Abstract
Humans are exposed to ionizing radiation (IR) under various circumstances, e.g. cosmic radiation, diagnostic X-rays and radiotherapy for cancer. It has been shown that IR can impair spermatogenesis and can cause mutations in germ cells. However, the mutagenic responses of germ cells exposed to IR at different stages of testicular maturation have not been examined by directly assessing the mutant frequency in defined spermatogenic cell types. This study was performed to address whether preadult exposure to IR can increase mutations in adult germ cells that could in turn have a major impact on adult reproductive function and the health of ensuing offspring. Male Lac I transgenic mice were irradiated with a single dose of 2.5 Gy of γ-ray at different ages before adulthood, reflecting different stages of testicular maturation, and then mutant frequency (MF) was determined directly in spermatogenic cell types emanating from the irradiated precursor cells. The results showed that (1) preadult exposure to IR did not significantly increase MF in adult epididymal spermatozoa; (2) spermatogenic stages immediately following the irradiated stage(s) displayed an elevated mutant frequency, but (3) the mutant frequency was restored to unirradiated levels in later stages of spermatogenesis. These findings provide evidence that there is a mechanism(s) to prevent spermatogenic cells with elevated mutant frequencies from progressing through spermatogenesis.
Keywords: ionizing radiation, mutagenesis, spermatogenic cells, Lac I mouse
1. Introduction
Germline mutagenesis induced by ionizing radiation (IR) is of importance for human health risk assessment. Extrapolation from animal data has been used in an effort to understand IR-induced germline mutations in humans due to the inherent difficulties in human epidemiological studies involving germ cells [1]. Several rodent assays are capable of detecting germline mutation, including the specific locus (SL) test [2], heritable translocation (HT) assay [3], dominant lethal (DL) assay [4], expanded simple tandem repeats (ESTRs) assay [5], and transgenic rodent assays [6,7]. The traditional mutation assays, e.g. SL test, DL assay and HT assay determine germline mutant frequency in the offspring of IR-exposed mice. Thus, while highly informative for understanding how mutagenic treatment of germ cells can give rise to offspring carrying mutations, these assays don’t directly measure mutations in specific spermatogenic cell types and are limited in their ability to reveal information about changes in mutant frequency during spermatogenesis.
The transgenic rodent and ESTRs assays are capable of directly monitoring IR-induced germline mutations [8, 9]. In a Lac I transgenic mouse system, the bacteriophage λ genome carrying the Lac I repressor gene from the prokaryotic Lac operon was introduced into the mouse genome [7]. The λ DNA is recovered from mouse genomic DNA and used to infect Escherichia coli carrying a Lac Z (β-galactosidase) gene (ΔLacZ M15), but lacking a functional Lac I gene. Phage carrying mutations in the Lac I gene are identified as blue plaques on E. coli lawns plated on agarose containing the chromogenic substrate 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal). The LacI transgenic rodent model is being applied in increasing numbers of in vivo mutagenesis studies [7,10–15]. However, there is a paucity of information regarding IR-induced germline mutagenesis in Lac I transgenic mice. One study has reported that treatment of 8- to 10-week-old mice with 1 Gy of γ-rays did not significantly increase mutant frequency in whole testis when mutant frequency was assayed 35 days after exposure in Lac I transgenic mice [8]. However, mutant frequency in defined spermatogenic cell types has not been directly investigated in Lac I transgenic mice after exposure to IR.
Spermatogenesis is a well-organized and sequential developmental and differentiation process. Postnatal spermatogenesis is initiated when prospermatogonia differentiate into spermatogonia. In the mouse, this occurs between birth and 5-days-old (d/o), and in human between birth and 6 months of age [16]. Some spermatogonia divide mitotically to become germline stem cells and most differentiate into type B spermatogonia [17]. Type B spermatogonia then undergo spermatogenesis and give rise sequentially to spermatocytes, spermatids and eventually mature spermatozoa. In the mouse, spermatozoa first appear in the lumen of seminiferous tubules by day 35 [16]. Initiating at puberty, germline stem cells (within the type A spermatogonial population) continue to replicate and ultimately to produce mature spermatozoa throughout the entire reproductive life of the male. However, spermatogenesis can be impaired by endogenous and exogenous agents. The testis is one of the most radiosensitive organs and the spermatogonia are the most radiosensitive cell types in adult testes in terms of cell killing by IR [18–22]. For example, aspermia was observed in male patients with Hodgkin’s disease after receiving lower trunk fractionated irradiation therapy [23]. Further, spermatids are susceptible to IR-induced mutations using the SL test [24], dominant lethal assay [25] and ESTRs [26]. However, to date, IR-induced cell type dependent germline mutant frequencies were deduced largely from the mutant frequency in the offspring of exposed mice [24–26]. By directly measuring mutant frequency in spermatogenic cells in Lac I transgenic mice, our laboratory has found that different spermatogenic cell types have different spontaneous mutant frequencies with the highest among the tested cell types being detected in primitive type A spermatogonia [27]. This indicates that different spermatogenic cell types have different capabilities of maintaining their genomic integrity that in turn may affect the response to IR. Thus, it is essential to directly measure IR-induced mutagenesis in defined spermatogenic cell types to better understand the mechanisms of IR-induced germline mutation.
In addition to the germ cell type dependent sensitivity to IR [18–22], age dependent radiosensitivity of the testis has been reported [22,28,29]. However, contrasting results have been observed with respect to the radiosensitivity of spermatogonia during postnatal development [22,30–33]. Children are among the IR exposed population and the consequence of such irradiation may impact germ cells. Whether the biochemical mechanisms that serve to ameliorate DNA damage in adults are sufficiently efficient in childhood to counteract environmental or therapeutic agents is a subject of growing interest. This is particularly important for reproductive cells because germline mutations occurring during childhood have a greater likelihood of being transmitted to offspring after children mature and reach adulthood since germline stem cells in children will have more opportunity to produce mutant mature germ cells than germline stem cells mutagenized in adults. Thus, it is a significant concern as to whether pubertal exposure to environmental agents, such as IR, will increase mutant frequency in germ cells.
In the present study, the Lac I transgenic mouse model was used to address the knowledge gap concerning the mutagenic responses in defined spermatogenic cells to IR and whether exposure to IR prior to adulthood would differentially impact the mutant frequency in spermatogenic cell types subsequently obtained from mature animals. Our results did not show a significantly increased mutant frequency in epididymal spermatozoa, the cell type that transmits genetic information to next generation. However, a greater mutant frequency was detected in spermatogenic cell types emanating from IR-treated stages of spermatogenesis. These data indicate there is a mechanism for lowering the mutant frequency within the spermatogenic cell population.
2. Materials and Methods
2.1 Animals
Mice homozygous for the LacI transgene (C57BL/6) were housed in the American Association of Accreditation for Laboratory Animal Care (AAALAC) accredited University of Texas Health Science Center at San Antonio animal facility. The animals were maintained ad libitum on standard food and water and were specific-pathogen-free. All animal procedures were approved by the institutional animal care and use committee prior to implementation.
2.2 Ionizing Radiation (IR) Resources
A GammaCell 40 irradiator (Atomic Energy of Canada Limited, Ontario, Canada) 137Cs source was used for exposures at a dose rate of 1.30 Gy per minute.
2.3 IR dose determination
The IR dose for mutagenesis experiments was determined by whole body exposure to 0, 1, 3, or 5 Gy of IR with 3 male mice (8-day-old (d/o)) in each treatment group. The mice were aged until 30 d/o at which time testes were removed and fixed in formalin then embedded in paraffin. H&E stained sections were examined for morphological indications of disruption of spermatogenesis. Substantial disruption was detected in all three mice exposed to 5 Gy, but only one mouse displayed a very minor disruption at 3 Gy of exposure to IR. No disruption was detected at 0 or 1 Gy. Therefore, 2.5 Gy of IR was used to deliver a dose just below the threshold of morphologically detectable disruption of spermatogenesis.
2.4 IR exposure
Male mice at different ages, neonatal (6 d/o or 8 d/o), or pubertal (18 d/o or 25 d/o), were placed in a pie-shaped, acrylic holding rack and the acrylic holding rack placed in the exposure chamber of an irradiator. A minimum of 15 male mice were whole-body irradiated for the collection of type A and type B spermatogonia from 8 d/o mice, 8 male mice for collection of pre-leptotene, leptotene/zygotene, and early pachytene spermatocytes from 18 d/o mice, 4–5 male mice for the collection of pachytene spermatocytes and round spermatids from 30 d/o mice, and 2–3 male mice for the collection of pachytene spermatocytes, round spermatids and spermatozoa from 60 d/o mice. Three replicates for each treatment group were performed each involving the numbers of mice just described. Mock exposed mice were placed in the acrylic holding rack and into the exposing chamber but the chamber was not lowered to the radiation source for exposure. Mice were mock exposed for a period of time equal to the time required for a treatment of 2.5 Gy based on the 137Cs source activity on the day of mock treatment. The experimental design is shown in Figure 1.
Figure 1.
Experimental design. Mice were irradiated at different ages and then sacrificed at later ages. For example, mice were sacrificed at 8-d/o, 18-d/o, 30-d/o or 60-d/o after irradiation at 6-d/o. The predominant spermatogenic cell types present at irradiation are shown above the ages of irradiation. The cell types collected are shown below the collection ages. A and B, type A and type B spermatogonia; P-L, pre-leptotene; L/Z, leptotene/zygotene; P, pachytene spermatocytes; RS, round spermatids; SP, spermatozoa.
2.5 Preparation of defined spermatogenic cell types
Mice were anesthetized at the appropriate ages (Figure 1) with isoflurane (Abbott Laboratories, Chicago, IL) followed by cervical dislocation. Specific populations of spermatogenic cell types (Figure 1) were prepared using a standard Sta Put gradient system as previously described [34]. Cells were identified morphologically by phase contrast microscopy. Cells were centrifuged at 1000 × g for 10 minutes, frozen in a dry ice/ethanol bath, and stored at −80°C until use.
2.6 Genomic DNA isolation
High molecular weight genomic DNA was prepared using the RecoverEase™ DNA isolation kit according to the manufacturer’s recommendations (Stratagene; La Jolla, CA) with slight modifications. Briefly, spermatogenic cells were suspended in 4 ml ice-cold lysis buffer (140 mM NaCl, 3 mM KCl, 350 mM sucrose, 800 μM EDTA, 1% Triton X-100, 10 mM Tris-HCl (pH 8.3)) in a 50 ml conical tube and vortexed gently 3 times for 5 seconds each. Lysates were centrifuged at 1000 × g for 15 minutes at 4°C to pellet nuclei. Supernatants were discarded and the nuclear pellets suspended in 100 μl digestion solution (12 mM Na2HPO4, 137 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 10 mM EDTA, 20 μl/ml RNace-It™ ribonuclease cocktail (Stratagene)). Samples were placed in a 50°C water bath and 100 μl of pre-warmed proteniase K solution (2 mg/ml) was added to each. The nuclear pellets were mixed gently to release high molecular weight DNA and incubated for 2 hours with gentle swirling every 15 minutes. The genomic DNA was transferred to the surface of a 10,000 MWC dialysis cup provided or a 10,000 MWC dialysis disk (Millipore). The DNA was dialyzed against 500 ml TE buffer (10 mM Tris-HCl (pH 7.5), 1 mM EDTA) for 24–48 hours at 4°C. Recovered DNA was stored at 4°C until use.
2.7 Mutagenesis assay
Lambda phage shuttle vectors harboring the bacterial LacI gene were recovered from high molecular weight genomic DNA samples using Stratagene’s Transpack in vitro packaging extracts (La Jolla, CA). Packaged phage was mixed with E. coli SCS-8 cells and added to top agarose containing 5-bromo-4-chloro-3-indoyl-betagalactopyranoside (X-gal) and plated on NZY agar assay trays. After incubation overnight at 37°C, plaque-forming units (pfus) were counted. Blue mutant plaques were visually identified. All putative mutant plaques were cored and replated at low density under the same incubation conditions. Mutant frequency (MF) was determined by dividing the number of confirmed mutant plaques by the total number of pfus obtained. A minimum of three in vitro packaging reactions were performed on each DNA sample. The summary of total pfus and confirmed mutants in different spermatogenic cell types are shown in Table 1.
Table 1.
Plaque forming units (Pfus) and confirmed mutants in different spermatogenic cell types
| Group | Cell (age) | Pfu | Confirmed Mutants | Mutant Frequency (Mean ± SEM, × 10−) |
|---|---|---|---|---|
| Mock | Type A (8 d/o) | 951,300 | 20 | 2.10 ± 0.47a |
| Type B (8 d/o) | 992,800 | 9 | 0.91 ± 0.30 | |
| Prelep/Lep (18d/o) | 1,212,600 | 9 | 0.74 ± 0.25 | |
| Lep/Zyg (18 d/o) | 1,039,900 | 6 | 0.58 ± 0.24 | |
| Pach (18 d/o) | 1,089,500 | 7 | 0.64 ± 0.24 | |
| RS (30 d/o) | 1,137,300 | 7 | 0.62 ± 0.23 | |
| RS (60 d/o) | 1,466,380 | 8 | 0.54 ± 0.19 | |
| Spermatozoa | 1,339,000 | 9 | 0.67 ± 0.22 | |
|
| ||||
| 6 d/o | Type A (8 d/o) | 1,716,825 | 89 | 5.18 ± 0.55a,b |
| Type B (8 d/o) | 2,847,750 | 47 | 1.65 ± 0.24a | |
| Prelep/Lep (18d/o) | 2,258,630 | 18 | 0.80 ± 0.22 | |
| Lep/Zyg (18 d/o) | 2,188,800 | 24 | 1.10 ± 0.22 | |
| Pach (18 d/o) | 2,102,100 | 20 | 0.95 ± 0.21 | |
| Pach (30 d/o) | 1,211,6450 | 12 | 0.98 ± 0.28 | |
| Pach (60 d/o) | 1,539,400 | 12 | 0.78 ± 0.22 | |
| RS (30 d/o) | 1,255,470 | 11 | 0.88 ± 0.26 | |
| RS (60 d/o) | 1,480,000 | 12 | 0.81 ± 0.23 | |
| Spermatozoa | 1,327,400 | 10 | 0.75 ± 0.24 | |
|
| ||||
| 8 d/o | Prelep/Lep (18d/o) | 1,045,720 | 26 | 2.49 ± 0.49a,b |
| Lep/Zyg (18 d/o) | 1,126,800 | 35 | 3.10 ± 0.52a,b | |
| Pach (18 d/o) | 1,267,750 | 19 | 1.50 ± 0.34a | |
| Pach (30 d/o) | 982,855 | 15 | 1.52 ± 0.39 a,b | |
| Pach (60 d/o) | 1,342,300 | 13 | 0.97 ± 0.27 a,b | |
| RS (30 d/o) | 971,185 | 10 | 1.03 ± 0.32 | |
| RS (60 d/o) | 1,371,550 | 14 | 1.02 ± 0.27 | |
| Spermatozoa | 952,140 | 8 | 0.84 ± 0.30 | |
|
| ||||
| 18 d/o | RS (30 d/o) | 1,563,450 | 17 | 1.09 ± 0.26 |
| RS (60 d/o) | 1,078,500 | 10 | 0.93 ± 0.29 | |
| Spermatozoa | 1,417,000 | 9 | 0.64 ± 0.21 | |
| Pach (30 d/o) | 1,348,215 | 27 | 2.00 ± 0.38 a,b | |
| Pach (60 d/o) | 1,159,900 | 21 | 1.81 ± 0.40 a,b | |
|
| ||||
| 25 d/o | RS (30 d/o) | 1,697,500 | 19 | 1.12 ± 0.26 |
| RS (60 d/o) | 1,266,480 | 14 | 1.10 ± 0.30 | |
| Spermatozoa | 1,070,120 | 7 | 0.65 ± 0.25 | |
d/o, day old; type A, type A spermatogonia; type B, type B spermatogonia; prelep, pre-leptotene spermatocytes; lep, leptotene spermatocytes; zyg, zygotene spermatocytes; pach, pachytene spermatocytes; RS, round spermatids; SEM, standard error of mean. a=significantly different from other cell types, same treatment. b=significantly different from mock treated, same cell type.
2.8 Doubling dose of IR
The doubling dose (DD) is the dose of IR required to double the spontaneous mutant frequency. The DDs were estimated using the method proposed previously [35]. The DD was dms estimated as , where ms is spontaneous mutant frequency, mIR is the mutant frequency after irradiation (mi = mIR - ms is the estimated induced mutant frequency) and d is the dose of radiation, i.e. 2.5 Gy in the present study. The DD was computed only when the IR-induced MF was significantly different from the spontaneous MF in the same cell type.
2.8 Statistical analysis
Data for germ cells were analyzed separately. The Chi-square test was used to compare results among radiation exposure, cell type, and age. If expected frequencies were small, StatXact software (Cytel Corp., Cambridge, MA) was used to carry out an exact test.
Confidence intervals (CI) for DD were calculated using Fieller’s theorem [36]. CIs do not exist if the denominator is not significantly different from zero and thus the DD was computed only when the IR-induced MF was significantly different from the spontaneous MF in the same cell type.
Testis weights were analyzed by analysis of variance (ANOVA). The data were transformed by a square root transformation in order to better satisfy the assumptions underlying the ANOVA. Comparisons among means were Bonferroni adjusted. Means and standard errors were computed using untransformed data.
3. Results
3.1 Spontaneous mutant frequencies (MF) in defined spermatogenic cell types from IR-mock treated mice
The highest spontaneous MF observed in the mock treated control group was found in type A spermatogonia, 2.10 ± 0.47 × 10−5. This MF was significantly higher (P=0.001) than that for all subsequent spermatogenic cell types (Table 1, Figure 2).
Figure 2.
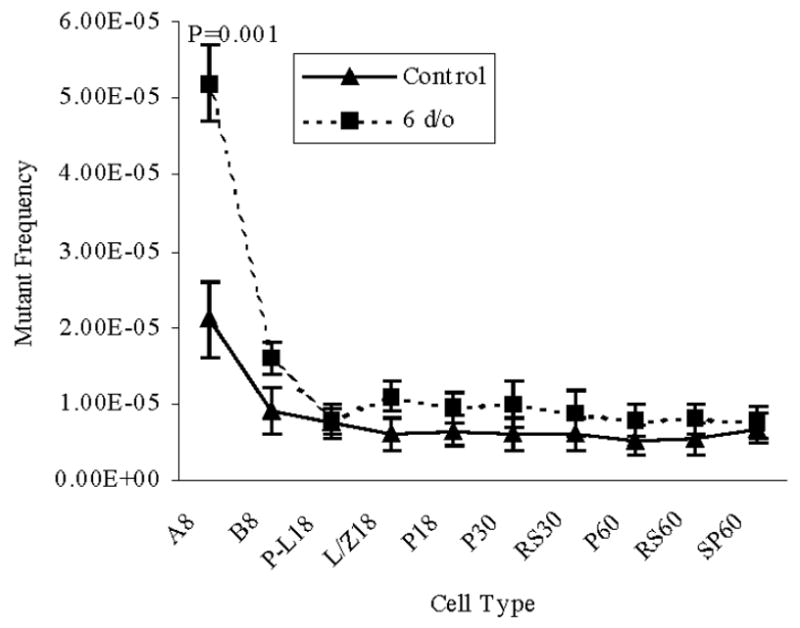
Mutant frequencies in spermatogenic cell types collected from mice irradiated at 6-d/o. The numbers following the cell type indicate the age of mice when the cells were collected. Data are presented as means ± SEM. P values represent significant differences between the IR-treated group and the control group, same cell type and same age. A and B, type A and B spermatogonia; P-L, pre-leptotene spermatocytes; L/Z, leptotene plus zygotene spermatocytes; P, pachytene spermatocytes; RS, round spermatids; SP, epididymal spermatozoa.
3.2 Neonatal exposure to 2.5Gy of IR
Mice at 6- or 8-d/o were used to examine the mutagenic consequences of neonatal male germ cell exposure to IR (Figure 1). Defined spermatogenic cell types were subsequently analyzed for MF from mice that were neonatal (8-d/0), or pubertal (18- or 30-d/o), or mature (60-d/o) after irradiation when the mice were 6-d/o, a time when primitive type A spermatogonia are the predominant germ cell type in the immature mouse testis. The MF was significantly elevated in type A spermatogonia (P=0.001) and in type B spermatogonia (P=0.001) recovered from 8-d/o mice compared to later spermatogenic cell types i.e. preleptotene, leptotene plus zygotene spermatocytes, 18-, 30-, and 60-d/o pachytene spermatocytes, 30- and 60-d/o round spermatids and 60-d/o epididymal spermatozoa obtained from irradiated mice. Type A spermatogonia obtained from irradiated mice displayed a higher MF (P=0.001) than the same cell type obtained from mock treated control mice (Figure 2, Table 1).
The MFs were also determined in spermatogenic cell types following irradiation at 8-d/o, a time when types A and B spermatogonia are the predominant germ cell types in the immature mouse testis. Pre-leptotene and leptotene plus zygotene primary spermatocytes displayed a significantly elevated (P=0.001) MF compared to later stages of spermatogenesis obtained from irradiated mice and from pre-leptotene and leptotene plus zygotene spermatocytes obtained from mock irradiated mice (Figure 3, Table 1). Although lower than pre-leptotene and leptotene plus zygotene spermatocytes, 18- and 30-d/o pachytene spermatocytes obtained from mice irradiated at 8-d/o, displayed a MF significantly greater (P=0.05) than subsequent stages of spermatogenesis obtained from irradiated mice. Comparison with 18-d/o pachytene spermatocytes obtained from mock irradiated mice was not significantly different while the MF for 30-d/o pachytene spermatocytes was significant (P=0.003;, Table 1, Figure 3).
Figure 3.
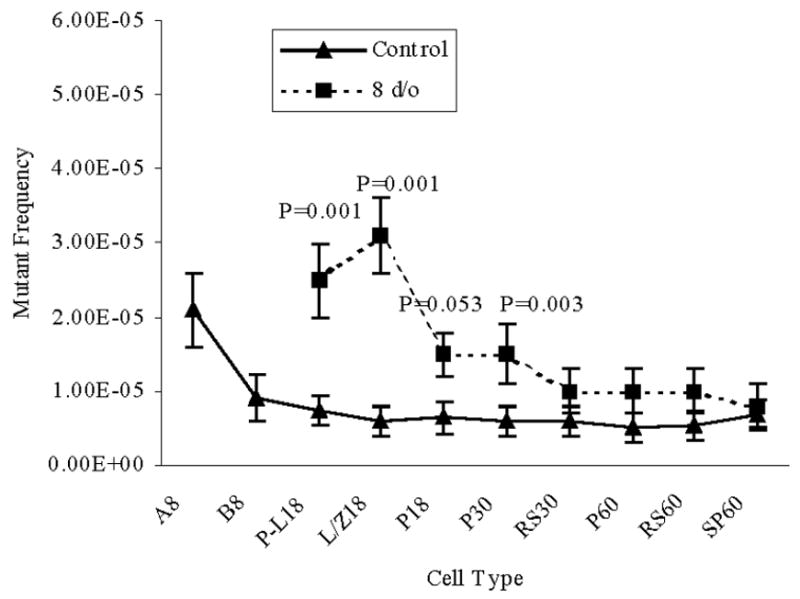
Mutant frequencies in spermatogenic cell types collected from mice irradiated at 8-d/o. The numbers following the cell type indicate the age of mice when the cells were collected. Data are presented as means ± SEM. P values represent significant differences between the IR-treated group and the control group, same cell type and same age. A and B, type A and B spermatogonia; P-L, pre-leptotene spermatocytes; L/Z, leptotene plus zygotene spermatocytes; P, pachytene spermatocytes; RS, round spermatids; SP, epididymal spermatozoa.
3.3 Pubertal exposure to 2.5 Gy of IR
Mice were irradiated at 18- or 25-d/o to determine the mutagenic consequences on spermatogenic cell types when exposed to IR during puberty. Predominant cell types present in the testis are primary spermatocytes including early pachytene spermatocytes (18-d/o) or pachytene spermatocytes and post-meiotic haploid round spermatids (25-d/o). As shown in Figure 4, MFs in pachytene spermatocytes obtained from 30- and 60-d/o mice after irradiation were significantly elevated compared to later cell types in the same treatment group (P=0.001). Pachytene spermatocytes obtained from 30-d/o irradiated mice displayed a greater MF than the same cell type from mock treated control mice (P=0.003). Similarly, pachytene spermatocytes from 60-d/o irradiated mice had a greater MF than those in mock-irradiated mice (P=0.001).
Figure 4.
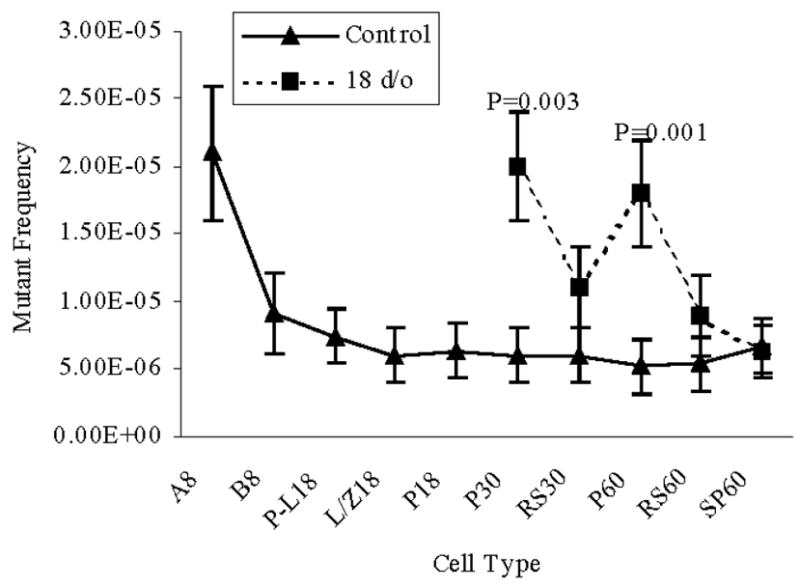
Mutant frequencies in spermatogenic cell types collected from mice irradiated at 18-d/o. The numbers following the cell type indicate the age of mice when the cells were collected. Data are presented as means ± SEM. P values represent significant differences between the IR-treated group and the control group, same cell type and same age. A and B, type A and B spermatogonia; P-L, pre-leptotene spermatocytes; L/Z, leptotene plus zygotene spermatocytes; P, pachytene spermatocytes; RS, round spermatids; SP, epididymal spermatozoa.
Figure 5 shows the MFs were significantly elevated in 30- and 60-d/o pachytene spermatocytes after IR exposure at 25-d/o compared to 30- and 60-d/o round spermatids and epididymal spermatozoa obtained from irradiated mice at 25 d/o (P=0.02) and compared to mock irradiated 30- and 60-d/o pachytene spermatocytes (P=0.003 and P=0.001, respectively).
Figure 5.

Mutant frequencies in spermatogenic cell types collected from mice irradiated at 25-d/o. The numbers following the cell type indicate the age of mice when the cells were collected. Data are presented as means ± SEM. P values represent significant differences between the IR-treated group and the control group, same cell type and same age. A and B, type A and B spermatogonia; P-L, pre-leptotene spermatocytes; L/Z, leptotene plus zygotene spermatocytes; P, pachytene spermatocytes; RS, round spermatids; SP, epididymal spermatozoa.
3.4 IR-induced mutagenesis is reduced during spermatogenesis
A significant decline in MF was detected between type A spermatogonia and type B spermatogonia in IR-treated and control groups (Figure 2). In IR treated and mock treated groups similar MFs were observed among type B spermatogonia and later spermatogenic cell types (Figure 2). Irradiation of 8-d/o type A and type B spermatogonia significantly increased MFs in preleptotene and leptotene plus zygotene spermatocytes compared to the mock group (Figure 3). A reduction in MF was observed between leptotene plus zygotene spermatocytes and pachytene spermatocytes collected from 18 d/o mice. When mice were irradiated at 18 d/o (Figure 4) or 25 d/o (Figure 5), the decline in MF occurred by the time epididymal spermatozoa were collected. Epididymal spermatozoa from IR treated mice displayed a MF similar to the epididymal spermatozoa from the control group.
3.5 IR dose used did not significantly impair mouse testis development
Significant age-related increases in testicular weight were observed in all groups (Table 2). For mock treated mice, the testis weights increased from 5.4 ± 0.2 mg at 8-d/o to 145.8 ± 6.4 mg at 60-d/o. In the mice irradiated at 6 d/o, the testis weights increased from 5.0 ± 0.2 mg at 8 d/o to 144.3 ± 4.1 mg at 60-d/o (Table 2). Comparison of testis weights between the mock treated group and IR exposed groups revealed that testis collected at 30-d/o from IR treated mice weighed significantly more than mock treated testes from the same aged mice. In addition, testis removed from 60-d/o mice that had been irradiated at 25-d/o, the testes weighed significantly less than those in the mock and other IR treated groups (Table 2).
Table 2.
Paired testis weight (mg) from mice exposed to IR at different ages
| Age at Collection | Treatment group
|
||||
|---|---|---|---|---|---|
| mock | 6 d/o | 8 d/o | 18 d/o | 25 d/o | |
| 8 d/o | 5.4 ± 0.2 | 5.0 ± 0.2 | |||
| 18 d/o | 15.1 ± 0.2 | 14.3 ± 0.4 | 14.4 ± 0.8 | ||
| 30 d/o | 40.8 ± 0.3* | 50.3 ± 1.8 | 61.1 ± 6.5 | 54.0 ± 1.4 | 67.5 ± 3.2 |
| 60 d/o | 145.8 ± 6.4 | 144.3 ± 4.1 | 151.0 ± 5.3 | 144.5 ± 7.3 | 119.2 ± 5.2* |
Data are presented as the average paired testis weights (mean ± SEM) for all mice for a particular age and treatment group.
Significantly different from other groups, same age at collection (same row comparison). d/o, day old. Comparisons among different ages at collect are significantly different from each other (same column comparison).
3.6 Sensitivity of spermatogenic cells to mutagenesis by IR
Germ cell type specific sensitivity to IR-induced mutation was estimated by calculating doubling doses (DD) (Table 3). The time (day) between defined spermatogenic stages was adopted from the literature [34,37] and was used to estimate the irradiated precursors of collected cell types. All cell types collected from mice treated at 6-d/o were from irradiated primitive type A spermatogonia. However, an IR-induced mutagenic effect was only observed in type A spermatogonia collected from 8-d/o mice (Table 1, Figure 2). The DD in type A spermatogonia is 1.71 Gy (95% CI is 0.71 – 4.18 Gy) (Table 3). In mice irradiated at 8-d/o, a mutagenic effect was observed in pre-leptotene and leptotene plus zygotene spermatocytes collected 10 days after irradiation and pachytene spermatocytes collected 10 and 22 days after irradiation (Table 1, Figure 3). The DDs varied among these spermatogenic cell types originating from type A spermatogonia, however, no significant difference was observed among the DDs. Irradiation of type A spermatogonia in 18-d/o or 25-d/o mice induced a mutagenic effect in pachytene spermatocytes collected from 30-d/o and 60-d/o mice (Table 1, Figure 4–5). The DDs were similar in pachytene spermatocytes collected at 30-d/o and 60-d/o, P>0.05 (Table 3). DDs were not calculated for round spermatids because the mutant frequencies were not significantly greater in IR treated versus mock-treated cells.
Table 3.
Doubling dose of different spermatogenic cell types.
| Age at irradiation (d/o) | Cell type (age-d/o) collected for MF | Irradiated cell types | Doubling dose (Gy) | 95% confidence interval |
|---|---|---|---|---|
| 6 | A (8) | Primitive A | 1.71 | 0.71 – 4.18 |
| 8 | P-L (18) | A | 1.06 | 0.28 – 3.76 |
| 8 | L/Z (18) | A | 0.57 | 0.10 – 1.54 |
| 8 | P (18) | A | 1.88 | 0.30 – 73.77 |
| 8 | P (30) | A | 1.50 | 0.26 – 26.73 |
| 18 | P (30) | A | 1.00 | 0.19 – 3.65 |
| 18 | P (60) | A | 1.02 | 0.20 – 4.40 |
| 25 | P (30) | P | 1.61 | 0.27 – 55.55 |
| 25 | P (60) | A | 0.95 | 0.19 – 3.93 |
d/o, day old. A, type A spermatogonia; primitive A, primitive type A spermatogonia; P-L, pre-leptotene spermatocytes; L/Z, leptotene plus zygotene spermatocytes, P, pachytene spermatocytes.
4. Discussion
This study was designed to examine directly the mutagenic impact, with regard to point mutations, emanating from neonatal or pubertal exposure to IR on defined spermatogenic cell types. A single exposure to γ-ray was delivered at different ages before or during puberty and did not significantly increase the mutant frequency in epididymal spermatozoa. However, γ-ray exposure did increase the mutant frequency in spermatogenic cell types immediately following from the irradiated cell type. Furthermore, the mutant frequency declined as spermatogenesis progressed such that a low mutant frequency, similar to that observed for untreated control samples, was achieved by the time that mature spermatozoa were produced from the irradiated precursors.
Spermatogonia are reported to be the most sensitive spermatogenic cells to killing by IR in the adult testis [18–22]. For induction of germline mutation by IR, however, spermatogonia in adult testes were found less susceptible than pachytene spermatocytes and spermatids in indirect germline mutation assays [24–26,38,39]. In these germline mutation assays, germline mutation is assessed in offspring of IR-treated male mice. Thus, mutant frequency for spermatozoa is inferred based on mutations detected in offspring. This approach has been enormously informative, but it leaves a knowledge gap. Assessing mutant frequencies in offspring does not provide a mechanism to determine directly mutant frequencies in defined spermatogenic cell types or to identify changes in mutant frequency during spermatogenesis because only the fully differentiated and successful spermatozoa are ascertained. Mating at different times after irradiation can be used to examine effects of treatment of different spermatogenic cell types using the SLT and related approaches. Again however, the interpretation is inferred because the spermatogenic cells are not examined directly and only mutations in mature and functional spermazoa are captured.
In the present study, epididymal spermatozoa were obtained from adult mice (60-d/o) that had previously been exposed to IR at 6-, 8-, 18- or 25-d/o. Consequently epididymal spermatozoa obtained from 60-d/o mice were derived from irradiated primitive type A spermatogonia, type A spermatogonia, differentiating spermatogonia or primary spermatocytes, respectively. The mutant frequencies in epididymal spermatozoa were not significantly elevated compared to the mock treated mice. If these male mice were mated with untreated female mice, we would not expect an increased mutant frequency in the offspring unless extant DNA damage in spermatozoa is converted to mutations in the one-cell embryo. As such, the results are consistent with a previous study in which mutant frequency was not increased in testis 35 days after irradiation [8]. Further, analyses of mutations in microsatellite DNA obtained from children born to atomic bomb survivors has demonstrated similar mutant frequencies among these children and children born to nonexposed parents [40,41]. An analysis of proteins by starch gel electrophoresis also found similar levels of mutation in offspring of atomic bomb survivors and children born to control parents [42].
Without assessing the mutant frequency for spermatogenic cell types arising between the IR-exposed cell types and spermatozoa, it would appear that the IR exposed spermatogenic cell types are poorly susceptible to IR-induced mutagenesis involving one or a few bases. Our results clearly demonstrate that IR does increase the frequency of point mutations in irradiated germ cells emanating by differentiation from the exposed precursor cell type (Figure 2). Regardless, the mutant frequency in epididymal spermatozoa returned to the levels observed for untreated control mice whether the mice were irradiated at neonatal or pubertal ages (Figure 2–5). Therefore, the findings in the present study fill a knowledge gap about IR-induced point mutations in differentiating spermatogenic cell types prior to the completion of spermatogenesis.
Sensitivity to IR-induced mutagenesis was quantitatively estimated by calculating the doubling doses in the later stages of spermatogenesis (Table 3). Doubling doses were higher in irradiated premeiotic and meiotic spermatogenic cell types in the present study compared to those reported from other germline mutation assays [43–46]. Notably, the Lac I mutation reporter system is effective in reporting point mutations, but is not as effective in reporting larger deletions, insertions or rearrangements. This could contribute to the differences in doubling doses found between the present study and previous studies using alternate mutation reporting systems.
Consistent with previous results [27], the spontaneous mutant frequency in type A spermatogonia is significantly greater than that in the later spermatogenic cell types and the reduction in mutant frequency takes place between type A spermatogonia and type B spermatogonia (Table 1). However, after IR treatment the return to the low spontaneous mutant frequency occurred later in spermatogenesis (Figures 2–5). Together with our previous study [27], it is clearly shown that spontaneous and IR-induced mutant frequencies can be reduced during spermatogenesis. The mechanism by which mutant frequency is reduced is not yet identified. One possibility is that germ cells carrying mutations may be removed through apoptosis or other venues of cell death. The mechanism that would signal cell death is not clear at this time. Another possibility is that of intrachromosomal recombination. The LacI transgene resides on mouse chromosome four in a head-to-tail concatemer of approximately 40 copies [7]. It is however, difficult to rationalize how the mutated copy is consistently and preferentially removed through this mechanism. Contributions from both mechanisms are also possible.
Testis weight provides a good indication of the abundance of germ cells and a loss in weight generally indicates a loss of spermatogenic cells. The present study was designed intentionally to employ a dose of IR that would not cause a massive disruption of spermatogenesis and loss of spermatogenic cells. Neonatal [47] or pubertal exposure to a single dose of γ-ray did not result in significantly reduced testis weight, except for testes collected from 60 d/o mice irradiated at 25 day old (Table 2). Other studies have reported reduced testes weights after irradiation of fetal [48,49], pubertal [50] and adult mice [51,52], with IR doses ranging from 0.25 Gy to 6.0 Gy. The different mouse stains (C57BL/6 in the present study vs. CBA/P [52]), animal ages (prepuberty vs. fetal [48,49] or adult [51,52]) could partly explain the discrepancy. The reduced weight may represent a mild disruption of spermatogenesis. Testes collected from 30 d/o mice, all treatment groups, weighed significantly more than controls. One study [53] described edema in testes after irradiation in rats. The consistent increase in testicular weight at 30 d/o in all treatment groups may reflect edema.
These studies described herein produced two major observations. First, premeiotic, meiotic and postmeiotic spermatogenic cells are susceptible to IR-induced mutagenesis. To detect the increased mutant frequency, it is necessary to examine directly spermatogenic cell types emanating from the irradiated precursor cell type. Secondly, and perhaps most importantly, there is/are a mechanism(s) for reducing mutant frequency during spermatogenesis such that spermatozoa do not reflect the increased mutant frequency detected in earlier spermatogenic cell types. This suggests strongly that there is a sensitive mechanism for monitoring genetic integrity during spermatogenesis and that cells with a greater mutant frequency can be removed and/or restored to a low mutant frequency. The implications are apparent. Germline genome integrity is stringently monitored and regulated, presumably as a means to balance species survival with evolution and diversity.
Acknowledgments
This work was supported by NIH grants AG24364 and AG21163 to Dr. CA Walter. The contents are solely the responsibility of the authors, and do not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.UNSCEAR. Hereditary effects of radiations. United Nations, New York: 2001. [Google Scholar]
- 2.Russell WL. X-ray-induced mutations in mice. Cold Spring Harb Symp Quant Biol. 1951;16:327–336. doi: 10.1101/sqb.1951.016.01.024. [DOI] [PubMed] [Google Scholar]
- 3.Generoso WM, Bishop JB, Gosslee DG, Newell GW, Sheu CJ, von Halle E. Heritable translocation test in mice. Mutat Res. 1980;76:191–215. doi: 10.1016/0165-1110(80)90010-x. [DOI] [PubMed] [Google Scholar]
- 4.Green S, Lavappa KS, Manandhar M, Sheu CJ, Whorton E, Springer JA. A guide for mutagenicity testing using the dominant lethal assay. Mutat Res. 1987;189:167–174. doi: 10.1016/0165-1218(87)90022-x. [DOI] [PubMed] [Google Scholar]
- 5.Dubrova YE, Nesterov VN, Krouchinsky NG, Ostapenko VA, Neumann R, Neil DL, Jeffreys AJ. Human minisatellite mutation rate after the Chernobyl accident. Nature. 1996;380:683–686. doi: 10.1038/380683a0. [DOI] [PubMed] [Google Scholar]
- 6.Gossen JA, de Leeuw WJ, Tan CH, Zwarthoff EC, Berends F, Lohman PH, Knook DL, Vijg J. Efficient rescue of integrated shuttle vectors from transgenic mice: a model for studying mutations in vivo. Proc Natl Acad Sci USA. 1989;86:7971–7975. doi: 10.1073/pnas.86.20.7971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohler SW, Provost GS, Fieck A, Kretz PL, Bullock WO, Sorge JA, Putman DL, Short JM. Spectra of spontaneous and mutagen-induced mutations in the lacI gene in transgenic mice. Proc Natl Acad Sci USA. 1991;88:7958–7962. doi: 10.1073/pnas.88.18.7958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoyes KP, Wadeson PJ, Sharma HL, Hendry JH, Morris ID. Mutation studies in lacI transgenic mice after exposure to radiation or cyclophosphamide. Mutagenesis. 1998;13:607–612. doi: 10.1093/mutage/13.6.607. [DOI] [PubMed] [Google Scholar]
- 9.Yauk CL, Dubrova YE, Grant GR, Jeffreys AJ. A novel single molecule analysis of spontaneous and radiation-induced mutation at a mouse tandem repeat locus. Mutat Res. 2002;500:147–156. doi: 10.1016/s0027-5107(02)00005-2. [DOI] [PubMed] [Google Scholar]
- 10.Huamani J, McMahan CA, Herbert DC, Reddick R, McCarrey JR, MacInnes MI, Chen DJ, Walter CA. Spontaneous mutagenesis is enhanced in Apex heterozygous mice. Mol Cell Biol. 2004;24:8145–8153. doi: 10.1128/MCB.24.18.8145-8153.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katoh M, Horiya N, Valdivia RP. Mutations induced in male germ cells after treatment of transgenic mice with ethylnitrosourea. Mutat Res. 1997;388:229–237. doi: 10.1016/s1383-5718(96)00121-0. [DOI] [PubMed] [Google Scholar]
- 12.Provost GS, Rogers BJ, Dycaico MJ, Carr G. Evaluation of the transgenic Lambda/LacI mouse model as a short-term predictor of heritable risk. Mutat Res. 1997;388:129–136. doi: 10.1016/s1383-5718(96)00109-x. [DOI] [PubMed] [Google Scholar]
- 13.Provost GS, Short JM. Characterization of mutations induced by ethylnitrosourea in seminiferous tubule germ cells of transgenic B6C3F1 mice. Proc Natl Acad Sci USA. 1994;91:6564–6568. doi: 10.1073/pnas.91.14.6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Putman DL, Ritter AP, Carr GJ, Young RR. Evaluation of spontaneous and chemical-induced lacI mutations in germ cells from lambda/lacI transgenic mice. Mutat Res. 1997;388:137–143. doi: 10.1016/s1383-5718(96)00110-6. [DOI] [PubMed] [Google Scholar]
- 15.Winegar RA, Carr G, Mirsalis JC. Analysis of the mutagenic potential of ENU and MMS in germ cells of male C57BL/6 lacI transgenic mice. Mutat Res. 1997;388:175–178. doi: 10.1016/s1383-5718(96)00114-3. [DOI] [PubMed] [Google Scholar]
- 16.Print CG, Loveland KL. Germ cell suicide: new insights into apoptosis during spermatogenesis. Bioessays. 2000;22:423–430. doi: 10.1002/(SICI)1521-1878(200005)22:5<423::AID-BIES4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 17.Dym M. Spermatogonial stem cells of the testis. Proc Natl Acad Sci U S A. 1994;91:11287–11289. doi: 10.1073/pnas.91.24.11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oakberg EF. Sensitivity and time of degeneration of spermatogenic cells irradiated in various stages of maturation in the mouse. Radiat Res. 1955;2:369–391. [PubMed] [Google Scholar]
- 19.Oakberg EF. Initial depletion and subsequent recovery of spermatogonia of the mouse after 20 r of gamma rays and 100, 300, and 600 r of x-rays. Radiat Res. 1959;11:700–719. [PubMed] [Google Scholar]
- 20.van Beek ME, Davids JA, van de Kant HJ, de Rooij DG. Response to fission neutron irradiation of spermatogonial stem cells in different stages of the cycle of the seminiferous epithelium. Radiat Res. 1984;97:556–569. [PubMed] [Google Scholar]
- 21.Meistrich ML, Finch M, Lu CC, de Ruiter-Bootsma AL, de Rooij DG, Davids JA. Strain differences in the response of mouse testicular stem cells to fractionated radiation. Radiat Res. 1984;97:478–487. [PubMed] [Google Scholar]
- 22.Shaver SL. X-irradiation injury and repair in the germinal epithelium of male rats. II. Injury and repair in immature rats. Am J Anat. 1953;92:433–449. doi: 10.1002/aja.1000920304. [DOI] [PubMed] [Google Scholar]
- 23.Speiser B, Rubin P, Casarett G. Aspermia following lower truncal irradiation in Hodgkin’s disease. Cancer. 1973;32:692–698. doi: 10.1002/1097-0142(197309)32:3<692::aid-cncr2820320323>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 24.Searle A. Mutation induction in mice. Advances in Radiation Biology. 1974;4:131–207. [Google Scholar]
- 25.Ehling UH. Comparison of radiation-and chemically-induced dominant lethal mutations in male mice. Mutat Res. 1971;11:35–44. doi: 10.1016/0027-5107(71)90030-3. [DOI] [PubMed] [Google Scholar]
- 26.Fan YJ, Wang Z, Sadamoto S, Ninomiya Y, Kotomura N, Kamiya K, Dohi K, Kominami R, Niwa O. Dose-response of a radiation induction of a germline mutation at a hypervariable mouse minisatellite locus. Int J Radiat Biol. 1995;68:177–183. doi: 10.1080/09553009514551081. [DOI] [PubMed] [Google Scholar]
- 27.Walter CA, Intano GW, McCarrey JR, McMahan CA, Walter RB. Mutation frequency declines during spermatogenesis in young mice but increases in old mice. Proc Natl Acad Sci USA. 1998;95:10015–10019. doi: 10.1073/pnas.95.17.10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beaumont HM. Changes in the radiosensitivity of the testis during foetal development. Int J Radiat Biol. 1960;2:247–256. doi: 10.1080/09553006014550291. [DOI] [PubMed] [Google Scholar]
- 29.Hughes G. Radiosensitivity of male germ-cells in neonatal rats. Int J Radiat Biol. 1962;4:511–519. doi: 10.1080/09553006214550291. [DOI] [PubMed] [Google Scholar]
- 30.Delic JI, Hendry JH, Morris ID, Shalet SM. Dose and time related responses of the irradiated prepubertal rat testis. II. Seminiferous epithelial function. Int J Androl. 1985;8:484–496. doi: 10.1111/j.1365-2605.1985.tb00861.x. [DOI] [PubMed] [Google Scholar]
- 31.Delic JI, Hendry JH, Morris ID, Shalet SM. Seminiferous epithelial function in the pubertal rat following local testicular irradiation. Radiother Oncol. 1986;5:39–45. doi: 10.1016/s0167-8140(86)80007-x. [DOI] [PubMed] [Google Scholar]
- 32.Erickson BH, Blend MJ. Response of the Sertoli cell and stem germ cell to 60Co gamma-radiation (dose and dose rate) in testes of immature rats. Biol Reprod. 1976;14:641–650. doi: 10.1095/biolreprod14.5.641. [DOI] [PubMed] [Google Scholar]
- 33.Harding LK. The survival of germ cells after irradiation of the neonatal male rat. Int J Radiat Biol. 1961;3:539–551. doi: 10.1080/09553006114550711. [DOI] [PubMed] [Google Scholar]
- 34.Bellve AR. Purification, culture, and fractionation of spermatogenic cells. Methods Enzymol. 1993;225:84–113. doi: 10.1016/0076-6879(93)25009-q. [DOI] [PubMed] [Google Scholar]
- 35.Sankaranarayanan K, Chakraborty R. Ionizing radiation and genetic risks. XI. The doubling dose estimates from the mid-1950s to the present and the conceptual change to the use of human data on spontaneous mutation rates and mouse data on induced mutation rates for doubling dose calculations. Mutat Res. 2000;453:107–127. doi: 10.1016/s0027-5107(00)00108-1. [DOI] [PubMed] [Google Scholar]
- 36.Finney D. Statistical Methods in Biological Assay. Hafner Press; New York: 1971. [Google Scholar]
- 37.Oakberg EF. Duration of spermatogenesis in the mouse and timing of stages of the cycle of the seminiferous epithelium. Am J Anat. 1956;99:507–516. doi: 10.1002/aja.1000990307. [DOI] [PubMed] [Google Scholar]
- 38.Auerbach C, Slizynski BM. Sensitivity of the mouse testis to the mutagenic action of X-rays. Nature. 1956;177:934–935. doi: 10.1038/177934b0. [DOI] [PubMed] [Google Scholar]
- 39.Russell WL, Bangham JW, Russell LB. Differential response of mouse male germ-cell stages to radiation-induced specific-locus and dominant mutations. Genetics. 1998;148:1567–1578. doi: 10.1093/genetics/148.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kodaira M, Izumi S, Takahashi N, Nakamura N. No evidence of radiation effect on mutation rates at hypervariable minisatellite loci in the germ cells of atomic bomb survivors. Radiat Res. 2004;162:350–356. doi: 10.1667/rr3243. [DOI] [PubMed] [Google Scholar]
- 41.Kodaira M, Satoh C, Hiyama K, Toyama K. Lack of effects of atomic bomb radiation on genetic instability of tandem-repetitive elements in human germ cells. Am J Hum Genet. 1995;57:1275–1283. [PMC free article] [PubMed] [Google Scholar]
- 42.Neel JV, Satoh C, Hamilton HB, Otake M, Goriki K, Kageoka T, Fujita M, Neriishi S, Asakawa J. Search for mutations affecting protein structure in children of atomic bomb survivors: preliminary report. Proc Natl Acad Sci USA. 1980;77:4221–4225. doi: 10.1073/pnas.77.7.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dubrova YE, Plumb M, Brown J, Fennelly J, Bois P, Goodhead D, Jeffreys AJ. Stage specificity, dose response, and doubling dose for mouse minisatellite germ-line mutation induced by acute radiation. Proc Natl Acad Sci USA. 1998;95:6251–6255. doi: 10.1073/pnas.95.11.6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luning KG, Searle AG. Estimates of the genetic risks from ionizing irradiation. Mutat Res. 1971;12:291–304. doi: 10.1016/0027-5107(71)90017-0. [DOI] [PubMed] [Google Scholar]
- 45.Russell WL, Kelly EM. Mutation frequencies in male mice and the estimation of genetic hazards of radiation in men. Proc Natl Acad Sci USA. 1982;79:542–544. doi: 10.1073/pnas.79.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singer TM, Lambert IB, Williams A, Douglas GR, Yauk CL. Detection of induced male germline mutation: correlations and comparisons between traditional germline mutation assays, transgenic rodent assays and expanded simple tandem repeat instability assays. Mutat Res. 2006;598:164–193. doi: 10.1016/j.mrfmmm.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 47.Tegelenbosch RA, de Rooij DG. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat Res. 1993;290:193–200. doi: 10.1016/0027-5107(93)90159-d. [DOI] [PubMed] [Google Scholar]
- 48.Vergouwen RP, Huiskamp R, Bas RJ, Roepers-Gajadien HL, Davids JA, de Rooij DG. Radiosensitivity of testicular cells in the fetal mouse. Radiat Res. 1995;141:66–73. [PubMed] [Google Scholar]
- 49.Moreno SG, Dutrillaux B, Coffigny H. High sensitivity of rat foetal germ cells to low dose-rate irradiation. Int J Radiat Biol. 2001;77:529–538. doi: 10.1080/09553000010030211. [DOI] [PubMed] [Google Scholar]
- 50.Vergouwen RP, Huiskamp R, Bas RJ, Roepers-Gajadien HL, de Jong FH, van Eerdenburg FJ, Davids JA, de Rooij DG. Radiosensitivity of testicular cells in the prepubertal mouse. Radiat Res. 1994;139:316–326. [PubMed] [Google Scholar]
- 51.Kochar NK, Bateman AJ. Post-irradiation changes in Sertoli cells. J Reprod Fertil. 1969;18:265–273. doi: 10.1530/jrf.0.0180265. [DOI] [PubMed] [Google Scholar]
- 52.Haines GA, Hendry JH, Daniel CP, Morris ID. Germ cell and dose-dependent DNA damage measured by the comet assay in murine spermatozoaa after testicular X-irradiation. Biol Reprod. 2002;67:854–861. doi: 10.1095/biolreprod.102.004382. [DOI] [PubMed] [Google Scholar]
- 53.Porter KL, Shetty G, Meistrich ML. Testicular edema is associated with spermatogonial arrest in irradiated rats. Endocrinology. 2006;147:1297–1305. doi: 10.1210/en.2005-0890. [DOI] [PubMed] [Google Scholar]



