JNK MAPKs and the IKKβ pathways.16,17,18 These in turn activate the various transcription factors involved in TNF expression and monocyte/macrophage activation including Elk-1, c-jun, c-Fos, ATF-1, ATF-2, SRF, CREB, and NF-κΒ. The ability of 4-HNE to inhibit monocyte/macrophage activation and subsequent TNF production has implications in the regulation of chronic inflammatory responses in diseases such as rheumatoid arthritis in which anti-TNF antibody therapy is currently practiced.19
To gain better insights into the potential role of 4-HNE as a regulator of pathogenesis and its relationship to the anti-inflammatory properties of 15-HPETE, we have examined its mechanism of action on human monocyte/macrophage TNF production at the pre- and posttranscriptional level in particular its effects on the MAP kinase pathways, because these are known to play a role in production of TNF by monocytes.20 We present evidence that its prime action is at the pretranscriptional level involving inhibition of selective members of the MAPK family.
Materials and Methods
Leukocytes
Mononuclear leukocytes (MNLs) were prepared from the blood of healthy volunteers using an adaptation of the Hypaque/Ficoll method.21 Briefly, blood was carefully layered onto a solution of Angiograffin (Schering, Munich, Germany)/Ficoll400 (Pharmacia Biotech, Uppsala, Sweden)/sodium diatrizoate (Sigma Chemical Co., St. Louis, MO) of density 1.114. After centrifugation at 600 × g for 30 to 35 minutes, the leukocytes resolved into two bands. The lower density MNLs in the top band were harvested and washed twice with RPMI medium (JRH Biosciences, Lenexa, KS).
Monocytes/macrophages were enriched from the MNL fraction by density gradient centrifugation.22 Briefly, MNLs were layered onto a 46% iso-osmotic Percoll gradient and centrifuged at 600 × g for 30 minutes at room temperature. The monocytes formed a band at the interface of the gradient and the lymphocytes formed a pellet at the bottom of the tube. Once harvested, the monocytes were washed twice in ice-cold RPMI medium with 1 mmol/L ethylenediaminetetraacetic acid by centrifugation at 4°C.
The human monocytic cell line MonoMac 6 was maintained at 37°C with 5% CO2 air and high humidity at a density of 1 × 106cells/ml in 250-ml culture flasks. Cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum (JRH Biosciences), 4 mmol/L l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin (CSL, Victoria, Australia), 1× nonessential amino acids solution, and 1 mmol/L sodium pyruvate (Sigma Chemical Co.).
Stimulation of Leukocytes
MNLs/monocytes were plated on a 96-well U-bottom plate (Nalge Nunc International, Rochester, NY) at 2 × 105 cells/well. Cells were pretreated as stated in the figure legends, by the addition of 50 μl of MNLs (4 × 106/ml) and 50 μl of the appropriate solution of 4-HNE (Cayman Chemical Co., Ann Arbor, MI) in RPMI 1640 medium and incubated at 37°C with 5% CO2 air and high humidity for the appropriate time. After pretreatment with 4-HNE, cells were stimulated by the addition of 100 μl of LPS (Sigma Chemical Co.) in RPMI 1640 medium supplemented with 5% heat-inactivated AB serum. Stimulation was allowed to proceed for 72 hours at 37°C with 5% CO2 air and high humidity. The cell-free supernatant was removed and stored at −70°C for cytokine analysis via enzyme-linked immunosorbent assay.
Quantitation of TNF and Interleukin (IL)-1β in Culture Supernatants
Cytokine levels in cell-free supernatants were measured using a four step enzyme-linked immunosorbent assay.23 Readings were made from TNF/IL-1β standards (NIBSC, Hertfordshire, UK) on a standard curve.
TNF mRNA Measurements and Determination of Rate of Decay
The transformation of TNF insert plasmid into competent cells and probe purification was done as previously described.24 MonoMac 6 cells (1 × 106) were induced as described in the figure legends.24 In all cases, cells were stimulated with 10 ng/ml of LPS in the presence of 10% (v/v) heat-inactivated FCS (final concentrations). Total cellular RNA for the slot blots was prepared by the Trizol (Invitrogen, Carlsbad, CA) method and analyzed as previously described.24 Rates of TNF mRNA were measured essentially as described previously using Actinomycin D to block further transcription.3
Measurement of MAP Kinase Activation
Measurement of p38 and ERK activity was performed as described previously.25 Briefly, precleared lysates were incubated with anti-p38 or anti-ERK2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA). After precipitation with protein A Sepharose, kinase activity was conducted in the presence of 32P-ATP. A solid phase assay for JNK activity was used as described previously.25 Briefly, lysates were incubated with GST-jun (1 to 79), which is coupled to glutathione Sepharose. After extensive washing, the beads were isolated for kinase assays in the presence of 32P-ATP. To determine MAP kinase phosphorylation, lysates were subjected to Western blotting as described.25 The blots were probed with anti-ACTIVE ERK antibody (Promega Corp., Madison, WI) and sequentially stripped and reprobed with anti-ACTIVE p38 (Promega Corp.), anti-phospho JNK (Santa Cruz Biotechnology), and finally anti-ERK2 antibody (Santa Cruz Biotechnology), to assess loading. The order in which the anti-active MAP kinase antibodies was used varied between experiments.
Measurement of NF-κB Activation
The activation of NF-κB was assessed by measuring the degradation of cytoplasmic IκB-α by Western blotting. Monocytes were pretreated with 4-HNE and stimulated with 10 ng/ml of LPS, after stimulation (15 minutes) cells were lysed and the level of IκB-α analyzed by Western blotting as previously described.26
LPS Binding Assay
The binding of LPS to monocytes was measured using flow cytometry. Monocytes were treated as described in the figure legends before the addition of fluorescein isothiocyanate-LPS (Sigma Chemical Co.) (1 μg/ml) for 30 minutes at 4°C. The cells were washed twice with 1 ml of Isoton II (Coulter, Brookvale, Australia) and resuspended in 500 μl of Isoton II. The fluorescence intensity of the samples was measured immediately using a flow cytometer (Becton Dickinson, Mountain View, CA).
Statistical Analysis
All data were analyzed for significance using the Tukey or Student-Newman-Keuls test for multiple comparisons. All graphical and statistical analyses were done using Graphpad Prism version 3.00 (Graphpad Software, San Diego, CA).
Results
4-HNE Inhibits TNF and IL-1β Production in Human Monocytes
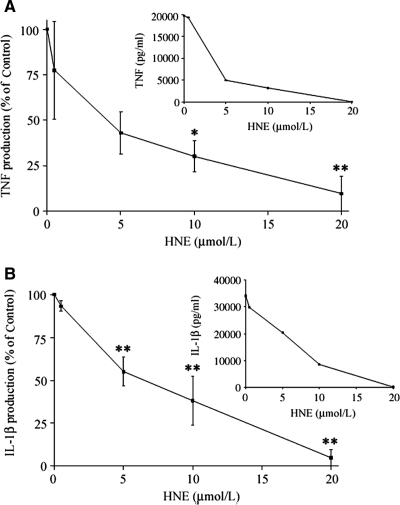
Previously it has been reported that 4-HNE inhibited LPS-induced TNF production in THP-1 cells.7To ensure that this was not specific to a macrophage cell line we examined effects of 4-HNE on peripheral blood monocyte TNF and IL-1β production. The cells were pretreated with varying concentrations of 4-HNE (5 to 20 μmol/L) and then stimulated with 10 ng/ml of LPS. 4-HNE was found to inhibit the production of TNF induced by LPS in a concentration-dependent manner (Figure 1). 4-HNE attenuated both TNF and IL-1β production with IC50 of 5.1 and 6.8 μmol/L, respectively.
Figure 1.
A: The effect of 4-HNE on TNF production in LPS-stimulated monocytes. Cells were pretreated with varying concentration of 4-HNE for 60 minutes and then challenged with 10 ng/ml of LPS. After 72 hours the culture supernatant was collected and TNF concentrations measured by enzyme-linked immunosorbent assay. Results are presented as percentage of TNF produced in the presence of 4-HNE relative to nontreated control. These are expressed as a mean ± SEM of four experiments. *P < 0.05, **P < 0.01. The inset graph shows a representative experiment in which the results are shown as pg/ml of cytokine produced. B: The effect of 4-HNE on IL-1β production in LPS-stimulated monocytes. Cells were pretreated with varying concentration of 4-HNE for 60 minutes and then challenged with 10 ng/ml of LPS. After 72 hours the culture supernatant was collected and IL-1β concentrations measured by enzyme-linked immunosorbent assay. Results are expressed as percentage of IL-1β produced in the presence of 4-HNE relative to nontreated control. These are presented as the mean ± SEM of four experiments. *P < 0.05, **P < 0.01. The inset graph shows a representative experiment in which results are presented as pg/ml of cytokine produced.
4-HNE Does Not Influence TNF mRNA Stability and Targets Pretranscriptional Pathways
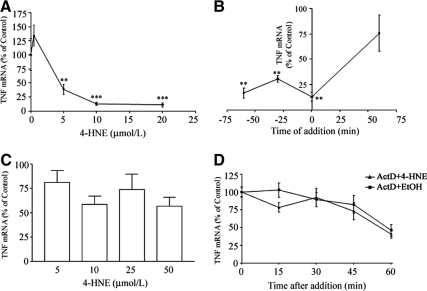
MonoMac 6 cells pretreated with 4-HNE showed a similar concentration-related inhibitory effect on TNF mRNA production (Figure 2A) to that seen with monocytes and MNLs, with an IC50 of 4.2 μmol/L. To ascertain whether 4-HNE also accelerated the decay of TNF mRNA, MonoMac 6 cells were treated with 10 μmol/L 4-HNE for different times relative to LPS stimulation (Figure 2B.) The aldehyde inhibited TNF mRNA when added before or at the same time as LPS but not if added after LPS addition. This suggests that it does not affect posttranscriptional events such as TNF mRNA stability. Not surprisingly, unlike 15-HPETE our data showed that 4-HNE had no effect on the level of TNF mRNA if added 60 minutes after stimulation with LPS (Figure 2C). Even when the concentration of 4-HNE was increased to 50 μmol/L, there was no effect on TNF mRNA levels. This indicates that there is no effect of 4-HNE on posttranscriptional events, at least at this level. To ascertain that 4-HNE did not influence TNF mRNA stability we examined the effects of treating LPS-prestimulated cells with 4-HNE in the presence of Actinomycin D. Then the levels of TNF mRNA were followed throughout time. The results in Figure 2D show that the rates of TNF mRNA decay are the same in the presence and absence of 4-HNE.
Figure 2.
The effect of 4-HNE on pretranscriptional and posttranscriptional events. A: MonoMac 6 cells were pretreated with varying concentrations of 4-HNE 60 minutes before stimulation with 10 ng/ml of LPS. After a further 90-minute incubation the level of TNF mRNA was determined by slot blot analysis. B: The effect of 4-HNE on TNF mRNA production in relation to time of LPS addition. Cells were treated with 10 μmol/L of 4-HNE at 60 minutes, 30 minutes, and 0 minutes before LPS addition as well as 60 minutes after LPS addition. C: Effect of varying the concentration of 4-HNE after treatment on TNF mRNA production. Cells were stimulated with LPS and after 60 minutes were treated with 5 to 50 μmol/L of 4-HNE. D: The effects of 4-HNE on rates of TNF mRNA decay. Cells were treated with LPS. Ninety minutes later they were treated with 10 μmol/L of 4-HNE in the presence of Actinomycin D (5 μg/ml). The TNF mRNA levels were followed. The results are expressed as percentage of TNF mRNA levels in the presence of 4-HNE relative to the nontreated control. These are expressed as mean ± SEM of six experiments. Differences between test and controls showed *P < 0.05, **P < 0.01, and ***P < 0.001.
Effect of HNE on LPS Binding to Monocytes
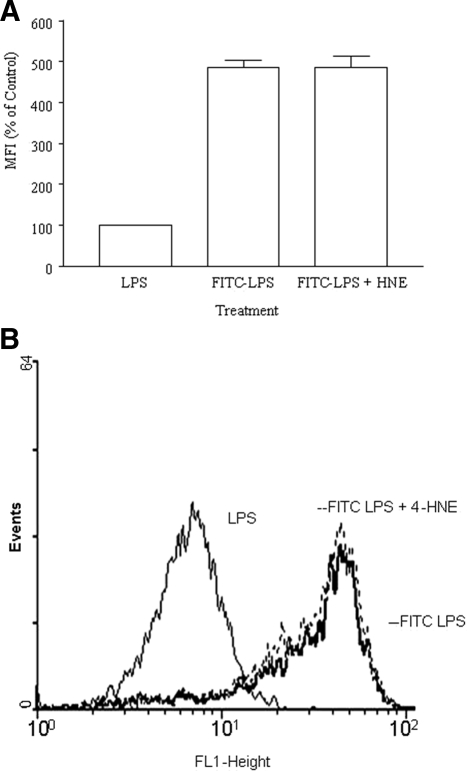
A starting point in dissecting the pretranscriptional effect of 4-HNE is to examine effects at the LPS binding step. The effects of 4-HNE on LPS binding to the human monocytes was assessed. Cells were pretreated for 1 hour with 20 μmol/L 4-HNE before the addition of 1 μg/ml of fluorescein isothiocyanate-labeled LPS. 4-HNE did not affect LPS binding to the monocytes (Figure 3).
Figure 3.
The effect 4-HNE on LPS binding to monocytes. Monocytes were pretreated with 4-HNE (20 μmol/L) and then incubated with 1 μg/ml of fluorescein isothiocyanate-LPS and the fluorescence intensity measured using a flow cytometer. A: Data are presented as a percentage of mean fluorescence intensity (MFI) in the 4-HNE-treated relative to the nontreated control. These are expressed as a mean ± SEM of three experiments. B: A representative histogram of the above results.
Effects of 4-HNE on p38, ERK, and JNK
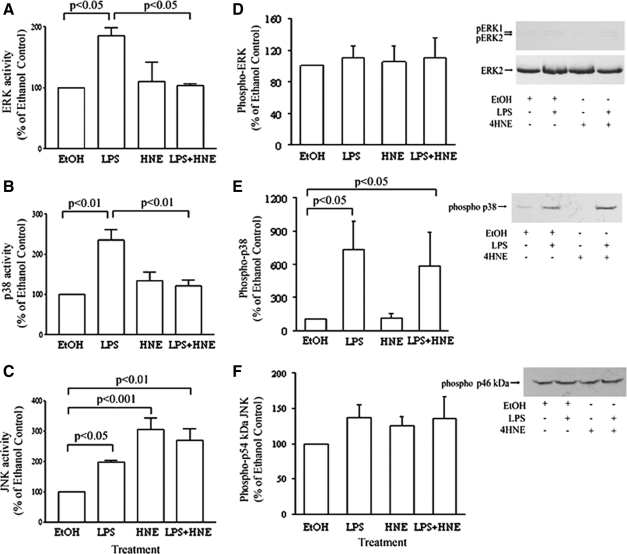
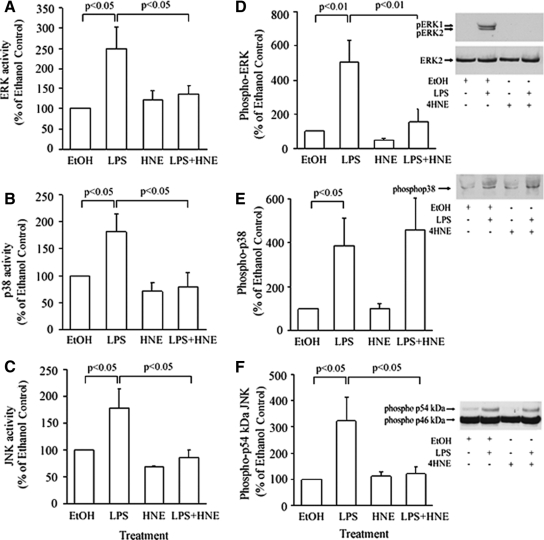
LPS is known to use MAP kinases to promote the production of TNF.20 Thus, the effects of 4-HNE on LPS-induced activation of these intracellular signaling pathways was examined using MonoMac 6 cells. These were pretreated with 10 μmol/L 4-HNE for 1 hour and then stimulated with LPS (10 ng/ml) for 15 minutes before assessing kinase activity, measured by the radioactive kinase assay. 4-HNE was shown to significantly inhibit p38 and ERK activity induced by LPS (Figure 4, A and B, respectively). In contrast to this, not only was LPS-induced JNK activity not inhibited, but at the same concentration, 4-HNE was able to stimulate JNK activity (Figure 4C).
Figure 4.
The effect 4-HNE on ERK (A), p38 (B), and JNK (C) activity and phosphorylation of the three kinases (D–F) in LPS-stimulated MonoMac 6 cells. Cells were pretreated with 10 μmol/L 4-HNE for 60 minutes and then stimulated with LPS. Kinase activity was then measured by a radioactive kinase assay. For Western blotting, lysates were fractionated on 12% SDS gels, and proteins were transferred to nitrocellulose. The blots were probed with anti-ACTIVE ERK antibody and sequentially stripped and reprobed with an antibody against the phosphorylated forms of either p38 or JNK and finally anti-ERK2 antibody, to assess loading. The results are expressed as percentage of the response of nonstimulated cells and as means ± SEM of three to four experiments.
To determine whether the effects of 4-HNE were exerted at an upstream kinase, we examined whether or not the aldehyde affected the phosphorylation of the MAP kinases under the same conditions as above. The results on ERK1/ERK2 showed that LPS did not stimulate phosphorylation of the kinase and 4-HNE had no effect on this (Figure 4D). The aldehyde did not inhibit the LPS-induced phosphorylation of p38 (Figure 4E). Surprisingly, unstimulated MonoMac 6 cells showed a high baseline phosphorylation of the 46-kDa form of JNK (Figure 4F). Phosphorylation of the 54-kDa form of JNK was not observed, even on prolonged exposure to the film. What is interesting and unexplained is that JNK activity was affected by the treatments without a significant change in the degree of phosphorylation of the p46 and p54 JNK forms (Figure 4F). Since Zhang and colleagues27 showed that 4-HNE increased the phosphorylation of ERK1/ERK2 and p38 at earlier time points, we examined the effect of the aldehyde at these earlier time points. The results showed that 4-HNE also caused an increase in the levels of phospho-ERK1/ERK2 and p38 in MonoMac 6 cells at 5, 10, and 30 minutes (data not presented). In contrast, no change in phospho-JNK was observed (data not shown).
Because our work extended to the human peripheral blood monocytes, it was relevant to see if these changes in intracellular signaling were correlated in normal cells. Interestingly with monocytes, the results showed that 4-HNE inhibited the LPS-induced increase in enzymatic activity of all three MAP kinases (Figure 5, A–C). Although both ERK and JNK phosphorylation induced by LPS were inhibited by 4-HNE, there was no effect on p38 phosphorylation (Figure 5, D–F). This suggests that 4-HNE may directly inhibit p38 kinase activity in primary monocytes. As observed with MonoMac 6 cells, there was a high baseline phosphorylation of the p46 kDa form of JNK but the treatments predominantly affected the phosphorylation status of the p54 kDa JNK.
Figure 5.
The effect 4-HNE on ERK (A), p38 MAPK (B), and JNK (C) activity and phosphorylation of the three kinases (D–F) in LPS-stimulated peripheral blood monocytes. Cells were pretreated with 10 μmol/L 4-HNE for 60 minutes and then stimulated with LPS. Kinase activity was then measured by a radioactive kinase assay, and lysates were blotted as described in legend to Figure 4. The results are expressed as percentage of the response of nonstimulated cells and are mean ± SEM of three to four experiments.
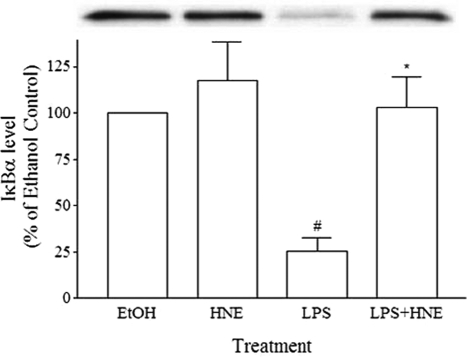
4-HNE Inhibits LPS-Mediated NF-κB Activation
Although it has been previously reported that 4-HNE inhibits NF-κB activation in THP-1 cells, it was evident that these cells show constitutive nuclear NF-κB.28 We found similar results with our studies on MonoMac 6 cells (data not presented). Because this raises the issue as to the validity of these results when relating these to normal cells, we examined the effects of 4-HNE on LPS-induced NF-κB activation in monocytes, by quantitating the levels of cytoplasmic IκB-α. Treatment with 10 μmol/L 4-HNE significantly inhibited the LPS-induced IκB-α degradation (Figure 6).
Figure 6.
The effect of 4-HNE on IκB-α degradation in human monocytes. Monocytes were pretreated for 1 hour with 10 μmol/L 4-HNE for 15 minutes and then stimulated with LPS (10 ng/ml). After stimulation, cells were lysed, and the level of IκB-α was analyzed by Western blotting. Inset shows band intensity of Western blot results from a representative experimental run. Data presented are the mean ± SEM of three experiments. *P < 0.05 with respect to stimulated cells, #P < 0.05 with respect to unstimulated cells.
Discussion
Previously, studies on the effects of 4-HNE on mononuclear phagocyte function were restricted to the monocytic cell line, THP-1.7 We have extended this study by showing that human monocyte TNF production can also be inhibited by 4-HNE when these cells are stimulated with LPS, with an IC50 of ∼5 μmol/L for both cell types. We also found that the inhibitory effect of 4-HNE was not restricted to TNF because similar effects were observed in the ability to produce IL-1β. At these concentrations 4-HNE was not toxic to either monocytes or MonoMac 6 cells.
These effective concentrations are attainable in vivo during an inflammatory reaction suggesting that 4-HNE may regulate monocyte/macrophage function. Under noninflammatory conditions, sub-μmol/L levels of 4-HNE in the plasma have been reported and are suggested to be the resting physiological concentrations of 4-HNE. However, it is relevant that this can reach up to 45 μmol/L during inflammation/pathophysiology,1 and 1 mmol/L in cellular membranes.2 Up to 100 μmol/L of 4-HNE has been reported in hepatocytes. Others have estimated that 4-HNE concentrations of 150 mmol/L are present in the lipid phase of strongly oxidized LDL.29 Human monocytes exposed to hemozoin, a malarial parasite pigment, generate ∼50 μmol/L of 4-HNE.30 Our findings demonstrate that 4-HNE has anti-inflammatory effects at levels generated in cells, tissues, and fluids (5 to 20 μmol/L).
To attempt to increase our understanding of the mechanism involved we examined whether the action of 4-HNE was at a pre- or posttranscriptional level. Unlike the previously reported effects of 15-HPETE,3 4-HNE acted only at a pretranscriptional level. No evidence was found to suggest that it acted at a posttranscriptional level, the aldehyde had no effects on TNF mRNA stability. Thus, although 15-HPETE can be converted to 4-HNE, it is evident that the effects of this hydroperoxide on TNF mRNA stability cannot be accounted for by the generation of 4-HNE. Previously Page and colleagues7 reported that 4-HNE inhibited NF-κB activation in THP-1. Here we demonstrate in human monocytes that 4-HNE can indeed inhibit the NF-κB pathway, a transcriptional factor involved in the production of TNF. Difficulties were encountered with measurement of NF-κB using the MonoMac 6 cell line, in that high levels of baseline activation were detected (data not presented). Levels of cytoplasmic IκB-α were significantly lower in MonoMac 6 cells than in monocytes, suggesting a high level of baseline activation. The ability of 4-HNE to inhibit LPS-induced NF-κB is likely to contribute to the inhibition of TNF production.
The research has identified other key mechanisms of the regulation of macrophage function by 4-HNE, summarized in Table 1. Consistent with previous data showing that 4-HNE transiently increased p38 phosphorylation in the murine macrophage cell line, RAW264.7,10,11 our data show that the aldehyde caused a transient increase in the phosphorylation of p38 and ERK1/ERK2 in MonoMac 6 cells (data not presented). Although pretreatment with 4-HNE did not affect LPS-induced p38 phosphorylation, the aldehyde inhibited LPS-stimulated increase in p38 activity in the human monocytic cell line MonoMac 6. LPS-induced increase in p38 activity in monocytes was also inhibited without an accompanying reduction in p38 phosphorylation. This suggests a direct inhibitory effect of 4HNE on p38 kinase activity. Furthermore, we found that 4-HNE inhibits LPS-stimulated ERK activity in MonoMac 6 and primary monocytes. Although 4-HNE inhibited LPS-stimulated JNK activity in peripheral blood monocytes, JNK activity in MonoMac 6 cells was increased by the aldehyde. Previous reports have established that inhibition of ERK1/ERK2, p38, or JNK alone is sufficient to inhibit cytokine production.31,32,33 For example blocking p38 inhibits production of TNF in monocytes. Our data suggest that the combined effects of the inhibition of p38, ERK, and JNK is likely to be responsible for the profound inhibitory effect of 4-HNE on the production of TNF in human macrophages.
Table 1.
Summary of the Effects of 4-HNE on Phosphorylation and Activity Levels of ERK1/ERK2, p38, and JNK MAP Kinases in Nonstimulated and LPS-Stimulated MonoMac 6 and Monocytes
| MonoMac 6
|
Monocytes
|
|||||||
|---|---|---|---|---|---|---|---|---|
| MAP kinase | Phospho−
|
Activity
|
Phospho−
|
Activity
|
||||
| − | +LPS | − | +LPS | − | +LPS | − | +LPS | |
| ERK | Undetectable | − | ↓ | − | ↓ | − | ↓ | |
| P38 | − | − | − | ↓ | − | − | − | ↓ |
| JNK | Undetectable | ↑ | − | − | ↓ | − | ↓ | |
Undetectable, indicates that there was no observable change in the level of phosphoMAP kinase in the absence and presence of LPS; ↓ indicates that the response to LPS was depressed by 4-HNE; ↑ indicates an increased basal level by 4-HNE; − indicates no change in levels.
It has been shown previously that 4-HNE activates JNK in primary human hepatic stellate cells by a direct interaction with JNK itself.34 The current data show that 4-HNE stimulates JNK activity in MonoMac 6, perhaps by a similar mechanism because we found that phospho-JNK levels were not increased. This is consistent with lack of effect of 4-HNE on JNK phosphorylation in hepatic stellate cells,34 unlike what had been observed in the human bronchial epithelial cell-line, HBE1.35 The data in HBB1 cells would be consistent with the observation that 4-HNE-stimulated JNK activity by stimulating the activity of the upstream SEK1.36 Thus, there may be a number of ways through which 4-HNE affects JNK activity. Further dissociation between MAP kinase activity and phosphorylation was observed in MonoMac 6 cells. Although LPS increased the activities of the three MAP kinases, only the phosphorylation of p38 was enhanced by LPS. It is possible that the levels of phospho-ERK and phospho-p54 kDa JNK were too low to be detected by the anti-ACTIVE/phospho-MAP kinase antibodies. Consistent with this suggestion, the same antibodies detected the phosphorylated forms of the MAP kinases in LPS-stimulated peripheral blood monocytes. The presence of ERK2 on the blots demonstrates that there was a sufficient amount of MAP kinase on the membrane and this is further confirmed by the ability to detect phospho-p38 on the same blots. In the case of ERK, it has previously been reported that LPS did not increase its phosphorylation in the monocytic cell line, THP-1.37
Our data highlight the need to exercise reservations on results from cell lines, as already previously reported.38 In comparison to MonoMac 6 cells, we found 4-HNE to cause the inhibition of all three kinases in response to LPS in monocytes. The monocytes did show an increase in levels of phospho-ERK and phospho-p54 kDa JNK in response to LPS. For ERK1/ERK2 and JNK the decrease in activity caused by 4-HNE correlated with the levels of phosphokinases, suggesting that 4-HNE acts on an upstream kinase. However with p38, no change in levels of LPS-induced phospho-p38 was induced by 4-HNE. This suggests that 4-HNE may act directly on p38 to inhibit its activity.
Although the effects of 4-HNE could be mediated through a depletion in the intracellular GSH pool, being metabolized to 4-HNE-GSH conjugate,39 this is unlikely. In human monocytes, the activation of ERK1/ERK2 by LPS is not influenced by the intracellular GSH status, unlike the phosphorylation of p38, which varies with GSH levels.37,40 In fact it has been reported that subjecting cells to H2O2, which depletes GSH41actually stimulates MAP kinases.42
4-HNE at concentrations used in this study can inhibit the activation of certain PKC isozymes by inhibiting the ability of these isozymes to translocate to the membrane fraction, most probably by interfering with the interaction between the PKC isozymes and membrane phospholipids.43,44 An interaction between 4-HNE and phospholipids has also been reported44 and 4-HNE forms Michael adducts with alkenylphospholipids, especially the O-alk-1-enyl form of phosphatidylethanolamine.45 This could interfere with the functioning of phospholipid-dependent signaling molecules because of a perturbation in the microenvironment in which these signaling molecules are activated by or interact with their respective phospholipid regulators. Thus, it is also likely that there are upstream signaling molecules that are dependent on membrane phospholipids for activation involved in the effects of 4-HNE. We found no evidence that 4-HNE prevented the binding of LPS to its receptor, suggesting that signaling molecules proximal to TLR4 may be affected by these changes in the lipid environment. Although there is also the possibility that 4-HNE directly modifies the MAP kinases through a Michael addition reaction, this is unlikely to contribute to the observed changes in MAP kinase activity in the MonoMac 6 given that the effects of 4-HNE on the three MAP kinases are not only incongruent but there are also dissimilarities between MonoMac 6 and peripheral blood monocytes for the same MAP kinases, eg, JNK kinase activity.
Microbial invasion is likely to lead to the release of 4-HNE that could thus modify innate immunity and in this manner even influence the adaptive immune response. It is also widely appreciated that excessive monocyte/macrophage activation and overexpression of TNF and its relative IL-1β lies at the heart of many inflammatory diseases such as rheumatoid arthritis and septic shock. Therefore endogenous inhibitors of TNF/IL-1β production and monocyte/macrophage activation, such as 4-HNE, could provide a novel pathway for the development of novel pharmaceuticals. Furthermore, the dual nature of 4-HNE in possessing both protective and destructive properties may need to be given careful consideration when anti-oxidant supplementation is being practiced.
Acknowledgments
We thank Dr. Yong Qin Li for her assistance in the running of the assays for the signaling molecules and Mrs. Renee Mackenzie for excellent secretarial assistance in the preparation of the manuscript.
Footnotes
Address reprint requests to Professor Antonio Ferrante, Department of Immunopathology, Children, Youth, and Women’s Health Services; 72 King William Rd., North Adelaide, SA, 5006. E-mail: antonio.ferrante@adelaide.edu.au.
Supported by the National Health and Medical Research Council of Australia, the National Heart Foundation of Australia and WCH Foundation.
References
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res. 2003;42:318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- Ferrante JV, Ferrante A. Novel role of lipoxygenases in the inflammatory response: promotion of TNF mRNA decay by 15-hydroperoxyeicosatetraenoic acid in a monocytic cell line. J Immunol. 2005;174:3169–3172. doi: 10.4049/jimmunol.174.6.3169. [DOI] [PubMed] [Google Scholar]
- Williams MV, Lee SH, Blair IA. Liquid chromatography/mass spectrometry analysis of bifunctional electrophiles and DNA adducts from vitamin C mediated decomposition of 15-hydroperoxyeicosatetraenoic acid. Rapid Commun Mass Spectrom. 2005;19:849–858. doi: 10.1002/rcm.1854. [DOI] [PubMed] [Google Scholar]
- Schneider C, Porter NA, Brash AR. Autoxidative transformation of chiral omega6 hydroxy linoleic and arachidonic acids to chiral 4-hydroxy-2E-nonenal. Chem Res Toxicol. 2004;17:937–941. doi: 10.1021/tx049913n. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Benedetti A, Lang J, Fulceri R, Fauler G, Comporti M. Studies on the mechanism of formation of 4-hydroxynonenal during microsomal lipid peroxidation. Biochim Biophys Acta. 1986;876:154–166. doi: 10.1016/0005-2760(86)90329-2. [DOI] [PubMed] [Google Scholar]
- Page S, Fischer C, Baumgartner B, Haas M, Kreusel U, Loidl G, Hayn M, Ziegler-Heitbrock HW, Neumeier D, Brand K. 4-Hydroxynonenal prevents NF-kappaB activation and tumor necrosis factor expression by inhibiting IkappaB phosphorylation and subsequent proteolysis. J Biol Chem. 1999;274:11611–11618. doi: 10.1074/jbc.274.17.11611. [DOI] [PubMed] [Google Scholar]
- Benedetti A, Comporti M, Fulceri R, Esterbauer H. Cytotoxic aldehydes originating from the peroxidation of liver microsomal lipids. Identification of 4,5-dihydroxydecenal. Biochim Biophys Acta. 1984;792:172–181. doi: 10.1016/0005-2760(84)90219-4. [DOI] [PubMed] [Google Scholar]
- Leonarduzzi G, Scavazza A, Biasi F, Chiarpotto E, Camandola S, Vogel S, Dargel R, Poli G. The lipid peroxidation end product 4-hydroxy-2,3-nonenal up-regulates transforming growth factor beta1 expression in the macrophage lineage: a link between oxidative injury and fibrosclerosis. FASEB J. 1997;11:851–857. doi: 10.1096/fasebj.11.11.9285483. [DOI] [PubMed] [Google Scholar]
- Kumagai T, Matsukawa N, Kaneko Y, Kusumi Y, Mitsumata M, Uchida K. A lipid peroxidation-derived inflammatory mediator: identification of 4-hydroxy-2-nonenal as a potential inducer of cyclooxygenase-2 in macrophages. J Biol Chem. 2004;279:48389–48396. doi: 10.1074/jbc.M409935200. [DOI] [PubMed] [Google Scholar]
- Kumagai T, Nakamura Y, Osawa T, Uchida K. Role of p38 mitogen-activated protein kinase in the 4-hydroxy-2-nonenal-induced cyclooxygenase-2 expression. Arch Biochem Biophys. 2002;397:240–245. doi: 10.1006/abbi.2001.2601. [DOI] [PubMed] [Google Scholar]
- Müller K, Hardwick SJ, Marchant CE, Law NS, Waeg G, Esterbauer H, Carpentar KL, Mitchinson MJ. Cytotoxic and chemotactic potencies of several aldehydic components of oxidised low density lipoprotein for human monocyte-macrophages. FEBS Lett. 1996;388:165–168. doi: 10.1016/0014-5793(96)00559-5. [DOI] [PubMed] [Google Scholar]
- Sakuma S, Fujimoto Y, Tagano S, Tsunomori M, Nishida H, Fujita T. Effects of nonanal, trans-2-nonenal and 4-hydroxy-2,3-trans-nonenal on cyclooxygenase and 12-lipoxygenase metabolism of arachidonic acid in rabbit platelets. J Pharm Pharmacol. 1997;49:150–153. doi: 10.1111/j.2042-7158.1997.tb06770.x. [DOI] [PubMed] [Google Scholar]
- Minekura HKT, Kawamoto Y, Nara F, Uchida K. 4-Hydroxy-2-nonenal is a powerful endogenous inhibitor of endothelial response. Biochem Biophys Res Commun. 2001;282:557–561. doi: 10.1006/bbrc.2001.4586. [DOI] [PubMed] [Google Scholar]
- Beutler B, Cerami A. The common mediator of shock, cachexia, and tumor necrosis. Adv Immunol. 1988;42:213–231. doi: 10.1016/s0065-2776(08)60846-9. [DOI] [PubMed] [Google Scholar]
- Fujihara M, Muroi M, Tanamoto K, Suzuki T, Azuma H, Ikeda H. Molecular mechanisms of macrophage activation and deactivation by lipopolysaccharide: roles of the receptor complex. Pharmacol Ther. 2003;100:171–194. doi: 10.1016/j.pharmthera.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Palsson-McDermott EM, O'Neill LA. Signal transduction by the lipopolysaccharide receptor. Toll-like receptor-4. Immunology. 2004;113:153–162. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13:85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- van den Berg WB. Anti-cytokine therapy in chronic destructive arthritis. Arthritis Res. 2001;3:18–26. doi: 10.1186/ar136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudhope SJ, Finney-Hayward TK, Nicholson AG, Mayer RJ, Barnette MS, Barnes PJ, Donnelley LE. Different MAP-kinase dependent cytokine responses in cells of the monocyte lineage. J Pharmacol Exp Ther. 2008;326:306–312. doi: 10.1124/jpet.107.127670. [DOI] [PubMed] [Google Scholar]
- Ferrante A, Thong YH. A rapid one-step procedure for purification of mononuclear and polymorphonuclear leukocytes from human blood using a modification of the Hypaque-Ficoll technique. J Immunol Methods. 1978;24:389–393. doi: 10.1016/0022-1759(78)90143-6. [DOI] [PubMed] [Google Scholar]
- Seager Danciger J, Lutz M, Hama S, Cruz D, Castrillo A, Lazaro J, Phillips R, Premack B, Berliner J. Method for large scale isolation, culture and cryopreservation of human monocytes suitable for chemotaxis, cellular adhesion assays, macrophage and dendritic cell differentiation. J Immunol Methods. 2004;288:123–134. doi: 10.1016/j.jim.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Ferrante A, Staugas RE, Rowan-Kelly B, Bresatz S, Kumaratilake LM, Rzepczyk CM, Adolf GR. Production of tumor necrosis factors alpha and beta by human mononuclear leukocytes stimulated with mitogens, bacteria, and malarial parasites. Infect Immun. 1990;58:3996–4003. doi: 10.1128/iai.58.12.3996-4003.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante JV, Huang ZH, Nandoskar M, Hii CS, Robinson BS, Rathjen DA, Poulos A, Morris CP, Ferrante A. Altered responses of human macrophages to lipopolysaccharide by hydroperoxy eicosatetraenoic acid, hydroxy eicosatetraenoic acid, and arachidonic acid. Inhibition of tumor necrosis factor production. J Clin Invest. 1997;99:1445–1452. doi: 10.1172/JCI119303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hii CS, Huang ZH, Bilney A, Costabile M, Murray AW, Rathjen DA, Der CJ, Ferrante A. Stimulation of p38 phosphorylation and activity by arachidonic acid in HeLa cells. HL60 promyelocytic leukemic cells, and human neutrophils. Evidence for cell type-specific activation of mitogen-activated protein kinases. J Biol Chem. 1998;273:19277–19282. doi: 10.1074/jbc.273.30.19277. [DOI] [PubMed] [Google Scholar]
- Ferrante A, Robinson BS, Singh H, Jersmann HP, Ferrante JV, Huang ZH, Trout NA, Pitt MJ, Rathjen DA, Easton CJ, Poulos A, Prager RH, Lee FS, Hii CS. A novel beta-oxa polyunsaturated fatty acid down-regulates the activation of the IkappaB kinase/nuclear factor kappaB pathway, inhibits expression of endothelial cell adhesion molecules, and depresses inflammation. Circ Res. 2006;99:34–41. doi: 10.1161/01.RES.0000231292.66084.cd. [DOI] [PubMed] [Google Scholar]
- Zhang H, Dickinson DA, Liu RM, Forman HJ. 4-Hydroxynonenal increases gamma-glutamyl transpeptidase gene expression through mitogen-activated protein kinase pathways. Free Radic Biol Med. 2005;38:463–471. doi: 10.1016/j.freeradbiomed.2004.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenberger M, Pforete A, Sternsdorf T, Passlick B, Baeuerle PA, Ziegler-Heitbrock HW. Constitutive nuclear NFκB in cells of the monocyte lineage. Biochem J. 1994;304:87–94. doi: 10.1042/bj3040087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterbauer H, Ramos P. Chemistry and pathophysiology of oxidation of LDL. Rev Physiol Biochem Pharmacol. 1996;127:31–64. doi: 10.1007/BFb0048264. [DOI] [PubMed] [Google Scholar]
- Schwarzer E, Muller O, Arese P, Siems WG, Grune T. Increased levels of 4-hydroxynonenal in human monocytes fed with malarial pigment hemozoin. A possible clue for hemozoin toxicity. FEBS Lett. 1996;388:119–122. doi: 10.1016/0014-5793(96)00523-6. [DOI] [PubMed] [Google Scholar]
- Adams JL, Boehm JC, Gallagher TF, Kassis S, Webb EF, Hall R, Sorenson M, Garigipati R, Griswold DE, Lee JC. Pyrimidinylimidazole inhibitors of p38: cyclic N-1 imidazole substituents enhance p38 kinase inhibition and oral activity. Bioorg Med Chem Lett. 2001;11:2867–2870. doi: 10.1016/s0960-894x(01)00570-4. [DOI] [PubMed] [Google Scholar]
- Scherle PA, Jones EA, Favata MF, Daulerio AJ, Covington MB, Nurnberg SA, Magolda RL, Trzaskos JM. Inhibition of MAP kinase kinase prevents cytokine and prostaglandin E2 production in lipopolysaccharide-stimulated monocytes. J Immunol. 1998;161:5681–5686. [PubMed] [Google Scholar]
- Zhu J, Krishnegowda G, Gowda DC. Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: the requirement of extracellular signal-regulated kinase, p38, c-Jun N-terminal kinase and NFkappaB pathways of expression of proinflammatory cytokines and nitric oxide. J Biol Chem. 2005;280:8617–8627. doi: 10.1074/jbc.M413539200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parola M, Robino G, Marra F, Pinzani M, Bellomo G, Leonarduzzi G, Chiarugi P, Camandola S, Poli G, Waeg G, Gentilini P, Dianzani MU. HNE interacts directly with JNK isoforms in human hepatic stellate cells. J Clin Invest. 1998;102:1942–1950. doi: 10.1172/JCI1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson DA, Iles KE, Watanabe N, Iwamoto T, Zhang H, Krzywanski DM, Forman HJ. 4-hydroxynonenal induces glutamate cysteine ligase through JNK in HBE1 cells. Free Radic Biol Med. 2002;33:974–978. doi: 10.1016/s0891-5849(02)00991-7. [DOI] [PubMed] [Google Scholar]
- Song BJ, Soh Y, Bae M, Pie J, Wan J, Jeong K. Apoptosis of PC12 cells by 4-hydroxy-2-nonenal is mediated through selective activation of the c-Jun N-terminal protein kinase pathway. Chem Biol Interact. 2001;130–132:943–954. doi: 10.1016/s0009-2797(00)00247-7. [DOI] [PubMed] [Google Scholar]
- Utsugi M, Dobashi K, Ishizuka T, Endou K, Hamuro J, Murata Y, Nakazawa T, Mori M. c-Jun N-terminal kinase negatively regulates lipopolysaccharide-induced IL-12 production in human macrophages: role of mitogen-activated protein kinase in glutathione redox regulation of IL-12 production. J Immunol. 2003;171:628–635. doi: 10.4049/jimmunol.171.2.628. [DOI] [PubMed] [Google Scholar]
- Rao KMK. MAP kinase activation in macrophages. J Leukoc Biol. 2001;69:3–10. [PubMed] [Google Scholar]
- Siems W, Grune T. Intracellular metabolism of 4-hydroxynonenal. Mol Aspects Med. 2003;24:167–175. doi: 10.1016/s0098-2997(03)00011-6. [DOI] [PubMed] [Google Scholar]
- Utsugi M, Dobashi K, Koga Y, Shimizu Y, Ishizuka T, Iizuka K, Hamuro J, Nakazawa T, Mori M. Glutathione redox regulates lipopolysaccharide-induced IL-12 production through p38 mitogen-activated protein kinase activation in human monocytes: role of glutathione redox in IFN-gamma priming of IL-12 production. J Leukoc Biol. 2002;71:339–347. [PubMed] [Google Scholar]
- Erlemann K-R, Cossette C, Gravel S, Lesimple A, Lee GJ, Saha G, Rokach J, Powell WS. Airway epithelial cells synthesize the lipid mediator 5-oxo-ETE in response to oxidative stress. Free Radic Biol Med. 2007;42:654–664. doi: 10.1016/j.freeradbiomed.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyton KZ, Liu Y, Gorospe M, Xu Q, Holbrook NJ. Activation of mitogen-activated protein kinase by H2O2. Role in cell survival following oxidant injury. J Biol Chem. 1996;271:4138–4142. doi: 10.1074/jbc.271.8.4138. [DOI] [PubMed] [Google Scholar]
- Nitti M, Domenicotti C, d'Abramo C, Assereto S, Cottalasso D, Melloni E, Poli G, Biasi F, Marinari UM, Pronzato MA. Activation of PKC-beta isoforms mediates HNE-induced MCP-1 release by macrophages. Biochem Biophys Res Commun. 2002;294:547–552. doi: 10.1016/S0006-291X(02)00512-0. [DOI] [PubMed] [Google Scholar]
- Chiarpotto E, Domenicotti C, Paola D, Vitali A, Nitti M, Pronzato MA, Biasi F, Cottalasso D, Marinari UM, Dragonetti A, Cesaro P, Isidoro C, Poli G. Regulation of rat hepatocyte protein kinase C beta isoenzymes by the lipid peroxidation product 4-hydroxy-2,3-nonenal: a signaling pathway to modulate vesicular transport of glycoproteins. Hepatology. 1999;29:1565–1572. doi: 10.1002/hep.510290510. [DOI] [PubMed] [Google Scholar]
- Bacot S, Bernoud-Hubac N, Baddas N, Chantegrel B, Deshayes C, Doutheau A, Lagarde M, Guichardant M. Covalent binding of hydroxy-alkenals 4-HDDE, 4-HHE, and 4-HNE to ethanolamine phospholipid subclasses. J Lipid Res. 2003;44:917–926. doi: 10.1194/jlr.M200450-JLR200. [DOI] [PubMed] [Google Scholar]