Abstract
A concise total asymmetric synthesis of the tetrahydronaphthridine alkaloid (−)-normalindine has been accomplished via the addition of a laterally metallated 4-methyl-3-cyanopyridine to a sulfinimine (N-sulfinyl imine) as the key step.
Enantiopure tetrahydroisoquinolines and tetrahydronaphthyridines represent a large class of naturally occurring alkaloids which possess a broad range of biological activities.1,2 Generally the synthesis of these compounds involves an intramolecular electrophilic aromatic cyclization step to form the nitrogen containing ring of the isoquinoline; i.e. Bischler-Napieralski,3 Pictet-Spengler,4 and Pomeranz Fritch5 reactions. Although these methods have been extensively exploited for this purpose, the success of these procedures is greatly enhanced when the aromatic ring is electron rich. Furthermore, these procedures often require harsh reaction conditions and suffer from unreliable regio- and stereoselectivity. Side reactions involving the reactive nitrilium ion intermediates can also be an issue. While some methods have been devised to circumvent these limitations, these procedures are largely target specific.6
An attractive alternative to the Bischler-Napieralski and Pictet-Spengler protocols is the addition of laterally lithiated amides and nitriles 1 to imines such as enantiopure sulfinimines (N-sulfinyl imines) 2 (Scheme 1).7 Indeed, this methodology has the promise of overcoming many of the problems of the electrophilic cyclization processes and to provide isoquinolines with substitution patterns not easily accessible by other means. This is because the nitrogen-containing ring is formed via a nucleophilic cyclization step of the sulfinamide intermediate 3 with the aromatic ring amide or nitrile substituent. The resulting isoquinolone 4 and cyclic imine 5 can be readily elaborated to tetrahydroisoquinolines. Using this methodology, we have described highly stereoselective asymmetric syntheses of (2R,4S)-(−)-6-methoxy-N-methyl-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-ol (6),8 trans-(1R,3R)-(−)-6,8-dimethoxy-1,3-dimethyl-1,2,3,4-tetrahydroisoquinoline (7),9 the isoquinoline segment of the anti-HIV michellamines, and (−)-xylopinine (8), the prototypical member of the protoberberine alkaloids.10 In these studies, it was found that the addition of laterally lithiated nitriles to enantiopure sulfinimines was preferred over laterally amides because the cyclic imine is formed in one pot. Furthermore, the formation of atropisomers in the sulfinamide derived from the laterally lithiated amides made it difficult to determine the diastereoselectivity of the reaction.
Scheme 1.
(−)-Normalindine (9) is an unusual 1,3-cis-disubstituted tetrahydronaphthridine alkaloid which was isolated from the root bark of Strychnos johnsonii.2a This alkaloid has been the subject of several racemic syntheses,11 but only one asymmetric synthesis. In their preparation of (−)-9, Ohba and co-workers employed an intramolecular oxazole-olefin Diels-Alder cyclization to form the key tetrahydronaphthyridine core.12 Although their synthesis confirmed the absolute configuration of the natural product, it required 16 steps with an overall yield of less than 0.1%. Here we describe a highly efficient asymmetric synthesis of (−)-9, requiring 8 steps with an overall yield of 14%, using our laterally lithiated nitrile sulfinimine technology.
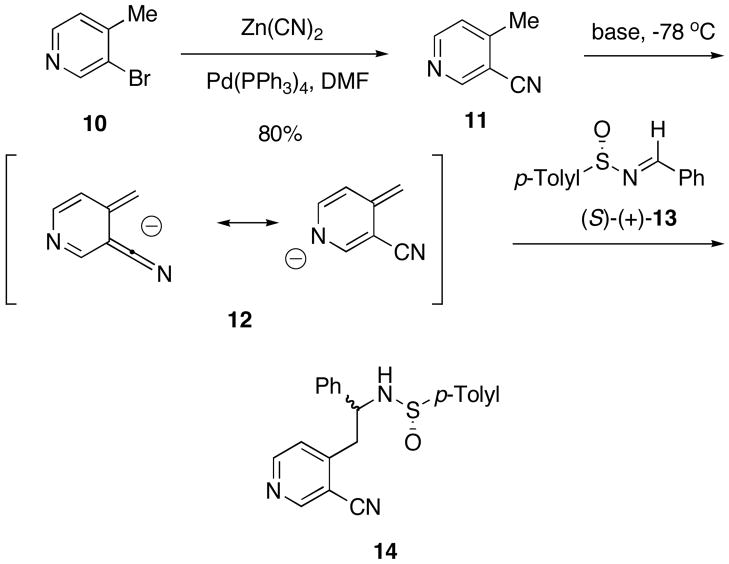
In earlier work, we attributed the high diastereoselectivities for the addition of laterally lithiated ortho-tolylnitriles to sulfinimines based on the o-quinonedimethane structure which was chelated through a lithium cation to the sulfinyl oxygen.9 Based on this hypothesis, one would predict that the diastereoselectivity for sulfinimine addition could be poor (Scheme 2) if in the o-quinonedimethane structure 12 generated from 3-cyano-4-methylpyridine (11) the anion is localized at the pyridine nitrogen. For this reason we first carried out a model study for the addition of 12 to sulfinimine (S)-(+)-13 before pursuing the synthesis (−)-normalindine (9).
Scheme 2.
3-Cyano-4-methylpyridine (11) was prepared in 80% yield by the catalytic palladium (0) coupling of zinc cyanide with 3-bromo-4-methylpyridine (10) (Scheme 2).10 The laterally metallated species were generated by the addition of 11 to a solution of base in THF at −78 °C and resulted in an intense orange colored solution of the anion. After 0.5 h, (S)-(+)-13 was added dropwise to the base solution. The reaction was complete as determined by TLC of the solution (typically 15 minutes) and was quenched with a saturated aqueous NH4Cl solution. Flash chromatography afforded the sulfinamide products 14 as an inseparable mixture of diastereomers. These results are summarized in Table 1.
Table 1.
Addition of metallated 11 to (S)-(+)-13 at −78 °C.
| entry | basea | solvent | 14 % isolated yieldb (%de)c |
|---|---|---|---|
| 1 | LDA | THF | 56 (18) |
| 2 | LDA | Toluene | No reaction |
| 3 | LDA | Ether | 23 (16) |
| 4 | LiHMDS | THF | 32 (16) |
| 5 | NaHMDS | THF | 22 (60) |
| 6 | NaHMDSd | THF | 87 (58) |
| 7 | KHMDS | THF | 68 (34) |
Anion aged for 30 min before adding to 13.
Yields correspond to the diastereomeric mixtures.
Determined by 1H NMR on the crude reaction mixtures.
Anion aged for 2 min before adding to 13.
The results summarized in Table 1 reveal that LDA, the base of choice for the generation of laterally lithiated o-tolunitriles,6b,c gave poor de’s of the desired product with 11 (Table 1, entry 1). Diethyl ether gave lower yields, probably due to the poor solubility of the laterally lithiated species in this solvent (Table 1, entries 3). No improvement in the yield or de was noted with LiHMDS or KHMDS (Table 1, entries 4 and 7). However, NaHMDS resulted in a de of 60%, but the yield was poor (Table 1, entry 5). The isolated yield was dramatically improved when the anion was formed and subsequently quenched with sulfinimine within two minutes. Apparently longer reaction times resulted in product decomposition. Although results using NaHMDS as the base were promising, the product diastereoisomers 14 proved to be inseparable. However, an advantage of our synthetic methodology is that modification of the sulfinimine often not only improves the diastereoselectivity but results in chromatographically separable products.
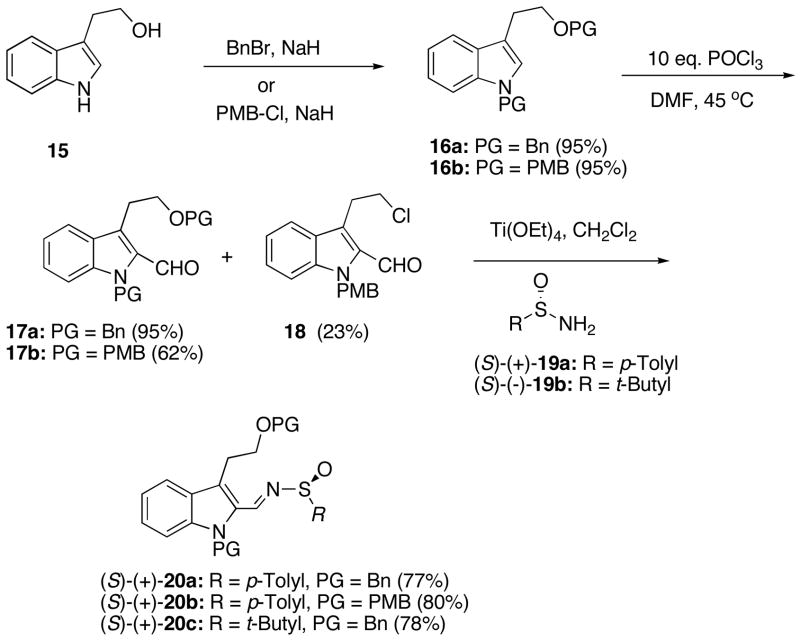
The synthesis of (−)-normalindine (9) begins with the preparation of the requisite sulfinimines 20a–c as outlined in Scheme 3. Commercially available tryptophol (15) was protected with either a benzyl or p-methoxy benzyl (PMB) to give 16a and 16b, respectively, in excellent yields. The Vilsmeier formulation reaction of the protected indoles 16 with POCl3 in DMF afforded the corresponding aldehydes 17a and 17b in moderate to excellent yields. 14 Apparently, in the course of this reaction with 17b, some hydrolysis of the PMB group occurs resulting in the formation of alkyl chloride 18, which was isolated in 23% yield (Scheme 3). The optimal conditions for the Vilsmeier reaction proved to be 10 equiv of POCl3 in DMF at 45 °C for 3 h. Sulfinimines 20a–c were prepared in 77–80% yields in the usual manner by condensing the aldehydes with either (S)-(+)-p-toluensulfinamide (19a) or (S)-(−)-tert-butanesulfinamide (19b) in the presence of Ti(OEt)4 in refluxing CH2Cl2 for 24 hrs.15
Scheme 3.
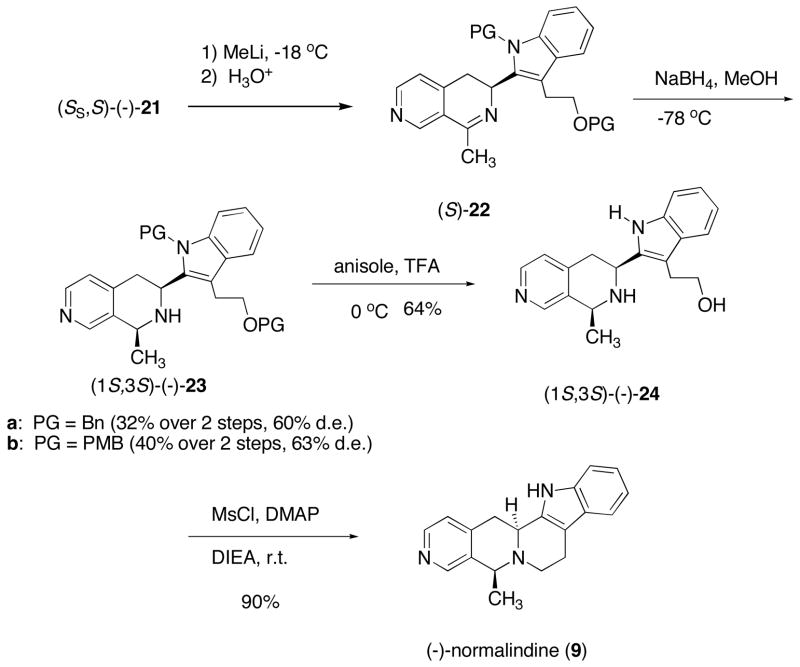
Next, 3-cyano-4-methylpyridine (11) was treated with the appropriate base at −78 °C in THF affording the orange colored solution of the anion (Scheme 4). Addition of the sulfinimines 20a–c to the anion solution quenched the color producing the diastereomeric sulfinamides 21a–b (Table 2). The results revealed in Table 2 indicated that while LiHMDS and KHMDS gave moderated de values of 40 and 60%, respectively, the yields were poor to modest (Table 2, entries 1 and 2). However, when NaHMDS was employed as the base the de’s were better than 80%. Furthermore, the diastereoisomers were easily separated affording the major isomers (SS, S)-(−)-21a and (SS, S)-(−)-21b in 68 and 81% isolated yields, respectively (Table 2, entries 3, 4). Decomposition occurred upon addition of the anion of 11 to the (S)-(−)-tert-butanesulfinimine 20c (Table 2, entry 5). The major diastereoisomer is predicted to have the (S)-configuration at the newly created chiral center in sulfinamide 21, based on our earlier proposed chelated transition state hypothesis.8,9 Indeed, this was confirmed in the total synthesis of (−)-normalidine (9).
Scheme 4.
Table 2.
Addition of the anion of 11 to (+)-20 at −78 °C in THF.
| entry | (+)-20 R = | (+)-20, PG = | Conditions, base, timea | sulfinamide (−)-21 % isolated yieldb (% de)c |
|---|---|---|---|---|
| 1 | 20a (p-Tolyl) | Bn | LiHMDS, 30 min | 27 (40) |
| 2 | 20a (p-Tolyl) | Bn | KHMDS, 30 min | 50 (64) |
| 3 | 20a (p-Tolyl) | Bn | NaHMDS, 2 min | 68 (80) |
| 4 | 20b (p-Tolyl) | PMB | NaHMDS, 2 min | 81 (82) |
| 5 | 20c (t-Bu) | Bn | NaHMDS, 2 min | No reaction |
Time for anion formation, before adding 20.
Isolated yield of major diastereoisomer.
Determined by 1H NMR on the crude reaction mixture.
Reaction of sulfinamides (−)-21 with MeLi, followed by addition of aq. HCl accomplishes four operations in one pot: (i) installation of the 1-methyl group, (ii) removal of the sulfinyl auxiliary, and (iii) hydrolysis of the resulting ketimine to the methyl ketone, and (iv) formation of the cyclic imines 22 (Scheme 5). The resulting imines were unstable to silica gel chromatography and therefore were immediately reduced with sodium borohydride to give the corresponding disubstituted tetrahydronaphthyridines (1S, 3S)-(−)-23a and (1S, 3S)-(−)-23b in modest de, 60 and 63% de, respectively.16 Other reducing reagents such as Super-Hydride or LiBH4 failed to improve the diastereoselectivity. Chromatographic separation of the diastereoisomers afforded (−)-22a and (−)-22b in 46 and 65% isolated yield, respectively, isolated yields for the two-step reaction sequence (Scheme 5).
Scheme 5.
Removal of the alcohol and indole benzyl protecting groups from (−)-23a was explored next. Dissolving metal reduction with sodium and liquid ammonia led to only trace amounts of (−)-24. Catalytic hydrogenation with 10% Pd-C with or without acetic acid at 350 psi in a Parr high pressure reactor produced none of the desired product and starting materials were recovered. Aluminium chloride, anisole mediated deprotection of the benzyl groups led to molecular destruction, again with only trace amounts of 24 being detected.
Fortunately, we were able to remove the PMB protecting groups in (−)-23b using a modification of the procedure reported by Forbes and co-workers.17 They reported that PMB deprotection of a protected indole could be accomplished in high yield with trifluoroacetic acid (TFA) in the presence of concentrated sulfuric acid and anisole. However, when 23b was submitted to these reaction conditions, only low yields of the desired product (−)-24 were obtained; ca 15% and numerous other impurities were observed. The elimination of sulfuric acid led to cleaner conversion to 24. Here the TFA and 10 equiv of anisole solution were cooled to 0 °C followed by addition of 23b. The reaction turned deep green and after 30 min was complete, affording (−)-24 in 64% isolated yield. Monitoring the reaction by TLC indicated that deprotection of the alcohol occurred first followed by a slower removal of the protecting group on the indole nitrogen. This represents the first simultaneous deprotection of an alcohol and indole protecting groups.
Cyclization was easily accomplished by reaction of (−)-24 with methanesulfonyl chloride, dimethylaminopyridine and Hunigs base resulting in a 90% yield of (−)-normalindine (9). This concise synthesis provided (−)-9 in 8 steps with an overall yield of 14% starting from commercially available starting materials. The spectral properties, specific rotation, and melting point of (−)-normalindine (1) were in agreement with literature values.2f Importantly this methodology suggests that it will find applications in the asymmetric synthesis of other electron-deficient tetrahydroisoquinolines and tetrahydronaphthyridines.
EXPERIMENTAL SECTION
(S)-(−)-N-(Benzylidene)-p-toluenesulfinamide (13) was prepared as previously described.15
(SS, S)-(+)-N-[(1)-2-(3-Cyanopyridin-4-yl)-1-phenylethyl]-4 methylbenzenesulfinamide (14)
In a 100-mL, 3-necked oven dried round-bottom flask equipped with a magnetic stirring bar, and rubber septum under argon was placed anhydrous THF (8 mL) and NaHMDS (8.47 mL, 1.0 M in THF, 8.47 mmol). The solution was cooled to −78 °C and 4-methyl-3-cyanopyridine (11) (0.50 g, 4.2 mmol) in THF (3 mL) was added dropwise while keeping the internal temperature less than −76 °C. To the yellow solution was immediately added (S)-(+)-13 (1.04 g, 4.23 mmol) in THF (5 mL) dropwise. After stirring for 2 min the reaction mixture was quenched with aqueous NH4Cl (10 mL) at −78 °C and the solution was warm to rt. After dilution with water (10 mL) and EtOAc (20 mL), the phases were separated, the aqueous layer was extracted with EtOAc (20 mL), and the combined organic phases were dried (Na2SO4), filtered, and concentrated. Flash chromatography (CHCl3/MeOH, 98:2) afforded 1.1 g (87%) of a mixture of diastereomers (79:21); [α]20D +9.5 (c 1.0, MeOH); 1H NMR of the major diastereomer (DMSO D6) δ 8.78 (s, 1 H), 8.67 (d, J = 5.2 Hz, 1 H,), 7.49 (m, 2 H), 7.30 (d, J = 5.2 Hz, 1 H,), 7.27 (5 H, m), 7.12 (2 H, m), 4.75 (1 H, m), 4.45 (d, J = 5.8 Hz, 1 H), 3.59 (dd, J = 7.2, 13.8 Hz, 1 H), 3.39 (dd, J = 7.6, 13.7 Hz, 1 H), 2.42 (s, 3 H). 13C NMR (DMSO) δ 153.1, 151.1, 142.2, 140.9, 137.9, 129.1, 128.0, 127.1, 126.5, 125.5, 116.0, 110.3, 57.1, 42.3, 21.2. ES HRMS calcd for C21H19N3OS (M + H) 362.1319. Found: 362.1321.
1-Benzyl-3-(2-(benzyloxy)ethyl)-1H-indole (16a)
In a 500-mL, round-bottom flask equipped with a stirring bar and rubber septum under an argon atmosphere, was placed bromobenzene (13.26 g, 77.5 mmol), and NaH (1.48 g, 61.8 mmol) in DMF (250 mL) at 0 °C. Tryptophol (15) (20 mL, 15.5 M in DMF, 31.0 mmol) was then added to the reaction mixture at 0 °C. The reaction mixture was warmed to rt, stirred for 12 h, and quenched with water (100 mL). The organic phase was extracted with Et2O (3 × 50 mL), dried (Na2SO4), and concentrated. Purification by flash chromatography (hexane/Et2O 10:1) gave 7.30 g (95%) of an oil; 1H NMR (CDCl3) δ 7.71 (d, J = 7.2 Hz, 1 H), 7.38 (m, 8 H), 7.22 (m, 4 H), 7.07 (s, 1 H), 5.34 (s, 2 H), 4.65 (s, 2 H), 3.89 (d, J = 7.5 Hz, 2 H), 3.20 (d, J = 7.0 Hz, 2 H); 13C NMR (CDCl3) δ 138.6, 137.8, 136.6, 128.8, 128.4, 128.3, 127.8, 127.6, 126.9, 126.3, 121.8, 119.1, 119.0, 112.2, 109.7, 73.0, 70.8, 50.0, 25.9. HRMS calcd for C24H23NO (M + H) 342.1893. Found: 342.1835.
1-(4-Methoxybenzyl)-3-(2-(4-methoxybenzyloxy)ethyl)-1H-indole (16b)
In a 250 mL, round-bottom flask equipped with a stirring bar and rubber septa under an argon atmosphere was placed p-methoxybenzyl chloride (2.43 g, 15.51 mmol) and NaH (0.37 g 77.5 mmol) in DMF (100 mL) at 0 °C. To the reaction mixture was added dropwise tryptophol (15) (10 mL, 6.2 M in DMF, 6.2 mmol) at 0 °C, the solution was warmed to rt, and stirred for 12 h. At this time the reaction mixture was quenched with water (30 mL), the organic phase was extracted with Et2O (3 × 25 mL), dried (Na2SO4), and concentrated. Purification by flash chromatography (hexane/Et2O, 10:1) gave 2.18 g (95%) of an oil; 1H NMR (CDCl3) δ 7.67 (d, J = 8.4 Hz, 1 H), 7.35 (m, 4 H), 7.19 (m, 4 H), 7.03 (s, 1 H), 6.92 (m, 4 H), 5.28 (s, 2 H), 4.57 (s, 2 H), 3.88 (s, 2 H), 3.85 (s, 3 H), 3.83 (t, J = 7.2 Hz, 2 H), 3.16 (t, J = 7.1 Hz, 2 H); 13C NMR (CDCl3) δ 159.2, 159.1, 136.5, 130.7, 129.8, 129.4, 128.3, 128.3, 126.1, 121.7, 119.1, 118.9, 114.1, 113.8, 112.1, 109.7, 72.7, 70.5, 55.4, 55.3, 49.4, 25.9. HRMS Calcd. for C26H27NO3 (M + H) 402.2064. Found 402.2054.
1-Benzyl-3-(2-(benzyloxy)ethyl)-1H-indole-2-carbaldehyde (17a)
In a 250-mL round-bottom flask equipped with a stirring bar and rubber septum under an argon atmosphere was placed phosphoryl chloride (53.9 g, 102.5 mmol) and 16a (7.30 g, 21.39 mmol) in DMF (150 mL). The reaction mixture was heated to 60 °C and stirred for 12 h, cooled to 0 °C, and 1 M NaOH (1 M, 250 mL) was slowly added. The solution was warmed to rt, stirred for 1 h, and sat. NH4Cl (100 mL) was added. The organic phase was extracted with Et2O (3 × 70 mL), dried (Na2SO4) and concentrated. Purification by flash chromatography (hexane/EtOAc 10:1) gave 7.84 g (95%) of a yellow oil; 1H NMR (CDCl3) δ 10.25 (s, 1 H), 7.84 (d, J = 6.1 Hz, 1 H), 7.31 (m, 13 H), 5.91 (s, 2 H), 4.60 (s, 2 H), 3.86 (t, J = 7.1 Hz, 2 H), 3.52 (t, J = 6.8 Hz, 2 H); 13C NMR (CDCl3) δ 182.0, 138.1, 131.4, 128.7, 128.6, 128.5, 128.0, 127.7, 127.7, 127.6, 127.5, 127.4,127.3,126.7,126.6, 126.5, 121.5, 121.1, 120.7, 111.2, 111.0, 73.2, 70.7, 47.9, 24.8. HRMS Calcd for C25H23NO2 (M + H) 370.1802. Found: 370.1786.
1-(4-Methoxybenzyl)-3-(2-(4-methoxybenzyloxy)ethyl)-1H-indole-2-carbaldehyde (17b)
In a 50-mL round-bottom flask equipped with a stirring bar and rubber septum under an argon atmosphere, was placed phosphoryl chloride (0.95 g, 6.2 mmol) and 16b (0.25 g, 0.62 mmol) in DMF (1.5 mL, 0.4 molar) at rt. The reaction mixture was heated to 45 °C for 4.5 h. At this time, the reaction mixture was cooled to 0 °C in an ice bath and NaOH (3 mL, 1M) was added, and the solution was stirred for 1 h at rt. At this time sat. NH4Cl (3 mL) was added, the organic phase was extracted with Et2O (3 × 15 mL), dried (Na2SO4), and concentrated. Purification by flash chromatography (hexane:EtOAc, 10:1) gave 0.16 g (62%) of a yellow oil; 1H NMR (CDCl3) δ 10.21 (s, 1 H), 7.78 (dd, J = 1.2, 8.1 Hz, 1 H), 7.46 (m, 2 H), 7.55 (m, 1 H), 7.25 (m, 3 H), 7.13 (d, J = 8.1 Hz, 2 H), 6.87 (m, 4 H), 5.82 (s, 2 H), 4.51 (s, 2 H), 3.87 (m, 3 H), 3.81 (m, 6 H), 3.47 (t, J = 6.6 Hz, 2 H); 13C NMR (CDCl3) δ 182.0, 159.2, 158.8, 139.6, 131.3, 130.3, 130.2, 129.2, 128.0, 128.0, 127.4, 126.7, 121.4, 120.6, 114.0, 114.0, 113.8, 111.0, 72.8, 70.4, 55.3, 55.3, 47.3, 24.8. HRMS Calcd for C27H27NO4 (M + Na) 452.1838. Found 452.1829.
1-(4-Methoxybenzyl)-3-(2-chloroethyl)-1H-indole-2-carbaldehyde (18)
Purification by flash chromatography (hexane/EtOAc, 10:1) gave 0.63 g (24%) of a clear oil; 1H NMR (CDCl3) δ 10.25 (s, 1 H), 7.84 (d, J = 6.1 Hz, 1 H), 7.31 (m, 13 H), 5.91 (s, 2 H), 4.60 (s, 2 H), 3.86 (t, J = 7.1 Hz, 2 H), 3.52 (t, J = 6.8 Hz, 2 H); 13C NMR (CDCl3) δ 182.0, 138.1, 131.4, 128.7, 128.6, 128.5, 128.0, 127.7, 127.7, 127.6, 127.5, 127.4, 127.3, 126.7, 126.6, 126.5, 121.5, 121.1, 120.7, 111.2, 111.0, 73.2, 70.7, 47.9, 24.8. HRMS Calcd. for C19H18ClNO2 (M + H) 328.1099. Found 328.1105.
(S)-(+)-N-((1E)-{1-Benzyl-3-[2-(benzyloxy)ethyl]-1H-indol-2-yl}methylidene)-4-methylbenzenesulfinamide (20a)
In a 250-mL oven dried round-bottom flask equipped with a reflux condenser, magnetic stirring bar, and rubber septum under argon, was placed 17a (3.57 g, 9.66 mmol) in anhydrous CH2Cl2 (75 mL). To the solution was added Ti(OEt)4 (22.0 g, 96.6 mmol) and p-toluenesulfinamide (S)-(+)-19a (1.82 g, 11.60 mmol). The solution was refluxed for 20 h, cooled to rt, and was slowly poured into a 1 L beaker containing ice water (200 mL) and CH2Cl2 (100 mL) with vigorously stirring. The resulting white solids were collected by vacuum filtration, and the organic and aqueous phases were added to a separatory funnel. The aqueous phase was extracted with CH2Cl2 (200 mL) and the combined organic layers were dried (Na2SO4), and concentrated. Purification by flash chromatography (hexane:EtOAc, 10:1) gave 3.75 g (76%) as a light yellow oil; [α]20D 28.2 (c 2.2, CHCl3); 1H NMR (CDCl3) δ 9.19 (s, 1 H), 7.86 (d, J = 8.0 Hz, 1 H), 7.52 (d, J = 8.0 Hz, 2 H), 7.42-7.29 (m, 13 H), 7.09 (d, J = 6.2 Hz, 2 H), 6.06 (d, J = 17.2 Hz, 1 H), 5.87 (d, J = 16 Hz, 1 H), 4.65 (s, 2 H), 3.86 (m, 2 H), 3.53 (m, 2 H); 2.50 (s, 3 H); 13C NMR (CDCl3) δ 150.0, 142.6, 141.4, 140.0, 138.5, 138.3, 130.0, 129.9, 128.6, 128.5, 127.6, 127.6, 127.2, 127.1, 126.5, 126.4, 124.8, 124.8, 121.0, 120.6, 110.6, 73.1, 70.6, 48.2, 25.3, 21.6. HRMS calcd for C32H30N2O2S (M + H) 507.2101. Found: 507.2076.
(SS)-(E)-N-((1-(4-Methoxybenzyl)-3-(2-(4-methoxybenzyloxy)ethyl)-1H-indol-2-yl)methylene)-4-methylbenzenesulfinamide (20b). Prepared from 1-(4-methoxybenzyl)-3-(2-(4-methoxybenzyloxy)ethyl)-1H-indole-2-carbaldehyde (17b)
Purification by flash chromatography (hexane/EtOAc, 10:3) gave 0.14 g (80%) of a light oil; [_]20D 137.8 (c 1.0, CHCl3); 1H NMR (CDCl3) δ 9.01 (s, 1 H), 7.28 (m, 15 H), 6.75 (d, J = 6.9 Hz, 1 H), 5.44 (s, 2 H), 4.53 (s, 2 H), 3.86 (m, 8 H), 3.34 (t, J = 6.9 Hz, 2 H), 2.50 (s, 3 H); 13C NMR (CDCl3) δ 159.4, 159.2, 137.6, 135.5, 130.4, 130.3, 130.2, 129.8, 129.3, 129.2, 128.4, 127.6, 126.4, 126.2, 125.5, 124.4, 121.1, 121.0, 120.9, 120.3, 114.3, 113.8, 113.8, 110.8, 109.0, 72.7, 69.5, 55.3, 55.2, 48.5, 26.1, 21.5. HRMS calcd. for C34H34N2O4 (M + H) 567.2312. Found: 567.2308.
(SS)-(E)-N-((1-Benzyl-3-(2-(benzyloxy)ethyl)-1H-indol-2-yl)methylene)-2-methylpropane-2-sulfinamide (20c). Prepared from tert-butlysulfinamide (S)-(−)-19b and 1-benzyl-3-(2-(benzyloxy)ethyl)-1H-indole-2-carbaldehyde (17a)
Purification by flash chromatography (hexane/EtOAc 10:2) gave 0.95 g (80%) of a yellow oil; mp 147–150 °C; [α]20D 35.6 (c 2.1, CHCl3); 1H NMR (CDCl3) δ 8.91 (s, 1 H), 7.79 (d, J = 8.1 Hz, 1 H), 7.36-7.18 (m, 11 H), 6.12 (d, J = 16.9 Hz, 1 H), 5.75 (d, J = 16.8 Hz, 1 H), 4.57 (s, 2 H), 3.81 (m, 2 H), 3.47 (m, 2 H); 1.097 (s, 9 H); 13C NMR (CDCl3) δ 152.3, 140.0, 138.7, 138.4, 130.3, 128.9, 128.6, 127.8, 127.7, 127.5, 127.3, 126.5, 126.0, 124.4, 121.2, 120.7, 110.6, 73.2, 70.9, 57.6, 48.3, 25.5, 22.5. HRMS calcd for C29H32N2O2S (M + H) 473.2257. Found 473.2232
(SS,S)-(−)-N-[(1S)-1-{1-Benzyl-3-[2-(benzyloxy)ethyl]-1H-indol-2-yl}-2-(3-cyanopyridin-4-yl)ethyl]-4-methylbenzenesulfinamide (21a)
In a 100-mL 3-necked, oven dried round-bottom flask equipped with a magnetic stirring bar and rubber septum under argon was placed NaHMDS (1.69 mL, 1.0 M in THF, 1.69 mmol) in THF (10 mL). The solution was cooled to −78 °C and 4-methyl-3-cyanopyridine (11) (0.10 g, 0.85 mmol) in THF (1 mL) was added dropwise while keeping the internal temperature less than −76 °C. To the yellow solution was immediately added dropwise (S)-(+)-20a (0.43 g, 0.85 mmol) in dry THF (3 mL). After stirring for 2 min, the reaction mixture was quenched with sat. NH4Cl (10 mL) at −78 °C and the solution was warmed to rt. After dilution with water (10 mL), EtOAc (20 mL) was added, the organic phase was separated, and the aqueous phase was extracted with EtOAc (2 × 20 mL). The combined organic phases were dried (Na2SO4) and concentrated. Flash chromatography (10–40% acetone/hexanes) afforded 0.36 g (68%) of the major diastereomer as a white solid mp 49–51 °C; [α]20D -44.0 (c .43, CHCl3); 1H NMR (CDCl3) δ 8.59 (s, 1 H), 8.38 (d, J = 5.2 Hz, 1 H), 7.53 (d, J = 8 Hz, 1 H), 7.37 (d, J = 8 Hz, 2 H), 7.29-7.13 (m, 14 H), 6.71 (d, J = 7.2 Hz, 2 H), 6.60 (bs, 1 H), 5.25-5.20 (m, 1 H), 4.81-4.70 (m, 2 H), 4.26 (d, J = 12 Hz, 1 H), 4.23 (d, J = 11.6 Hz, 1 H), 3.89-3.86 (m, 1 H), 3.70 (t, J = 8 Hz, 1 H), 3.65-3.60 (m, 1 H), 3.24-3.19 (m, 3 H), 2.36 (s, 3 H); 13C NMR (CDCl3) δ 153.0, 152.6, 150.6, 141.8, 141.3, 138.3, 137.8, 137.3, 130.0, 129.4, 128.8, 128.4, 128.2, 127.9, 127.9, 126.2, 126.2, 125.0, 123.1, 120.1, 119.0, 116.4, 111.9, 111.8, 110.2, 73.1, 70.0, 50.3, 46.3, 41.1, 25.9, 21.3. HRMS calcd for C39H36N4O2S (M + H) 625.2632. Found: 625.2659.
(SS, S)-(−)-N-[(1S)-2-(3-Cyanopyridin-4-yl)-1-(1-(4-methoxybenzyl)-3-{2-[(4-methoxybenzyl)oxy]ethyl}-1 H-indol-2-yl)ethyl]-4-methylbenzenesulfinamide (21b)
In a 1000-mL 3-necked oven dried round-bottom flask equipped with a magnetic stirring bar and rubber septum under argon, was placed THF (250 mL) and NaHMDS (21.2 mL, 1.0 M in THF, 21.2 mmol). The solution was cooled to −78 °C and 11 (1.38 g, 11.7 mmol) in THF (10 mL) was added dropwise while keeping the internal temperature less than −76 °C. To the yellow solution was immediately added dropwise (S)-(+)-20b (6.0 g, 10.6 mmol) in THF (25 mL). After stirring for 5 min the reaction mixture was quenched with aqueous NH4Cl (200 mL) at −78 °C and the solution was warm to rt. After dilution with water (100 mL) EtOAc (200 mL) was added and the phases were separated. The aqueous phase was extracted with EtOAc (2 × 200 mL) and the combined organic phases were dried (Na2SO4), and concentrated. Flash chromatography (12.5–40% acetone:hexanes) afforded 5.9 g (81%) as a white solid; mp 55–58 °C; [〈]20D -47.3 (c 1.35, CHCl3); 1H NMR (CDCl3) δ 8.55 (s, 1 H), 8.35 (d, J = 4.8 Hz, 1 H), 7.48 (d, J = 8.0 Hz, 1 H), 7.36 (d, J = 7.6 Hz, 2 H), 7.20 (m, 4 H), 7.12 (m, 1 H), 7.03 (d, 2 H), 6.77-6.66 (m, 5 H), 6.57 (d, J = 8.4 Hz, 2 H), 5.14 (bm, 1 H), 4.71 (bs, 2 H), 4.19 (d, J = 11.6 Hz, 1 H), 4.16 (d, J = 11.6 Hz, 1 H) 3.81 (m, 1 H), 3.77 (s, 3 H), 3.73 (s, 3 H), 3.65-3.55 (m, 2 H), 3.25-3.11 (m, 3 H), 2.39 (s, 3 H); 13C NMR (CDCl3) δ 159.7, 159.3, 152.9, 152.5, 150.6, 141.7, 141.4, 137.2, 135.3, 130.1, 130.0, 129.9, 127.7, 127.4, 126.2, 125.7, 125.0, 123.0, 119.9, 118.9, 116.3, 114.7, 114.1, 111.9, 111.7, 110.1, 73.1, 70.1, 55.7, 55.6, 50.5, 46.2, 41.4, 26.3, 21.7. ES HRMS calcd for C41H40N4O4S (M + H) 685.2843. Found: 685.2835. Anal. Calcd for C41H40N4O4S: C, 71.91; H, 5.89; N, 8.18. Found: C, 71.56; H, 5.65; N, 8.04.
(1S,3S)-(−)-3-{1-Benzyl-3-[2-(benzyloxy)ethyl]-1H-indol-2-yl}-1-methyl-1,2,3,4-tetrahydro-2,7-naphthyridine (23a)
In a 250-mL oven dried round-bottom flask equipped with a magnetic stirring bar, rubber septum, was placed (−)-21a (1.0 g, 1.6 mmol) in toluene (30 mL). The solution was cooled to −18 °C and MeLi (9.97 mL, 1.6 M in ether, 15.95 mmol) was added. After 2 h the reaction mixture was quenched with 1 N HCl (27 mL) and stirring was continued for 1.5 h at 0 °C. At this time sat. bicarbonate solution (50 mL) was added to neutralize the acid (pH 7.0). After dilution with water (50 mL), the solution was extracted with EtOAc (2 × 50 mL), the combined organic phases were dried (Na2SO4), and concentrated to afford a yellow solid of 22a. Since this material was unstable to chromatography and slowly air oxidized it was immediately taken to the reduction step.
In a separate 100-mL, oven dried round-bottom flask equipped with a magnetic stirring bar, rubber septum, was placed anhydrous MeOH (25 mL) was placed crude imine (S)-22a (0.70 g, 1.44 mmol) and NaBH4 (0.11 g, 2.88 mmol) at −78 °C. After 2 h the reaction mixture was quenched with aqueous NH4Cl (30 mL) and EtOAc (75 mL) was added. At this time the solution was extracted with EtOAc (2 × 50 mL), the combined organic phases were dried (Na2SO4), and concentrated. Flash chromatography (hexanes:acetone, 70:30) afforded 0.32 g (46%) over two steps of a yellow solid, mp 55–58 °C; [α]20D −47.3 (c 1.35, CHCl3); 1H NMR (CDCl3) δ 8.44 (s, 1 H), 8.31 (d, J = 4.8 Hz, 1 H), 7.65 (m, 1 H), 7.29-7.24 (m, 8 H), 7.18-7.15 (m, 3 H), 6.93 (d, J = 6.4 Hz, 2 H), 6.78 (d, J = 5.0 Hz, 1 H), 5.92 (d, J = 17.2 Hz, 1 H), 5.59 (d, J = 17.2 Hz, 1 H), 4.51 (m, 2 H), 4.06 (q, J = 6 Hz, 1 H), 3.78 (t, J = 6.8 Hz, 2 H), 3.60 (t, J = 6.8 Hz, 2 H), 3.20-3.13 (dd, 11.6, 17.2 Hz, 1 H), 2.69-2.64 (dd, 3.4, 17.0 Hz, 1 H), 1.34 (d, J = 6.4, 3 H); 13C NMR (CDCl3) δ 147.6, 147.2, 144.1, 139.1, 138.8, 137.7, 137.5, 135.9, 129.1, 128.7, 128.1, 128.0, 127.4, 126.1, 123.9, 122.4, 119.8, 119.0, 110.7, 110.4, 73.6, 71.4, 52.0, 51.2, 48.3, 35.6, 25.7, 22.0. ES HRMS calcd for C33H33N3O (M + H) 488.2697. Found: 488.2716.
(1S,3S)-(−)-3-(1-(4-Methoxybenzyl)-3-{2-[(4-methoxybenzyl)oxy]ethyl}-1H-indol-2-yl)-1-methyl-1,2,3,4-tetrahydro-2,7-naphthyridine (23b)
In a 500-mL oven dried 3-necked round-bottom flask equipped with a magnetic stirring bar, rubber septa, was placed 21b (2.47 g, 3.60 mmol) in ether (100 mL). The solution was cooled to −18 °C (salt water ice bath) and MeLi (22.5 mL, 1.6 M in ether, 36.0 mmol) was added dropwise. Upon addition of MeLi the solution became heterogeneous and turned yellow. After 1 h the reaction mixture was quenched with 1 N HCl (72 mL), stirring was continued for 1.5 h at 0 °C, and sat. bicarbonate solution (200 mL) was added. At this time the solution was extracted with EtOAc (2 × 200 mL), the combined organic phases were dried (Na2SO4), and concentrated to give crude imine (S)-22b.
In a separate 100-mL oven dried round-bottom flask equipped with a magnetic stirring bar, rubber septa, was placed crude imine (S)-22b (0.92 g, 1.69 mmol) and sodium borohydride (0.128 g, 3.37 mmol) in anhydrous MeOH (25 mL) at −78 °C. After stirring for 1 h, the reaction mixture was quenched with sat. aqueous NH4Cl (30 mL) and EtOAc (75 mL) was added. The solution was extracted with EtOAc (2 × 50 mL), the combined organic layers were dried (Na2SO4), and concentrated. Flash chromatography (CH2Cl2/MeOH, 98:2) afforded 0.60 g (65%) and an orange solid, mp 66–70 °C; [α]20D −43.3 (c 1.0, CHCl3); 1H NMR (CDCl3) δ 8.41 (s, 1 H), 8.27 (d, J = 5.1 Hz, 1 H), 7.59 (m, 1 H), 7.17-7.09 (m, 5 H), 6.85-6.75 (m, 7 H), 5.80 (d, J = 17.1 Hz, 1 H), 5.47 (d, J = 17.4 Hz, 1 H), 4.50-4.45 (dd, J = 3.6, 11.4 Hz, 1 H), 4.40 (m, 2 H), 4.03 (q, J = 6.9 Hz, 1 H), 3.78 (s, 3 H), 3.75 (s, 3 H), 3.71 (t, J = 6.6 Hz, 2 H), 3.20 (t, J = 6.6 Hz, 2 H), 3.13 (m, 1 H), 2.63 (dd J = 3.6, 16.9 Hz, 1 H), 1.90 (bs, 1 H), 1.33 (d J = 6.3 Hz, 3 H); 13C NMR (CDCl3) δ 159.5, 159.0, 147.6, 147.2, 144.2, 137.6, 137.5, 136.0, 131.1, 130.9, 129.7, 128.2, 127.2, 123.9, 122.4, 119.7, 119.0, 114.4, 114.1, 110.7, 110.4, 73.2, 71.1, 55.6, 55.6, 52.0, 51.2, 47.7, 35.6, 25.7, 22.1. ES HRMS calcd for C35H37N3O3 (M + H) 548.2913. Found 548.2901.
2-{2-[(1S,3S)-1-Methyl-1,2,3,4-tetrahydro-2,7-naphthyridin-3-yl]-1 H-indol-3-yl}ethanol (24)
In a 50-mL oven dried round-bottom flask equipped with a magnetic stirring bar, rubber septa was placed 23b (0.28 g, 0.51 mmol), cold TFA (9 mL) was added followed by anisole (0.28 mL, 2.53 mmol). After 0.5 h the green solution was slowly poured into ice cold sat. sodium bicarbonate solution (50 mL), and EtOAc (50 mL) was added. The solution was extracted with EtOAc (2 × 50 mL) and the combined organic phases were dried (Na2SO4), and concentrated. Flash chromatography (MeOH:CHCl3, 2%–8%) afforded 0.99 g (64%) of a white powder, mp 203–205 °C; [α]20D −13.0 (c 1.0, CHCl3); 1H NMR (CD3OD) δ 8.66 (s, 1 H), 8.47 (d, J = 5.1 Hz, 1 H), 7.69 (d, J = 7.8 Hz, 1 H), 7.51 (d, J = 8.1 Hz, 1 H), 7.41 (d, J = 5.4 Hz, 1 H), 7.27 (t, J = 6.9 Hz, 1 H), 7.09 (t, J = 6.9 Hz, 1 H), 4.63 (dd, J = 3.0, 11.7 Hz, 1 H), 4.58-4.52 (q, J = 6.3 Hz, 1 H), 3.96 (t, J = 6.6 Hz, 2 H), 3.54 (m, 1 H), 3.23-3.14 (m, 3 H), 1.75 (d, J = 6.9 Hz, 3 H); 13C NMR (CD3OD) δ 146.2, 146.0, 145.4, 136.2, 136.0, 135.6, 127.9, 124.1, 121.3, 118.5, 117.9, 110.7, 109.0, 62.1, 51.2, 49.4, 34.3, 27.3, 19.8. ES HRMS calcd for C19H21N3O(M + H) 308.1758. Found 308.1729.
(−)-Normalindine (9)
In an oven dried 11 dram vial equipped with a magnetic stirring bar and rubber septum was added 24 (0.02 g, 0.065 mmol) in a 1:1 CH2Cl2/DMF (2 mL). Mesyl chloride (5 L, 0.065 mmol) and DMAP (0.002 g, 0.02 mmol) were added and the reaction mixture was stirred at 0 0C for 1.5 h. At this time water (5 mL) and EtOAc (5 mL) were added and the solution was extracted with EtOAc (5 × 5 mL). The combined organic phases were dried (Na2SO4), and concentrated. Flash chromatography (MeOH:CH2Cl2, 4%) afforded 0.010 g (90%) of a light yellow solid, mp 120–124 °C [lit.2f 131–136 °C]; [α]20D −204.0 (c 0.4, CHCl3) [lit2f. [α]20D −210 (c 0.1, CHCl3)]; 1H NMR (CHCl3) δ 8.52 (s, 1 H), 8.37 (d, J = 5.1 Hz, 1 h), 7.94 (bs, 1 H), 7.52 (d J = 7.2 Hz, 1 H), 7.33 (d, J = 7.5 Hz, 1 H), 7.20-7.07 (m, 3 H), 3.82 (m, 2 H), 3.60 (ddd, J = 1.8, 5.3, 11.4 Hz, 1 H), 3.09-2.94 (m, 3 H), 2.84 (m, 1 H), 2.58 (td, J = 3.7, 11.4 Hz, 1 H), 1.64 (d, J = 6.3 Hz, 3 H); 13C NMR (CDCl3) δ 148.7, 146.6, 142.6, 136.5, 136.0, 134.3, 127.1, 123.1, 121.8, 119.6, 118.4, 110.9, 109.2, 57.3, 55.1, 48.8, 34.8, 22.4, 22.1. ES HRMS calcd for C19H19N3 (M + H) 290.1652. Found 290.1656.
Supplemental Material
Acknowledgments
This work was supported by a grant from the National Institutes of Health (GM51982) and by Merck through a fellowship to JYM. Mass spectra were provided by Charles Ross and Joan Murphy at Merck.
Footnotes
Supporting Information Available. 1H and 13C NMR spectra for all new compounds and (−)-9. This material is available free of charge via the Internet hhtp://pubs.acs.org.
References
- 1.(a) Martin SF. In: The Alkaloids. Brossi A, editor. Vol. 30. Academic Press; New York: 1987. p. 252. [Google Scholar]; (b) Bringman G, Pokorny F. In: The Alkaloids. Cordell GA, editor. Vol. 46. Academic Press; New York: 1995. p. 128. [Google Scholar]; (c) Bhakuni DS, Jain S. In: The Alkaloids. Brossi A, editor. Vol. 28. Academic Press; New York: 1986. p. 95. [Google Scholar]
- 2.(a) Massiot G, Thepenier P, Jacquier MJ, Men-Olivier LL, Verpoorte R, Delaude C. Phytochemistry. 1987;26:2839. [Google Scholar]; (b) Jahangir Brook MA, MacLean DB, Holland HL. Tetrahedron. 1987;43:5761. [Google Scholar]; (c) Phillipson JD, Hemingway SR. Phytochemistry. 1974;13:973. [Google Scholar]; (d) Erdelmeier CA, Regenass U, Rali T, Sticher O. Planta medica. 1992;58:43. doi: 10.1055/s-2006-961387. [DOI] [PubMed] [Google Scholar]; (e) Abreu P, Pereira A. Heterocycles. 1998;48:885. [Google Scholar]; (f) Arbain D, Putra DP, Sargent MV. Aust J Chem. 1993;46:977. [Google Scholar]; (g) Caprasse M, Tavernier D, Anteunis MJO, Angenot L. Planta medica. 1984;50:27. doi: 10.1055/s-2007-969613. [DOI] [PubMed] [Google Scholar]; (h) Arbain D, Lajis NH, Putra DP, Sargent MV, Skelton BW, White AH. Aust J Chem. 1993;46:969. [Google Scholar]; (i) Arbain D, Byrne LT, Dachriyanus Sargent MV. Aust J Chem. 1997;50:1109. [Google Scholar]
- 3.Whaley WM, Govindachari TR. Org React. 1951;6:74. [Google Scholar]
- 4.Whaley WM, Govindachari TR. Org React. 1951;6:151. [Google Scholar]
- 5.Gensler WJ. Org React. 1951;6:191. [Google Scholar]
- 6.(a) Mendelson WL, Spainhour CB, Jr, Jones SS, Lam BL, Wert KL. Tetrahedron Lett. 1980;21:1393. [Google Scholar]; (b) Subimoto A, Shinba-Tanaka H, Ishikawa M. Synthesis. 1995:431. [Google Scholar]; (c) Quallich GJ, Makowski TW, Sander AF, Urban FJ, Vazquez E. J Org Chem. 1998;63:4116. [Google Scholar]
- 7.For a reviews on lateral lithiation reactions see: Clark RD, Jahangir A. Org React. 1995;47:5.Snieckus V. Chem Rev. 1990;90:879.
- 8.Davis FA, Andemichael YW. J Org Chem. 1999;64:8627. [Google Scholar]
- 9.Davis FA, Mohanty PK, Burns DM, Andemichael YW. Org Lett. 2000;2:3901. doi: 10.1021/ol006654u. [DOI] [PubMed] [Google Scholar]
- 10.Davis FA, Mohanty PK. J Org Chem. 2002;67:1290. doi: 10.1021/jo010988v. [DOI] [PubMed] [Google Scholar]
- 11.(a) Maiti BC, Giri VS, Pakrashi SC. Heterocycles. 1990;31:847. [Google Scholar]; (b) Rey AW, Szarek WA, MacLean DB. Heterocycles. 1991;32:1143. [Google Scholar]
- 12.(a) Ohba M, Kubo H, Fujii T, Ishibashi H, Sargent MV, Arbain D. Tetrahedron Lett. 1997;38:6697. [Google Scholar]; (b) Ohba M, Kubo H, Ishibashi H. Tetrahedron. 2000;56:7751. [Google Scholar]
- 13.Tschaen DM, Desmond R, King AO, Fortin MC, Pipik B, King S, Verhoeven TR. Synth Comm. 1994;24:887. [Google Scholar]
- 14.Walkup RE, Linder J. Tetrahedron Lett. 1985;26:2155. [Google Scholar]
- 15.Davis FA, Zhang Y, Andemichael Y, Fang T, Fanelli DL, Zhang H. J Org Chem. 1999;64:1403. [Google Scholar]
- 16.Bringmann G, Weirich R, Reuscher H, Jansen JR, Kinzinger L, Ortmann T. Liebigs Ann Chem. 1993:877. [Google Scholar]
- 17.Forbes IT, Johnson CN, Thompson M. J Chem Soc, Perk Trans 1. 1992:275. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.