Abstract
To learn the extent of human exposure to polyfluoroalkyl compounds (PFCs) in a remote fishing population, we measured, in Faroese children and pregnant women, the serum concentrations of nine PFCs, including perfluorooctane sulfonate (PFOS), perfluorooctanoate (PFOA), and perfluorononanoate (PFNA), by using on-line solid-phase extraction coupled to isotope dilution high-performance liquid chromatography-tandem mass spectrometry. The serum samples analyzed had been collected between 1993 and 2005 from 103 children 7 years of age, 79 of these children at 14 years of age, and from 12 pregnant women and their children 5 years later. PFOS was detected in all samples analyzed, and both PFOA and PFNA in all but one of the samples. The concentrations found are comparable to those reported elsewhere. Correlations between paired concentrations were poor. However, PFOS and PFNA concentrations correlated well with the frequency of pilot whale dinners and with concentrations of mercury and polychlorinated biphenyls. One whale meal every two weeks increased the PFOS concentration in 14-year olds by about 25% and PFNA by 50%. The high frequency of detection of most PFCs suggests widespread exposure in the Faroe Islands already by the early 1990s, with whale meat being an important source.
Synopsis: Pilot whale meat may have been an important source of dietary exposure to PFOS and PFNA among Faroe Islands residents since the 1990s
Keywords: Biomonitoring, dietary intake, environmental exposure, PFOS, PFOA
Introduction
Polyfluoroalkyl compounds (PFCs) owe many of their unique properties to the remarkable strength of the carbon-fluorine bond. PFCs have been used in a variety of commercial applications, such as in water, oil, soil, and grease repellents for fabric, leather, rugs, carpets, stone, and tile; in fire-fighting foams; in alkaline cleaners; in floor polish; in sizing agents for packaging and paper products (to resist the spreading and penetration of liquids); and in leveling agents for coatings (1). Some PFCs have demonstrated developmental, reproductive, genotoxic, and carcinogenic effects in laboratory animal studies (1–3) although at serum concentrations that are orders of magnitude higher than those observed in the general population (4, 5).
Because several PFCs resist hydrolysis, photolysis, and biodegradation in the environment, PFCs are emerging as a new group of persistent organic pollutants. Two of these PFCs, PFOS and perfluorooctanoate (PFOA), have been found around the world in wildlife (1, 6, 7), in humans (8–11), and in the environment (1, 12, 12). Although environmental sources and routes of human exposure to PFCs have not been clearly identified (12–16), crustaceans, molluscs, fish and marine mammals may represent important sources of dietary intake (9, 17–20). Assessing human exposure to PFCs in diverse geographical areas may provide useful information for understanding the pathways of exposure to PFCs, so that strategies for reducing exposure to these compounds and their precursors may be implemented.
The Faroe Islands are located in the North Atlantic Ocean, between Norway and Iceland. The Faroese are a fairly homogeneous population mainly of Scandinavian origin with a standard of living comparable to the Danes and other Scandinavians. Based on their need to be self-sufficient with the resources readily available on the islands, the Faroese have depended on a traditional diet that includes fish, pilot whale, sheep, and birds as staples. Blood/serum concentrations of mercury and persistent organic pollutants in Faroese residents are primarily linked to dietary intake of pilot whale (21–23), but data on the serum concentrations of PFCs are not available. To determine the prevalence and magnitude of exposure to PFCs in the Faroe Islands, we measured the serum concentrations of 9 PFCs in two Faroese population groups.
Materials and methods
Study populations
One group of participants included 12 pregnant women, for whom blood samples were collected in 2000 at the last antenatal examination, and their 5-year-old children examined in 2005. The mothers were selected to represent a wide range of seafood diets, four of them not eating pilot whale. The second group of participants was a subset of children from a prospective birth cohort established in 1986–1987. At approximately 7 years of age (1993–1994) and 14 years (2000–2001), the cohort members were invited for a thorough health examination. The examinations included a questionnaire about intake of any type of fish (number of dinners per week) and whale meat (number of dinners per month), and blood was collected for analysis for environmental chemicals. A total of 917 7-year-olds and 795 of the 14-year-olds completed the examination. Previous analyses included the mercury concentration in whole blood and the total concentration of polychlorinated biphenyls in serum lipids and showed the impact of pilot whale consumption on these contaminant concentrations (21, 22). Due to limited blood volume collected, the number of serum samples available for PFC measurements was 103 for the 7-year-olds, 79 of whom were also examined at age 14 years. A majority of 60 boys (compared to 43 girls) were included at age 7 years (44 boys and 35 girls at age 14 years), but the sub-sample did not differ from the rest of the cohort in regard to age, diet, and exposure to methylmercury and polychlorinated biphenyls.
All protocols were reviewed and approved by the Faroese ethical review committee and the institutional review board in the United States and were found to comply with international institutional guidelines for the protection of human subjects. The project was exempt from additional human subjects review at CDC, because all serum samples were coded.
Laboratory measurements
After collection, the blood was allowed to clot for approximately 30 minutes and then spun at 2000 g for 10 minutes to separate the serum portion of the blood. The serum was transferred to clean cryovials, frozen, and stored at −70 °C until analysis. At the CDC laboratory, by using an analytical method previously described in detail (24), we measured the following analytes: perfluorooctane sulfonamide (PFOSA), 2-(N-ethyl-perfluorooctane sulfonamido) acetic acid (Et-PFOSA-AcOH), 2-(N-methyl-perfluorooctane sulfonamido) acetic acid (Me-PFOSA-AcOH), perfluorohexane sulfonic acid (PFHxS), perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA), perfluorononanoic acid (PFNA), perfluorodecanoic acid (PFDeA), and perfluorododecanoic acid (PFDoA). Briefly, we added 250 μL of 0.1 M formic acid and 25 μL of internal standard solution to 100 μL of serum, and the spiked serum was vortex-mixed and sonicated. The samples were placed on a Symbiosis online SPE system (Spark Holland, Plainsboro, NJ) for the preconcentration of the analytes on a HySphere HD C18 cartridge (7 μm, 10 mm × 1 mm; Spark Holland). The analytes were transferred onto a Betasil C8 HPLC column (3 mm × 50 mm, 5 μm; ThermoHypersil Keystone, Bellefonte, PA), separated by HPLC (mobile phase A: 20 mM ammonium acetate in water, pH = 4; mobile phase B: methanol), and detected by negative-ion TurboIonspray-MS/MS on an API 4000 mass spectrometer (Applied Biosystems, Foster City, CA). The following isotope-labeled internal standards were used for quantification: 18O2-PFOS, 13C2-PFOA, 13C5-PFNA, 13C2-PFDeA, 18O2-PFOSA, D3-Me-PFOSA-AcOH, and D5-Et-PFOSA-AcOH. To compensate for the lack of isotope-labeled internal standards for PFHxS and PFDoA and to account for matrix effects, the calibration standards were spiked into calf serum.
The limits of the detection (LODs) were 0.1 ng/mL for PFHxS, PFOSA, PFOA and PFNA, 0.2 ng/mL for PFOS, Me-PFOSA-AcOH, Et-PFOSA-AcOH, PFDeA, and PFDoA. The concentrations of PFOS reported correspond to the sum of linear and branched isomers. Standard accuracies (77%–109%), their relative standard deviations (5%–24%), and the precision of the method (6%–16%) have been reported before (24). To ensure the accuracy and reliability of the data, quality control materials of low- and high-concentration, prepared from a base calf serum pool and characterized as described previously (24), were included in each analytical batch with the Faroese samples, calibration standards, and reagent and serum blanks. Furthermore, as part of its quality assurance program, the CDC laboratory successfully participated in the 1st and 2nd Interlaboratory Study on PFCs for human biological matrices conducted in 2005 and 2006 (1, 25). Since 2006, the laboratory also participates in the ongoing German External Quality Assessment Scheme for PFOS and PFOA in serum, managed by the University of Erlangen-Nuremberg (Erlangen, Germany) (26).
Statistical methods
We performed the statistical analyses by using SAS software (SAS Institute, Cary, NC, version 9.1). For concentrations below the LOD, we used a value equal to the LOD divided by the square root of 2. PFOS and PFOA showed only slight deviations from Gaussian distribution; parametric statistics were therefore used throughout.
Results
High frequencies of detection were obtained for most of the PFCs (Tables 1 and 2), and PFOS was detected in all samples analyzed. By contrast, PFDoA was detected infrequently—in less than 25% of the samples—and will not be discussed further. Correlations between paired results from the 7 and 14-year-old subjects (N = 79) were relatively poor, with Pearson’s r values of 0.144 for PFOS and 0.168 for PFOA. Likewise, concentrations in the pregnant mothers (N = 12) were poorly associated with those seen in their children five years later.
Table 1.
Serum concentrations (in ng/mL) in 12 Faroese mothers (collected in 2000) and their 5 year old children (collected in 2005)
| Analyte | Frequency of detection (%) | Mean (Median) | Range |
|---|---|---|---|
| Mothers | |||
| PFOS | 100 | 25.5 (23.7) | 16.4,38.3 |
| PFOA | 100 | 2.6 (2.4) | 0.1, 4.0 |
| PFOSA | 100 | 0.6 (0.6) | 0.2, 1.1 |
| Me-PFOSA-AcOH | 75 | 0.7 (0.9) | <LOD, 1.3 |
| Et-PFOSA-AcOH | 42 | 0.2 (<LOD) | <LOD, 0.6 |
| PFHxS | 25 | 0.6 (<LOD) | <LOD, 3.6 |
| PFNA | 100 | 0.6 (0.6) | 0.1, 1.2 |
| PFDeA | 67 | 0.3 (0.3) | <LOD, 0.6 |
|
| |||
| Children | |||
| PFOS | 100 | 16.3 (16.3) | 8.3, 25.5 |
| PFOA | 100 | 5.1 (4.5) | 2.8, 9.9 |
| PFOSA | 17 | <LOD (<LOD) | <LOD, 0.2 |
| Me-PFOSA-AcOH | 58 | 0.2 (0.3) | <LOD, 0.7 |
| Et-PFOSA-AcOH | 25 | 0.2 (<LOD) | <LOD, 1.7 |
| PFHxS | 100 | 0.7 (0.6) | 0.2, 1.5 |
| PFNA | 100 | 1.4 (1.3) | 0.6, 3.0 |
| PFDeA | 67 | 0.3 (0.3) | <LOD, 0.8 |
Table 2.
Serum concentrations (in ng/mL) in the Faroese 7-year-old children (n = 103, collected in 1993–1994) and in the 14-year-old children (n=79, collected in 2000–2001)
| Analyte | Frequency of detection (%) | Mean (Median) | Range | |||
|---|---|---|---|---|---|---|
| 7 | 14 | 7 | 14 | 7 | 14 | |
| PFOS | 100 | 100 | 29.0 (26.3) | 33.0 (31.2) | 5.3,109.0 | 16.7, 65.2 |
| PFOA | 99 | 100 | 5.5 (5.0) | 4.4 (4.2) | <LOD, 16.1 | 2.3, 7.3 |
| PFOSA | 99 | 84 | 1.5 (1.3) | 0.4 (0.3) | <LOD, 6.6 | <LOD, 1.4 |
| Me-PFOSA-AcOH | 79 | 94 | 0.6 (0.4) | 0.5 (0.4) | <LOD, 4.9 | <LOD, 1.8 |
| Et-PFOSA-AcOH | 96 | 100 | 1.8(1.4) | 1.1 (1.0) | <LOD, 11.6 | 0.2, 4.2 |
| PFHxS | 98 | 100 | 0.8 (0.4) | 3.0 (2.9) | <LOD, 5.1 | 1.1, 5.5 |
| PFNA | 99 | 100 | 0.9 (0.8) | 0.9 (0.8) | <LOD, 4.8 | 0.4, 4.8 |
| PFDeA | 79 | 90 | 0.5 (0.3) | 0.4 (0.3) | <LOD, 5.2 | <LOD, 1.2 |
The highest average concentrations were for PFOS (with one child at age 7 years exceeding 100 ng/mL), followed by PFOA and the rest of the PFCs (Tables 1 and 2). The PFOS mean increased from 29 ng/mL to 33 ng/mL (p = 0.09) between 7 and 14 years of age, and a 3-fold increase was seen in the PFHxS (p < 0.001, paired t-test) concentrations. However, decreases were observed for PFOA (p = 0.001) and PFOSA (p < 0.001), and Et-PFOSA-AcOH between 7 and 14 years (p < 0.001) (Table 2). During the same period, the children did not change their frequency of fish dinners, but whale meat dinners decreased from an average of 1.9 per month at age 7 years to 0.8 at age 14.
We found statistically significant correlations between the concentrations of PFOS and PFOA (r = 0.632, p < 0.0001 for the 7-year-old children and r = 0.494, p < 0.0001 for the 14-year-olds), and between the concentrations of PFOA and PFNA (r = 0.401 for the 7-year-old children, p < 0.0001). The correlation between the concentrations of PFOA and PFNA for the 14-year-olds was not as strong (r = 0.269). Furthermore, the PFOS concentrations correlated well with the combined concentrations of fluorooctanyl sulfonamide derivatives (i.e., PFOSA, Me-PFOSA-AcOH, and Et-PFOSA-AcOH, which are considered precursors of PFOS (15)), with r = 0.546 (p < 0.0001) for the 7-year-old children and r = 0.635 (p<0.0001) for the 14-year-olds.
In the Faroese paired mother-children samples, mothers had higher PFOS concentrations than their children (p = 0.002; paired t test), but the children had higher concentrations of PFOA (p < 0.001) and PFNA (p = 0.007) than their mothers had five years previously.
Four of the 12 pregnant mothers did not eat pilot whale at all, and their concentrations of Me-PFOSA-AcOH and PFDeA were all <LOD, and the same was true for Et-PFOSA-AcOH for three mothers. Results <LOD were obtained for PFDeA and Et-PFOSA-AcOH only for mothers not eating whale. In children aged 7 years, a tendency of higher PFC concentrations was seen in those who included whale in their diet (data not shown). At age 14 years, PFOS, PFDeA, and PFNA were significantly associated with the frequency of pilot whale dinners (Table 3). Fish and whale intakes were poorly correlated (r = 0.07), and the PFCs were not associated with the frequency of fish dinners, except for PFHxS at age 14 (r = 0.24; p < 0.05); adjustment for whale intake decreased the r to 0.19. There was no association with sex.
Table 3.
Mean serum concentrations (in ng/mL) in 79 Faroese adolescents at age 14 years (in 2000–2001) in relation to the monthly number of pilot whale dinners (numbers in parenthesis).
| Analyte | <0.2 (19) | 0.2–0.5 (21) | 1.0 (26) | ≥1.5 (16) | r |
|---|---|---|---|---|---|
| PFOS | 30.1 | 29.0 | 33.1 | 38.8 | 0.26* |
| PFOA | 4.6 | 4.2 | 4.0 | 4.3 | −0.10 |
| PFOS precursors | 2.1 | 1.7 | 2.0 | 1.9 | −0.02 |
| PFOSA | 0.43 | 0.42 | 0.31 | 0.37 | −0.12 |
| Me-PFOSA-AcOH | 0.52 | 0.37 | 0.47 | 0.54 | 0.06 |
| Et-PFOSA-AcOH | 1.15 | 0.98 | 1.22 | 1.03 | −0.01 |
| PFHxS | 2.6 | 2.8 | 2.9 | 3.2 | 0.16 |
| PFNA | 0.78 | 0.83 | 0.90 | 1.13 | 0.26* |
| PFDeA | 0.28 | 0.33 | 0.40 | 0.55 | 0.35** |
p < 0.05;
p < 0.01
On a relative scale, a high intake of two pilot whale dinners per month is associated with increases in the 14-year serum concentrations of PFOS, PFNA, and PFDeA by almost 25%, 50%, and 100%, when compared to concentrations in subjects eating little or no whale at all (Table 3). Fish dinners had a much weaker effect, although each weekly fish dinner augmented the PFHxS concentration by about 10%.
Discussion
This study observed a high frequency of detection of eight of the nine PFCs measured, with PFOS as the main PFC occurring at higher concentrations than PFOA and the sum of other PFCs in the samples analyzed. This pattern is similar to the one previously reported in other Western populations (8–11). Although the significance of likely exposure sources is unknown, the Faroe Islands may well share some routes of PFC exposure with other regions around the world. In addition, in this fishing community, marine food may contribute to dietary exposures (9, 17, 27–30).
The origin and transport pathways of PFCAs into the Northern Hemisphere water bodies have not been clearly established, and several theories exist. One explanation for the presence of PFOA and other PFCAs in the Northern Hemisphere is the atmospheric oxidation of volatile fluorotelomer precursors (13, 31–33). Another explanation is the oceanic transport of directly emitted PFCAs (34, 35). Any of these sources and transport pathways or a combination of them may have contributed to the exposure of Faroese residents to PFCs.
The correlations between the serum concentrations of PFOS and PFOA and of PFOA and PFNA are in agreement with data reported previously (10, 36, 37). As a possible explanation for such correlations, PFOA and other perfluorocarboxylates (PFCAs, e.g., PFNA) might both be formed from the biodegradation of the volatile fluorotelomer alcohols (14). The transformation of certain PFOS-related sulfonamides to PFOS and potentially to PFOA in the atmosphere could also represent a common mechanism for formation of both PFOS and PFOA.
Seafood has previously been identified as a major source of exposure to PFCs (18, 28), and concentrations of some PFCs in marine mammals appear to be increasing (38). Our results suggest that traditional whale meat consumption in the Faroes may be a major contributor to PFOS, PFNS, and PFDeA exposures, while fish dinners appear less important, except in regard to PFHxS. The latter PFC showed a noteworthy 3-fold increase over the 7-year interval between the two examinations. However, the sensitivity of the present study to detect dietary associations was limited by the crude information available, without details on the type of fish and portion sizes. Although imprecise, dietary intake levels at age 14 years may reflect long-term food habits and thereby indicate possible PFC accumulation from seafood. PFOS and PFHxS deserve particular attention, because serum concentrations tended to increase during the 7-year interval between examinations of the children, despite the decrease of whale meat consumption by over 50% during the same period and fish intake remaining constant.
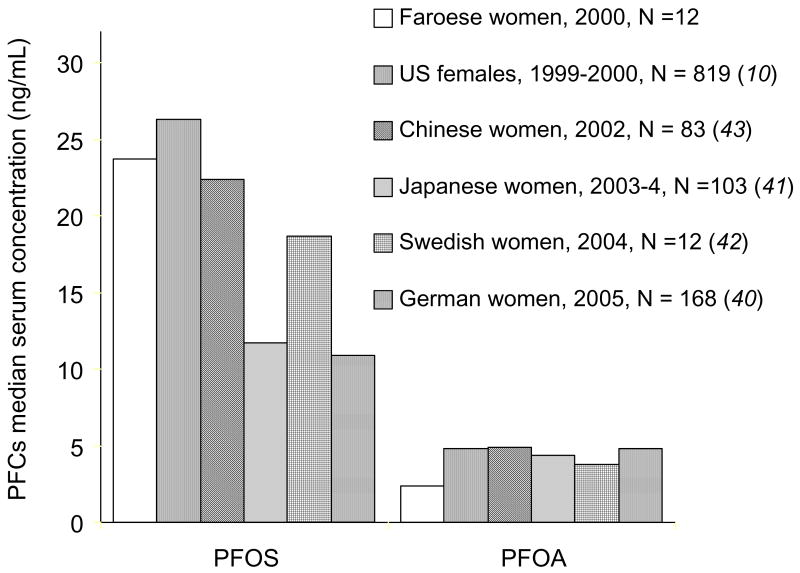
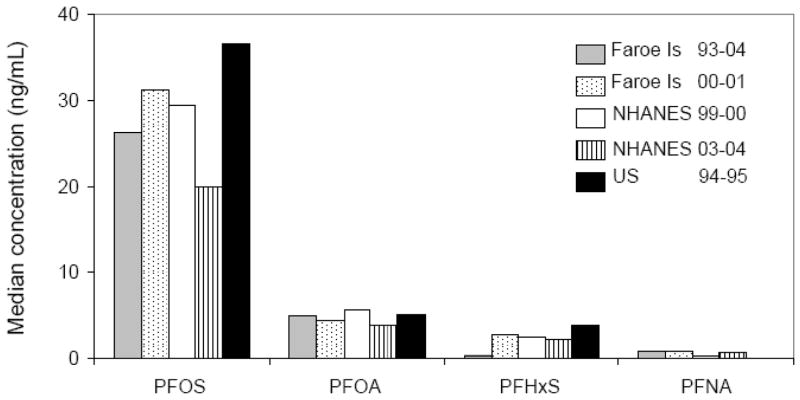
The mean concentrations of PFOA and PFOS in the mothers in this study are slightly below average concentrations reported in Danish pregnant women (39) and similar to average concentrations in US females during 1999 – 2000 (10) and in women in other countries (40–43) (Figure 1). Data on PFC concentrations in children are available only for the US (10, 36, 44). The mean concentrations of PFCs among the 14-year-old subjects in this study were similar to the results for 12 –19 year old adolescents from the general US population during 1999 – 2000 and 2003–04 (10) (Figure 2). The mean concentrations of PFOS and PFHxS in the 7-year-old children in this study were lower than those in younger children in the US (44).
Figure 1.
PFOS and PFOA among selected female populations
Figure 2.
Comparison of serum median concentrations among Faroe Island residents (7 years of age [N ) 103] and 14 years of age [N ) 79]), the U.S. general population aged 12–19 years (National Health and Nutrition Examination Survey [NHANES] 1999–2000, N ) 543 (10), and NHANES 2003–2004, N ) 640 (36)), and U.S. children aged 2–12 years (1994–1995, N ) 598 (44)). PFNA for children 2–12 years old was not available.
The collection of the children’s samples analyzed for this study spanned over a period of 12 years starting in 1993–4, when the samples for the 7 year old children were collected. We found detectable concentrations of most PFCs, regardless of year of collection (Tables 1–2). These concentration data, the first reported for Faroese residents, suggest that children from this North Atlantic community have been exposed to PFCs at least since the early 1990s. While estimated serum elimination half-lives of three of the best characterized PFCs range from approximately 4 to 7 years (PFHxS, 7.3 years; PFOA, 3.5 years and PFOS, 4.8 years) (45), the correlation between paired PFC concentrations among the Faroese children was rather poor. This finding would suggest that exposure sources were changing with age, or changing with time. In addition, the correlations–and lack thereof–between PFCs deserve to be further explored in regard to possible sources. In this light, the associations with whale meat consumption is noteworthy and suggests that other Northern populations whose traditional diets include marine mammals may also be exposed to PFCs trough their diet.
Acknowledgments
The authors thank Dr. Jack Reidy and Xavier Bryant for technical assistance. This research was supported in part by an appointment (A.W.) to the Research Participation Program at the Centers for Disease Control and Prevention (CDC), National Center for Environmental Health, Division of Laboratory Sciences, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and CDC. The cohort studies at the Faroe Islands were supported by grants from the US National Institute of Environmental Health Sciences (ES06112, ES09797 and ES12199), the U.S. Environmental Protection Agency (R830758) and the Danish Environmental Protection Agency.
Footnotes
Publisher's Disclaimer: Disclaimer
The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention, and the contents of this paper do not represent the official views of the NIEHS, NIH or any other funding agency.
References
- 1.Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: A review of monitoring and toxicological findings. Toxicol Sci. 2007;99:366–394. doi: 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy GL, Butenhoff JL, Olsen GW, O’Connor JC, Seacat AM, Perkins RG, Biegel LB, Murphy SR, Farrar DG. The toxicology of perfluorooctanoate. Crit Rev Toxicol. 2004;34:351–384. doi: 10.1080/10408440490464705. [DOI] [PubMed] [Google Scholar]
- 3.Kudo N, Kawashima Y. Toxicity and toxicokinetics of perfluorooctanoic acid in humans and animals. J Toxicol Sci. 2003;28:49–57. doi: 10.2131/jts.28.49. [DOI] [PubMed] [Google Scholar]
- 4.Butenhoff JL, Kennedy GL, Frame SR, O’Connor JC, York RG. The reproductive toxicology of ammonium perfluorooctanoate (APFO) in the rat. Toxicology. 2004;196:95–116. doi: 10.1016/j.tox.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Luebker DJ, York RG, Hansen KJ, Moore JA, Butenhoff JL. Neonatal mortality from in utero exposure to perfluorooctanesulfonate (PFOS) in Sprague-Dawley rats: Dose-response, and biochemical and pharamacokinetic parameters. Toxicology. 2005;215:149–169. doi: 10.1016/j.tox.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 6.Giesy JP, Kannan K. Global distribution of perfluorooctane sulfonate in wildlife. Environ Sci Technol. 2001;35:1339–1342. doi: 10.1021/es001834k. [DOI] [PubMed] [Google Scholar]
- 7.Houde M, Martin JW, Letcher RJ, Solomon KR, Muir DCG. Biological monitoring of polyfluoroalkyl substances: A review. Environ Sci Technol. 2006;40:3463–3473. doi: 10.1021/es052580b. [DOI] [PubMed] [Google Scholar]
- 8.Kannan K, Corsolini S, Falandysz J, Fillmann G, Kumar KS, Loganathan BG, Mohd MA, Olivero J, Van Wouwe N, Yang JH, Aldous KM. Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environ Sci Technol. 2004;38:4489–4495. doi: 10.1021/es0493446. [DOI] [PubMed] [Google Scholar]
- 9.Taniyasu S, Kannan K, Horii Y, Hanari N, Yamashita N. A survey of perfluorooctane sulfonate and related perfluorinated organic compounds in water, fish, birds, and humans from Japan. Environ Sci Technol. 2003;37:2634–2639. doi: 10.1021/es0303440. [DOI] [PubMed] [Google Scholar]
- 10.Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Tully JS, Needham LL. Serum concentrations of 11 polyfluoroalkyl compounds in the US population: Data from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Sci Technol. 2007;41:2237–2242. doi: 10.1021/es062686m. [DOI] [PubMed] [Google Scholar]
- 11.Olsen GW, Church TR, Miller JP, Burris JM, Hansen KJ, Lundberg JK, Armitage JB, Herron RM, Medhdizadehkashi Z, Nobiletti JB, O’Neill EM, Mandel JH, Zobel LR. Perfluorooctanesulfonate and other fluorochemicals in the serum of American Red Cross adult blood donors. Environ Health Perspect. 2003;111:1892–1901. doi: 10.1289/ehp.6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prevedouros K, Cousins IT, Buck RC, Korzeniowski SH. Sources, fate and transport of perfluorocarboxylates. Environ Sci Technol. 2006;40:32–44. doi: 10.1021/es0512475. [DOI] [PubMed] [Google Scholar]
- 13.Wallington TJ, Hurley MD, Xia J, Wuebbles DJ, Sillman S, Ito A, Penner JE, Ellis DA, Martin J, Mabury SA, Nielsen OJ, Andersen MPS. Formation of C7F15COOH (PFOA) and other perfluorocarboxylic acids during the atmospheric oxidation of 8: 2 fluorotelomer alcohol. Environ Sci Technol. 2006;40:924–930. doi: 10.1021/es051858x. [DOI] [PubMed] [Google Scholar]
- 14.Ellis DA, Martin JW, De Silva AO, Mabury SA, Hurley MD, Sulbaek Andersen MP, Wallington TJ. Degradation of fluorotelomer alcohols: A likely atmospheric source of perfluorinated carboxylic acids. Environ Sci Technol. 2004;38:3316–3321. doi: 10.1021/es049860w. [DOI] [PubMed] [Google Scholar]
- 15.Tomy GT, Tittlemier SA, Palace VP, Budakowski WR, Braekevelt E, Brinkworth L, Friesen K. Biotransformation of N-ethyl perfluorooctanesulfonamide by rainbow trout (Onchorhynchus mykiss) liver microsomes. Environ Sci Technol. 2004;38:758–762. doi: 10.1021/es034550j. [DOI] [PubMed] [Google Scholar]
- 16.Dinglasan-Panlilio MJA, Mabury SA. Significant residual fluorinated alcohols present in various fluorinated materials. Environ Sci Technol. 2006;40:1447–1453. doi: 10.1021/es051619+. [DOI] [PubMed] [Google Scholar]
- 17.Kannan K, Corsolini S, Falandysz J, Oehme G, Focardi S, Giesy JP. Perfluorooctanesulfonate and related fluorinated hydrocarbons in marine mammals, fishes, and birds from coasts of the Baltic and the Mediterranean Seas. Environ Sci Technol. 2002;36:3210–3216. doi: 10.1021/es020519q. [DOI] [PubMed] [Google Scholar]
- 18.Tittlemier SA, Pepper K, Seymour C, Moisey J, Bronson R, Cao XL, Dabeka RW. Dietary exposure of Canadians to perfluorinated carboxylates and perfluorooctane sulfonate via consumption of meat, fish, fast foods, and food items prepared in their packaging. Journal of agricultural and food chemistry. 2007;55:3203–3210. doi: 10.1021/jf0634045. [DOI] [PubMed] [Google Scholar]
- 19.Tomy GT, Budakowski W, Halldorson T, Helm PA, Stern GA, Friesen K, Pepper K, Tittlemier SA, Fisk AT. Fluorinated organic compounds in an eastern Arctic marine food web. Environ Sci Technol. 2004;38:6475–6481. doi: 10.1021/es049620g. [DOI] [PubMed] [Google Scholar]
- 20.Van Leeuwen SPJ, van der Veen I, Leonards PEG, De Boer J. Perfluorinated compounds in edible Dutch fish. Organohalogen Compd. 2006;68:535–538. [Google Scholar]
- 21.Barr DB, Weihe P, Davis MD, Needham LL, Grandjean P. Serum polychlorinated biphenyl and organochlorine insecticide concentrations in a Faroese birth cohort. Chemosphere. 2006;62:1167–1182. doi: 10.1016/j.chemosphere.2005.06.063. [DOI] [PubMed] [Google Scholar]
- 22.Debes F, Budtz-Jorgensen E, Weihe P, White RF, Grandjean P. Impact of prenatal methylmercury exposure on neurobehavioral function at age 14 years. Neurotoxicology and Teratology. 2006;28:536–547. doi: 10.1016/j.ntt.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Grandjean P, Budtz-Jorgensen E, Steuerwald U, Heinzow B, Needham LL, Jorgensen PJ, Weihe P. Attenuated growth of breast-fed children exposed to increased concentrations of methylmercury and polychlorinated biphenyls. Faseb Journal. 2003;17:699–701. doi: 10.1096/fj.02-0661fje. [DOI] [PubMed] [Google Scholar]
- 24.Kuklenyik Z, Needham LL, Calafat AM. Measurement of 18 perfluorinated organic acids and amides in human serum using on-line solid-phase extraction. Anal Chem. 2005;77:6085–6091. doi: 10.1021/ac050671l. [DOI] [PubMed] [Google Scholar]
- 25.Van Leeuwen SPJ, Karrman A, van Bavel B, De Boer J, Lindstrom G. Struggle for quality in determination of perfluorinated contaminants in environmental and human samples. Environ Sci Technol. 2006;40:7854–7860. doi: 10.1021/es061052c. [DOI] [PubMed] [Google Scholar]
- 26.German External Quality Assessment Scheme (G-EQUAS) Available at http://www.g-equas.de/
- 27.Butt CM, Mabury SA, Muir DCG, Braune BM. Prevalence of long-chained perfluorinated carboxylates in seabirds from the canadian arctic between 1975 and 2004. Environ Sci Technol. 2007;41:3521–3528. doi: 10.1021/es062710w. [DOI] [PubMed] [Google Scholar]
- 28.Falandysz J, Taniyasu S, Gulkowska A, Yamashita N, Schulte-Oehlmann U. Is fish a major source of fluorinated surfactants and repellents in humans living on the Baltic coast? Environ Sci Technol. 2006;40:748–751. doi: 10.1021/es051799n. [DOI] [PubMed] [Google Scholar]
- 29.Van de Vijver KI, Hoff P, Das K, Brasseur S, Van Dongen W, Esmans E, Reijnders P, Blust R, De Coent W. Tissue distribution of perfluorinated chemicals in harbor seals (Phoca vitulina) from the Dutch Wadden Sea. Environ Sci Technol. 2005;39:6978–6984. doi: 10.1021/es050942+. [DOI] [PubMed] [Google Scholar]
- 30.Verreault J, Houde M, Gabrielsen GW, Berger U, Haukas M, Letcher RJ, Muir DCG. Perfluorinated alkyl substances in plasma, liver, brain, and eggs of glaucous gulls (Larus hyperboreus) from the Norwegian Arctic. Environ Sci Technol. 2005;39:7439–7445. doi: 10.1021/es051097y. [DOI] [PubMed] [Google Scholar]
- 31.Shoeib M, Harner T, Vlahos P. Perfluorinated chemicals in the Arctic atmosphere. Environ Sci Technol. 2006;40:7577–7583. doi: 10.1021/es0618999. [DOI] [PubMed] [Google Scholar]
- 32.Stock NL, Furdui VI, Muir DCG, Mabury SA. Perfluoroalkyl contaminants in the canadian arctic: Evidence of atmospheric transport and local contamination. Environ Sci Technol. 2007;41:3529–3536. doi: 10.1021/es062709x. [DOI] [PubMed] [Google Scholar]
- 33.Young CJ, Furdui VI, Franklin J, Koerner RM, Muir DCG, Mabury SA. Perfluorinated acids in arctic snow: New evidence for atmospheric formation. Environ Sci Technol. 2007;41:3455–3461. doi: 10.1021/es0626234. [DOI] [PubMed] [Google Scholar]
- 34.Armitage J, Cousins IT, Buck RC, Prevedouros K, Russell MH, MacLeod M, Korzeniowski SH. Modeling global-scale fate and transport of perfluorooctanoate emitted from direct sources. Environ Sci Technol. 2006;40:6969–6975. doi: 10.1021/es0614870. [DOI] [PubMed] [Google Scholar]
- 35.Wania F. A global mass balance analysis of the source of perfluorocarboxylic acids in the Arctic ocean. Environ Sci Technol. 2007;41:4529–4535. doi: 10.1021/es070124c. [DOI] [PubMed] [Google Scholar]
- 36.Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL. Polyfluoroalkyl chemicals in the US population: Data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ Health Perspect. 2007;115:1596–1602. doi: 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olsen GW, Mair DC, Reagen WK, Ellefson ME, Ehresman DJ, Butenhoff JL, Zobel LR. Preliminary evidence of a decline in perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) concentrations in American Red Cross blood donors. Chemosphere. 2007;68:105–111. doi: 10.1016/j.chemosphere.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 38.Dietz R, Bossi R, Riget FF, Sonne C, Born EW. Increasing Perfluoroalkyl Contaminants in East Greenland Polar Bears (Ursus maritimus): A New Toxic Threat to the Arctic Bears. Environ Sci Technol. doi: 10.1021/es7025938. in press Web Release Date: 29–Feb-2008. [DOI] [PubMed] [Google Scholar]
- 39.Fei CY, McLaughlin JK, Tarone RE, Olsen J. Perfluorinated chemicals and fetal growth: A study within the Danish National Birth Cohort. Environ Health Perspect. 2007;115:1677–1682. doi: 10.1289/ehp.10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fromme H, Midasch O, Twardella D, Angerer J, Boehmer S, Liebl B. Occurrence of perfluorinated substances in an adult German population in southern Bavaria. Int Arch Occup Environ Health. 2007;80:313–319. doi: 10.1007/s00420-006-0136-1. [DOI] [PubMed] [Google Scholar]
- 41.Harada K, Koizumi A, Saito N, Inoue K, Yoshinaga T, Date C, Fujii S, Hachiya N, Hirosawa I, Koda S, Kusaka Y, Murata K, Omae K, Shimbo S, Takenaka K, Takeshita T, Todoriki H, Wada Y, Watanabe T, Ikeda M. Historical and geographical aspects of the increasing perfluorooctanoate and perfluorooctane sulfonate contamination in human serum in Japan. Chemosphere. 2007;66:293–301. doi: 10.1016/j.chemosphere.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 42.Karrman A, Ericson I, van Bavel B, Darnerud PO, Aune M, Glynn A, Lignell S, Lindstrom G. Exposure of perfluorinated chemicals through lactation: Levels of matched human milk and serum and a temporal trend, 1996–2004, in Sweden. Environ Health Perspect. 2007;115:226–230. doi: 10.1289/ehp.9491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin YH, Saito N, Harada KH, Inoue K, Koizumi A. Historical trends in human serum levels of perfluorooctanoate and perfluorooctane sulfonate in Shenyang, China. Tohoku Journal of Experimental Medicine. 2007;212:63–70. doi: 10.1620/tjem.212.63. [DOI] [PubMed] [Google Scholar]
- 44.Olsen GW, Church TR, Hansen KJ, Burris JM, Butenhoff JL, Mandel JH, Zobel LR. Quantitative Evaluation of Perfluorooctanesulfonate (PFOS) and Other Fluorochemicals in the Serum of Children. J Childr Health. 2004;2:53–76. [Google Scholar]
- 45.Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, Zobel LR. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect. 2007;115:1298–1305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]