Abstract
Fingolimod is a sphingosine-1-phosphate (S1P) analogue that has been used in clinical trials as a systemic immunomodulatory therapy for multiple sclerosis. Fingolimod readily accesses the central nervous system, raising the issue of its direct effects on neural cells. We assessed the effects of active fingolimod on dissociated cultures of mature, myelin-producing oligodendrocytes (OLGs) derived from adult human brain. Human OLGs express S1P receptor transcripts in relative abundance of S1P5>S1P3>S1P1, with undetectable levels of S1P4. Low doses of fingolimod (100 pmol/L to 1 nmol/L) induced initial membrane elaboration (2 days), subsequent retraction (4 days), and recurrence of extension with prolonged treatment (8 days). Higher doses (10 nmol/L to 1 μmol/L) caused the opposite modulation of membrane dynamics. Retraction was rescued by co-treatment with the S1P3/S1P5 pathway antagonist, suramin, and was associated with RhoA-mediated cytoskeletal signaling. Membrane elaboration was mimicked using the S1P1 agonist SEW2871. Fingolimod rescued human OLGs from serum and glucose deprivation-induced apoptosis, which was reversed with suramin co-treatment and mimicked using an S1P5 agonist. High doses of fingolimod induced an initial down-regulation of S1P5 mRNA levels relative to control (4 hours), subsequent up-regulation (2 days), and recurrent down-regulation (8 days). S1P1 mRNA levels were inversely regulated compared with S1P5. These results indicate that fingolimod modulates maturity- and species-specific OLG membrane dynamics and survival responses that are directly relevant for myelin integrity.
Myelin is a membrane that ensheaths axons to permit rapid saltatory conduction of action potentials.1 Within the central nervous system, myelin is produced by oligodendrocytes (OLGs) and continues to undergo a turnover throughout life.2,3,4 Myelin and OLGs are the proposed targets of the immune-mediated injury that underlies the development of the autoimmune-disease multiple sclerosis (MS). OLGs are lost early in the disease process in specific subtypes of MS; in chronic MS lesions, there is universal loss of OLGs.5 Fingolimod is being assessed for MS therapy because of its anti-inflammatory properties.6 Unlike currently prescribed MS therapies that have limited access to the central nervous system, orally administered fingolimod can penetrate the blood-brain barrier.7 Once it has penetrated the brain parenchyma, fingolimod is rapidly phosphorylated by sphingosine kinase 2 (SphK2)-expressing neural cells to its biologically active metabolite (fingolimod-phosphate), a lipophilic sphingosine-1-phosphate (S1P) analogue.8 Although the active form of the drug demonstrates poor permeability across the blood-brain barrier, fingolimod is not primarily phosphorylated in the circulation given the low blood levels of the sphingosine kinases.8 Autoradiographical analysis after 1 week of oral treatment with C14-labeled fingolimod has demonstrated that both the parent drug and active phosphate metabolite are present at higher concentrations in the brain relative to the blood, and are localized to myelin membranes.9 In vitro studies have demonstrated the capacity of fingolimod-phosphate to impact known cell survival and cytoskeleton-associated signaling pathways10,11,12; together these findings indicate the potential of fingolimod to influence myelin and OLGs.
Fingolimod-phosphate is an agonist for G-protein-coupled receptors S1P1, -3, -4, and -5, which belong to the endothelial differentiation gene-related (EDG) family.13,14 EDG receptor mRNA levels are up-regulated at the perimeter of MS lesions.15 The immunomodulatory effects of fingolimod are consequent to its binding to S1P1 on circulating naïve lymphocytes, subsequent endocytosis of the bound receptor,16,17 down-regulation of S1P1 mRNA levels,18 and resulting blockade of S1P1-dependent efflux from secondary lymph nodes to target organs.19,20
Rodent-derived mature OLGs express S1P1, S1P3, and S1P5 isoforms in vitro and in vivo.21,22 Generally, binding to S1P3 and S1P5 lead to G12/13 activation and membrane retraction via RhoA GTPase,23,24 whereas S1P1 linkage to Gi/o is associated with Rac1 and Ras GTPase activation and subsequent membrane extension and survival.11,17 Previous studies have demonstrated that Rho GTPase-dependent signaling is crucial in regulating oligodendroglial process extension and myelin integrity.25,26 Contrary to observations with rat pre-OLGs, treatment of mature rat OLGs with either fingolimod-phosphate or the endogenous ligand S1P does not induce cytoskeletal retraction yet induces a prosurvival response.11,24
Differing receptor affinities and potencies between fingolimod-phosphate and S1P suggest the possibility of inducing distinct responses in target cells.27 We have previously demonstrated that fingolimod-phosphate can impact cytoskeletal dynamics and survival responses in human oligodendrocyte progenitor cells (OPCs),10 which are the cell type shown to be responsible for myelin repair in experimental animal systems.28,29 Fingolimod-phosphate treatment of human OPCs induced an initial retraction and subsequent extension of the cytoskeleton, and a prosurvival response in a death-promoting environment.10 Our objective was to determine whether fingolimod could impact these same processes in human mature OLGs, and whether these cells have a distinct response profile relative to their progenitor counterparts.
For our studies, we used OLGs isolated from the adult human central nervous system; these are postmitotic cells that express all markers associated with a mature myelinating phenotype.30 Unlike their rodent counterparts, such cells survive in vitro for prolonged periods even in the absence of defined growth factors.30 Also in contrast to these human cells, most rodent-based studies of mature OLGs, such as those cited above, involve progenitor cells differentiated in culture. We investigated the effect of the active phosphorylated form of fingolimod on dissociated cultures of human adult mature OLGs with respect to morphology and survival responses implicated in myelin maintenance, and linked responses with relative S1P receptor levels and associated signaling pathways. We used concentrations of fingolimod-phosphate that are comparable to those used to modulate immune function31,32,33 and that have been measured in the brain parenchyma and cerebrospinal fluid of treated animals.9
Materials and Methods
Fingolimod Biological Activity and Lymphocyte Culture
To demonstrate the biological activity of fingolimod-phosphate in vitro, we assessed the effects of the drug on S1P receptor levels in lymphocytes isolated from healthy human adults. Previous studies performed with lymphocytes derived from fingolimod-treated rodents demonstrate decreased levels of S1P1 receptor mRNA levels.18 We tested the ability of fingolimod-phosphate to down-regulate lymphocyte S1P1 mRNA by treating CD8 T cells with fingolimod-phosphate and performing real-time quantitative polymerase chain reaction (PCR) for S1P1. Peripheral blood was drawn and layered over a Ficoll-Paque (Pharmacia Biotech, Baie d’Urfe, Canada). Cells were incubated on ice with microbead-conjugated anti-CD8 antibody (Miltenyi Biotech, Auburn, CA), washed with MACS buffer (2 mmol/L/L ethylenediaminetetraacetic acid, 0.5% fetal calf serum in phosphate-buffered saline), and separated using positive selection columns (Miltenyi Biotech) according to the manufacturer’s instructions. The CD8+ lymphocyte fraction was resuspended in serum-free RPMI (Sigma, Oakville, Canada) with or without 1 μmol/L phosphorylated-fingolimod for 15 minutes. RNA was extracted and S1P1 mRNA modulation was assessed as described below.
Human Adult Mature OLG Culture
Tissue was obtained from surgical resections performed as treatment for nontumor-related intractable epilepsy in accordance with the guidelines set by the Biomedical Ethics Unit of McGill University. Mature OLGs were isolated as previously described.34 After removal of blood clots, tissue was digested with 0.25% trypsin (Invitrogen, Burlington, Canada) and 25 μg/ml of DNase I (Roche, Laval, Canada) for 30 minutes at 37°C. Cells were mechanically dissociated with a nylon mesh and separated on a linear 30% Percoll density gradient (Pharmacia Biotech) to remove myelin debris. Two overnight rounds of differential adhesion in uncoated tissue culture flasks were used to isolate the floating OLG fraction and reduce the proportion of contaminating microglia. Cells were plated in poly-l-lysine-coated glass chamber slides (Nalge Nunc International, Naperville, IL) in minimal essential medium with 5% fetal calf serum (Sigma), 1% penicillin-streptomycin, 1% glutamine, 0.1% glucose (all from Invitrogen), at a density of 105 cells per well. The purity of these cultures has been previously characterized.35,36
Human Fetal OPC Culture
Human fetal OPC cultures were derived as described10 from central nervous system tissue obtained from 19- to 23-week-old embryos, provided by the Human Fetal Tissue Repository (Albert Einstein College of Medicine, Bronx, NY). These studies were approved by their and our institutional review boards. Diced brain tissue was incubated with 0.25% trypsin (Invitrogen, Grand Island, NY) and 25 μg/ml of DNase I (Roche Diagnostics) at 37°C for 30 minutes and passed through a mesh. A2B5+ cells were isolated by immunomagnetic bead separation using an A2B5 IgM antibody (from a hybridoma), and a microbead-conjugated rat anti-mouse IgM (Miltenyi Biotech). Cells were resuspended in Dulbecco’s modified Eagle’s medium-F12 supplemented with 1% penicillin-streptomycin, 1% glutamine (all from Invitrogen), N1 supplement (1×; Sigma, St. Louis, MO), thyroid hormone T3 (2 ng/ml, Sigma), basic fibroblast growth factor (20 ng/ml, Sigma), and platelet-derived growth factor (20 ng/ml, Sigma), and plated on a confluent bed of lysed and washed human fetal astrocytes grown on a plastic poly-l-lysine-coated coverslip (Nunc, Rochester, NY). The purity of the cultures has been previously characterized.37
Pharmacological Studies
The active phosphorylated form of fingolimod (provided by Novartis, Basel, Switzerland) was dissolved in dimethyl sulfoxide/50 mmol/L HCl and diluted in culture media before each experiment. To identify the receptors and associated signaling pathways activated by fingolimod-phosphate, cultures were treated with the S1P1-specific agonist SEW2871 (100 nmol/L; Calbiochem, San Diego, CA), the S1P5-specific agonist (10 nmol/L, provided by Novartis), or co-treated with fingolimod-phosphate and the S1P3/S1P5 pathway antagonist suramin (1 nmol/L, 100 nmol/L; EMD Bioscience, San Diego, CA). l-α-lysophosphatidic acid (LPA) (10 μmol/L, Sigma) was used as a positive control for RhoA GTPase activation.38 Treatments were replaced every 2 days. No effects were observed when the OLGs were treated with the appropriate vehicles used for reconstitution of these products.
Functional Assays
Cytoskeletal Dynamics
To assess process extension of mature OLGs, we determined the area of myelin-associated glycoprotein (MAG) staining (1:200, Chemicon, Temecula, CA) or phalloidin-Alexa 488 staining (1:400, Invitrogen-Molecular Probes) per cell (μm2) using a calibrated optical density image (Scion Image software NIH, Bethesda, MD) and divided it by the total number of nuclei in the image. Cell numbers were quantified with ImageJ software (NIH, Bethesda, MD) using the watershed tool, to give area of staining per cell (μm2/cell). These cultures had an average of ∼75 cells per field.
To assess activation of cytoskeletal modulators downstream of Rho GTPases in response to pharmacological agents, we immunostained cells with rabbit polyclonal anti-sera against phospho-myosin light chain (MLC)-II (1:50, Thr18/Ser19; Santa Cruz Biotechnology, Santa Cruz, CA). Phosphorylation levels were determined by assessing the immunostaining intensity in individual MAG-positive cells using the histogram function in Adobe Photoshop (San Jose, CA). Fifteen cells per field in each ×20 objective image were quantified; four images per condition were taken for each of the three independent samples resulting in 180 cells being quantified for MLC II phosphorylation levels.
Survival
To induce apoptosis in OLGs, cells were cultured in media deprived of glucose and serum for 4 days. Only samples in which the deprivation condition increased the percentage of apoptotic cells by at least twofold over basal media levels were used for analysis. Fingolimod-phosphate, SEW2871, or the S1P5 agonist were diluted in the deprivation media and applied for the entire 4 days. Apoptosis was assessed by incubating fixed cells with the recombinant terminal deoxynucleotidyl transferase kit (TUNEL; Promega Corp., Madison, WI) with biotin-16-2′-deoxy-uridine-5′-triphosphate (Biotin-16-dUTP, Roche) for 1 hour at 37°C, and incubating with streptavidin-fluorescein isothiocyante (1:1000; Jackson ImmunoResearch, West Grove, PA) for 30 minutes at 37°C. The percentage of TUNEL and MAG double-immunopositive cells was determined by manually counting positive cells in a blinded manner.
Immunocytochemistry
Cells were fixed with 2% paraformaldehyde and blocked with HHG (1 nmol/L HEPES, 2% heat-inactivated horse serum, 10% heat-inactivated goat serum, Hanks’ balanced salt solution) for 20 minutes. Primary antibodies were applied for 1 hour at 4°C. The secondary antibodies goat anti-mouse Cy3 (1:500, Jackson ImmunoResearch), and goat anti-rabbit fluorescein isothiocyanate (1:100; Biosource, Camarillo, CA) were applied for 30 minutes at 4°C. Primary antibody isotype controls showed low nonspecific staining. Cell nuclei were identified using the Hoechst dye (bis-benzimide, 1:1000; Molecular Probes, Carlsbad, CA), and slides were mounted with Fluoromount-G (EMS, Hatfield, PA).
RNA-Based S1P Receptor Modulation Studies
After cell lysis with TRIzol (Invitrogen), total RNA was extracted with the Minielute Qiagen RNeasy mini kit, and samples were treated with DNase (Qiagen, Mississauga, Canada). Reverse transcription (RT) was performed on 2 μg of RNA, and cDNA was generated using random hexaprimers (Roche) and the Moloney murine leukemia virus-RT enzyme (Invitrogen) in a thermocycler at 42°C, 75°C for 60 minutes, 4°C for 10 minutes. For real-time qPCR, cDNA from CD8 T cells isolated from normal donors and Jurkat T cells were used to create a standard curve of serial 10-fold dilutions for S1P1, S1P4, S1P5, and S1P3, respectively,39,40 using primers, TaqMan, and probes from Assays on Demand by Applied Biosystems (Melbourne, Australia). Transcript levels were assessed using the ABI Prism 7000 sequence detection system (Applied Biosystems) using default temperature settings (2 minutes at 50°C, 10 minutes at 95°C, followed by 40 cycles of 15 seconds at 95°C, 1 minute at 60°C). Water controls indicated undetectable levels of noise (data not shown). β-Actin transcript levels were used as endogenous controls for the amount of RNA transcribed, and primers/probes were designed using the PRIMER express software (Applied Biosystems).41 All S1P receptor levels were normalized to the β-actin levels in the corresponding sample. Because of poor reactivity/specificity of commercially available human reactive anti-S1P receptor antibodies, we were unable to demonstrate the specificity of these antibodies on our cells using various methods, and were thus unable to directly assess expression and modulation of S1P receptors at the protein level.
Statistical Analyses
Four ×20 objective images were captured for each condition for one experiment, and each experiment was repeated with at least three different samples. Results are presented as fold difference greater than control normalized to the mean value in untreated culture conditions at the respective time point. Comparisons between conditions were made using one-way analysis of variance. Probability values <0.05 were considered statistically significant.
Results
Fingolimod Is Functionally Active
Fingolimod-phosphate treatment of CD8+ T lymphocytes for 15 minutes induced a down-regulation of S1P1 mRNA levels to 0.62 ± 0.006 of control. This is consistent with the finding that ex vivo T lymphocytes isolated from fingolimod-treated animals have lower levels of S1P1 transcripts,18 and that in vitro fingolimod-phosphate treatment induces down-regulation of transfected receptors.31,42 Our findings support the functional activity of the fingolimod used in our studies.
Adult Human Mature OLGs Have S1P Receptor Transcripts
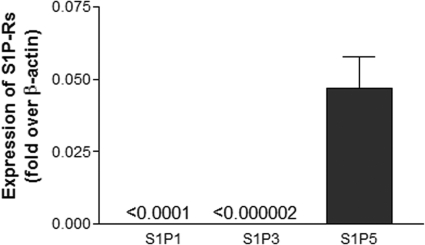
Using real-time quantitative PCR, we characterized the relative abundance of transcripts for S1P receptors that can bind fingolimod-phosphate in the OLG cultures under basal culture conditions. We found that human OLGs express high levels of S1P5, relatively lower levels of S1P3 and S1P1, and undetectable levels of S1P4 (Figure 1). This is in contrast to the relatively high S1P1 and low S1P5 transcript levels previously observed in human OPCs.10
Figure 1.
Human adult mature OLGs have transcripts for sphingosone 1-phosphate (S1P) receptors. qPCR levels for S1P receptors were normalized to β-actin levels in the respective sample and then normalized to control levels. Human mature OLGs have S1P receptor transcripts in the relative abundance of S1P5>S1P1>S1P3, and undetectable S1P4.
Dose- and Treatment Duration-Dependent Modulation of Mature OLG Cytoskeleton by Fingolimod
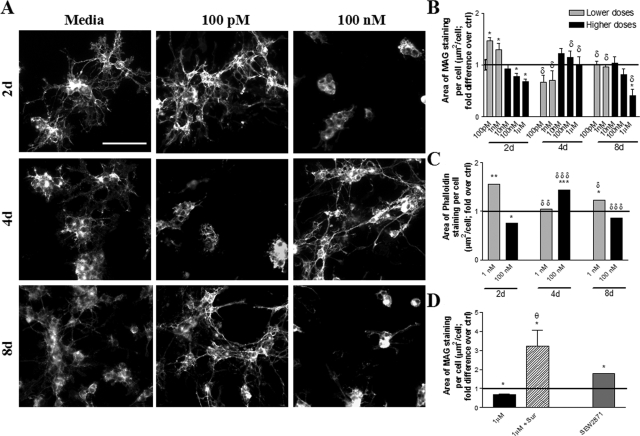
We observed that fingolimod-phosphate induced cyclic modulation of mature OLG process dynamics throughout time in a dose-dependent manner. Untreated cultures had progressive and maintained membrane elaboration throughout time (8-day area of MAG staining per cell was 1.84 ± 0.19-fold greater than 2 day cultures). Very low doses of 1 to 10 pmol/L did not have significant effects on membrane elaboration (data not shown). Low doses of fingolimod-phosphate (<100 nmol/L) had no significant effects on process dynamics of mature OLGs at 1 day of treatment; higher dose treatment (1 μmol/L), however, was associated with a significant process extension (see Supplemental Figure 1 at http://ajp.amjpathol.org). When mature OLGs were treated with low doses of fingolimod-phosphate for 2 days (100 pmol/L, 1 nmol/L), we observed a significant elaboration of membrane (area of MAG staining was 1.46 ± 0.06-fold and 1.42 ± 0.02-fold over control, respectively) (Figure 2, A and B). Conversely, higher doses (100 nmol/L, 1 μmol/L) caused a reduction in membrane elaboration relative to control (area of MAG staining was 0.78 ± 0.06-fold and 0.68 ± 0.05-fold greater than control) (Figure 2, A and B).
Figure 2.
Fingolimod regulates human mature OLG cytoskeleton dynamics in a dose- and treatment duration-dependent manner. A: Representative images of cultures immunostained against MAG. Untreated cultures showed maintenance of membrane elaboration throughout time (left). Treatment with a low dose of fingolimod-phosphate (100 pmol/L, middle) induced an initial membrane elaboration (2 days), subsequent retraction (4 days), and recurrent extension (8 days). Conversely, treatment with a high dose of fingolimod-phosphate (100 nmol/L, right) induced initial membrane retraction (2 days), subsequent elaboration (4 days), and ensuing retraction (8 days). B: Quantification of membrane elaboration expressed as mean area of MAG staining per cell (μm2/cell) normalized to untreated culture values at the respective time point. Treatment with low doses of fingolimod-phosphate (100 pmol/L to 1 nmol/L) induced a cyclic regulation of membrane elaboration; treatment with higher doses (100 nmol/L to 1 μmol/L) provoked a reciprocal modulation of membrane dynamics. *P < 0.05 relative to control; δP < 0.05 relative to previous time point. C: Quantification of cytoskeletal modulation expressed as mean area of phalloidin staining per cell (μm2/cell) normalized to untreated culture values at the respective time point. *P < 0.05, **P < 0.01 relative to control; δP < 0.05, δδP < 0.01, δδδP < 0.001 relative to previous time point. Results confirm those obtained from quantification of area of MAG staining and suggest modifications at the level of the actin cytoskeleton. D: Co-treatment of cultures with the S1P3/5 pathway antagonist, suramin (100 nmol/L), antagonized the process retraction observed with 1 μmol/L fingolimod-phosphate at 2 days of treatment. Treatment with the S1P1-specific agonist, SEW2871 (100 nmol/L), for 2 days induced significant membrane elaboration relative to control. *P < 0.05 relative to control; θP < 0.05 relative to fingolimod alone. Scale bar = 50 μm.
By 4 days of fingolimod-phosphate treatment, 100 pmol/L- and 1 nmol/L-treated cultures showed membrane retraction relative to 2-day-treated cultures (area of MAG staining was 0.67 ± 0.14 and 0.71 ± 0.18 of control, respectively). In contrast, a recovery in cytoskeletal elaboration was observed with 100 nmol/L and 1 μmol/L, with no significant difference in comparison to control (area of MAG staining was 1.15 ± 0.12-fold and 1.00 ± 0.16 of control, respectively) (Figure 2, A and B).
By 8 days of treatment, there was a significant recovery of membrane in cultures treated with lower doses (area of MAG staining was 1.01 ± 0.06-fold greater than control for 0.1 nmol/L and 0.97 ± 0.04-fold for 1 nmol/L), and recurrence of retraction in cultures treated with 1 μmol/L fingolimod-phosphate (area of MAG staining of 0.42 ± 0.12 of control). Phalloidin staining indicated that observed responses were associated with an extension or retraction of filamentous actin-positive processes, rather than a loss of MAG staining (Figure 2C).
We had previously observed that treatment of human OPCs with high doses of fingolimod-phosphate (10 nmol/L to 1 μmol/L) induced initial cytoskeletal retraction and subsequent extension. We next sought to determine whether OPCs also have the potential to respond to fingolimod in a dose-dependent manner as observed in mature OLGs. Unlike the mature OLGs, OPCs responded to low doses of fingolimod-phosphate (100 pmol/L, 1 nmol/L) in a similar manner than to higher doses (See Supplemental Figure 2 at http://ajp.amjpathol.org).8 This suggests that OPCs and mature OLGs can potentially exhibit cell-type-specific and differing cytoskeletal responses to fingolimod based on the dose to which they are exposed.
To assess the functional basis behind the fingolimod-induced modulations in human mature OLG cytoskeletal dynamics, we treated cultures with S1P receptor- or signaling pathway-specific agonists and antagonists. The membrane retraction observed with 1 μmol/L fingolimod-phosphate at 2 days was significantly antagonized by co-treatment with suramin (100 nmol/L) (Figure 2D), an S1P3/S1P5 pathway antagonist that uncouples these receptors from their G protein.43,44 Suramin alone had no significant effect on membrane elaboration. These findings suggest that fingolimod-phosphate induced membrane retraction via S1P3 or S1P5 signaling. The ability of the S1P1-specific agonist SEW2871 (100 nmol/L) to induce membrane elaboration at 2 days suggests that S1P1-mediated signaling is sufficient in causing such a response (Figure 2D).
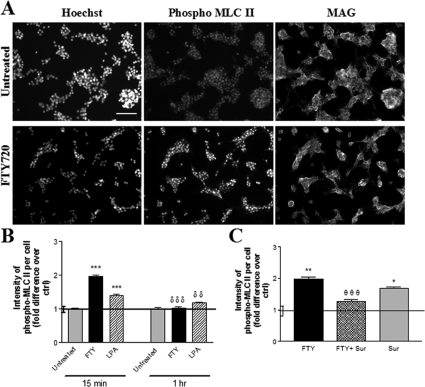
Functional signaling downstream of S1P receptors was assessed by evaluating the expected activation of cytoskeletal modulators. We associated the observed membrane retraction observed initially with 1 μmol/L fingolimod-phosphate with RhoA GTPase-associated myosin light chain (MLC) II phosphorylation.45 We used another EDG receptor ligand, LPA, as a positive control because of its ability to induce RhoA GTPase activation.38,46 A significant increase in MLC II phosphorylation was observed at 15 minutes of treatment with fingolimod-phosphate (1.81 ± 0.05-fold greater than control) or LPA (1.40 ± 0.05-fold greater than control) (Figure 3, A and B). At 1 hour of fingolimod-phosphate or LPA treatment, MLC II phosphorylation decreased relative to 15 minutes (1.03 ± 0.04-fold and 1.18 ± 0.04-fold of control, respectively) (Figure 3B), demonstrating an expected drop in activation throughout time.45 The fingolimod-induced increase in phospho-MLC II signal was significantly reversed by co-treatment with suramin (Figure 3C), suggesting an S1P3/S1P5-dependent mechanism. Intensity of phospho-MLC II signal did not change in untreated cultures throughout time (Figure 3B).
Figure 3.
Fingolimod-induced activation of a cytoskeletal modulator in mature OLGs indicative of functional signaling downstream of S1P receptors. A: Representative images of untreated (top) and fingolimod-phosphate-treated (FTY, 1 μmol/L; bottom) mature OLG cultures immunostained for the nuclear stain Hoechst (left), phospho-myosin light chain (MLC) II (middle), and MAG, demonstrate that at 15 minutes of treatment fingolimod-phosphate increases the intensity of phospho-MLC II staining in OLGs relative to control. B: Quantification of phospho-MLC II staining intensity per cell normalized to untreated control at the respective time point. fingolimod-phosphate (1 μmol/L) and the positive control LPA induce a significant increase in phospho-MLC II staining intensity at 15 minutes, with an expected drop at a later time point (1 hour). ***P < 0.001 relative to control; δδδP < 0.001 relative to previous time point; δδP < 0.01 relative to previous time point. C: The fingolimod-induced increase in intensity of phospho-MLC II staining per cell at 15 minutes was negated by co-treatment with the S1P3/5 pathway antagonist, suramin (sur, 100 nmol/L), whereas the increase in intensity was still observed when suramin was applied alone. *P < 0.05, **P < 0.01 relative to control; θθθP < 0.001 relative to fingolimod-phosphate alone.
Fingolimod Promotes Mature OLG Survival under Apoptotic Conditions
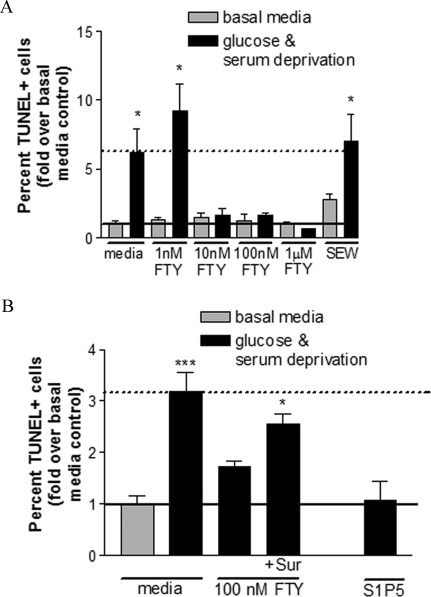
Our previous studies indicated the potential of fingolimod to promote cell survival signaling in human OPCs under apoptotic conditions.10 We induced cell death in mature OLGs by serum and glucose deprivation throughout 4 days to determine whether fingolimod could also support the survival of these cells. Deprivation conditions increased the percentage of TUNEL-positive mature OLGs to 6.18 ± 1.7-fold greater than basal media control (Figure 4A). The low dose of 1 nmol/L was not able to rescue the cells from apoptosis. However, higher doses of fingolimod-phosphate (10 nmol/L to 1 μmol/L) significantly decreased the proportion of apoptotic cells such that it was not significantly different from basal media control levels (Figure 4A). Treatment with the S1P1 agonist SEW2871 was not able to rescue OLGs from apoptosis (Figure 4A). This contrasts with the finding that SEW2871 is sufficient in rescuing human OPCs from apoptosis.10 The fingolimod rescue effect was reversed when cultures were co-treated with the S1P3/5 pathway antagonist, suramin, and was mimicked by application of an S1P5-specific agonist (Figure 4B). This suggests that fingolimod can promote cell survival signaling in OPCs and OLGs via different S1P receptors, and also reveals cell-type-specific dependencies on S1P receptors for survival signaling.
Figure 4.
Fingolimod rescues human adult mature OLGs from serum and glucose deprivation-induced cell death. A: Deprivation of culture supplements for 4 days (black) significantly increased the proportion of apoptotic TUNEL-positive mature OLGs to 6.2 ± 1.7-fold greater than basal media levels (gray). A low dose of fingolimod-phosphate (FTY, 1 nmol/L) was unable to rescue OLGs from apoptosis. Higher doses of fingolimod-phosphate (10 nmol/L to 1 μmol/L) significantly decreased the percentage of apoptotic cells to control levels. Treatment with the S1P1-specific agonist, SEW23871 (SEW, 100 nmol/L) was not sufficient in mimicking this rescue effect. *P < 0.05 relative to basal media condition. B: The prosurvival effect of fingolimod-phosphate was antagonized by co-treatment with the S1P3/5 pathway antagonist, suramin (100 nmol/L) and was mimicked by a S1P5-specific agonist (10 nmol/L). ***P < 0.001 and *P < 0.05 relative to basal media condition.
Fingolimod Modulates S1P Receptor mRNA Levels
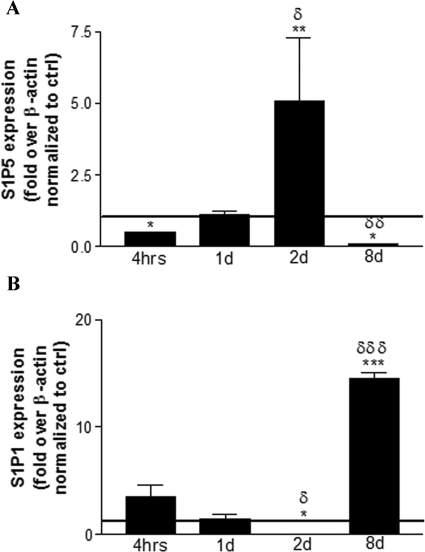
CD8+ T lymphocytes were used as positive controls for S1P1, S1P4, and S1P5 receptor mRNA levels39 and Jurkat T cells were used as a control for S1P3 levels.40 Prolonged culture of mature OLGs caused a progressive decrease in S1P receptor mRNA levels (data not shown), likely reflecting the impact of S1P in the serum-supplemented media on receptor modulation. After fingolimod-phosphate treatment, we observed a treatment duration-dependent regulation of S1P1 and S1P5 mRNA levels in the mature OLG cultures. Exposure to 1 μmol/L fingolimod-phosphate caused S1P5 levels to be significantly down-regulated at 4 hours relative to control; levels were subsequently up-regulated at 2 days then recurrently down-regulated at 8 days (Figure 5A). S1P1 mRNA levels were oppositely modulated at all time points (Figure 5B). This suggests that these two receptors, which likely regulate opposing signaling pathways, are cyclically and oppositely regulated in mature OLGs in response to fingolimod-phosphate. In all conditions and at all time points, S1P3 levels remained low, and undetectable levels of S1P4 persisted.
Figure 5.
Fingolimod reciprocally modulates S1P1 and S1P5 receptor transcripts in human adult mature OLGs in a cyclic manner. qPCR results for S1P5 (A) and S1P1 (B) were normalized to β-actin in the respective sample and normalized to untreated cultures at the respective time point. At 4 hours of treatment with fingolimod-phosphate (1 μmol/L), S1P5 is down-regulated and S1P1 is concomitantly up-regulated relative to control. By 2 days of treatment, S1P1 is down-regulated and S1P5 is up-regulated. The opposite is observed at 8 days of treatment. At all time points, S1P3 levels remained low and S1P4 remained undetectable. S1P receptor levels were progressively decreased throughout time in untreated cultures likely because of the presence of S1P in the serum-supplemented media. *P < 0.05 relative to control; δP < 0.05, δδP < 0.01, δδδP < 0.001 relative to previous time point.
Discussion
Our in vitro studies reveal that the biologically active form of fingolimod can exert dose- and treatment duration-dependent effects on adult human mature OLG morphology and survival, with associated modulation of S1P receptor transcripts. As discussed below, our results with the human OLGs indicate that there may be substantial differences compared to rodent cells. These studies suggest that interpretations drawn from rodent in vitro studies may not be readily extrapolated to human cells and disease. Furthermore, the direct neurobiological effects of fingolimod in in vivo animal models, specifically an animal model of MS, experimental autoimmune encephalomyelitis, are difficult to dissociate from potential indirect effects mediated via modulation of the systemic immune cell responses.
Fingolimod Modulates Human OLG Membrane Elaboration in a Dose- and Treatment Duration-Dependent Manner
Fingolimod-phosphate induced a cycling of cytoskeletal responses in human mature OLGs in a dose- and treatment duration-dependent manner. Treatment with lower doses caused an initial membrane extension, subsequent membrane retraction, and recurring extension with prolonged treatment. Higher doses produced an opposite modulation of responses. Dose-dependent responses may reflect differing affinities for S1P receptors. Lower doses of phosphorylated-fingolimod may be more prone to bind higher affinity S1P1 to induce initial extension, whereas higher doses may also bind lower affinity S1P3/S1P5 to trigger retraction. Rat and human OLG progenitors also show transient cytoskeletal responses on S1P receptor engagement,10,24 although to our knowledge we are the first to demonstrate a cycling of responses in mature OLGs. Conversely, neonatal rat OLGs matured in vitro do not retract membranes on S1P treatment.24 We documented similar lack of responses with 1 and 2 days of fingolimod-phosphate treatment of rat matured OLGs (data not shown), suggesting species-specific differences in responsiveness to fingolimod. However, disparities between rodent and human OLG responses may be attributable to differences in S1P receptor affinities for the ligand,47 receptor levels and coupling to associated signaling pathways, and sample age/cell maturity.
Membrane retraction in human mature OLGs was associated with increased phosphorylation of myosin light chain II, indicative of RhoA GTPase signaling.45 Both the retraction and the increase in phospho-MLC II signal were antagonized by co-treatment with the S1P3/S1P5 pathway antagonist, suramin. Our data support that fingolimod-phosphate initiates membrane retraction in an S1P3/S1P5- and RhoA-dependent manner. S1P5-associated signaling can induce Rho GTPase-dependent membrane retraction in rodent24 and human fetal OPCs.10 S1P1-mediated signaling was sufficient to induce membrane extension in human mature OLGs, similar to previous observations in human OPCs10 and rodent neurons.23 The importance of Rho GTPase-dependent modulation of the cytoskeleton in regulating oligodendroglial process outgrowth and myelin integrity has been previously established in vitro and in vivo.25,26
Fingolimod Promotes Human Mature OLG Survival
We observed that treatment with high doses of fingolimod-phosphate were able to rescue human mature OLGs from serum and glucose deprivation-induced cell death at 4 days of treatment. The S1P1 agonist SEW2871 was not able to promote cell survival in the OLGs, eliminating the possibility that S1P1 signaling is sufficient to stimulate survival responses in these cells. The fingolimod-induced survival response was antagonized by suramin co-treatment, and mimicked with the S1P5 agonist, suggesting that fingolimod-phosphate may signal through S1P5 to rescue human mature OLGs from apoptosis. This suggests that there is a dissociation between S1P receptor signaling regulating survival and cytoskeletal responses in human mature OLGs. Accordingly, S1P and fingolimod-phosphate treatment of neonatal rat mature OLGs differentiated in vitro rescues cells from serum withdrawal-induced apoptosis via S1P5, without any associated morphological changes.11,24 This study found that fingolimod-phosphate was not able to rescue rat pre-OLGs from apoptosis but instead promoted process retraction via S1P5, suggestive of either a developmental switch in G-protein or a change in coupling efficiency to the S1P receptors with oligodendroglial maturation.24 The ability of fingolimod-phosphate to enhance the survival of human OPCs in death-inducing environments via S1P1 and not S1P3/510 suggests maturity-dependent responses to human S1P receptor signaling in the context of cell survival.
Fingolimod Modulates Relative S1P Receptor mRNA Levels in Human Adult Mature OLGs
We demonstrated that human mature OLGs have relative S1P receptor mRNA levels of S1P5>S1P3>S1P1, and undetectable levels of S1P4. This is consistent with the relative mRNA levels of S1P receptor subtypes in neonatal rat OLGs.22,48 The comparative levels of S1P receptor subtypes may influence which ones are preferentially bound by fingolimod-phosphate and the ensuing cellular response.
Quantitative real-time PCR revealed that human mature OLGs reciprocally regulate S1P5 and S1P1 mRNA levels in a cyclic manner in response to fingolimod-phosphate. Treatment with a high dose of fingolimod-phosphate induced an initial down-regulation of S1P5, subsequent up-regulation, and recurrent down-regulation with prolonged treatment. Interestingly, S1P1 levels were oppositely regulated at all time points. Given that fingolimod-phosphate binding to a given S1P receptor subtype initiates its down-regulation at the mRNA level,18 we interpreted this down-regulation as a readout of which receptor was bound at a given time.
The initial down-regulation of S1P5 mRNA levels at 4 hours of treatment with 1 μmol/L fingolimod-phosphate would be associated with the observed S1P3/5- and RhoA-dependent membrane retraction observed with this dose at 2 days of treatment. The concomitant up-regulation of S1P1 mRNA levels at 4 hours of treatment with 1 μmol/L fingolimod-phosphate likely increased the availability of S1P1 receptors to bind fingolimod, thereby causing S1P1 down-regulation by 2 days of treatment and a switch in responses to membrane elaboration by 4 days of exposure to fingolimod. The subsequent up-regulation of S1P5 mRNA levels at 2 days of treatment likely promoted its binding and ensuing down-regulation at 8 days of treatment, which would be associated with the process retraction observed with this dose at this time point.
These real-time PCR results and the assessment of receptor-specific associated responses and signaling together support changes in S1P receptor protein expression. Our previous findings that human OPCs also demonstrate this cyclic regulation of S1P receptors10 suggests that chronic fingolimod treatment can stimulate continuous signaling in cells of the human oligodendroglial lineage by way of reciprocal cycling of S1P receptors with potentially opposing signaling pathways.
Conclusion
Our studies reveal the capacity of fingolimod to induce continuous and cyclic functional effects on human OLG membrane elaboration and survival responses in a dose-dependent manner. These findings imply that chronic fingolimod therapy may impact cellular events that are implicated in the maintenance of myelin. Our observations are relevant not only in the context of treatment of MS, but also for other neurological conditions such as stroke and trauma, in which myelin integrity can be severely compromised.
Acknowledgments
We thank neurosurgeons Dr. Andre Olivier for providing adult human brain tissue from surgical specimens; Novartis (Basel, Switzerland) for providing the active phosphorylated form of fingolimod and the S1P5-specific agonist; Sathyanath Rajasekharan for providing rat mature oligodendrocytes; and Manon Blain, Philippe Saikali, and Caroline Lambert for technical support.
Footnotes
Address reprint requests to Dr. Jack P. Antel, Room 111, Neuroimmunology Unit, Montreal Neurological Institute, 3801 University St., Montreal, QC, Canada, H3A 2B4. E-mail: jack.antel@mcgill.ca.
Supported by the Foundation of the Multiple Sclerosis Society of Canada (to J.P.A. and T.E.K.), the Fondation de Recherche en Sante du Quebec (to T.E.K.), the Canadian Institutes of Health Research (studentship to V.E.M.), and the National Multiple Sclerosis Society (to B.S. and J.P.A.).
Supplemental material for this article can be found on http:// ajp.amjpathol.org.
References
- Sherman DL, Brophy PJ. Mechanisms of axon ensheathment and myelin growth. Nat Rev Neurosci. 2005;6:683–690. doi: 10.1038/nrn1743. [DOI] [PubMed] [Google Scholar]
- Smith ME. The turnover of myelin in the adult rat. Biochim Biophys Acta. 1968;164:285–293. doi: 10.1016/0005-2760(68)90154-9. [DOI] [PubMed] [Google Scholar]
- Agrawal HC, Trotter JL, Burton RM, Mitchell RF. Metabolic studies on myelin. Evidence for a precursor role of a myelin subfraction. Biochem J. 1974;140:99–109. doi: 10.1042/bj1400099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajtha A, Toth J, Fujimoto K, Agrawal HC. Turnover of myelin proteins in mouse brain in vivo. Biochem J. 1977;164:323–329. doi: 10.1042/bj1640323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann H, Bruck W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210–218. doi: 10.1111/j.1750-3639.2007.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem. 2004;92:913–922. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- Kappos L, Antel J, Comi G, Montalban X, O'Connor P, Polman CH, Haas T, Korn AA, Karlsson G, Radue EW. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med. 2006;355:1124–1140. doi: 10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]
- Billich A, Bornancin F, Devay P, Mechtcheriakova D, Urtx N, Baumruker T. Phosphorylation of the immunomodulatory drug FTY720 by sphingosine kinases. J Biol Chem. 2003;278:47408–47415. doi: 10.1074/jbc.M307687200. [DOI] [PubMed] [Google Scholar]
- Foster CA, Howard LM, Schweitzer A, Persohn E, Hiestand PC, Belatoni B, Reuschel R, Beerli C, Schwartz M, Billich A. Brain penetration of the oral immunomodulatory drug FTY720 and its phosphorylation in the central nervous system during experimental autoimmune encephalomyelitis: consequences for mode of action in multiple sclerosis. J Pharmacol Exp Ther. 2007;323:469–475. doi: 10.1124/jpet.107.127183. [DOI] [PubMed] [Google Scholar]
- Miron VE, Jung CG, Kim HJ, Kennedy TE, Soliven B, Antel JP. FTY720 modulates human oligodendrocyte progenitor process extension and survival. Ann Neurol. 2008;63:61–71. doi: 10.1002/ana.21227. [DOI] [PubMed] [Google Scholar]
- Jung CG, Kim HJ, Miron VE, Cook S, Kennedy TE, Foster CA, Antel JP, Soliven B. Functional consequences of S1P receptor modulation in rat oligodendroglial lineage cells. Glia. 2007;55:1656–1667. doi: 10.1002/glia.20576. [DOI] [PubMed] [Google Scholar]
- Coelho RP, Payne SG, Bittman R, Spiegel S, Sato-Bigbee C. The immunomodulator FTY720 has a direct cytoprotective effect in oligodendrocyte progenitors. J Pharmacol Exp Ther. 2007;323:626–635. doi: 10.1124/jpet.107.123927. [DOI] [PubMed] [Google Scholar]
- Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, Rosenbach M, Hale J, Lynch CL, Rupprecht K, Parsons W, Rosen H. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- Davis MD, Clemens JJ, Macdonald TL, Lynch KR. Sphingosine 1-phosphate analogs as receptor antagonists. J Biol Chem. 2005;280:9833–9841. doi: 10.1074/jbc.M412356200. [DOI] [PubMed] [Google Scholar]
- Beer MS, Stanton JA, Salim K, Rigby M, Heavens RP, Smith D, Mcallister G. EDG receptors as a therapeutic target in the nervous system. Ann NY Acad Sci. 2000;905:118–131. doi: 10.1111/j.1749-6632.2000.tb06544.x. [DOI] [PubMed] [Google Scholar]
- Liu CH, Thangada S, Lee MJ, Van Brocklyn JR, Spiegel S, Hla T. Ligand-induced trafficking of the sphingosine-1-phosphate receptor EDG-1. Mol Biol Cell. 1999;10:1179–1190. doi: 10.1091/mbc.10.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl EJ, Rosen H. Regulation of immunity by lysosphingolipids and their G protein-coupled receptors. J Clin Invest. 2004;114:1531–1537. doi: 10.1172/JCI23704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicka E, Dubois G, Jarai G, Edwards M, Thomas M, Nicholls A, Albert R, Newson C, Brinkmann V, Walker C. The sphingosine 1-phosphate receptor agonist FTY720 differentially affects the sequestration of CD4+/CD25+ T-regulatory cells and enhances their functional activity. J Immunol. 2005;175:7973–7980. doi: 10.4049/jimmunol.175.12.7973. [DOI] [PubMed] [Google Scholar]
- Webb M, Tham CS, Lin FF, Lariosa-Willingham K, Yu N, Hale J, Mandala S, Chun J, Rao TS. Sphingosine 1-phosphate receptor agonists attenuate relapsing-remitting experimental autoimmune encephalitis in SJL mice. J Neuroimmunol. 2004;153:108–121. doi: 10.1016/j.jneuroim.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Kataoka H, Ohtsuki M, Shimano K, Mochizuki S, Oshita K, Murata M, Sugahara K, Sato N, Hoshino Y, Chiba K. Immunosuppressive activity of FTY720, sphingosine 1-phosphate receptor agonist: II. Effect of FTY720 and FTY720-phosphate on host-versus-graft and graft-versus-host reaction in mice. Transplant Proc. 2005;37:107–109. doi: 10.1016/j.transproceed.2004.12.287. [DOI] [PubMed] [Google Scholar]
- Terai K, Soga T, Takahashi M, Kamohara M, Ohno K, Yatsugi S, Okada M, Yamaguchi T. Edg-8 receptors are preferentially expressed in oligodendrocyte lineage cells of the rat CNS. Neuroscience. 2003;116:1053–1062. doi: 10.1016/s0306-4522(02)00791-1. [DOI] [PubMed] [Google Scholar]
- Yu N, Lariosa-Willingham KD, Lin FF, Webb M, Rao TS. Characterization of lysophosphatidic acid and sphingosine-1-phosphate-mediated signal transduction in rat cortical oligodendrocytes. Glia. 2004;45:17–27. doi: 10.1002/glia.10297. [DOI] [PubMed] [Google Scholar]
- Toman RE, Payne SG, Watterson KR, Maceyka M, Lee NH, Milstien S, Bigbee JW, Spiegel S. Differential transactivation of sphingosine-1-phosphate receptors modulates NGF-induced neurite extension. J Cell Biol. 2004;166:381–392. doi: 10.1083/jcb.200402016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillard C, Harrison S, Stankoff B, Aigrot MS, Calver AR, Duddy G, Walsh FS, Pangalos MN, Arimura N, Kaibuchi K, Zalc B, Lubetzki C. Edg8/S1P5: an oligodendroglial receptor with dual function on process retraction and cell survival. J Neurosci. 2005;25:1459–1469. doi: 10.1523/JNEUROSCI.4645-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M, Trotter J. Wrapping it up: the cell biology of myelination. Curr Opin Neurobiol. 2007;17:533–540. doi: 10.1016/j.conb.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Sloane JA, Vartanian TK. WAVE1 and regulation of actin nucleation in myelination. Neuroscientist. 2007;13:486–491. doi: 10.1177/1073858407299423. [DOI] [PubMed] [Google Scholar]
- Gardell SE, Dubin AE, Chun J. Emerging medicinal roles for lysophospholipid signaling. Trends Mol Med. 2006;12:65–75. doi: 10.1016/j.molmed.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Franklin RJ. Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci. 2002;3:705–714. doi: 10.1038/nrn917. [DOI] [PubMed] [Google Scholar]
- Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19:197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- Kim SU, Moretto G, Ruff B, Shin DH. Culture and cryopreservation of adult human oligodendrocytes and astrocytes. Acta Neuropathol. 1984;64:172–175. doi: 10.1007/BF00695582. [DOI] [PubMed] [Google Scholar]
- Gräler MH, Goetzl EJ. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. FASEB J. 2004;18:551–553. doi: 10.1096/fj.03-0910fje. [DOI] [PubMed] [Google Scholar]
- Lan YY, De Creus A, Colvin BL, Abe M, Brinkmann V, Coates PT, Thomson AW. The sphingosine-1-phosphate receptor agonist FTY720 modulates dendritic cell trafficking in vivo. Am J Transplant. 2005;5:2649–2659. doi: 10.1111/j.1600-6143.2005.01085.x. [DOI] [PubMed] [Google Scholar]
- Park SI, Felipe CR, Machado PG, Garcia R, Skerjanec A, Schmouder R, Tedesco-Silva JH, Medina-Pestana JO. Pharmacokinetic/pharmacodynamic relationships of FTY720 in kidney transplant recipients. Braz J Med Biol Res. 2005;38:683–694. doi: 10.1590/S0100-879X2005000500005. [DOI] [PubMed] [Google Scholar]
- Yong VW, Antel JP. Culture of glia cells from human brain biopsies. Federoff S, Richardson A, editors. New York: Humana,; Protocols for Neural Cell Culture. 2001:pp 81–96. [Google Scholar]
- D'Souza SD, Bonetti B, Balasingam V, Cashman NR, Barker PA, Troutt AB, Raine CS, Antel JP. Multiple sclerosis: fas signaling in oligodendrocyte cell death. J Exp Med. 1996;184:2361–2370. doi: 10.1084/jem.184.6.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wosik K, Ruffini F, Almazan G, Olivier A, Nalbantoglu J, Antel JP. Resistance of human adult oligodendrocytes to AMPA/kainate receptor-mediated glutamate injury. Brain. 2004;127:2636–2648. doi: 10.1093/brain/awh302. [DOI] [PubMed] [Google Scholar]
- Miron VE, Rajasekharan S, Jarjour AA, Zamvil SS, Kennedy TE, Antel JP. Simvastatin regulates oligodendroglial process dynamics and survival. Glia. 2007;55:130–143. doi: 10.1002/glia.20441. [DOI] [PubMed] [Google Scholar]
- Dawson J, Hotchin N, Lax S, Rumsby M. Lysophosphatidic acid induces process retraction in CG-4 line oligodendrocytes and oligodendrocyte precursor cells but not in differentiated oligodendrocytes. J Neurochem. 2003;87:947–957. doi: 10.1046/j.1471-4159.2003.02056.x. [DOI] [PubMed] [Google Scholar]
- Graeler M, Shankar G, Goetzl EJ. Cutting edge: suppression of T cell chemotaxis by sphingosine 1-phosphate. J Immunol. 2002;169:4084–4087. doi: 10.4049/jimmunol.169.8.4084. [DOI] [PubMed] [Google Scholar]
- Jin Y, Knudsen E, Wang L, Bryceson Y, Damaj B, Gessani S, Maghazachi AA. Sphingosine 1-phosphate is a novel inhibitor of T-cell proliferation. Blood. 2003;101:4909–4915. doi: 10.1182/blood-2002-09-2962. [DOI] [PubMed] [Google Scholar]
- Jack CS, Arbour N, Manusow J, Montgrain V, Blain M, McCrea E, Shapiro A, Antel JP. TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol. 2005;175:4320–4330. doi: 10.4049/jimmunol.175.7.4320. [DOI] [PubMed] [Google Scholar]
- Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- Ancellin N, Hla T. Differential pharmacological properties and signal transduction of the sphingosine 1-phosphate receptors EDG-1, EDG-3, and EDG-5. J Biol Chem. 1999;274:18997–19002. doi: 10.1074/jbc.274.27.18997. [DOI] [PubMed] [Google Scholar]
- Niedernberg A, Blaukat A, Schoneberg T, Kostenis E. Regulated and constitutive activation of specific signalling pathways by the human S1P5 receptor. Br J Pharmacol. 2003;138:481–493. doi: 10.1038/sj.bjp.0705055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alabed YZ, Grados-Munro E, Ferraro GB, Hsieh SH, Fournier AE. Neuronal responses to myelin are mediated by rho kinase. J Neurochem. 2006;96:1616–1625. doi: 10.1111/j.1471-4159.2006.03670.x. [DOI] [PubMed] [Google Scholar]
- Lee CW, Rivera R, Dubin AE, Chun J. LPA(4)/GPR23 is a lysophosphatidic acid (LPA) receptor utilizing G(s)-, G(q)/G(i)-mediated calcium signaling and G(12/13)-mediated Rho activation. J Biol Chem. 2007;282:4310–4317. doi: 10.1074/jbc.M610826200. [DOI] [PubMed] [Google Scholar]
- Niedernberg A, Scherer CR, Busch AE, Kostenis E. Comparative analysis of human and rat S1P(5) (edg8): differential expression profiles and sensitivities to antagonists. Biochem Pharmacol. 2002;64:1243–1250. doi: 10.1016/s0006-2952(02)01289-3. [DOI] [PubMed] [Google Scholar]
- Novgorodov AS, El Alwani M, Bielawski J, Obeid LM, Gudz TI. Activation of sphingosine-1-phosphate receptor S1P5 inhibits oligodendrocyte progenitor migration. FASEB J. 2007;21:1503–1514. doi: 10.1096/fj.06-7420com. [DOI] [PubMed] [Google Scholar]