Abstract
Hospitalized infants (4,618) were studied for lower respiratory infections from 1989 through 2000 by routine immunofluorescence assay and viral isolation. The hospitalization rate for respiratory syncytial virus (RSV) averaged 2% per year. The fatality rate was 0.1%. Monthly RSV detection varied from 14 to 88%, and epidemics lasted 3.5 to 6 months. From 1994 high-early versus low-late epidemic patterns alternately were observed, the first influenced by a group B strain.
Acute respiratory infections are a major worldwide health problem because of associated high morbidity and mortality rates. In Chile acute lower respiratory infections (ALRI) are the primary cause of hospital admissions during infancy. Respiratory syncytial virus (RSV) is the principal cause of ALRI, causing yearly winter epidemics that frequently challenge health resources (3, 4, 5, 9, 12, 15, 21). The expanded use of new techniques has facilitated the local identification of etiologic agents, allowing the comparison of clinical and epidemiological features with biological agent characteristics (2, 6, 7, 14, 16, 17, 19). The aim of this study is to present an overview of RSV infection causing pediatric admissions for ALRI in Chile over a long enough period of time to support recommendations for better clinical and epidemiological management. We present 12 years of data from immunofluorescence assays and isolation for routine viral diagnosis in the pediatric context.
A total of 4,618 out of 21,912 children under 2 years of age admitted for ALRI to the Roberto del Rio Children's Hospital, Santiago, Chile, were studied prospectively for RSV from January 1989 to December 2000. Exclusion criteria were prematurity; recurrent wheezing or asthma; and underlying chronic pulmonary, cardiac, or neurological diseases. During the cold season (April to September) two ALRI admissions were randomly selected per day in order to collect 40 to 60 cases per month. During the warm season (October to March) almost all patients were enrolled. For each patient, clinical features, personal and family history of asthma, and the admission diagnosis were obtained from hospital records.
Nasopharyngeal aspirates (NPA) were routinely obtained within the first 48 h after admission and were immediately transported to the laboratory for viral isolation and indirect immunofluorescence assay (IFA). Weekday specimens were processed on the same day, but those obtained on weekends were kept at 4°C until processing. Samples were inoculated into HEp-2 and MDCK cells and observed for cytopathic effect (CPE) for 1 week. Confirmatory IFA for RSV, adenovirus, influenza A and B viruses, and parainfluenza viruses 1 to 3 were performed for cultures with and without CPE. Standard IFA was done using monoclonal antibodies provided by L. Anderson, Centers for Disease Control and Prevention (CDC), Atlanta, Ga., and P. Pothier, Dijon, France, as previously described (3, 17). From 1994 to 2000, RSV strains from NPA or positive cultures were grouped by IFA with monoclonal antibodies 133-1H and 93-11C (CDC) and 2B8 (P. Pothier) for group A typing and 102-10B (CDC) for group B typing (1).
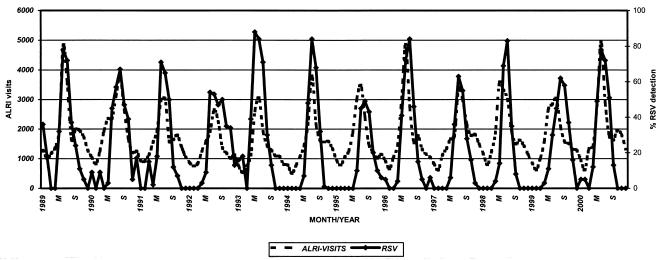
During the 12-year survey, an annual mean of 1,826 children less than 2 years old were admitted for ALRI, out of an estimated population of 26,000 children. RSV was detected at admission in 29% (1,337 of 4,618) of the enrolled patients. Thus, the mean burden of hospitalizations for RSV disease was 2% per year. The impact of respiratory infections demonstrated by the monthly number of outpatient visits and RSV detection in hospitalized cases is presented in Fig. 1. The total number of outpatient visits to emergency facilities during each RSV epidemic presented a nonsignificant yearly variation (data not shown), as did the mean annual RSV detection (Table 1).
FIG. 1.
RSV detection in hospitalized infants and outpatient visits to the Emergency Service for ALRI at Roberto del Rio Children's Hospital from January 1989 to December 2000. M, May; S, September.
TABLE 1.
Acute respiratory infections in Roberto del Rio Children's Hospital from 1989 to 2000
| Yr | No. of outpatient visits for ALRIa | No. of hospitalized patients enrolled | No. of RSV-positive cases | % RSV positive |
|---|---|---|---|---|
| 1989 | 23,728 | 270 | 105 | 39 |
| 1990 | 23,431 | 260 | 85 | 33 |
| 1991 | 19,974 | 502 | 95 | 19 |
| 1992 | 16,801 | 421 | 115 | 27 |
| 1993 | 16,451 | 315 | 124 | 39 |
| 1994 | 18,757 | 452 | 146 | 32 |
| 1995 | 19,679 | 448 | 82 | 18 |
| 1996 | 21,554 | 326 | 121 | 37 |
| 1997 | 20,943 | 337 | 92 | 27 |
| 1998 | 22,600 | 448 | 103 | 23 |
| 1999 | 20,395 | 450 | 125 | 27 |
| 2000 | 23,440 | 389 | 144 | 37 |
| Total | 247,753 | 4,618 | 1,337 | 29 |
Chi square for linear trend: 0.01 (P = 0.978).
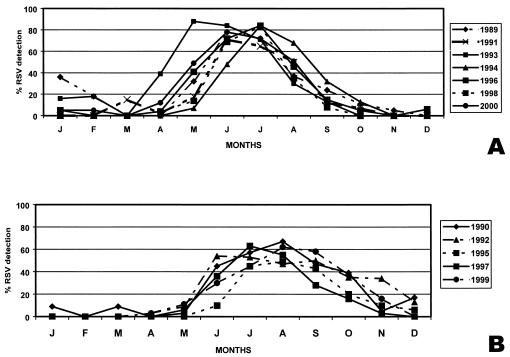
With a detection rate of over 20% as the definition of an epidemic, the mean duration of RSV epidemics was 4.5 months and the durations ranged from 3.5 (1995 and 1998) to 6 months (1992). The peak incidence was June or July in 8 out of 12 years. The shape of the epidemics varied. In 7 out of 12 years the outbreaks were characterized by monthly detection rates over 75%; from 1994 on they occurred in alternate years (Fig. 1). In 4 out of 7 years with high rates of RSV detection (1989, 1991, 1993, and 2000), the epidemics started early, in late April (Fig. 2A). In 4 out of 5 years with low rates of RSV detection, the outbreaks started in late May; furthermore, for this group, in two RSV seasons the peak occurred in mid-August (Fig. 2B).
FIG. 2.
Monthly RSV detection in infants hospitalized for ALRI at Roberto del Rio Children's Hospital from January 1989 to December 2000. (A) Years with epidemic peaks of over 75% RSV detection. (B) Years with epidemic peaks of below 75% RSV detection. Months are sequential from left to right, starting with January (J).
Typing of RSV strains A and B from 1994 to 2000 showed a general cocirculation of both groups, with predominance of A. Grouping was not achieved in 259 of 792 (33%) samples due to low number of positive cells or to cross-reactivity. In the even-numbered years a significant proportion of strain B isolates, associated with high-early RSV epidemics, were detected (Table 2).
TABLE 2.
RSV A and B grouping by immunofluorescence of strains recovered from infants and young children hospitalized for ALRI in Santiago from January 1989 to December 2000
| RSV groupa | No. of RSV strains in:
|
% | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1994 | 1995 | 1996 | 1997 | 1998 | 1999 | 2000 | Total | ||
| A | 4 | 63 | 62 | 49 | 37 | 61 | 35 | 311 | 58 |
| B | 97 | 4 | 30 | 4 | 54 | 7 | 26 | 222 | 42 |
| Total | 101 | 67 | 92 | 53 | 91 | 68 | 61 | 533 | 100 |
Chi square: 225.75 (P < 0.01).
Infants younger than 6 months of age accounted for 64% of all the enrolled cases. Ten percent (465) were younger than 1 month. The mean RSV detection rate was 29%, and it decreased significantly with increasing age from 16% for children over 12 months to 32% for children under six months. Pneumonia and wheezing plus pneumonia were the most frequent clinical diagnoses at admission. Among patients positive for RSV, 59 and 62% had clinical signs of pneumonia or wheezing, respectively. These clinical features confirmed our previous experience (3). During this period five deaths from ALRI occurred, implying a fatality rate of 0.1% (5 of 4,607). Only one case was RSV positive.
Our aim has been to study previously healthy children in order to assess the burden of respiratory viral epidemics in the community. A 20% positive level in hospital patients was used to determine the duration of epidemics. Anecdotally we observed that the beginning of an outbreak is marked by an increase above this level and that the end of one is marked by a decrease below it. The surveillance policy of collecting 25 to 50 samples per month from inpatients with ALRI is as efficient as taking a greater number of outpatient samples. IFA is sufficiently sensitive and easy to implement for routine RSV detection (3, 17).
The leading role of RSV as an etiologic agent of epidemic ALRI and the clinical presentation of cases have been previously described (3, 4, 15). The low lethality rate reported seems to be strongly influenced by the enrollment criteria, which included only previously healthy patients. We have frequently detected RSV in autopsies of infants that died from pneumonia, but RSV was found most often in handicapped patients (L. F. Avendaño, V. Luchsinger, and M. A. Palomino, unpublished data). Thus, the clinical background appears to be a major outcome factor to consider in the management of RSV disease.
The seasonality of the RSV epidemics herein reported agrees with data from abroad (7, 8, 11). The annual burden of RSV infection in Santiago, measured by outpatient visits for ALRI to emergency facilities, showed no significant yearly variation, which is consistent with the observation that over 60% of the infants acquire the infection in the first year of life (8, 10). The RSV hospitalization burden also stayed the same during the study period. To our knowledge, this is the first report that shows the occurrence of two different epidemic patterns, with an annual variation in intensity and duration. The early epidemics usually cause more public health problems than those occurring later, in spite of the fact that the total RSV season burden is the same annually. Temporal and geographical variations have been associated with circulation of genomic and antigenic variants and could explain the differences in epidemic features, but not the clinical severity (7, 11, 18, 20, 22). In this study, the presence of B strains was associated with high-early epidemics, and its influence could be explained through fluctuations in herd immunity and contacts among children. We assume that strain diversity is responsible for differences in outbreak presentation, while host determinants account for the clinical outcome.
Chilean health authorities implemented the “winter campaigns” to handle the increased ambulatory and hospital demand for medical care. Mortality rates for pneumonia in infants decreased from 242.9/100,000 inhabitants in 1990 to 105.7/100,000 inhabitants in 1999 in the capital (9). The implementation of routine RSV screening in patients admitted for ALRI will contribute to improved disease management. Unless an RSV vaccine is developed, seasonal respiratory outbreaks will continue to challenge the resources allocated for pediatric patients.
Acknowledgments
This work was supported by Chilean (FONDECYT no. 194 0527 and 198 0892) and European Community (proposal ERB 3514 PL97 2371) grants.
We thank Maria Inés Espinoza and Inés Orellana for technical assistance, S. Rose for advise in preparing the manuscript, and S. A. Barton for revising the language.
REFERENCES
- 1.Anderson, L. J., J. C. Hierholzer, C. Tsou, M. Hendry, B. F. Fernie, I. Stone, and K. McIntosh. 1985. Antigenic characterization of respiratory syncytial virus strains with monoclonal antibodies. J. Infect. Dis. 151:626-633. [DOI] [PubMed] [Google Scholar]
- 2.Arens, M. 1999. Methods for subtyping and molecular comparison of human viral genomes. Clin. Microbiol. Rev. 12:612-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avendaño, L. F., C. Larrañaga, M. A. Palomino, A. Gaggero, G. Montaldo, M. Suárez, and A. Díaz. 1991. Community- and hospital-acquired respiratory syncytial virus infection in Chile. Pediatr. Infect. Dis. J. 10:564-568. [DOI] [PubMed] [Google Scholar]
- 4.Avendaño, L. F., A. Céspedes, X. Stecher, and M. A. Palomino. 1999. Influencia de virus respiratorios, frío y contaminación aérea en la infección respiratoria aguda baja del lactante. Rev. Med. Chile 127:1075-1079. [PubMed] [Google Scholar]
- 5.Bedoya, V. I., V. Abad, and H. Trujillo. 1996. Frequency of respiratory syncytial virus in hospitalized infants with lower acute respiratory tract illness in Colombia. Pediatr. Infect. Dis. J. 15:1123-1124. [DOI] [PubMed] [Google Scholar]
- 6.Cane, P., and C. R. Pringle. 1992. Molecular epidemiology of respiratory syncytial virus: rapid identification of subgroup A lineages. J. Virol. Methods 40:297-306. [DOI] [PubMed] [Google Scholar]
- 7.Cane, P. 2001. Molecular epidemiology of syncytial virus. Rev. Med. Virol. 11:103-116. [DOI] [PubMed] [Google Scholar]
- 8.Collins P. L., K. McIntosh, and R. M. Chanock. 1996. Respiratory syncytial virus, p. 1313-1351. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 9.Girardi, B., O. Astudillo, and F. Zúñiga. 2001. El programa IRA en Chile: hitos e historia. Rev. Chil. Pediatr. 72:292-300. [Google Scholar]
- 10.Glezen, W. P., L. H. Taber, A. L. Frank, and J. A. Kasel. 1986. Risk of primary infection and reinfection with respiratory syncytial virus. Am. J. Dis. Child. 140:543-546. [DOI] [PubMed] [Google Scholar]
- 11.Hall, C. B., E. E. Walsh, K. C. Schnabel, C. E. Long, K. M. McConnochie, S. W. Hildreth, and L. J. Anderson. 1990. Occurrences of groups A and B of respiratory syncytial virus over 15 years: associated epidemiologic and clinical characteristics in hospitalized and ambulatory children. J. Infect. Dis. 162:1283-1290. [DOI] [PubMed] [Google Scholar]
- 12.Kaempfer, A. M., and E. Medina. 2000. Análisis de la mortalidad infantil y factores condicionantes. Chile 1998. Rev. Chil. Pediatr. 71:405-412. [Google Scholar]
- 13.Kajon, A. E., A. S. Mistchenko, C. Videla, M. Hortal, G. Wadell, and L. F. Avendaño. 1996. Molecular epidemiology of adenovirus acute lower respiratory infections of children in the south cone of South America (1991-1994). J. Med. Virol. 48:151-156. [DOI] [PubMed] [Google Scholar]
- 14.Kajon, A. E., C. Larrañaga, M. Suarez, G. Wadell, and L. F. Avendaño. 1994. Genome type analysis of Chilean adenovirus strains isolated in children's hospital between 1988 and 1990. J. Med. Virol. 42:16-21. [DOI] [PubMed] [Google Scholar]
- 15.Lagos, R., L. F. Avendaño, and M. Levine. 1999. Vigilancia sistemática de virus influenza, respiratorio sincicial, parainfluenza y adenovirus en niños ambulatorios con infecciones agudas respiratorias. Rev. Med. Chile 127:1063-1072. [PubMed] [Google Scholar]
- 16.Larrañaga, C., A. Kajon, E. Villagra, and L. F. Avendaño. 2000. Adenovirus surveillance on children hospitalized for acute lower respiratory infections in Chile (1988-1996). J. Med. Virol. 60:342-346. [PubMed] [Google Scholar]
- 17.Palomino, M. A., C. Larrañaga, and L. F. Avendaño. 2000. Hospital-acquired adenovirus 7h infantile respiratory infection in Chile. Pediatr. Infect. Dis. J. 19:527-531. [DOI] [PubMed] [Google Scholar]
- 18.Peret, T. C., C. B. Hall, G. W. Hammond, P. A. Piedra, G. A. Storch, W. A. Sullender, C. Tsou, and L. J. Anderson. 2000. Circulation patterns of group A and B human respiratory syncytial virus genotypes in 5 communities in North America. J. Infect. Dis. 181:1891-1896. [DOI] [PubMed] [Google Scholar]
- 19.Román, M., W. J. Calhoun, L. F. Avendaño, A. M. Escobar, and P. Díaz. 1997. Respiratory syncytial virus infection in infants is associated with predominant Th-2-like response. Am. J. Resp. Crit. Care Med. 156:190-195. [DOI] [PubMed] [Google Scholar]
- 20.Sullender, W. M. 2000. Respiratory syncytial virus genetic and antigenic diversity. Clin. Microbiol. Rev. 13:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Videla C., G. Carballal, A. Misirlian, and M. Aguilar. 1998. Acute lower respiratory infections due to respiratory syncytial virus and adenovirus among hospitalized children from Argentina. Clin. Diagn. Virol. 10:17-23. [DOI] [PubMed] [Google Scholar]
- 22.Waris M. 1991. Pattern of respiratory syncytial virus epidemics in Finland: two-year cycles with alternating prevalence of groups A and B. Infect. Dis. J. 163:464-469. [DOI] [PubMed] [Google Scholar]