Abstract
MyoD and Myf5 belong to the family of basic helix-loop-helix transcription factors that are key operators in skeletal muscle differentiation. MyoD and Myf5 genes are selectively activated during development in a time and region-specific manner and in response to different stimuli. However, molecules that specifically regulate the expression of these two genes and the pathways involved remain to be determined. We have recently shown that the serum response factor (SRF), a transcription factor involved in activation of both mitogenic response and muscle differentiation, is required for MyoD gene expression. We have investigated here whether SRF is also involved in the control of Myf5 gene expression, and the potential role of upstream regulators of SRF activity, the Rho family G-proteins including Rho, Rac, and CDC42, in the regulation of MyoD and Myf5. We show that inactivation of SRF does not alter Myf5 gene expression, whereas it causes a rapid extinction of MyoD gene expression. Furthermore, we show that RhoA, but not Rac or CDC42, is also required for the expression of MyoD. Indeed, blocking the activity of G-proteins using the general inhibitor lovastatin, or more specific antagonists of Rho proteins such as C3-transferase or dominant negative RhoA protein, resulted in a dramatic decrease of MyoD protein levels and promoter activity without any effects on Myf5 expression. We further show that RhoA-dependent transcriptional activation required functional SRF in C2 muscle cells. These data illustrate that MyoD and Myf5 are regulated by different upstream activation pathways in which MyoD expression is specifically modulated by a RhoA/SRF signaling cascade. In addition, our results establish the first link between RhoA protein activity and the expression of a key muscle regulator.
INTRODUCTION
The formation of skeletal muscle results from the determination of mesodermal cells into myoblasts, which then will differentiate into mature skeletal muscle. These two processes of muscle cell determination and differentiation are orchestrated by a family of muscle-regulatory factors (MRFs) belonging to the basic helix-loop-helix protein family and include MyoD, Myf5, myogenin, and MRF4 (Weintraub et al., 1991; Rudnicki and Jaenisch, 1995). All four MRFs are characterized by their ability to convert a variety of nonmuscle cells into myocytes expressing muscle-specific genes (Weintraub et al., 1991; Olson and Klein, 1994). Among these myogenic factors, MyoD and Myf5 are the only two MRFs expressed in dividing myoblasts before the onset of differentiation, implying that they must play important roles in early muscle determination. Indeed, mice lacking MyoD and Myf5 are devoid of muscle precursor cells and muscle fibers (Rudnicki et al., 1993). Interestingly, mice lacking either Myf5 or MyoD, although capable of muscle formation (Braun et al., 1992; Rudnicki et al., 1992), show specific phenotypes indicating that these two genes control different aspects of muscle development: Myf5 has a fundamental role in correct muscle cell positioning (Tajbakhsh et al., 1996) and activation of MyoD in parallel with Pax3 (Maroto et al., 1997; Tajbakhsh et al., 1997), whereas MyoD regulates muscle cell regeneration (Megeney et al., 1996). Part of these specificities of action between MyoD and Myf5 may lie in different spatio-temporal expressions. Myf5 is the first myogenic factor to be expressed in the dorso-medial part of the myotome, whereas MyoD is detectable only 1–2 d after Myf5 in a more lateral location (Weintraub et al., 1991; Cossu et al., 1996). One explanation to these observations would be that Myf5 and MyoD are activated by different upstream signaling pathways. In vitro muscle cell culture showed that ligand-activated nuclear receptors of thyroid hormone family and insulin-like growth factors (IGFs) regulate MyoD expression without having any effects on Myf5 gene expression (Carnac et al., 1992; Montarras et al., 1996). Moreover, a recent report implicated the glucocorticoid receptor and AP1 in a positive regulation of Myf5 expression in myogenic cell line (Auradéet al., 1997). Interestingly, in vivo experiments in mice showed that the dorsal neural tube releases specific factor(s) capable of activating Myf5, whereas MyoD is under the control of factor(s) secreted from adjacent dorsal ectoderm (Cossu et al., 1996). However, such endogenous diffusible factors are still unidentified. In conclusion, a picture emerged where part of muscle specification could be the result of a selective activation of MyoD or Myf5 gene expression. The identity of molecules that activate MyoD and Myf5 expression and of their downstream molecular components remains to be established.
We have shown that the serum response factor (SRF), a DNA-binding protein containing a highly conserved DNA-binding/dimerization domain termed the MADS box (reviewed by Treisman, 1990), is required for both in vitro muscle differentiation (Vandromme et al., 1992) and MyoD gene expression (Gauthier-Rouviere et al., 1996; Soulez et al., 1996). However, these studies did not investigate whether SRF is also required for Myf5 gene expression or whether SRF-dependent pathway is peculiar to MyoD. In addition, we wished to identify potential upstream regulators of this SRF/MyoD-regulatory cascade. One signaling pathway recently shown to be involved in the activation of SRF is mediated by the Rho family GTPases (Hill et al., 1995). The mammalian Rho GTPases form a subgroup of Ras family GTP-binding proteins including RhoA, B, C, D, E, and G; Rac1 and 2; Rac E; CDC42Hs, and TC10 (reviewed by Van Aelst and D’Souza-Schorey, 1997). Rho GTPases play crucial roles in diverse cellular events such as actin cytoskeletal organization, cell growth control, and membrane trafficking. The role of Rho GTPases in actin cytoskeleton rearrangement (Tapon and Hall, 1997) raised the question of their potential implication in muscle differentiation. The Drosophila homologues of Rac1, Rac2, and CDC42 are highly expressed in mesoderm cells (Luo et al., 1994). When Rac1 mutant proteins were expressed in Drosophila muscle precursor cells, myoblasts failed to fuse properly. In contrast, overexpression of CDC42 mutant proteins did not perturb myoblasts fusion but seemed to control their migration (Luo et al., 1994). In conclusion, it was postulated that Rac and CDC42 may regulate muscle development most likely through their effects on fusion and actin cytoskeleton rearrangement. There have been no reports on a role of Rho in skeletal muscle differentiation. Recent reports revealed that Rho protein family members also play a crucial role in regulating nuclear signaling: RhoA is required for SRF activation whereas Rac1 and CDC42Hs can activate C-jun N-terminal kinases (JNK)/stress-activated protein kinase (SAPK) and P38 Kinase (Coso et al., 1995; Hill et al., 1995; Minden et al., 1995). Here we demonstrate that specific inactivation of SRF did not affect Myf5 gene expression while MyoD was inhibited efficiently. We further show that this specificity of regulation resides upstream of SRF. Indeed, blocking the small G-protein RhoA, but not CDC42 and Rac, also resulted in the extinction of MyoD expression without affecting Myf5 expression. These data clearly show that SRF and the small G-protein RhoA can act as molecular determinants of a specific pathway that controls MyoD, but not Myf5, gene expression.
MATERIALS AND METHODS
Reagents
Ham’s-F12, G418 (geneticin) were purchased from Life Technologies/BRL (Cergy-Pontoise, France). DMEM came from ICN (Orsay, France). Calf serum came from DAP (Neuf-Brisach, France). Lovastatin was a generous gift from Merck Sharp and Dohme Laboratory (West Point, PA). Botulinum C3 was a gift from Dr. P. Bocquet (INSERM U452, faculte de Medicine, Nice 06107, France).
Cell Culture
C2.7 myoblasts (Pinset et al., 1988) and L6G7 subclone (Vandromme et al., 1992) were routinely grown in proliferation medium (a 1:1 mixture of Ham’s-F12/DMEM) supplemented with 10% FCS (vol/vol) and subcultured twice a week. For lovastatin and clostridium botulinum experiments, myoblasts were plated at 4000 cells per cm2 on plastic dishes and grown for 2 d in proliferation medium before treatments.
Control C2CL2 myoblasts and C2CL2 SRF antisense clone 6 (Soulez et al., 1996) were plated at a density of 60,000 cells per 60-mm-diameter dish, in DMEM plus 10% FCS. They were grown for 3 d in presence or absence of 10−6 M dexamethasone.
Microinjection
For microinjection studies, L6 and C2–7 cells were grown in proliferation medium at a density of 10,000 cells/cm2. Forty eight hours after plating, cells were microinjected with purified DNA-binding domain of SRF protein (SRF-DB) at 0.5 mg/ml in the needle (Gauthier-Rouviere et al., 1993) in a solution containing mouse markers IgGs (0.5 mg/ml in the needle). After microinjection, cells were kept in the same medium and returned to the incubator; 6 h later, cells were fixed and stained for Myf5 expression and the presence of the marker antibodies.
Transfections
1. Stable Transfection of MyoD Promoter.
C2–7 cells were cotransfected using Lipofectamin (Life Technologies/BRL) as described by the supplier with a chimeric construct containing DRR and the PRR regions of MyoD promoter driving βgal expression (Tapscott et al., 1992) and PSV2neo DNA carrying the neomycin marker (M ratio between MyoD promoter and PSV2neo was 15:1). The transfected cells were selected in the presence of 800 μg/ml G418 (geneticin, Life Technologies/BRL). Pools of clones were isolated after 10 d, passaged into stable cell lines, and then analyzed for βgal activity as previously described (Nielsen et al., 1983).
2. Transient Transfection of −630 MLC1A, −630(mSRF)MLC1A Promoter Gene.
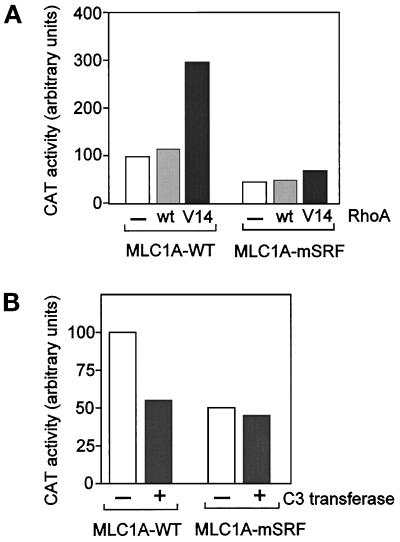
C2–7 cells were cotransfected using Lipofectamin (Life Technologies/BRL) as described by the supplier with 1 μg of chimeric construct containing the first 630 base pairs (bp) of MLC1A promoter, −630 MLC1A, or its mutated form in the CArG box, −630 (mSRF)MLC1A (Catala et al., 1995; kindly provided by M. Buckingham, Institut Pasteur, Paris) with either 0.8 μg of empty vector cytomegalovirus (CMV), CMVRhoA-WT or CMVRhoA-Val14, and CMVβgal. Forty eight hours after transfection, chloramphenicol acetyltransferase (CAT) activity was measured as described by Nielsen et al. (1989) and corrected with respect to βgal activity (Figure 6A). For C3 transferase treatments, C2 cells were treated 24 h after transfection with 4 μg/ml C3 transferase (or not treated, as indicated) for a further 24 h before assaying for CAT as above.
Figure 6.
RhoA requires a functional SRF-binding site to regulate the activity of a reporter construct containing the MLC1A gene promoter in C2 myoblasts. (A) C2 cells plated in 60-mm dishes were transfected with 0.8 μg of the reporter constructs, either MLC1AWT-CAT or MLC1A(mSRF)-CAT, together with either 0.8 μg of empty vector (CMV), CMVRhoA-WT or CMVRhoA-Val14, and 0.4 μg of CMVβgal. Forty-eight hours after transfection, CAT activity was measured and corrected with respect to βgal activity. (B) C2 cells plated in 60-mm dishes were transfected with 1 μg of MLC1AWT-CAT or MLC1A(mSRF)-CAT together with 1 μg of CMVβgal. Twenty-four hours after transfection, cells were treated with C3 transferase at 4 μg/ml (or not, as indicated) for 24 h. CAT activity was then determined as in panel A. CAT activities are expressed relative to that of MLC1AWT-CAT transfected with the empty vector set as 100%.
3. Transient Transfection of CMVβgal, Myc-tagged CDC42Hs-N17, Rac1-N17, RhoA-N19.
C2 cells were plated at 10,000 cells/cm2 (in 35-mm dishes) in proliferation medium. After 24 h, transfection of plasmid DNA was performed using DOSPER lipids (Boehringer Mannheim, Indianapolis, IN) as described by the supplier. One microgram of CMVβgal (Stratagene, La Jolla, CA), Myc-tagged CDC42Hs-N17, and Rac1-N17 or RhoA-N19 expression vectors (a generous gift of N. Lamarche and A. Hall) were used for each condition. Forty eight hours after transfection of CMVβgal, cells were treated with 4 μg/ml C3 transferase and βgal activities were measured as previously described (Nielsen et al., 1983); 24 h after transfection of CMVβgal, Myc-tagged CDC42Hs-N17, Rac1-N17, or RhoA-N19 cells were fixed and processed for immunofluorescence analysis.
Immunofluorescence
Cells were fixed for 5 min in 3.7% formalin in PBS followed by a 30-s extraction in −20°C acetone and rehydratation in PBS containing 0.5% BSA. Cells were stained for injected mouse monoclonal marker antibody by using fluorescein-conjugated anti-mouse antibody (1:50; Cappel, Velizy, France). Expression of Myf5, MyoD, and βgal were analyzed by using a rabbit polyclonal anti-Myf5 antibody (directed against the N-terminal protein (Primig, Tajbakhsh, and Buckingham, manuscript in preparation; diluted 1:300), a mouse monoclonal antibody against MyoD diluted 1:5 (Dako/Novocastra, Burlingame, CA), and a mouse monoclonal antibody against βgal (Boehringer Mannheim). Primary antibody diluted in PBS/BSA was incubated for 1 h at 37°C, and then washed in PBS, followed by a 30-min incubation with biotinylated anti-rabbit or anti-mouse antibody (1:200, Amersham, Les Ulis, France). Staining was finally revealed after an incubation of 30 min with streptavidin-Texas red (1:200; Amersham). DNA was stained with Hoechst (0.1 μg/ml; Sigma Chemical, St. Louis, MO).
Immunoblotting
Cells cultured in 35- or 60-mm dishes were rinsed twice in cold PBS and solubilized into Laemmli sample buffer (40 mM Tris-HCl, pH 6.8; 5 mM DTT, 1% SDS, 7.5% glycerol; 0.01% bromophenol blue) by direct addition to the dish. After scraping and boiling, the sample (50–100 μg of proteins) was loaded on a 10% polyacrylamide gel. After electrophoresis, protein were transferred to nitrocellulose. The membrane was saturated in PBS containing 5% dry milk for 1 h and subsequently incubated with the primary antibody for 1 h. The following antibodies were used: rabbit polyclonal antibodies directed against Myf5 C-terminal protein, diluted 1:500 (Primig, Tajbakhsh, and Buckingham, manuscript in preparation); polyclonal anti-MyoD, diluted 1:400 (C-20 from Santa Cruz Biotechnology, Santa Cruz, CA); anti-annexin diluted 1:2000 (Rothut et al., 1995); and mouse monoclonal antibody anti-α-tubulin diluted 1:10,000 (clone DMA1A). Membranes were washed and incubated with a peroxidase-conjugated secondary antibody (Amersham) at a dilution of 1:5000. After several washes, membranes were incubated with chemoluminescence reagents. Autoradiographs were scanned to determine MyoD and Myf5 protein levels, which were corrected for variations in the amount of protein loaded on each track using annexin or α-tubulin levels.
RESULTS
Inactivation of SRF Inhibits MyoD but Does Not Alter Myf5 Gene Expression in Muscle Cell Lines
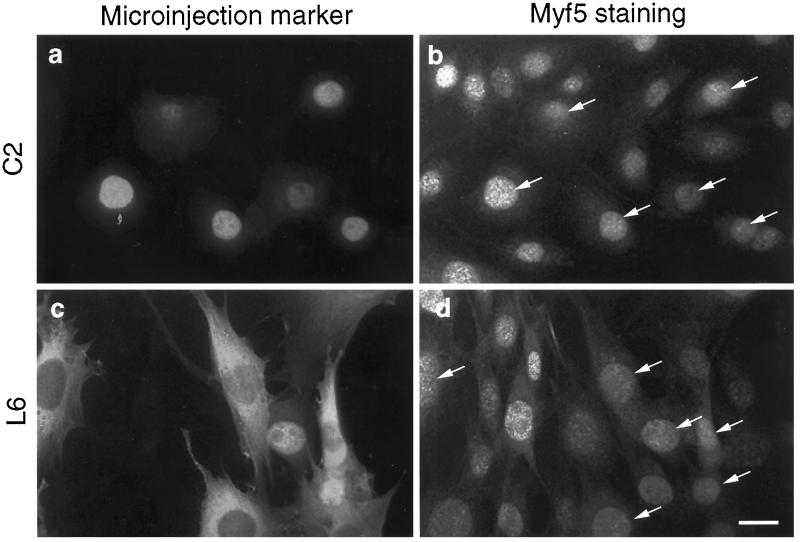
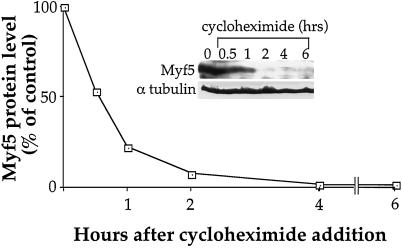
We have shown previously that inhibition of SRF activity or expression in mouse myogenic cell lines rapidly abolishes MyoD gene expression (Gauthier-Rouvière et al., 1996). To assess the specificity of such regulation, we examined the effect of SRF inhibition on the expression of Myf5 gene, another member of the MyoD gene family expressed, like MyoD, at the myoblast stage in myogenic C2 cells. Inhibition of SRF in mouse C2 myoblasts was first effected as previously described by microinjection of purified dominant negative SRF proteins, SRF-DB (Gauthier-Rouvière et al., 1993), which results in the rapid extinction of MyoD expression in mouse C2 myoblasts (Gauthier-Rouvière et al., 1996). We therefore examined the effect of SRF inhibition on Myf5 gene expression by immunofluorescence using a Myf5 polyclonal antibody (Primig, Tajbakhsh, and Buckingham, manuscript in preparation; see also MATERIALS AND METHODS). C2 myoblasts were grown at subconfluence under proliferation conditions and microinjected with purified SRF-DB. Six hours after injection, cells were fixed and processed for immunofluorescence analysis. As shown in Figure 1 (panels a and b), injected C2 cells present a level of Myf5 protein (95%, n = 60) comparable to noninjected control cells. These data show that inhibition of SRF in C2 cells does not seem to affect the expression of Myf5, whereas under the same conditions, we observed a complete loss of MyoD expression (Carnac et al., unpublished results). It was reported previously that down-regulation of MyoD resulted in increased levels of Myf5 both in vivo and in vitro (Montarras et al., 1996; Rudnicki et al., 1992). Therefore, to avoid a potential cross-regulation between MyoD and Myf5 expression patterns, we conducted the same experiment in rat L6 cells, which are devoid of MyoD but express high levels of Myf5. The same result was obtained with L6 cells in which all injected cells show a level of Myf5 protein comparable to noninjected control cells (98%, n = 55; Figure 1, c and d). These data suggest that inhibition of SRF activity does not affect the expression of Myf5, whereas it rapidly abolishes MyoD expression. However, the possibility remains that Myf5 protein could be more stable than MyoD protein. We therefore measured the turnover of Myf5 protein. For this purpose, C2 cells were treated with cycloheximide (CHX) at a concentration of 15 μg/ml, which blocks protein synthesis, and Myf5 protein level was analyzed by Western blotting at different times after CHX addition. As shown in Figure 2, the half-life of Myf5 protein is fairly short, ∼30–40 min. Such a half-life is similar to that of MyoD protein (45 min; Thayer et al., 1989) and another basic helix-loop-helix protein, E12 (60 min; Kho et al., 1997). Therefore, the lack of effect of SRF inhibition on Myf5 gene expression cannot be due to an extended half-life of Myf5 protein.
Figure 1.
Inhibition of SRF through microinjection of purified SRF-DB does not modify Myf5 expression in muscle cell lines. Mouse C2 (upper panels) and rat L6 (lower panels) cells were cultured in proliferation medium. They were injected with a solution containing mouse marker antibodies and purified SRF-DB proteins, a dominant negative form of SRF corresponding to the DNA-binding region of SRF but lacking the transactivation domain (Gauthier-Rouvière et al., 1993). Six hours after microinjection, cells were fixed and double stained for microinjected markers with biotinylated anti-mouse IgGs followed by streptavidin-Texas red (both from Amersham) (panels a and c) and Myf5 expression with rabbit anti-Myf5 polyclonal antibody followed by fluorescein-conjugated anti-rabbit IgGs (panels b and d). In both cases, injected cells (marked by arrows) present a level of Myf5 protein comparable to noninjected control surrounding cells.
Figure 2.
Myf5 has a short half-life. C2 cells were cultured for 48 h in proliferation medium before being treated with 15 μg/ml CHX added to the medium. Myf5 and α-tubulin protein levels were followed by immunoblot analysis at the indicated time after CHX addition. Immunoblots were quantified by densitometric scanning, and Myf5 protein levels were expressed as the ratio of Myf5/α-tubulin signals.
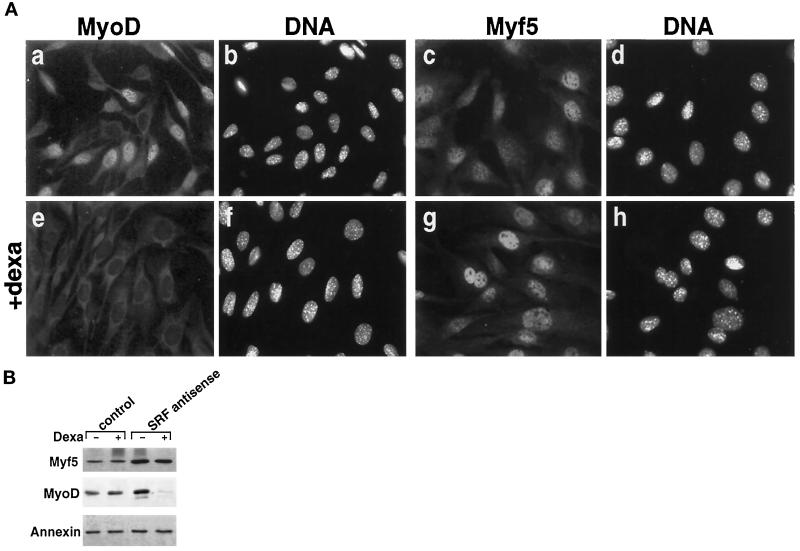
Inhibition of SRF can be also effected by a different approach, with an SRF antisense strategy. Indeed, using a C2 cell line derivative stably transfected to express glucocorticoid-inducible SRF antisense mRNA (Soulez et al., 1996), we showed that induction of SRF antisense after dexamethasone treatment down-regulates SRF expression and abolishes MyoD expression (Gauthier-Rouvière et al., 1996). To verify that the suppression of SRF expression, like the inhibition of SRF activity, would not affect Myf5 expression, we used this antisense-SRF-inducible cell line. Cells were grown in proliferation medium in the presence of 10−6 M dexamethasone to induce the production of SRF antisense mRNA. After 3 d, cells were fixed and analyzed for MyoD and Myf5 expression by immunofluorescence as detailed in Figure 3A. Induction of antisense SRF (after dexamethasone treatment) resulted in a complete inhibition of MyoD gene expression as previously described (panels a and e). In contrast, Myf5 levels remain constant whatever the conditions (Figure 3A, panels c and g). To confirm that the expression of Myf5 was not affected by antisense SRF, Western blot experiments were performed in control cells and in inducible antisense SRF C2 myoblasts. In this experiment, annexin level was used as an internal loading control. Immunoblot analysis shown in Figure 3B confirms the data obtained by immunofluorescence: cells induced with antisense SRF present barely detectable levels of MyoD proteins (see also Soulez et al., 1996), whereas Myf5 protein levels remained constant or slightly higher (Figure 3B). Thus, by using two different approaches to inhibit SRF, we established that SRF is not required for Myf5 gene expression.
Figure 3.
Inhibition of SRF expression by SRF antisense does not inhibit Myf5 expression while blocking efficiently MyoD expression. Control C2 cells (stably transfected with the glucocorticoid receptor only) and SRF antisense C2 cells (stably transfected with the glucocorticoid receptor and dexamethasone-inducible antisense SRF) (see Soulez et al., 1996) were cultured in proliferation medium for 3 d in the presence or absence of 10−6 M dexamethasone to induce the production of SRF antisense. (A) cells were fixed and stained for MyoD (a and e) or Myf5 (c and g) and for DNA with Hoechst dye (b, d, f, and h) after 3 d of culture in the absence (a, b, c, and d) or presence (e, f, g, and h) of 10−6 M dexamethasone (Dexa). (B) Culture conditions are the same as the one described above. Three days after plating, proteins were extracted and immunoblot analyses were performed with rabbit anti-MyoD antibodies, rabbit anti-Myf5 antibodies, and rabbit anti-annexin antibodies as described in MATERIALS AND METHODS.
In conclusion, taken together with our previous reported results (Gauthier-Rouvière et al., 1996), these data clearly show that SRF is involved in a specific pathway that controls MyoD, but not Myf5, gene expression.
Inactivation of Rho GTPase Activities Represses MyoD, but Not Myf5, Gene Expression
Recently, the Rho family of GTP-binding proteins, including Rho, Rac, and CDC42 subfamilies, has been implicated as a regulator of SRF activity (Hill et al., 1995). To determine whether the Rho family of GTPases can also participate in a regulatory pathway affecting specifically MyoD, we inactivated these small G-proteins using several methods.
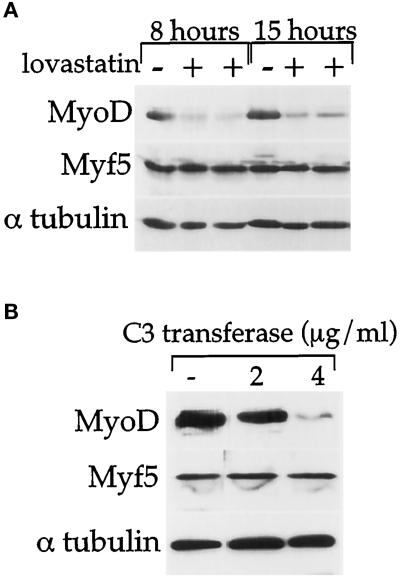
Blocking synthesis of isoprenyl moieties with drugs such as lovastatin has been found to be an effective way of inactivating the small GTP-binding proteins (Fenton et al., 1993). More specifically, the exoenzyme C3 transferase inactivates Rho A, B, and C proteins by ADP-ribosylation but not CDC42 and Rac (for a review, Aktories and Hall, 1989). C3 exoenzyme can be introduced into cells by simple incubation in the culture medium (Morii and Narumiya, 1995). C2 cells were grown in proliferation medium in the presence of 50 μM lovastatin for 8 and 15 h or increasing concentrations of C3 transferase for 24 h. Total proteins were subsequently analyzed by Western blotting for expression of MyoD, Myf5, and α-tubulin as internal loading control. Western blot analysis revealed that addition of lovastatin reduced MyoD protein level by threefold after 8 h and fourfold after 15 h (Figure 4A). C3 transferase strongly repressed MyoD gene expression by 20-fold at 4 μg/ml (Figure 4B). In contrast, the level of Myf5 protein remained constant throughout lovastatin or C3 transferase treatments (Figure 4, A and B). It is worth noting that SRF protein level (as assessed by Western blot analysis) remained unchanged after treatments with C3 transferase (our unpublished results).
Figure 4.
A lovastatin-/C3 transferase-sensitive G-protein is required for MyoD, but not for Myf5, gene expression. C2 cells were cultured for 48 h in proliferation medium and 50 μM lovastatin (A) or 2–4 μg/ml C3 transferase (B) was added to the medium. (A) Eight and 15 h after lovastatin treatment, proteins were analyzed by Western blot for MyoD, Myf5, and α-tubulin expression. Two different protein samples of lovastatin-treated cells were loaded. (B) Twenty-four hours after C3 transferase treatment, proteins were analyzed by Western blot for MyoD, Myf5, and α-tubulin expression.
In conclusion, a lovastatin-/C3 transferase-sensitive G-protein activity, most likely Rho, appears to be crucial for MyoD, but not for Myf5, gene expression.
A Dominant Negative Form of RhoA Efficiently Inhibits MyoD, but Not Myf5, Gene Expression
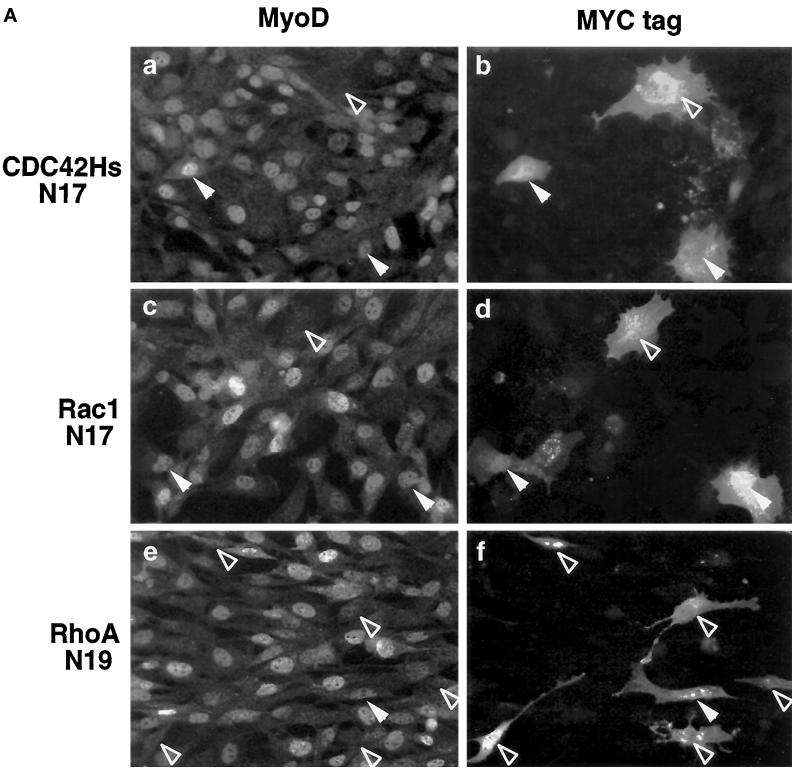
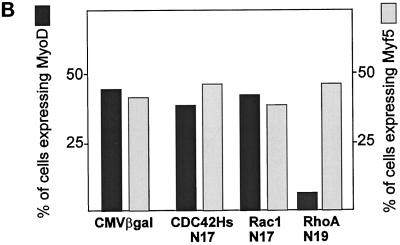
In Swiss 3T3 fibroblasts, CDC42, Rac, and Rho proteins have been placed in a hierarchical cascade where CDC42 activates Rac, which in turn activates Rho (Nobes and Hall, 1995). However, activation of SRF by CDC42 and Rac occurs independently of Rho, suggesting that at least two distinct signaling pathways converge on SRF (Hill et al., 1995). To determine whether the Rho family G-proteins differ in their effects on MyoD gene expression, we overexpressed dominant negative inhibitor constructs of Rho proteins known as CDC42Hs-N17, Rac1-N17, and RhoA-N19: such variants of Rho proteins have point mutations that sequester GTP exchange factors and act as dominant negative on endogenous Rho proteins (Ridley and Hall, 1992; Ridley et al., 1992; Nobes and Hall, 1995). C2 myoblasts were transiently transfected with plasmids encoding CMV-driven Myc-tagged CDC42Hs-N17, Rac1-N17, or RhoA-N19. As a control, we transiently overexpressed CMVβgal. Twenty four hours after transfections, cells were fixed and analyzed by coimmunofluorescence for expression of Myc-tagged or βgal proteins and MyoD (Figure 5, A and B). Overexpression of CDC42Hs-N17, Rac1-N17, or the control plasmid CMVβgal resulted in similar levels of MyoD expression: between 40 and 50% CDC42Hs-N17 (n = 291), Rac1-N17 (n = 296) (Figure 5, A and B), or βgal-positive cells (n = 124) (Figure 5B) expressed MyoD. In contrast, transient overexpression of dominant negative RhoA proteins strongly inhibited MyoD: only 5–10% of the myoblasts expressing Myc-tagged RhoA-N19 coexpressed MyoD (n = 225; Figure 5, A and B). These results show that, among the members of Rho family G-proteins, RhoA, but not CDC42 or Rac, appears to be involved in MyoD gene regulation. To test whether Rho protein family might be involved in Myf5 gene regulation, experiments were conducted as previously described, but cells were analyzed for Myf5 expression after transfection of Myc-tagged G-proteins. We found that overexpression of CMVβgal, CDC42Hs-N17, Rac1-N17, or RhoA-N19 had minimal effects on Myf5 (Figure 5B). Together, these results strongly support that RhoA is a genuine member of a specific pathway required for MyoD, but not Myf5, gene expression.
Figure 5.
Only RhoA-dominant negative mutant selectively inhibits MyoD expression with no effect on Myf5. C2 cells were plated in proliferation medium 16 h before transfection: 1 μg of CMVβgal, CMV Myc-tagged CDC42HsN17, Rac1N17, or RhoAN19 was transiently transfected with DOSPER lipids. Twenty four hours after transfections, cells were fixed and analyzed by coimmunofluorescence for the expression of either βgal or Myc-tagged proteins together with either MyoD or Myf5. (A) The staining for Myc-tagged dominant negative G proteins is shown as indicated (b, d, and f) and for MyoD protein (a, c, and e). Open arrows, MyoD-negative cells; solid arrows, MyoD-positive cells. (B) Summary of the quantification for both MyoD and Myf5 expression in cells transfected with either βgal or Myc-tagged CDC42HsN17-, Rac1N17-, and RhoAN19-encoding plasmids.
RhoA Biological Activities Are Dependent on a Functional SRF in Muscle Cells
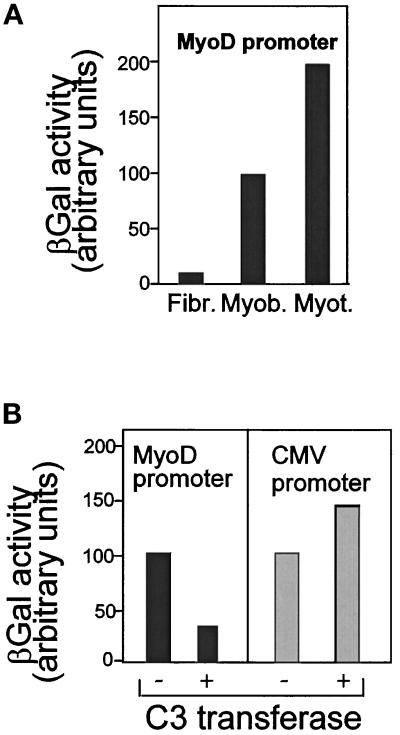
Taken together with the data of Hill et al. (1995), our results support a model in which RhoA protein regulates MyoD gene expression by controlling SRF activity. To test the hypothesis that the effects of RhoA are dependent on functional SRF in muscle cells, we carried out experiments using CAT reporter constructs under the control of a 630-bp sequence of myosin light chain 1A (MLC1A) 5′-promoter (Catala et al., 1995). This promoter has been shown to contain a functional binding site for SRF, a CArG box contained within the 630-bp sequence. The involvement of this CArG box in muscle-specific regulation of MLC1A promoter was shown to occur through SRF binding, and a mutation in the CArG box that abrogates this binding significantly reduced muscle-specific activity of this construct (Catala et al., 1995). To test whether RhoA activity could regulate the activity of this construct in its wild-type and CArG-mutated form, we transfected into C2 myoblasts constructs of MLC1A promoter containing either the wild-type CArG box (−630 MLC1A) or the mutated CArG box (−630(mSRF)MLC1A) driving CAT reporter gene expression. As previously reported, the mutation in the CArG box reduces by about twofold the activity of MLC1A gene reporter (Figure 6A; see also Catala et al., 1995). Coexpression of a construct encoding RhoA wild-type (RhoAWT) did not significantly affect the activity of either MLC1A wild-type promoter or its CArG-mutated form. However, overexpression of a constitutively activated RhoA (RhoA-Val14) enhanced by threefold the activity of the −630 wild-type MLC1A promoter, whereas it had little effect on the −630 mutated CArG MLC1A promoter (Figure 6A). These data show, therefore, that only MLC1A construct containing a functional SRF-binding site is responsive to activation by the constitutively active form of RhoA.
We next used C3 transferase treatment to test whether inhibition of endogenous RhoA would affect MLC1A promoter activity. C2 myoblasts were transfected with wild-type MLC1A promoter construct or its CArG-mutated form in the presence of C3 transferase for 24 h. As shown in Figure 6B, addition of C3 transferase reduced the activity of the wild-type MLC1A promoter by about twofold and in contrast did not affect the activity of the MLC1A construct mutated in its CArG box, showing that only the construct containing a functional CArG box was sensitive to inhibition of RhoA by C3 transferase. Together, these experiments show that RhoA-mediated transcriptional activation required functional SRF in C2 muscle cells.
C3 Transferase Represses MyoD Promoter Function
We raise the question of how Rho protein might control MyoD expression. Simply stated, inhibiting Rho protein activity could repress MyoD promoter activity and, consequently, MyoD protein accumulation. To examine the effect of Rho GTPases on MyoD promoter activity, a chimeric construct containing MyoD promoter proximal and distal regulatory sequences (PRR and DRR) driving βgal expression was stably integrated into C2 myoblasts (MyoD promoter requires chromosomal integration to be fully activated; Tapscott et al., 1992). As previously reported, MyoD promoter activity was detected in muscle cells but not in 10T1/2 fibroblast cells, and this activity increased with the differentiation status (Figure 7A; Tapscott et al., 1992). C2 myoblasts stably expressing MyoD promoter were cultured in proliferation medium for 48 h before treatment with C3 transferase. As shown in Figure 7B, addition of C3 transferase reduced MyoD promoter activity by more than threefold. In contrast, C3 transferase did not affect the activity of the viral CMV promoter, demonstrating the specificity of such regulation. Therefore, C3 transferase can inhibit MyoD expression through inactivation of MyoD promoter function.
Figure 7.
C3 transferase represses MyoD promoter activity. MyoD promoter (DRR and PRR regions driving βgal reporter gene, Tapscott et al., 1992) was stably transfected in mouse C2 myoblasts and mouse 10T1/2 fibroblasts in the presence of PSV2neo encoding a gene for resistance to geneticin (G418). After selection with G418, a pool of clones was cultured as a permanent cell line. (A) Shown is βgal activity in myoblasts (Myob.), in fibroblasts (Fibr.), and after the onset of differentiation (Myot.). βgal values are expressed relative to that of myoblasts set as 100%. (B) To determine the effect of C3 transferase on MyoD promoter activity, cells stably transfected with MyoD promoter were grown for 48 h in proliferation medium and then treated for 24 h with 4 μg/ml C3 transferase. As a control, CMVβgal was transiently transfected in myoblasts cells in the presence or not of C3 transferase. βgal values determined in nontreated cells were fixed at 100 for each case, and those obtained after C3 treatment were expressed relative to their respective control.
DISCUSSION
The data reported in this study shed light on a new regulatory pathway that controls myogenic gene expression. We showed that inactivation of the SRF selectively inhibited MyoD and not Myf5 expression. We further found that an upstream regulator of SRF activity, the small G-protein RhoA, can also specifically regulate MyoD: blocking RhoA but not Rac or CDC42 protein activity inhibited MyoD promoter activity and also endogenous MyoD expression while not affecting Myf5. Thus, these data substantiate that MyoD and Myf5 are regulated by different upstream activation pathways in which MyoD expression is controlled by a RhoA/SRF signaling cascade.
SRF Regulation on MyoD: A Key Step in SRF Effects on Myogenesis
We have shown previously that SRF acts very early in the process of muscle differentiation: inhibition of SRF activity in mouse myogenic cell lines prevented MyoD gene expression at the myoblast stage and myoblast/myotube transition (Vandromme et al., 1992; Gauthier-Rouviere et al., 1996; Soulez et al. 1996). Several observations established a positive correlation between the level of the muscle-regulatory gene MyoD and the ability of myogenic cells to terminally differentiate (Pinset et al., 1988; Brennan et al., 1990; Montarras et al., 1991, 1996). It was therefore tempting to speculate that the regulation of MyoD gene expression by SRF was a key step in the control exerted by SRF on myogenesis. However, mouse C2 cells at myoblast stage express not only MyoD but also Myf5, a member of the MyoD gene family, believed to be involved in early events of myogenesis (Tajbakhsh et al., 1996). Here, we have shown that SRF is not involved in the control of Myf5 gene expression. Indeed, inactivation of SRF through microinjection of SRF dominant negative proteins or by constitutive expression of SRF antisense prevents MyoD gene expression but leaves intact Myf5 protein levels. Recent reports have demonstrated functional and physical interactions between SRF and MyoD proteins (Catala et al., 1995; Groisman et al., 1996). As MyoD can activate its own expression (Thayer et al., 1989), it is tempting to speculate that SRF might also interfere with the MyoD-autoregulatory loop. Together, these data support that SRF regulation on MyoD gene expression and protein activity may confer skeletal muscle specificity to SRF.
Rho GTPases and SRF Define a Specific Pathway Required for MyoD Expression in Skeletal Muscle Cells
Molecules that link Rho GTPases to nuclear signaling pathways have begun to be identified. CDC42 and Rac, but not Rho, can activate JNK/SAPK and P38 kinase (Coso et al., 1995; Minden et al., 1995). Rho does not regulate the JNK pathway. However, Rho, but also CDC42 and Rac, can mediate SRF transcriptional activation by serum or lysophosphatidic acid establishing SRF as the target of a novel nuclear signaling pathway mediated by Rho family GTPases (Hill et al., 1995). SRF dimers are known to form complexes with a ternary complex factor (TCF) on their DNA-binding site (named SRE or CArG). In such a complex, TCF binds a DNA sequence (called ets) localized 5′ of the CArG box (Treisman, 1990). It appears that different independent signaling pathways converge on the c-fos promoter SRF-binding site: a Ras/MAP kinase pathway specifically activates the TCF-dependent SRF transcriptional activity, whereas a Rho-mediated pathway is shown to activate SRF in a TCF-independent manner, (Hill et al., 1995; reviewed in Van Aelst and D’Souza-Schorey, 1997). In this respect, it is interesting to note that most SRF-fixation sites present in muscle genes do not have a 5′-adjacent site for TCF fixation (Catala et al., 1995; Croissant et al., 1996; Galvagni et al., 1997).
Here, we have shown that inhibition of SRF activity or RhoA- but not Rac- or CDC42-dependent pathways led to a selective inhibition of MyoD gene expression, which did not interfere with the MyoD-related protein, Myf5. Interestingly, most if not all extracellular signals (serum, lysophosphatidic acid, 12-O-tetradecanoylphorbol 13-acetate, AIF4−) known to activate SRF are inhibited by C3-transferase, thus establishing RhoA as a key effector of SRF transcriptional activation (Hill et al., 1995). Additionally, we show that, in our muscle cell system, only the expression of a MLC1A gene promoter construct containing an intact SRF-binding site, and not a mutated one, is stimulated by cotransfection with a constitutively active form of RhoA and inhibited by C3 transferase (Figure 6), further supporting that RhoA effects are mediated through SRF (Catala et al., 1995; Figure 6). Together with these data, our results imply that RhoA and SRF act in the same regulatory pathway in muscle cells.
We show that although inhibition of RhoA can prevent MyoD expression, overexpression of a constitutively active form of RhoA (RhoV14) cannot activate endogenous MyoD (our unpublished results), thus demonstrating that RhoA-dependent signals are necessary but not sufficient for activation of endogenous MyoD. Recently, Alberts et al. (1998) reported that even though a constitutively active form of RhoA induces expression of extrachromosomal SRF reporter gene, it fails to regulate chomosomal SRF reporter gene unless acetylation-linked signaling pathways were activated (Alberts et al., 1998). Similarly, cooperation between RhoA and acetylation signaling pathways might be required to activate endogenous MyoD gene expression.
We show here that SRF- and Rho GTPase-mediated regulation of MyoD expression appears to take place at the transcriptional level. Demonstrating how Rho and SRF proteins act to regulate MyoD transcription will require the identification of their site(s) of action on MyoD-regulatory sequences: since RhoA is an upstream regulator of SRF, RhoA and SRF must regulate MyoD transcriptional activity by targeting the same DNA sequence(s) on MyoD promoter region. Indeed, MyoD promoter region (PRR and DRR, Tapscott et al., 1992) contains several putative CArG boxes that diverge more or less from the consensus CArG sequence CC(A/T)6GG, one of which is identical to the SRF-binding site shown to be functional in MLC1A gene (Catala et al., 1995) and used in our study (Figure 6).
Potential Upstream Factors of the RhoA/SRF Signaling Cascade in Muscle Cells
The identification of a RhoA/SRF-specific pathway upstream of MyoD raises the question of how this signaling cascade itself is activated. It is generally accepted that Rho and SRF protein activities are dependent on growth factors (for reviews: Treisman, 1990; Van Aelst and D’Souza-Schorey, 1997; see also Hill et al., 1995). Several growth factors are known to affect the differentiation of muscle cells including members of fibroblast growth factors, TGFs, and IGFs (Florini et al., 1991a; Filvaroff et al., 1994; Floss et al., 1997). IGFs emerged from this list since they are required for muscle differentiation and for MyoD but not for Myf5 gene expressions (Florini et al., 1991b; Montarras et al., 1996). Thus, inactivation of IGFs, SRF, or Rho proteins have similar consequences: a dramatic decrease of MyoD expression and the maintenance of Myf5 expression. The link between IGFs and Rho was established from studies on signal transduction pathways of type 1 IGF receptors. It is becoming clear that myogenic effects of ligand-activated IGF receptor 1 are due to stimulation of a phosphatidylinositol 3-kinase (PI 3-kinase) pathway but not of Ras/MAP kinase pathway (Kalinam et al., 1996; Pinset et al., 1997). Furthermore, Ras is a strong inhibitor of myogenesis and MyoD expression (Lassar et al., 1989). Several groups have now provided evidence that PI 3-kinase and Rho GTPases operate in hierarchy, where activated PI 3-kinase triggers membrane ruffles and stress fibers in a Rac- and Rho-dependent manner (Nobes et al., 1995; Reif et al., 1996). It is therefore tempting to speculate that IGFs, Rho, and SRF may lie on the same linear signal transduction cascade. However, this appealing hypothesis is challenged by different observations: 1) Overexpression of constitutively active CDC42, Rac, or Rho proteins failed to restore MyoD expression in differentiation-deficient myoblasts unlike insulin (our unpublished observation); 2) Activation of GTPase is not the sole result of PI 3-kinase activation (Cohen et al., 1997); 3) Other growth factors, namely TGFβ, are important for muscle cell differentiation and can interact with Rho GTPases (Zentella and Massague, 1992; Filvaroff et al., 1994; Mucsi et al., 1996; Afti et al., 1997). Thus, further studies will be required to piece together members of the Rho/SRF/MyoD signaling cascade in muscle cells. In this respect, the identification of RhoA protein as a specific effector of a pathway that controls the expression of the key muscle regulator MyoD will be useful to examine the transduction pathways that link growth factors and myogenic gene expression.
ACKNOWLEDGMENTS
We thank Dr. Margaret Buckingham (Institut Pasteur, Paris, France) and Dr. A. Kahn (Institut Cochin de Génétique Moléculaire, Paris, France) for their interest in this work and their support to M.P. and D.T., respectively. We thank Drs. S.J. Tapscott (Fred Hutchinson Cancer Research Center, Washington, D.C.) for plasmids encoding MyoD promoter, N. Lamarche and A. Hall (Medical Research Council, London, England) for plasmids encoding CDC42Hs-N17, Rac1-N17, RhoA-N19, RhoAWT, and RhoAV14, and Dr. Margaret Buckingham for plasmids encoding contructs of MLC1A gene promoter termed −630 MLC1A-TKCAT and −630 (mSRF)MLC1A-TKCAT. We also thank Dr. P. Bocquet (INSERM U452, Nice, France) and Merck Sharp and Dohme Laboratory (West Point, PA) for their generous gift of Botulinum C3 and lovastatin, respectively. We are grateful to Drs. M. Vandromme, A. Bonnieu, and A. Debant for many helpful discussions and critical reading of the manuscript. This work was supported by grants from Association Française contre les Myopathies (A.F.M.) and the Ligue Nationale contre le Cancer. G.C. and M.P. are recipients of postdoctoral fellowships of A.F.M.
REFERENCES
- Afti A, Djelloul S, Chastre E, Davis R, Gespach C. Evidence for a role of Rho-like GTPases and stress-activated protein kinase/c-jun N-terminal kinase (SAPK/JNK) in transforming Growth factor β-mediated signalling. J Biol Chem. 1997;272:1429–1432. doi: 10.1074/jbc.272.3.1429. [DOI] [PubMed] [Google Scholar]
- Aktories K, Hall A. Botulinum ADP-ribosyl transferase: a new tool to study low molecular weight GTP-binding proteins. Trends Pharmacol Sci. 1989;10:415–418. doi: 10.1016/0165-6147(89)90191-0. [DOI] [PubMed] [Google Scholar]
- Alberts AS, Geneste O, Treisman R. Activation of SRF-regulated chromosomal templates by Rho-family GTPases requires a signal that also induces H4 hyperacetylation. Cell. 1998;92:475–487. doi: 10.1016/s0092-8674(00)80941-1. [DOI] [PubMed] [Google Scholar]
- Auradé F, Pfarr CM, Lindon C, Garcia A, Primig M, Montarras D, Pinset C. The glucocorticoid receptor and AP-1 are involved in a positive regulation of the muscle regulatory gene Myf5 in cultured myoblasts. J Cell Sci. 1997;110:2771–2779. doi: 10.1242/jcs.110.22.2771. [DOI] [PubMed] [Google Scholar]
- Brennan TJ, Edmonson DG, Olson EN. Aberrant regulation of MyoD1 contributes to the partially defective myogenic phenotype of BC3H1 cells. J Cell Biol. 1990;110:929–937. doi: 10.1083/jcb.110.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun T, Rudnicki MA, Arnold HH, Jaenisch R. Targeted inactivation of the muscle regulatory gene Myf5 results in abnormal ribs development and perinatal death. Cell. 1992;71:369–382. doi: 10.1016/0092-8674(92)90507-9. [DOI] [PubMed] [Google Scholar]
- Carnac G, Albagli-Curiel O, Vandromme M, Pinset C, Montarras D, Laudet V, Bonnieu A. 3, 5, 3′-Triiodothyronine positively regulates both MyoD1 gene transcription and terminal differentiation in C2 myoblast. Mol Endocrinol. 1992;6:1185–1194. doi: 10.1210/mend.6.8.1406697. [DOI] [PubMed] [Google Scholar]
- Catala F, Wanner R, Barton P, Cohen A, Wright W, Buckingham M. A skeletal muscle-specific enhancer regulated by factor binding to E and CArG Boxes is present in the promoter of the mouse myosin light-chain 1A gene. Mol Cell Biol. 1995;14:4585–4596. doi: 10.1128/mcb.15.8.4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Alessi DR, Cross. DA. PDK1, one of the missing links in insulin signal transduction? FEBS Lett. 1997;410:3–10. doi: 10.1016/s0014-5793(97)00490-0. [DOI] [PubMed] [Google Scholar]
- Coso OA, Chiariello M, Yu JC, Teramoto H, Crespo P, Xu N, Miki T, Gutkind JS. The small GTP-binding proteins Rac1 and CDC42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- Cossu G, Kelly R, Tajbakhsh S, Di Donna S, Vivarelli E, Buckingham M. Activation of different myogenic pathways: myf-5 is induced by the neural tube and MyoD by the dorsal ectoderm in mouse paraxial mesoderm. Development. 1996;122:429–437. doi: 10.1242/dev.122.2.429. [DOI] [PubMed] [Google Scholar]
- Croissant JD, Kim JH, Eichele G, Goering L, Lough J, Prywes R, Schwartz RJ. Avian serum reponse factor expression restricted primarily to muscle cell lineages is required for α-actin gene transcription. Dev Biol. 1996;177:250–264. doi: 10.1006/dbio.1996.0160. [DOI] [PubMed] [Google Scholar]
- Fenton RG, Kung HF, Longo DL, Smith MR. Regulation of intracellular actin polymerisation by prenylated cellular proteins. J Cell Biol. 1993;117:347–356. doi: 10.1083/jcb.117.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filvaroff EH, Ebner R, Derynck R. Inhibition of myogenic differentiation in myoblast expressing a truncated type II TGF-β receptor. Development. 1994;120:1085–1095. doi: 10.1242/dev.120.5.1085. [DOI] [PubMed] [Google Scholar]
- Florini JR, Ewton DZ, Magri KA. Hormones, growth factors, and myogenic differentiation. Annu Rev Physiol. 1991a;53:201–216. doi: 10.1146/annurev.ph.53.030191.001221. [DOI] [PubMed] [Google Scholar]
- Florini JR, Magri KA, Ewton DZ, James PL, Grindstaff K, Rotwein PS. “Spontaneous” differentiation of skeletal myoblasts is dependent upon autocrine secretion of insulin-like growth factor II. J Biol Chem. 1991b;266:15917–15923. [PubMed] [Google Scholar]
- Floss T, Arnold HH, Braun T. A role for FGF-6 in skeletal muscle regeneration. Genes Dev. 1997;11:2040–2051. doi: 10.1101/gad.11.16.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvagni F, Lestingi M, Cartocci E, Oliviero S. Serum response factor and protein-mediated DNA bending contribute to transcription of the dystrophin muscle-specific promoter. Mol Cell Biol. 1997;17:1731–1743. doi: 10.1128/mcb.17.3.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier-Rouvière C, Caï QQ, Lautredou N, Fernandez A, Blanchard JM, Lamb N. Expression and purification of the DNA binding domain of SRF: SRF-DB, a part of a DNA-binding protein which can act as a dominant negative mutant in vivo. Exp Cell Res. 1993;209:208–215. doi: 10.1006/excr.1993.1303. [DOI] [PubMed] [Google Scholar]
- Gauthier-Rouvière C, Vandromme M, Tuil D, Lautredou N, Morris M, Soulez M, Kahn A, Fernandez A, Lamb N. Expression and activity of serum response factor is required for expression of the muscle-determining factor MyoD in both dividing and differentiationg mouse C2C12 myoblasts. Mol Cell Biol. 1996;5:719–729. doi: 10.1091/mbc.7.5.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman R, Masutani H, Leibovitch MP, Robin P, Soudant I, Trouche D, Harel-bellan A. Physical interaction between the mitogen-responsive serum response factor and myogenic basic-helix-loop-helix proteins. J Biol Chem. 1996;271:5258–5264. doi: 10.1074/jbc.271.9.5258. [DOI] [PubMed] [Google Scholar]
- Hill CS, Wynne J, Treisman R. The Rho family GTPases RhoA, Rac1 and CDC42Hs regulate transcriptional activation by SRF. Cell. 1995;81:1159–1170. doi: 10.1016/s0092-8674(05)80020-0. [DOI] [PubMed] [Google Scholar]
- Kalinam P, Vinals F, Testard X, Palacin M, Zorzano A. Phosphatidylinositol 3-kinase inhibitors block differentiation of skeletal muscle cells. J Biol Chem. 1996;271:19146–19151. doi: 10.1074/jbc.271.32.19146. [DOI] [PubMed] [Google Scholar]
- Kho CJ, Huggins GS, Endege WO, Hsieh CM, Lee ME, Haber E. Degradation of E2A proteins through a ubiquitin-conjugating enzyme, UbcE2A. J Biol Chem. 1997;272:3845–3851. doi: 10.1074/jbc.272.6.3845. [DOI] [PubMed] [Google Scholar]
- Lassar AB, Thayer MT, Overell RW, Weintraub H. Transformation by activated ras or fos prevents myogenesis by inhibiting expression of MyoD. Cell. 1989;58:659–667. doi: 10.1016/0092-8674(89)90101-3. [DOI] [PubMed] [Google Scholar]
- Luo L, Liao J, Jan JL, Jan YN. Distinct morphogenetic functions of similar GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 1994;8:1787–1802. doi: 10.1101/gad.8.15.1787. [DOI] [PubMed] [Google Scholar]
- Maroto M, Reshef R, Munsterberg AE, Koester S, Goulding M, Lassar AB. Ectopic pax-3 activates MyoD and myf-5 expression in embryonic mesoderm and neural tissue. Cell. 1997;89:139–148. doi: 10.1016/s0092-8674(00)80190-7. [DOI] [PubMed] [Google Scholar]
- Megeney LA, Kablar B, Garrett K, Anderson JE, Rudnicki MA. MyoD is required for myogenic stem cell function in adult skeletal muscle. Genes Dev. 1996;10:1173–1183. doi: 10.1101/gad.10.10.1173. [DOI] [PubMed] [Google Scholar]
- Minden A, Lin A, Claret FX, Abo A, Karin M. Selective activation of the JNK signaling cascade and c-jun transcriptional activity by the small GTPases Rac and CDC42Hs. Cell. 1995;81:1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- Montarras D, Aurade F, Johnson T, Ilan J, Gros F, Pinset C. Autonomous differentiation in the mouse myogenic cells line, C2, involves a mutual positive control between insulin-like growth factor II and MyoD, operating as early as the myoblast stage. J Cell Sci. 1996;109:551–560. doi: 10.1242/jcs.109.3.551. [DOI] [PubMed] [Google Scholar]
- Montarras D, Chelly J, Bober E, Arnold H, Ott MO, Gros F, Pinset C. Developmental patterns in the expression of Myf5, MyoD, myogenin, and MRF4 during myogenesis. New Biol. 1991;3:592–600. [PubMed] [Google Scholar]
- Morii N, Narumiya S. Preparation of native and recombinant Clostridium botulinum C3 ADP-ribosyltransferase and identification of Rho proteins by ADP-ribosylation. Methods Enzymol. 1995;256:196–207. doi: 10.1016/0076-6879(95)56024-6. [DOI] [PubMed] [Google Scholar]
- Mucsi I, Skorecki KL, Goldberg HJ. Extracellular signal-regulated kinase and the small GTP-binding protein Rac contribute to the effects of transforming growth factor-β1 on gene expression. J Biol Chem. 1996;271:16567–16572. doi: 10.1074/jbc.271.28.16567. [DOI] [PubMed] [Google Scholar]
- Nielsen DA, Chang TC, Shapiro DJ. A highly sensitive, mixed phase assay for chloramphenicol acetyl transferase activity in transfected cells. Anal Biochem. 1989;179:19–23. doi: 10.1016/0003-2697(89)90193-0. [DOI] [PubMed] [Google Scholar]
- Nielsen DA, Chou J, Mackrell AJ, Casabadan MJ, Steiner DF. Expression of preproinsulin β-galactosidase gene fusion in mammalian cells. Proc Natl Acad Sci USA. 1983;80:5198–5202. doi: 10.1073/pnas.80.17.5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, Rac, and CDC42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hawkins P, Stephens L, Hall. A. Activation of the small GTP-binding proteins rho and Rac by growth factor receptors. J Cell Sci. 1995;108:225–233. doi: 10.1242/jcs.108.1.225. [DOI] [PubMed] [Google Scholar]
- Olson E, Klein WH. bHLH factors in muscle development: dead lines and commitments, what to leave in and what to leave out. Genes Dev. 1994;8:1–8. doi: 10.1101/gad.8.1.1. [DOI] [PubMed] [Google Scholar]
- Pinset C, Garcia A, Rousse S, Dubois C, Montarras D. Wortmannin inhibits IGF-dependent differentiation in the mouse myogenic cell line. C2. C R Acad Sci. 1997;320:367–374. doi: 10.1016/s0764-4469(97)85024-x. [DOI] [PubMed] [Google Scholar]
- Pinset C, Montarras D, Chenevert J, Minty A, Barton P, Laurent C, Gros F. Control of myogenesis in the mouse myogenic C2 cell line by medium composition and by insulin: characterisation of permissive and inducible C2 myoblasts. Differentiation. 1988;38:28–34. doi: 10.1111/j.1432-0436.1988.tb00588.x. [DOI] [PubMed] [Google Scholar]
- Reif K, Nobes CD, Thomas G, Hall A, Cantrell DA. Phosphatidylinositol 3-kinase signals activate a selective subset of Rac/Rho- dependent effector pathways. Curr Biol. 1996;6:1445–1455. doi: 10.1016/s0960-9822(96)00749-x. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Caroline LJ, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Rothut B, Dubois T, Feliers D, Russo-Marie F, Oudinet JP. Inhibitory effect of annexin V on protein kinase C activity in mesanglia cells lysates. Eur J Biochem. 1995;232:865–872. [PubMed] [Google Scholar]
- Rudnicki MA, Braun T, Hinuma S, Jaenisch R. Inactivation of MyoD in mice leads to up-regulation of the myogenic HLH gene Myf-5 and results in apparently normal muscle development. Cell. 1992;71:383–390. doi: 10.1016/0092-8674(92)90508-a. [DOI] [PubMed] [Google Scholar]
- Rudnicki MA, Jaenisch R. The MyoD family of transcription factors and skeletal myogenesis. Bioessays. 1995;17:203–209. doi: 10.1002/bies.950170306. [DOI] [PubMed] [Google Scholar]
- Rudnicki MA, Schnegelsberg PN, Stead RH, Braun T, Arnold H, Jaenish R. MyoD or Myf5 is required for the formation of skeletal muscle. Cell. 1993;75:1351–1359. doi: 10.1016/0092-8674(93)90621-v. [DOI] [PubMed] [Google Scholar]
- Soulez M, Gauthier-Rouviere C, Chafey P, Hentzen D, Vandromme M, Lautredou N, Lamb N, Kahn A, Tuil D. Growth and differentiation of myogenic cells are dependent on serum response factor. Mol Cell Biol. 1996;16:6065–6074. doi: 10.1128/mcb.16.11.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajbakhsh S, Rocancourt D, Buckingham M. Muscle progenitor cells failing to respond to positional cues adopt non-myogenic fates in myf-5 null mice. Nature. 1996;384:266–270. doi: 10.1038/384266a0. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S, Rocancourt D, Cossu G, Buckingham M. Redefining the genetic hierarchies controlling skeletal myogenesis: Pax-3 and Myf-5 act upstream of MyoD. Cell. 1997;89:127–138. doi: 10.1016/s0092-8674(00)80189-0. [DOI] [PubMed] [Google Scholar]
- Tapon N, Hall A. Rho, Rac and CDC42 regulate the organisation of the actin cytoskeleton. Curr Opin Cell Biol. 1997;9:86–92. doi: 10.1016/s0955-0674(97)80156-1. [DOI] [PubMed] [Google Scholar]
- Tapscott SJ, Lassar AB, Weintraub H. A novel myoblast enhancer element mediates MyoD transcription. Mol Cell Biol. 1992;12:4994–5003. doi: 10.1128/mcb.12.11.4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer MJ, Tapscott SJ, Davis RL, Wright WE, Lassar AB, Weintraub H. Positive autoregulation of the myogenic determination gene MyoD1. Cell. 1989;58:241–248. doi: 10.1016/0092-8674(89)90838-6. [DOI] [PubMed] [Google Scholar]
- Treisman R. The SRE: a growth factor responsive transcriptional regulator. Semin Cancer Biol. 1990;1:47–58. [PubMed] [Google Scholar]
- Van Aelst L, D’Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- Vandromme M, Gauthier-Rouviere C, Carnac G, Lamb N, Fernandez A. Serum response factor p67SRF is expressed and required during myogenic differentiation of both mouse C2 and rat L6 muscle cell lines. J Cell Biol. 1992;118:1489–1500. doi: 10.1083/jcb.118.6.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H, et al. The MyoD gene family: nodal point during specification of the muscle lineage. Science. 1991;251:761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- Zentella A, Massague J. Transforming growth factor β induces myoblast differentiation in the presence of mitogens. Proc Natl Acad Sci USA. 1992;89:5176–5180. doi: 10.1073/pnas.89.11.5176. [DOI] [PMC free article] [PubMed] [Google Scholar]