Abstract
Peptidylarginine deiminases (PADs), which are a group of posttranslational modification enzymes, are involved in protein citrullination (deimination) by the conversion of peptidylarginine to peptidylcitrulline in a calcium concentration-dependent manner. Among the PADs, PAD2 is widely distributed in various tissues and is the only type that is expressed in brain. To elucidate the involvement of protein citrullination by PAD2 in the pathogenesis of brain-specific prion diseases, we examined the profiles of citrullinated proteins using the brains of scrapie-infected mice as a prion disease model. We found that, compared with controls, increased levels of citrullinated proteins of various molecular weights were detected in different brain sections of scrapie-infected mice. In support of this data, expression levels of PAD2 protein as well as its enzyme activity were significantly increased in brain sections of scrapie-infected mice, including hippocampus, brain stem, and striatum. Additionally, the expression levels of PAD2 mRNA were increased during scrapie infection. Moreover, PAD2 immunoreactivity was increased in scrapie-infected brains, with staining detected primarily in reactive astrocytes. Using two-dimensional electrophoresis and matrix-assisted laser desorption/ionization-time of flight mass spectrometry, various citrullinated proteins were identified in the brains of scrapie-infected mice, including glial fibrillary acidic protein, myelin basic protein, enolases, and aldolases. This study suggests that accumulated citrullinated proteins and abnormal activation of PAD2 may function in the pathogenesis of prion diseases and serve as potential therapeutic targets.
Accumulation of misfolded proteins, posttranslational modification of proteins, alteration of free ion distribution, and perturbation of cellular redox homeostasis are general features of progressive neurodegenerative disorders. These changes have been observed consistently as part of the neuropathogenesis and neuropathology of prion diseases. Prion diseases are characterized by various neurological symptoms and common histopathological features such as spongiform degeneration of the central nervous system, reactive gliosis, neuronal loss, and, in some cases, formation of amyloid plaques.1 It has been reported that all prion diseases are associated with the aberrant metabolism of prion protein (PrP). Conversion of the cellular prion protein (PrPC) into an abnormal, protease-resistant and infectious isoform (PrPSc) is believed to be a principal molecular basis of prion diseases,2 and the accumulation of PrPSc in the central nervous system is thought to be responsible for neuronal loss and/or astrocytosis.3 In general, the pathogenic mechanisms of neurodegenerative disorders are not fully delineated; prion diseases are no exception to this uncertainty.
Alteration of intracellular calcium (Ca2+) distribution and Ca2+-related proteins have a critical role in synaptic dysfunction and neuronal cell death in neurodegenerative diseases. In cultured cells, prion infection induced abnormalities in Ca2+ homeostasis by altering receptor-mediated intracellular Ca2+ responses,4,5 suggesting a possible role of Ca2+ in the neuronal cell death seen in prion diseases. Peptidylarginine deiminases (PADs) are known to be directly affected by Ca2+ homeostasis and convert peptidylarginine to peptidylcitrulline (protein citrullination or deimination) in a Ca2+ concentration-dependent manner.6,7 This modification of proteins decreases their positive charge resulting in changing the functions of native proteins.8 PADs are found as five different isoforms in various mammalian tissues such as brain, spinal cord, spleen, and skeletal muscle.9 Among them, only PAD type II (PAD2) is expressed in adult rat brains and its cellular localization was found in glial cells.10,11,12 In a very recent report, PAD2 expression was detected in cultured Schwann cells.13 Previous reports indicate that PAD2 is involved in the citrullination of various cerebral proteins under neurodegenerative conditions.14 Recently, it has been reported that the abnormal accumulation of citrullinated proteins including glial fibrillary acidic protein (GFAP) and vimentin were found in Alzheimer’s disease (AD)-afflicted hippocampus; increased expression of PAD2 and its enzyme activity were detected during neurodegenerative changes and were accompanied by impairment of intracellular Ca2+ homeostasis.15 In multiple sclerosis (MS) patients, previous studies have revealed that citrullinated myelin basic protein (MBP) was increased to 45% of total MBP compared to healthy adults16 and has been implicated in the pathological mechanism of MS.17 Therefore, PAD and citrullinated proteins can be used as important factors for the diagnosis of various human diseases.7
To our knowledge, there are no data available regarding citrullination by PAD2 in prion diseases. Here we report for the first time that increased citrullinated proteins including GFAP, MBP, and several newly identified proteins were found in the brains of scrapie-infected mice along with increased expression of PAD2 protein and its enzyme activity. These findings suggest a possible role of citrullination in the induction of pathological changes in the brains of scrapie-infected mice.
Materials and Methods
Animals and Scrapie Strain
C57BL/6J mice, 4 to 6 weeks of age, were obtained from the Experimental Animal Center of Hallym University. The original stock of ME7 scrapie strain was kindly provided by Dr. Alan Dickinson of Agriculture and Food Research Council and Medical Research Council Institute (Edinburgh, UK): this scrapie strain was maintained by serial intracerebral passage of brain homogenate from terminally affected mice. Mice were inoculated intracerebrally with 30 μl of 1% (w/v) brain homogenate in 0.01 mol/L phosphate-buffered saline (PBS) prepared from ME7-injected C57BL mice or from control mice that had been injected with normal brain homogenate. The mice were then sacrificed under 16.5% urethane at 150 ± 10 days after inoculation with ME7 scrapie strain, a time when clinical manifestations of disease were evident. To perform a time course study, brains were also collected at different time points (50, 100, and 150 days after inoculation). Mice inoculated with normal brain homogenate remained healthy throughout the same period. For immunohistochemistry, mice were perfused transcardially with PBS followed by 4% paraformaldehyde in PBS (pH 7.4). The brains were removed immediately, postfixed in the same fixative for 2 hours at room temperature, rinsed with PBS, dehydrated with ethanol, and embedded in paraffin.
Western Blot Analysis
Brains from control and scrapie-infected mice were homogenized in modified RIPA buffer containing 50 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 2 mmol/L ethylenediaminetetraacetic acid, 1% Triton X-100, 1% Nonidet P-40, 0.25% sodium deoxycholate, and protease inhibitors.18 The homogenates were rocked at 4°C for 1 hour and centrifuged at 18,000 × g at 4°C for 10 minutes to remove cell debris. The supernatant was collected and protein concentration was determined with a BCA protein assay kit (Pierce, Rockford, IL). Citrullinated proteins were detected by Western blot analysis as described previously.19 Briefly, equal amounts of protein (50 μg/lane) were subjected to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membrane using an electrotransfer system (Bio-Rad, Hercules, CA). For detection of citrullinated proteins, citrulline residues on the polyvinylidene difluoride membrane were chemically modified by overnight incubation at 37°C in modification reagent [1 vol of 1% diacetyl monoxime/0.5% antipyrine/1 N acetic acid, and 2 vol of a mixture of 85% H3PO4/98% H2SO4/H2O (20/25/55) containing 0.025% FeCl3].20 The membrane was then blocked with 5% nonfat dry milk in PBST (8 mmol/L Na2HPO4, 2 mmol/L KH2PO4, 138 mmol/L NaCl, 2.7 mmol/L KCl, pH 7.4, 0.05% Tween 20) for 2 hours at room temperature, then probed with an anti-modified citrulline antibody at 1:1000 (Upstate, Lake Placid, NY) in PBST overnight at 4°C. For the detection of other target proteins, the transferred polyvinylidene difluoride membranes were directly probed with either monoclonal anti-PAD2 antibody (1:2000), mouse monoclonal anti-neuron-specific enolase (1:2000) (AbFrontier, Seoul, Republic of Korea), rabbit polyclonal anti-aldolase C (1:1000) (Santa Cruz Biotechnology, Santa Cruz, CA), mouse monoclonal anti-MBP (1:3000) (Abcam, Cambridge, MA), or mouse monoclonal anti-β actin (1:10,000) (Sigma, St. Louis, MO) in 5% nonfat dry milk in PBST. These membranes were then incubated with the appropriate secondary antibody-conjugated to horseradish peroxidase. Bound antibodies were visualized by chemiluminescent substrate as described by the manufacturer (Amersham Biosciences, Piscataway, NJ).
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted from brain samples of control and scrapie-infected mice using TRI-reagent (Sigma) according to the manufacturer’s protocols. Complementary DNA (cDNA) was generated using the Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI) according to the instructions of the manufacturer. RT-PCR was performed using primers specific for the PAD2 (665 bp; forward, 5′-CTGCGGTCTCTGGGTCCTTCCTGTA-3′ and reverse, 5′-GACCAGGCGAGAGAACAGAAATAGC-3′) and β-actin (196 bp; forward, 5′-TGTGATGGACTCCGGTGACGG-3′ and reverse, 5′- ACA GCTTCTCTTTGATGTCACGC-3′) genes. The PCR products were separated by electrophoresis on a 1% agarose gel and visualized under UV light.
Measurement of PAD2 Activity
The PAD2 activity was determined as described previously.9 Briefly, the reaction mixture containing 100 mmol/L Tris-HCl, pH 7.5, 10 mmol/L CaCl2, 5 mmol/L dithiothreitol, with or without 10 mmol/L benzoyl-l-arginine ethyl ester (Sigma), and 0.5 mg of brain protein in a final volume of 120 μl was incubated at 50°C for 1 hour. After incubation, the reaction was stopped by adding final 1 mol/L perchloric acid. Samples were cooled down on ice for 20 minutes and then centrifuged at 18,000 × g for 5 minutes at room temperature. Aliquots of 120 μl of supernatant were mixed with 380 μl of dH2O and 500 μl of color developing reagent and incubated at 95°C for 15 minutes. The samples were cooled to room temperature and then the absorbance was measured at 540 nm by enzyme-linked immunosorbent assay reader (VersaMax; Molecular Devices, Sunnyvale, CA). Quantification of citrulline was determined by comparison with appropriate standards. One unit of the enzyme is defined as the amount of enzyme that deiminates 1 μmol/L of the substrate (benzoyl-l-arginine ethyl ester) in 1 minute at 50°C.
Immunohistochemistry and Double-Immunofluorescence Staining
Immunohistochemical procedures were performed using the ABC kit (Vector, Burlingame, CA) by a modification of the avidin-biotin-peroxidase method. Briefly, 6-μm sections of brain were deparaffinized with xylene and hydrated in a graded ethanol series, and then treated with 0.3% hydrogen peroxide in methyl alcohol for 20 minutes to block endogenous peroxidase. The sections were exposed to normal donkey serum (Jackson ImmunoResearch, West Grove, PA), and then incubated with mouse monoclonal antibody for PAD2 (1:500) overnight at 4°C. After washing, the sections were treated sequentially with biotinylated anti-mouse IgG and avidin-biotin-peroxidase complex, developed with diaminobenzidine-hydrogen peroxide solution (0.003% 3,3-diaminobenzidine and 0.03% hydrogen peroxide in 50 mmol/L Tris buffer), and finally counterstained with hematoxylin. For staining of citrullinated proteins, the sections were incubated in modification reagent for 2 hours at 37°C before initiation of the immunohistochemistry protocol. After three washes in PBS buffer, the sections were exposed to 10% normal donkey serum for 1 hour at room temperature and then rabbit polyclonal antibody to modified citrulline (1:4000) for 1 hour at 37°C. The subsequent procedures were performed as described above for PAD2 staining. For double-immunofluorescence staining, the sections were incubated in the following order: 10% normal donkey serum in PBS for 1 hour, rabbit polyclonal anti-PAD2 (1:100)21 overnight at 4°C, lissamine rhodamine sulfonyl chloride (LRSC)-conjugated donkey anti-rabbit IgG (1:200) (Jackson ImmunoResearch) for 1 hour at room temperature, washed, and blocked with 10% normal goat serum in PBS for 1 hour at room temperature and then incubated with various primary antibodies as follows: mouse monoclonal anti-GFAP antisera (1:400; DAKO, Copenhagen, Denmark), mouse monoclonal anti-NeuN (1:50) (Chemicon, Temecula, CA), mouse monoclonal anti-MBP (1:3000) overnight at 4°C and finally washed and incubated with fluorescein isothiocyanate-conjugated goat anti-mouse IgG (1:200) (Jackson ImmunoResearch). For microglia staining, the lectin Griffonia simplicifolia (GSA) was optimized by incubating the sections in 0.5 mg/ml of trypsin in 0.05 mol/L Tris-buffered saline containing 1 mmol/L CaCl2 (pH 7.6) for 5 minutes at 37°C. Biotinylated lectin GSA B4-isolectin (Sigma) was then added for 1 hour at room temperature, followed by fluorescein isothiocyanate-labeled streptavidin (Zymed, San Francisco, CA). The sections were examined with a LSM 510 confocal laser-scanning microscope (Carl Zeiss, Oberkochen, Germany).
Two-Dimensional Gel Electrophoresis (2-DE) and Proteome Analysis
Protein extraction and 2-DE were performed as reported previously.22 Briefly, 200 μg of brain protein of control and ME7 scrapie-infected mice was dissolved in a rehydration buffer containing 8 mol/L urea, 2% CHAPS, 65 mmol/L dithiothreitol, 0.5% immobilized pH gradient (IPG) buffer (Bio-Rad), 40 mmol/L Tris-HCl, and 0.002% bromophenol blue. The brain homogenates were applied to the IPG Readystrip, 7 cm, pH 3 to 10 linear gradient (Bio-Rad). The IPG strips were rehydrated for 16 hours at 20°C using the PROTEAN isoelectric focusing cell (Bio-Rad) according to the manufacturer’s instructions. Briefly, isoelectric focusing was conducted at 250 V for 15 minutes, linearly increased throughout 2 hours to a maximum of 4000 V, and then run to accumulate a total of 20,000 Vhours. The gel strips were equilibrated before second dimensional electrophoresis for 15 minutes in 50 mmol/L Tris-HCl (pH 8.8) containing 6 mol/L urea, 30% glycerol, 2% sodium dodecyl sulfate, 0.002% bromophenol blue, and 80 mmol/L dithiothreitol or 0.025% iodoacetamide. The gel strips were then separated in 12% polyacrylamide gels to perform the second dimensional electrophoresis. The 2-DE gels were then exposed to Coomassie Brilliant Blue G-250 (CBBG-250) or silver staining. Duplicated 2-DE gels were also transferred on polyvinylidene difluoride membrane and then used for the detection of citrullinated proteins using an antibody to modified citrulline. The protein spots of immunoblotting-matched citrullinated proteins were subjected to in-gel trypsin digestion, and digested peptides were analyzed by matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry (the Proteomics Service by Genomine, Pohang, Republic of Korea). The obtained peptide mass fingerprints spectra were analyzed by searching the Genomine local database and the National Centre for Biotechnology Information, nonredundant protein database with ProFound (http://prowl. rockefeller.edu/prowl-cgi/profound.exe).
Statistical Analysis
Quantitative results were expressed as means ± SEM. The probability of statistical differences between control and scrapie-infected groups was determined by a one-way analysis of variance test as indicated. Differences were considered significant at P < 0.001, P < 0.01, and P < 0.05.
Results
Increased Accumulation of Citrullinated Proteins in Various Brain Regions of Scrapie-Infected Mice
We first used Western blot analysis as described in the Materials and Methods to examine the citrullinated proteins from different brain regions of control and ME7 scrapie-infected mice. Total proteins were extracted from whole brains as well as from various dissected brain regions including cerebral cortex, hippocampus, striatum, cerebellum, and brain stem of control and scrapie-infected mice. In general, citrullinated proteins in most brain sections of scrapie-infected mice were markedly increased compared to controls (Figure 1, asterisks). In all brain regions, most bands from 10 kDa to 100 kDa were increased in scrapie. Those bands between 100 kDa and 150 kDa in whole brain, cerebellum, and brain stem were increased during scrapie infection, whereas no difference in citrullination was seen in other regions.
Figure 1.
Detection of citrullinated proteins in brains from control and ME7 scrapie-infected mice. Whole brains and various brain sections of control (lanes 1 to 3) and ME7-infected mice (lanes 4 to 6) were homogenized, and then citrullinated proteins were detected by Western blot analysis using a polyclonal anti-modified citrulline antibody. Note that citrullinated proteins were increased in scrapie-infected regions compared to controls. Each lane shows the results for a homogenate obtained from brains of each individual mouse. Asterisks indicate the citrullinated proteins. These results are representative of at least three separate experiments.
Next, we performed immunohistochemical staining of citrullinated proteins in different brain sections of control and scrapie-infected mice. As shown in Figure 2, citrullinated proteins (indicated by arrows) were more prominent in the scrapie-infected brains (Figure 2, F–J) than in control brains (Figure 2, A–E). These results correlated with the increased citrullinated proteins observed in scrapie-infected mice using Western blot analysis (Figure 1). We also observed similar results in the brains of 87V scrapie-infected mice (data not shown), indicating the occurrence of increased citrullination after infection is a general phenomenon.
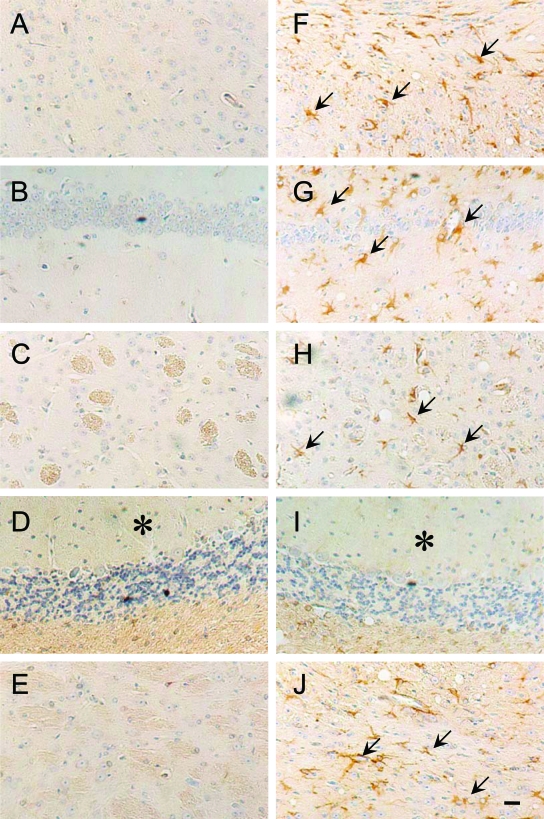
Figure 2.
Immunohistochemical staining of citrullinated proteins in brain sections from control and ME7 scrapie-infected mice. Citrullinated proteins were detected in the brains of control (A–E) and scrapie-infected (F–J) mice at 160 days after inoculation. In the scrapie brain, citrullinated proteins (arrows) were more frequent than in control brains: cerebral cortex (F), hippocampus (G), striatum (H), cerebellum (I), and brain stem (J). Asterisks indicate the position of cerebellar molecular layer. Scale bar = 20 μm.
Up-Regulation of PAD2 Expression in Various Brain Regions from Scrapie-Infected Mice
Posttranslational modification of protein arginine residues to citrulline is mediated by PAD2 enzyme in the brain. Therefore, we examined whether the expression levels of PAD2 in the brains are increased after ME7 scrapie infection. As can be seen in Figure 3A, the expression levels of PAD2 protein appeared to be increased in scrapie-infected mice in the following brain sections: cerebral cortex, hippocampus, striatum, and brain stem. However the expression level of PAD2 in cerebellum did not appear to be different between control and infected mice. Densitometric analysis of the gels showed that the relative intensity of the PAD2 bands was significantly higher in all sections of scrapie-infected brains compared to controls except in the cerebellum (Figure 3B).
Figure 3.
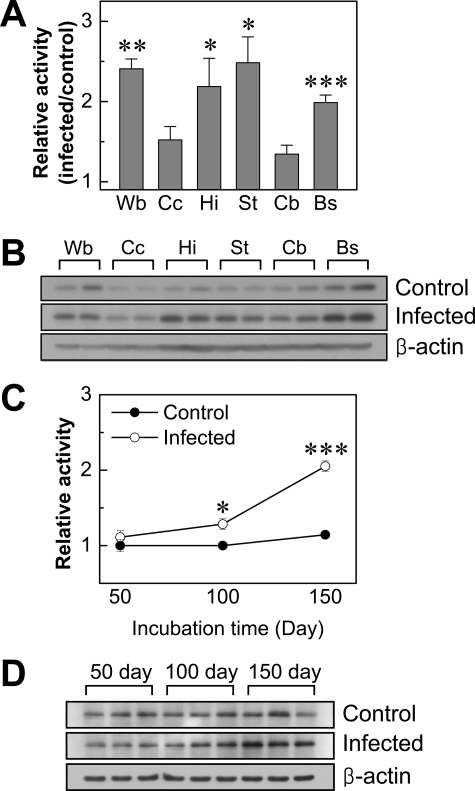
Comparison of expression level of PAD2 in brains from control and ME7 scrapie-infected mice. A: Expression level of PAD2 protein in whole brain and various brain sections of control and ME7-infected mice were analyzed by Western blot using a monoclonal anti-PAD2 antibody. Each lane shows the results for a homogenate obtained from dissected brain of each individual mouse. B: Densitometric analysis of bands in A normalized with β-actin. Wb, whole brain; Cc, cerebral cortex; Hi, hippocampus; St, striatum; Cb, cerebellum; Bs, brain stem. C: In whole brains, mRNA levels of PAD2 were analyzed by RT-PCR using three individuals of each group. Error bars represent SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
In the next experiment, we measured expression levels of PAD2 mRNA in whole brains of both control and ME7 scrapie-infected mice using PAD2-specific primers as described in the Materials and Methods. As can be seen in Figure 3C, RT-PCR analysis showed increased PAD2 mRNA in the whole brain of ME7 scrapie-infected mice compared to levels in controls. This result suggests that up-regulated expression of PAD2 protein seen in most brain regions is caused by an increase of gene expression in scrapie-infected mice.
Increased Immunofluorescence Staining of PAD2 in Reactive Astrocytes in Brains of Scrapie-Infected Mice
Next, to confirm the increased PAD2 expression and to determine the cellular localization of PAD2, immunohistochemical analysis was performed using various brain sections from control and scrapie-infected mice (Figure 4, A–E and F–J, respectively). In scrapie-infected brains, PAD2 immunoreactivity was more intense compared to control brains and these results correlated with the expression patterns of PAD2 protein in results of Western blot analysis (Figure 3A). According to previous reports, PAD2 is mainly expressed in glial cells such as astrocytes, oligodendrocytes, and microglial cells.10,11,12 To further characterize the subcellular localization of PAD2, we performed double-immunofluorescence staining using GFAP, MBP, NeuN, and B4-isolectin as a marker for astrocytes, oligodendrocytes, neurons, and microglia, respectively. Interestingly, immunoreactivity was mainly observed in activated astrocytes in the brains of scrapie-infected mice. As can be seen in Figure 5, PAD2 was mainly co-localized with GFAP-positive astrocytes and in a few B4-isolectin-positive microglia, but not with MBP or NeuN. These data suggest that up-regulation of PAD2 expression in reactive astrocytes is responsible for the increased citrullinated proteins in the brains of scrapie-infected mice.
Figure 4.
Expression of PAD2 in various brain sections. Immunohistochemical staining of PAD2 was performed using a monoclonal anti-PAD2 antibody in various brain sections from control (A–E) and ME7 scrapie-infected (F–J) mice. PAD2 was observed in dissected control and scrapie-infected brains at 160 days after inoculation with the ME7 scrapie strain. PAD2-positive cells were strongly immunostained in scrapie-infected brains and its immunoreactivity was observed in reactive astrocytes (arrows). A and F: Cerebral cortex; B and G: hippocampus; C and H: striatum; D and I: cerebellum; E and J: brain stem. Asterisks indicate the position of cerebellar molecular layer. Scale bar = 20 μm.
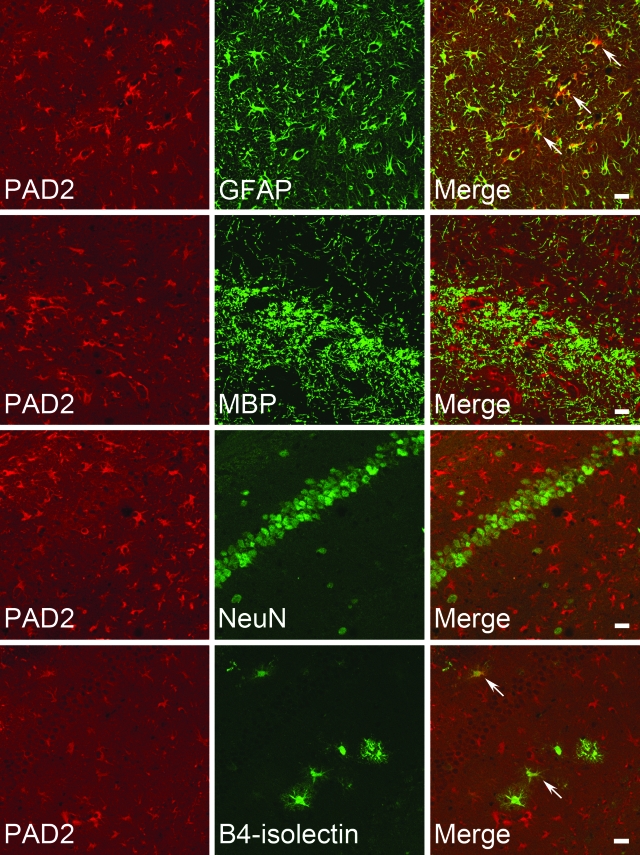
Figure 5.
Cellular localization of PAD2 in scrapie-infected brains. The brain sections were doubly immunostained with anti-PAD2 and one of the following antibodies: astrocyte-specific GFAP, oligodendrocyte-specific MBP, neuron-specific NeuN, or microglia-specific B4-isolectin antibodies. Slides were examined under confocal laser-scanning microscopy. Note that PAD2-positive cells were strongly co-localized in cells positive for GFAP (arrows) with very few B4-isolectin-positive microglia (arrows). Scale bars = 20 μm.
Elevated PAD2 Enzyme Activity in the Brains of Scrapie-Infected Mice
To investigate whether the increase in citrullinated proteins is attributable to higher levels of PAD2 enzyme activity and whether the increased PAD2 expression is correlated with its enzyme activity in scrapie-infected mice, we analyzed PAD2 enzyme activity in the brain homogenates of various sections from both control and ME7 scrapie-infected mice using benzoyl-l-arginine ethyl ester as a substrate as previously described.9 PAD2 enzyme activity was significantly increased approximately twofold in whole brains as well as in hippocampus, striatum, and brain stem of scrapie-infected brains compared to controls (Figure 6A): whole brain [2.41-fold, control: 0.322 ± 0.052 (mean ± SEM, units); infected: 0.776 ± 0.039], hippocampus (2.19-fold, control: 0.342 ± 0.081; infected: 0.748 ± 0.120), striatum (2.48-fold, control: 0.256 ± 0.009; infected: 0.636 ± 0.082), and brain stem (1.99-fold, control: 0.589 ± 0.028; infected: 1.172 ± 0.055). The difference from controls was not significant in cerebral cortex (control: 0.193 ± 0.022; infected: 0.294 ± 0.032) or in cerebellum (control: 0.312 ± 0.041; infected: 0.419 ± 0.035). To determine whether the amount of PAD2 protein is associated with changes in enzyme activity, we compared the expression level of PAD2 protein in 50 μg of total protein from whole brain and from different brain regions of control and scrapie-infected mice. As shown in Figure 6B, the expression levels of PAD2 protein in cerebral cortex and in cerebellum were lower than other regions but still higher than controls. It should be noted that all regions showed increased levels of citrullinated proteins in scrapie-infected brains compared to controls (Figures 1 and 2). The results suggest that demonstration of PAD2 enzyme activity (Figure 6A) may require a certain amount of PAD2 protein to see the difference of enzyme activity and its citrullination between control and scrapie-infected brains in our in vitro assay. In addition, to determine whether the enzyme activity and protein expression of PAD2 are correlated with the progress of this disease, we also examined PAD2 enzyme activity and its protein expression at different time points during scrapie incubation period using whole brains of scrapie-infected and control mice (Figure 6, C and D). In Figure 6C, no difference between scrapie-infected and control brains was observed at 50 days after inoculation. However, the PAD2 enzyme activity tended to increase relative to the stage of scrapie development and was significantly increased at 100 and 150 days after inoculation; this time corresponds to marked accumulation of PAD2 (Figure 6D) and citrullinated proteins (data not shown). These results suggest that increased PAD activity and its protein expression might be responsible for the increased citrullinated proteins at end stage of scrapie infection.
Figure 6.
Measurement of PAD2 enzyme activity in mouse brains. A: PAD2 enzyme activity in the brains of control and ME7-infected mice. PAD2 enzyme activity was determined as described in the Materials and Methods. The results are presented as a column graph of values in infected samples relative to controls. Values are mean ± SEM obtained from separate assays of three mouse brains (n = 3). B: Comparison of the expression levels of PAD2 protein in various brain sections of scrapie-infected mice. Wb, whole brain; Cc, cerebral cortex; Hi, hippocampus; St, striatum; Cb, cerebellum; Bs, brain stem. C: PAD2 enzyme activity was determined at different time points during scrapie incubation period using the whole brains of control (filled circle) and ME7-infected mice (open circle) as indicated (n = 3). D: Expression level of PAD2 protein in whole brains of control and scrapie-infected mice at 50, 100, and 150 days after inoculation was analyzed by Western blotting. Each experiment was repeated at least three times, and similar results were obtained. *P < 0.05, **P < 0.01, ***P < 0.001.
Identification of Citrullinated Proteins by 2-DE and MALDI-TOF Mass Spectrometry
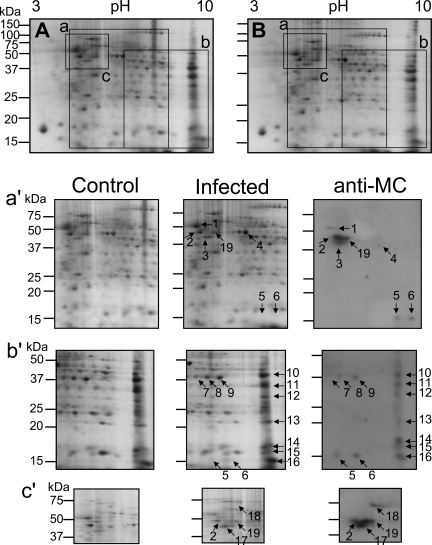
For identification of various citrullinated proteins in scrapie-infected brains, we performed 2-DE analysis using whole brains as well as three different brain regions: hippocampus, cerebellum, and brain stem. We chose these three regions because i) hippocampus is the most damaged brain region, ii) cerebellum showed a marked increase in citrullinated bands between 100 kDa and 150 kDa, and iii) brain stem has shown the highest expression of PAD2 after infection with the ME7 scrapie strain. Figure 7 shows representative 2-DE gels for the whole brain from control and ME7 scrapie-infected mice. The rectangular areas (Figure 7, a–c) in 2-DE gels were representative immunoblotting for the whole brain (Figure 7a′), cerebellum (Figure 7b′), and brain stem (Figure 7c′). Using an antibody to modified citrulline, we detected numerous citrullinated spots in dissected brain regions of scrapie-infected brains that were not seen in controls (Figure 7, a′–c′; right). In case of hippocampus, citrullinated spots detected were the same as those seen in whole brain and brain stem (data not shown).
Figure 7.
Detection of citrullinated proteins from two-dimensional electrophoresis. The 2-DE gels show CBBG-250 staining of proteins from brains of control and infected mice (A and B, respectively). The rectangular areas (a–c) of the 2-DE gels represent a part of the 2-DE gels in each region of control and infected brains (left and middle, respectively) and citrullinated proteins (anti-MC) assayed by Western blot (right) from whole brain (a′), cerebellum (b′), brain stem (c′). Serial numbers in each infected region were used to distinguish between the identified citrullinated proteins in each region. The second spot in whole brain was the same as the one in brain stem, the fifth and sixth spots in whole brain were equal to the spots in cerebellum. The 19th spot was also detected in hippocampus (data not shown). Each experiment was repeated at least three times, and similar results were obtained.
To identify the citrullinated spots, we analyzed peptide spots by MALDI-TOF mass spectrometer. Identified citrullinated proteins in the brains of scrapie-infected mice are summarized in Table 1. We have found some known citrullinated proteins including GFAP and MBP as well as new substrates for PAD2 including neuron-specific enolase (NSE; 2-phospho-d-glycerate hydrolase), fructose 1,6-bisphosphate aldolase A and C (ALDC), and mitochondrial malate dehydrogenase 2 (MDH2), and voltage-dependent anion channel 1 (VDAC1).
Table 1.
Summary of identified citrullinated proteins in ME7 scrapie-infected mice
| Spot No. | Identification | Sequence coverage (%) | pI | kDa | NCBI accession no. | #Z-value |
|---|---|---|---|---|---|---|
| 1 | Tubulin, beta 2 | 24 | 4.8 | 50.29 | NP_033476 | 2.07 |
| 2 | Enolase 2, neuron-specific | 40 | 5.0 | 47.62 | NP_038537 | 2.37 |
| 3 | Glial fibrillary acidic protein | 50 | 5.0 | 46.57 | AAK56090 | 2.15 |
| 4 | Enolase 1, alpha | 42 | 6.4 | 47.47 | P17182 | 2.33 |
| 5 | Myelin basic protein | 36 | 11.8 | 14.19 | AAA39497 | 1.68 |
| 6 | Myelin basic protein | 56 | 11.8 | 14.19 | AAA39497 | 1.69 |
| 7 | Aldolase 3, C isoform | 36 | 6.7 | 38.78 | P05063 | 2.41 |
| 8 | Aldolase 3, C isoform | 39 | 6.7 | 38.78 | P05063 | 2.39 |
| 9 | Aldolase 3, C isoform | 35 | 6.7 | 38.78 | P05063 | 2.24 |
| 10 | Aldolase 1, A isoform | 45 | 8.8 | 39.79 | NP_031464 | 2.40 |
| 11 | Malate dehydrogenase 2 | 54 | 9.4 | 36.05 | NP_032643 | 2.37 |
| 12 | Voltage-dependent anion channel 1 | 36 | 8.7 | 32.50 | Q60932 | 2.29 |
| 13 | Peroxiredoxin 1 | 39 | 8.6 | 22.39 | NP_035164 | 2.08 |
| 14 | Cofilin 1 | 51 | 8.5 | 18.77 | NP_031713 | 2.33 |
| 15 | Peptidylprolyl isomerase A | 36 | 7.9 | 18.12 | NP_032933 | 1.80 |
| 16 | Myelin basic protein | 61 | 11.8 | 14.19 | AAA39497 | 2.41 |
| 17 | Glial fibrillary acidic protein | 41 | 5.0 | 46.57 | AAK56090 | 1.53 |
| 18 | Heat shock protein 8 | 28 | 5.3 | 71.08 | AAH66191 | 2.38 |
| 19 | Glial fibrillary acidic protein | 28 | 5.0 | 46.57 | AAK56090 | 2.38 |
Citrullinated spots were identified from spots 1–6 in whole brain, spots 7–16 in cerebellum, and spots 17–19 in brain stem. The 19th spot was also identified in hippocampus from ME7 scrapie-infected mice. #Z-value and its corresponding confidence are following: 1.282, 90.0%; 1.645, 95.0%; 2.326, 99.0%; 3.090, 99.9%.
Expression Levels of Identified Citrullinated Proteins; Aldolase C, Neuron-Specific Enolase, MBP, and GFAP
Based on the result of MALDI-TOF analysis, we performed Western blot analysis to determine the expression pattern of identified citrullinated proteins in control and scrapie-infected brains. As shown in Figure 8, interestingly, ALDC, a newly identified citrullinated protein in this study was significantly increased in scrapie-infected brains compared to controls. It has been known that ALDC is mainly expressed in astrocytes and Purkinje cells.23,24 In immunohistochemical staining of ALDC in the brains of control and infected mice, we also observed highly intense staining of ALDC, mainly in astrocytes of hippocampus (data not shown). The results for NSE showed slightly decreased levels in hippocampus (37%) and cerebellum (17%) of scrapie brain compared to controls, whereas the quantities in other brain regions appeared to be very similar in infected and controls (Figure 8). Strikingly, MBP was decreased in scrapie-positive mice compared to control animals in several regions, including cerebral cortex, hippocampus, striatum, and brain stem; the difference from control was quite marked in hippocampus and striatum. In accordance with previous reports, GFAP was increased in all infected brain regions that reflects the pathological finding of astrocytosis.
Figure 8.
Expression levels of several identified citrullinated proteins in brains of control and scrapie-infected mice. Proteins that had been identified as citrullinated were analyzed by Western blot in homogenates from whole brain, cerebral cortex, hippocampus, striatum, cerebellum, and brain stem. The proteins included ALDC, NSE, GFAP, MBP, and actin as a control stain. Note that ALDC and GFAP were significantly increased in scrapie-infected mice. In contrast, NSE was slightly decreased in whole brain, hippocampus, and cerebellum and MBP was significantly decreased in cerebral cortex, brain stem, hippocampus, and striatum in brains from scrapie-infected mice compared to controls.
Discussion
It has been postulated that citrullination of proteins has important effects on their structure and functions. Citrullination, an irreversible posttranslational modification, has been associated with the pathogenesis of several neurodegenerative diseases including AD and MS.15,17 Citrullinated proteins in these diseases are thought to play a role during disease initiation and/or disease progression. The mouse models of neurodegenerative diseases provide a rapid and inexpensive means of studying pathogenesis and pathology of human diseases.
In the present study, we demonstrated for the first time that protein citrullination occurs in a widely used model of prion diseases, ie, ME7 scrapie-infected mice. PAD enzymes have been shown to increase in various abnormal conditions in vivo including transgenic animal models of demyelinating diseases,25 optic nerve damage in glaucoma,26 and in hippocampi of AD patients.15 However, the regulatory mechanism of PAD2 expression and of the induction of citrullinated proteins involved in the pathogenesis of various diseases has not been clarified. Using a model system of scrapie-infected mice provides a number of advantages in that scrapie strains have specific parameters of incubation period, location of histopathological changes, and mouse strain susceptibility. Therefore, the combination of scrapie strain-mouse strain may give an area-specific difference in the expression pattern of PAD2 and hypercitrullinated proteins that can be correlated with histopathological changes in each brain region. The situation is more complex with human disease because of different or even unknown causes and the inability to assess brain changes during the course of the disease. However, studies with the mouse models of scrapie can provide a basis for studying citrullination in postmortem tissue from a variety of diseases.
An important aspect of citrullination in vivo and in vitro is that PAD enzymes require ∼100-fold higher than the normal intracellular Ca2+ level for its activation.7 PrPSc is associated with elevation of intracellular Ca2+ released from endoplasmic reticulum in neuroblastoma cells.27 PrP 106-126 peptide that shares several characteristics with PrPSc induces Ca2+ release from endoplasmic reticulum and mitochondria.28,29 These reports suggest that intracellular Ca2+ is increased in a variety of cells in the brains of scrapie-infected mice. The increase in intracellular Ca2+ during scrapie infection would augment the activity of PAD2, which, in turn, would lead to citrullination of various proteins. In addition, malfunctioning of some of these citrullinated proteins could play a role in the pathogenesis of the pathological changes that are seen in prion diseases. This scenario is supported by our findings of increased PAD2 and citrullinated proteins combined with the above-noted reports on the relationship between Ca2+ metabolism and scrapie.
Because the current knowledge of the molecular features of PAD enzymes is limited, it is still unclear whether activation of PAD2 is a cause of or an effect of the progression of prion diseases. In a previous article, PAD2 was increased and accumulated in the hippocampus during the progress of AD.15 This finding suggests that because PAD2 was increased even during early stages of AD, PAD2 activation may be related to onset of neurodegenerative change in this disease. It has been suggested that the activation of PADs is induced by elevation of intracellular calcium levels or leakage of the enzyme to the extracellular space as a result of cell membrane disruption.30,31 Although the increased intracellular calcium levels appear to be the major regulator of PADs, it has been shown that some hormones, including estrogen, progesterone, and insulin, and other molecules that induce differentiation of cells regulate the activity of PAD2 and other PAD isotypes.32 Moreover, PAD2 activity is enhanced in a reducing environment,33,34 indicating that PAD activity can be up-regulated by modulation of disulfide bonds. Thus, these data show that the regulation of PAD2 activity is highly complex with multiple interlocking control points.
Results of Western blot analysis using an antibody to citrullinated proteins have shown a variety of strong bands between 10 kDa to 150 kDa. A number of these were markedly increased in scrapie compared to control brain regions. We analyzed homogenates of whole brain, brain stem, and cerebellum from control and scrapie-infected mice by 2-DE and MALDI-TOF mass spectrometry analyses that yielded 13 distinct citrullinated proteins (Table 1). We could not establish the identity of the bands in brain stem and cerebellum between 100 kDa to 150 kDa using 2-DE. Three distinct citrullinated proteins were previously demonstrated: GFAP in AD,15 MBP in MS,35 and α-enolase in rheumatoid arthritis (RA).36 These proteins relate to energy production pathway and to cellular structure.
GFAP and MBP are well known to show increases in citrullination in degenerative disorders. In ME7-infected mice, there were pronounced increases in the levels of citrullinated GFAP compared to controls in all brain regions tested (Figure 1). Citrullinated spots were seen at 50 kDa and at 15 kDa, which are the molecular weight designations for GFAP and MBP, respectively. GFAP and MBP are arginine-rich proteins that have 47 arginine residues in GFAP and 19 residues in MBP, in both human and mouse. Any arginine residue can be citrullinated, and a number of specific rules of citrullination have been formulated.8 The expression level of GFAP was increased in every brain region tested; in contrast, there was a marked decrease in MBP in all regions except cerebellum (Figure 8). These results may reflect the unfolding of citrullinated MBP and subsequent rapid degradation by cathepsin D,37 an enzyme that is increased in scrapie.38 A decrease of MBP may evoke demyelinating changes in prion diseases.39,40 In scrapie-infected mice, there is decreased expression of MBP, but the MBP present is citrullinated abundantly (Figures 1 and 8 and Table 1). A high proportion of GFAP-positive cells contain citrullinated proteins, although there are cells that appear to be negative for staining. NSE and MBP were also shown to be positive for citrullination, indicating that neurons and oligodendrocytes are also affected. Neurons could also be affected indirectly in that citrullination of proteins in astrocytes could affect their role in physiological support of neurons; this, in turn, could lead to neuron degeneration and loss.
Interestingly, we found that energy regulation-related proteins such as enolases, aldolases, MDH2, and VDAC1 can be citrullinated by PAD2 in scrapie. α-Enolase, a glycolytic enzyme, has been identified as an autoantigen in diseases such as MS41 and Hashimoto’s encephalopathy.42 NSE has been used as a pathogenic marker in various neurological diseases including MS,43 acute ischemic stroke,44 and RA.36,45 In addition, previous reports showed an early elevation of NSE in cerebrospinal fluid of Creutzfeldt-Jakob disease (CJD) patients, which decreased with disease duration, and this early elevation is considered to support the diagnosis of CJD.46,47,48 We also examined the expression level of NSE in brains of scrapie-infected mice and the results showed slightly decreased levels in hippocampus and cerebellum of scrapie brain compared to controls; levels in other brain regions were similar in control and scrapie (Figure 8).
Aldolase A is suggested as a candidate autoantigen in RA45 and in AD,49 and its deficiency is associated with a hemolytic anemia.50 ALDC has been termed “scrapie-responsive protein 2 (scrg2),” and its mRNA is increased in scrapie infection.51,52 We also confirmed an increase of ALDC in scrapie-infected mice using Western blot (Figure 8) and immunohistochemical analyses (data not shown). In addition, we are first to report that ALDC is a new candidate citrullinated protein and that it was mainly expressed in reactive astrocytes of scrapie-infected brain. Although ALDC is a predominant type in brain, the total aldolase activity was not significantly changed by scrapie infection (data not shown). It is possible that citrullination of ALDC leads to its accumulation, but generates its inactive form because there were no significant changes of enzyme activity. MDH2, an enzyme in the citric acid cycle, is thought to play a key role in the pathophysiology of schizophrenia.53 However, thus far, the evidence of MDH2 involvement in disease is weak. VDAC1 is a mitochondrial membrane protein that plays a role in the transport of various metabolites across the outer mitochondrial membrane and that regulates mitochondrial Ca2+ homeostasis54 and apoptosis in conjunction with the Bcl-2 family.55 Previous reports have demonstrated that several divalent metal ions including Ca2+ are increased in mitochondrial membrane fractions of scrapie-infected mice.56 Assuming that citrullination of VDAC affects its role in membrane permeability, this in turn could lead to changes in Ca2+ homeostasis and in the induction of neuronal cell death in prion diseases.
Finally, we have identified more candidate citrullinated proteins such as cofilin 1, peptidylprolyl isomerase A, and peroxiredoxin 1. Cofilin 1 is an actin-binding protein that modulates neuronal actin dynamics and synaptic plasticity.57,58 Peptidylprolyl isomerase A has multiple roles in protein refolding, signal transduction, and cell cycle regulation.59 Peroxiredoxin 1 is a thiol-specific multifunctional antioxidant enzyme whose major role is a defense against oxidative stress through peroxidase activity.60 In a recent report, the expression of this gene was increased by ∼30% in 139A scrapie-infected mice.61 Further investigations are required to characterize these candidate citrullinated proteins and their physiological functions.
Herein we demonstrated the increased expression of PAD2 and identified a number of citrullinated proteins in the ME7-infected mouse model of prion diseases. Whether the citrullination of these proteins has physiological consequences remains unknown. Nevertheless, PAD2 may play a role in the onset and progression of prion diseases by abnormal disruption of Ca2+ homeostasis, and/or by increasing citrullinated proteins. Furthermore, citrullinated proteins may serve as a useful marker for human neurodegenerative diseases. The further study of these citrullinated proteins may provide more definitive answers about molecular functions and pathological mechanisms in prion diseases, including human neurodegenerative disorders.
Footnotes
Address reprint requests to Professor Eun-Kyoung Choi, Laboratory of Cellular Aging and Neurodegeneration, Ilsong Institute of Life Science, Hallym University, Anyang, Gyeonggi-do 431-060, Republic of Korea. E-mail: ekchoi@hallym.ac.kr.
Supported by the Korea Research Foundation grant funded by Korean Government (MOEHRD, Basic Research Promotion Fund) (KRF-2006- 331-E00287).
B.J. and E.K. contributed equally to this article.
References
- Prusiner SB. Prions. Proc Natl Acad Sci USA. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguzzi A, Heikenwalder M, Polymenidou M. Insights into prion strains and neurotoxicity. Nat Rev Mol Cell Biol. 2007;8:552–561. doi: 10.1038/nrm2204. [DOI] [PubMed] [Google Scholar]
- Cronier S, Laude H, Peyrin JM. Prions can infect primary cultured neurons and astrocytes and promote neuronal cell death. Proc Natl Acad Sci USA. 2004;101:12271–12276. doi: 10.1073/pnas.0402725101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K, Qiu Y, Hyun W, Nixon R, VanCleff J, Sanchez-Salazar J, Prusiner SB, DeArmond SJ. Decreased receptor-mediated calcium response in prion-infected cells correlates with decreased membrane fluidity and IP3 release. Neurology. 1996;47:741–750. doi: 10.1212/wnl.47.3.741. [DOI] [PubMed] [Google Scholar]
- Takenouchi T, Iwamaru Y, Imamura M, Kato N, Sugama S, Fujita M, Hashimoto M, Sato M, Okada H, Yokoyama T, Mohri S, Kitani H. Prion infection correlates with hypersensitivity of P2X7 nucleotide receptor in a mouse microglial cell line. FEBS Lett. 2007;581:3019–3026. doi: 10.1016/j.febslet.2007.05.057. [DOI] [PubMed] [Google Scholar]
- Inagaki M, Takahara H, Nishi Y, Sugawara K, Sato C. Ca2+-dependent deimination-induced disassembly of intermediate filaments involves specific modification of the amino-terminal head domain. J Biol Chem. 1989;264:18119–18127. [PubMed] [Google Scholar]
- Vossenaar ER, Zendman AJ, van Venrooij WJ, Pruijn GJ. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. Bioessays. 2003;25:1106–1118. doi: 10.1002/bies.10357. [DOI] [PubMed] [Google Scholar]
- Tarcsa E, Marekov LN, Mei G, Melino G, Lee SC, Steinert PM. Protein unfolding by peptidylarginine deiminase. Substrate specificity and structural relationships of the natural substrates trichohyalin and filaggrin. J Biol Chem. 1996;271:30709–30716. doi: 10.1074/jbc.271.48.30709. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Akiyama K, Hikichi K, Ohtsuka R, Okuyama A, Senshu T. Combined biochemical and immunochemical comparison of peptidylarginine deiminases present in various tissues. Biochim Biophys Acta. 1988;966:375–383. doi: 10.1016/0304-4165(88)90088-8. [DOI] [PubMed] [Google Scholar]
- Vincent SR, Leung E, Watanabe K. Immunohistochemical localization of peptidylarginine deiminase in the rat brain. J Chem Neuroanat. 1992;5:159–168. doi: 10.1016/0891-0618(92)90041-n. [DOI] [PubMed] [Google Scholar]
- Asaga H, Senshu T. Combined biochemical and immunocytochemical analyses of postmortem protein deimination in the rat spinal cord. Cell Biol Int. 1993;17:525–532. doi: 10.1006/cbir.1993.1094. [DOI] [PubMed] [Google Scholar]
- Akiyama K, Sakurai Y, Asou H, Senshu T. Localization of peptidylarginine deiminase type II in a stage-specific immature oligodendrocyte from rat cerebral hemisphere. Neurosci Lett. 1999;274:53–55. doi: 10.1016/s0304-3940(99)00678-3. [DOI] [PubMed] [Google Scholar]
- Keilhoff G, Prell T, Langnaese K, Mawrin C, Simon M, Fansa H, Nicholas AP. Expression pattern of peptidylarginine deiminase in rat and human Schwann cells. Dev Neurobiol. 2008;68:101–114. doi: 10.1002/dneu.20578. [DOI] [PubMed] [Google Scholar]
- Asaga H, Ishigami A. Protein deimination in the rat brain after kainate administration: citrulline-containing proteins as a novel marker of neurodegeneration. Neurosci Lett. 2001;299:5–8. doi: 10.1016/s0304-3940(00)01735-3. [DOI] [PubMed] [Google Scholar]
- Ishigami A, Ohsawa T, Hiratsuka M, Taguchi H, Kobayashi S, Saito Y, Murayama S, Asaga H, Toda T, Kimura N, Maruyama N. Abnormal accumulation of citrullinated proteins catalyzed by peptidylarginine deiminase in hippocampal extracts from patients with Alzheimer’s disease. J Neurosci Res. 2005;80:120–128. doi: 10.1002/jnr.20431. [DOI] [PubMed] [Google Scholar]
- Moscarello MA, Wood DD, Ackerley C, Boulias C. Myelin in multiple sclerosis is developmentally immature. J Clin Invest. 1994;94:146–154. doi: 10.1172/JCI117300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscarello MA, Mastronardi FG, Wood DD. The role of citrullinated proteins suggests a novel mechanism in the pathogenesis of multiple sclerosis. Neurochem Res. 2007;32:251–256. doi: 10.1007/s11064-006-9144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi EK, Zaidi NF, Miller JS, Crowley AC, Merriam DE, Lilliehook C, Buxbaum JD, Wasco W. Calsenilin is a substrate for caspase-3 that preferentially interacts with the familial Alzheimer’s disease-associated C-terminal fragment of presenilin 2. J Biol Chem. 2001;276:19197–19204. doi: 10.1074/jbc.M008597200. [DOI] [PubMed] [Google Scholar]
- Senshu T, Sato T, Inoue T, Akiyama K, Asaga H. Detection of citrulline residues in deiminated proteins on polyvinylidene difluoride membrane. Anal Biochem. 1992;203:94–100. doi: 10.1016/0003-2697(92)90047-b. [DOI] [PubMed] [Google Scholar]
- Rothnagel JA, Rogers GE. Citrulline in proteins from the enzymatic deimination of arginine residues. Methods Enzymol. 1984;107:624–631. doi: 10.1016/0076-6879(84)07046-4. [DOI] [PubMed] [Google Scholar]
- Ishigami A, Ohsawa T, Asaga H, Akiyama K, Kuramoto M, Maruyama N. Human peptidylarginine deiminase type II: molecular cloning, gene organization, and expression in human skin. Arch Biochem Biophys. 2002;407:25–31. doi: 10.1016/s0003-9861(02)00516-7. [DOI] [PubMed] [Google Scholar]
- Toda T, Satoh M, Sugimoto M, Goto M, Furuichi Y, Kimura N. A comparative analysis of the proteins between the fibroblasts from Werner’s syndrome patients and age-matched normal individuals using two-dimensional gel electrophoresis. Mech Ageing Dev. 1998;100:133–143. doi: 10.1016/s0047-6374(97)00131-0. [DOI] [PubMed] [Google Scholar]
- Kumanishi T, Watabe K, Washiyama K. An immunohistochemical study of aldolase C in normal and neoplastic nervous tissues. Acta Neuropathol (Berl) 1985;67:309–314. doi: 10.1007/BF00687817. [DOI] [PubMed] [Google Scholar]
- Buono P, D'Armiento FP, Terzi G, Alfieri A, Salvatore F. Differential distribution of aldolase A and C in the human central nervous system. J Neurocytol. 2001;30:957–965. doi: 10.1023/a:1021828421792. [DOI] [PubMed] [Google Scholar]
- Moscarello MA, Pritzker L, Mastronardi FG, Wood DD. Peptidylarginine deiminase: a candidate factor in demyelinating disease. J Neurochem. 2002;81:335–343. doi: 10.1046/j.1471-4159.2002.00834.x. [DOI] [PubMed] [Google Scholar]
- Bhattacharya SK, Bhat MB, Takahara H. Modulation of peptidyl arginine deiminase 2 and implication for neurodegeneration. Curr Eye Res. 2006;31:1063–1071. doi: 10.1080/02713680600991437. [DOI] [PubMed] [Google Scholar]
- Hetz C, Russelakis-Carneiro M, Maundrell K, Castilla J, Soto C. Caspase-12 and endoplasmic reticulum stress mediate neurotoxicity of pathological prion protein. EMBO J. 2003;22:5435–5445. doi: 10.1093/emboj/cdg537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan CN, Tobin D, Cotter TG. Prion protein fragment PrP-(106-126) induces apoptosis via mitochondrial disruption in human neuronal SH-SY5Y cells. J Biol Chem. 2001;276:43516–43523. doi: 10.1074/jbc.M103894200. [DOI] [PubMed] [Google Scholar]
- Ferreiro E, Resende R, Costa R, Oliveira CR, Pereira CM. An endoplasmic-reticulum-specific apoptotic pathway is involved in prion and amyloid-beta peptides neurotoxicity. Neurobiol Dis. 2006;23:669–678. doi: 10.1016/j.nbd.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Takahara H, Okamoto H, Sugawara K. Calcium-dependent properties of peptidylarginine deiminase from rabbit skeletal muscle. Agric Biol Chem. 1986;50:2899–2904. [Google Scholar]
- Nijenhuis S, Zendman AJ, Vossenaar ER, Pruijn GJ, van Venrooij WJ. Autoantibodies to citrullinated proteins in rheumatoid arthritis: clinical performance and biochemical aspects of an RA-specific marker. Clin Chim Acta. 2004;350:17–34. doi: 10.1016/j.cccn.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Méchin MC, Sebbag M, Arnaud J, Nachat R, Foulquier C, Adoue V, Coudane F, Duplan H, Schmitt AM, Chavanas S, Guerrin M, Serre G, Simon M. Update on peptidylarginine deiminases and deimination in skin physiology and severe human diseases. Int J Cosmet Sci. 2007;29:147–168. doi: 10.1111/j.1467-2494.2007.00377.x. [DOI] [PubMed] [Google Scholar]
- Fujisaki M, Sugawara K. Properties of peptidylarginine deiminase from the epidermis of newborn rats. J Biochem (Tokyo) 1981;89:257–263. doi: 10.1093/oxfordjournals.jbchem.a133189. [DOI] [PubMed] [Google Scholar]
- Kubilus J, Baden HP. Purification and properties of a brain enzyme that deiminates proteins. Biochim Biophys Acta. 1983;745:285–291. doi: 10.1016/0167-4838(83)90060-2. [DOI] [PubMed] [Google Scholar]
- Wood DD, Bilbao JM, O'Connors P, Moscarello MA. Acute multiple sclerosis (Marburg type) is associated with developmentally immature myelin basic protein. Ann Neurol. 1996;40:18–24. doi: 10.1002/ana.410400106. [DOI] [PubMed] [Google Scholar]
- Kinloch A, Tatzer V, Wait R, Peston D, Lundberg K, Donatien P, Moyes D, Taylor PC, Venables PJ. Identification of citrullinated alpha-enolase as a candidate autoantigen in rheumatoid arthritis. Arthritis Res Ther. 2005;7:R1421–R1429. doi: 10.1186/ar1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritzker LB, Joshi S, Gowan JJ, Harauz G, Moscarello MA. Deimination of myelin basic protein. 1. Effect of deimination of arginyl residues of myelin basic protein on its structure and susceptibility to digestion by cathepsin D. Biochemistry. 2000;39:5374–5381. doi: 10.1021/bi9925569. [DOI] [PubMed] [Google Scholar]
- Diedrich JF, Minnigan H, Carp RI, Whitaker JN, Race R, Frey W, II, Haase AT. Neuropathological changes in scrapie and Alzheimer’s disease are associated with increased expression of apolipoprotein E and cathepsin D in astrocytes. J Virol. 1991;65:4759–4768. doi: 10.1128/jvi.65.9.4759-4768.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld MY, Josiphov J, Korczyn AD. Demyelinating peripheral neuropathy in Creutzfeldt-Jakob disease. Muscle Nerve. 1992;15:1234–1239. doi: 10.1002/mus.880151103. [DOI] [PubMed] [Google Scholar]
- Kovács T, Arányi Z, Szirmai I, Lantos PL. Creutzfeldt-Jakob disease with amyotrophy and demyelinating polyneuropathy. Arch Neurol. 2002;59:1811–1814. doi: 10.1001/archneur.59.11.1811. [DOI] [PubMed] [Google Scholar]
- Almeras L, Lefranc D, Drobecq H, de Seze J, Dubucquoi S, Vermersch P, Prin L. New antigenic candidates in multiple sclerosis: identification by serological proteome analysis. Proteomics. 2004;4:2184–2194. doi: 10.1002/pmic.200300732. [DOI] [PubMed] [Google Scholar]
- Yoneda M, Fujii A, Ito A, Yokoyama H, Nakagawa H, Kuriyama M. High prevalence of serum autoantibodies against the amino terminal of alpha-enolase in Hashimoto’s encephalopathy. J Neuroimmunol. 2007;185:195–200. doi: 10.1016/j.jneuroim.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Forooghian F, Cheung RK, Smith WC, O'Connor P, Dosch HM. Enolase and arrestin are novel nonmyelin autoantigens in multiple sclerosis. J Clin Immunol. 2007;27:388–396. doi: 10.1007/s10875-007-9091-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand N, Stead LG. Neuron-specific enolase as a marker for acute ischemic stroke: a systematic review. Cerebrovasc Dis. 2005;20:213–219. doi: 10.1159/000087701. [DOI] [PubMed] [Google Scholar]
- Ukaji F, Kitajima I, Kubo T, Shimizu C, Nakajima T, Maruyama I. Serum samples of patients with rheumatoid arthritis contain a specific autoantibody to “denatured” aldolase A in the osteoblast-like cell line MG-63. Ann Rheum Dis. 1999;58:169–174. doi: 10.1136/ard.58.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimi T, Wakayama Y, Shibuya S, Nakata H, Tomaru T, Takahashi Y, Kosaka K, Asano T, Kato K. High levels of nervous system-specific proteins in cerebrospinal fluid in patients with early stage Creutzfeldt-Jakob disease. Clin Chim Acta. 1992;211:37–46. doi: 10.1016/0009-8981(92)90103-w. [DOI] [PubMed] [Google Scholar]
- Evers S, Droste DW, Lüdemann P, Oberwittler C. Early elevation of cerebrospinal fluid neuron-specific enolase of cerebrospinal fluid neuron-specific enolase in Creutzfeldt-Jakob disease. J Neurol. 1998;145:52–53. doi: 10.1007/s004150050176. [DOI] [PubMed] [Google Scholar]
- Kropp S, Zerr I, Schulz-Schaeffer WJ, Riedemann C, Bodemer M, Laske C, Kretzschmar HA, Poser S. Increase of neuron-specific enolase in patients with Creutzfeldt-Jakob disease. Neurosci Lett. 1999;261:124–126. doi: 10.1016/s0304-3940(98)00992-6. [DOI] [PubMed] [Google Scholar]
- Mor F, Izak M, Cohen IR. Identification of aldolase as a target antigen in Alzheimer’s disease. J Immunol. 2005;175:3439–3445. doi: 10.4049/jimmunol.175.5.3439. [DOI] [PubMed] [Google Scholar]
- Kishi H, Mukai T, Hirono A, Fujii H, Miwa S, Hori K. Human aldolase A deficiency associated with a hemolytic anemia: thermolabile aldolase due to a single base mutation. Proc Natl Acad Sci USA. 1987;84:8623–8627. doi: 10.1073/pnas.84.23.8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandoy-Dron F, Benboudjema L, Guillo F, Jaegly A, Jasmin C, Dormont D, Tovey MG, Dron M. Enhanced levels of scrapie responsive gene mRNA in BSE-infected mouse brain. Brain Res Mol Brain Res. 2000;76:173–179. doi: 10.1016/s0169-328x(00)00028-0. [DOI] [PubMed] [Google Scholar]
- Dandoy-Dron F, Guillo F, Benboudjema L, Deslys JP, Lasmézas C, Dormont D, Tovey MG, Dron M. Gene expression in scrapie. Cloning of a new scrapie-responsive gene and the identification of increased levels of seven other mRNA transcripts. J Biol Chem. 1998;273:7691–7697. doi: 10.1074/jbc.273.13.7691. [DOI] [PubMed] [Google Scholar]
- La Y, Wan C, Zhu H, Yang Y, Chen Y, Pan Y, Ji B, Feng G, He L. Hippocampus protein profiling reveals aberration of malate dehydrogenase in chlorpromazine/clozapine treated rats. Neurosci Lett. 2006;408:29–34. doi: 10.1016/j.neulet.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Gincel D, Zaid H, Shoshan-Barmatz V. Calcium binding and translocation by the voltage-dependent anion channel: a possible regulatory mechanism in mitochondrial function. Biochem J. 2001;358:147–155. doi: 10.1042/0264-6021:3580147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y, Shimizu S. The voltage-dependent anion channel: an essential player in apoptosis. Biochimie. 2002;84:187–193. doi: 10.1016/s0300-9084(02)01370-6. [DOI] [PubMed] [Google Scholar]
- Jin JK, Kim NH, Min DS, Kim JI, Choi JK, Jeong BH, Choi SI, Choi EK, Carp RI, Kim YS. Increased expression of phospholipase D1 in the brains of scrapie-infected mice. J Neurochem. 2005;92:452–461. doi: 10.1111/j.1471-4159.2004.02881.x. [DOI] [PubMed] [Google Scholar]
- Carlier MF, Ressad F, Pantaloni D. Control of actin dynamics in cell motility. Role of ADF/cofilin. J Biol Chem. 1999;274:33827–33830. doi: 10.1074/jbc.274.48.33827. [DOI] [PubMed] [Google Scholar]
- Jang DH, Han JH, Lee SH, Lee YS, Park H, Lee SH, Kim H, Kaang BK. Cofilin expression induces cofilin-actin rod formation and disrupts synaptic structure and function in Aplysia synapses. Proc Natl Acad Sci USA. 2005;102:16072–16077. doi: 10.1073/pnas.0507675102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göthel SF, Marahiel MA. Peptidyl-prolyl cis-trans isomerases, a superfamily of ubiquitous folding catalysts. Cell Mol Life Sci. 1999;55:423–436. doi: 10.1007/s000180050299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immenschuh S, Baumgart-Vogt E. Peroxiredoxins, oxidative stress, and cell proliferation. Antioxid Redox Signal. 2005;7:768–777. doi: 10.1089/ars.2005.7.768. [DOI] [PubMed] [Google Scholar]
- Zabel C, Sagi D, Kaindl AM, Steireif N, Klare Y, Mao L, Peters H, Wacker MA, Kleene R, Klose J. Comparative proteomics in neurodegenerative and non-neurodegenerative diseases suggest nodal point proteins in regulatory networking. J Proteome Res. 2006;5:1948–1958. doi: 10.1021/pr0601077. [DOI] [PubMed] [Google Scholar]