Abstract
Endothelial cells acquire distinctive molecular signatures in their transformation to an angiogenic phenotype that are indicative of changes in cell behavior and function. Using a rat mesentery model of inflammation-induced angiogenesis and a panel of known endothelial markers (CD31, VE-cadherin, BS-I lectin), we identified a capillary sprout-specific endothelial phenotype that is characterized by the marked down-regulation of CD36, a receptor for the anti-angiogenic molecule thrombospondin-1 (TSP-1). TSP-1/CD36 interactions were shown to regulate angiogenesis in this model as application of TSP-1 inhibited angiogenesis and blockade of both TSP-1 and CD36 accelerated angiogenesis. Vascular endothelial growth factor, which was up-regulated in the in vivo model, elicited a dose- and time-dependent down-regulation of CD36 (ie, to a CD36low phenotype) in cultured human umbilical vein endothelial cells. Human umbilical vein endothelial cells that had been conditioned to a CD36low phenotype with VEGF were found to be refractory to anti-proliferative TSP-1 signaling via a CD36-dependent mechanism. The loss of exposure to wall shear stress, which occurs in vivo when previously quiescent cells begin to sprout, also generated a CD36low phenotype. Ultimately, our results identified the regulation of endothelial cell CD36 expression as a novel mechanism through which VEGF stimulates and sustains capillary sprouting in the presence of TSP-1. Additionally, CD36 was shown to function as a potential molecular linkage through which wall shear stress may regulate both microvessel sprouting and quiescence.
Angiogenesis, the growth of new blood vessels from existing vessels, is an integral component of many physiological and pathological conditions such as wound healing, inflammation, and tumor growth.1 Endothelial cells involved in angiogenesis acquire distinctive structural and molecular characteristics in the transformation from a quiescent to an angiogenic phenotype.2,3 We previously identified a novel angiogenic phenotype in vivo that is marked by the absence of the OX-43 antigen, a glycoprotein of unknown identity and function, and is specific to capillary sprouts during inflammation-induced angiogenesis.4 Interestingly, there is evidence that suggests that the OX-43 antigen could show a similar expression pattern as the thrombospondin (TSP-1) receptor CD36,5,6 which has an important role in the regulation of angiogenesis.
Neovascularization is critically dependent on endothelial cell survival, which in turn, is regulated by opposing signals from angiogenic growth factors and inhibitors.7,8 TSP-1 is a matricellular protein that inhibits growth factor-induced endothelial proliferation and migration in vitro9,10,11 and suppresses neovascularization in vivo.9,12,13,14,15 Systemic application of a TSP-1-derived peptide dose-dependently inhibits tumor growth and, as a result, is currently being tested in clinical trials.16,17 Several studies have shown that CD36 mediates the anti-angiogenic actions of TSP-1,18,19 and there is clear evidence that, on TSP-1 binding, CD36 complexes initiate caspase-dependent apoptotic signaling cascades.19,20
Although CD36 is a critical anti-angiogenic receptor, and its mechanisms of action are defined, the endogenous regulation of endothelial CD36 expression during angiogenesis is poorly understood. Here, based on our previous findings that the OX-43 antigen marks a capillary sprout-specific endothelial phenotype4 and circumstantial evidence that the OX43 antigen could be CD36,5,6 we examined endothelial CD36 expression during inflammation-induced angiogenesis and present the first evidence that CD36 expression is absent from capillary sprout endothelial cells in vivo. We then tested whether TSP-1 and CD36 regulate inflammation-induced angiogenesis, whether vascular endothelial growth factor (VEGF) and shear stress regulate endothelial CD36 expression, and whether conditioning endothelial cells with VEGF to a CD36low phenotype attenuates endothelial responses to anti-angiogenic TSP-1 signaling.
Materials and Methods
Inflammation-Induced Angiogenesis Models
All animal studies were approved by the institutional animal care and use committee. Angiogenesis was studied in two distinct rat (Sprague-Dawley, ∼250 g body weight) mesentery models of inflammation. The first was chronic exposure to compound 48/80, a mast cell degranulator that was administered as described.4,21 The second involved surgically exteriorizing and mechanically irritating the tissue under sterile conditions as described.4,22 In some groups, immediately after tissue irritation, TSP-1 (5 μg/ml; Sigma, St. Louis, MO) or neutralizing monoclonal antibodies to CD36 (20 μg/ml, clone UA009; Cell Sciences, Canton, MA) or TSP-1 (20 μg/ml, clone A6.1; Lab Vision, Freemont, CA) were applied to a single mesenteric window while intravital images of the area of tissue vascularization were acquired. Reagents were dissolved in sterile saline and applied directly to the isolated window for 30 minutes, while keeping the rest of the exteriorized mesenteric tissue hydrated with saline. An isotype-matched control antibody was used as a control for anti-CD36 and anti-TSP-1 treatments, and bovine serum albumin was used as an inactive control protein for TSP-1. Treated windows were marked by inserting sutures into adjacent fat pads of the mesenteric tissue for identification before tissue harvest. The tissue was then returned to the peritoneum and allowed to recover for 3 days before processing for immunochemistry.
Tissue Harvest and Immunochemistry
Before tissue harvest, the animals were anesthetized by an intramuscular injection of ketamine (80 mg/kg body weight) and xylazine (8 mg/kg body weight) and then euthanized. Mesenteries were dissected, fixed in ice-cold 100% methanol, and subsequently double-labeled for CD36 and other endothelial markers. Primary antibodies were monoclonal mouse anti-rat CD36 (1:200, Cell Sciences), biotinylated monoclonal mouse anti-rat CD31 (1:200; PharMingen, San Diego, CA), and polyclonal goat anti-rat VE-cadherin (1:50; Santa Cruz Biotechnology, Santa Cruz, CA). BS-I lectin (1:200, Sigma) was also used.
Imaging and Specimen Analysis
Fluorescently labeled specimens were observed with a Nikon TE-300 inverted microscope and confocal scanner (Nikon, Melville, NY). The percentage of capillary sprout tips, characterized by positive staining for CD31, that were also CD36-positive was quantified, and capillary sprout lengths were measured. At least two mesenteric windows, defined as the translucent tissue regions bordered by the ileal resistance vessels and the intestine, were observed per animal. Several hundred sprouts were analyzed per window. For groups in which TSP-1, anti-TSP-1, or anti-CD36 were applied at the time of mechanical irritation, the percent change in vascularized tissue area and the vessel length density were measured. The percent change in vascularized tissue area, defined as the ratio of vascularized tissue area to total tissue area, was measured from intravital images at the time of surgery (day 0) and at tissue harvest (day 3). Vessel length density, defined as total microvessel length per unit vascularized area, was determined from confocal images of CD31 expression.
VEGF Expression in the Mesentery
Mesenteric windows were harvested, fixed in paraformaldehyde (4% in phosphate-buffered saline), and immunolabeled using a rabbit anti-rat polyclonal antibody for VEGF-A (clone 147, Santa Cruz Biotechnology) followed by a CY3-conjugated goat anti-rabbit IgG. A nonspecific rabbit IgG (Sigma) was used in place of the primary antibody as a negative staining control. A rat VEGF enzyme-linked immunosorbent assay (ELISA) kit (RayBio, Norcross, GA) was used for the quantitative measurement of VEGF in tissue lysates.
Primary Endothelial Cell Isolation and Culture
Human umbilical vein endothelial cells (HUVECs) were isolated from primary cords and maintained on flasks coated with 0.1% gelatin in M-199 medium (Cambrex, Walkersville, MD) supplemented with 10% fetal bovine serum (Life Technologies, Inc., Grand Island, NY), 100 U/ml penicillin-G + 100 μg/ml streptomycin, 2 mmol/L l-glutamine (Life Technologies, Inc.), 5 μg/ml endothelial cell growth supplement (Biomedical Technologies, Stoughton, MA), and 10 μg/ml heparin (Sigma). Second passage cells were used for all experiments. For each set of experimental comparisons, cells were taken from the same primary cell culture.
Endothelial Cell Stimulation by VEGF165 and Shear Stress
HUVECs were plated at confluence on cell culture grade plastic coated with 0.1% gelatin. After 24 hours, confluent cells were treated with soluble VEGF165 (5 to 50 ng/ml; Biovision, Mountain View, CA) or exposed to flow. A cone and plate flow apparatus was used to induce steady flow at 2 dyn/cm2 shear stress. Fresh culture media consisting of M-199 with 2% dextran (General Electric, Crotonville, NY), 2% fetal bovine serum, 100 U/ml penicillin-G + 100 μg/ml streptomycin, 2 mmol/L l-glutamine, 5 μg/ml endothelial cell growth supplement, and 100 μg/ml heparin was added to the cells before exposure to flow cone and plate apparatus and to corresponding static cultures.
CD36 Immunoprecipitation, Western Blot, and Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
CD36 protein was immunoprecipitated from cell lysates (150 μg of total protein) with a rabbit anti-CD36 antibody (1:100, clone H-300; Santa Cruz Biotechnology), resolved on sodium dodecyl sulfate gels, and immunoblotted with rabbit anti-CD36 antibody (1:500) in Tris-buffered saline and 1% bovine serum albumin. For RT-PCR, total RNA was extracted using the PureLink Microto-Midi total RNA purification system (Invitrogen, Carlsbad, CA) and reverse-transcribed using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Primers were designed using Beacon Designer 2.0 (Biosoft International, Palo Alto, CA) for CD36 (forward 5′-TGCTGTCCTGGCTGTGTTTG-3′, reverse 5′-GCTGCTGTTCATCATCACTTCC-3′). The expression of mRNA was analyzed using AmpliTaq Gold (Applied Biosystems, Foster City, CA), SYBR Green (Invitrogen), and an iCycler (Bio-Rad). Expression of CD36 was normalized to β2-microglobulin, which is endogenously expressed by many cell types, including endothelial cells. β2-Microglobulin expression is not altered by many stimuli, including shear stress and growth factor stimulation, and serves as an excellent housekeeping gene for comparison between experimental conditions.23,24,25
Endothelial Cell Proliferation and Chemotaxis Assays
CD36high cells were obtained by culturing confluent HUVECs in complete media for 24 hours as described, whereas a CD36low phenotype was generated by supplementing media with 50 ng/ml of VEGF165 for 24 hours. Proliferation was quantified by MTS assay according to the manufacturer’s instructions (Promega, Madison, WI). Chemotaxis was assessed with modified Boyden chambers with 8-μm pore membranes (Costar, Cambridge, MA). For some experiments, the activity of CD36 was neutralized with a monoclonal anti-human CD36 antibody (10 μg/ml, clone FA6-152; Cell Sciences). For each assay, experiments were repeated a minimum of three times with different primary cells.
Results
CD36 Expression Is Markedly Reduced in Capillary Sprout Endothelial Cells during Inflammation-Induced Angiogenesis
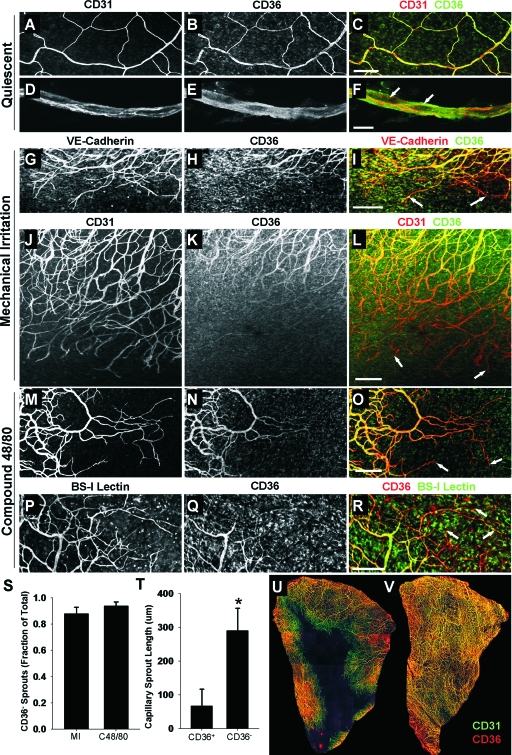
Rat mesenteries immunolabeled for CD36 and selected endothelial markers are shown in Figure 1. Endothelial cells in quiescent capillaries co-express CD36 and CD31 throughout unstimulated microvascular networks, as shown in Figure 1, A–F. Compound 48/80 and mechanical irritation elicit robust angiogenic responses, as evidenced by increased vascular density and capillary sprouting 3 days after stimulation (Figure 1, G–R). In contrast to quiescent endothelial cells, capillary sprout endothelial cells exhibit progressively less CD36 expression along the length of the sprout, with CD36 usually becoming completely undetectable at capillary sprout tips (Figure 1, I, L, O, and R). This result was independent of whether endothelial cells were labeled for VE-cadherin (Figure 1, G–I), CD31 (Figure 1, J–O), or BS-I lectin (Figure 1, P–R). More than 90% of capillary sprout tips were CD36− (Figure 1S). The designation of sprout tips as CD36− or CD36+ was strongly correlated with sprout length, with CD36+ sprouts being significantly shorter than CD36− sprouts (Figure 1T). Although CD36 expression was typically absent from capillary sprouts invading the avascular tissue during the peak phase of sprouting (Figure 1U), CD36 expression was restored throughout the microvasculature when sprouting ceased and the tissue was fully vascularized (Figure 1V), thereby providing evidence that the CD36− phenotype is restricted to sprouts and CD36 expression is restored in nascent capillaries.
Figure 1.
CD36 is differentially expressed by endothelial cells during inflammation-induced angiogenesis. A–F: Confocal images of microvascular networks labeled for CD31 (A, D) and CD36 (B, E). Merge panels (C, F) show co-expression of CD31 (red fluorescence) and CD36 (green fluorescence) with arrows in F denoting nuclei. G–R: Immunolabeled networks from mechanically irritated (G–L) and compound 48/80-stimulated (M–R) specimens 3 days after stimulus application. Microvessels are labeled for the endothelial markers VE-cadherin (G), CD31 (J, M), and BS-I lectin (P), as well as CD36 (H, K, N, Q). Merge panels (I, L, O, R) show diminishing CD36 expression along capillary sprouts. Arrows denote CD36− capillary sprout tips. S: Fraction of CD31+ capillary sprout tips that are CD36−. Values are means ± SD. MI, mechanical irritation; C48/80, compound 48/80. T: Mean lengths of CD36− and CD36+ sprouts. Values are means ± SD. *Significantly different from CD36+ (P < 0.05) by unpaired Student’s t-test. U and V: Confocal images of CD31 (green) and CD36 (red) expression in mesenteric tissues during peak sprouting (U) and when the tissue is fully vascularized (V). Note that endothelial cells leading capillary sprouts into the avascular tissue space are predominantly CD36− during sprouting, whereas CD36 is restored when the tissue is fully vascularized. Scale bars: 200 μm (A–C, G–R); 10 μm (D–F).
TSP-1 and CD36 Regulate Inflammation-Induced Angiogenesis in Vivo
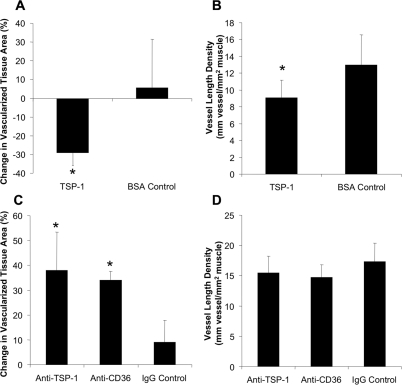
To investigate whether TSP-1 and CD36 regulate inflammation-induced angiogenesis, either TSP-1 or a function neutralizing antibody to either TSP-1 or CD36 was applied at the time of mechanical irritation. Importantly, at the time of application, capillary sprouting was minimal and endothelial cells were predominantly CD36+. TSP-1 induced a significant regression in vascularized tissue area (Figure 2A) and decreased vessel length density by 30% (Figure 2B). Although vessel length density was unchanged after blocking TSP-1 and CD36 (Figure 2D), a significant increase in vascularized tissue area was observed for both interventions (Figure 2C), indicating that angiogenesis into the avascular tissue space was markedly accelerated.
Figure 2.
TSP-1 and CD36 regulate the angiogenic response to mechanical irritation. A and B: Bar graphs showing influence of TSP-1 on vascularized tissue area (A) and vessel length density (B) after mechanical irritation. C and D: Bar graphs showing influence of TSP-1 and CD36 blockade on vascularized tissue area (C) and vessel length density (D). *Significantly different from control (P < 0.05) by Student’s t-test. All values are means ± SD.
VEGF Is Up-Regulated during Inflammation-Induced Angiogenesis
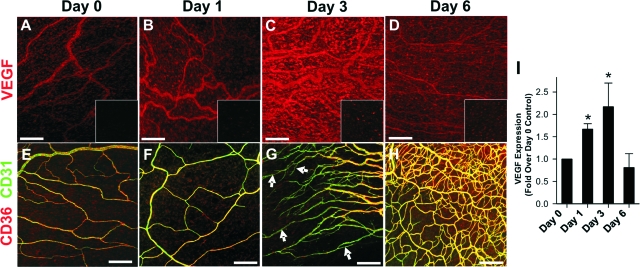
Immunochemistry and ELISA assays were used to determine the time course of VEGF-A expression after mechanical irritation (Figure 3). Confocal images of mesenteric tissues labeled with an antibody specific for VEGF-A are shown in Figure 3, A–D. By immunochemistry, VEGF-A expression was low at day 0, increased at days 1 and 3, and back to control levels by day 6. VEGF-A antibody specificity is demonstrated by the substantial increase in fluorescence in VEGF-labeled tissues compared to nonspecific IgG control tissues, which are shown in the insets of Figure 3, A–D. For comparison, a time-matched series of CD36/CD31-immunolabeled mesenteries is shown in Figure 3, E–H. Significant sprouting is not seen until after day 1. Peak sprouting occurs at approximately day 3, whereas network restitution begins to occur at approximately day 6. ELISA analysis revealed a 1.7-fold increase in VEGF-A at day 1 and a 2.2-fold increase at day 3, but no significant difference between day 0 and day 6 (Figure 3I). Note that VEGF-A expression was significantly increased before significant sprouting occurred, indicating that enhanced VEGF-A expression precedes endothelial CD36 down-regulation in capillary sprouts.
Figure 3.
VEGF expression in the mechanical irritation model of angiogenesis. A–D: Mesenteric tissues labeled for VEGF-A (red) or a negative staining control IgG (insets). Increased red fluorescence in B and C illustrates the increased expression of VEGF-A. E–H: Time-matched series of images from mesenteries labeled for CD36 (red) and CD31 (green) showing tissue before sprouting (days 0 and 1), during sprouting (day 3), and after sprouting (day 6). Arrows in G show CD31+/CD36− sprout stalks. I: ELISA for VEGF-A expression at days 0, 1, 3, and 6. Values are means ± SD. *Significantly different (P < 0.05) than day 0 by one-way analysis of variance and Tukey’s t-test. Scale bars = 200 μm.
VEGF Dose- and Time-Dependently Down-Regulates CD36 Expression in Endothelial Cells
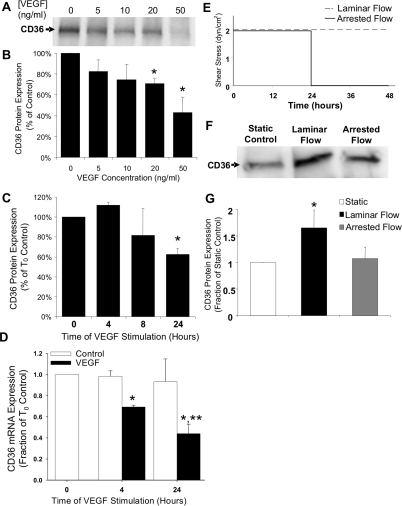
After revealing that VEGF is up-regulated in our inflammatory model of angiogenesis, we exposed confluent HUVECs, which are routinely used to investigate endothelial cell responses to angiogenic stimuli and exhibit phenotypic changes consistent with those exhibited by microvascular endothelial cells,26,27,28,29 to soluble VEGF to determine whether VEGF regulates endothelial expression of CD36. VEGF-A165 elicited a dose-dependent decrease in CD36 protein levels after 24 hours as shown in Figure 4A and quantified by densitometry in Figure 4B. CD36 protein was maximally reduced to 41% of control at 50 ng/ml VEGF-A165 (Figure 4B). CD36 protein expression was unchanged after 4 hours of exposure to 50 ng/ml of VEGF, but increasingly down-regulated as exposure time increased to 24 hours (Figure 4C). RT-PCR analysis showed that CD36 mRNA levels were reduced by 30% after 4 hours and by 60% after 24 hours of exposure to VEGF (Figure 4D), indicating that VEGF regulates transcriptional levels of CD36.
Figure 4.
Endothelial CD36 expression is regulated by VEGF and shear stress. A: Western blot for CD36 expression in HUVECs 24 hours after VEGF treatment. B: Bar graph of densitometric analysis of CD36 expression throughout a range of VEGF concentrations. *Significantly different from 0 ng/ml (P < 0.05) by one-way analysis of variance and Tukey’s t-test. C: Bar graph of densitometric analysis of CD36 expression throughout a range of VEGF application times. *Significantly different from 0 hours (P < 0.05) by one-way analysis of variance and Tukey’s t-test. D: Bar graph of CD36 mRNA expression by RT-PCR. Data are normalized to the β2-microglobulin (B2M) housekeeping gene and then to control cells at 0 hours. *Significantly different from T0 control (P < 0.05) by Student’s t-test. *,**Significantly different from time-matched control (P < 0.05) by two-way analysis of variance and Tukey’s t-test. E: Experimental protocol for shear stress experiments. Quiescent capillary conditions were simulated by 48 hours of flow at 2 dynes/cm2, whereas sprout conditions were simulated by 24 hours of flow followed by 24 hours of static conditions. F: Western blot for CD36 expression in HUVECs exposed to static, laminar flow, and arrested flow conditions. G: Bar graph of CD36 expression in HUVECs exposed to static, steady flow, and arrested flow conditions. *Significantly different (P < 0.05) than static and arrested flow groups by Student’s t-test. All values are means ± SD.
CD36 Expression Is Reduced when Shear Stress Is Withdrawn from Shear Preconditioned Endothelial Cells
To assess how changes in shear stress affect endothelial CD36 expression, steady flow exerting a shear stress of 2 dyne/cm2 was applied to confluent endothelial monolayers in two distinct flow patterns (Figure 4E) intended to simulate either quiescent conditions (steady flow = 48 hours of shear stress) or sprouting conditions (arrested flow = 24 hours of shear stress followed by 24 hours of no-flow). CD36 protein levels were increased after 48 hours of steady flow relative to static controls (Figure 4, F and G). Equal levels of CD36 expression were exhibited in static controls taken at the onset of flow and in time-matched static controls taken after 48 hours of culture in flow media (data not shown), demonstrating that increased CD36 expression was attributable to exposure to flow rather than time spent in culture. Endothelial exposure to flow induced an equivalent increase in CD36 expression after 24 and 48 hours, indicating that CD36 was maximally up-regulated by 24 hours and was sustained throughout prolonged exposure to laminar flow (data not shown). On the other hand, cells exposed to flow for 24 hours showed significantly diminished CD36 expression 24 hours after the arrest of flow (Figure 4, F and G), with flow arrest specifically reducing CD36 protein to 62% of the level observed in cells exposed to 48 hours of laminar flow (Figure 4G).
VEGF Preconditioned Endothelial Cells Become Insensitive to Anti-Proliferative TSP-1 Signaling via a CD36-Dependent Mechanism
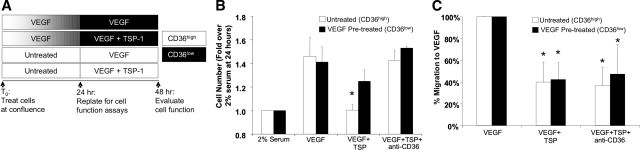
To determine whether the reduced expression of CD36 in endothelial cells exposed to VEGF-A165 attenuates endothelial responsiveness to anti-angiogenic TSP-1 signaling, proliferation and chemotaxis assays were performed for both control (CD36high) and VEGF (50 ng/ml for 24 hours)-pretreated (CD36low) HUVECs. The experimental design is diagrammed in Figure 5A. Here, open bars indicate high CD36 expression in untreated HUVECs and shaded bars illustrate the loss of CD36 expression throughout the 24-hour course of VEGF pretreatment. CD36high (open bars) and CD36low (shaded bars) cells were then replated and HUVEC proliferation and chemotaxis in response to VEGF was assessed in the presence and absence of TSP-1 for 24 hours. Neutralizing antibodies to CD36 were also used to determine whether altered sensitivity to TSP-1 was indeed dependent on CD36.
Figure 5.
HUVECs preconditioned to a CD36low phenotype become insensitive to anti-proliferative TSP-1 signaling via a CD36-dependent mechanism. A: Experimental design for testing TSP-1-mediated inhibition of angiogenic function in VEGF pretreated (CD36low, black shading) and control (CD36high, white shading) HUVECs. B: Bar graph of cell number after 24 hours of stimulation with 5 ng/ml of VEGF represented as a fold increase over stimulation with 2% serum. *Significantly different from CD36low cells in VEGF+TSP group and CD36high cells in both the VEGF and VEGF+TSP+anti-CD36 groups (P < 0.05) by Student’s t-test. C: Bar graph of HUVEC migration expressed as a percentage of maximum migration induced by VEGF (20 ng/ml). *Significantly different (P < 0.05) than same cell type in VEGF group by Student’s t-test. All values are means ± SD.
In the absence of TSP-1, VEGF-A elicited a significant and equivalent amount of proliferation for control (CD36high) and VEGF-pretreated (CD36low) HUVECs (Figure 5B). As expected, the proliferation of control (CD36high) cells was then significantly inhibited by TSP-1. However, TSP-1 was significantly less effective in inhibiting the proliferation of cells conditioned to a CD36low phenotype by VEGF-A. Blocking CD36 completely restored the proliferation of both CD36low and CD36high cells in the presence of TSP-1, indicating that anti-proliferative TSP-1 signaling was markedly attenuated in VEGF preconditioned cells because of reduced CD36 expression. TSP-1 also significantly inhibited endothelial chemotaxis stimulated by VEGF-A165 in CD36high cells; however, in contrast to the proliferation studies, the inhibitory action of TSP-1 was not attenuated in VEGF-A preconditioned CD36low cells (Figure 5C). Blocking CD36 had no effect on TSP-1-mediated chemotaxis inhibition, suggesting that, in this model, TSP-1 inhibits endothelial migration to VEGF primarily through a CD36-independent mechanism(s).
Discussion
CD36, a functional receptor for the anti-angiogenic protein TSP-1, is commonly thought to be constitutively expressed by microvascular endothelial cells. However, a major finding in this study is that CD36 is typically not detectable in capillary sprout endothelial cells during inflammation-induced angiogenesis. We observed this pattern of CD36 expression in a tissue in which CD36 and TSP-1 regulate angiogenesis; therefore, these results are consistent with the hypothesis that CD36 down-regulation in capillary sprout endothelial cells facilitates angiogenesis in the presence of TSP-1. To then explore whether the CD36low sprout phenotype could be regulated by extrinsic factors acting on sprouting endothelial cells, we exposed endothelial cells to VEGF, which is markedly up-regulated during inflammation-induced angiogenesis, and to the withdrawal of shear stress, which occurs when quiescent cells that are initially exposed to blood flow begin to sprout into the tissue. We found that both stimuli strongly down-regulate endothelial CD36 expression. Finally, we determined that endothelial cells that had been preconditioned to a CD36low phenotype with VEGF became insensitive to anti-proliferative TSP-1 signaling. Importantly, this loss of TSP-1 sensitivity was dependent on CD36 expression changes and not attributable to the VEGF-induced altered expression and/or function of another TSP-1 receptor(s). Ultimately, these results suggest that the regulation of endothelial cell CD36 expression is a novel mechanism through which VEGF stimulates and maintains endothelial proliferation in the presence of TSP-1, and they provide evidence that shear stress regulates microvessel sprouting and quiescence via control of endothelial CD36 expression.
Implications of Differential CD36 Expression in Inflammation-Induced Angiogenesis
We determined via immunofluorescence that CD36 is expressed ubiquitously by quiescent endothelial cells, but absent from most capillary sprout endothelial cells. Once sprouting ceased and the tissue was fully vascularized, endothelial CD36 expression was restored, suggesting that CD36 is transiently down-regulated in endothelial cells migrating into avascular tissue. Transient changes in CD36 expression have also been identified during the ovarian cycle30; however, this expression pattern was derived from whole tissue and not specific to endothelial cells.30 During tumor growth, CD36 is expressed by most microvessels31; however, sprout-specific changes in CD36 have not been reported.
Neovascularization is governed by the local balance of pro- and anti-angiogenic signals.7,8 CD36 plays a critical role in endothelial cell fate because it mediates the anti-angiogenic activity of TSP.18,19,20 Here, TSP-1/CD36 signaling during angiogenesis was assessed by applying neutralizing anti-CD36 or anti-TSP-1 antibodies to the mesentery at the time of mechanical irritation, when the endothelial cells are predominantly CD36+. Blocking either TSP-1 or CD36 accelerated vascular growth, consistent with numerous reports that TSP-1 is an endogenous angiogenesis inhibitor.32,33,34,35 When considered in the context of the in vivo CD36 expression pattern (Figure 1), these results provide evidence that the induction of CD36low capillary sprout endothelial cells sustains angiogenesis in a TSP-1-rich environment.
CD36 Regulation as a Mechanism through which VEGF Sustains Endothelial Cell Proliferation in the Presence of TSP-1
VEGF-A expression, which was enhanced by mechanical irritation (Figure 3), may be induced by inflammatory cytokines and has been implicated in chronic inflammation disorders.36,37 VEGF is known to elicit changes in several genes that impact angiogenesis,38 and we show here that VEGF causes a dose- and time-dependent decrease in CD36 mRNA and protein expression (Figure 4). Our immunolabeling studies show that VEGF is up-regulated throughout the mesenteric tissue (Figure 3, A–D), suggesting that both CD36+ and CD36− sprout cells are exposed to elevated VEGF. However, we have shown that CD36 is further decreased with longer VEGF exposure times, and this could explain why CD36+ sprouts tend to be shorter than CD36− sprouts. Specifically, if we assume an approximately constant sprouting speed, we can postulate that long sprouts are both typically older than short sprouts and usually CD36− because of a longer duration of VEGF exposure.
Our finding that VEGF induces a CD36low endothelial phenotype motivated us to explore the functional consequences of this phenotype and ultimately demonstrate that the attenuation of inhibitory TSP-1 signaling via CD36 down-regulation is a novel mechanism through which VEGF stimulates and sustains endothelial cells in the presence of TSP-1. Consistent with previous reports,10,11,20,39,40 the proliferation of control (CD36high) cells was abrogated by TSP-1; however, anti-proliferative TSP-1 signaling was inhibited in VEGF pretreated (CD36low) cells. In addition, because blocking CD36 negated the inhibitory effect of TSP-1, we showed that anti-proliferative TSP-1 signaling depended on CD36. Thus, the distinct responses to TSP-1 for the two endothelial cell populations were a direct consequence of differential CD36 expression. In contrast, differential CD36 expression did not influence anti-migratory TSP-1 signaling. Both CD36low and CD36high cells were susceptible to the inhibition of migration by TSP-1. Importantly, blocking CD36 induced no changes in TSP-1-mediated migration inhibition, indicating that anti-migratory TSP-1 signaling is CD36-independent. TSP-1 binds multiple receptors on endothelial cells, including several integrins, and β1 integrins are known to mediate endothelial responses to TSP-1.41,42 Specifically, the TSR domain of TSP-1, which contains the CD36 binding site, inhibits HUVEC migration to VEGF via β1 integrin.42 Therefore, it is not surprising that changes in CD36 expression did not affect endothelial cell migration in the presence of TSP-1.
Endothelial CD36 Expression, Shear Stress, and Microvessel Sprouting and Stability
Capillary sprout endothelial cells are removed from contact with blood flow as they migrate away from microvessels. Hemodynamic forces determine endothelial cell structure, function, and phenotype.23,24 We have shown that shear stress withdrawal leads to reduced CD36 expression (Figure 4), whereas low levels of steady shear induce a twofold increase in CD36 expression, consistent with a recent report.43 After preconditioning cells with shear for 24 hours, we found that exposing cells to static conditions for an additional 24 hours led to a 62% decrease in CD36 expression compared to cells that were exposed to shear continuously for 48 hours. Because CD36 levels were equal after 24 and 48 hours of shear, reduced CD36 expression after flow cessation was a direct consequence of shear withdrawal, rather than a time-dependent increase in CD36 from sustained shear exposure from 24 to 48 hours. Also, static control cells were kept in the flow device and exposed to recirculating media to ensure that CD36 expression changes were not attributable to secreted biochemical factors. Overall, our in vitro shear stress results are consistent with the hypothesis that the CD36low phenotype in sprout endothelial cells is regulated, at least in part, by exposure to blood flow. Furthermore, our in vivo observation that endothelial CD36 expression is fully restored after the tissue has been fully vascularized by newly formed capillaries that have presumably reacquired blood flow also suggests a role for hemodynamics in CD36 regulation. Future studies will be needed to determine whether CD36low shear-withdrawn endothelial cells exhibit the same reduced sensitivity to anti-proliferative TSP-1 signaling as VEGF-conditioned CD36low endothelial cells; however, the ability of shear stress to regulate endothelial CD36 expression in a manner that is entirely consistent with its in vivo expression pattern identifies CD36 as a potential molecular linkage between hemodynamic forces, capillary sprouting, and microvessel quiescence.
Footnotes
Address reprint requests to Richard J. Price, Ph.D., Associate Professor, Department of Biomedical Engineering, University of Virginia, Box 800759, UVA Health System, Charlottesville, VA 22908. E-mail: rprice@virginia.edu.
Supported by the National Institutes of Health (grant HL74082 to R.J.P.).
References
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Li DY, Sorensen LK, Brooke BS, Urness LD, Davis EC, Taylor DG, Boak BB, Wendel DP. Defective angiogenesis in mice lacking endoglin. Science. 1999;284:1534–1537. doi: 10.1126/science.284.5419.1534. [DOI] [PubMed] [Google Scholar]
- Anderson CR, Ponce AM, Price RJ. Absence of OX-43 antigen expression in invasive capillary sprouts: identification of a capillary sprout-specific endothelial phenotype. Am J Physiol. 2004;286:H346–H353. doi: 10.1152/ajpheart.00772.2003. [DOI] [PubMed] [Google Scholar]
- Robinson AP, White TM, Mason DW. MRC OX-43: a monoclonal antibody which reacts with all vascular endothelium in the rat except that of brain capillaries. Immunology. 1986;57:231–237. [PMC free article] [PubMed] [Google Scholar]
- Asch AS, Barnwell J, Silverstein RL, Nachman RL. Isolation of the thrombospondin membrane receptor. J Clin Invest. 1987;79:1054–1061. doi: 10.1172/JCI112918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Benjamin LE. The controls of microvascular survival. Cancer Metastasis Rev. 2000;19:75–81. doi: 10.1023/a:1026552415576. [DOI] [PubMed] [Google Scholar]
- Good DJ, Polverini PJ, Rastinejad F, Le Beau MM, Lemons RS, Frazier WA, Bouck NP. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci USA. 1990;87:6624–6628. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolsma SS, Volpert OV, Good DJ, Frazier WA, Polverini PJ, Bouck N. Peptides derived from two separate domains of the matrix protein thrombospondin-1 have anti-angiogenic activity. J Cell Biol. 1993;122:497–511. doi: 10.1083/jcb.122.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel T, Guo NH, Krutzsch HC, Blake DA, Hartman J, Mendelovitz S, Panet A, Roberts DD. Modulation of endothelial cell proliferation, adhesion, and motility by recombinant heparin-binding domain and synthetic peptides from the type I repeats of thrombospondin. J Cell Biochem. 1993;53:74–84. doi: 10.1002/jcb.240530109. [DOI] [PubMed] [Google Scholar]
- Iruela-Arispe ML, Lombardo M, Krutzsch HC, Lawler J, Roberts DD. Inhibition of angiogenesis by thrombospondin-1 is mediated by 2 independent regions within the type 1 repeats. Circulation. 1999;100:1423–1431. doi: 10.1161/01.cir.100.13.1423. [DOI] [PubMed] [Google Scholar]
- Volpert OV, Lawler J, Bouck NP. A human fibrosarcoma inhibits systemic angiogenesis and the growth of experimental metastases via thrombospondin-1. Proc Natl Acad Sci USA. 1998;95:6343–6348. doi: 10.1073/pnas.95.11.6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Fraipont F, Nicholson AC, Feige JJ, Van Meir EG. Thrombospondins and tumor angiogenesis. Trends Mol Med. 2001;7:401–407. doi: 10.1016/s1471-4914(01)02102-5. [DOI] [PubMed] [Google Scholar]
- Streit M, Velasco P, Riccardi L, Spencer L, Brown LF, Janes L, Lange-Asschenfeldt B, Yano K, Hawighorst T, Iruela-Arispe L, Detmar M. Thrombospondin-1 suppresses wound healing and granulation tissue formation in the skin of transgenic mice. EMBO J. 2000;19:3272–3282. doi: 10.1093/emboj/19.13.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiher FK, Volpert OV, Jimenez B, Crawford SE, Dinney CP, Henkin J, Haviv F, Bouck NP, Campbell SC. Inhibition of tumor growth by systemic treatment with thrombospondin-1 peptide mimetics. Int J Cancer. 2002;98:682–689. doi: 10.1002/ijc.10247. [DOI] [PubMed] [Google Scholar]
- Westphal JR. Technology evaluation: ABT-510. Abbott Curr Opin Mol Ther. 2004;6:451–457. [PubMed] [Google Scholar]
- Dawson DW, Pearce SF, Zhong R, Silverstein RL, Frazier WA, Bouck NP. CD36 mediates the in vitro inhibitory effects of thrombospondin-1 on endothelial cells. J Cell Biol. 1997;138:707–717. doi: 10.1083/jcb.138.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med. 2000;6:41–48. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]
- Guo N, Krutzsch HC, Inman JK, Roberts DD. Thrombospondin 1 and type I repeat peptides of thrombospondin 1 specifically induce apoptosis of endothelial cells. Cancer Res. 1997;57:1735–1742. [PubMed] [Google Scholar]
- Norrby KA, Jakobsson, Sorbo J. Mast-cell-mediated angiogenesis: a novel experimental model using the rat mesentery. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;52:195–206. doi: 10.1007/BF02889963. [DOI] [PubMed] [Google Scholar]
- Ponce AM, Price RJ. Angiogenic stimulus determines the positioning of pericytes within capillary sprouts in vivo. Microvasc Res. 2003;65:45–48. doi: 10.1016/s0026286202000146. [DOI] [PubMed] [Google Scholar]
- Garcia-Cardeña G, Comander J, Anderson KR, Blackman BR, Gimbrone MA. Biomechanical activation of vascular endothelium as a determinant of its functional phenotype. Proc Natl Acad Sci USA. 2001;98:4478–4485. doi: 10.1073/pnas.071052598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vaughn S, Blackman BR, Kamm RD, García-Cardeña G, Gimbrone MA. Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc Natl Acad Sci USA. 2004;101:14871–14876. doi: 10.1073/pnas.0406073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings NE, Simmers MB, McDonald OG, Wamhoff BR, Blackman BR. Atherosclerosis-prone hemodynamics differentially regulates endothelial and smooth muscle cell phenotypes and promotes pro-inflammatory priming. Am J Physiol. 2007;93:C1824–C1833. doi: 10.1152/ajpcell.00385.2007. [DOI] [PubMed] [Google Scholar]
- Mader JS, Smyth D, Marshall J, Hoskin DW. Bovine lactoferricin inhibits basic fibroblast growth factor- and vascular endothelial growth factor165-induced angiogenesis by competing for heparin-like binding sites on endothelial cells. Am J Pathol. 2006;169:1753–1766. doi: 10.2353/ajpath.2006.051229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai J, Jones MK, Tarnawski AS. Serum response factor is a critical requirement for VEGF signaling in endothelial cells and VEGF-induced angiogenesis. FASEB J. 2004;18:1264–1266. doi: 10.1096/fj.03-1232fje. [DOI] [PubMed] [Google Scholar]
- Williams CK, Li JL, Murga M, Harris AL, Tosato G. Up-regulation of the Notch ligand Delta-like 4 inhibits VEGF-induced endothelial cell function. Blood. 2006;107:931–939. doi: 10.1182/blood-2005-03-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tressel SL, Huang RP, Tomsen N, Jo H. Laminar shear inhibits tubule formation and migration of endothelial cells by an angiopoietin-2 dependent mechanism. Arterioscler Thromb Vasc Biol. 2007;27:2150–2156. doi: 10.1161/ATVBAHA.107.150920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrik JJ, Gentry PA, Feige JJ, LaMarre J. Expression and localization of thrombospondin-1 and -2 and their cell-surface receptor, CD36, during rat follicular development and formation of the corpus luteum. Biol Reprod. 2002;67:1522–1531. doi: 10.1095/biolreprod.102.007153. [DOI] [PubMed] [Google Scholar]
- Sund M, Hamano Y, Sugimoto H, Sudhakar A, Soubasakos M, Yerramalla U, Benjamin LE, Lawler J, Kieran M, Shah A, Kalluri R. Function of endogenous inhibitors of angiogenesis as endothelium-specific tumor suppressors. Proc Natl Acad Sci USA. 2005;102:2934–2939. doi: 10.1073/pnas.0500180102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpert OV, Zaichuk T, Zhou W, Reiher F, Ferguson TA, Stuart PM, Amin M, Bouck NP. Inducer-stimulated Fas targets activated endothelium for destruction by anti-angiogenic thrombospondin-1 and pigment epithelium-derived factor. Nat Med. 2002;8:349–357. doi: 10.1038/nm0402-349. [DOI] [PubMed] [Google Scholar]
- DiPietro LA, Nebgen DR, Polverini PJ. Down-regulation of endothelial cell thrombospondin 1 enhances in vitro angiogenesis. J Vasc Res. 1994;31:178–185. doi: 10.1159/000319585. [DOI] [PubMed] [Google Scholar]
- Chen D, Asahara T, Krasinski K, Witzenbichler B, Yang J, Magner M, Kearney M, Frazier WA, Isner JM, Andrés V. Antibody blockade of thrombospondin accelerates reendothelialization and reduces neointima formation in balloon-injured rat carotid artery. Circulation. 1999;100:849–854. doi: 10.1161/01.cir.100.8.849. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Manzaneque JC, Lane TF, Ortega MA, Hynes RO, Lawler J, Iruela-Arispe ML. Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proc Natl Acad Sci USA. 2001;98:12485–12490. doi: 10.1073/pnas.171460498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch AE, Harlow LA, Haines GK, Amento EP, Unemori EN, Wong WL, Pope RM, Ferrara N. Vascular endothelial growth factor. A cytokine modulating endothelial function in rheumatoid arthritis. J Immunol. 1994;152:4149–4156. [PubMed] [Google Scholar]
- Detmar M, Brown LF, Claffey KP, Yeo KT, Kocher O, Jackman RW, Berse B, Dvorak HF. Overexpression of vascular permeability factor/vascular endothelial growth factor and its receptors in psoriasis. J Exp Med. 1994;180:1141–1146. doi: 10.1084/jem.180.3.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- Taraboletti G, Roberts D, Liotta LA, Giavazzi R. Platelet thrombospondin modulates endothelial cell adhesion, motility, and growth: a potential angiogenesis regulatory factor. J Cell Biol. 1990;111:765–772. doi: 10.1083/jcb.111.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagavandoss P, Wilks JW. Specific inhibition of endothelial cell proliferation by thrombospondin. Biochem Biophys Res Commun. 1990;170:867–872. doi: 10.1016/0006-291x(90)92171-u. [DOI] [PubMed] [Google Scholar]
- Calzada MJ, Zhou L, Sipes JM, Zhang J, Krutzsch HC, Iruela-Arispe ML, Annis DS, Mosher DF, Roberts DD. Alpha4beta1 integrin mediates selective endothelial cell responses to thrombospondins 1 and 2 in vitro and modulates angiogenesis in vivo. Circ Res. 2004;94:462–470. doi: 10.1161/01.RES.0000115555.05668.93. [DOI] [PubMed] [Google Scholar]
- Short SM, Derrien A, Narsimhan RP, Lawler J, Ingber DE, Zetter BR. Inhibition of endothelial cell migration by thrombospondin-1 type-1 repeats is mediated by beta1 integrins. J Cell Biol. 2005;168:643–653. doi: 10.1083/jcb.200407060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhu Y, Rannou F, Lee TS, Formentin K, Zeng L, Yuan X, Wang N, Chien S, Forman BM, Shyy JY. Laminar flow activates peroxisome proliferator-activated receptor-gamma in vascular endothelial cells. Circulation. 2004;110:1128–1133. doi: 10.1161/01.CIR.0000139850.08365.EC. [DOI] [PubMed] [Google Scholar]