Abstract
Recent in vitro evidence suggests that the antioxidant lycopene can prevent alcohol-induced oxidative stress and inflammation. However, knowledge of possible interactions in vivo between escalating doses of lycopene and chronic alcohol ingestion are lacking. In this study, we investigated potential interactions between alcohol ingestion and lycopene supplementation and their effect on hepatic lycopene concentration, cytochrome P4502E1 (CYP2E1) induction, and inflammation. Fischer 344 rats (6 groups, n = 10 per group) were fed either a liquid ethanol Lieber-DeCarli diet or a control diet (isocaloric maltodextrin substituted for ethanol) with or without lycopene supplementation at 2 doses (1.1 or 3.3 mg·kg body weight−1·d−1) for 11 wk. Plasma and hepatic concentrations of lycopene isomers were assessed by HPLC analysis. We examined expressions of hepatic CYP2E1 and tumor necrosis factor-α (TNFα) and the incidence of hepatic inflammatory foci. Both plasma and hepatic lycopene concentrations were greater in alcohol-fed rats than in control rats supplemented with identical doses of lycopene. In contrast, alcohol-fed rats had a lower percentage of lycopene cis isomers in the plasma and the liver compared with control rats fed the same dose of lycopene. Notably, lycopene supplementation at the higher dose significantly induced hepatic CYP2E1 protein, TNFα mRNA, and the incidence of inflammatory foci in the alcohol-fed rats but not in the control rats. These data indicate an interaction between chronic alcohol ingestion and lycopene supplementation and suggest a need for caution among individuals consuming high amounts of both alcohol and lycopene.
Introduction
Chronic and excessive alcohol intake is associated with increased oxidative stress (1), a decrease in antioxidant enzymes (2), and induction of the cytochrome P4502E1 (CYP2E1)3 enzyme (3,4). The absence of antioxidants has been shown to further promote alcohol-induced oxidative stress, which could lead to an inflammatory state (5). Chronic alcohol intake also induces bacterial endotoxin (6), which is associated with activation of the nuclear factor-κB inflammatory pathway, leading to the activation of several proinflammatory cytokines, including tumor necrosis factor-α (TNFα), and increased hepatic infiltration by inflammatory cells. Whereas some antioxidants such as tea polyphenols are protective against alcohol-induced changes in the liver (7,8), others such as vitamin E have no beneficial effect (9).
Whereas the antioxidant properties of lycopene have been demonstrated both in vivo and in vitro (10), recent evidence suggests that it also functions as an antiinflammatory agent (11,12). It has been demonstrated that lycopene can inhibit the expression of inflammatory cytokines and reverse the loss of antioxidant enzymes induced by inflammation caused by either injecting with lipopolysaccharide or by exposure to iron (11,13,14). Although the data for lycopene’s role both as an antioxidant and an antiinflammatory agent in various models are compelling, such effects have not been demonstrated in an in vivo model of alcohol-induced oxidative stress and inflammation. One human study has shown that lycopene was inversely associated with concentrations of alcohol-induced γ-glutamyl-transferase, an indicator of liver damage, in moderate and heavy drinkers (15). An in vitro study demonstrated that in addition to decreasing alcohol-induced oxidative stress, lycopene could also ameliorate alcohol-modified hepatic biomarkers (16). In that study, incubating hepatic HepG2 cells that overexpressed CYP2E1 with lycopene resulted in decreased alcohol-induced apoptosis and hydrogen peroxide formation and restoration of mitochondrial glutathione levels (16). However, no in vivo studies have examined the effect of lycopene on alcohol-induced oxidative stress and inflammation, particularly with regard to dose responsiveness.
The goal of this study was to investigate potential interactions between alcohol feeding and lycopene supplementation in vivo and to determine the effects of escalating doses of lycopene on alcohol-induced hepatic biomarkers.
Materials and Methods
Animal study
Sixty male Fischer 344 rats (2 mo old, weighing 130–140 g) were obtained from Charles River Laboratories and were housed individually in cages in an American Association of Accreditation of Laboratory Animal Care accredited animal facility at the Human Nutrition Research Center on Aging at Tufts University. The rats were housed in a room with controlled temperature (68–72°F), humidity (45–55%), and light (12-h-light/-dark cycles). They were acclimatized over 1 wk on a nonpurified diet (Harlan Teklad LM-485 diet), the composition of which has been previously described (17). The rats were then randomly divided into 6 groups: control + placebo, control + low dose lycopene, control + high dose lycopene, alcohol + placebo, alcohol + low dose lycopene, and alcohol + high dose lycopene and were adapted to the nutritionally adequate Lieber-DeCarli liquid control and alcohol diets (Dyets) over a 2-wk period. The composition of the diets used has been previously published (18); both diets provided 18% of energy from protein and 35% from fat. The control diet provided 47% of energy from carbohydrates. In the ethanol diet, 36% of energy was provided by ethanol and the remaining 11% was provided by carbohydrates. The amount of alcohol ingested in rats fed the ethanol diet is approximately equal to an intake of 100 g/d of alcohol in a human consuming a 2000-kcal (8.4 MJ) diet (varies from 80 to 150 g/d of alcohol depending on energy intake). Neither the control nor the alcohol placebo diets contained lycopene. For dietary lycopene supplementation, water soluble lycopene beadlets, containing 10% wt:wt lycopene (BASF) were blended into the liquid diets [1.1 mg·kg body weight (BW)−1·d−1 or 3.3 mg·kg BW−1·d−1] and were fed to the rats. The placebo beadlets (BASF) contained the same constituents as the lycopene beadlets except the lycopene. The lower dose (1.1 mg·kg BW−1·d−1) is equivalent to a 15 mg/d intake in a 70-kg man based on our previous studies in ferrets (19), and the observed similarity between rats and ferrets in terms of lycopene absorption (20). This low dose is above the average intake of lycopene (9.4 ± 0.3 mg/d) in humans (21). The higher dose (3.3 mg·kg BW−1·d−1) is equivalent to a 45 mg/d intake in a 70-kg man and is achievable by using supplements. For the period of the study, the rats were group pair-fed to the alcohol + placebo group with their respective diets and were given ~70 mL/d of the diet. The diets were prepared twice per week and were stored at 4°C in opaque bottles to prevent degradation of the lycopene. BW were recorded once per week for the period of the study. After 11 wk of treatment, the rats were killed by terminal exsanguination under deep anesthesia. Blood and livers were collected for future analyses. This study protocol was reviewed and approved of by the Animal Care and Use Committee at the Human Nutrition Research Center on Aging at Tufts University.
HPLC analyses
Liver and plasma concentrations of all-trans and cis isomers (5-cis, 13-cis, and 9-cis) of lycopene were determined by HPLC. A Water’s separation system fitted with a C30 column was used to separate lycopene and its isomers. The HPLC system, the mobile phase, and the gradient procedures used have been previously described (22). Liver tissue (100 mg) or plasma (1 mL) was homogenized in 3 mL of saline and ethanol (1:2 ratio). Lycopene was then extracted from the samples using 5 mL of hexane and ether (1:1 ratio) by vortexing for 3 min, centrifuging at 2000 × g for 10 min at 4°C, and collecting the upper layer. Samples were extracted 3 times and were evaporated under nitrogen gas, after which they were reconstituted with 100 μL of ethanol and ether (2:1). A 50-μL sample of the final extract was injected into the system to measure lycopene isomer concentrations. In this HPLC system, 13-cis lycopene, 9-cis lycopene, all-trans lycopene, and 5-cis lycopene were eluted at 28.4, 32.0, 35.0, and 35.3 min, respectively. Retinyl acetate and echinenone were used as internal controls to determine the efficiency of extraction. Extraction efficiency > 80% was considered adequate to calculate concentrations of all-trans, 13-cis, 9-cis, and 5-cis lycopene. All procedures were conducted under red light.
Western blotting
Tissue homogenates were prepared from frozen liver tissue by homogenizing with ice-cold, whole-cell lysate buffer containing inhibitors. Protein concentrations were determined by spectrophotometry using Coomassie Blue and proteins in the tissue homogenates were resolved on a 10% SDS polyacrylamide gel, after which they were transferred onto nitrocellulose membranes. The membranes were blocked using 5% milk and were incubated with the CYP2E1 primary antibody (Chemicon) for 2 h at room temperature followed by the secondary antibody for 1 h at room temperature. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Chemicon) was used as a loading control. Results were quantified using a densitometer and are expressed as fold of the control group.
Histopathology
Liver sections were fixed in buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin for examination. The number of clusters of infiltrating mononuclear cells (foci of inflammation) on each slide (2 liver sections) was examined at both 10× and 40× magnification and counted by 2 independent investigators who were unaware of the treatment. The mean number of clusters in each group was compared.
Real-time PCR
mRNA levels of TNFα were determined by real-time quantitative PCR. Liver RNA was extracted using TriPure reagent (Roche Applied Science) according to the manufacturer’s instructions and cDNA was synthesized using random primer Moloney murine leukemia virus reverse transcriptase (Invitrogen). Primers for rat TNFα and GAPDH were designed using the Primer Express version 2.0 (Applied Biosystems) software. The sequences for TNFα (accession no. NM-012675.2) were as follows: sense, 5′-CCAGACCCTCACACTCA-GATCA-3′ and antisense, 5′-TCCGCTTGGTGGTTTGCTA-3′. The sequences for GAPDH (accession no. NM-017008.2) were as follows: sense, 5′-AGTGCCAGCCTCGTCTCATAG-3′ and antisense, 5′-CCTTG-ACTGTGCCGTTGAACT-3′. Real-time PCR was performed using the SYBR Green qPCR kit (Invitrogen) according to the manufacturer’s instructions on an Applied Biosystems 7000 sequence detection system. Gene expression was quantified relative to the values of the control group after adjusting for GAPDH by the 2−ΔΔCT method as described previously (23). Results were expressed as fold of the control group.
Statistical analyses
SPSS (version 14.0) and Graph Pad PRISM (version 3.0) software were used for statistical analyses. Samples identified as outliers by the SPSS statistical software were excluded from the analyses. Two-way ANOVA and trend analyses were used to analyze the data. After determining the presence of significant interactions between alcohol feeding and lycopene supplementation for hepatic lycopene concentrations and all the biomarkers assessed, we used 1-way ANOVA followed by Tukey’s honestly significant difference test to test for differences between means of multiple groups for results discussed in this study. Differences between means were considered significant at P < 0.05. Results are expressed as means ± SEM.
Results
Body and liver weights
After 11 wk of treatment, the alcohol-fed rats had significantly lower BW and liver weights than the control groups (Fig. 1) as previously documented (24). Lycopene supplementation at the 2 doses did not affect either BW or liver weight. The liver weight:BW ratio was constant between the groups (ranging from 0.029 to 0.031), indicating that decreased liver weight was consistent with the decreased BW in the alcohol-fed rats.
Figure 1.
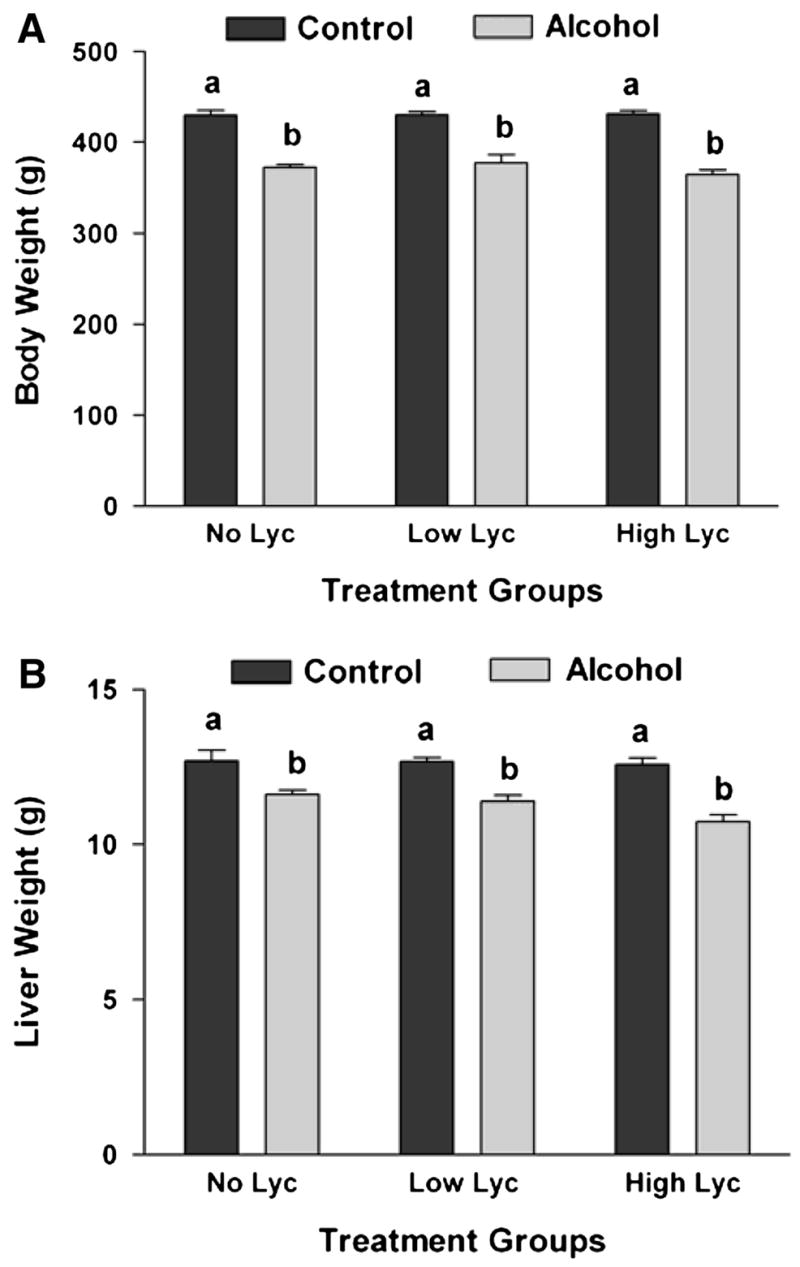
BW (A) and liver weight (B) of rats after 11 wk of feeding either control or alcohol diets with or without lycopene (Lyc) supplementation. Values are means ± SEM, n = 10. Bars without a common letter differ, P < 0.05.
Plasma and liver lycopene concentrations
Lycopene isomers were detected in both the plasma and liver of rats supplemented with lycopene but not in the rats supplemented with the placebo. Although we detected only the all-trans and 5-cis isomers in the plasma, the liver contained the all-trans, 5-cis, 9-cis, and 13-cis isomers of lycopene. In the plasma, we observed a significant dose-dependent increase in lycopene isomer concentrations in alcohol-fed rats (Table 1). In the liver, however, a similar increase was observed in both the control and the alcohol-fed rats (Table 1). Plasma lycopene concentrations increased 48 and 127% (low dose and high dose, respectively) in rats that were fed alcohol vs. rats fed the control diet with the same doses of lycopene. Similarly, total hepatic lycopene concentrations increased 74 and 157% (low dose and high dose, respectively) in rats that were fed alcohol vs. rats fed the control diet with the same doses of lycopene. Both plasma and hepatic lycopene isomer concentrations analyzed by 2-way ANOVA analyses showed that the interaction between alcohol feeding and lycopene supplementation was significant for all the lycopene isomers detected (P < 0.05; Table 1). This difference was supported by significant differences between means using 1-way ANOVA analyses and by adjusting for multiple comparisons using Tukey’s honestly significant difference test (Table 1).
TABLE 1.
Plasma and hepatic lycopene isomer concentrations in rats fed diets with or without alcohol and lycopene for 11 wk1–4
| Control
|
Alcohol
|
P of main effects
|
|||||
|---|---|---|---|---|---|---|---|
| Low Lyc | High Lyc | Low Lyc | High Lyc | Alcohol | Lyc | P of interaction | |
| Plasma, nmol/L | |||||||
| Total lycopene | 3.82 ± 0.67a | 5.37 ± 0.61a | 5.67 ± 0.66a | 12.22 ± 1.36b | <0.0001 | <0.0001 | 0.0068 |
| All-trans | 1.75 ± 0.36a | 2.27 ± 0.29a | 3.12 ± 0.48a | 6.45 ± 0.63b | <0.0001 | 0.0001 | 0.0038 |
| All-trans, % | 46 | 42 | 55 | 53 | |||
| 5-Cis | 2.07 ± 0.33a | 3.10 ± 0.34a | 2.55 ± 0.28a | 5.77 ± 0.79b | 0.0022 | <0.0001 | 0.0276 |
| 5-Cis, % | 54 | 58 | 45 | 47 | |||
| Liver, nmol/g | |||||||
| Total lycopene | 1.07 ± 0.10a | 2.84 ± 0.24b | 1.87 ± 0.18a | 7.30 ± 0.31c | <0.0001 | <0.0001 | <0.0001 |
| All-trans | 0.42 ± 0.05a | 1.47 ± 0.16b | 1.00 ± 0.10b | 4.50 ± 0.21c | <0.0001 | <0.0001 | <0.0001 |
| All-trans, % | 39 | 52 | 54 | 62 | |||
| 5-Cis | 0.34 ± 0.03a | 0.90 ± 0.08b | 0.51 ± 0.05a | 1.98 ± 0.09c | <0.0001 | <0.0001 | <0.0001 |
| 9-Cis | 0.15 ± 0.01a | 0.23 ± 0.01b | 0.15 ± 0.01a | 0.34 ± 0.01c | 0.0009 | <0.0001 | 0.0016 |
| 13-Cis | 0.16 ± 0.01a | 0.25 ± 0.01b | 0.20 ± 0.01a,b | 0.46 ± 0.02c | <0.0001 | <0.0001 | <0.0001 |
| Cis isomers, % | 61 | 49 | 46 | 38 | |||
Values are means ± SEM, n = 9–10. Means in a row without a common superscript differ, P < 0.05 (1-way ANOVA followed by Tukey’s test for differences between means).
Lycopene (Lyc) was not detected in placebo-fed rats with or without alcohol.
P-values of main effects and the interaction were determined by 2-way ANOVA.
One outlier from each of the alcohol + low Lyc and alcohol + high Lyc groups was excluded from final analyses.
Based on our HPLC analyses, the lycopene beadlet supplement contained 75 ± 3% of lycopene as the all-trans isomer and the remaining 25 ± 3% was present as cis isomers. In the livers of rats supplemented with lycopene, however, we detected a higher proportion of lycopene cis isomers (between 38 and 61%) compared with what was provided in the diet (Table 1). In the lycopene-supplemented control groups, the percentage of cis isomers of lycopene was greater than or almost equal to that of the all-trans isomers. In the alcohol-fed rats, however, the percentage of cis isomers was less than the all-trans isomers of lycopene and was lower than that detected in the control rats supplemented with the same dose of lycopene. In both the control and alcohol-fed groups, the percentage of lycopene cis isomers decreased with increasing lycopene dose in the liver but not in the plasma (Table 1). Apo-8′- and 12′-lycopenals, metabolites of lycopene, were previously detected in the livers of lycopene-supplemented rats (25). In our study, we did not detect apo-8′-, 10′-, or 12′-lycopenals in the liver tissue of rats after lycopene supplementation, although some unidentified lycopene metabolites were detected during HPLC analyses (retention time between 11.6 and 12.2 min).
Hepatic CYP2E1 protein
We assessed the effect of alcohol and lycopene supplementation on hepatic protein levels of CYP2E1. Alcohol feeding significantly increased expression of CYP2E1 compared with control rats (Fig. 2) as previously documented (26). Lycopene supplementation alone did not affect CYP2E1 expression. However, in the presence of alcohol, we observed a trend of increasing CYP2E1 protein expression with an increasing dose of lycopene (P-trend = 0.003). High dose lycopene with alcohol feeding increased hepatic CYP2E1 expression compared with rats fed alcohol alone without lycopene (P of interaction between alcohol feeding and lycopene supplementation = 0.005).
Figure 2.
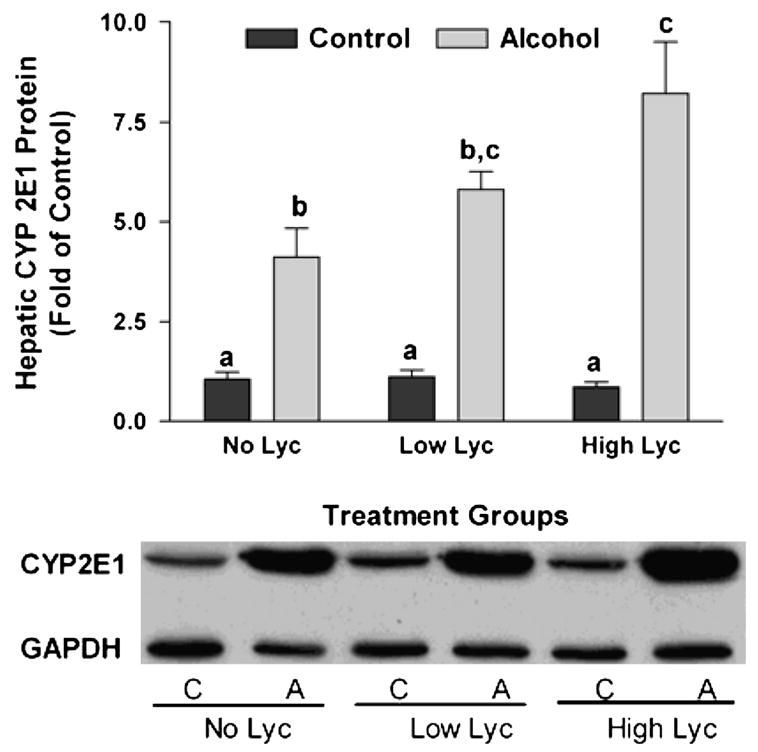
Induction of hepatic CYP2E1 protein in rats after 11 wk of feeding either control (C) or alcohol (A) diets with or without lycopene (Lyc) supplementation. Representative western blot of CYP2E1 and GAPDH protein expression (inset). Values are means ± SEM, n = 10. Bars without a common letter differ, P < 0.05.
Hepatic inflammation
Because chronic alcohol intake is associated with significantly increased hepatic TNFα expression and inflammation (27), we assessed hepatic levels of TNFα mRNA (Fig. 3). We did not detect any significant increase of hepatic TNFα mRNA in alcohol-fed rats supplemented with placebo or low dose lycopene vs. control rats treated similarly. However, lycopene supplementation at the high dose induced the expression of TNFα in the liver of alcohol-fed rats compared with the other groups (P of interaction between alcohol feeding and lycopene supplementation = 0.032). Lycopene supplementation alone, in the absence of alcohol, at either dose did not affect TNFα expression. We further investigated the effects of lycopene supplementation on hepatic infiltration by mononuclear inflammatory cells (Fig. 4). Alcohol feeding or lycopene supplementation alone did not affect the incidence of hepatic inflammatory foci. However, in the presence of alcohol, high dose lycopene supplementation was associated with an increased incidence of inflammatory foci in the liver (P of interaction between alcohol feeding and lycopene supplementation = 0.036).
Figure 3.
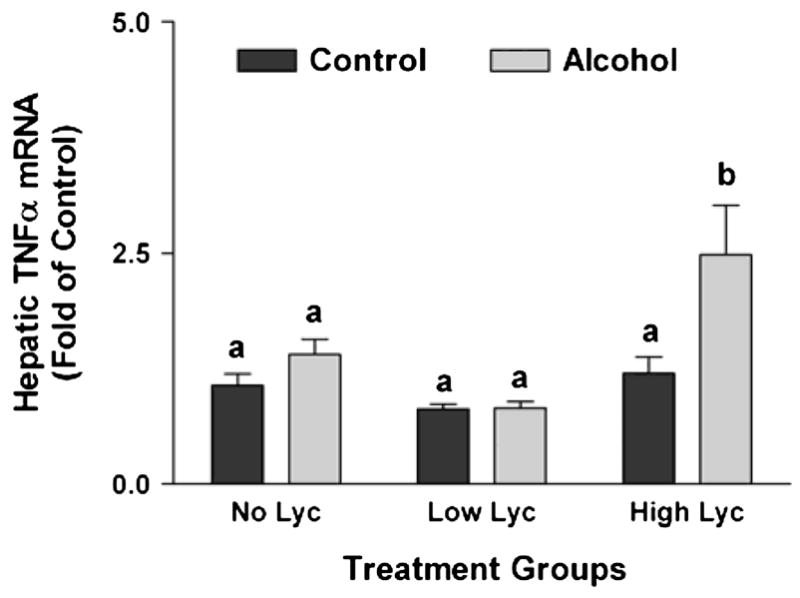
Expression of TNFα mRNA in the livers of rats after 11 wk of feeding either control or alcohol diets with or without lycopene (Lyc) supplementation. Values are means ± SEM, n = 10. Bars without a common letter differ, P < 0.05.
Figure 4.
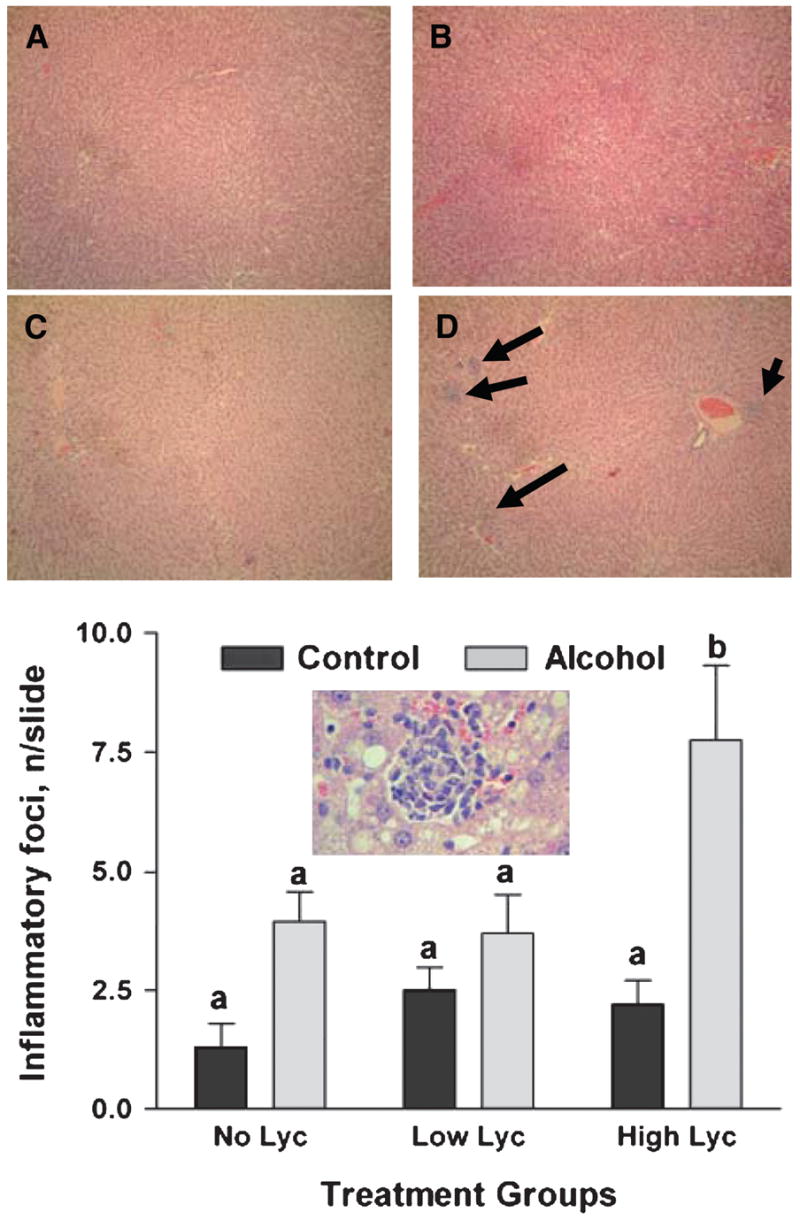
Incidence of inflammatory foci in the livers of rats after 11 wk of feeding either control or alcohol diets with or without lycopene (Lyc) supplementation. Values are means ± SEM, n = 10. Bars without a common letter differ, P < 0.05. Upper panel: Representative image of hepatic inflammatory foci (arrows) from the control (A), control + high lycopene (B), alcohol (C), and alcohol + high dose lycopene group (D) at 10× magnification and 40× magnification (inset).
Discussion
This study clearly shows an interaction between in vivo lycopene supplementation and alcohol intake in a rat model and indicates the need for caution among alcohol drinkers who also consume high amounts of supplementary lycopene. In the present study, we tested 2 doses of lycopene (1.1 and 3.3 mg·kg BW−1·d−1), which are approximately equivalent to 15 mg and 45 mg/d lycopene in a 70-kg adult man. The average American diet provides ~9.4 mg/d of lycopene (21); therefore, both the experimental doses were higher than the usual intake. However, these doses are currently present in dietary supplements and are being tested in prostate cancer clinical trials (28–30), emphasizing the importance of our observations from the current animal study. Furthermore, the hepatic lycopene concentrations that we detected (1.07–7.30 nmol/g) in the rats are within the range normally seen in humans (0.1–20.7 nmol/g) (31,32), indicating that the interaction between chronic alcohol intake and supplementary lycopene and their combined effect on hepatic biomarkers takes place at physiologically relevant tissue concentrations of lycopene. Plasma concentrations in the rats (3.82–12.22 nmol/L), however, were much lower than those observed in humans (260–900 nmol/L) (33).
Alcohol-fed rats accumulated more lycopene than control animals supplemented with the same dose of lycopene. Interestingly, chronic alcohol intake is also associated with increased plasma (34) and hepatic concentrations (35) of another carotenoid, β-carotene. The accumulation of β-carotene in the liver has been attributed to the inhibition of its catabolism to retinoids by chronic alcohol intake (36). In the case of lycopene, however, the mechanism(s) involved is unknown at present. Multiple mechanisms could be involved; e.g. alcohol intake could increase the solubility and/or bioavailability of lycopene for absorption, or it could decrease lycopene catabolism. Recently, we have shown that the carotene 9′,10′-oxygenase can cleave cis isomers of lycopene into apo-10′-lycopenoid metabolites (37) and other investigators have reported apo-8′-and 12′-lycopenals in rat livers after lycopene supplementation (25). However, we did not detect any apolycopenoids in the livers of rats with lycopene supplementation and observed no changes in hepatic carotene 9′,10′-oxygenase protein levels or in the formation of the unidentified polar metabolites in alcohol-fed rats vs. the control rats (S. Veeramachaneni and X-D. Wang, unpublished data). It is, therefore, unlikely that alcohol altered lycopene degradation, suggesting that the increased concentrations of lycopene in vivo might result from enhanced absorption in the gut due to alcohol ingestion. However, this hypothesis needs to be addressed in future studies using intestinal perfusion models as previously described (38).
Though the lycopene beadlet predominantly contained all-trans lycopene, the rat livers had a higher accumulation of cis lycopene compared with that in the diet. This observation is similar to what is normally seen in humans, where higher concentrations of lycopene cis isomers are detected in the body although dietary lycopene is predominantly in the all-trans form (39). In the current study, the percentage of cis isomers of lycopene decreased in the livers, but not in the plasma, with increasing lycopene dose (Table 1) in both the control and the alcohol-fed groups. This suggests that the ratio of cis/trans lycopene isomers in the circulation may not be reflective of isomerization of the all-trans isomer to cis isomers in the liver. Interestingly, the alcohol-fed rats had a lower percentage of cis lycopene accumulation than the control rats fed the same dose of lycopene. It is unknown whether this decrease in cis lycopene accumulation is a result of either decreased absorption of cis isomers in the gut or decreased isomerization of the all-trans isomer to cis isomers by chronic alcohol ingestion.
Chronic alcohol ingestion can induce the levels of CYP2E1 (26), which plays an important role in metabolizing excess amounts of alcohol in the liver via the microsomal ethanol oxidizing system (26). During this process, CYP2E1 generates several reactive oxygen species, including hydrogen peroxide and superoxide, leading to increased oxidative stress (1,3,40). Reactive oxygen species generated by CYP2E1 induce mitochondrial damage and induce cell membrane damage by increasing lipid peroxidation and lipid aldehyde formation. Alcohol regulates CYP2E1 levels both by inducing its transcription (41) and by stabilizing the protein against degradation (42,43). In the present study, in addition to the expected induction of CYP2E1 in response to alcohol treatment, we observed a trend of increased CYP2E1 protein with increasing lycopene dose in the alcohol-fed rats. This increase was significantly greater when lycopene was supplemented at the high dose in rats compared with the rats that were supplemented with the placebo. Although the mechanisms for these results are currently unknown, our observation suggests that lycopene supplementation at a high dose may potentiate the harmful effects of excessive alcohol intake. This notion is further supported by the following observations. First, it has been previously documented that chronic alcohol intake was associated with increased incidence of inflammatory foci in the livers of alcohol-fed rats compared with control rats (44,45). Although we observed no significant increase in inflammatory foci with alcohol feeding, we found that rats fed both alcohol and high dose lycopene had a significantly higher incidence of hepatic inflammatory foci compared with the other groups. Second, previous evidence has shown that alcohol induces the expression of TNFα in rats (27), and patients with alcoholic liver disease have increased levels of several proinflammatory cytokines, including TNFα (46). In our model, hepatic TNFα mRNA did not differ between the control and alcohol-fed groups supplemented with placebo. This is in agreement with earlier in vivo data showing no significant differences in TNFα expression in the livers of mice fed control or alcohol Lieber-DeCarli liquid diets (47). However, our observations in Fischer rats are contradictory to findings from another study using alcohol-fed Sprague-Dawley rats (27). Although the exact reasons for the differences in response to alcohol feeding are not known, it is possible that different strains of rats react differently to chronic alcohol feeding. Rats fed both alcohol and high dose lycopene had significantly higher expression of TNFα compared with all other groups. Interestingly, rats fed alcohol and low dose lycopene (equal to one-third of the dose supplemented to the high dose groups) had no significant induction of TNFα expression. Lycopene has been shown to act as a prooxidant or an antioxidant depending on the dose at which it is administered (48,49). In this study, although neither dose was protective, lycopene supplementation at the higher dose had a more pronounced effect on alcohol-related changes compared with the lower dose. Further studies are needed to understand both the interaction between alcohol and lycopene and the mechanisms by which high doses of lycopene, in the presence of alcohol, modulate hepatic inflammation and CYP2E1 expression.
Acknowledgments
We thank Kang-Quan Hu for assistance with HPLC analyses and Senait Assefa for assistance with animal care and handling.
Footnotes
Supported by grant R01CA104932 and R01AA12682 from the NIH, Bethesda, MD and by the USDA under agreement no. 1950-51000-064. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of the USDA.
Author disclosures: S. Veeramachaneni, L. M. Ausman, R. M. Russell, S. W. Choi, and X.-D. Wang, no conflicts of interest.
Abbreviations used: BW, body weight; CYP2E1, cytochrome P4502E1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; TNFα, tumor necrosis factor α.
Literature Cited
- 1.Dey A, Cederbaum AI. Alcohol and oxidative liver injury. Hepatology. 2006;43:S63–74. doi: 10.1002/hep.20957. [DOI] [PubMed] [Google Scholar]
- 2.Zima T, Kalousova M. Oxidative stress and signal transduction pathways in alcoholic liver disease. Alcohol Clin Exp Res. 2005;29:S110–5. doi: 10.1097/01.alc.0000189288.30358.4b. [DOI] [PubMed] [Google Scholar]
- 3.Cederbaum AI. CYP2E1: biochemical and toxicological aspects and role in alcohol-induced liver injury. Mt Sinai J Med. 2006;73:657–72. [PubMed] [Google Scholar]
- 4.Wu D, Cederbaum AI. Alcohol, oxidative stress, and free radical damage. Alcohol Res Health. 2003;27:277–84. [PMC free article] [PubMed] [Google Scholar]
- 5.Kessova IG, Ho YS, Thung S, Cederbaum AI. Alcohol-induced liver injury in mice lacking Cu, Zn-superoxide dismutase. Hepatology. 2003;38:1136–45. doi: 10.1053/jhep.2003.50450. [DOI] [PubMed] [Google Scholar]
- 6.Nanji AA, Khettry U, Sadrzadeh SM. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease) Proc Soc Exp Biol Med. 1994;205:243–7. doi: 10.3181/00379727-205-43703. [DOI] [PubMed] [Google Scholar]
- 7.Kaviarasan S, Ramamurthy N, Gunasekaran P, Varalakshmi E, Anuradha CV. Epigallocatechin-3-gallate(-) protects Chang liver cells against ethanol-induced cytotoxicity and apoptosis. Basic Clin Pharmacol Toxicol. 2007;100:151–6. doi: 10.1111/j.1742-7843.2006.00036.x. [DOI] [PubMed] [Google Scholar]
- 8.Luczaj W, Siemieniuk E, Roszkowska-Jakimiec W, Skrzydlewska E. Protective effect of black tea against ethanol-induced oxidative modifications of liver proteins and lipids. J Stud Alcohol. 2006;67:510–8. doi: 10.15288/jsa.2006.67.510. [DOI] [PubMed] [Google Scholar]
- 9.Sadrzadeh SM, Meydani M, Khettry U, Nanji AA. High-dose vitamin E supplementation has no effect on ethanol-induced pathological liver injury. J Pharmacol Exp Ther. 1995;273:455–60. [PubMed] [Google Scholar]
- 10.Wertz K, Siler U, Goralczyk R. Lycopene: modes of action to promote prostate health. Arch Biochem Biophys. 2004;430:127–34. doi: 10.1016/j.abb.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 11.Kim GY, Kim JH, Ahn SC, Lee HJ, Moon DO, Lee CM, Park YM. Lycopene suppresses the lipopolysaccharide-induced phenotypic and functional maturation of murine dendritic cells through inhibition of mitogen-activated protein kinases and nuclear factor-kappaB. Immunology. 2004;113:203–11. doi: 10.1111/j.1365-2567.2004.01945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siler U, Barella L, Spitzer V, Schnorr J, Lein M, Goralczyk R, Wertz K. Lycopene and vitamin E interfere with autocrine/paracrine loops in the Dunning prostate cancer model. FASEB J. 2004;18:1019–21. doi: 10.1096/fj.03-1116fje. [DOI] [PubMed] [Google Scholar]
- 13.Reifen R, Nissenkorn A, Matas Z, Bujanover Y. 5-ASA and lycopene decrease the oxidative stress and inflammation induced by iron in rats with colitis. J Gastroenterol. 2004;39:514–9. doi: 10.1007/s00535-003-1336-z. [DOI] [PubMed] [Google Scholar]
- 14.Riso P, Visioli F, Grande S, Guarnieri S, Gardana C, Simonetti P, Porrini M. Effect of a tomato-based drink on markers of inflammation, immunomodulation, and oxidative stress. J Agric Food Chem. 2006;54:2563–6. doi: 10.1021/jf053033c. [DOI] [PubMed] [Google Scholar]
- 15.Sugiura M, Nakamura M, Ikoma Y, Yano M, Ogawa K, Matsumoto H, Kato M, Ohshima M, Nagao A. High serum carotenoids are inversely associated with serum gamma-glutamyltransferase in alcohol drinkers within normal liver function. J Epidemiol. 2005;15:180–6. doi: 10.2188/jea.15.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Y, Leo MA, Lieber CS. Lycopene attenuates alcoholic apoptosis in HepG2 cells expressing CYP2E1. Biochem Biophys Res Commun. 2003;308:614–8. doi: 10.1016/s0006-291x(03)01435-9. [DOI] [PubMed] [Google Scholar]
- 17.Baez-Saldana A, Ortega E. Biotin deficiency blocks thymocyte maturation, accelerates thymus involution, and decreases nose-rump length in mice. J Nutr. 2004;134:1970–7. doi: 10.1093/jn/134.8.1970. [DOI] [PubMed] [Google Scholar]
- 18.Kim CI, Leo MA, Lowe N, Lieber CS. Differential effects of retinoids and chronic ethanol consumption on membranes in rats. J Nutr. 1988;118:1097–103. doi: 10.1093/jn/118.9.1097. [DOI] [PubMed] [Google Scholar]
- 19.Liu C, Lian F, Smith DE, Russell RM, Wang XD. Lycopene supplementation inhibits lung squamous metaplasia and induces apoptosis via up-regulating insulin-like growth factor-binding protein 3 in cigarette smoke-exposed ferrets. Cancer Res. 2003;63:3138–44. [PubMed] [Google Scholar]
- 20.Ferreira AL, Yeum KJ, Liu C, Smith D, Krinsky NI, Wang XD, Russell RM. Tissue distribution of lycopene in ferrets and rats after lycopene supplementation. J Nutr. 2000;130:1256–60. doi: 10.1093/jn/130.5.1256. [DOI] [PubMed] [Google Scholar]
- 21.Institute of Medicine. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: 2000. [Google Scholar]
- 22.Liu C, Russell RM, Wang XD. Lycopene supplementation prevents smoke-induced changes in p53, p53 phosphorylation, cell proliferation, and apoptosis in the gastric mucosa of ferrets. J Nutr. 2006;136:106–11. doi: 10.1093/jn/136.1.106. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-[Delta][Delta]CT method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Lian F, Chung J, Russell RM, Wang X-D. Alcohol-reduced plasma IGF-I levels and hepatic IGF-I expression can be partially restored by retinoic acid supplementation in rats. J Nutr. 2004;134:2953–6. doi: 10.1093/jn/134.11.2953. [DOI] [PubMed] [Google Scholar]
- 25.Gajic M, Zaripheh S, Sun F, Erdman JW., Jr Apo-8′-lycopenal and apo-12′-lycopenal are metabolic products of lycopene in rat liver. J Nutr. 2006;136:1552–7. doi: 10.1093/jn/136.6.1552. [DOI] [PubMed] [Google Scholar]
- 26.Lieber CS. Microsomal ethanol-oxidizing system (MEOS): the first 30 years (1968–1998): a review. Alcohol Clin Exp Res. 1999;23:991–1007. [PubMed] [Google Scholar]
- 27.Lin HZ, Yang SQ, Zeldin G, Diehl AM. Chronic ethanol consumption induces the production of tumor necrosis factor-alpha and related cytokines in liver and adipose tissue. Alcohol Clin Exp Res. 1998;22:S231–7. doi: 10.1097/00000374-199805001-00004. [DOI] [PubMed] [Google Scholar]
- 28.Clark PE, Hall MC, Borden LS, Jr, Miller AA, Hu JJ, Lee WR, Stindt D, D’Agostino R, Jr, Lovato J, et al. Phase I–II prospective dose-escalating trial of lycopene in patients with biochemical relapse of prostate cancer after definitive local therapy. Urology. 2006;67:1257–61. doi: 10.1016/j.urology.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 29.Vaishampayan U, Hussain M, Banerjee M, Seren S, Sarkar FH, Fontana J, Forman JD, Cher ML, Powell I, et al. Lycopene and soy isoflavones in the treatment of prostate cancer. Nutr Cancer. 2007;59:1–7. doi: 10.1080/01635580701413934. [DOI] [PubMed] [Google Scholar]
- 30.Jatoi A, Burch P, Hillman D, Vanyo JM, Dakhil S, Nikcevich D, Rowland K, Morton R, Flynn PJ, et al. A tomato-based, lycopene-containing intervention for androgen-independent prostate cancer: results of a Phase II study from the North Central Cancer Treatment Group. Urology. 2007;69:289–94. doi: 10.1016/j.urology.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 31.Schmitz HH, Poor CL, Wellman RB, Erdman JW., Jr Concentrations of selected carotenoids and vitamin A in human liver, kidney and lung tissue. J Nutr. 1991;121:1613–21. doi: 10.1093/jn/121.10.1613. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan LA, Lau JM, Stein EA. Carotenoid composition, concentrations, and relationships in various human organs. Clin Physiol Biochem. 1990;8:1–10. [PubMed] [Google Scholar]
- 33.Gerster H. The potential role of lycopene for human health. J Am Coll Nutr. 1997;16:109–26. doi: 10.1080/07315724.1997.10718661. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed S, Leo MA, Lieber CS. Interactions between alcohol and beta-carotene in patients with alcoholic liver disease. Am J Clin Nutr. 1994;60:430–6. doi: 10.1093/ajcn/60.3.430. [DOI] [PubMed] [Google Scholar]
- 35.Leo MA, Aleynik SI, Aleynik MK, Lieber CS. β-Carotene beadlets potentiate hepatotoxicity of alcohol. Am J Clin Nutr. 1997;66:1461–9. doi: 10.1093/ajcn/66.6.1461. [DOI] [PubMed] [Google Scholar]
- 36.Leo MA, Lieber CS. Alcohol, vitamin A, and beta-carotene: adverse interactions, including hepatotoxicity and carcinogenicity. Am J Clin Nutr. 1999;69:1071–85. doi: 10.1093/ajcn/69.6.1071. [DOI] [PubMed] [Google Scholar]
- 37.Hu KQ, Liu C, Ernst H, Krinsky NI, Russell RM, Wang XD. The biochemical characterization of ferret carotene-9′,10′-monooxygenase catalyzing cleavage of carotenoids in vitro and in vivo. J Biol Chem. 2006;281:19327–38. doi: 10.1074/jbc.M512095200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang XD, Krinsky NI, Marini RP, Tang G, Yu J, Hurley R, Fox JG, Russell RM. Intestinal uptake and lymphatic absorption of beta-carotene in ferrets: a model for human beta-carotene metabolism. Am J Physiol. 1992;263:G480–6. doi: 10.1152/ajpgi.1992.263.4.G480. [DOI] [PubMed] [Google Scholar]
- 39.Boileau TW, Boileau AC, Erdman JW., Jr Bioavailability of all-trans and cis-isomers of lycopene. Exp Biol Med (Maywood) 2002;227:914–9. doi: 10.1177/153537020222701012. [DOI] [PubMed] [Google Scholar]
- 40.Caro AA, Cederbaum AI. Oxidative stress, toxicology, and pharmacology of CYP2E1. Annu Rev Pharmacol Toxicol. 2004;44:27–42. doi: 10.1146/annurev.pharmtox.44.101802.121704. [DOI] [PubMed] [Google Scholar]
- 41.Ronis MJ, Huang J, Crouch J, Mercado C, Irby D, Valentine CR, Lumpkin CK, Ingelman-Sundberg M, Badger TM. Cytochrome P450 CYP 2E1 induction during chronic alcohol exposure occurs by a two-step mechanism associated with blood alcohol concentrations in rats. J Pharmacol Exp Ther. 1993;264:944–50. [PubMed] [Google Scholar]
- 42.Roberts BJ, Song BJ, Soh Y, Park SS, Shoaf SE. Ethanol induces CYP2E1 by protein stabilization. Role of ubiquitin conjugation in the rapid degradation of CYP2E1. J Biol Chem. 1995;270:29632–5. doi: 10.1074/jbc.270.50.29632. [DOI] [PubMed] [Google Scholar]
- 43.Roberts BJ, Shoaf SE, Jeong KS, Song BJ. Induction of CYP2E1 in liver, kidney, brain and intestine during chronic ethanol administration and withdrawal: evidence that CYP2E1 possesses a rapid phase half-life of 6 hours or less. Biochem Biophys Res Commun. 1994;205:1064–71. doi: 10.1006/bbrc.1994.2774. [DOI] [PubMed] [Google Scholar]
- 44.Sampey BP, Korourian S, Ronis MJ, Badger TM, Petersen DR. Immunohistochemical characterization of hepatic malondialdehyde and 4-hydroxynonenal modified proteins during early stages of ethanol-induced liver injury. Alcohol Clin Exp Res. 2003;27:1015–22. doi: 10.1097/01.ALC.0000071928.16732.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jarvelainen HA, Fang C, Ingelman-Sundberg M, Lindros KO. Effect of chronic coadministration of endotoxin and ethanol on rat liver pathology and proinflammatory and anti-inflammatory cytokines. Hepatology. 1999;29:1503–10. doi: 10.1002/hep.510290508. [DOI] [PubMed] [Google Scholar]
- 46.Hanck C, Glatzel M, Singer MV, Rossol S. Gene expression of TNF-receptors in peripheral blood mononuclear cells of patients with alcoholic cirrhosis. J Hepatol. 2000;32:51–7. doi: 10.1016/s0168-8278(00)80189-0. [DOI] [PubMed] [Google Scholar]
- 47.Fleming S, Toratani S, Shea-Donohue T, Kashiwabara Y, Vogel SN, Metcalf ES. Pro- and anti-inflammatory gene expression in the murine small intestine and liver after chronic exposure to alcohol. Alcohol Clin Exp Res. 2001;25:579–89. [PubMed] [Google Scholar]
- 48.Lowe GM, Booth LA, Young AJ, Bilton RF. Lycopene and beta-carotene protect against oxidative damage in HT29 cells at low concentrations but rapidly lose this capacity at higher doses. Free Radic Res. 1999;30:141–51. doi: 10.1080/10715769900300151. [DOI] [PubMed] [Google Scholar]
- 49.Yeh S, Hu M. Antioxidant and pro-oxidant effects of lycopene in comparison with beta-carotene on oxidant-induced damage in Hs68 cells. J Nutr Biochem. 2000;11:548–54. doi: 10.1016/s0955-2863(00)00117-0. [DOI] [PubMed] [Google Scholar]


