Abstract
Gestational trophoblastic disease includes choriocarcinoma, a frankly malignant tumor, and hydatidiform mole (HM), which often leads to the development of persistent gestational trophoblastic neoplasia and requires chemotherapy. NANOG is an important transcription factor that is crucial for maintaining embryonic stem cell self-renewal and pluripotency. We postulated that NANOG is involved in the pathogenesis of gestational trophoblastic disease. In this study, significantly higher NANOG mRNA and protein expression levels, by quantitative PCR and immunoblotting, respectively, were demonstrated in HMs, particularly those that developed persistent disease, when compared with normal placentas. In addition, significantly increased nuclear NANOG immunoreactivity was found by immunohistochemistry in HMs (P < 0.001) and choriocarcinoma (P = 0.002). Higher NANOG expression levels were demonstrated in HMs that developed persistent disease, as compared with those that regressed (P = 0.025). Nuclear localization of NANOG was confirmed by confocal microscopy and immunoblotting in choriocarcinoma cell lines. There was a significant inverse correlation between NANOG immunoreactivity and apoptotic index assessed by M30 CytoDeath antibody (P = 0.012). After stable knockdown of NANOG in the choriocarcinoma cell line JEG-3 by an shRNA approach, increased apoptosis was observed in relation to with enhanced caspases and poly(ADP-ribose) polymerase activities. NANOG knockdown was also associated with decreased mobility and invasion of JEG-3 and down-regulation of matrix metalloproteases 2 and 9. These findings suggest that NANOG is involved in the pathogenesis and clinical progress of gestational trophoblastic disease, likely through its effect on apoptosis, cell migration, and invasion.
Gestational trophoblastic disease (GTD) is a heterogeneous group of diseases that arises from the placental trophoblasts. It includes lesions such as hydatidiform mole (HM), which may be considered as abnormal placenta that is prone to malignant transformation, and frankly malignant tumors such as choriocarcinoma. Most HM will spontaneously regress after suction evacuation. However, about 8% to 30% will develop persistent gestational trophoblastic neoplasia with metastatic potential requiring chemotherapy. The pathogenesis of GTD remains a controversial issue.1,2,3
NANOG is one of the core transcription factors found in pluripotent embryonic stem cells.4 NANOG is found to be essential for maintaining self-renewal and pluripotency of both human and mouse embryonic stem cells.5,6,7,8 On implantation of blastocysts, Nanog mRNA is detected exclusively in the epiblast of the mouse embryo, is finally restricted to primordial germ cells, and becomes undetectable in adult tissues.9,10
Embryonic stem cells and cancer cells may be considered to share some similarities in phenotypes. They have the potential to grow rapidly and display high telomerase expression, which is responsible for maintaining their immortality.11,12 Trophoblasts and cancer cells may also be comparable regarding their proliferative and invading potential. In HM, activation of telomerase is associated with the development of aggressive gestational trophoblastic neoplasia.13,14
Recently, NANOG expression has been reported in human neoplasms, including germ cell tumors,15,16,17,18 breast carcinomas,18 and osteosarcoma.19 Furthermore, ectopic expression of Nanog induced an oncogenic potential in NIH3T3.20 We postulate that NANOG is important in the development and malignant progression of GTD. In the present study, we attempted to examine the expression of NANOG in GTD in association with clinical outcome and to characterize the in vitro functions of NANOG.
Materials and Methods
Clinical Sample Selection
A total of 38 fresh-frozen trophoblast samples, including 9 first trimester placentas, 8 term placentas, 14 HMs that spontaneously regressed (regressive moles), and 7 HMs that subsequently progressed to persistent gestational trophoblastic neoplasia (persistent moles), were collected at Queen Mary Hospital, the University of Hong Kong (study approval had been obtained from the Institutional Research Board). Fifty formalin-fixed paraffin-embedded tissues, including 7 first trimester placentas, 6 term placentas, 20 regressive moles, 11 persistent moles, and 6 choriocarcinomas, were also retrieved. The tissues of HMs and choriocarcinomas were obtained from specimens of uterine evacuate and/or hysterectomy. First trimester and term placentas were collected, after induced abortion by suction evacuation and normal delivery, respectively. Persistent gestational trophoblastic neoplasia was diagnosed if there was a plateau in human chorionic gonadotrophin level for 4 weeks or a further rise in human chorionic gonadotrophin for three consecutive weeks after evacuation, according to internationally accepted criteria.21 The histological features of all these cases in H&E-stained sections were determined using generally agreed and accepted diagnostic criteria.1,2,3 Most HM cases had previously been assessed for apoptotic activity by M30 CytoDeath antibody22 and ploidy analysis by fluorescent microsatellite genotyping after microdissection and chromosome in situ hybridization.23,24
Cell Lines, Cell Culture, and Subcellular Protein Extraction
A normal extravillous trophoblast cell line (TEV-1)25 and three choriocarcinoma cell lines, JEG-3, JAR, and BeWo (American Type Culture Collection, Rockville, MD), were cultured in Minimum Essential Medium Eagle (Sigma, St. Louis, MO) supplemented with 10% fetal bovine serum (JRH Biosciences, Lenexa, KS), and 100 U/ml penicillin and streptomycin (Invitrogen, San Diego, CA). Isolation of cytoplasmic and nuclear extracts from JEG-3 was performed using the NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce Biotechnology, Rockford, IL).26 Twenty μg protein was used for detecting NANOG in subcellular protein fractions.
Quantitative Real-Time PCR
Total RNA was extracted using Trizol reagent (Invitrogen). cDNA was synthesized from 2.5 μg total RNA by SuperScript Reverse Transcriptase (Invitrogen). Primers and probes used are listed in Table 1. Quantitative PCR (qPCR) was performed with the ABI PRISM 7700 Sequence Detection System. The expression of NANOG was normalized with respect to that of TBP, a housekeeping gene.27,28 The expression of matrix metalloprotease (MMP)-2, MMP-9, vascular endothelial growth factor, and urokinase-type plasminogen activator was determined by 2−ΔΔCT method after normalization with house keeping gene.29
Table 1.
Primers and Probes Used for Quantitative Real-Time PCR
| Gene | Primer direction | Sequences (5′-3′) | References |
|---|---|---|---|
| NANOG | Forward | 5′-CCATCCTTGCAAATGTCTTCTG-3′ | Design |
| Reverse | 5′-CTTTGGGACTGGTGGAAGAATC-3′ | ||
| TaqMan probe | 5′-AGATGCCTCACACGGAGA-3′ | ||
| TBP | Forward | 5′-ACGAACCACGGCACTGAT-3′ | 27 |
| Reverse | 5′-AACCCAACTTCTGTACAACTCTAGCA-3′ | ||
| TaqMan probe | 5′-ACAGTCCAGACTGGCAGCAAGAAAATA-3′ | ||
| MMP-2 | Forward | 5′-GGCCCTGTCACTCCTGAGAT-3′ | 48 |
| Reverse | 5′-GGCATCCAGGTTATGGGGGA-3′ | ||
| MMP-9 | Forward | 5′-CAACATCACCTATTGGATCC-3′ | 48 |
| Reverse | 5′-CGGGTGTAGAGTCTCTCGCT-3′ | ||
| VEGF | Forward | 5′-AACCATGAACTTTCTGCTGTCTTG-3′ | 49 |
| Reverse | 5′-TTCACCACTTCGTGATGATTCTG-3′ | ||
| uPA | Forward | 5′-TCACCACCAAAATGCTGTGT-3′ | 50 |
| Reverse | 5′-AGGCCATTCTCTTCCTTGGT-3′ |
Immunoblotting
Total protein lysate was extracted with lysis buffer (0.125 M Tris, pH 6.8 at 22°C containing 1% NP-40 [v/v], 2 mmol/L EDTA, 2 mmol/L N-ethylmaleimide, 2 mmol/L phenylmethylsulfonyl fluoride, 1 mmol/L sodium orthovanadate, and 0.1 μmol/L sodium okadate). Protein concentration was determined by detergent compatible protein assay (Bio-Rad Laboratories, Hercules, CA). Fifty μg of protein was resolved by SDS-polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membrane, and probed with corresponding antibodies.30 Antibodies used in the present study are listed in Table 2.
Table 2.
Primary Antibodies Used for Immunoblotting, Immunofluorescence Microscopy, and Immunohistochemistry
| Target protein | Animal source* | Catalog# | Usage** | Working dilution | Vendor |
|---|---|---|---|---|---|
| NANOG | Goat | AF1997 | IB | 1:300 | R&D systems (Minneapolis, MN) |
| NANOG | Rabbit | ab21603 | IF, IHC | 1:50 (IF, IHC) | Abcam (Cambridge, MA) |
| M30 CytoDeath | Mouse | 2140349 | IHC | 1:100 | Boehringer (Mannheim, Germany) |
| a Tubulin | Mouse | sc-8035 | IB | 1:200 | Santa Cruz biotechnology, Inc (Santa Cruz, CA) |
| Histone H1 | Mouse | sc-8030 | IB | 1:200 | Santa Cruz biotechnology, Inc |
| Caspase-3 | Rabbit | 9662 | IB | 1:1000 | Cell Signaling (Beverly, MA) |
| Cleaved caspase-3 | Rabbit | 9661 | IB | 1:1000 | Cell signaling |
| Caspase-7 | Rabbit | 9492 | IB | 1:1000 | Cell signaling |
| Cleaved Caspase−7 | Rabbit | 9491 | IB | 1:1000 | Cell signaling |
| Caspase-9 | Rabbit | 9502 | IB | 1:1000 | Cell signaling |
| Cleaved Caspase-9 | Rabbit | 9501 | IB | 1:1000 | Cell signaling |
| PARP | Rabbit | 9542 | IB | 1:1000 | Cell signaling |
| Cleaved PARP | Rabbit | 9541 | IB | 1:1000 | Cell signaling |
| Actin | Rabbit | A5060 | IB | 1:1000 | Sigma |
Antibodies prepared in corresponding host animals.
IB: immunoblotting; IF: immunofluorescence microscopy; IHC: immunohistochemistry.
Confocal Microscopy
JEG-3 cultured on coverslips were fixed with 3.7% paraformaldehyde in PBS for 10 minutes, permeabilized with 0.2% Triton X-100 for 10 minutes, blocked in 3% bovine serum albumin for 1 hour, and incubated subsequently with an anti-NANOG antibody (as listed in Table 2) overnight and Alexa 488-labeled secondary antibody (1:500; Molecular Probes, Eugene, OR) for 30 minutes. Immunofluorescence images were acquired in a Zeiss LSM510 laser scanning confocal microscope (Carl Zeiss Microimaging Inc., Thornwood. NY).31
Immunohistochemistry
Formalin-fixed paraffin sections were stained with anti-NANOG antibody and anti-M30 CytoDeath antibody (as listed in Table 2) by EnVision+ Dual Link System (K4061; Dako, Carpinteria, CA). Antigen retrieval was performed by heating in a pressure cooker using 10 mmol/L EDTA (pH 8.0) (for NANOG) or in a microwave using 10 mmol/L citric acid buffer (pH 6) (for M30 CytoDeath). Omission or replacement of the primary antibody with pre-immune IgG serum was used as a negative control. Images were captured through a ×20 objective. For NANOG, only nuclear staining was considered as positive. Staining intensity was scored as 0 (negative), 1 (weak), 2 (moderate), and 3 (intense). The percentage of positive staining was rated as 0 (negative), 1 (<25%), 2 (26% to 50%), 3 (51% to 75%), and 4 (>75%). The immunoreactivity score was given as a sum of the values of the two parameters, giving the range of 0 to 7.26 For M30 CytoDeath, cells having intense cytoplasmic staining were considered as positive.22
Stable Knockdown of NANOG in JEG-3
For stable silencing, cells transfected with a set of shRNA constructs (containing a puromycin-resistant gene) against human NANOG, pRS-shNANOG (Origene, Rockville, MD) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) were selected with puromycin (1.875 μg/ml). The pRS vector was used as control. Stable clones were picked 2 weeks after selection.32,33 Specific knockdown of NANOG was determined by qPCR and immunoblotting analysis.
TdT-Mediated dUTP Nick End Labeling Assay
TdT-mediated dUTP nick end labeling assay (TUNEL) assay was performed using an In Situ Cell Death Detection kit (Roche Biochemical, Indianapolis, IN).22 By fluorescence microscopy, the number of TUNEL-positive cells in control and in JEG-3 after stable knockdown of NANOG was counted in five different fields at ×40 magnification.
Flow Cytometry
Harvested cells were washed in PBS, fixed in 70% ethanol, and stained with propidium iodide (1 μg/ml) (Sigma). Green fluorescent protein-positive cells were analyzed by flow cytometry using FACSCalibur system (BD, Franklin Lakes, NJ) and WinMDI software.
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide Assay
Cells were seeded in 96-well plates with 2000 cells/well. At day 5, 10 μl 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) was added to each well. Plates were then incubated at 37°C for 4 hours, to be followed by the addition of 100 μl dimethyl sulfoxide to each well for dye extraction. Cell viability was determined by measuring the absorbance of samples at 570 nm with 630 nm as the reference wavelength.
Wound Healing Assay
Cells grown in twelve-well plates with 90% confluence were cut with a sterile 200-μl pipette tip. Fresh culturing medium was added. Photos were retaken at the same position of the wound after 48 hours.
In Vitro Migration and Invasion Assays
Migration and invasion assays were performed as previously described.34 Briefly, cells were plated on the upper compartment of a Transwell chamber. For migration assays, cells were migrated through an 8-μm pore size membrane. For invasion assays, cells were invaded through a Matrigel-coated membrane. After 48 hours, cells at the upper side of the membrane were removed and the migrated or invaded cells were fixed, stained, and counted.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 3.0 for Windows (GraphPad Software, San Diego, CA). Non-parametric unpaired t-test (Mann-Whitney test) was used for continuous data. Correlation analysis was performed using Spearman’s rho test. P values <0.05 were considered as statistically significant.
Results
NANOG Was Overexpressed in Nuclei of HMs and Choriocarcinomas and Correlated with Clinical Outcome
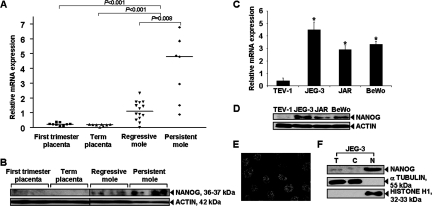
By qPCR, significantly increased NANOG mRNA was found in HMs when compared with that in normal first trimester (P < 0.001) and term placentas (P < 0.001) that showed barely detectable expression (Figure 1A). Significantly higher NANOG mRNA was detected in the HMs that developed persistent gestational trophoblastic neoplasia requiring chemotherapy compared with HMs that spontaneously regressed (P = 0.008) (Figure 1A). Such differential expression pattern was also detected at protein level by immunoblot analysis (Figure 1B). Furthermore, higher NANOG mRNA (P < 0.05) and protein were found to be expressed in all three choriocarcinoma cell lines, JEG-3, JAR, and BeWo, than that in normal trophoblast cell line TEV-1 (Figure 1, C and D). At the subcellular level, nuclear localization of NANOG was also detected in choriocarcinoma cells by confocal microscopy (Figure 1E) and by immunoblot analysis using subcellular protein fractions (Figure 1F).
Figure 1.
mRNA and protein expression of NANOG in GTD clinical samples and cell-lines. A: A comparison of NANOG mRNA expression in GTD clinical samples by qPCR. NANOG expression was normalized with that of TBP and analyzed by Mann-Whitney test. B: Representative images from immunoblot analysis of NANOG in randomly selected GTD clinical samples. C and D: mRNA and protein expression, respectively, of NANOG in a normal extravillous trophoblast cell line (TEV-1) and three choriocarcinoma cell lines, JEG-3, JAR, and BeWo, as determined by qPCR (Bars = means ± SD of three experiments; *P < 0.05 as compared with TEV-1, Mann-Whitney test) or immunoblotting analysis, respectively. E: Immunofluorescence of NANOG in JEG-3. F: NANOG in subcellular protein fractions (T: total cell lysate; C: cytoplasmic fraction; N: nuclear fraction) of JEG-3.
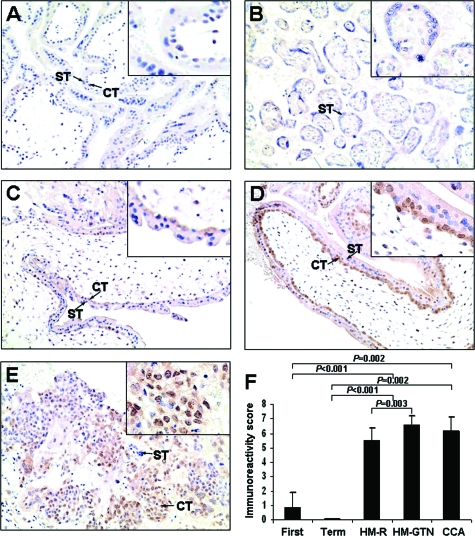
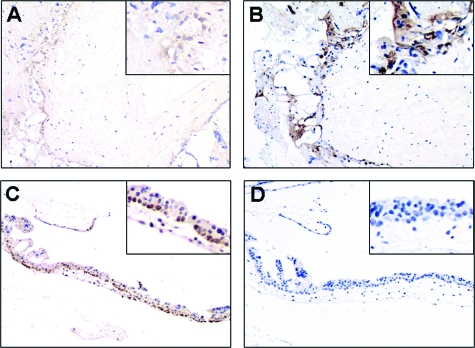
Immunohistochemical studies show that immunoreactivity of NANOG was mainly detected in the nuclei of cytotrophoblasts and villous intermediate trophoblasts in first trimester placentas and GTD samples (Figure 2). The expression in implantation site intermediate trophoblasts was focal, whereas no immunoreactivity was detected in syncytiotrophoblast. The nuclear NANOG immunoreactivity in chorionic-type extravillous intermediate trophoblast was very weak. In term placentas, nuclear expression of NANOG was thus almost non-detectable (Figure 2B). Significantly higher NANOG nuclear immunoreactivity was detected in HMs (P < 0.001) and choriocarcinomas (P = 0.002), as compared with normal placentas (Figures 2A, C–F). Among HMs, significantly higher nuclear immunoreactivity of NANOG was found in the 11 HMs that subsequently developed persistent gestational trophoblastic neoplasia than the 20 HMs that spontaneously regressed (P = 0.003) (Figure 2, C, D, and F). Furthermore, nuclear NANOG expression was found to correlate inversely with apoptosis assessed by M30 CytoDeath antibody in the group of HMs (correlation coefficient = −0.504, P = 0.012, Spearman’s rho test). Such inverse expression patterns of NANOG and M30 CytoDeath Antibody was shown in Figure 3.
Figure 2.
Immunoreactivity of NANOG in first trimester placenta (A), term placenta (B), HM that regressed (C), HM that subsequently developed persistent trophoblastic neoplasia (D), and choriocarcinoma (E). NANOG immunoreactivity was mainly detected in the nuclei of cytotrophoblast (CT) and was absent in syncytiotrophoblast (ST) (see arrows). Insets highlighting selected regions of the trophoblasts at higher magnification are included. F: Bar chart of nuclear immunoreactivity of NANOG in first trimester placentas (First), term placentas (Term), HM that regressed (HM-R), HM that subsequently developed persistent trophoblastic neoplasia (HM-GTN), and choriocarcinomas (CCA). Nuclear immunoreactivity of NANOG was analyzed by Mann-Whitney test.
Figure 3.
Inverse relation of NANOG expression and M30 CytoDeath Antibody staining in HMs. Weak NANOG expression (A) and strong M30 CytoDeath Antibody staining (B) in the same case of HM. Strong NANOG expression (C) and weak M30 CytoDeath Antibody staining (D) in the other case of HM.
Knockdown of NANOG Increased Apoptosis of Choriocarcinomas Cells in Relation to Caspases and Poly (ADP-Ribose) Polymerase Cleavage Activities
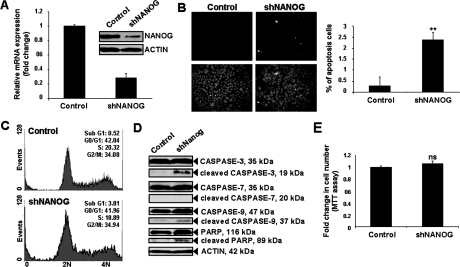
The possible functional role of NANOG was investigated after stable knockdown of NANOG in JEG-3 by short hairpin RNA (shRNA) approach. After confirming the specific knockdown of NANOG mRNA and protein (Figure 4A), the effect on apoptosis was assessed by TUNEL assay. Stable knockdown of NANOG (shNANOG) in JEG-3 led to increase in proportion of apoptotic cells to 2.4% compared with 0.3% in control (Figure 4B). In addition to TUNEL results, further analysis by flow cytometry also showed a significant increase in the percentage of apoptosis cells from 0.52% to 3.81% after knockdown of NANOG (P < 0.05) (Figure 4C). Increased cleavage of caspase-3, caspase-9, and poly (ADP-ribose) polymerase, central regulators of apoptosis, was demonstrated by immunoblot analysis (Figure 4D).
Figure 4.
Stable knockdown of NANOG promoted apoptosis and activation of caspase-3, caspase-9, and poly (ADP-ribose) polymerase in JEG-3. A: Specific knockdown of NANOG (shNANOG) mRNA and protein expression by qPCR and immunoblotting analysis (inset) respectively. NANOG mRNA expression in control was arbitrarily set as 1. Bars = means ± SD of three experiments. B: Left panel: photographed representative fields of TUNEL assay. Right panel: percentage of apoptotic cells (apoptotic cells/total cells counted). Bars = mean ± SD of three experiments; **P < 0.005, Mann-Whitney test. C: Representative examples of cell cycle analysis by flow cytometry in control and shNANOG JEG-3. Sub-G1 peak represented the apoptotic cells. Cell populations’ sizes were given as percentage of total populations. D: The expression of total and cleaved forms of caspases-3, -7, and -9, and poly (ADP-ribose) polymerase in control and shNANOG JEG-3 as determined by immunoblot analysis. E: Cell proliferation rate in control and shNANOG JEG-3 as determined by MTT assay. The absorbance of control was arbitrarily set as 1. Bars = means ± SD of three experiments; ns, not significantly different, Mann-Whitney test.
Knockdown of NANOG Had No Effect on Proliferation Rate and Cell-Cycle of Choriocarcinoma Cells
MTT proliferation assay showed no significant difference in the proliferation rate of control and shNANOG JEG-3 (Figure 4E). Flow cytometry analysis also confirmed that stable knockdown of NANOG had no significant effect on the proportion of cells at G0/G1, S, and G2/M (Figure 4C).
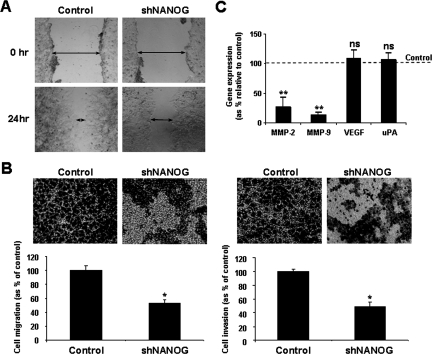
Knockdown of NANOG Reduced Choriocarcinoma Cell Migration and Invasion and Down-Regulated MMP-2 and MMP-9
The effect of NANOG on cell motility and invasion was further assessed. By wound healing assay, slower migration rate was found in shNANOG JEG-3 than in control (Figure 5A). By Transwell migration and invasion assays, significantly reduced migration and invasion (P < 0.05) in shNANOG JEG-3 was observed, implicating that down-regulation of NANOG slowed down JEG-3 migration and invasion (Figure 5B). Furthermore, qPCR showed significantly reduced expression of MMP-2 and MMP-9 mRNA (P < 0.005), whereas the levels of vascular endothelial growth factor and urokinase-type plasminogen activator mRNA (P > 0.05) remained virtually unchanged in control and shNANOG JEG-3 (Figure 5C).
Figure 5.
Stable knockdown of NANOG-retarded JEG-3 cell migration and invasion through the down-regulation of MMP-2 and MMP-9 mRNA expression. A: Wound healing assay in control and shNANOG JEG-3. B: In vitro migration (left panel) and invasion assays (right panel) involving transwell membrane without or with Matrigel coating respectively. Upper panels: representative images showing cells migrated or invaded to the lower chamber after 48 hours. Lower panels: Bars = mean ± SD of cell migration or invasion as percentage of control in five fields of triplicate wells from three experiments; *P < 0.05, Mann-Whitney test. C: qPCR analysis on the mRNA levels of MMP-2, MMP-9, vascular endothelial growth factor, and urokinase-type plasminogen activator in control and shNANOG JEG-3. Bars = means ± SD of three experiments; **P < 0.005, Mann-Whitney test.
Discussion
In this study, we found significantly higher NANOG mRNA and protein expression in HMs and choriocarcinomas as compared with normal placentas. An association between higher NANOG expression and aggressive behavior of HMs was also demonstrated. Such findings suggested that NANOG may participate in the pathogenesis of GTD, including clinical progress of gestational trophoblastic neoplasia and tumorigenesis of choriocarcinoma. Our observations concur with the presence of NANOG expression in other human malignancies,15,16,17,18 further providing evidence on the role of NANOG in human neoplasms.
To explore the function of NANOG in choriocarcinoma, we have adopted a shRNA approach to specific knockdown of NANOG in JEG-3. The anti-apoptosis effect of NANOG on JEG-3 was found, probably mediated through the regulation of caspase-3, caspase-9, and poly (ADP-ribose) polymerase cleavages, suggesting that overexpression of NANOG in choriocarcinoma may contribute to the prolonged survival of cancer cells. To the best of our knowledge, this is the first report demonstrating such an anti-apoptotic effect of NANOG on cancer cells. Such an anti-apoptotic ability of NANOG is not surprising since regulation of apoptosis is necessary to maintain the self-renewal state of embryonic stem cells.35,36 In mouse embryonic stem cells, suppression of Nanog expression by the tumor suppressor p53 was associated with the differentiation of mouse embryonic stem cells into other cell types that undergo efficient p53-dependent cell-cycle arrest and apoptosis.37 Recent reports also demonstrated that Oct-4, another stem cell-related gene, is essential for protecting mouse embryonic stem cells from stress-related apoptosis.38 Furthermore, the anti-apoptotic effect of NANOG can also explain the association of higher NANOG expression in HMs that progress to gestational trophoblastic neoplasia, because our earlier studies have demonstrated that HMs with lower apoptotic activity tend to progress and require chemotherapy.22,39 In the present study, nuclear NANOG expression was inversely correlated with an apoptotic index evaluated by M30 CytoDeath antibody, further supporting the notion that NANOG plays a role in determining the progress of HM through regulation of its apoptotic activity.
The present study also revealed that knock-down of NANOG decreased mobility and invasion of choriocarcinoma cells. Such finding suggested that NANOG may be involved in the malignant progression of trophoblasts. In fact, Nanog has been suggested to have effect on cell migration in studies of migrating primordial germ cells in the mouse.40 Moreover, ectopic expression of Nanog has been found to promote transformation and growth rate in NIH3T3 cells.20,41 Such effects of NANOG on cell invasion seems to be better demonstrated in choriocarcinoma than in extravillous implantation site intermediate trophoblasts (a trophoblast subtype associated with invasive capacity) in normal placentas, because only focal NANOG immunoreactivity was found in this trophoblast subtype. On the other hand, higher NANOG nuclear immunoreactivity was detected in choriocarcinoma, a frankly malignant tumor, as compared with normal placentas. These observations suggested that the effect of NANOG on cell invasion may be cell-type specific. Indeed, different from malignant tumor cells, the invasion of implantation site intermediate trophoblasts occurs in normal placentas during early pregnancy, under precise control and is restricted to the implantation site.42,43,44 In the choriocarcinoma cell line, we further found that the cell-invasion effect of NANOG was likely to be mediated through the down-regulation of MMP-2 and MMP-9, two MMPs essential for choriocarcinoma cell invasion.45 In fact, the promoters of these two likely targets of NANOG have been found to be occupied by NANOG in embryonic stem cells.46 These observations help to explain the mechanisms regarding the role of NANOG in tumorigenesis.
Cytotrophoblasts act as germinal cells that proliferate and differentiate into either syncytiotrophoblasts or villous intermediate trophoblasts, that subsequently proliferate in the proximal site of the cell columns, and eventually differentiate into implantation site intermediate trophoblasts in the implantation site, or chorionic-type intermediate trophoblast in the chorion leave (fetal membrane).47 Thus, the observation of nuclear NANOG immunoreactivity in cytotrophoblasts and villous intermediate trophoblasts in first trimester placentas and HMs tends to suggest that NANOG may have an effect on trophoblast proliferation. Involvement of Nanog in cellular proliferation in NIH3T3 after its ectopic expression has indeed been reported.20 However, by in vitro studies on choriocarcinoma cells, no change in the proliferation rate after stable knock-down of NANOG could be detected. Our findings suggested that NANOG may not be involved in choriocarcinoma cell proliferation in an in vitro setting. NANOG may be executing its functional roles differently in different environments.
In summary, our findings provide new insights into the role of NANOG in the pathogenesis of GTD, particularly its correlation with the clinical progress of HM, as well as the possible effect of NANOG on apoptosis, migration, and invasion in choriocarcinoma cells. The mechanism regulating NANOG expression in GTD is not yet known. Indeed, we have attempted to investigate the possible effect of histone deacetylation on NANOG mRNA expression by treating JEG-3 and JAR with Trichostatin A. Our qPCR results showed that Trichostatin A could not induce NANOG mRNA expression in JEG-3 and JAR (unpublished data), implicating that histone acetylation is not involved in the increase expression of NANOG in choriocarcinoma cells. It is also important to determine whether a change in NANOG expression is a primary causal or secondary event in the pathogenesis of GTD. This query needs to be elucidated by more studies in the future. Such findings may assist to define NANOG as a potential therapeutic target in GTD.
Footnotes
Address reprint requests to Annie N. Y. Cheung, M.D., FRCPath., Department of Pathology, The University of Hong Kong, Queen Mary Hospital, Pokfulam Road, Hong Kong, China. E-mail: anycheun@hkucc.hku.hk.
See related Commentary on page 911
Supported by grants from the Research Grants Council of the Hong Kong Special Administrative Region (HKU 7309/04M) and the Conference and Research Council grant from the University of Hong Kong.
References
- Shih Ie M. Gestational trophoblastic neoplasia–pathogenesis and potential therapeutic targets. Lancet Oncol. 2007;8:642–650. doi: 10.1016/S1470-2045(07)70204-8. [DOI] [PubMed] [Google Scholar]
- Paradinas FJ, Elston CW. Gestational trophoblastic disease. Fox H, Wells M, editors. Edinburgh: Churchill Livingstone; 2003:pp. 1359–1430. [Google Scholar]
- Cheung AN. Pathology of gestational trophoblastic diseases. Best Pract Res Clin Obstet Gynaecol. 2003;17:849–868. doi: 10.1016/s1521-6934(03)00094-4. [DOI] [PubMed] [Google Scholar]
- Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, Bourque G, George J, Leong B, Liu J, Wong KY, Sung KW, Lee CW, Zhao XD, Chiu KP, Lipovich L, Kuznetsov VA, Robson P, Stanton LW, Wei CL, Ruan Y, Lim B, Ng HH. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–440. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- Wang J, Rao S, Chu J, Shen X, Levasseur DN, Theunissen TW, Orkin SH. A protein interaction network for pluripotency of embryonic stem cells. Nature. 2006;444:364–368. doi: 10.1038/nature05284. [DOI] [PubMed] [Google Scholar]
- Rao S, Orkin SH. Unraveling the transcriptional network controlling ES cell pluripotency. Genome Biol. 2006;7:230. doi: 10.1186/gb-2006-7-8-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan G, Thomson JA. Nanog and transcriptional networks in embryonic stem cell pluripotency. Cell Res. 2007;17:42–49. doi: 10.1038/sj.cr.7310125. [DOI] [PubMed] [Google Scholar]
- Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- Odorico JS, Kaufman DS, Thomson JA. Multilineage differentiation from human embryonic stem cell lines. Stem Cells. 2001;19:193–204. doi: 10.1634/stemcells.19-3-193. [DOI] [PubMed] [Google Scholar]
- Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- Cheung AN, Zhang DK, Liu Y, Ngan HY, Shen DH, Tsao SW. Telomerase activity in gestational trophoblastic disease. J Clin Pathol. 1999;52:588–592. doi: 10.1136/jcp.52.8.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HW, Tsao SW, Cheung AN. Current understandings of the molecular genetics of gestational trophoblastic diseases. Placenta. 2002;23:20–31. doi: 10.1053/plac.2001.0744. [DOI] [PubMed] [Google Scholar]
- Hoei-Hansen CE, Almstrup K, Nielsen JE, Brask Sonne S, Graem N, Skakkebaek NE, Leffers H, Rajpert-De Meyts E. Stem cell pluripotency factor NANOG is expressed in human fetal gonocytes, testicular carcinoma in situ and germ cell tumours. Histopathology. 2005;47:48–56. doi: 10.1111/j.1365-2559.2005.02182.x. [DOI] [PubMed] [Google Scholar]
- Hart AH, Hartley L, Parker K, Ibrahim M, Looijenga LH, Pauchnik M, Chow CW, Robb L. The pluripotency homeobox gene NANOG is expressed in human germ cell tumors. Cancer. 2005;104:2092–2098. doi: 10.1002/cncr.21435. [DOI] [PubMed] [Google Scholar]
- Santagata S, Ligon KL, Hornick JL. Embryonic stem cell transcription factor signatures in the diagnosis of primary and metastatic germ cell tumors. Am J Surg Pathol. 2007;31:836–845. doi: 10.1097/PAS.0b013e31802e708a. [DOI] [PubMed] [Google Scholar]
- Ezeh UI, Turek PJ, Reijo RA, Clark AT. Human embryonic stem cell genes OCT4. NANOG, STELLAR, and GDF3 are expressed in both seminoma and breast carcinoma. Cancer. 2005;104:2255–2265. doi: 10.1002/cncr.21432. [DOI] [PubMed] [Google Scholar]
- Gibbs CP, Kukekov VG, Reith JD, Tchigrinova O, Suslov ON, Scott EW, Ghivizzani SC, Ignatova TN, Steindler DA. Stem-like cells in bone sarcomas: implications for tumorigenesis. Neoplasia. 2005;7:967–976. doi: 10.1593/neo.05394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wang X, Chen B, Suo G, Zhao Y, Duan Z, Dai J. Expression of Nanog gene promotes NIH3T3 cell proliferation. Biochem Biophys Res Commun. 2005;338:1098–1102. doi: 10.1016/j.bbrc.2005.10.071. [DOI] [PubMed] [Google Scholar]
- Ngan HY. The practicability of FIGO 2000 staging for gestational trophoblastic neoplasia. Int J Gynecol Cancer. 2004;14:202–205. doi: 10.1111/j.1048-891X.2004.014236.x. [DOI] [PubMed] [Google Scholar]
- Chiu PM, Ngan YS, Khoo US, Cheung AN. Apoptotic activity in gestational trophoblastic disease correlates with clinical outcome: assessment by the caspase-related M30 CytoDeath antibody. Histopathology. 2001;38:243–249. doi: 10.1046/j.1365-2559.2001.01065.x. [DOI] [PubMed] [Google Scholar]
- Lai CY, Chan KY, Khoo US, Ngan HY, Xue WC, Chiu PM, Tsao SW, Cheung AN. Analysis of gestational trophoblastic disease by genotyping and chromosome in situ hybridization. Mod Pathol. 2004;17:40–48. doi: 10.1038/modpathol.3800010. [DOI] [PubMed] [Google Scholar]
- Cheung AN, Khoo US, Lai CY, Chan KY, Xue WC, Cheng DK, Chiu PM, Tsao SW, Ngan HY. Metastatic trophoblastic disease after an initial diagnosis of partial hydatidiform mole: genotyping and chromosome in situ hybridization analysis. Cancer. 2004;100:1411–1417. doi: 10.1002/cncr.20107. [DOI] [PubMed] [Google Scholar]
- Feng HC, Choy MY, Deng W, Wong HL, Lau WM, Cheung AN, Ngan HY, Tsao SW. Establishment and characterization of a human first-trimester extravillous trophoblast cell line (TEV-1). J Soc Gynecol Investig. 2005;12:e21–32. doi: 10.1016/j.jsgi.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Chan HY, Siu MK, Zhang HJ, Wong E, Ngan HY, Chan YK, Cheung AN. Activated Stat3 expression in gestational trophoblastic disease: correlation with apoptotic indices and clinicopathological parameters. Histopathology. 2008;53:139–146. doi: 10.1111/j.1365-2559.2008.03089.x. [DOI] [PubMed] [Google Scholar]
- Chan KY, Ozcelik H, Cheung AN, Ngan HY, Khoo US. Epigenetic factors controlling the BRCA1 and BRCA2 genes in sporadic ovarian cancer. Cancer Res. 2002;63:4151–4156. [PubMed] [Google Scholar]
- Shen DH, Chan KYK, Khoo US, Ngan HYS, Xue WC, Chiu PM, Ip PPC, Cheung ANY. Epigenetic and genetic alterations of p33ING1b in ovarian cancer. Carcinogenesis. 2005;26:855–863. doi: 10.1093/carcin/bgi011. [DOI] [PubMed] [Google Scholar]
- Feng HC, Tsao SW, Ngan HY, Xue WC, Chiu PM, Cheung AN. Differential expression of insulin-like growth factor binding protein 1 and ferritin light polypeptide in gestational trophoblastic neoplasia: combined cDNA suppression subtractive hybridization and microarray study. Cancer. 2005;104:2409–2416. doi: 10.1002/cncr.21483. [DOI] [PubMed] [Google Scholar]
- Feng H, Cheung AN, Xue WC, Wang Y, Wang X, Fu S, Wang Q, Ngan HY, Tsao SW. Down-regulation and promoter methylation of tissue inhibitor of metalloproteinase 3 in choriocarcinoma. Gynecol Oncol. 2004;94:375–382. doi: 10.1016/j.ygyno.2004.04.019. [DOI] [PubMed] [Google Scholar]
- Siu MK, Wong CH, Lee WM, Cheng CY. Sertoli-germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J Biol Chem. 2005;280:25029–25047. doi: 10.1074/jbc.M501049200. [DOI] [PubMed] [Google Scholar]
- Chan VS, Chan KY, Chen Y, Poon LL, Cheung AN, Zheng B, Chan KH, Mak W, Ngan HY, Xu X, Screaton G, Tam PK, Austyn JM, Chan LC, Yip SP, Peiris M, Khoo US, Lin CL. Homozygous L-SIGN (CLEC4M) plays a protective role in SARS coronavirus infection. Nat Genet. 2006;38:38–46. doi: 10.1038/ng1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez PC, Quiceno DG, Ochoa AC. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood. 2007;109:1568–1573. doi: 10.1182/blood-2006-06-031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu PM, Feng HC, Benbrook DM, Ngan HY, Khoo US, Xue WC, Tsao SW, Chan KW, Cheung AN. Effect of all-trans retinoic acid on tissue dynamics of choriocarcinoma cell lines: an organotypic model. J Clin Pathol. 2006;59:845–850. doi: 10.1136/jcp.2005.025833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval D, Trouillas M, Thibault C, Dembele D, Diemunsch F, Reinhardt B, Mertz AL, Dierich A, Boeuf H. Apoptosis and differentiation commitment: novel insights revealed by gene profiling studies in mouse embryonic stem cells. Cell Death Differ. 2006;13:564–575. doi: 10.1038/sj.cdd.4401789. [DOI] [PubMed] [Google Scholar]
- Pyle AD, Lock LF, Donovan PJ. Neurotrophins mediate human embryonic stem cell survival. Nature Biotechnol. 2006;24:344–350. doi: 10.1038/nbt1189. [DOI] [PubMed] [Google Scholar]
- Lin T, Chao C, Saito S, Mazur SJ, Murphy ME, Appella E, Xu Y. p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nat Cell Biol. 2005;7:165–171. doi: 10.1038/ncb1211. [DOI] [PubMed] [Google Scholar]
- Guo Y, Mantel C, Hromas RA, Broxmeyer HE. Oct 4 is critical for survival/antiapoptosis of murine embryonic stem cells subjected to stress. Effects associated with STAT3/survivin. Stem Cells. 2007;26:30–34. doi: 10.1634/stemcells.2007-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SY, Ngan HY, Chan CC, Cheung AN. Apoptosis in gestational trophoblastic disease is correlated with clinical outcome and Bcl-2 expression but not Bax expression. Mod Pathol. 1999;12:1025–1033. [PubMed] [Google Scholar]
- Yamaguchi S, Kimura H, Tada M, Nakatsuji N, Tada T. Nanog expression in mouse germ cell development. Gene Expr Patterns. 2005;5:639–646. doi: 10.1016/j.modgep.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Piestun D, Kochupurakkal BS, Jacob-Hirsch J, Zeligson S, Koudritsky M, Domany E, Amariglio N, Rechavi G, Givol D. Nanog transforms NIH3T3 cells and targets cell-type restricted genes. Biochem Biophys Res Commun. 2006;343:279–285. doi: 10.1016/j.bbrc.2006.02.152. [DOI] [PubMed] [Google Scholar]
- Bischof P, Meisser A, Campana A. Paracrine and autocrine regulators of trophoblast invasion–a review. Placenta. 2000;21 Suppl A:S55–S60. doi: 10.1053/plac.2000.0521. [DOI] [PubMed] [Google Scholar]
- Lala PK, Chakraborty C. Factors regulating trophoblast migration and invasiveness: possible derangements contributing to pre-eclampsia and fetal injury. Placenta. 2003;24:575–587. doi: 10.1016/s0143-4004(03)00063-8. [DOI] [PubMed] [Google Scholar]
- Shih Ie M, Hsu MY, Oldt RJ, 3rd, Herlyn M, Gearhart JD, Kurman RJ. The role of E-cadherin in the motility and invasion of implantation site intermediate trophoblast. Placenta. 2002;23:706–715. doi: 10.1016/s0143-4004(02)90864-7. [DOI] [PubMed] [Google Scholar]
- Vegh GL, Selcuk Tuncer Z, Fulop V, Genest DR, Mok SC, Berkowitz RS. Matrix metalloproteinases and their inhibitors in gestational trophoblastic diseases and normal placenta. Gynecol Oncol. 1999;75:248–253. doi: 10.1006/gyno.1999.5564. [DOI] [PubMed] [Google Scholar]
- Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, Jaenisch R, Young RA. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurman RJ: Pathology of trophoblast. Monogr Pathol 1991, 195–227 [PubMed] [Google Scholar]
- Janowska-Wieczorek A, Marquez LA, Nabholtz JM, Cabuhat ML, Montano J, Chang H, Rozmus J, Russell JA, Edwards DR, Turner AR. Growth factors and cytokines up-regulate gelatinase expression in bone marrow CD34(+) cells and their transmigration through reconstituted basement membrane. Blood. 1999;93:3379–3390. [PubMed] [Google Scholar]
- Zhang L, Yang N, Katsaros D, Huang W, Park JW, Fracchioli S, Vezzani C, Rigault de la Longrais IA, Yao W, Rubin SC, Coukos G. The oncogene phosphatidylinositol 3′-kinase catalytic subunit alpha promotes angiogenesis via vascular endothelial growth factor in ovarian carcinoma. Cancer Res. 2003;63:4225–4231. [PubMed] [Google Scholar]
- Helenius MA, Saramaki OR, Linja MJ, Tammela TL, Visakorpi T. Amplification of urokinase gene in prostate cancer. Cancer Res. 2001;61:5340–5344. [PubMed] [Google Scholar]