Abstract
Delayed-type hypersensitivity (DTH) reactions are characterized by a strong cellular infiltrate, including neutrophils, macrophages, and T lymphocytes. In all these cell types, both E- and P-selectin-dependent adhesion pathways play a significant role in recruitment into the inflamed skin. Accordingly, inhibition of selectin-mediated interactions (eg, by antibodies) results in impairment of acute DTH reactions. However, whether inhibition of a specific cell type is responsible for the anti-inflammatory effect or whether all leukocytes are affected remains unclear. To address this question, we used fucosyltransferase-VII knockout mice that lack functional selectin ligands as either donors or recipients in a DTH model elicited by Th1 cell and antigen transfer. We found that selectin-mediated adhesion is required by Th1 effector cells to enter the DTH reaction site and, additionally, to elicit the DTH reaction. On the other hand, elimination of selectin binding in the recipient’s neutrophils and macrophages by use of fucosyltransferase-deficient mice receiving wild-type Th1 effector cells resulted in a strongly reduced infiltration of neutrophils and macrophages but unimpaired footpad swelling. These findings demonstrate a major role for both E- and P-selectin in the recruitment of different leukocyte cell types. However, only the presence of selectin ligands on T cells was critical for the inflammatory reaction. These findings reveal T cells as the predominant targets for selectin blockade that aim to suppress skin inflammation.
A classic hallmark of inflammation is the local infiltration by leukocytes. The cellular components of the infiltrate depend on the type of immune reaction, eg, Th1-type immune reactions are characterized by infiltration of T cells, granulocytes, and macrophages, whereas a Th2-driven inflammation usually contains eosinophils beside T cells. The infiltrates are initiated by the local, inflammation-induced release of chemoattractants and by up-regulation of adhesion molecules on endothelial cells within inflamed sites.
The initial step of the adhesion cascade, rolling and tethering, is predominantly mediated by selectins.1,2,3 E- and P-selectin are expressed on inflamed vessels within nonlymphoid sites, notably within the skin. Because of the preferential accumulation of CLA/E-selectin ligand-positive T cells within the skin, E-selectin is considered as a skin-specific adhesion molecule.4 In contrast, P-selectin has been shown to support homing not only to inflamed skin sites, but also into inflamed peritoneal and mucosal sites.5,6 E- and P-selectin have overlapping and partially redundant function in T-cell recruitment into inflamed sites.7
Selectin ligands are constitutively expressed on neutrophils and macrophages.8 On T cells, they are induced on differentiation from naïve to effector/memory T cells.9 In vitro, IL-12 is a major inductor of E- and P-selectin ligands on CD4+ and CD8+ T cells.10,11,12 In vivo, selectin ligand expression is found on both interferon (IFN)-γ and interleukin (IL)-4-producing T cells and also on subsets of regulatory T cells,13,14,15,16 and it is regulated by tissue-specific signals.17 Selectin ligands are composed of carrier proteins, such as PSGL-1, which are posttranslationally modified by glycosyltransferases. For the generation of selectin ligands in T cells fucosyltransferase (FucT)-VII and core-2 β1,6-glucosaminyltransferase (C2GlcNAT)-I play a critical role.18,19,20 Deficiency in either of these transferases abolishes binding of T cells to E- and P-selectin. FucT-IV has a role partially overlapping that of FucT-VII; P-selectin ligands generated by FucT-VII are crucial for initial leukocyte tethering, whereas E-selectin ligands that permit maximal slowing require simultaneous expression of FucT-IV and FucT-VII.21 Inflammation-dependent leukocyte recruitment and lymphocyte homing, which are retained to a minor degree in FucT-VII-deficient mice, are completely lost in doubly-deficient mice.22 T-cell-dependent immune reactions, such as the DTH or cutaneous hypersensitivity reactions, are impaired after E- and P-selectin blockade and reduced in FucT-VII and FucT-IV/VII mice.19,23,24 Studies in selectin-deficient mice showed the redundancy of E- and P-selectin: whereas blockade of P-selectin by antibody treatment abrogated delayed-type hypersensitivity (DTH) in the skin in E-selectin-deficient mice the same treatment had no effect in wild-type mice.25 In contrast, a study by Subramaniam and colleagues26 suggested an important role of P-selectin alone in cutaneous hypersensitivity reactions because a strong reduction of cellular infiltration was seen in P-selectin-deficient mice. Although these studies provided compelling evidence for a major role of selectin-mediated interactions in inflammation, the question as to the crucial cellular components depending on selectin function remained unanswered.
We have recently developed a DTH model in which a strong local inflammation is induced by transfer of in vitro-generated antigen-specific Th1 effector cells into recipient mice that are challenged by local application of antigen.27 By transfer of Th1 cells from either wild-type or FucT knockout (KO) mice we were able to determine the impact of selectin-mediated adhesion on both the recruitment of T cells into the DTH reaction and the induction of the DTH reaction. In addition, transfer of wild-type Th1 cells into FucT KO recipients allowed us to elucidate the impact of selectin-mediated adhesion on myeloid cell recruitment and inflammatory function.
Our results show that the infiltration of both T cells and leukocytes is to a large degree dependent on the selectin pathway, yet the reduced number of leukocytes recruited in a selectin-independent manner is still sufficient to mount a strong inflammatory reaction. In contrast, selectin ligand deficiency on the inducer T cells critically influences the severity of the local immune response.
Materials and Methods
Mice
BALB/c, DO11.10, C57/BL6, and OT-II mice were purchased from the BfR (Bundesinstitut fuer Risikobewertung, Berlin, Germany). FucT-VII−/− and FucT-IV/VII−/− were on a BL6 background. FucT-VII−/−mice were backcrossed to BALB/c at least seven times. Then, these mice were crossed with DO11.10 mice to generate FucT-VII−/−xDO11.10 mice. FucT-IV/VII−/− were left on C57/BL6 background. FucT-VII−/−, FucT-IV/VII−/−, and FucT-VII−/−xDO11.10 mice were bred under specific pathogen-free conditions in our animal facility. All mice were used at an age of 8 to 10 weeks for the experiments. All animal experiments were performed in accordance with institutional, state, and federal guidelines.
Antibodies, Staining, and Sorting Reagents
The following antibodies were produced in our laboratory: anti-IL-4 (11B11), fluorescein isothiocyanate- and Cy5-labeled anti-CD4 (GK1.5), Cy5-labeled anti-OVA T-cell receptor (KJ1.26), fluorescein isothiocyanate-labeled anti-CD4-F(ab) (GK1.5), and fluorescein isothiocyanate-labeled anti-IFN-γ (AN18.17.25). As anti-P-selectin antibody, either RB-40 or RMP-1, and as anti-E-selectin antibody, RME-1 or UZ-4 were used, with similar results. The recombinant P-selectin human IgG fusion protein was kindly provided by D. Vestweber (Munster, Germany). Phycoerythrin-labeled anti-human IgG antibodies were obtained from Jackson ImmunoResearch (West Grove, PA). All microbeads were obtained from Miltenyi Biotec (Gladbach, Germany).
Flow Cytometry
Cytometric analysis was performed using a FACSCalibur and CellQuest software (BD Biosciences, Franklin Lakes, NJ). Dead cells were excluded by staining with 4,6-diamidino-2-phenylindol or propidium iodide (Sigma, St. Louis, MO). P-selectin ligands were detected by P-selectin human IgG chimeric protein and phycoerythrin-conjugated anti-human IgG antibody as secondary reagent in Hanks’ balanced salt solution containing Ca2+ and Mg2+. OVA-TCR transgenic (OVA-TCRtg) CD4+ T cells were identified using the clonotype-specific antibody KJ1.26. For intracellular cytokine staining cells were stimulated with phorbol myristate acetate/ionomycin (10 ng/ml, 500 ng/ml; Sigma) for 4 hours with addition of Brefeldin A (10 μg/ml, Sigma) for the last 2 hours. After stimulation, cells were surface-stained for tg TCR and CD4. Afterward cells were fixed in 2% paraformaldehyde (Sigma), permeabilized by 0.5% saponin (Sigma), and stained intracellularly for IFN-γ.
Th1-Mediated DTH Model
OVA-specific Th1 cells were generated in vitro by antigen-specific activation of naive CD4+ T cells from DO11.10 (BALB/c background) or FucT-VII−/−xDO11.10 mice (BALB/c background) as previously described.28 FucT-IV/VII−/− mice were only available on BL6-background; in this case, Th1 cells were generated from OT-II mice (BL6 background). This protocol results in generation of an effector cell population containing 50 to 80% cells producing IFN-γ and less than 1% producing IL-4. For induction of the DTH response, a total of 5 × 105 OVA-TCRtg Th1 cells were injected intravenously into recipient mice. Twenty-four hours later, 250 ng of OVA323-339 peptide in 5 μl of incomplete Freund’s adjuvant (IFA, Sigma) were injected subcutaneously into the left footpad. Phosphate-buffered saline (PBS)/IFA was injected into the right footpad as a control. This treatment induces a strong DTH reaction within the footpad containing OVA323-339 peptide. Depending on the background, slight differences in the kinetics of the reaction are observed, ie, in BALB/c mice reaction peaks at 24 hours whereas in BL6 mice the initial peak occurs at 48 hours. The strength of inflammation was determined by measuring the footpad swelling using an Oditest micrometer gauge (Kroeplin Laengenmesstechnik, Schlüchtern, Germany).
For blocking experiments mice were treated with 200 μg/mouse anti-P-selectin antibody (RMP-1) and 200 μg/mouse anti-E-selectin antibody (RME-1) at the time point of cell transfer and again on day 2 after induction of the DTH. For depletion experiments 150 μg/mouse anti-Gr-1 antibody (RB6 8C5; BD Biosciences) were administered intraperitoneally on days −2 and +1 of the DTH induction, ie, antigen administration. Efficiency of depletion was confirmed by fluorescence-activated cell sorting analysis of whole blood cells showing less than 1% Gr-1-positive cells in anti-Gr-1 monoclonal antibody (mAb)-treated mice 24 hours after antibody treatment. This was confirmed by morphological analysis in blood smears.
Homing of Adoptively Transferred Th1 Cells
The homing assay was performed as previously described.28 Briefly, in vitro-generated Th1 effector cells were labeled with 51chromium (Amersham Buchler, Braunschweig, Germany) at 37°C (2 × 107 cells/ml, 20 μCi/ml) followed by 2 hours of incubation at 37°C in fresh medium and removal of dead cells with gradient centrifugation (17.1% isotonic Nycodenz; Oslo Nyegaard, Norway). Labeled cells were adoptively transferred into recipient animals on day 1 after DTH induction. Twenty-four hours after transfer, animals were sacrificed and the indicated tissues were removed. Measurement of recovered radioactivity was done using a γ-counter (Wallac, Turku, Finland). In addition to the analysis of the migration behavior, 51Cr-labeling allows the determination of the global survival rate of cells in vivo because dying cells release 51Cr, which is rapidly excreted from the animal.29 By counting the radioactivity of a sample of the transferred cell population and detection of the overall radioactivity within a mouse, the percentage of recovery corresponding to the survival of the transferred cells can be determined.
Histology and Immunohistochemistry of Tissue Sections
Histological evaluation of inflamed footpad tissue was performed on days 1 or 2 after DTH induction. For immunostaining, 4-μm-thick sections of formalin-fixed, paraffin-embedded tissue were cut, deparaffinized, and subjected to a heat-induced epitope retrieval step. Slides were rinsed in cool running water and washed in Tris-buffered saline (pH 7.4) before incubation with primary antibodies against CD3 (clone N1580; DAKO, Glostrup, Denmark), myeloperoxidase (MPO, clone A0398; DAKO), and F4/80 (eBioscience, San Diego, CA) for 30 minutes. For detection biotinylated donkey anti-rat (Dianova, Hamburg, Germany) secondary antibody, and/or the streptavidin AP kit (K5005, DAKO) was used. Alkaline phosphatase was revealed by Fast Red as chromogen. Evaluation and grading of hematoxylin and eosin (H&E)-stained samples were performed by microscopic analysis by an experienced pathologist after examination of five high-power fields (hpf) per sample. For this, an Olympus AX70 microscope with JVC KY-F70 camera (JVC, Yokohama, Japan) and DISKUS software (Koenigswinter, Germany) were used. Image processing was performed using Adobe Photoshop 7.0 (Adobe, San Jose, CA). For overall infiltration and tissue damage a 0 to 4 score was used (footpad score): 0, no infiltrates, no edema; 1, loose phlegmonous infiltrates, little edema; 2, moderately dense predominantly phlegmonous infiltrates within subcutaneous fatty tissue, moderate edema; 3, abscess formation within subcutaneous fatty tissue, extensive edema; 4, abscess formation, infiltrates in skeletal muscle, extensive edema. Neutrophil and macrophage infiltrates were counted according to cell-specific staining in five hpf per sample.
Statistics
Data are presented as mean ± SD. Nonparametrical tests (Wilcoxon log rank test, Mann-Whitney U-test) were used for DTH responses, homing experiments, and histological analysis. Differences were considered statistically significant at P < 0.05 and highly significant at P < 0.01.
Results
E- and P-Selectin Blockade Prevents the Development of an Acute DTH Reaction
The adoptive transfer of 5 × 105 OVA-TCRtg Th1 cells into BALB/c recipients followed by injection of OVA323-339 peptide in IFA into the footpad generates a strong DTH reaction showing peak swelling at 24 hours after antigen challenge. In this model of inflammation, a few effector T cells activated by antigen act as pioneer cells and elicit in an autofeedback loop the accumulation of large numbers of T cells as well as of other leukocytes (S. Ghani et al, in preparation).
To determine the role of E- and P-selectin-dependent adhesion mechanisms for this type of DTH reaction we treated recipient mice with antibodies directed against E- and P-selectin before antigen challenge and again on day 2 after induction of the DTH response. In the OVA-treated footpads, blocking of E- and P-selectin almost completely abolished the antigen-specific footpad swelling compared with mice treated with control IgG (Figure 1; P < 0.01 for each time point), confirming the crucial role of selectins in an acute inflammation.
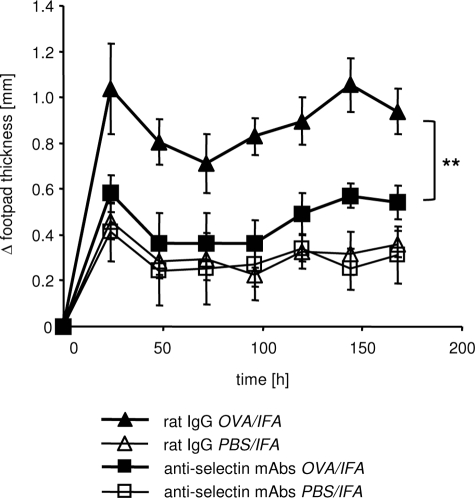
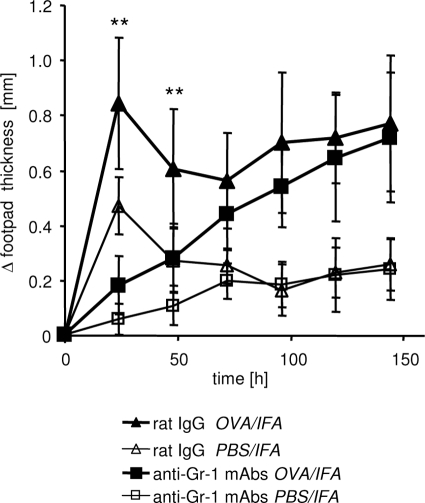
Figure 1.
Blockade of E- and P-selectin abolishes DTH response. The DTH reaction was induced by intravenous transfer of OVA-TCRtg Th1 effector cells and subsequent (24 hours later) subcutaneous injection of OVA323-339 peptide in IFA into footpads of WT recipient mice. Anti-E- and P-selectin antibodies or rat IgG were injected intravenously at the time of Th1 cell transfer and again intraperitoneally on day 2 after induction of the DTH. Footpad swelling of the OVA323-339 peptide-containing site (OVA/IFA) or control site (PBS/IFA) was determined at the indicated time points (shown as Δ footpad thickness). A significant reduction of the antigen-specific response (OVA/IFA site) could be measured throughout time in anti-E- and P-selectin mAb-treated mice compared with the rat IgG-treated control group. **P < 0.01; Mann-Whitney; n = 5 to 6.
T-Effector Cells Require Selectin Ligands for Entry into an Established DTH Reaction
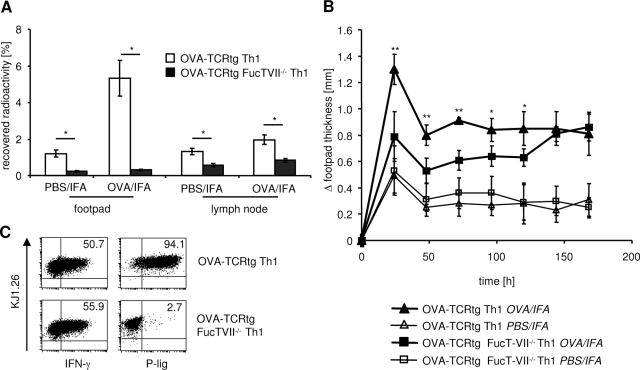
To determine the importance of selectin-dependent adhesion pathways for the accumulation of T-effector cells in the inflamed skin we measured the migration of radioactively labeled Th1 cells into an established DTH reaction. Therefore, we compared FucT-VII-deficient cells lacking functional selectin ligands to wild-type (WT) cells. Th1 cells from FucT-VII−/− × DO11.10 mice or DO11.10 mice were radioactively labeled and injected into mice that had received unlabeled OVA-TCRtg Th1 cells intravenously and OVA/IFA into the footpad 48 hours and 24 hours before to induce DTH. Radioactively labeled cells were allowed to home to inflamed tissues for 4 hours and 24 hours. Immigration of FucT-VII-deficient Th1 cells into inflamed sites and, to a lesser extent, into draining lymph nodes was dramatically reduced after periods of 4 hours (data not shown) and 24 hours of homing when compared with WT control cells (Figure 2A). This was not attributable to impaired survival because the recovery of radioactivity, corresponding to cell survival, was similar, in fact even slightly higher using OVA-TCRtg FucT-VII−/− Th1 (68.8%) compared to OVA-TCRtg Th1 cells (55.1%). Immigration of FucT-VII−/− Th1 cells into control sites, where some unspecific inflammation caused by the adjuvant was observed, was also reduced. Trafficking of FucT-VII−/− Th1 cells to large organs such as spleen, liver, and lung was unimpaired, in line with the inflammation-specific role of E/P-selectins. These data demonstrate again that expression of selectin ligands on T-effector cells is a prerequisite for their immigration into the inflamed skin. In addition, these data provide evidence that, in T cells, the presence of FucT-IV cannot compensate for the lack of FucT-VII.19,30
Figure 2.
Selectin ligand deficiency of Th1 effector cells results in reduced recruitment into inflammatory sites and inhibits induction of DTH response. A: The DTH reaction was induced as described in Figure 1. Radioactively labeled indicator OVA-TCRtg Th1 effector cells derived from DO11.10 or FucT-VII−/− × DO11.10 mice were transferred on day 1 of the DTH response into WT recipients. After 24 hours, accumulation of these cells was determined by measurement of radioactivity in large organs (not shown) and the inflamed (OVA/IFA) and control (PBS/IFA) footpads as well as corresponding draining lymph nodes (n = 4; *P < 0.05; Mann-Whitney test). B: The DTH was induced by OVA-TCRtg Th1 effector cells derived either from DO11.10 or FucT-VII−/− × DO11.10 mice and injection of OVA323-339 peptide in IFA into the footpad. Footpad swelling of the OVA peptide-containing site (OVA/IFA) or control site (PBS/IFA) was determined at the indicated time points. Significant reduction of the OVA-specific response was measured from days 1 to 5 in mice that had received OVA-TCRtg Th1 cells derived from FucT-VII−/− × DO11.10 mice compared with WT OVA-TCRtg Th1 cells. (n = 5; *P < 0.05; **P < 0.01; Mann-Whitney test; one of two independent experiments). C: In vitro-generated OVA-TCRtg Th1 cells from DO11.10 and FucT-VII−/−× DO11.10 mice were stained for KJ1.26, functional selectin ligand expression (P-lig), and IFN-γ production.
Selectin-Dependent Migration of Pioneer T Cells Is Instrumental in the Induction of a DTH Reaction
To determine whether the efficient suppression of the early DTH response by anti-selectin Abs is caused by the inability of the T-effector cells to reach the inflamed site we used OVA-TCRtg FucT-VII-deficient Th1 cells in comparison to OVA-TCRtg WT Th1 cells to elicit the DTH reaction. FucT-VII deficiency resulted in a reduction of the antigen-specific DTH response at the initial peak at 24 hours by 70% (mean, 68.8 ± 20.5%; mean of individual data from two independent experiments) compared with WT Th1 effector cells (Figure 2B). However, the effect was not as profound and long lasting as that observed with in vivo treatment with antibodies directed against E- and P-selectin and as one might expect according to the dramatically impaired recruitment of FucT-VII−/− T cells into the DTH. This might suggest a potential contribution of selectin-dependent recruitment of neutrophils or macrophages to the pathology of an early DTH reaction. To exclude the possibility that the observed differences between FucT-VII-deficient Th1 cells and WT Th1 cells are because of differences in Th1 differentiation, namely in production of IFN-γ required for tissue conditioning, we determined IFN-γ production in FucT-VII−/− and WT Th1 cells. No impairment of IFN-γ production was found in FucT-VII−/− Th1 cells lacking selectin binding (Figure 2C). This shows that selectin-dependent immigration of T-effector cells into the site of antigen challenge is of major importance, yet the small residual, FucT-VII-independent recruitment nevertheless allows for a slow development of the inflammatory reaction.
Unimpaired DTH Reaction after Transfer of WT Th1 Effector Cells into Selectin Ligand-Deficient Mice Despite Reduced Leukocyte Infiltration
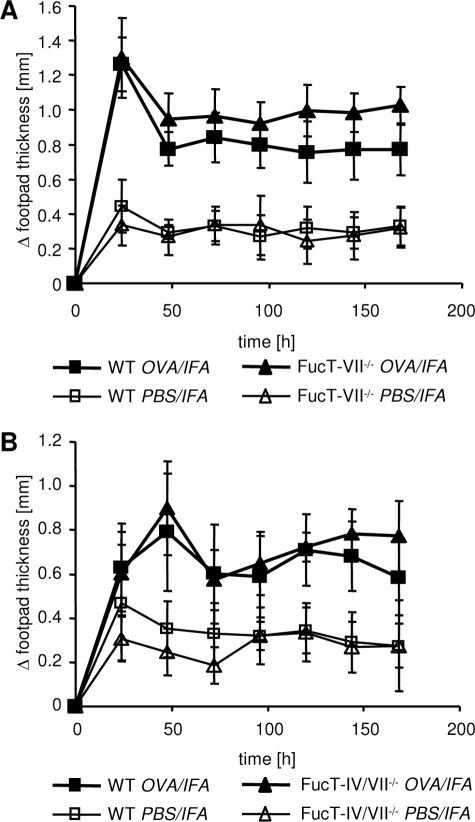
The above findings could suggest that the anti-inflammatory effect of selectin blockade also acts via other leukocytes. Myeloid cells such as neutrophils and macrophages also express E- and P-selectin ligands and can use selectin-dependent adhesion mechanisms to enter acutely inflamed regions.8 To determine the impact of selectin-dependent recruitment of these cells on the development of the DTH, we induced the reaction in FucT-VII-deficient mice by transfer of WT Th1 cells and antigen challenge. Surprisingly, FucT-VII deficiency on recipient cells, including the recipients’ myeloid cells, did not significantly affect the induction of the DTH reaction (Figure 3A). To exclude the possibility that the remaining FucT-IV-dependent selectin ligands might support leukocyte entry into the inflamed sites we performed similar experiments using FucT-IV/VII double KO mice as recipients.22,31 As shown in Figure 3B, we observed an unimpaired course of the DTH response in FucT-IV/VII−/− mice similar to FucT-VII-deficient mice (Figure 3B).
Figure 3.
Deficiency of functional selectin ligand expression in recipient mice does not impair the course of DTH response. OVA-TCRtg Th1 effector cells were transferred into WT and FucT-VII−/− (A; n = 10) or FucT-IV/VII−/− mice (B; n = 9), respectively. The DTH reaction was induced by subsequent subcutaneous injection of OVA323-339 peptide in IFA into the footpad and footpad swelling was measured at the indicated time points.
The data above might suggest that neutrophils and macrophages do not use selectin-dependent adhesion at all to enter early the DTH reaction. To clarify this, we performed histological analysis of antigen-challenged footpads from WT and FucT-IV/VII double KO mice. As shown in Figure 4A, infiltrates in WT mice are primarily dominated by neutrophils and macrophages, as identified by MPO7 and F4/80 staining, respectively (Figure 4A). In line with the peak of swelling at 48 hours after antigen challenge in BL6 mice, infiltration of lymphocytes, neutrophils, and macrophages increased up to this time point. Interestingly, in FucT-IV/VII-deficient recipients that showed an unimpaired, strong footpad swelling, overall infiltration and tissue damage at the antigen-challenged site (OVA/IFA) was significantly lower than in WT mice (Figure 4B, histological score) and even lower than within the PBS/IFA site in WT mice. Between 2- and 10-fold decreased numbers of infiltrating neutrophils and macrophages were recorded at the antigen-containing and at the control site of FucT-IV/VII-deficient mice in comparison with WT mice (MPO7 and F4/80, Figure 4B). T-lymphocyte infiltration was comparable in both recipient strains as shown by CD3 staining (Figure 4, A and B).
Figure 4.
Infiltration of neutrophils and macrophages is reduced in selectin ligand-deficient mice. The DTH reaction was induced by transfer of OVA-TCRtg Th1 effector cells into WT and FucT-IV/VII−/− mice, followed by injection of OVA323-339 peptide in IFA into the footpad. Histological analysis was done 24 hours and 48 hours after antigen challenge. A: Infiltrates were characterized by H&E, CD3, MPO7, and F4/80 staining in inflamed tissue (OVA/IFA) and control site (PBS/IFA). Representative samples are shown for WT and FucT-IV/VII−/− mice. B: Histological footpad scores are shown for inflamed footpads and control sites of WT and FucT-IV/VII−/− mice after 24 hours (top) and 48 hours (bottom). Grading was done after examination of five hpf per sample. Infiltrating cells in inflamed footpads and control sites of WT and FucT-IV/VII−/− mice were counted in five hpf per sample according to cell-specific staining. Four to five mice were included per group and time point (*P < 0.05, **P < 0.01; Mann-Whitney test). Original magnifications: ×200 (A); ×400 (A, insets).
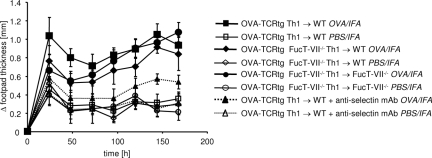
To determine possible synergistic effects between the selectin-ligand deficiency on T cells and selectin-ligand deficiency on myeloid cells we additionally transferred Th1 cells generated from FucT-VII−/−xDO11.10 mice into FucT-VII−/− recipients. This setting resulted in a DTH course similar to a setting in which only the transferred Th1 cells were deficient in selectin ligands; surprisingly, no significant synergism between FucT-VII deficiency in T cells and in recipient leukocytes was observed, as one would have expected from the antibody inhibition experiment done in parallel (Figure 5). Together, these results show that the recruitment of myeloid cells, as that of T cells, is primarily dependent on the expression of functional selectin ligands. However, if myeloid cells are required at all, residual leukocyte accumulation is sufficient to allow a vigorous swelling reaction; apparently, rather small numbers of recruited neutrophils or macrophages than can do the job.
Figure 5.
Selectin ligand deficiency of both Th1 effector and myeloid cells shows no additive effect over selectin ligand deficiency of Th1 effector cells alone in the DTH response. OVA-TCRtg Th1 effector cells derived from either DO11.10 or FucT-VII−/− × DO11.10 mice were transferred into WT and FucT-VII−/− mice, respectively. The DTH reaction was induced by subsequent subcutaneous injection of OVA323-339 peptide in IFA into the footpad, and footpad swelling was measured at the indicated time points (OVA-TCRtg Th1 → WT, n = 6; OVA-TCRtg FucT-VII−/− Th1 → WT/FucT-VII−/−, n = 4). For comparison, the DTH response with anti-selectin blocking is shown in dotted lines (see also Figure 1).
Complete Impairment of Early DTH Response by Depletion of Neutrophils
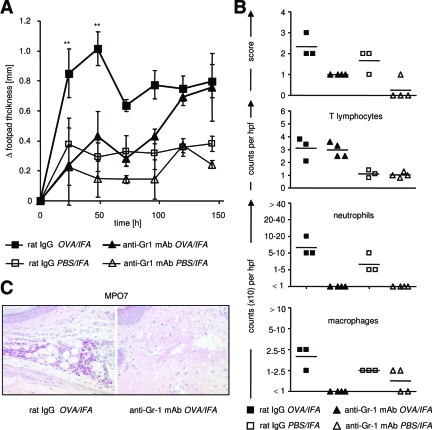
To test whether neutrophils are indeed required in the skin inflammation model used here, we depleted them before induction of DTH in WT mice by intraperitoneal injection of anti-Gr-1 mAb. This treatment almost completely inhibited the acute DTH reaction compared with control mice, although again, in the later phase, inflammation flared up and persisted as in WT mice (Figure 6). Even in FucT-IV/VII-deficient mice, in which only minimal infiltration by neutrophils and macrophages was observed, depletion of granulocytes by anti-Gr-1 antibody treatment led to a complete and slightly prolonged suppression of early inflammation compared with untreated mice (Figure 7A). Under these conditions, almost no infiltration of neutrophils and macrophages was detectable at early time points (Figure 7, B and C). Gr-1 depletion did not impair T-lymphocyte infiltration because untreated and anti-Gr-1 treated mice showed comparable numbers of CD3+ cells in the OVA-treated footpad (Figure 7B). Together, these data suggest that the small numbers of granulocytic cells that invade despite the lack of selectin ligands are required but also sufficient to induce an acute DTH reaction.
Figure 6.
Depletion of neutrophils completely abolishes early DTH response. WT recipient mice were treated with anti-Gr-1 mAb intraperitoneally on days −2 and +1 of the DTH reaction. Control mice received rat IgG. The DTH reaction was induced by transfer of OVA-TCRtg Th1 effector cells and subsequent injection of OVA323-339 peptide in IFA into the footpads. Significant reduction of the OVA-specific response was measured on days 1 and 2 in mice that had received anti-Gr-1 mAb compared with rat IgG-treated mice (n = 8; **P < 0.01, Mann-Whitney test).
Figure 7.
Inhibition of early DTH reaction in selectin-deficient recipient mice after depletion of neutrophils. A: FucT-IV/VII-deficient recipient mice were treated with anti-Gr-1 mAb as described in Figure 6. The DTH reaction was induced by transfer of OVA-TCRtg Th1 effector cells and subsequent injection of OVA323-339 peptide in IFA into the footpads. Significant reduction of the OVA-specific response was measured on days 1 and 2 in mice that had received anti-Gr-1 mAb compared with rat IgG-treated mice (n = 8 per treated group, from days 3 to 6 of the DTH response, n = 4 per treated group; **P < 0.01, Mann-Whitney test). B: Histological assessment of OVA-containing and control tissue of anti-Gr-1 and rat IgG-treated mice was performed on day 2. Shown are the histological footpad scores as well as counts of T lymphocytes, neutrophils, and macrophages (five hpf per sample; three to four mice per group). C: MPO7 staining of representative samples of OVA-challenged tissue from anti-Gr-1- and rat IgG-treated mice on day 2 after antigen application is shown. Original magnifications, ×200.
Discussion
E- and P-selectin regulate the recruitment of leukocytes into inflamed tissues. In contrast to other adhesion molecules, a major role in homeostatic cell trafficking is not observed. Because of this inflammation-specific role, this pathway appears as an interesting target for anti-inflammatory strategies. However, to what extent different effector cell populations use these selectins and what impact this has on the inflammatory reaction has not been studied in detail. Therefore, we here investigated the role of different leukocyte subsets and their dependency on selectin-mediated recruitment in a well-defined DTH model (S. Ghani et al, in preparation),27 in which an inflammation is initiated by antigen challenge of adoptively transferred TCR-transgenic CD4+ T-effector cells.
In this model, antigen-specific T cells serve both as pioneer cells paving the way for massive cell recruitment as well as effector cells acting directly and indirectly via other leukocytes as shown here. The data of this study confirm that E- and P-selectin-dependent adhesion is critical for T cells to enter the inflamed skin as shown by the strongly reduced ability of selectin ligand-deficient T cells to accumulate in the antigen-challenged site. Interestingly, the deficiency of FucT-VII−/− T cells in homing into the inflamed skin was much more pronounced than the reduction in the DTH-inducing capacity. A study by Hwang and colleagues32 using intravital microscopy in a contact hypersensitivity model provided evidence that E- and P-selectin-mediated adhesion is especially important for the recruitment of the pioneer T cells in the first 2 hours after antigen challenge, but is dispensable a few hours later when other adhesion molecules take over, a finding that could explain this discrepancy. However, in the DTH model used here, FucT-VII−/− T-effector cells were almost unable to enter the established DTH site for at least 24 hours after antigen challenge, and infiltration of neutrophils was drastically reduced in FucT-VII−/− and FucT-IV/VII−/− mice 24 hours as well as 48 hours after challenge, suggesting that most cells permanently depend on selectin mechanisms to enter the inflamed site. This does not exclude that other adhesion molecules and chemotactic signals, such as C5a or VLA-4 were required in addition, as shown elsewhere.33,34 The fact that the number of leukocytes infiltrating into the inflamed tissue can strongly be decreased by defective selectin adhesion whereas immunopathology as measured by footpad swelling is preserved points to the importance of functional activation of the cellular players, resulting in a nonlinear relationship between local cell number and pathogenic effect.
The use of the adoptive transfer model in selectin ligand-deficient mice allowed us to study the sequential requirement of different cell types within the antigen-challenged site for the development of a DTH reaction. Previously it was not entirely clear, to what extent antigen-specific Th1 cells, which by themselves secrete a number of pro-inflammatory cytokines, are sufficient to initiate a full inflammatory reaction. The data presented here illustrate that antigen-specific T cells have to enter the site to initiate the DTH response; yet, also depletion of neutrophils completely suppresses the acute phase of the reaction, indicating that neutrophils are necessary during this phase to elicit the overt symptoms of inflammation and act as downstream effectors, eg, by inducing enhanced vascular permeability and edema formation.
A contribution of neutrophils to antigen-induced DTH reactions has been described before.35,36,37 However, the finding that even the small number of neutrophils localizing within the inflamed tissue of FucT-VII−/− and FucT-IV/VII−/− mice is sufficient to evoke a full footpad swelling was surprising and shows the potency of this cell type. Moreover, their number was significantly lower under these conditions than in adjuvant-only injected control sites of WT mice. Incomplete Freund’s adjuvant is a mild irritant, which was found to cause a significant infiltration of leukocytes, mostly neutrophils. Yet, tissue swelling and damage in the control footpad are much less pronounced and shorter lasting than in the presence of T cell activated by antigen. This demonstrates a so far underestimated role of the cross talk between activated T cells and neutrophils. It is tempting to speculate that IFN-γ is a major player in the interaction between T cells and neutrophils, since it has recently been shown to prolong neutrophil survival and to potentiate their function.38 Neutrophils recruited only by the unspecific signals of adjuvant rather occur as a silent, nonpathogenic infiltrate in the absence of stimulating signals from T cells.
The capacity of small numbers of neutrophils to elicit a DTH reaction on T-cell-generated activation might also be the reason why deficiency in selectin ligands on both T cells and myeloid cells was not superior in reducing the DTH compared to T-cell deficiency alone, according to the experiments combining FucT KO T cells with FucT KO recipients. Despite the strong reduction of both neutrophils and macrophages in mice lacking fucosyltransferase the effects on the footpad swelling were minor. In contrast, antibody blockade resulted in stronger and longer lasting inhibition of footpad swelling, a discrepancy that cannot be easily explained. Alternative explanations could assume either signaling induced by antibody binding to the endothelial cells, exerting a suppressive signal (although the effects were not dependent on a specific mAb clone), or, cells originated in a FucT KO animal might have acquired compensatory mechanisms, not unraveled by the functional tests applied.
It has been reported that mast cells play an important role for the recruitment of neutrophils in a DTH reaction elicited by trinitrochlorobenzene.39 In the DTH model used here, we found in experiments using mast cell-deficient WBB6F1-KitW/KitW-v mice as recipients that mast cells are dispensable for elucidation of a full DTH response (unpublished data). Although some accumulation of mast cells was observed on challenge of the footpad, cell recruitment was irrespective of antigen presence or selectin-ligand expression (unpublished data). A recent study by Norman and colleagues40 showed that the presence of mast cells affects the differentiation of T cells in a contact hypersensitivity reaction. Considering the missing effect of mast cells in our model that relies on the in vitro differentiation of Th1 cells the modulation of T-cell priming might be the dominant effect of mast cells in T-cell-dependent skin reactions.
Interestingly, at a later time point footpad swelling occurred in neutrophil-depleted mice and reached levels comparable to those in untreated mice on days 3 to 5 after antigen challenge. Staining of blood from neutrophil-depleted mice confirmed that Gr-1+ cells are still absent from the circulation on day 3 after induction of the DTH reaction (data not shown), suggesting that neutrophils are dispensable for the late occurring immune response and might be replaced by macrophages as secondary effectors, activated by the antigen-specific T cells.
In summary, this study shows that selectins play a major role in the recruitment of T cells, neutrophils, and macrophages in T-cell-initiated DTH reactions. In this model, T cells are instrumental in initiation, whereas neutrophils and other leukocytes are downstream effectors of T-cell-generated signals. Global blocking of selectins by monoclonal antibodies is effective in preventing the development of the T-cell-dependent inflammation. Whereas lack of selectin ligands on effector T cells results in suppression of the early phase of inflammation, defective selectin binding of neutrophils has much less impact on the inflammatory reaction. These findings unravel T cells as the predominant targets for selectin blockade aiming to suppress skin inflammation.
Acknowledgments
We thank Simone Spiekermann, Uta Lauer, and Kerstin Schlawe for excellent technical assistance; Doerte Huscher for help in statistical analyses; and Heidi Schliemann, Heidi Hecker-Kia, and Tuula Geeske for providing us with mAbs.
Footnotes
Address reprint requests to Dr. Uta Syrbe, Charité, Campus Benjamin Franklin, Medizinische Klinik I, 12200 Berlin, Germany. E-mail address: uta.syrbe@charite.de.
Supported by the Deutsche Forschungsgemeinschaft (grants SFB421 and SY31/2–1).
J.H. and U.S. share senior authorship.
Current address of K.S.: Swiss Institute of Allergy and Asthma Research, Davos, Switzerland.
References
- Sperandio M. Selectins and glycosyltransferases in leukocyte rolling in vivo. FEBS J. 2006;273:4377–4389. doi: 10.1111/j.1742-4658.2006.05437.x. [DOI] [PubMed] [Google Scholar]
- Lowe JB. Glycan-dependent leukocyte adhesion and recruitment in inflammation. Curr Opin Cell Biol. 2003;15:531–538. doi: 10.1016/j.ceb.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Schottelius AJ, Hamann A, Asadullah K. Role of fucosyltransferases in leukocyte trafficking: major impact for cutaneous immunity. Trends Immunol. 2003;24:101–104. doi: 10.1016/s1471-4906(03)00024-3. [DOI] [PubMed] [Google Scholar]
- Chu A, Hong K, Berg EL, Ehrhardt RO. Tissue specificity of E- and P-selectin ligands in Th1-mediated chronic inflammation. J Immunol. 1999;163:5086–5093. [PubMed] [Google Scholar]
- Xie H, Lim YC, Luscinskas FW, Lichtman AH. Acquisition of selectin binding and peripheral homing properties by CD4(+) and CD8(+) T cells. J Exp Med. 1999;189:1765–1776. doi: 10.1084/jem.189.11.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad W, Cooper CJ, Zhang Z, Brown JB, Zhu Y, Issekutz A, Fuss I, Lee HO, Kansas GS, Barrett TA. P-selectin and P-selectin glycoprotein ligand 1 are major determinants for Th1 cell recruitment to nonlymphoid effector sites in the intestinal lamina propria. J Exp Med. 2003;198:369–377. doi: 10.1084/jem.20020691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietz W, Allemand Y, Borges E, Laer DV, Hallmann R, Vestweber D, Hamann A. CD4+ T-cells only migrate into inflamed skin if they express ligands for E- and P-selectin. J Immunol. 1998;161:963–970. [PubMed] [Google Scholar]
- Ley K. The role of selectins in inflammation and disease. Trends Mol Med. 2003;9:263–268. doi: 10.1016/s1471-4914(03)00071-6. [DOI] [PubMed] [Google Scholar]
- Lowe JB. Glycosylation in the control of selectin counter-receptor structure and function. Immunol Rev. 2002;186:19–36. doi: 10.1034/j.1600-065x.2002.18603.x. [DOI] [PubMed] [Google Scholar]
- Wagers AJ, Waters CM, Stoolman LM, Kansas GS. Interleukin 12 and interleukin 4 control T cell adhesion to endothelial selectins through opposite effects on alpha1, 3-fucosyltransferase VII gene expression. J Exp Med. 1998;188:2225–2231. doi: 10.1084/jem.188.12.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YC, Henault L, Wagers AJ, Kansas GS, Luscinskas FW, Lichtman AH. Expression of functional selectin ligands on Th cells is differentially regulated by IL-12 and IL-4. J Immunol. 1999;162:3193–3201. [PubMed] [Google Scholar]
- White SJ, Underhill GH, Kaplan MH, Kansas GS. Cutting edge: differential requirements for Stat4 in expression of glycosyltransferases responsible for selectin ligand formation in Th1 cells. J Immunol. 2001;167:628–631. doi: 10.4049/jimmunol.167.2.628. [DOI] [PubMed] [Google Scholar]
- Teraki Y, Picker LJ. Independent regulation of cutaneous lymphocyte-associated antigen expression and cytokine synthesis phenotype during human CD4+ memory T cell differentiation. J Immunol. 1997;159:6018–6029. [PubMed] [Google Scholar]
- Kretschmer U, Bonhagen K, Debes GF, Mittrucker HW, Erb KJ, Liesenfeld O, Zaiss D, Kamradt T, Syrbe U, Hamann A. Expression of selectin ligands on murine effector and IL-10-producing CD4(+) T cells from non-infected and infected tissues. Eur J Immunol. 2004;34:3070–3081. doi: 10.1002/eji.200424972. [DOI] [PubMed] [Google Scholar]
- Huehn J, Siegmund K, Lehmann J, Siewert C, Haubold U, Feuerer M, Debes GF, Lauber J, Frey O, Przybylski GK, Niesner U, Rosa MDL, Schmidt CA, Bräuer R, Buer J, Scheffold A, Hamann A. Developmental stage, phenotype and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J Exp Med. 2004;199:303–313. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syrbe U, Hoffmann U, Schlawe K, Liesenfeld O, Erb K, Hamann A. Microenvironment-dependent requirement of STAT4 for the induction of P-selectin ligands and effector cytokines on CD4+ T cells in healthy and parasite-infected mice. J Immunol. 2006;177:7673–7679. doi: 10.4049/jimmunol.177.11.7673. [DOI] [PubMed] [Google Scholar]
- Campbell DJ, Butcher EC. Rapid acquisition of tissue-specific homing phenotypes by CD4+ T cells activated in cutaneous or mucosal lymphoid tissues. J Exp Med. 2002;195:135–141. doi: 10.1084/jem.20011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maly P, Thall A, Petryniak B, Rogers CE, Smith PL, Marks RM, Kelly RJ, Gersten KM, Cheng G, Saunders TL, Camper SA, Camphausen RT, Sullivan FX, Isogai Y, Hindsgaul O, von Andrian UH, Lowe JB. The alpha(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell. 1996;86:643–653. doi: 10.1016/s0092-8674(00)80137-3. [DOI] [PubMed] [Google Scholar]
- Smithson G, Rogers CE, Smith PL, Scheidegger EP, Petryniak B, Myers JT, Kim DS, Homeister JW, Lowe JB. Fuc-TVII is required for T helper 1 and T cytotoxic 1 lymphocyte selectin ligand expression and recruitment in inflammation, and together with Fuc-TIV regulates naive T cell trafficking to lymph nodes. J Exp Med. 2001;194:601–614. doi: 10.1084/jem.194.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapp KR, Heitzig CE, Ellies LG, Marth JD, Kansas GS. Differential requirements for the O-linked branching enzyme core 2 beta1–6-N-glucosaminyltransferase in biosynthesis of ligands for E-selectin and P-selectin. Blood. 2001;97:3806–3811. doi: 10.1182/blood.v97.12.3806. [DOI] [PubMed] [Google Scholar]
- Weninger W, Ulfman LH, Cheng G, Souchkova N, Quackenbush EJ, Lowe JB, von Andrian UH. Specialized contributions by alpha(1,3)-fucosyltransferase-IV and FucT- VII during leukocyte rolling in dermal microvessels. Immunity. 2000;12:665–676. doi: 10.1016/s1074-7613(00)80217-4. [DOI] [PubMed] [Google Scholar]
- Homeister JW, Thall AD, Petryniak B, Maly P, Rogers CE, Smith PL, Kelly RJ, Gersten KM, Askari SW, Cheng G, Smithson G, Marks RM, Misra AK, Hindsgaul O, von Andrian UH, Lowe JB. The alpha(1,3)fucosyltransferases FucT-IV and FucT-VII exert collaborative control over selectin-dependent leukocyte recruitment and lymphocyte homing. Immunity. 2001;15:115–126. doi: 10.1016/s1074-7613(01)00166-2. [DOI] [PubMed] [Google Scholar]
- Catalina MD, Estess P, Siegelman MH. Selective requirements for leukocyte adhesion molecules in models of acute and chronic cutaneous inflammation: participation of E- and P- but not L-selectin. Blood. 1999;93:580–589. [PubMed] [Google Scholar]
- Erdmann I, Scheidegger EP, Koch FK, Heinzerling L, Odermatt B, Burg G, Lowe JB, Kundig TM. Fucosyltransferase VII-deficient mice with defective E-, P-, and L-selectin ligands show impaired CD4+ and CD8+ T cell migration into the skin, but normal extravasation into visceral organs. J Immunol. 2002;168:2139–2146. doi: 10.4049/jimmunol.168.5.2139. [DOI] [PubMed] [Google Scholar]
- Labow MA, Norton CR, Rumberger JM, Lombard Gillooly KM, Shuster DJ, Hubbard J, Bertko R, Knaack PA, Terry RW, Harbison ML. Characterization of E-selectin-deficient mice: demonstration of overlapping function of the endothelial selectins. Immunity. 1994;1:709–720. doi: 10.1016/1074-7613(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Subramaniam M, Saffaripour S, Watson SR, Mayadas TN, Hynes RO, Wagner DD. Reduced recruitment of inflammatory cells in a contact hypersensitivity response in P-selectin-deficient mice. J Exp Med. 1995;181:2277–2282. doi: 10.1084/jem.181.6.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerer M, Eulenburg K, Loddenkemper C, Hamann A, Huehn J. Self-limitation of Th1-mediated inflammation by IFN-γ. J Immunol. 2006;176:2857–2863. doi: 10.4049/jimmunol.176.5.2857. [DOI] [PubMed] [Google Scholar]
- Siegmund K, Feuerer M, Siewert C, Ghani S, Haubold U, Dankof A, Krenn V, Schön MP, Scheffold A, Lowe J, Hamann A, Syrbe U, Huehn J. Migration matters: regulatory T cell compartmentalization determines suppressive activity in vivo. Blood. 2005;106:3097–3104. doi: 10.1182/blood-2005-05-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher EC, Ford WL. Following cellular traffic: methods of labelling lymphocytes and other cells to trace their migration in vivo. Weir DM, Herzenberg LA, Blackwell C, editors. Handbook of Experimental Immunology 4th ed, Volume 2: Cellular Immunology. :1–57. [Google Scholar]
- Piccio L, Rossi B, Colantonio L, Grenningloh R, Gho A, Ottoboni L, Homeister JW, Scarpini E, Martinello M, Laudanna C, D'Ambrosio D, Lowe JB, Constantin G. Efficient recruitment of lymphocytes in inflamed brain venules requires expression of cutaneous lymphocyte antigen and fucosyltransferase-VII. J Immunol. 2005;174:5805–5813. doi: 10.4049/jimmunol.174.9.5805. [DOI] [PubMed] [Google Scholar]
- Huang MC, Zollner O, Moll T, Maly P, Thall AD, Lowe JB, Vestweber D. P-selectin glycoprotein ligand-1 and E-selectin ligand-1 are differentially modified by fucosyltransferases fuc-TIV and fuc-TVII in mouse neutrophils. J Biol Chem. 2000;275:31353–31360. doi: 10.1074/jbc.M005449200. [DOI] [PubMed] [Google Scholar]
- Hwang JM, Yamanouchi J, Santamaria P, Kubes P. A critical temporal window for selectin-dependent CD4+ lymphocyte homing and initiation of late-phase inflammation in contact sensitivity. J Exp Med. 2004;199:1223–1234. doi: 10.1084/jem.20032016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman MU, Hulliger S, Colarusso P, Kubes P. Multichannel fluorescence spinning disk microscopy reveals early endogenous CD4 T cell recruitment in contact sensitivity via complement. J Immunol. 2008;180:510–521. doi: 10.4049/jimmunol.180.1.510. [DOI] [PubMed] [Google Scholar]
- Ferguson TA, Mizutani H, Kupper TS. Two integrin-binding peptides abrogate T cell-mediated immune responses in vivo. Proc Natl Acad Sci USA. 1991;88:8072–8076. doi: 10.1073/pnas.88.18.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo C, Yamashita T, Terashita M, Sendo F. Modulation of in vivo immune response by selective depletion of neutrophils using a monoclonal antibody. RP-3 II inhibition by RP-3 treatment of mononuclear leukocyte recruitment in delayed-type hypersensitivity to sheep red blood cells in rats. J Immunol. 1993;150:3739–3746. [PubMed] [Google Scholar]
- Terashita M, Kudo C, Yamashita T, Gresser I, Sendo F. Enhancement of delayed-type hypersensitivity to sheep red blood cells in mice by granulocyte colony-stimulating factor administration at the elicitation phase. J Immunol. 1996;156:4638–4643. [PubMed] [Google Scholar]
- Tumpey TM, Fenton R, Molesworth-Kenyon S, Oakes JE, Lausch RN. Role for macrophage inflammatory protein 2 (MIP-2), MIP-1alpha, and interleukin-1alpha in the delayed-type hypersensitivity response to viral antigen. J Virol. 2002;76:8050–8057. doi: 10.1128/JVI.76.16.8050-8057.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T, Takahashi M. IFN-{gamma}-mediated survival enables human neutrophils to produce MCP-1/CCL2 in response to activation by TLR ligands. J Immunol. 2007;179:1942–1949. doi: 10.4049/jimmunol.179.3.1942. [DOI] [PubMed] [Google Scholar]
- Biedermann T, Kneilling M, Mailhammer R, Maier K, Sander CA, Kollias G, Kunkel SL, Hultner L, Rocken M. Mast cells control neutrophil recruitment during T cell-mediated delayed-type hypersensitivity reactions through tumor necrosis factor and macrophage inflammatory protein 2. J Exp Med. 2000;192:1441–1452. doi: 10.1084/jem.192.10.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman MU, Hwang J, Hulliger S, Bonder CS, Yamanouchi J, Santamaria P, Kubes P. Mast cells regulate the magnitude and the cytokine microenvironment of the contact hypersensitivity response. Am J Pathol. 2008;172:1638–1649. doi: 10.2353/ajpath.2008.070559. [DOI] [PMC free article] [PubMed] [Google Scholar]