Abstract
Interleukin (IL)-33 is a novel member of the IL-1 family of cytokines that promotes Th2 responses in lymphocytes as well as the activation of both mast cells and eosinophils via the ST2 receptor. Additionally, IL-33 has been proposed to act as a chromatin-associated transcriptional regulator in both endothelial cells of high endothelial venules and chronically inflamed vessels. Here we show that nuclear IL-33 is expressed in blood vessels of healthy tissues but down-regulated at the earliest onset of angiogenesis during wound healing; in addition, it is almost undetectable in human tumor vessels. Accordingly, IL-33 is induced when cultured endothelial cells reach confluence and stop proliferating but is lost when these cells begin to migrate. However, IL-33 expression was not induced by inhibiting cell cycle progression in subconfluent cultures and was not prevented by antibody-mediated inhibition of VE-cadherin. Conversely, IL-33 knockdown did not induce detectable changes in either expression levels or the cellular distribution of either VE-cadherin or CD31. However, activation of endothelial cell cultures with either tumor necrosis factor-α or vascular endothelial growth factor and subcutaneous injection of these cytokines led to a down-regulation of vascular IL-33, a response consistent with both its rapid down-regulation in wound healing and loss in tumor endothelium. In conclusion, we speculate that the proposed transcriptional repressor function of IL-33 may be involved in the control of endothelial cell activation.
Interleukin (IL)-33 (also known as C9ORF26, DVS27,1 NF-HEV,2 and IL-1F113) is a novel member of the IL-1 family of proinflammatory cytokines that also includes IL-1α, IL-1β, and IL-18. IL-33 expression has been found in a broad specter of tissues and cells.1,2,3,4,5,6 Like IL-1 and IL-18, IL-33 is synthesized as a 31-kDa precursor and may be cleaved by caspase-1 in vitro to form a mature 18-kDa protein.3 Mature, recombinant IL-33 signals through the IL-1 receptor-related protein ST2 (also known as IL-33Rα, IL1RL1, T1, DER-4, Fit-1, or IL1R4) and involves heterodimerization with the IL-1 receptor accessory protein (IL-1RAcP)7,8 as well as activation of nuclear factor (NF)-κB and MAP kinases.3,5 Mature IL-33 was recently found to drive T-helper type 2 (Th2) responses in lymphocytes3; to act as a Th2 chemoattractant9; and to induce blood eosinophilia, splenomegaly, increased serum levels of IgE, IgA, IL-5, and IL-13, as well as severe pathology in mucosal organs on IL-33 treatment in mice.3 Moreover, mature IL-33 also induces maturation and proinflammatory cytokine production in mast cells,7,10,11,12 degranulation and survival in eosinophils,13 as well as a reduction in the development of atherosclerosis.6
All biological effects of IL-33 described to this point appear associated with its mature and, in most cases, recombinant 18-kDa form. However, in common with IL-1α, the full-length precursor IL-33 can also act as a nuclear factor with transcriptional regulatory properties.4 Although precursor IL-1α acts as a proinflammatory activator of transcription,14 possibly by interacting with histone acetyltransferases involved in transcriptional activation,15,16 precursor IL-33 associates with heterochromatin and has transcriptional repressor properties,4 most likely associated with its evolutionary conserved homeodomain-like helix-turn-helix motif in its N-terminus.2,4
To date, nuclear IL-33 expression has been described in the endothelial cells of high endothelial venules in secondary lymphoid organs,2 in chronically inflamed venules in tissues from patients with rheumatoid arthritis and Crohn’s disease,4 and in lesions of atherosclerosis.6 To get a better understanding of the expression of nuclear IL-33 in vascular endothelial cells, we initiated an immunohistochemical screen in several different tissues, and found that nuclear, endothelial IL-33 is generally expressed in blood vessels of normal tissues. Moreover, activation of endothelial cells appeared associated with a down-regulation of IL-33, because it was rapidly down-regulated in the proinflammatory/angiogenic environment of wound healing and virtually undetectable in the angiogenic/immature vessels of tumors. Accordingly, IL-33 was induced in high-density endothelial cell cultures but down-regulated when cells broke out of confluence or when exposed to tumor necrosis factor (TNF)-α, IL-1β, or vascular endothelial growth factor (VEGF).
Materials and Methods
Reagents
Recombinant human (rh) or rat (rr) IL-1β, epidermal growth factor, basic fibroblast growth factor, TNF-α, and interferon (IFN)-γ were obtained from R&D Systems (Abingdon, UK); VEGF from Peprotech (London, UK); and rrIL-33 from Alexis (Lausen, Switzerland). Fetal bovine serum, gentamicin, fungizone, l-glutamine, MCDB 131, and Opti-MEM I were purchased from Invitrogen Life Technologies (Paisley, UK); gelatin, citraconic anhydride and fumagillin from Sigma-Aldrich (Oslo, Norway); trypsin-EDTA from BioWhittaker (Walkersville, MD); and siPORTAmine from Ambion (now Applied Bioystems, Oslo, Norway).
Cell Culture
Umbilical cords were obtained from the Department of Gynecology and Obstetrics at the Rikshospitalet Medical Center following a protocol approved by the Regional Committee for Research Ethics (S-05152). Human umbilical vein-derived endothelial cells (HUVECs) were isolated as described by Jaffe and colleagues17 and cultured in MCDB 131 containing 7.5% fetal bovine serum, 10 ng/ml epidermal growth factor, 1 ng/ml basic fibroblast growth factor, 1 μg/ml hydrocortisone, 50 μg/ml gentamicin, and 250 ng/ml fungizone. The cells were maintained at 37°C in humid 95% air/5% CO2 atmosphere and split at a ratio of 1:3 before reaching confluence. The cultures were used at passage level one to five. Medium was changed when the cells reached confluence (usually 3 to 4 days after splitting) and the experiment started 1 day thereafter (cells were then superconfluent). Superconfluent HUVEC monolayers were activated for the indicated times and concentrations with rhIFN-γ, rhTNF-α, rhIL-1β, or rhVEGF. Endothelial cells from human skin, nasal polyps, and intestine were cultured as described elsewhere.18,19,20
Cell Cycle Inhibition
To study the effect of cell cycle inhibition in confluent cells, monolayers were either treated with fumagillin in complete medium for 16 hours or full growth medium was replaced by growth factor- and serum-free MCDB 131 for 24 hours. To inhibit the cell cycle in subconfluent cells, they were seeded in complete growth medium at a density of 1.9 × 104 cells/cm2, either in the presence of fumagillin (16 hours) or cells were allowed to attach for 4 hours before replacing the complete medium by growth factor- and serum-free MCDB 131 and incubating them for another 24 hours. To assess the percentage of cells positive for cell-cycle markers or IL-33, fixed cells were double stained for IL-33 and Ki-67 or hyperphosphorylated retinoblastoma protein (ppRb). The fraction of positive cells (defined as having a homogenous nuclear signal) were enumerated and normalized by setting the control value to 100 in each independent experiment.
Immunocytochemistry and Histochemistry
Affinity-purified IL-33Nter rabbit polyclonal antibody was generated by Eurogentec (Seraing, Belgium), using a peptide antigen derived from the first 15 amino acids of human IL-33 (MKPKMKYSTNKISTA). Other primary antibodies and all working concentrations are specified in Table 1. Fluorescein isothiocyanate-conjugated Ulex europaeus agglutinin I (UEA) (used at 5 μg/ml), biotinylated horse anti-mouse IgG (used at 7.5 μg/ml), and biotinylated goat anti-rabbit IgG (used at 7.5 μg/ml) antibodies were purchased from Vector Laboratories (Burlingame, CA), streptavidin-Cy2 conjugate (used at 1 μg/ml) was purchased from Amersham Biosciences (now GE Health Care, Piscataway, NJ), Cy3-conjugated donkey anti-mouse IgG (used at 2 μg/ml) and streptavidin-Cy3 (used at 1.5 μg/ml) were from Jackson ImmunoResearch Laboratories (Newmarket, UK), and Hoechst nuclear dye (used at 0.5 μg/ml) was from Invitrogen Life Technologies.
Table 1.
Primary Antibodies Used in This Study
| Specificity | Designation | Working concentration | Specification | Source |
|---|---|---|---|---|
| IL-33 | IL-33 Nter | 1.8 μg/ml | Rabbit | Eurogentec |
| IL-33 | Nessy-1 | 1 μg/ml | Mouse IgG1 | Alexis |
| IL-33 | ALX-210-447 | 1 μg/ml | Rabbit | Alexis |
| IL-33 | ALX-210-993 | 1 μg/ml | Rabbit | Alexis |
| CD31 | HEC7 | 1 μg/ml | Mouse IgG2a | Fitzgerald |
| CD31 | RB-10333 | 0.4 μg/ml | Rabbit | LabVision |
| PCNA | PC10 | 1.25 μg/ml | Mouse IgG2a | DAKO |
| Phospho-Rb (Ser807/811) | #9308 | 1:100 | Rabbit | Cell signaling |
| Ki67 | MIB-1 | 10 μg/ml | Mouse IgG1 | DAKO |
| Ki67 | ab15580 | 5 μg/ml | Rabbit | Abcam |
| Lymphatic EC | D2-40 | 0.26 μg/ml | Mouse IgG1 | DAKO |
| VE-cadherin | 4.8.G | 1:1000 | Mouse IgG1 | O. Bakke |
| VE-cadherin | BV9 | 10 μg/ml | Mouse IgG2a | Hycult biotechnology |
| Actin | sc-8432 | 1:500 | Mouse IgG1 | Santa Cruz |
| Histone H1 | sc-10806 | 1 μg/ml | Rabbit | Santa Cruz |
| Irrelevant control | H0892 | Matched | Rabbit | Sigma |
| Irrelevant control | MOPC 21 | Matched | Mouse IgG1 | Sigma |
HUVECs grown on Lab-Tek chamber slides (Nalge Nunc International, Hereford, UK) coated with 1% (w/v) gelatin type A from porcine skin, were briefly submerged in prewarmed phosphate-buffered saline (PBS) (37°C) and either fixed for 10 minutes in methanol and air-dried or prewarmed 4% paraformaldehyde (37°C, pH 7.4) for 10 minutes before washing 2 × 5 minutes in PBS. Formalin- or methanol-fixed, paraffin-embedded tissue samples are part of the diagnostic biobank at the Division of Pathology and were used according to guidelines approved by the Regional Committee for Research Ethics (S-05152). Tissue sections were deparaffinized using standard laboratory procedures. Fixed cells or tissue sections were boiled for 20 minutes in PBS containing 0.05% citraconic anhydride (pH 7.4), incubated with primary antibodies diluted in PBS with 0.1% saponin for permeabilization overnight at 4°C, followed by secondary and tertiary reagents for 1.5 hours each at room temperature. Irrelevant concentration-matched primary antibodies were used as negative controls. Double stainings using UEA were performed by adding the lectin to the secondary antibody or streptavidin incubation step. Double stainings with mouse and rabbit reagents were performed by mixing the primary antibodies and mixing fluorochrome-conjugated reagents, respectively. Microscopy was performed with a Nikon (Tokyo, Japan) Eclipse E-800 fluorescence microscope equipped with Nikon Plan-Fluor objective lenses and an F-VIEW digital camera controlled by AnalySIS 3.2 software (Soft Imaging System, Münster, Germany).
Quantitative Polymerase Chain Reaction (PCR)
Total RNA from cytokine-treated or siRNA-transfected cells was isolated using the RNeasy mini kit with on-column DNase treatment according to instructions of the manufacturer (Qiagen, Oslo, Norway). Total RNA was reverse-transcribed using Oligo(dT) and Superscript III reverse transcriptase (Invitrogen Life Technologies). Gene transcripts were quantified by real-time PCR using the Mx3000P system from Stratagene (now Agilent Technologies, La Jolla, CA). Transcript levels of IL-33 were normalized against transcript levels for GAPDH and HPRT. Primer sequences used are listed in Table 2.
Table 2.
Primers Used for Quantitative PCR
| Forward primer | Reverse primer | |
|---|---|---|
| IL-33 no. 1 | 5′-TGTCAACAGCAGTCTACTGTGGAGTGCT-3′ | 5′-GCAAAAGTAATGGATTGATCATTGTATGTGCT-3′ |
| IL-33 no. 2 | 5′-CAGACCAGGCCTTCTTTGTCCTTCAT-3′ | 5′-CTAAGTTTCAGAGAGCTTAAACAAGATATTTTCAGTACA-3′ |
| GAPDH | 5′-TGTTCGACAGTCAGCCGCATCTTCT-3′ | 5′-TGATGGCAACAATATCCACTTTACCAGAGTT-3′ |
| HPRT | 5′-AATACAAAGCCTAAGATGAGAGTTCAAGTTGAGTT-3′ | 5′-TTAGGAATGCAGCAACTGACATTTCTAAAGTAC-3′ |
Nuclear Fractionation
HUVECs were lysed in a buffer containing 0.1% Nonidet P-40, 10 nmol/L NaCl, 5 mmol/L MgCl2, 10 nmol/L NaH2PO4 pH = 7.4, 65 mmol/L Na-orthovanadate (S-6508, Sigma-Aldrich), and protease inhibitor cocktail (P-8340, 1:100; Sigma-Aldrich) and centrifuged at 900 × g for 10 minutes (4°C). Supernatant fluid containing the cytoplasmic fraction was harvested and the pellet containing the nuclear fraction was resuspended in a buffer containing 1 mmol/L EDTA, 3.5% sodium dodecyl sulfate, 10% glycerol, and 70 mmol/L Tris, pH = 6.8. Nuclear and cytoplasmic fractions were sonicated (4 × 10 seconds, pulse duration 0.8/second and 20% output of 400 W) and the protein concentration measured as described below. Samples were boiled in loading buffer (300 mmol/L Tris-HCl, pH 6.8, 2% sodium dodecyl sulfate, 0.1% bromphenol blue, 10% glycerol, and 50 mmol/L dithiothreitol) and 20 μg of protein were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Protein Extraction and Western Blot
Total protein was harvested from HUVECs by lysing them in 2× Laemmli buffer (4% sodium dodecyl sulfate, 20% glycerol, 10% 2-mercaptoethanol, 0.004% bromphenol blue, 0.125 mol/L Tris-HCl) protease inhibitor cocktail (P-8340, 1:100; Sigma-Aldrich), diluting the lysates 1:1 in PBS and subsequently boiling them for 10 minutes. Protein concentrations were determined using the RC/DC protein assay kit (Bio-Rad, Oslo, Norway) and up to 20 μg of protein were loaded per lane. For visualization of protein loading, membranes were stained with Ponceau S solution [0.1% (w/v) Ponceau S, 5% acetic acid in ddH2O]. The protein was blotted to a nitrocellulose membrane (Hybond-ECL, RPN303D; Amersham Biosciences). After blocking in PBS, Tween 0.05%, and 5% milk, IL-33 protein was detected by sequential incubation of blots with mouse anti-human IL-33 (Nessy-1, 1:1000), biotinylated horse anti-mouse IgG (BA-2000, 3 μg/ml; Vector Laboratories) and horseradish peroxidase-conjugated streptavidin (21124, 0.04 μg/ml; Pierce, Cramlington, UK). After IL-33 detection, the blots were reincubated with mouse anti-human actin (sc-8432, 1:500; Santa Cruz, Heidelberg, Germany) followed by the above-described secondary and detection methods. The rabbit anti-human histone H1 (FL-219, 1 μg/ml, sc-10806; Santa Cruz) was used as a loading control for nuclear extracts and detected by sequential incubation of blots with horseradish peroxidase-conjugated goat anti-rabbit IgG (NA934V, 1:3000; Amersham Biosciences). Horseradish peroxidase signal was then detected by enhanced chemiluminescence substrate (no. 32106, Pierce) and analyzed on a Kodak Image Station 4000R (Eastman-Kodak, Rochester, NY). The intensity of protein bands was quantified using Kodak Molecular Imaging Software (version 4.0.5).
Blocking of VE-Cadherin
Single cell suspensions of HUVECs harvested at subconfluence were incubated with 10 μg/ml of the monoclonal antibody to VE-cadherin (BV9) or vehicle (PBS) in the growth medium before seeding into Lab-Tek chamber slides (Nalge Nunc International) coated with 1% (w/v) gelatin type A from porcine skin. Medium with antibody or vehicle was changed daily until cells reached confluence. Slides were methanol-fixed and subsequently co-stained for VE-cadherin and IL-33 using another murine antibody to VE-cadherin (clone 4.8.G) and rabbit polyclonal antibody to IL-33 (IL-33Nter), followed by a biotinylated goat anti-rabbit IgG antibody (Vector Laboratories) and Cy3-conjugated goat anti-mouse IgG1 (Southern Biotechnology, Birmingham, AL) and Cy2-conjugated streptavidin (Jackson ImmunoResearch Laboratories). For consistency throughout the figures, the red and green channels in Figure 3 (below) were reversed.
Figure 3.
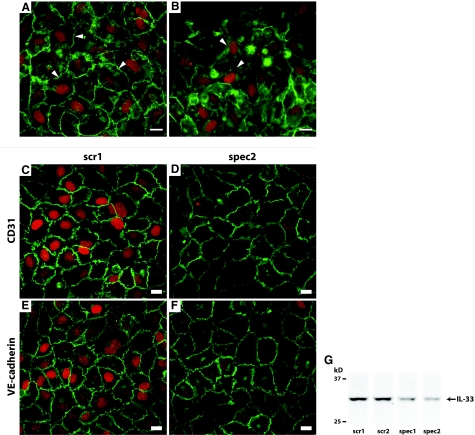
Inhibition of the junctional protein VE-cadherin does not alter the expression level of IL-33 and knockdown of IL-33 does not alter the cellular distribution or expression level of junctional proteins CD31 or VE-cadherin. Immunocytochemical double staining for IL-33 (IL-33Nter, red) and VE-cadherin (mouse IgG1, green) in HUVECs preincubated with vehicle (PBS, A) or anti-VE-cadherin (IgG2a, B) from point of seeding until fixation. Pictures were obtained using identical exposure times and image-enhancement parameters. Arrowheads point to areas of intercellular contact. Note that cells in B with redistribution or lowered expression level of VE-cadherin maintained expression of IL-33. C and D: Immunocytochemical double staining for IL-33 (IL-33Nter, red) and CD31 (green) in HUVECs transfected with mock (C) or specific siRNA (D). E and F: Immunocytochemical double staining for IL-33 (red) and VE-cadherin (green) in HUVECs transfected with mock (E) or specific siRNA (F). Pictures were obtained using identical exposure times and image enhancement parameters. G: Western blot of IL-33 in cell lysates of HUVEC monolayers transfected with either scrambled siRNA controls (scr1 and scr2) or concentration-matched IL-33 targeting siRNA (spec1 and spec2). Shown are lanes from the same experiment, which was independently repeated twice with similar results. Scale bars = 10 μm.
IL-33 Knockdown
Silencer predesigned siRNA for IL-33 (siRNA ID no. 131654, 131655, 35368, 35460, 35551) or negative controls (Silencer negative control siRNAs no. 1, 2, 3) were obtained from Ambion. Single cell suspensions of HUVECs harvested at subconfluence were incubated with a precomplexed mixture of siPORTAmine (Ambion) and 40 nmol/L siRNA oligos in OPTI-MEM (Invitrogen Life Technologies) and plated at a density of 7.2 × 104 cells/cm2. After 6 hours the medium was replaced by regular growth medium. At 24 hours after start of transfection the cells were supplied with fresh medium and harvested or fixed after an additional 24 hours.
Animal Experiments
Inbred BD-IX female rats (Charles River Laboratories, Lyon, France), age 6 months, were caged in groups of three animals in Macrolon III cages (Techniplast S.p.a, Buguggiate, Varese, Italy). Animal care and protocol were in accordance with national legislation and institutional guidelines. A combination of fentanyl/fluanisone and midazolam was used for anesthesia. To assess the fate of endothelial IL-33 during wound healing, a boat-shaped excision (1 × 0.5 cm) was made between the ears using scissors and closed with one suture. Biopsy specimens were harvested 1, 2, 4, 6, and 11 days after injury and the tissue samples fixed in formalin for 24 hours at 4°C before embedding in paraffin using standard procedures.
To analyze the in vivo response of IL-33 to TNF-α and VEGF, carrier-free recombinant rat (rr) cytokines or PBS controls were injected subcutaneously on the back. To trace the site of injection, Indian fountain ink (1:200; Pelikan, Hannover, Germany) was added to the vehicle before injection. Leukocyte infiltration served as positive control for rrTNF-α injections. Extravasation of Evans Blue dye served as a positive control for the injection of rrVEGF: one animal received 0.5 ml of a 1% Evans Blue in PBS solution intravenously injected 20 minutes before the rrVEGF injection. Overall expression levels of vascular IL-33 were assessed in a blinded manner after immunostaining of fixed specimens.
Results
IL-33 Is Generally Expressed in Blood Vessels of Healthy Tissue
Although IL-33 has been reported to be preferentially expressed in endothelial cells of high endothelial venules2 and more recently in vessels of chronic inflammation4 and atherosclerosis,6 a detailed assessment of expression in healthy tissues using several different antibodies (see Table 1 and supplemental Figure S1 at http://ajp.amjpathol.org) indicated to us that IL-33 is most likely globally present in endothelial cells of all blood vessels. By co-staining a collection of tissue samples from normal human organs for IL-33 and the pan-vascular marker Ulex europaeus agglutinin I (UEA), we found that IL-33 was expressed in endothelial cell nuclei of most vessels in normal skin (Figure 1A), small intestine (Figure 1B), umbilical vein (Figure 1C), and lung (Figure 1D) as well as in colon, mammary gland, and kidney (all visible in control areas of Figure 4, see below). Occasionally, we observed scattered thin-walled vessels that expressed only low or almost undetectable levels of IL-33 in their endothelial nuclei and considered them to be of lymphatic origin based on co-staining with the lymphatic marker D2-40 (data not shown). The staining pattern of all antibodies to IL-33 was abolished by preincubating them with recombinant hIL-33 or the respective IL-33-derived peptide (data not shown).
Figure 1.
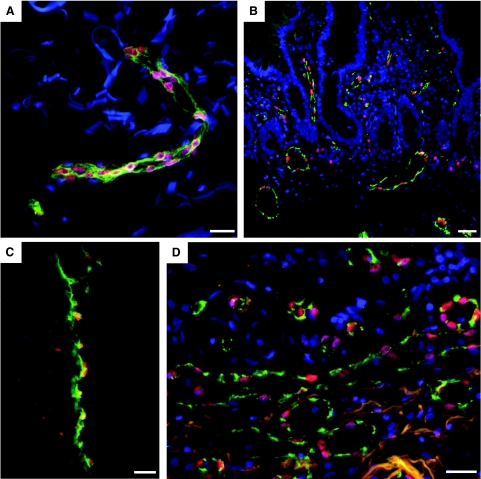
IL-33 is globally expressed in nuclei of vascular endothelium in normal human tissues. Endothelial cells in vessels of human skin (A), small intestine (B), umbilical vein (C), and lung (D) were stained for IL-33 (Nessy-1, red) and with Ulex europaeus agglutinin I (A, C, and D; green) or CD31 (B, green) and Hoechst dye (blue) to visualize endothelial cells and cell nuclei, respectively. Three-color fluorescence micrograph of fixed and permeabilized tissue sections are shown. Note of presence of IL-33 positive single tissue resident cells in (B) and (C). Scale bars = 50 μm.
Figure 4.
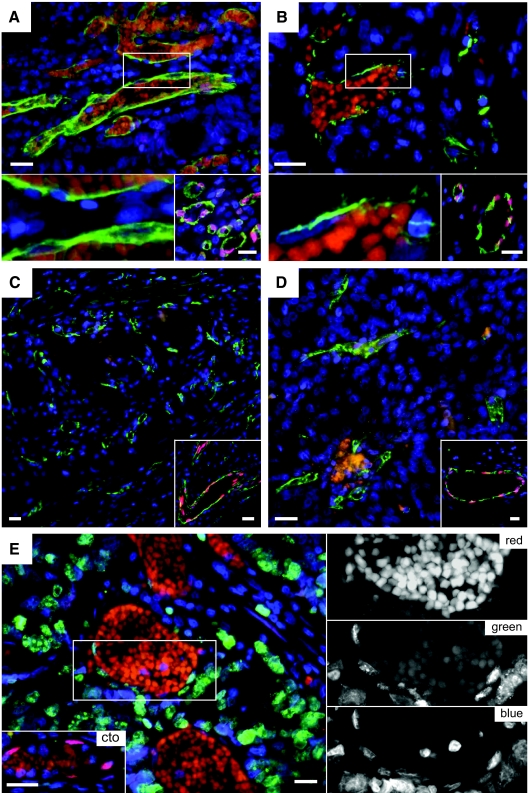
Vascular IL-33 expression is lost in tumor vessels. Endothelial cells in vessels of human colon adenocarcinoma (A), ductal mammary carcinoma (B), renal clear cell carcinoma (C), and renal papillary carcinoma (D) were stained for IL-33 (Nessy-1, red) and with Ulex europaeus lectin (green) and Hoechst dye (blue) to visualize endothelial cells and cell nuclei, respectively. (For consistency throughout the figures, the red and green channels in Figure 4 were reversed.) The bottom left panels in A and B show high-power magnifications of the respective boxed areas. Note presence of autofluorescent intravascular red blood cells in A and B as well as lack of IL-33 signal in endothelial cell nuclei. The bottom right panels in A to D and bottom left panel in E show IL-33-positive vessels in tissue surrounding the tumor in the same section. E: Human colon adenocarcinoma (different sample from A) was stained for IL-33 (Nessy-1, red), Ki-67 (green), and Hoechst dye (blue). Autofluorescent erythrocytes indicate blood vessels. Right: High-power magnifications of the boxed area in E separating the different colors. Note the lack of IL-33 signal in Ki-67-positive endothelial nuclei. Scale bars = 50 μm.
IL-33 Is Induced by Confluence and Lost on Cell Migration
Observing that IL-33 is a general constituent of resting endothelial cells in vivo, we aimed at defining in vitro conditions that would mimic properties of resting endothelium including the expression of IL-33. Careful assessment of IL-33 expression in cultured HUVECs revealed that IL-33 was absent in subconfluent cultures, but induced as the cell density increased and peaked when the cells formed a compact, high-density monolayer (Figure 2, A–C). At this point most of the cells featured a considerably higher ratio of nuclear versus cytoplasmic area because of space constrictions (Figure 2C). We defined cell monolayers of such density and appearance as superconfluent. Induction of IL-33 was not a feature particular to HUVECs, because it could also be induced in cultured endothelial cells from skin, nasal polyps, and small intestine (data not shown).
Figure 2.
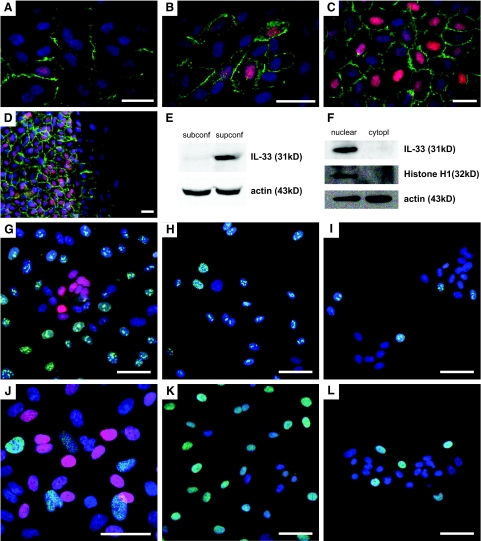
IL-33 in HUVECs responds to cell density signals and is localized to the nucleus in nonproliferating, superconfluent cells. A–D: Monolayers of HUVECs stained for IL-33 (red) using IL-33Nterm (A–C) and Nessy-1 (D). CD31 is stained in green and cell nuclei are visualized by Hoechst dye (blue). A and B: HUVECs induce expression of nuclear IL-33 and enhance the localization of CD31 to the intercellular areas when cell density increases. C: Almost all cells are IL-33-positive in a superconfluent monolayer. D: Superconfluent HUVEC monolayer 4 hours after scratch wounding. The red signal in the cytoplasm cannot be distinguished from the signal obtained with irrelevant antibodies (data not shown). Note loss of nuclear IL-33 and reduction of CD31 (green) in cells that migrate into the open area (right half of image). E and F: Western blot for IL-33 detection in whole cell lysates of subconfluent (subconf) or superconfluent (supconf) cells (E) or nuclear versus cytoplasmic (cytopl) fraction of a superconfluent HUVEC monolayer (F). Monolayers of HUVECs stained for IL-33 (IL-33Nter in G, Nessy-1 in H–L; all red), Ki-67 (G--I, green), and ppRb (J–L, green). Endothelial nuclei are visualized by Hoechst dye (blue). Note the lack of IL-33 expression in serum and growth factor starved cells in I and L. Scale bars: 25 μm (A–D); 30 μm (G–L).
The establishment of junctions as estimated by the translocation of CD31 to cell boundaries21 correlated with the induction of IL-33 because only cells with a pronounced CD31 staining at their cell-cell boundaries expressed IL-33 (Figure 2, A–C). Because IL-33 expression gets induced in the transition from confluence to superconfluence, we asked how the reverse change would affect IL-33 expression. To this end, we removed a 2-mm broad band of cells from superconfluent HUVEC monolayers and analyzed the cells that migrated into the open space. Within 4 hours, cells were observed that had left the confluent monolayer and down-regulated IL-33 to levels indistinguishable from background levels (Figure 2D and data not shown). Western blotting confirmed the induction of IL-33 (detection of a 31-kDa band) in total cell lysates of confluent HUVECs but not in lysates of subconfluent cultures (Figure 2E). Nuclear fractionation of superconfluent HUVECs confirmed that IL-33 is predominantly located in the nucleus because IL-33 was undetectable in the cytoplasmic fraction (Figure 2F). Moreover, none of the lysates showed expression of the cleaved, 18-kDa mature form of IL-333 (see below for whole cell lysates; also data not shown).
Establishment of a confluent monolayer also correlated with the loss of proliferative activity in HUVECs. To test whether expression of IL-33 might correlate with cell cycle progression, we co-stained for IL-33 and the proliferation marker Ki-6722 and observed that they were mutually exclusive (Figure 2G). Similar results were obtained with co-staining for IL-33 and proliferating cell nuclear antigen (proliferative cell nuclear antigen, data not shown).23 Moreover, we also found that expression of ppRb (hyperphosphorylated Rb protein, which allows progression from G1 to S phase24) and IL-33 were mutually exclusive (Figure 2J). To study further the influence of the cell cycle on IL-33 expression, proliferation was inhibited either by withdrawal of serum and growth factors or by addition of fumagillin, an Aspergillus-derived antibiotic that inhibits endothelial cell proliferation,25,26 most likely in late G1.27 Starvation of subconfluent cells reduced the fraction of Ki-67+ cells (Figure 2, H and I) by 66 to 87% (range of three independent experiments) and that of ppRb+ cells (Figure 2, K and L) by 73 to 90% (range of three independent experiments) but failed to induce nuclear IL-33. Moreover, fumagillin used in the range of 1 to 1000 nmol/L dose dependently reduced the fraction of Ki-67+ cells by 45 to 59% (range of three independent experiments at 100 nmol/L) and that of ppRb+ cells by 38 to 55% (range of three independent experiments at 100 nmol/L), respectively, again revealing no induction of nuclear IL-33 (data not shown). Moreover, fumagillin treatment could not rescue the down-regulation of IL-33 seen after reseeding IL-33-positive cells from superconfluent monolayers at low density, indicating that inhibition of proliferation does not permit maintained expression of IL-33 when contact inhibition is lost (data not shown). Inhibition of proliferation in superconfluent monolayers also reduced the fraction of Ki-67+ or ppRb+ cells but at the same time changed the density of the monolayer compared to control treatment and thus prevented an independent assessment of cell cycle on IL-33 expression in this setting.
We next examined whether VE-cadherin, a known molecular mediator of contact inhibition, might be involved in the induction of IL-33. Addition of the inhibitory antibody BV9 to HUVECs induced a redistribution of VE-cadherin away from the junctions, as described by Corada and colleagues,28 but failed to visibly reduce the expression level of IL-33 (Figure 3). Conversely, we asked if knockdown of IL-33 would have an effect on the establishment of cell-cell junctions. Using siRNA amounts below the toxicity level as judged from control oligos, we achieved a maximum reduction of IL-33 protein levels down to ∼15% of the control values using either single or pooled siRNA oligos (Figure 3G). Knockdown of IL-33, however, did not alter the expression levels or cellular distribution of VE-cadherin (Figure 3, E and F). Additionally, knockdown of IL-33 also failed to alter the distribution of CD31, another marker of junctional integrity (Figure 3, C and D). Taken together, these data imply that cultured HUVECs express IL-33 when the cells are nonproliferating and organized in a confluent, endothelial monolayer. IL-33 expression is apparently not regulated by the homophilic binding of VE-cadherin, nor does loss of IL-33 induce changes in the expression of VE-cadherin or CD31.
IL-33 Is Lost in Tumor Endothelium and during Wound Healing Angiogenesis
To address whether loss of IL-33 is also linked to the activation status of endothelial cells in vivo, we assessed the vasculature of several different tumors because such vessels are believed to be under the constant exposure to proangiogenic signals generated by tumor cells.29,30 We immunostained five different human tumor types including samples from colon (n = 5, Figure 4A), mammary gland (n = 3, Figure 4B), kidney (n = 1; Figure 4, C and D), and lung (n = 2, data not shown), finding in every sample a highly similar expression pattern. Although endothelial cell nuclei expressed IL-33 in surrounding healthy areas, all vessels inside the tumors contained little or no detectable IL-33 and proliferating, Ki-67-positive tumor endothelial cells were consistently negative for IL-33 (Figure 4E).
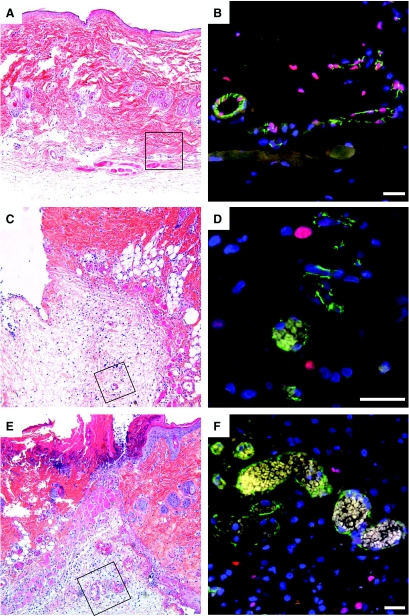
The almost complete absence of IL-33 from tumor vessels led us to assess the dynamics of IL-33 expression in the controlled setting of experimental wound healing, in which growth factors such as VEGF, TGF-β, epidermal growth factor, and fibroblast growth factor, as well as many proinflammatory cytokines drive an ordered sequence of events including angiogenesis.31 We therefore made excision wounds in rat skin and retrieved sequential biopsy specimens throughout the course of 11 days. IL-33 expression in rat skin was detected using the IL-33 (Nessy-1) antibody, and the resulting staining pattern was abolished by preincubation with rrIL-33 (data not shown). Although we found blood vessels of noninjured rat dermis positive for IL-33 (Figure 5B), we observed loss of IL-33 expression in vessels close to the wound edge as early as 24 hours after injury (Figure 5D). This pattern was also seen 2 days after injury (Figure 5F). Most of these vessels appeared to fulfill the criteria of microvessel-derived mother vessels that are characterized by enlargement, pericyte detachment, and basement membrane degradation.32,33 On days 4, 6, and 11 after injury IL-33 remained absent in vessels of the wound site whereas the number and density of small microvessels increased (data not shown).
Figure 5.
Vascular IL-33 expression is lost during wound healing. Biopsies from normal rat skin (A and B) and from wounds after 1 (C and D) or 2 days (E and F) were fixed and stained with H&E (A, C, and E) or immunostained (B, D, and F) for IL-33 (Nessy-1, red) and CD31 (green). A, C, and E show overviews of the wound area and the boxed areas indicate the position of vessels shown in B, D, and F, respectively. Cell nuclei are stained with Hoechst dye. Note presence of vessels without IL-33 and perivascular cell nuclei at days 1 and 2. Scale bars = 50 μm.
IL-33 Expression Is Lost on Cytokine Activation
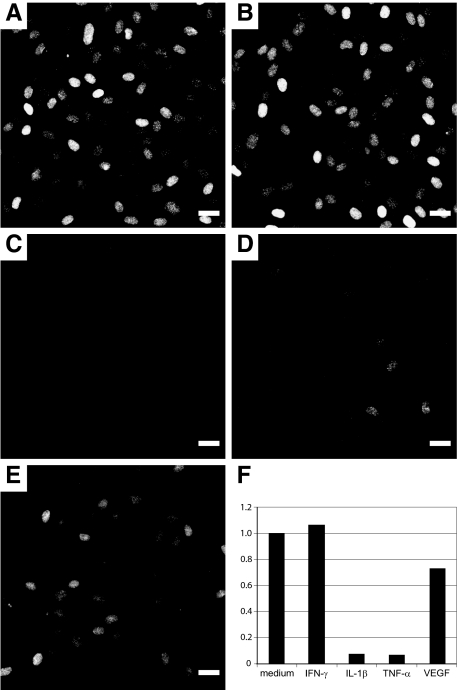
Given the rapid down-regulation of IL-33 in wound healing and its absence in tumor vessels, we next tested the effect of several different proinflammatory/angiogenic cytokines on IL-33 expression in HUVECs. The observed effects on IL-33 regulation were detectable by immunocytochemistry, revealing that the modulation of IL-33 involved the majority of cells rather than a subset. Although treatment of superconfluent HUVEC monolayers for 17 hours with IFN-γ (50 ng/ml) revealed no difference compared to control cells (Figure 6, B and A, respectively), IL-1β (500 pg/ml, Figure 6C) or TNF-α (500 pg/ml, Figure 6D) induced a dramatic drop in the number and intensity of stained cells. Moreover, a mild effect of VEGF treatment was discernible (10 ng/ml, Figure 6E). These responses were reflected at the transcription level: Although both IL-1β and TNF-α strongly reduced IL-33 mRNA expression at 7 hours, VEGF induced a reduction of ∼30% and IFN-γ had no discernible effect (Figure 6F).
Figure 6.
IL-33 expression is down-regulated by proinflammatory cytokines and VEGF. A--E: Response of superconfluent HUVEC monolayers exposed for 17 hours to medium alone (A), INF-γ (50 ng/ml) (B), IL-1β (0.5 ng/ml) (C), TNF-α (0.5 ng/ml) (D), or VEGF (10 ng/ml) (E). Immunostaining for IL-33 (IL-33Nter) after fixation of monolayers. Pictures were obtained using identical exposure times and image enhancement parameters. F: Quantitative PCR analysis of IL-33 expression in superconfluent HUVEC monolayers treated with cytokines for 7 hours at the same concentrations as given above. IL-33 transcript levels were obtained using primer pair IL-33 no. 2 and normalized to HPRT, data are given as ratio of transcript values in sample over those in medium-treated controls. Similar results were obtained when using a different primer pair for IL-33 detection or when normalizing against housekeeping gene GAPDH (data not shown). Scale bars = 25 μm.
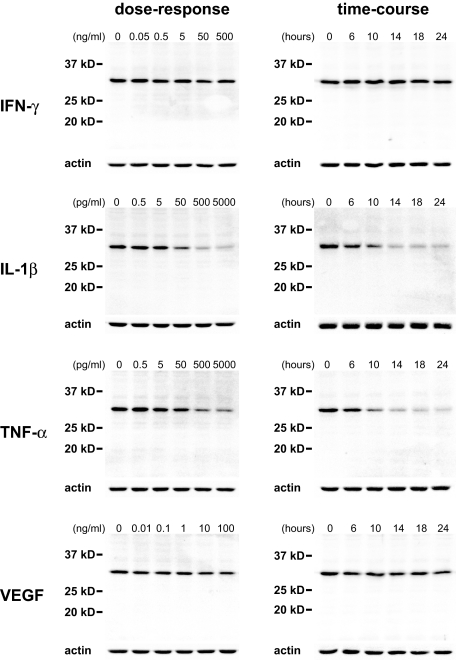
The sensitivity and time course of IL-33 down-regulation was next quantified. Whereas treatment of superconfluent HUVEC monolayers with IFN-γ up to 500 ng/ml had no discernible effect on IL-33 expression levels (Figure 7), IL-1β or TNF-α reduced IL-33 expression within 6 hours when given in the picogram range (Figure 7). Interestingly, even in the presence of such proinflammatory cytokines, we observed no induction of smaller IL-33 species in the blot that could indicate proteolytic cleavage and generation of mature IL-33. Because we found IL-33 expression to be absent in angiogenic tumor vessels with no obvious signs of proinflammatory activation, we asked if IL-33 expression might also be affected by exposing superconfluent HUVECs to VEGF, a powerful proangiogenic inducer34,35,36 in vivo and in vitro.37 To this end, we found that VEGF induced a reduction in IL-33 protein amounting to a reduction by 26 to 38% (range of three independent experiments, Figure 7). A similar level of reduction was also seen in an enzyme-linked immunosorbent assay performed on adherent, superconfluent cells (unpublished data).
Figure 7.
Dose-response and time-course of IL-33 expression in HUVECs. Western blot detection of IL-33 protein after cytokine treatment of superconfluent HUVEC monolayers for 18 hours with the indicated concentration of cytokines (left column) or for the indicated time with INF-γ (50 ng/ml), IL-1β (500 pg/ml), TNF-α (500 pg/ml), or VEGF (10 ng/ml) (right column). Note the sensitivity of IL-33 levels to IL-1β and TNF-α whereas VEGF has a mild effect.
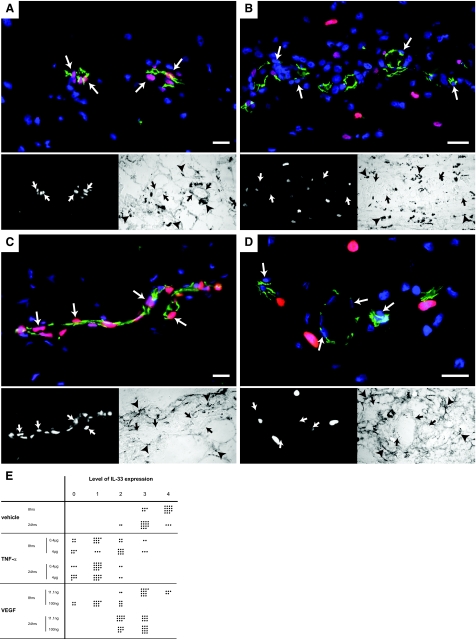
Having observed an effect of proinflammatory cytokines or proangiogenic growth factors on IL-33 expression levels in vitro, we next asked if such mediators can provide a signal for IL-33 down-regulation in vivo. To this end, we injected either rrTNF-α (2 μg or 0.2 μg) or rrVEGF-A (100 ng or 11.1 ng) into rat skin and harvested samples after 8 and 24 hours. We observed a strong reduction in endothelial IL-33 expression 8 and 24 hours after injection of rrTNF-α (Figure 8, B and E) compared to the control injection (Figure 8, A and E). By contrast, VEGF reduced IL-33 expression levels most strongly at 8 hours (Figure 8, D and C) and at the highest dose, whereas the expression levels returned close to control levels after 24 hours (Figure 8E).
Figure 8.
TNF-α and VEGF down-regulate IL-33 in vivo. Immunostaining for IL-33 (Nessy-1, red) and CD31 (green) of rat skin after subcutaneous injection of PBS (A, C) and cytokines (B, D). Cell nuclei are stained with Hoechst dye. The bottom left panels show the red channel and the bottom right panels the light microscopy images of the respective area. Twenty-four hours after the injection of 0.4 μg of rrTNF-α (B) or 8 hours after the injection of 100 ng of rrVEGF (D) the endothelial signal for IL-33 has decreased compared to the mock injection (A and C, respectively). Pictures were obtained using identical exposure times and image enhancement parameters for A and B as well as for C and D. Arrows indicate endothelial nuclei. Arrowheads indicate ink particles. E: Semiquantitative analysis of vascular IL-33 expression in rat skin after injection of vehicle (PBS), rrTNF-α, or rrVEGF. Endothelial cell nuclei were categorized as negative (0) or increasingly positive (levels 1 to 4) by a blinded observer, counting in each condition 20 nuclei. Scale bars = 50 μm.
Discussion
This study shows that full-length/precursor IL-33 is a nuclear factor generally expressed in resting endothelial cells in healthy tissues. IL-33 expression is induced by signals associated with building patent vessels, as reflected by its induction when cultured endothelial cells grow into a confluent endothelial monolayer and stop proliferating. Conversely, IL-33 is strongly down-regulated in the course of the endothelial cell activation seen in wound healing and tumor growth.
The general expression of IL-33 in resting endothelial cells and induction by cell density contrast but also explain previous conclusions from our laboratory that IL-33 is preferentially expressed in high endothelial venules of secondary lymphoid organs because freshly isolated high endothelial venule-derived endothelial cells were compared to cultured, subconfluent umbilical vein- and nasal polyp-derived endothelial cells.2 The use of less than superconfluent cultures may also explain the conclusion of a previous report that found no effects of TNF-α or IL-1β treatment on IL-33 expression levels in HUVECs1 and is therefore at variance with our findings that IL-1β or TNF-α strongly reduced IL-33 in HUVECs. In fact, endothelial cells (and bone marrow-derived murine dendritic cells3) respond quite differently from most other cell types, because the latter up-regulate IL-33 expression in response to proinflammatory activation.1,3,38 The observation that proinflammatory cytokines down-regulate IL-33 in endothelial cells also appears to contrast the recent report that vascular IL-33 expression is linked to the development of high endothelial venule-like vessels in chronic inflammation.4 We therefore stained tissue samples of Crohn’s disease, ulcerative colitis, and psoriasis in parallel with noninflamed controls. Surprisingly, we found the expression of IL-33 to be similar in chronically inflamed and healthy tissues, with the exception that some subepithelial blood vessels in psoriatic lesions were IL-33-negative (our unpublished observations), perhaps because of inflammation-induced angiogenesis. Given the known expression of TNF-α and IL-1β in such lesions, it is tempting to speculate that their down-regulating effects on IL-33 expression seen in this study may be counteracted by currently unknown factors.
Induction of nuclear IL-33 in endothelial cells appears to require the following conditions: first, cells must organize in a superconfluent monolayer and second, they have to be nonproliferating, because we never observed IL-33 in cells that had progressed through the restriction point within the G1 phase of the cell cycle, as indicated by the absence of cells double-positive for IL-33 and markers of proliferation. We were also unable to detect endogenous IL-33 associated with mitotic chromatin (A.M. Küchler et al, unpublished observation), a finding that contrasts the reported presence of ectopic IL-33 in mitotic chromosomes of nonendothelial cells.4 Furthermore, the inhibition of cycle progression by growth factor deprivation or fumagillin treatment failed to induce IL-33. We therefore conclude that the nonproliferative cell cycle state is necessary, but not sufficient for IL-33 induction.
The apparent association of IL-33 expression with the induction of a superconfluent monolayer also prompted us to assess the role of VE-cadherin known to affect the junctional integrity and permeability of HUVEC monolayers. However, efforts to manipulate contact inhibition by addition of a blocking antibody to VE-cadherin did not down-regulate IL-33, perhaps because such inhibition can be considered incomplete when compared to that obtainable under conditions of flow or in vivo.39,40 Moreover, the lack of change in VE-cadherin or CD31 expression levels or cellular localization observed after IL-33 knockdown may be attributable to the incompleteness of IL-33 knockdown. Taken together, the careful dissection of the interplay between density-associated signals, cell cycle, and the expression of IL-33 deserves future attention.
IL-33 is strongly down-regulated in endothelium undergoing tumor or wound healing angiogenesis. This could be explained by a cytokine profile containing those found to down-regulate IL-33 levels in vitro and in vivo in the present study. In fact, the presence of congested, thin-walled vessels in samples of both wound healing or tumor tissue can be mimicked by adenoviral delivery of VEGF33 and is also seen during VEGF-driven tumor angiogenesis.35 VEGF may also be a driving force during wound healing, as prestored VEGF may be released from platelets,41 and perhaps acts in concert with TNF-α released from tissue-resident mast cells42 to down-regulate vascular IL-33. Such effects may perhaps also explain the strong effect of VEGF induction in rat skin as opposed to the moderate effect of VEGF in vitro. Moreover, inflammatory mechanisms may also dominate the tumor microenvironment43,44,45,46 and contribute to the virtual absence of vascular IL-33 in the tumors examined.
Although nuclear, full-length/precursor IL-33 has been described to possess transcriptional repressor properties4 and also to interact with several nuclear proteins47 at least in vitro, the precise role of IL-33 in endothelial cells remains unclear. Nevertheless, our data make it tempting to speculate that IL-33 may act as repressor of proangiogenic activation and it will be interesting to precisely dissect the sequence of events that precede and follow loss of IL-33.
In conclusion, our data show that IL-33 is generally expressed in the nuclei of resting endothelium but is rapidly down-regulated during early angiogenic events in a process that appears to involve exposure to proinflammatory cytokines, proangiogenic VEGF, or loss of cell-cell contacts. The reduced expression of IL-33 seen in tumors and during wound healing may contribute to maintaining the angiogenic state of vessels. Future studies should focus on the elucidation of IL-33 function in endothelial cells.
Acknowledgments
We thank Finn-Eirik Johansen, Ole Petter Clausen, Ruth Holm, Finn P. Reinholt, and David Scheie for expert advice; Johanna Hol for helpful comments on the manuscript; Oddmund Bakke for sharing the 4.8.G supernatant; Aaste Aursjø, Kathrine Hagelsteen, Hogne Røed Nilsen, Linda Solfjell, and Vigdis Wendel for excellent technical assistance; Olav Schreurs for valuable suggestions on technical issues; and the staff at the Department of Gynecology and Obstetrics, Rikshospitalet, for assistance in collecting umbilical cords.
Footnotes
Address reprint requests to Guttorm Haraldsen, Department of Pathology, Rikshospitalet University Hospital 0027 Oslo, Norway. E-mail: guttorm.haraldsen@rr-research.no.
Supported by the Helse Sør-Øst (grant 2008139 to A.M.K. and J.S.), the Norwegian Cancer Society (grants PK01-2007-0335 T97426 and B02085), and the Research Council of Norway (grant 133924/300).
J.P. and J.B. are postdoctoral fellows of the Norwegian Cancer Society.
Disclosure: G.H. is inventor of patent WO 2004/056868 A2.
Supplemental material for this article can be found on http:// ajp.amjpathol.org.
References
- Onda H, Kasuya H, Takakura K, Hori T, Imaizumi T, Takeuchi T, Inoue I, Takeda J. Identification of genes differentially expressed in canine vasospastic cerebral arteries after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 1999;19:1279–1288. doi: 10.1097/00004647-199911000-00013. [DOI] [PubMed] [Google Scholar]
- Baekkevold ES, Roussigne M, Yamanaka T, Johansen FE, Jahnsen FL, Amalric F, Brandtzaeg P, Erard M, Haraldsen G, Girard JP. Molecular characterization of NF-HEV, a nuclear factor preferentially expressed in human high endothelial venules. Am J Pathol. 2003;163:69–79. doi: 10.1016/S0002-9440(10)63631-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Carriere V, Roussel L, Ortega N, Lacorre DA, Americh L, Aguilar L, Bouche G, Girard JP. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci USA. 2007;104:282–287. doi: 10.1073/pnas.0606854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007;117:1538–1549. doi: 10.1172/JCI30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AM, Xu D, Asquith DL, Denby L, Li Y, Sattar N, Baker AH, McInnes IB, Liew FY. IL-33 reduces the development of atherosclerosis. J Exp Med. 2008;205:339–346. doi: 10.1084/jem.20071868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S, Huber M, Kollewe C, Bischoff SC, Falk W, Martin MU. IL-1 receptor accessory protein is essential for IL-33-induced activation of T lymphocytes and mast cells. Proc Natl Acad Sci USA. 2007;104:18660–18665. doi: 10.1073/pnas.0705939104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chackerian AA, Oldham ER, Murphy EE, Schmitz J, Pflanz S, Kastelein RA. IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. J Immunol. 2007;179:2551–2555. doi: 10.4049/jimmunol.179.4.2551. [DOI] [PubMed] [Google Scholar]
- Komai-Koma M, Xu D, Li Y, McKenzie AN, McInnes IB, Liew FY. IL-33 is a chemoattractant for human Th2 cells. Eur J Immunol. 2007;37:2779–2786. doi: 10.1002/eji.200737547. [DOI] [PubMed] [Google Scholar]
- Moulin D, Donze O, Talabot-Ayer D, Mezin F, Palmer G, Gabay C. Interleukin (IL)-33 induces the release of pro-inflammatory mediators by mast cells. Cytokine. 2007;40:216–225. doi: 10.1016/j.cyto.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Ho LH, Ohno T, Oboki K, Kajiwara N, Suto H, Iikura M, Okayama Y, Akira S, Saito H, Galli SJ, Nakae S. IL-33 induces IL-13 production by mouse mast cells independently of IgE-Fc{epsilon}RI signals. J Leukoc Biol. 2007;82:1481–1490. doi: 10.1189/jlb.0407200. [DOI] [PubMed] [Google Scholar]
- Iikura M, Suto H, Kajiwara N, Oboki K, Ohno T, Okayama Y, Saito H, Galli SJ, Nakae S. IL-33 can promote survival, adhesion and cytokine production in human mast cells. Lab Invest. 2007;87:971–978. doi: 10.1038/labinvest.3700663. [DOI] [PubMed] [Google Scholar]
- Cherry WB, Yoon J, Bartemes KR, Iijima K, Kita H. A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J Allergy Clin Immunol. 2008;121:1484–1490. doi: 10.1016/j.jaci.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werman A, Werman-Venkert R, White R, Lee JK, Werman B, Krelin Y, Voronov E, Dinarello CA, Apte RN. The precursor form of IL-1alpha is an intracrine proinflammatory activator of transcription. Proc Natl Acad Sci USA. 2004;101:2434–2439. doi: 10.1073/pnas.0308705101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buryskova M, Pospisek M, Grothey A, Simmet T, Burysek L. Intracellular interleukin-1alpha functionally interacts with histone acetyltransferase complexes. J Biol Chem. 2004;279:4017–4026. doi: 10.1074/jbc.M306342200. [DOI] [PubMed] [Google Scholar]
- Nakatani Y. Histone acetylases—versatile players. Genes Cells. 2001;6:79–86. doi: 10.1046/j.1365-2443.2001.00411.x. [DOI] [PubMed] [Google Scholar]
- Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ades EW, Candal FJ, Swerlick RA, George VG, Summers S, Bosse DC, Lawley TJ. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol. 1992;99:683–690. doi: 10.1111/1523-1747.ep12613748. [DOI] [PubMed] [Google Scholar]
- Haraldsen G, Rugtveit J, Kvale D, Scholz T, Muller WA, Hovig T, Brandtzaeg P. Isolation and long-term culture of human intestinal microvascular endothelial cells. Gut. 1995;37:225–234. doi: 10.1136/gut.37.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnsen FL, Brandtzaeg P, Haye R, Haraldsen G. Expression of functional VCAM-1 by cultured nasal polyp-derived microvascular endothelium. Am J Pathol. 1997;150:2113–2123. [PMC free article] [PubMed] [Google Scholar]
- Muller WA, Ratti CM, McDonnell SL, Cohn ZA. A human endothelial cell-restricted, externally disposed plasmalemmal protein enriched in intercellular junctions. J Exp Med. 1989;170:399–414. doi: 10.1084/jem.170.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Kelman Z. PCNA: structure, functions and interactions. Oncogene. 1997;14:629–640. doi: 10.1038/sj.onc.1200886. [DOI] [PubMed] [Google Scholar]
- Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- Ingber D, Fujita T, Kishimoto S, Sudo K, Kanamaru T, Brem H, Folkman J. Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth. Nature. 1990;348:555–557. doi: 10.1038/348555a0. [DOI] [PubMed] [Google Scholar]
- Wang J, Lou P, Henkin J. Selective inhibition of endothelial cell proliferation by fumagillin is not due to differential expression of methionine aminopeptidases. J Cell Biochem. 2000;77:465–473. [PubMed] [Google Scholar]
- Abe J, Zhou W, Takuwa N, Taguchi J, Kurokawa K, Kumada M, Takuwa Y. A fumagillin derivative angiogenesis inhibitor, AGM-1470, inhibits activation of cyclin-dependent kinases and phosphorylation of retinoblastoma gene product but not protein tyrosyl phosphorylation or protooncogene expression in vascular endothelial cells. Cancer Res. 1994;54:3407–3412. [PubMed] [Google Scholar]
- Corada M, Liao F, Lindgren M, Lampugnani MG, Breviario F, Frank R, Muller WA, Hicklin DJ, Bohlen P, Dejana E. Monoclonal antibodies directed to different regions of vascular endothelial cadherin extracellular domain affect adhesion and clustering of the protein and modulate endothelial permeability. Blood. 2001;97:1679–1684. doi: 10.1182/blood.v97.6.1679. [DOI] [PubMed] [Google Scholar]
- Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- Paku S, Paweletz N. First steps of tumor-related angiogenesis. Lab Invest. 1991;65:334–346. [PubMed] [Google Scholar]
- Pettersson A, Nagy JA, Brown LF, Sundberg C, Morgan E, Jungles S, Carter R, Krieger JE, Manseau EJ, Harvey VS, Eckelhoefer IA, Feng D, Dvorak AM, Mulligan RC, Dvorak HF. Heterogeneity of the angiogenic response induced in different normal adult tissues by vascular permeability factor/vascular endothelial growth factor. Lab Invest. 2000;80:99–115. doi: 10.1038/labinvest.3780013. [DOI] [PubMed] [Google Scholar]
- Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- Feng D, Nagy JA, Dvorak AM, Dvorak HF. Different pathways of macromolecule extravasation from hyperpermeable tumor vessels. Microvasc Res. 2000;59:24–37. doi: 10.1006/mvre.1999.2207. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Chang YS, Munn LL, Hillsley MV, Dull RO, Yuan J, Lakshminarayanan S, Gardner TW, Jain RK, Tarbell JM. Effect of vascular endothelial growth factor on cultured endothelial cell monolayer transport properties. Microvasc Res. 2000;59:265–277. doi: 10.1006/mvre.1999.2225. [DOI] [PubMed] [Google Scholar]
- Hudson CA, Christophi GP, Gruber RC, Wilmore JR, Lawrence DA, Massa PT. Induction of IL-33 expression and activity in central nervous system glia. J Leukoc Biol. 2008;84:631–643. doi: 10.1189/jlb.1207830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnittler HJ, Puschel B, Drenckhahn D. Role of cadherins and plakoglobin in interendothelial adhesion under resting conditions and shear stress. Am J Physiol. 1997;273:H2396–H2405. doi: 10.1152/ajpheart.1997.273.5.H2396. [DOI] [PubMed] [Google Scholar]
- Corada M, Mariotti M, Thurston G, Smith K, Kunkel R, Brockhaus M, Lampugnani MG, Martin-Padura I, Stoppacciaro A, Ruco L, McDonald DM, Ward PA, Dejana E. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc Natl Acad Sci USA. 1999;96:9815–9820. doi: 10.1073/pnas.96.17.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möhle R, Green D, Moore MA, Nachman RL, Rafii S. Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc Natl Acad Sci USA. 1997;94:663–668. doi: 10.1073/pnas.94.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JR, Galli SJ. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature. 1990;346:274–276. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- Brigati C, Noonan DM, Albini A, Benelli R. Tumors and inflammatory infiltrates: friends or foes? Clin Exp Metastasis. 2002;19:247–258. doi: 10.1023/a:1015587423262. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal B. New insight into CCN3 interactions—nuclear CCN3: fact or fantasy? Cell Commun Signal. 2006;4:1–8. doi: 10.1186/1478-811X-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]