Abstract
Premature delivery occurs in 12% of all births and accounts for nearly half of long-term morbidity. Current therapeutic approaches to preterm delivery are ineffective and present serious risks to both mother and fetus. The single most common cause of preterm birth is infection. Previous in vitro investigations have shown that endothelin-1 (ET-1) is induced by inflammatory cytokines and that it increases myometrial smooth muscle tone. Furthermore, we have previously shown that both the endothelin-converting enzyme-1 (ECE-1) inhibitor, phosphoramidon, as well as a novel ET-1 receptor A antagonist synthesized by our group, control premature delivery in a mouse model of inflammation-associated preterm delivery. In the current work, we show that levels of both ET-1 and ECE-1 are increased in gestational tissues in E16.5 mice induced to deliver prematurely after lipopolysaccharide administration. We also show that premature delivery is controlled by treatment with the selective endothelin receptor A antagonist BQ-123 in a dose-dependent manner. Finally, we show here for the first time that premature delivery can be controlled using RNA silencing, by hydrodynamic transfection of E15 mice with ECE-1 RNAi. Taken together, these data support a critical role for the ECE-1/ET-1 system in inflammation-associated premature delivery. The ability to control premature delivery by antagonizing or silencing the ECE-1/ET-1 system offers a novel approach to an unmet clinical need.
Premature delivery, defined as delivery occurring before 37 weeks of gestation, occurs in 12% of all births, and accounts for nearly half of long-term neurological morbidity, and 60 to 80% of perinatal mortality, excluding infants with congenital anomalies.1 Despite the advances that have been made in obstetrics and neonatology, the rate of premature delivery has not decreased throughout the past 20 to 30 years. In fact, the National Center for Health Statistics (Centers for Disease Control and Prevention) has reported a 20% increase (from 10.6 to 12.7%) in the percentage of premature deliveries during the years 1990 to 2005. Sadly, the existing therapies for prevention of preterm labor are ineffective and introduce risks to the mother and fetus.2
Intrauterine infection is often associated with preterm labor. It has been suggested that at least 40% of all premature deliveries occur to mothers with intrauterine infection, and that gestational age is inversely related to the frequency of intrauterine infections.3 Considerable evidence suggests that the proinflammatory cytokine-prostaglandin cascade plays a central role in the pathogenesis of inflammation-associated premature delivery.4,5
The critical function of TLR-4 in the pathogenesis of inflammation-associated preterm labor has been demonstrated by at least three groups of investigators.6,7,8 Binding of TLR-4 is followed by a host inflammatory response involving several cytokines. Interleukin-1 and tumor necrosis factor, in particular, have been associated with infection-triggered preterm labor in humans9,10,11 as well as in other species.12,13,14,15 The inflammatory cytokines, in turn, induce later steps in the parturition cascade, such as increased prostaglandin synthesis, decreased prostaglandin catabolism, increased expression of contraction-associated proteins, and increased uterine contractile activity,16,17 culminating in the production of full-blown labor and delivery.
Previous in vitro investigations have shown that endothelin-1 (ET-1), an extremely potent vasoconstrictor peptide,18 increases myometrial smooth muscle tone.19,20,21 It has also been shown that infection and inflammatory cytokines stimulate ET-1 production22 and that ET-1, in turn, stimulates an inflammatory cytokine pathway through activation of the ETB receptor.23 We hypothesized, therefore, that ET-1 plays a critical role in the pathogenesis of inflammation-associated premature delivery.
In previous work, we have shown that treating lipopolysaccharide (LPS)-stimulated pregnant mice with the endothelin-converting enzyme-1 (ECE-1) inhibitor phosphoramidon decreases the incidence of premature delivery in a mouse model.24 In addition, we have recently found that treating LPS-stimulated pregnant mice with 6-alkoxy substituted-3-carboxybenzyl-N-benzyl-quinol-4-ones, designed and synthesized as novel endothelin receptor A antagonists by our group, results in decreased incidence of premature delivery.25 In the current work, we present biochemical, pharmacological, and molecular evidence that ET-1 has a critical function in an animal model simulating inflammation-associated premature delivery.
Materials and Methods
Mouse Models of Premature Delivery
C57BL/6 mice from Taconic Farms (Hudson, NY) were used for all experiments. Animals were housed in plastic cages in a temperature-controlled animal facility with alternating 12:12 hour light:dark cycles, with ad libitum access to food and water. All experimental protocols used were approved by the Animal Care and Utilization Committee of the College of Pharmacy and Allied Health Professions, St. John’s University, and the research was conducted according to the requirements of the National Institutes of Health Guide for the Care and Use of Laboratory Animals (revised 1996). A total of 21 timed pregnant mice, weighing 22 to 28 g, were used for the first series of experiments. Mice were randomly assigned to three groups: inflammation and labor (group I, n = 8), no inflammation and no labor (group II, n = 9), labor without inflammation (group III, n = 4). Group I mice received an intraperitoneal injection of 6.6 mg/kg of LPS (serotype 026:B6; Sigma Chemical Co., St Louis, MO) dissolved in 0.5 ml of phosphate-buffered saline (PBS) at E15.5 and delivered within 24 hours of the LPS injection. Group II mice (reference controls) received intraperitoneal injections of 0.5 ml of PBS at E15.5. Group III mice received an intraperitoneal injection of 6.7 mg/kg prostaglandin F2α (PGF-2α, Sigma) dissolved in 0.5 ml of PBS every 3 hours, beginning at E16.0, and delivered within 12 hours of the first injection of PGF-2α.
Tissue Collection and Preparation
Placentas were collected at E16.0 to E16.5, immediately after delivery, at hysterectomy 24 hours after LPS or PBS injection, or at hysterectomy 12 hours after the first injection of PGF-2α. Uteri were collected at E16.5, at hysterectomy, either 24 hours after LPS or PBS injection or 12 hours after the first injection of PGF-2α. Mice were sacrificed by CO2 asphyxiation and rapidly hysterectomized. All tissues were collected rapidly, frozen immediately, and stored at −80°C.
Western Blotting
Placental and uterine tissues were homogenized in 10 vol of ice-cold homogenization buffer (20 mmol/L Tris/HCl, pH 7.5, containing 5 mmol/L MgCl2), using a polytron homogenizer, for 90 seconds at 4°C. An equal volume of loading buffer was added to each sample. Samples were denatured by incubation for 7 minutes at 95°C. Proteins were separated by 16% Tricine gels (Invitrogen, Carlsbad, CA) and transferred to polyvinylidene difluoride membranes (Invitrogen). The membranes were wetted with TBS-T (Tris-buffered saline with 0.1% Tween-20, pH 7.8) for 10 minutes, and were blocked with Western blocker solution (Sigma) for 120 minutes. Then, the membranes were hybridized with primary antibodies diluted in the blocker solution for 120 minutes at room temperature. Anti-endothelin-converting enzyme-1 (ECE-1) (United States Biological, Swampscott, MA) was diluted to 0.5 μg/ml, and anti-β-actin (Sigma) and anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Sigma) were both diluted to 20 μg/ml dilution. The membranes were washed with TBS-T several times and incubated with secondary antibody, anti-rabbit IgG, horseradish peroxidase-linked whole antibody (GE Healthcare, Buckinghamshire UK), at a 1:2500 dilution in Western blocker solution at 4°C for 120 minutes. The membranes were washed again and treated with the ECL Plus Western blotting detection system (GE Healthcare) and chemifluorescence was detected by exposure to phosphor screen (GE Healthcare). Densitometric quantification of the Western blot signal intensity of the bands was performed by densitometric analysis program. The density values of GAPDH or actin were used for normalization. At least four different mice from each group were included in these experiments.
Immunohistochemistry
Mouse placental tissue was fixed in formaldehyde and embedded in paraffin. Serial sections (4 μm) of the placental labyrinth were mounted on glass slides. Representative sections were then deparaffinized, rehydrated, and washed. Sections were immersed in prewarmed (37°C) 3% H2O2 to quench endogenous peroxidase activity and then microwaved in antigen retrieval solution (8.2 mmol/L sodium citrate, 1.8 mmol/L citric acid, pH 5.7, containing 0.01% Triton X-100). Sections were incubated with 1:200 dilutions of rabbit anti-ET-1 antisera (Sigma). After 1 hour of incubation with primary antiserum, the sections were washed and incubated with biotinylated anti-rabbit Ig secondary antibody (DAKO, Carpinteria, CA). Immunostaining was achieved using an avidin-biotin complex developer kit (DAKO) and 3,30-diaminobenzidine as substrate.
In Vivo Assay of BQ-123 Effect on Premature Delivery
A total of 24 timed pregnant mice were randomly assigned to the following three groups: sham (n = 10), low dose of BQ-123 (n = 7), and high dose of BQ-123 (n = 7). All mice were injected intraperitoneally with 9.9 mg/kg of LPS diluted in 0.5 ml of PBS at E15.5. After 10 hours of LPS injection, 10 mice received a sham intraperitoneal injection of 500 μl of PBS, seven mice received a low dose (100 μg/200 μl of PBS) of the selective endothelin type A (ETA) receptor antagonist BQ-123 (Sigma), and seven mice received a high dose (200 μg/200 μl of PBS) of BQ-123. All animals were observed for preterm delivery. After 24 hours of LPS injection, mice were sacrificed to confirm pregnancy and to count the number of retained fetuses.
RNA Knockdown
The target sequence of ECE-1 siRNA (Dharmacon RNA Technologies, Lafayette, CO) used was 5′-NNCUUCCACAGCCCCCGGAGU-3′. The sense and anti-sense sequences of siRNAs were, respectively, 5′-CUUCCACAGCCCCCGGAGUdTdT-3′ and 5′-ACUCCGGGGGC UGUGGAAGdTdT-3′. Thirteen mice were randomly assigned to two groups: control group (n = 7) and RNA knockdown group (n = 6). All animals received an intraperitoneal injection of LPS (9.9 mg/kg) diluted in 0.5 ml of PBS at E15.5. Mice were anesthetized with Cetacaine topical anesthetic spray (Cetylite Industries, Inc., Pennsauken, NJ) through tail skin. Mice in the RNA knockdown group were rapidly injected in the tail vein with 500 μg of ECE-1 siRNA dissolved in 0.5 ml of PBS, using a hydrodynamic transfection method at scheduled intervals: 30 hours, 12 hours, and 3 hours before LPS injection. Control mice received 0.5 ml of PBS injected into the tail vein, using the same method and at the same scheduled times as the RNA knockdown group. All mice were observed for preterm delivery within 24 hours of LPS injection. Placental tissue was collected either immediately after delivery or at hysterectomy, 24 hours after LPS injection. Uteri were collected at hysterectomy 24 hours after LPS injection. All animals were sacrificed to confirm pregnancy and count retained pups.
Statistical Analysis
Statistical significance of changes in protein expression detected by Western blotting analysis was evaluated with the Student’s t-test. Statistical significance of effects of BQ-123 and ECE-1 RNAi on LPS-induced preterm labor and numbers of pups retained in utero were evaluated with Fisher’s exact test. Changes in ET-1 expression detected immunohistochemically were evaluated by χ2 analysis and Bartholomew’s trend test.
Results
LPS-Induced Premature Delivery Is Associated with Elevated ECE-1 Expression in the Placenta and Uterus
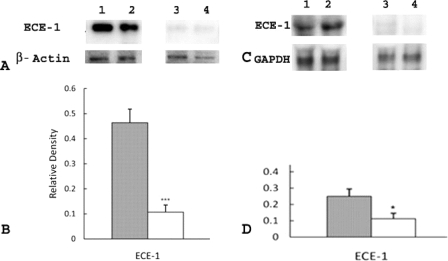
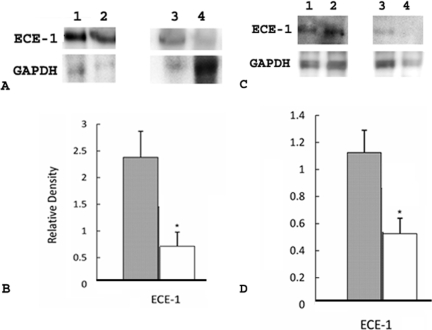
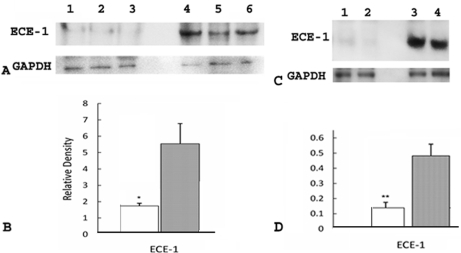
We investigated the involvement of the ECE-1/ET-1 pathway in LPS-induced premature delivery by examining the expression of ECE-1 in placental and uterine tissues in the following three groups of mice: group I (n = 8), mice that received LPS and developed premature delivery; group II (n = 9), mice that received PBS and did not develop premature delivery (control group); and group III (n = 4), mice that received PGF2α, which is predicted to act downstream of the ECE-1/ET-1 pathway, and developed premature delivery. Western blot analyses clearly showed increased elevation in ECE-1 expression in placentas of group I mice when compared to group II (P < 0.001) (Figure 1, A and B) and group III mice (P < 0.05) (Figure 1, C and D). Western blot analyses also showed significant elevation in ECE-1 expression in uteri of group I mice when compared to groups II (Figure 2, A and B) and III (Figure 2, C and D) (P < 0.05).
Figure 1.
Expression of ECE-1 in placenta in group I (mice that received LPS and developed premature delivery) versus group II (mice that received PBS and did not develop premature delivery) and group I mice versus group III mice (mice that received PGF2α and developed premature delivery). A: Each lane was loaded with 1.3 μg of placental tissue and samples were separated by 16% Tricine gel. Immunoblots were probed with antibodies directed against ECE-1 and β-actin, as indicated. Lanes 1 and 2, group I; lanes 3 and 4, group II. B: Levels of expression were determined by densitometry after normalization to β-actin. Filled bar, group I; open bar, group II. Values shown represent the mean ± SEM. ***P < 0.001 for ECE-1 expression between groups I and II. C: Each lane was loaded with 1.3 μg of placental tissue and samples were separated by 16% Tricine gel. Immunoblots were probed with antibodies directed against ECE-1 and GAPDH, as indicated. Lanes 1 and 2, group I; lanes 3 and 4, group III. D: Levels of expression were determined by densitometry after normalization to GAPDH. Filled bar, group I; open bar, group III. Values shown represent the mean ± SEM. *P < 0.05 ECE-1 expression between groups I and III.
Figure 2.
ECE-1 in uteri in group I (mice that received LPS and developed premature delivery) versus group II (mice that received PBS and did not develop premature delivery) and group I versus group III (mice that received PGF2α and developed premature delivery). A: Each lane was loaded with 1.3 μg of uterine tissue and samples were separated by 16% Tricine gel. Immunoblots were probed with antibodies directed against ECE-1 and GAPDH as indicated. Lanes 1 and 2, group I; lanes 3 and 4, group II. B: Levels of expression were determined by densitometry after normalization to GAPDH. Filled bar, group I; open bar, group II. Values shown represent the mean ± SEM. *P < 0.05 for ECE-1 expression between groups I and II. C: Each lane was loaded with 1.3 μg of uterine tissue and samples were separated by 16% Tricine gel. Immunoblots were probed with antibodies directed against ECE-1 and GAPDH, as indicated. Lanes 1 and 2, group I; lanes 3 and 4, group III. D: Levels of expression were determined by densitometry after normalization to GAPDH. Filled bar, group I; open bar, group III. Values shown represent the mean ± SEM. *P < 0.05 for ECE-1 expression between groups I and III.
LPS-Induced Premature Delivery Is Associated with Increased ET-1 Immunohistochemical Staining in the Placenta
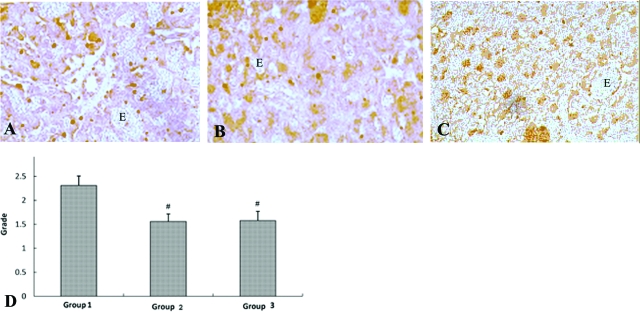
Levels of placental ET-1 expression in groups 1, 2, and 3 were examined by performing immunohistochemical staining on microscopic sections of the placental labyrinth. Immunohistochemistry was performed on sections prepared from four randomly chosen group 1 mice, three randomly chosen group 2 mice, and four randomly chosen group 3 mice. Representative sections of group 1, group 2, and group 3 placentas subjected to ET-1 immunohistochemical staining are shown in Figure 3, A–C, respectively. Staining was graded by two different blinded observers using an algorithm based on number of cells positive for immunoreactivity and staining intensity. Data were evaluated with Bartholomew’s trend test and group 1 mice had significantly more ET-1 immunoreactivity than either group 2 or group 3 mice (P < 0.001) (Figure 3).
Figure 3.
ET-1 immunohistochemical staining in group 1 (mice that received LPS and developed premature delivery), group 2 (mice that received PBS and did not develop premature delivery), and group III (mice that received PGF2α and developed premature delivery). Immunohistochemical staining of placental labyrinth was performed as described in the Materials and Methods. Four group 1, three group 2, and four group 3 mice were randomly chosen for immunohistochemical analysis. Grading of immunostaining was performed by two independent blinded observers, using an algorithm based on number of cells positive for staining and staining intensity and a four-point grading scale. Data were analyzed with Bartholomew’s trend test and placental ET-1 immunoreactivity was significantly increased in group I as compared to either group II or group III (#P < 0.001). A: Group 1. B: Group 2. C: Group 3. D: Statistical analysis. Erythrocytes in images indicated by E.
ETA Receptor Antagonist BQ-123 Inhibits LPS-Induced Premature Delivery
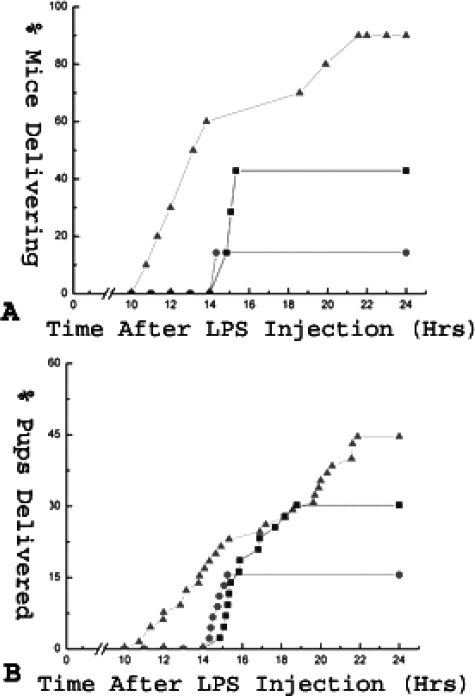
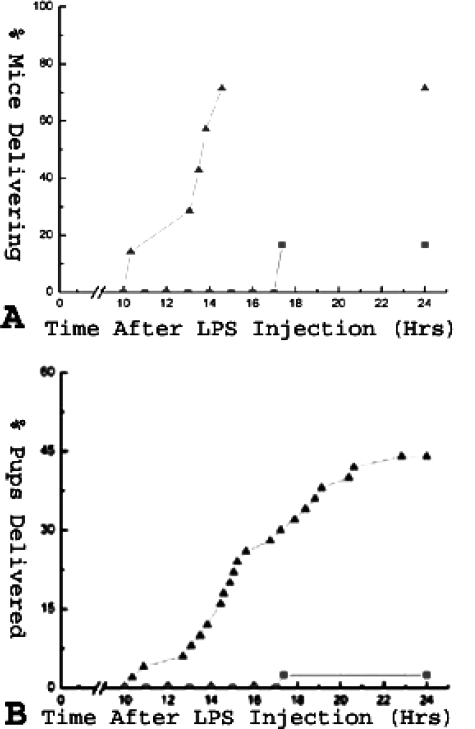
To elucidate whether the ECE-1/ET-1 pathway plays a role in LPS-induced premature delivery, we examined the effect of BQ-123, an ETA receptor antagonist, on pregnant mice induced to deliver prematurely with LPS. In the control group (n = 10), which contained mice that were treated with LPS followed by a sham injection of PBS at t = 10 hours (where t = 0 is time of LPS injection), 9 of 10 (90%) mice delivered within 24 hours of LPS injection. In the Lo-BQ-123 group (n = 7), which received LPS followed by 3.3 mg/kg of BQ-123 at t = 10 hours, three of seven mice (42.9%) delivered within 24 hours of LPS injection (P ≈ 0.06). In the Hi-BQ-123 group (n = 7), which received LPS followed by 6.7 mg/kg of BQ-123, one of seven (14.3%) delivered within 24 hours of LPS injection (P < 0.005) (Table 1 and Figure 4A). Delivery occurred without any maternal death in all cases. Additionally, BQ-123 also decreased the percentage of prematurely delivered pups in a dose-dependent manner. In the control group, 44.6% of the pups were delivered prematurely, in comparison to 30.2% (P ≈ 0.10) and 15.6% (P < 0.005), respectively, for the Lo- and Hi-BQ-123 groups (Table 1 and Figure 4B). Thus BQ-123 reduced the frequency of preterm delivery and prolonged the duration of pregnancy in a dose-dependent manner in this preterm labor mouse model.
Table 1.
Effect of BQ-123 in the LPS-Induced Preterm Delivery Mouse Model
| Group* | n | Number of mice delivering preterm | Percent of mice delivering preterm | Total number of pups | Number of pups delivered preterm | Percent of pups delivered preterm |
|---|---|---|---|---|---|---|
| Control | 10 | 9 | 90 | 65 | 29 | 44.6 |
| Hi BQ-123 | 7 | 1† | 14.3† | 45 | 7† | 15.6† |
| Lo BQ-123 | 7 | 3 | 42.9 | 43 | 13 | 30.2 |
Control group is treated with LPS and PBS; Hi BQ-123 group is treated with LPS and high dose (6.7 mg/k g) of BQ-123; Lo BQ-123 group is treated with LPS and low dose (3.3 mg/kg) of BQ-123.
P < 0.01 between control and Hi BQ-123 (Fisher’s exact test).
Figure 4.
Effect of BQ-123 on premature delivery in LPS-treated mice. LPS-stimulated (9.9 mg/kg, i.p.) mice were treated with an intraperitoneal injection of PBS (control, 200 μl), low (3.3 mg/kg), or high (6.7 mg/kg) dose of BQ-123 10 hours after LPS injection. All mice were observed for premature delivery within 24 hours of LPS injection. A: Percentage of delivering mice throughout time for the control, Lo BQ-123, and Hi BQ-123 groups is shown. B: Percentage of pups delivered prematurely throughout the time for the same three groups is shown. P < 0.01 for LPS-induced premature delivery between the control and Hi BQ-123 groups. Triangles: control group; squares: Lo BQ-123 group; circles: Hi BQ-123 group.
Knockdown of ECE-1 Expression by Its siRNA Inhibits LPS-Induced Premature Delivery
We investigated the involvement of the ECE-1/ET-1 pathway in inflammation-associated premature delivery by knocking down expression of ECE-1, the enzyme that catalyzes the rate-limiting step in the synthesis of ET-126 and the only enzyme that synthesizes ET-1 in vivo. The elimination of ECE-1 expression by its siRNA was verified by Western blot. In comparison to the control group, which received PBS and delivered prematurely, expression of ECE-1 (MW, 87.2 kDa) was significantly reduced in the RNAi group, which received siRNA for ECE-1 and did not develop premature delivery, in both placental (P < 0.05) (Figure 5, A and B) and uterine (P < 0.01) (Figure 5, C and D) tissues.
Figure 5.
Effect of siRNA for ECE-1 on the expression of ECE-1 in placenta and uteri. LPS-stimulated (9.9 mg/kg, i.p.) mice were treated with 0.5 ml of PBS (control) or 500 μg of siRNA for ECE-1 dissolved in 0.5 ml of PBS (RNAi) injected into tail vein at 30, 12, and 3 hours before LPS injection, using a hydrodynamic transfection method. A: Each lane was loaded with 1.3 μg of placental tissue and samples were separated by 16% Tricine gel. Immunoblots were probed with antibodies directed against ECE-1 and GAPDH, as indicated. Lanes 1 to 3: ECE-1 siRNA-treated; lanes 4 to 6: control. B: Levels of expression were determined by densitometry after normalization to GAPDH. Open bar, ECE-1 siRNA-treated; filled bar, control. Values shown represent the mean ± SEM. *P < 0.05 ECE-1 expression between the control and RNAi groups. C: Each lane was loaded with 1.3 μg of uterine tissue and samples were separated by 16% Tricine gel. Immunoblots were probed with antibodies directed against ECE-1 and GAPDH. D: Levels of expression were determined by densitometry after normalization to GAPDH. Values shown represent the mean ± SEM. Open bar, ECE-1 siRNA-treated; filled bar, control. **P < 0.01 for ECE-1 expression between the control and ECE-1 groups.
In the control group, which received PBS at 30, 12, and 3 hours before LPS injection, five of seven (71.4%) mice delivered the first pup within 24 hours of LPS injection. In the RNAi group, which received siRNA for ECE-1 (500 μg in 0.5 ml of PBS), only one of six (16.7%) mice delivered its first pup prematurely (P ≈ 0.08) (Table 2 and Figure 6A). Furthermore, all mice in the control group that delivered prematurely delivered the first pup within 14 hours of LPS injection, whereas the delivery time of the one delivering mouse in the RNAi group was at 17.4 hours. No maternal death was observed in either group. siRNA for ECE-1 also decreased the percentage of prematurely delivered pups within 24 hours of LPS injection: for the control group, 44% of pups were delivered prematurely, in comparison to only 2.4% for the RNAi group (P < 0.0005) (Table 2 and Figure 6B). These results suggest that reduction in production of ET-1 caused by siRNA for ECE-1 was associated with a decrease in premature delivery.
Table 2.
Effect of siRNA for ECE-1 in the LPS-Induced Preterm Delivery Mouse Model
| Group* | n | Number of mice delivering preterm | Percent of mice delivering preterm | Total number of pups | Number of pups delivered preterm | Percent of pups delivered preterm |
|---|---|---|---|---|---|---|
| Control | 7 | 5 | 71.4 | 50 | 22 | 44 |
| RNAi | 6 | 1 | 16.7 | 41 | 1† | 2.4† |
Control group is treated with LPS and PBS; RNAi group is treated with LPS and siRNA for ECE-1.
P < 0.001 between control and RNAi groups (Fisher’s exact test).
Figure 6.
Effect of siRNA for ECE-1 on PTL in LPS-treated mice. LPS-stimulated (9.9 mg/kg, i.p.) mice were treated with 0.5 ml of PBS (control) or 500 μg of siRNA for ECE-1 dissolved in 0.5 ml of PBS (RNAi) injected into tail vein at 30, 12, and 3 hours before LPS injection, using a hydrodynamic transfection method. All mice were observed for PTL within 24 hours of LPS injection. A: Percentage of delivering mice throughout time for both groups is shown. Near significant difference (P ≈ 0.08) is observed for the percentage of LPS-induced PTL between the control and RNAi groups. B: Percentage of prematurely delivered pups throughout time for both groups is shown. P < 0.001 for the difference in percentage of pups prematurely delivered between the control and RNAi groups. Triangles: control group; squares: RNAi group.
Discussion
In this study, we have shown that ET-1 and ECE-1 levels are increased in gestational tissues in pregnant mice induced to labor with LPS. We have also demonstrated that blockade of the ETA receptor with a specific receptor antagonist leads to control of premature labor and delivery in LPS-treated animals. Finally, we are the first group, to our knowledge, to apply RNA knockdown technology to the study of preterm labor and delivery. The data presented indicate that knockdown of ECE-1 mRNA leads to control of premature delivery in LPS-treated pregnant mice.
Although endothelin has now been implicated in a very broad spectrum of disease processes, ranging from cancer to cardiovascular disease, its role in the pathogenesis of reproductive disorders, such as premature delivery, has not been thoroughly investigated. The enthusiasm for endothelin as a therapeutic target in reproductive disorders is dampened by our understanding, from work with both the ET-1 and ECE-1 knockout mice,27 that blockade of ET-1 action early in gestation leads to severe craniofacial anomalies and death in the perinatal period. These abnormalities occur, however, when ET-1 is absent early in development. Although BQ-123 is a relatively small molecule, predicted to cross the placenta, none of the pups in the work presented here, which were all transiently viable E16.5 animals, demonstrated such abnormalities, probably because exposure to the ET-1 blocking agent was transient and relatively late in gestation, well after embryogenesis and organogenesis had been completed.
In previous work, we had shown that treatment with phosphoramidon, an ECE-1 inhibitor, leads to control of inflammation-associated premature delivery in our mouse model.24 Phosphoramidon, however, also inhibits neutral endopeptidase.28 Therefore another possible mechanism of the effect of phosphoramidon in controlling premature delivery seen in our previous work is through the inhibition of neutral endopeptidase and decreased degradation of gonadotropin-releasing hormone or any one of the myriad regulatory peptides degraded by neutral endopeptidase. Still another possible mechanism of phosphoramidon-dependent control of premature delivery is through inhibition of additional metalloproteases, especially the matrix metalloproteases, which we have recently shown to play an important role in the pathogenesis of premature delivery in our mouse model29 as well as in normal parturition.30 The uncertainty surrounding the mechanism of action of phosphoramidon in previous experiments led us to test whether a specific ET-1-blocking agent would have the same effect as the metalloprotease inhibitor. The data presented here provide strong evidence that phosphoramidon controls premature delivery through its inhibition of ECE-1.
A major shortcoming in the value of RNA knockdown technology for the treatment of chronic disease, is that siRNAs are unstable and need to be frequently reapplied.31 For an acute, short-lived disorder, such as preterm labor, however, the approach may be promising. In the current study, we were able to prevent premature delivery entirely in five of six mice with only three injections. Furthermore, because of the relative instability of the siRNAs, crossing of the placenta and knockdown of fetal ECE-1 mRNA to an extent significant enough to lead to teratogenic effects is unlikely. Examination of all 41 pups exposed to ECE-1 siRNA in fact revealed no anomalies.
The current therapeutic approaches to preterm labor and delivery remain unsatisfactory and often not without risk to either mother or fetus. At the same time, the incidence of premature delivery continues to rise. Ultimately, a combination of therapies most likely will prove the most useful in managing this important clinical disorder. The identification of specific components of the preterm parturition cascade, such as ET-1, will hopefully provide direction for the development of effective therapy.
Acknowledgments
We thank Helen Scaramell and the staff of the St. John’s University animal facility for their assistance with the breeding of the animals in this study, Zhi Shi for his help with RNA knockdown, and Herbert Tanowitz for his review of our manuscript.
Footnotes
Address reprint requests to Sandra E. Reznik, M.D., Ph.D., Associate Professor, Department of Pharmaceutical Sciences, College of Pharmacy and Allied Health Professions, St. John’s University, St. Albert Hall, SB-24B, 8000 Utopia Parkway, Queens, NY 11439. E-mail: rezniks@stjohns.edu.
Partially supported by the National Institute of Child Health and Human Development (grant 1K08HD01209 to S.E.R.).
References
- Rush RW, Keirse MJNC, Howat P, Baum JD, Anderson AB, Turnbull AC. Contribution of preterm delivery to perinatal mortality. BMJ. 1976;2:965–968. doi: 10.1136/bmj.2.6042.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simhan HN, Caritis SN. Prevention of preterm delivery. New Engl J Med. 2007;357:477–487. doi: 10.1056/NEJMra050435. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Hauth MC, Andrews WW. Intrauterine infection and preterm delivery. New Engl J Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- Lappas M, Permezel M, Georgiou HM, Rice GE. Nuclear factor kappa B regulation of proinflammatory cytokines in human gestational tissues in vitro. Biol Reprod. 2002;67:668–673. doi: 10.1095/biolreprod67.2.668. [DOI] [PubMed] [Google Scholar]
- Belt AR, Baldassare JJ, Molnar M, Romero R, Hertelendy F. The nuclear transcription factor NF-kappaB mediates interleukin-1beta-induced expression of cyclooxygenase-2 in human myometrial cells. Am J Obstet Gynecol. 1999;181:359–366. doi: 10.1016/s0002-9378(99)70562-4. [DOI] [PubMed] [Google Scholar]
- Wang H, Hirsch E. Bacterially-induced preterm labor and regulation of prostaglandin-metabolizing enzyme expression in mice: the role of toll-like receptor 4. Biol Reprod. 2003;69:1957–1963. doi: 10.1095/biolreprod.103.019620. [DOI] [PubMed] [Google Scholar]
- Elovitz M, Wang Z, Chien EK, Rychlik DF, Phillippe M. A new model for inflammation-induced preterm birth: the role of platelet-activating factor and toll-like receptor-4. Am J Pathol. 2003;163:2103–2111. doi: 10.1016/S0002-9440(10)63567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahams VM. Antagonizing toll-like receptors to antagonize preterm labor. Reprod Sci. 2008;15:108–109. doi: 10.1177/1933719108314574. [DOI] [PubMed] [Google Scholar]
- Romero R, Mazor M, Brandt F, Sepulveda W, Avila C, Cotton DB, Dinarello CA. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol. 1992;27:117–123. doi: 10.1111/j.1600-0897.1992.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Romero R, Mazor M, Sepulveda W, Avila C, Copeland D, Williams J. Tumor necrosis factor in preterm and term labor. Am J Obstet Gynecol. 1992;166:1576–1587. doi: 10.1016/0002-9378(92)91636-o. [DOI] [PubMed] [Google Scholar]
- Dudley DJ, Collmer D, Mitchell MD, Trautman MS. Inflammatory cytokine mRNA in human gestational tissue: implications for term and preterm labor. J Soc Gynecol Investig. 1996;3:328–335. [PubMed] [Google Scholar]
- Fidel PL, Romero R, Wolf N, Cutright J, Ramirez M, Araneda H, Cotton DB. Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am J Obstet Gynecol. 1994;170:1467–1475. doi: 10.1016/s0002-9378(94)70180-6. [DOI] [PubMed] [Google Scholar]
- McDuffie RS, Jr, Sherman MP, Gibbs RS. Amniotic fluid tumor necrosis factor-alpha and interleukin-1 in a rabbit model of bacterially induced preterm pregnancy loss. Am J Obstet Gynecol. 1992;167:1583–1588. doi: 10.1016/0002-9378(92)91745-v. [DOI] [PubMed] [Google Scholar]
- Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, Novy MJ. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol. 1994;171:1660–1667. doi: 10.1016/0002-9378(94)90418-9. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Blanchard R, Mehta SP. Differential fetal and maternal contributions to the cytokine milieu in a murine model of infection-induced preterm birth. Am J Obstet Gynecol. 1999;180:429–434. doi: 10.1016/s0002-9378(99)70227-9. [DOI] [PubMed] [Google Scholar]
- Brown NL, Alvi SA, Elder MG, Bennett PR. Interleukin-1beta and bacterial endotoxin change the metabolism of prostaglandins E2 and F2alpha in intact term fetal membranes. Placenta. 1998;19:625–630. doi: 10.1016/s0143-4004(98)90024-8. [DOI] [PubMed] [Google Scholar]
- Challis JRG. Mechanism of parturition and preterm labor. Obstet Gynecol Surv. 2000;55:650–660. doi: 10.1097/00006254-200010000-00025. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayishi Y, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Kaya T, Cetin A, Cetin M, Sarioglu Y. Effects of endothelin-1 and calcium channel blockers on contractions in human myometrium. A study on myometrial strips from normal and diabetic pregnant women. J Reprod Med. 1999;44:155–121. [PubMed] [Google Scholar]
- Yallampalli C, Garfield RE. Uterine contractile responses to endothelin-1 and endothelin receptors are elevated during labor. Biol Reprod. 1994;51:640–645. doi: 10.1095/biolreprod51.4.640. [DOI] [PubMed] [Google Scholar]
- Wolff K, Nisell H, Modin A, Lundberg JM, Lunell NO, Lindblom B. Contractile effects of endothelin 1 and endothelin 3 on myometrium and small intra-myometrial arteries of pregnant women at term. Gynecol Obstet Invest. 1993;36:166–171. doi: 10.1159/000292619. [DOI] [PubMed] [Google Scholar]
- Woods M, Mitchell JA, Wood EG, Barker S, Walcont NR, Rees GM, Warn TD. Endothelin-1 is induced by cytokines in human vascular smooth muscle cells: evidence for intracellular endothelin-converting enzyme. Mol Pharmacol. 1999;55:902–909. [PubMed] [Google Scholar]
- Motte S, McEntee K, Naeije R. Endothelin receptor antagonists. Pharmacol Ther. 2006;110:386–414. doi: 10.1016/j.pharmthera.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Koscica K, Sylvestre G, Reznik SE. The effect of phosphoramidon on inflammation-mediated preterm delivery in a mouse model. Am J Obstet Gynecol. 2004;190:528–531. doi: 10.1016/j.ajog.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Olgun N, Patel H, Wang W, Yen H, Stephani R, Reznik SE. The role of a 1,3,6-trisubstituted-2-carboxy-quinol-4-one, a novel putative selective ETA receptor antagonist, in controlling preterm labor in a mouse model. Can J Physiol Pharmacol. 2008;86:571–575. doi: 10.1139/Y08-057. [DOI] [PubMed] [Google Scholar]
- Turner AJ, Murphy LJ. Molecular pharmacology of endothelin converting enzymes. Biochem Pharmacol. 1996;51:91–102. doi: 10.1016/0006-2952(95)02036-5. [DOI] [PubMed] [Google Scholar]
- Yanagisawa H, Yanagisawa M, Kapur RP, Richardson JA, Williams SC, Cloutheir DE, de Wit D, Noriaki E, Hammer RE. Dual genetic pathways of endothelin-mediated intercellular signaling revealed by targeted disruption of endothelin converting enzyme-1 gene. Development. 1998;125:825–836. doi: 10.1242/dev.125.5.825. [DOI] [PubMed] [Google Scholar]
- Turner AJ, Isaac RE, Coates D. The neprilysin (NEP) family of zinc metalloendopeptidases: genomics and function. Bioessays. 2001;23:261–269. doi: 10.1002/1521-1878(200103)23:3<261::AID-BIES1036>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Koscica K, Ananth CV, Placido J, Reznik SE. The effect of a matrix metalloproteinase inhibitor on inflammation-mediated preterm delivery. Am J Obstet Gynecol. 2007;196:551.e1–551.e3. doi: 10.1016/j.ajog.2007.01.043. [DOI] [PubMed] [Google Scholar]
- Vu D, Feng Y, Placido J, Reznik SE. Placental matrix metalloproteinase-1 is up-regulated in labor. Reprod Sci. 2008;15:420–424. doi: 10.1177/1933719108314625. [DOI] [PubMed] [Google Scholar]
- Lewis DL, Hagstrom JE, Loomis AG, Wolff JA, Herweijer H. Efficient delivery of siRNA for inhibition of gene expression in post-natal mice. Nat Genet. 2002;32:107–108. doi: 10.1038/ng944. [DOI] [PubMed] [Google Scholar]